Bacteriophages for the Treatment of Graft Infections in Cardiovascular Medicine
Abstract
:1. Introduction
2. Pathogenic Bacteria and Vascular Graft Infections
3. Infections in Ventricular Assist Device (VAD) Therapy
4. Endocarditis in Patients with Prosthetic Heart Valves (PHV)
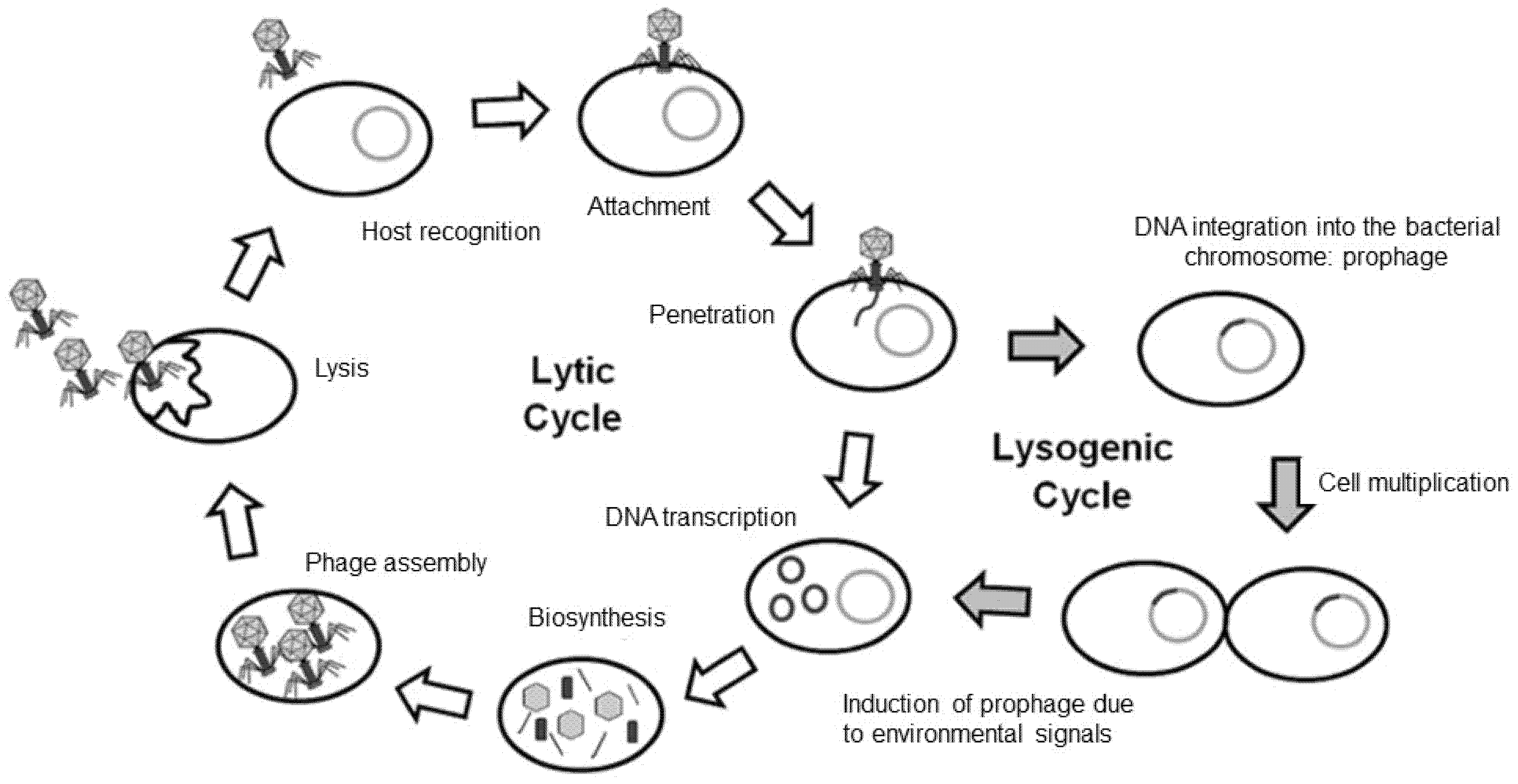
5. Bacteriophage Therapy
6. Challenges to Phage Therapy
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations and Acronyms
| AMR | Antimicrobial resistance |
| CT | Computed tomography |
| EVAR | Endovascular aortic repair |
| IE | Infective endocarditis |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| PET | Positron emission tomography |
| PHV | Prosthetic heart valve |
| SAVR | Surgical aortic valve replacement |
| TAVR | Transcatheter aortic valve replacement |
| TEVAR | Thoracic endovascular aortic repair |
| VGI | Vascular graft infection |
| VAD | Ventricular assist device |
References
- Mussa, F.F.; Hedayati, N.; Zhou, W.; El-Sayed, H.F.; Kougias, P.; Darouiche, R.O.; Lin, P.H. Prevention and treatment of aortic graft infection. Expert Rev. Anti-Infect. Ther. 2007, 5, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Gastmeier, P.; Schumacher, M.; Daschner, F.; Rüden, H. An analysis of two prevalence surveys of nosocomial infection in German intensive care units. J. Hosp. Infect. 1997, 35, 97–105. [Google Scholar] [CrossRef]
- Kilic, A.; Arnaoutakis, D.J.; Reifsnyder, T.; Black, J.H.; Abularrage, C.J.; Perler, B.A.; Lum, Y.W. Management of infected vascular grafts. Vasc. Med. 2016, 21, 53–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wienhold, S.-M.; Lienau, J.; Witzenrath, M. Towards Inhaled Phage Therapy in Western Europe. Viruses 2019, 11, 295. [Google Scholar] [CrossRef] [Green Version]
- Lorentzen, J.E.; Nielsen, O.M.; Arendrup, H.; Kimose, H.H.; Bille, S.; Andersen, J.; Jensen, C.H.; Jacobsen, F.; Røder, O.C. Vascular graft infection: An analysis of sixty-two graft infections in 2411 consecutively implanted synthetic vascular grafts. Surgery 1985, 98, 81–86. [Google Scholar] [PubMed]
- Stewart, P.S. Mechanisms of antibiotic resistance in bacterial biofilms. Int. J. Med. Microbiol. 2002, 292, 107–113. [Google Scholar] [CrossRef]
- Reisner, A.; Høiby, N.; Tolker-Nielsen, T.; Molin, S. Microbial pathogenesis and biofilm development. Contrib. Microbiol. 2005, 12, 114–131. [Google Scholar] [CrossRef] [PubMed]
- Hall-Stoodley, L.; Stoodley, P. Biofilm formation and dispersal and the transmission of human pathogens. Trends Microbiol. 2005, 13, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Döring, G.; Conway, S.P.; Heijerman, H.G.; Hodson, M.E.; Høiby, N.; Smyth, A.; Touw, D.J. Antibiotic therapy against Pseudomonas aeruginosa in cystic fibrosis: A European consensus. Eur. Respir. J. 2000, 16, 749–767. [Google Scholar] [CrossRef]
- Herten, M.; Idelevich, E.A.; Sielker, S.; Becker, K.; Scherzinger, A.S.; Osada, N.; Torsello, G.B.; Bisdas, T. Vascular Graft Impregnation with Antibiotics: The Influence of High Concentrations of Rifampin, Vancomycin, Daptomycin, and Bacteriophage Endolysin HY-133 on Viability of Vascular Cells. Med. Sci. Monit. Basic Res. 2017, 23, 250–257. [Google Scholar] [CrossRef] [Green Version]
- Kirklin, J.K.; Pagani, F.D.; Kormos, R.L.; Stevenson, L.W.; Blume, E.D.; Myers, S.L.; Miller, M.A.; Baldwin, J.T.; Young, J.B.; Naftel, D.C. Eighth annual INTERMACS report: Special focus on framing the impact of adverse events. J. Heart Lung Transplant. 2017, 36, 1080–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, J.G.; Pagani, F.D.; Tatooles, A.J.; Bhat, G.; Slaughter, M.S.; Birks, E.J.; Boyce, S.W.; Najjar, S.S.; Jeevanandam, V.; Anderson, A.S.; et al. Intrapericardial Left Ventricular Assist Device for Advanced Heart Failure. N. Engl. J. Med. 2017, 376, 451–460. [Google Scholar] [CrossRef]
- Gummert, J.F.; Haverich, A.; Schmitto, J.D.; Potapov, E.; Schramm, R.; Falk, V. Permanent Implantable Cardiac Support Systems. Dtsch. Arztebl. Int. 2019, 116, 843–848. [Google Scholar] [CrossRef] [PubMed]
- O’Horo, J.C.; Abu Saleh, O.M.; Stulak, J.M.; Wilhelm, M.P.; Baddour, L.M.; Rizwan Sohail, M. Left Ventricular Assist Device Infections: A Systematic Review. ASAIO J. 2018, 64, 287–294. [Google Scholar] [CrossRef]
- Gordon, R.J.; Weinberg, A.D.; Pagani, F.D.; Slaughter, M.S.; Pappas, P.S.; Naka, Y.; Goldstein, D.J.; Dembitsky, W.P.; Giacalone, J.C.; Ferrante, J.; et al. Prospective, multicenter study of ventricular assist device infections. Circulation 2013, 127, 691–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Feller, E.D.; Chen, W.; Liang, Y.; Dilsizian, V. FDG PET/CT for Early Detection and Localization of Left Ventricular Assist Device Infection: Impact on Patient Management and Outcome. JACC Cardiovasc. Imaging 2019, 12, 722–729. [Google Scholar] [CrossRef]
- Potapov, E.V.; Antonides, C.; Crespo-Leiro, M.G.; Combes, A.; Färber, G.; Hannan, M.M.; Kukucka, M.; de Jonge, N.; Loforte, A.; Lund, L.H.; et al. 2019 EACTS Expert Consensus on long-term mechanical circulatory support. Eur. J. Cardio Thorac. Surg. 2019, 56, 230–270. [Google Scholar] [CrossRef]
- Rojas, S.V.; Haverich, A. Kardiales Pumpversagen: Herzunterstützungssysteme und Herztransplantation. J. Chir. 2019, 90, 110–116. [Google Scholar] [CrossRef]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.-P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2015, 36, 3075–3128. [Google Scholar] [CrossRef]
- Baddour, L.M.; Wilson, W.R.; Bayer, A.S.; Fowler, V.G., Jr.; Tleyjeh, I.M.; Rybak, M.J.; Barsic, B.; Lockhart, P.B.; Gewitz, M.H.; Levison, M.E.; et al. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation 2015, 132, 1435–1486. [Google Scholar] [CrossRef]
- Østergaard, L.; Valeur, N.; Wang, A.; Bundgaard, H.; Aslam, M.; Gislason, G.; Torp-Pedersen, C.; Bruun, N.E.; Søndergaard, L.; Køber, L.; et al. Incidence of infective endocarditis in patients considered at moderate risk. Eur. Heart J. 2019, 40, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Butt, J.H.; Ihlemann, N.; de Backer, O.; Søndergaard, L.; Havers-Borgersen, E.; Gislason, G.H.; Torp-Pedersen, C.; Køber, L.; Fosbøl, E.L. Long-Term Risk of Infective Endocarditis After Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2019, 73, 1646–1655. [Google Scholar] [CrossRef]
- Tinica, G.; Tarus, A.; Enache, M.; Artene, B.; Rotaru, I.; Bacusca, A.; Burlacu, A. Infective endocarditis after TAVI: A meta-analysis and systematic review of epidemiology, risk factors and clinical consequences. Rev. Cardiovasc. Med. 2020, 21, 263–274. [Google Scholar] [CrossRef]
- Legout, L.; Sarraz-Bournet, B.; D’Elia, P.V.; Devos, P.; Pasquet, A.; Caillaux, M.; Wallet, F.; Yazdanpanah, Y.; Senneville, E.; Haulon, S.; et al. Characteristics and prognosis in patients with prosthetic vascular graft infection: A prospective observational cohort study. Clin. Microbiol. Infect. 2012, 18, 352–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiraev, T.; Barrett, S.; Heywood, S.; Mirza, W.; Hunter-Dickson, M.; Bradshaw, C.; Hardman, D.; Neilson, W.; Bradshaw, S. Incidence, Management, and Outcomes of Aortic Graft Infection. Ann. Vasc. Surg. 2019, 59, 73–83. [Google Scholar] [CrossRef]
- Cruse, P.J.E.; Foord, R. A five-year prospective study of 23,649 surgical wounds. Arch. Surg. 1973, 107, 206–210. [Google Scholar] [CrossRef]
- Diener, H.; Larena-Avellaneda, A.; Debus, E.S. Postoperative Komplikationen in der Gefässchirurgie. J. Chir. 2009, 80, 814–826. [Google Scholar] [CrossRef] [PubMed]
- Yashar, J.J.; Weyman, A.K.; Burnard, R.J.; Yashar, J. Survival and limb salvage in patients with infected arterial prostheses. Am. J. Surg. 1978, 135, 499–504. [Google Scholar] [CrossRef]
- Szilagyi, D.E.; Smith, R.F.; Elliott, J.P.; Vrandecic, M.P. Infection in arterial reconstruction with synthetic grafts. Ann. Surg. 1972, 176, 321–323. [Google Scholar] [CrossRef]
- Jamieson, G.G.; DeWeese, J.A.; Rob, C.G. Infected arterial grafts. Ann. Surg. 1975, 181, 850–852. [Google Scholar] [CrossRef]
- Jones, L.; Braithwaite, B.D.; Davies, B.; Heather, B.P.; Earnshaw, J.J. Mechanism of late prosthetic vascular graft infection. Cardiovasc. Surg. 1997, 5, 486–489. [Google Scholar] [CrossRef]
- Ilgenfritz, F.M.; Jordan, F.T. Microbiological monitoring of aortic aneurysm wall and contents during aneurysmectomy. Arch. Surg. 1988, 123, 506–508. [Google Scholar] [CrossRef] [PubMed]
- Batt, M.; Magne, J.-L.; Alric, P.; Muzj, A.; Ruotolo, C.; Ljungstrom, K.-G.; Garcia-Casas, R.; Simms, M. In situ revascularization with silver-coated polyester grafts to treat aortic infection: Early and midterm results. J. Vasc. Surg. 2003, 38, 983–989. [Google Scholar] [CrossRef] [Green Version]
- Koshiko, S.; Sasajima, T.; Muraki, S.; Azuma, N.; Yamazaki, K.; Chiba, K.; Tachibana, M.; Inaba, M. Limitations in the use of rifampicin-gelatin grafts against virulent organisms. J. Vasc. Surg. 2002, 35, 779–785. [Google Scholar] [CrossRef] [Green Version]
- Vicaretti, M.; Hawthorne, W.J.; Ao, P.Y.; Fletcher, J.P. An increased concentration of rifampicin bonded to gelatin-sealed Dacron reduces the incidence of subsequent graft infections following a staphylococcal challenge. Cardiovasc. Surg. 1998, 6, 268–273. [Google Scholar] [CrossRef]
- Domingos Hadamitzky, C.; Schulte, S.; Horsch, S. Vacuum assisted wound closure in postoperative periprosthetic groin infections: A new gold standard? J. Cardiovasc. Surg. 2007, 48, 477–483. [Google Scholar]
- Diener, H.; Chakfe, N.; Honig, S. Die europäischen Leitlinien zur Versorgung von Gefäßprothesen- und Stentgraftinfektionen. Gefässchirurgie 2020, 25, 632–642. [Google Scholar] [CrossRef]
- Summers, W.C. Félix Hubert d’Herelle (1873–1949): History of a scientific mind. Bacteriophage 2016, 6, e1270090. [Google Scholar] [CrossRef]
- Fernández, L.; Gutiérrez, D.; García, P.; Rodríguez, A. The Perfect Bacteriophage for Therapeutic Applications-A Quick Guide. Antibiotics 2019, 8, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doss, J.; Culbertson, K.; Hahn, D.; Camacho, J.; Barekzi, N. A Review of Phage Therapy against Bacterial Pathogens of Aquatic and Terrestrial Organisms. Viruses 2017, 9, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, H. Shiga-toxin-converting bacteriophages. Res. Microbiol. 2001, 152, 687–695. [Google Scholar] [CrossRef]
- Kuzminov, A. Recombinational repair of DNA damage in Escherichia coli and bacteriophage lambda. Microbiol. Mol. Biol. Rev. 1999, 63, 751–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; McDaniel, A.D.; Wolf, L.E.; Keusch, G.T.; Waldor, M.K.; Acheson, D.W.K. Quinolone antibiotics induce Shiga toxin-encoding bacteriophages, toxin production, and death in mice. J. Infect. Dis. 2000, 181, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Hassan, F.; Kamruzzaman, M.; Mekalanos, J.J.; Faruque, S.M. Satellite phage TLCφ enables toxigenic conversion by CTX phage through dif site alteration. Nature 2010, 467, 982–985. [Google Scholar] [CrossRef] [Green Version]
- Waldor, M.K.; Mekalanos, J.J. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science 1996, 272, 1910–1914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohde, C.; Wittmann, J.; Kutter, E. Bacteriophages: A Therapy Concept against Multi-Drug-Resistant Bacteria. Surg. Infect. 2018, 19, 737–744. [Google Scholar] [CrossRef] [Green Version]
- Malik, B.; Bhattacharyya, S. Antibiotic drug-resistance as a complex system driven by socio-economic growth and antibiotic misuse. Sci. Rep. 2019, 9, 9788. [Google Scholar] [CrossRef]
- De Kraker, M.E.A.; Stewardson, A.J.; Harbarth, S. Will 10 Million People Die a Year due to Antimicrobial Resistance by 2050? PLoS Med. 2016, 13, e1002184. [Google Scholar] [CrossRef] [Green Version]
- Expert Round Table on Acceptance and Re-Implementation of Bacteriophage Therapy; Sybesma, W.; Rohde, C.; Bardy, P.; Pirnay, J.-P.; Cooper, I.; Caplin, J.; Chanishvili, N.; Coffey, A.; de Vos, D.; et al. Silk Route to the Acceptance and Re-Implementation of Bacteriophage Therapy—Part II. Antibiotics 2018, 7, 35. [Google Scholar] [CrossRef] [Green Version]
- McCallin, S.; Sacher, J.C.; Zheng, J.; Chan, B.K. Current State of Compassionate Phage Therapy. Viruses 2019, 11, 343. [Google Scholar] [CrossRef] [Green Version]
- Jault, P.; Leclerc, T.; Jennes, S.; Pirnay, J.P.; Que, Y.-A.; Resch, G.; Rousseau, A.F.; Ravat, F.; Carsin, H.; Le Floch, R.; et al. Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): A randomised, controlled, double-blind phase 1/2 trial. Lancet Infect. Dis. 2019, 19, 35–45. [Google Scholar] [CrossRef]
- Pires, D.P.; Costa, A.R.; Pinto, G.; Meneses, L.; Azeredo, J. Current challenges and future opportunities of phage therapy. FEMS Microbiol. Rev. 2020, 44, 684–700. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [Green Version]
- Rubalskii, E.; Ruemke, S.; Salmoukas, C.; Boyle, E.C.; Warnecke, G.; Tudorache, I.; Shrestha, M.; Schmitto, J.D.; Martens, A.; Rojas, S.V.; et al. Bacteriophage Therapy for Critical Infections Related to Cardiothoracic Surgery. Antibiotics 2020, 9, 232. [Google Scholar] [CrossRef]
- Chan, B.K.; Turner, P.E.; Kim, S.; Mojibian, H.R.; Elefteriades, J.A.; Narayan, D. Phage treatment of an aortic graft infected with Pseudomonas aeruginosa. Evol. Med. Public Health 2018, 2018, 60–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Exarchos, V.; Tkhilaishvili, T.; Potapov, E.; Starck, C.; Trampuz, A.; Schoenrath, F. Successful bacteriophage treatment of infection involving cardiac implantable electronic device and aortic graft: A Trojan horse concept. Europace 2020, 22, 597. [Google Scholar] [CrossRef] [PubMed]
- Aslam, S.; Pretorius, V.; Lehman, S.M.; Morales, S.; Schooley, R.T. Novel bacteriophage therapy for treatment of left ventricular assist device infection. J. Heart Lung Transplant. 2019, 38, 475–476. [Google Scholar] [CrossRef]
- Mulzer, J.; Trampuz, A.; Potapov, E.V. Treatment of chronic left ventricular assist device infection with local application of bacteriophages. Eur. J. Cardiothorac. Surg. 2020, 57, 1003–1004. [Google Scholar] [CrossRef]
- Malik, D.J.; Sokolov, I.J.; Vinner, G.K.; Mancuso, F.; Cinquerrui, S.; Vladisavljevic, G.T.; Clokie, M.R.J.; Garton, N.J.; Stapley, A.G.F.; Kirpichnikova, A. Formulation, stabilisation and encapsulation of bacteriophage for phage therapy. Adv. Colloid Interface Sci. 2017, 249, 100–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merabishvili, M.; Pirnay, J.-P.; de Vos, D. Guidelines to Compose an Ideal Bacteriophage Cocktail. Methods Mol. Biol. 2018, 1693, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Leung, S.S.Y.; Parumasivam, T.; Gao, F.G.; Carter, E.A.; Carrigy, N.B.; Vehring, R.; Finlay, W.H.; Morales, S.; Britton, W.J.; Kutter, E.; et al. Effects of storage conditions on the stability of spray dried, inhalable bacteriophage powders. Int. J. Pharm. 2017, 521, 141–149. [Google Scholar] [CrossRef] [Green Version]
- González-Menéndez, E.; Fernández, L.; Gutiérrez, D.; Rodríguez, A.; Martínez, B.; García, P. Comparative analysis of different preservation techniques for the storage of Staphylococcus phages aimed for the industrial development of phage-based antimicrobial products. PLoS ONE 2018, 13, e0205728. [Google Scholar] [CrossRef] [PubMed]
- Rubalskii, E.; Ruemke, S.; Salmoukas, C.; Aleshkin, A.; Bochkareva, S.; Modin, E.; Mashaqi, B.; Boyle, E.C.; Boethig, D.; Rubalsky, M.; et al. Fibrin glue as a local drug-delivery system for bacteriophage PA5. Sci. Rep. 2019, 9, 2091. [Google Scholar] [CrossRef] [Green Version]
- Botka, T.; Pantůček, R.; Mašlaňová, I.; Benešík, M.; Petráš, P.; Růžičková, V.; Havlíčková, P.; Varga, M.; Žemličková, H.; Koláčková, I.; et al. Lytic and genomic properties of spontaneous host-range Kayvirus mutants prove their suitability for upgrading phage therapeutics against staphylococci. Sci. Rep. 2019, 9, 5475. [Google Scholar] [CrossRef]
- Drake, J.W. Ultraviolet mutagenesis in bacteriophage T4. II. Photoreversal of mutational lesions. J. Bacteriol. 1966, 92, 144–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dąbrowska, K. Phage therapy: What factors shape phage pharmacokinetics and bioavailability? Systematic and critical review. Med. Res. Rev. 2019, 39, 2000–2025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Beyrouti, H.; Lescan, M.; Doemland, M.; Mustafi, M.; Jungmann, F.; Jorg, T.; Halloum, N.; Dorweiler, B. Early results of a low-profile stent-graft for thoracic endovascular aortic repair. PLoS ONE 2020, 15, e0240560. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Junghans, S.; Rojas, S.V.; Skusa, R.; Püschel, A.; Grambow, E.; Kohlen, J.; Warnke, P.; Gummert, J.; Gross, J. Bacteriophages for the Treatment of Graft Infections in Cardiovascular Medicine. Antibiotics 2021, 10, 1446. https://doi.org/10.3390/antibiotics10121446
Junghans S, Rojas SV, Skusa R, Püschel A, Grambow E, Kohlen J, Warnke P, Gummert J, Gross J. Bacteriophages for the Treatment of Graft Infections in Cardiovascular Medicine. Antibiotics. 2021; 10(12):1446. https://doi.org/10.3390/antibiotics10121446
Chicago/Turabian StyleJunghans, Simon, Sebastian V. Rojas, Romy Skusa, Anja Püschel, Eberhard Grambow, Juliane Kohlen, Philipp Warnke, Jan Gummert, and Justus Gross. 2021. "Bacteriophages for the Treatment of Graft Infections in Cardiovascular Medicine" Antibiotics 10, no. 12: 1446. https://doi.org/10.3390/antibiotics10121446
APA StyleJunghans, S., Rojas, S. V., Skusa, R., Püschel, A., Grambow, E., Kohlen, J., Warnke, P., Gummert, J., & Gross, J. (2021). Bacteriophages for the Treatment of Graft Infections in Cardiovascular Medicine. Antibiotics, 10(12), 1446. https://doi.org/10.3390/antibiotics10121446






