Paediatric Antimicrobial Stewardship for Respiratory Infections in the Emergency Setting: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Focused Question
2.2. PICO Question
2.3. Search Strategy
2.4. Eligibility Criteria
2.5. Study Selection
2.6. Study Quality & Risk of Bias
2.7. Data Extraction
3. Results
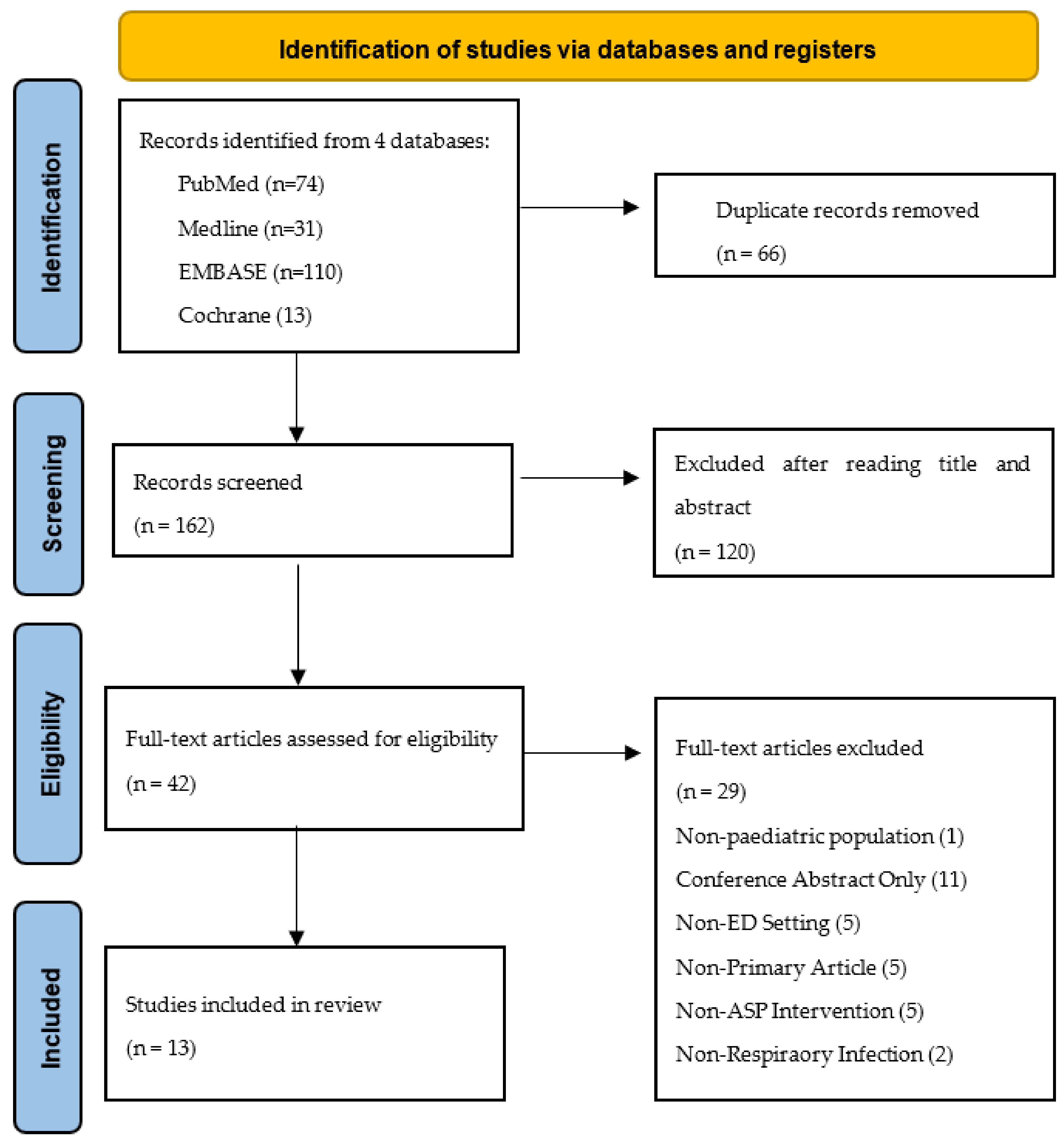
3.1. Search Results
3.2. Included Studies
3.3. Intervention
3.4. Primary Outcome
3.5. Efficacy of ASPs
3.5.1. Education-Based Interventions
3.5.2. Feedback for STUDY Participants
3.5.3. RRP Testing
3.5.4. Impact of Vaccinations/Enhanced Antimicrobial Control
3.6. Clinical Outcomes following ASP Search Results
3.7. Risk of Bias
4. Discussion
Strengths and Limitations
5. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Step | Search Terms |
| 1 | ‘antimicrobial stewardship’ |
| 2 | ‘antimicrobial control’ |
| 3 | ‘antibiotic control’ |
| 4 | ‘antibiotic stewardship’ |
| 5 | ‘child *’ |
| 6 | ‘paediatric’ |
| 7 | ‘pediatric’ |
| 8 | ‘infant’ |
| 9 | ‘neonat*’ |
| 10 | ‘respiratory tract infection’ |
| 11 | ‘chest infection’ |
| 12 | ‘lung infection’ |
| 13 | ‘pneumo *’ |
| 14 | ‘emergency’ |
| 15 | ‘emergency department’ |
| 16 | ‘acute care’ |
| 17 | ‘critical care’ |
| 18 | ‘urgent care’ |
| 19 | 1 OR 2 OR 3 OR 4 |
| 20 | 5 OR 6 OR 7 OR 8 OR 9 |
| 21 | 10 OR 11 OR 12 OR 13 |
| 22 | 14 OR 15 OR 16 OR 17 OR 18 |
| 23 | 19 AND 20 AND 21 AND 22 |
Appendix B
| Quality Criteria | Study Design ** | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Dimension | Specific Criteria * | RCT | CBA | CITS | NCITS | NCBA | CS | QUAL | |
| 1 | Clear aims and justification | a. Clear statement of the aims of the research? | YY | YY | YY | YY | YY | YY | YY |
| b. Rationale for number of pre- and postintervention points or adequate baseline measurement | N | N | Y | YY | YY | N | N | ||
| c. Explanation for lack of control group | N | N | N | Y | Y | N | N | ||
| d. Appropriateness of qualitative methodology | N | N | N | N | N | N | Y | ||
| e. Appropriate study design | N | N | N | N | N | N | YY | ||
| 2 | Managing bias in sampling or between groups | a. Sequence generation | YY | N | N | N | N | N | N |
| b. Allocation concealment | YY | N | N | N | N | N | N | ||
| c. Justification for sample choice | N | N | N | YY | YY | N | N | ||
| d. Intervention and control group selection designed to protect against systematic difference/selection bias | N | YY | N | N | N | N | N | ||
| e. Comparability of groups | N | N | N | N | N | YY | N | ||
| f. Sampling and recruitment | N | N | N | N | N | N | YY | ||
| 3 | Managing bias in outcome measurements and blinding | a. Blinding | YY | N | N | N | N | N | N |
| b. Baseline measurement and protection against selection bias | N | YY | N | N | N | N | N | ||
| c. Protection against contamination | N | YY | N | N | N | N | N | ||
| d. Protection against secular changes | N | N | YY | N | N | N | N | ||
| e. Protection against detection bias: Blinded assessment of primary outcome measures | Y | Y | Y | Y | Y | Y | N | ||
| f. Reliable primary outcome measures | Y | Y | Y | Y | Y | Y | Y | ||
| g. Comparability of outcomes | N | N | N | N | N | YY | N | ||
| 4 | Managing bias in follow-up | a. Follow-up of subjects (protection against exclusion bias) | Y | N | N | N | N | N | N |
| b. Follow-up of patients or episodes of care | Y | N | N | N | N | N | N | ||
| c. Incomplete outcome data addressed | Y | Y | Y | Y | Y | YY | Y | ||
| 5 | Managing bias in other study aspects | a. Protection against detection bias: Intervention unlikely to affect data collection | Y | Y | Y | Y | Y | N | N |
| b. Protection against information bias | N | N | N | N | N | Y | N | ||
| c. Data collection appropriate to address research aims | N | N | N | N | N | N | Y | ||
| d. Attempts to mitigate effects of no control | N | N | N | YY | YY | N | N | ||
| 6 | Analytical rigour | a. Sufficient data points to enable reliable statistical inference | N | N | YY | N | N | N | N |
| b. Shaping of intervention effect specified | N | N | Y | N | N | N | N | ||
| c. Analysis sufficiently rigorous/free from bias | Y | Y | Y | Y | Y | Y | Y | ||
| 7 | Managing bias in reporting/ethical considerations | a. Free of selective outcome reporting | Y | Y | Y | Y | Y | Y | Y |
| b. Limitations addressed | Y | Y | Y | Y | Y | Y | Y | ||
| c. Conclusions clear and justified | Y | Y | Y | Y | Y | Y | Y | ||
| d. Free of other bias | Y | Y | Y | Y | Y | Y | Y | ||
| e. Ethics issues addressed | Y | Y | Y | Y | Y | Y | Y | ||
Appendix C
| Study Design * | Mandatory Criteria | Minimum Score ** |
|---|---|---|
| RCT, cRCT | 1a, 2a, 2b, 3a | 22 |
| CBA | 1a, 2d, 3b, 3c | 18 |
| CITS | 1a, 3d, 6a | 18 |
| NCITS | 1a, 1b, 2c, 5d | 22 |
| NCBA | 1a, 1b, 2c, 5d | 22 |
| Cohort | 1a, 2e, 3g, 4c | 18 |
| Qualitative | 1a, 1e, 2f | 16 |
Appendix D
| Study | Study Design | Minimum Score Required | Study Score |
|---|---|---|---|
| Ambroggio et al. | Cohort | 18 | 28 |
| Forrest et al. | Cohort | 18 | 24 |
| Huang et al. | NCBA | 22 | 30 |
| May et al. | RCT | 22 | 35 |
| McDaniel et al. | NCBA | 22 | 34 |
| Ouldali et al. | CITS | 18 | 39 |
| Pernica et al. | RCT | 22 | 33 |
| Rutman et al. | Cohort | 18 | 32 |
| Shishido et al. | NCBA | 22 | 36 |
| Van de Maat et al. | RCT | 22 | 36 |
| Weddle et al. | NCBA | 22 | 26 |
References
- Davey, P.; Marwick, C.A.; Scott, C.L.; Charani, E.; McNeil, K.; Brown, E.; Gould, I.M.; Ramsay, C.R.; Michie, S. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst. Rev. 2017, 2, CD003543. [Google Scholar] [CrossRef] [Green Version]
- DeNegre, A.A.; Ndeffo Mbah, M.L.; Myers, K.; Fefferman, N.H. Emergence of antibiotic resistance in immunocompromised host populations: A case study of emerging antibiotic resistant tuberculosis in AIDS patients. PLoS ONE 2019, 14, e0212969. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shenoy, E.S.; Paras, M.L.; Noubary, F.; Walensky, R.P.; Hooper, D.C. Natural history of colonization with methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE): A systematic review. BMC Infect. Dis. 2014, 14, 177. [Google Scholar] [CrossRef]
- Peters, L.; Olson, L.; Khu, D.T.K.; Linnros, S.; Le, N.K.; Hanberger, H.; Hoang, N.T.B.; Tran, D.M.; Larsson, M. Multiple antibiotic resistance as a risk factor for mortality and prolonged hospital stay: A cohort study among neonatal intensive care patients with hospital-acquired infections caused by gram-negative bacteria in Vietnam. PLoS ONE 2019, 14, e0215666. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, M.; Pereira, B.; Lima, J.; Decq-Mota, J.; Vieira, J.D.; Costa, J.N. Predictors of in-hospital mortality in elderly patients with bacteraemia admitted to an Internal Medicine ward. Int. Arch. Med. 2011, 4, 33. [Google Scholar] [CrossRef]
- Medernach, R.L.; Logan, L.K. The Growing Threat of Antibiotic Resistance in Children. Infect. Dis Clin. N. Am. 2018, 32, 1–17. [Google Scholar] [CrossRef]
- Agiro, A.; Gautam, S.; Wall, E.; Hackell, J.; Helm, M.; Barron, J.; Zaoutis, T.; Fleming-Dutra, K.E.; Hicks, L.A.; Rosenberg, A. Variation in Outpatient Antibiotic Dispensing for Respiratory Infections in Children by Clinician Specialty and Treatment Setting. Pediatric Infect. Dis. J. 2018, 37, 1248–1254. [Google Scholar] [CrossRef]
- Gulliford, M.C.; Juszczyk, D.; Prevost, A.T.; Soames, J.; McDermott, L.; Sultana, K.; Wright, M.; Fox, R.; Hay, A.D.; Little, P.; et al. Electronically delivered interventions to reduce antibiotic prescribing for respiratory infections in primary care: Cluster RCT using electronic health records and cohort study. Health Technol. Assess 2019, 23, 1–70. [Google Scholar] [CrossRef]
- Martinez-Gonzalez, N.A.; Keizer, E.; Plate, A.; Coenen, S.; Valeri, F.; Verbakel, J.Y.J.; Rosemann, T.; Neuner-Jehle, S.; Senn, O. Point-of-care c-reactive protein testing to reduce antibiotic prescribing for respiratory tract infections in primary care: Systematic review and meta-analysis of randomised controlled trials. Antibiotics 2020, 9, 610. [Google Scholar] [CrossRef]
- Schuetz, P.; Wirz, Y.; Sager, R.; Christ-Crain, M.; Stolz, D.; Tamm, M.; Bouadma, L.; Luyt, C.E.; Wolff, M.; Chastre, J.; et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst. Rev. 2017, 8, 1297–1371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dona, D.; Barbieri, E.; Daverio, M.; Lundin, R.; Giaquinto, C.; Zaoutis, T.; Sharland, M. Implementation and impact of pediatric antimicrobial stewardship programs: A systematic scoping review. Antimicrob. Resist Infect. Control 2020, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Ouldali, N.; Bellettre, X.; Milcent, K.; Guedj, R.; De Pontual, L.; Cojocaru, B.; Soussan-Banini, V.; Craiu, I.; Skurnik, D.; Gajdos, V.; et al. Impact of Implementing National Guidelines on Antibiotic Prescriptions for Acute Respiratory Tract Infections in Pediatric Emergency Departments: An Interrupted Time Series Analysis. Clin. Infect. Dis. 2017, 65, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Launay, E.; Le Guen, C.G. Antibiotic prescription in paediatric emergency departments: Fear and reason. Lancet Infect. Dis. 2019, 19, 341–342. [Google Scholar] [CrossRef]
- Angoulvant, F.; Pereira, M.; Perreaux, F.; Soussan, V.; Pham, L.L.; Trieu, T.V.; Cojocaru, B.; Guedj, R.; Cohen, R.; Alberti, C.; et al. Impact of unlabeled French antibiotic guidelines on antibiotic prescriptions for acute respiratory tract infections in 7 Pediatric Emergency Departments, 2009–2012. Pediatr. Infect. Dis. J. 2014, 33, 330–333. [Google Scholar] [CrossRef]
- van de Maat, J.; van de Voort, E.; Mintegi, S.; Gervaix, A.; Nieboer, D.; Moll, H.; Oostenbrink, R.; Research in European Pediatric Emergency Medicine Study Group. Antibiotic prescription for febrile children in European emergency departments: A cross-sectional, observational study. Lancet Infect. Dis. 2019, 19, 382–391. [Google Scholar] [CrossRef]
- Aoybamroong, N.; Kantamalee, W.; Thadanipon, K.; Techasaensiri, C.; Malathum, K.; Apiwattanakul, N. Impact of an Antibiotic Stewardship Program on Antibiotic Prescription for Acute Respiratory Tract Infections in Children: A Prospective Before-After Study. Clin. Pediatr. 2019, 58, 1166–1174. [Google Scholar] [CrossRef]
- Giubilini, A. Antimicrobial resistance and antimicrobial stewardship programmes: Benefiting the patient or the population? J. Med. Ethics 2017, 43, 653–654. [Google Scholar] [CrossRef]
- Masterton, R.G. Antibiotic de-escalation. Crit. Care Clin. 2011, 27, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Owens, R.C., Jr. Antimicrobial stewardship: Concepts and strategies in the 21st century. Diagn. Microbiol. Infect. Dis. 2008, 61, 110–128. [Google Scholar] [CrossRef]
- Rajar, P.; Saugstad, O.D.; Berild, D.; Dutta, A.; Greisen, G.; Lausten-Thomsen, U.; Mande, S.S.; Nangia, S.; Petersen, F.C.; Dahle, U.R.; et al. Antibiotic Stewardship in Premature Infants: A Systematic Review. Neonatology 2020, 117, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Araujo da Silva, A.R.; Marques, A.; Di Biase, C.; Faitanin, M.; Murni, I.; Dramowski, A.; Hubner, J.; Zingg, W. Effectiveness of antimicrobial stewardship programmes in neonatology: A systematic review. Arch. Dis. Child. 2020, 105, 563–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zingg, W.; Castro-Sanchez, E.; Secci, F.V.; Edwards, R.; Drumright, L.N.; Sevdalis, N.; Holmes, A.H. Innovative tools for quality assessment: Integrated quality criteria for review of multiple study designs (ICROMS). Public Health 2016, 133, 19–37. [Google Scholar] [CrossRef]
- Ambroggio, L.; Thomson, J.; Kurowski, E.M.; Courter, J.; Statile, A.; Graham, C.; Sheehan, B.; Iyer, S.; Shah, S.S.; White, C.M. Quality improvement methods increase appropriate antibiotic prescribing for childhood pneumonia. Pediatrics 2013, 131, e1623–e1631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pernica, J.M.; Harman, S.; Kam, A.J.; Carciumaru, R.; Vanniyasingam, T.; Crawford, T.; Dalgleish, D.; Khan, S.; Slinger, R.S.; Fulford, M.; et al. Short-Course Antimicrobial Therapy for Pediatric Community-Acquired Pneumonia: The SAFER Randomized Clinical Trial. JAMA Pediatrics 2021, 175, 475–482. [Google Scholar] [CrossRef]
- Shishido, A.; Otake, S.; Kimura, M.; Tsuzuki, S.; Fukuda, A.; Ishida, A.; Kasai, M.; Kusama, Y. Effects of a nudge-based antimicrobial stewardship program in a pediatric primary emergency medical center. Eur. J. Pediatrics 2021, 180, 1933–1940. [Google Scholar] [CrossRef]
- May, L.; Tatro, G.; Poltavskiy, E.; Mooso, B.; Hon, S.; Bang, H.; Polage, C. Rapid multiplex testing for upper respiratory pathogens in the emergency department: A randomized controlled trial. Open Forum Infect. Dis. 2019, 6, ofz481. [Google Scholar] [CrossRef] [Green Version]
- van de Maat, J.S.; Peeters, D.; Nieboer, D.; van Wermeskerken, A.M.; Smit, F.J.; Noordzij, J.G.; Tramper-Stranders, G.; Driessen, G.J.A.; Obihara, C.C.; Punt, J.; et al. Evaluation of a clinical decision rule to guide antibiotic prescription in children with suspected lower respiratory tract infection in The Netherlands: A stepped-wedge cluster randomised trial. PLoS Med. 2020, 17, e1003034. [Google Scholar] [CrossRef] [Green Version]
- Yadav, K.; Meeker, D.; Mistry, R.D.; Doctor, J.N.; Fleming-Dutra, K.E.; Fleischman, R.J.; Gaona, S.D.; Stahmer, A.; May, L. A Multifaceted Intervention Improves Prescribing for Acute Respiratory Infection for Adults and Children in Emergency Department and Urgent Care Settings. Acad. Emerg. Med. 2019, 26, 719–731. [Google Scholar] [CrossRef]
- Forrest, C.L.; Verzone, A. Antibiotic stewardship: Improving patient-centered right care in urgent care using a shared decision aid and 5 Ds tool. J. Am. Assoc. Nurse Practitioners 2020, 33038113. [Google Scholar] [CrossRef]
- Rutman, L.; Wright, D.R.; O’Callaghan, J.; Spencer, S.; Lion, K.C.; Kronman, M.P.; Zhou, C.; Mangione-Smith, R. A Comprehensive Approach to Pediatric Pneumonia: Relationship between Standardization, Antimicrobial Stewardship, Clinical Testing, and Cost. J. Healthc. Qual. 2017, 39, e59–e69. [Google Scholar] [CrossRef]
- Huang, Y.C.; Lin, C.F.; Ting, P.J.; Tang, T.H.; Huang, F.L.; Chao, H.J.; Wang, C.L.; Chen, P.Y. Respiratory pathogens—Some altered antibiotic susceptibility after implementation of pneumococcus vaccine and antibiotic control strategies. J. Microbiol. Immunol. Infect. 2020, 53, 682–689. [Google Scholar] [CrossRef]
- McDaniel, C.E.; Haaland, W.; Parlaman, J.; Zhou, C.; Desai, A.D. A Multisite Intervention for Pediatric Community-acquired Pneumonia in Community Settings. Acad. Emerg. Med. 2018, 25, 870–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weddle, G.; Goldman, J.; Myers, A.; Newland, J. Impact of an Educational Intervention to Improve Antibiotic Prescribing for Nurse Practitioners in a Pediatric Urgent Care Center. J. Pediatric Health Care Off. Publ. Natl. Assoc. Pediatric Nurse Assoc. Pract. 2017, 31, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Sidiki, S.; Grider, B.; Fink, B.; Hubbard, N.; Mukundan, D. A Study of the Use and Outcomes from Respiratory Viral Testing at a Mid-Sized Children’s Hospital. Clin. Pediatrics 2019, 58, 185–190. [Google Scholar] [CrossRef]
- Pulia, M.; Redwood, R.; May, L. Antimicrobial Stewardship in the Emergency Department. Emerg. Med. Clin. North Am. 2018, 36, 853–872. [Google Scholar] [CrossRef]
- Xu, Y.L.; Hu, L.M.; Xie, Z.Z.; Dong, Y.W.; Dong, L. Impact of antimicrobial stewardship program on antimicrobial usage and detection rate of multidrug-resistant gram-negative bacteria. Zhonghua Er Ke Za Zhi = Chin. J. Pediatrics 2019, 57, 553–558. (In Chinese) [Google Scholar]
- Savoldi, A.; Foschi, F.; Kreth, F.; Gladstone, B.P.; Carrara, E.; Eisenbeis, S.; Buhl, M.; Marasca, G.; Bovo, C.; Malek, N.P.; et al. Impact of implementing a non-restrictive antibiotic stewardship program in an emergency department: A four-year quasi-experimental prospective study. Sci. Rep. 2020, 10, 8194. [Google Scholar] [CrossRef] [PubMed]
- May, L.; Nguyen, M.H.; Trajano, R.; Tancredi, D.; Aliyev, E.R.; Mooso, B.; Anderson, C.; Ondak, S.; Yang, N.; Cohen, S.; et al. A multifaceted intervention improves antibiotic stewardship for skin and soft tissues infections. Am. J. Emerg. Med. 2021, 46, 374–381. [Google Scholar] [CrossRef]
- Calo, F.; Onorato, L.; Macera, M.; Di Caprio, G.; Monari, C.; Russo, A.; Galdieri, A.; Giordano, A.; Cuccaro, P.; Coppola, N. Impact of an Education-Based Antimicrobial Stewardship Program on the Appropriateness of Antibiotic Prescribing: Results of a Multicenter Observational Study. Antibiotics 2021, 10, 314. [Google Scholar] [CrossRef]
- Penalva, G.; Fernandez-Urrusuno, R.; Turmo, J.M.; Hernandez-Soto, R.; Pajares, I.; Carrion, L.; Vazquez-Cruz, I.; Botello, B.; Garcia-Robredo, B.; Camara-Mestres, M.; et al. Long-term impact of an educational antimicrobial stewardship programme in primary care on infections caused by extended-spectrum beta-lactamase-producing Escherichia coli in the community: An interrupted time-series analysis. Lancet Infect. Dis. 2020, 20, 199–207. [Google Scholar] [CrossRef]
- Kjaersgaard, M.; Leth, R.A.; Udupi, A.; Ank, N. Antibiotic stewardship based on education: Minor impact on knowledge, perception and attitude. Infect. Dis. 2019, 51, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Fowler, S.; Weber, A.; Cooper, B.S.; Phimister, A.; Price, K.; Carter, Y.; Kibbler, C.C.; Simpson, A.J.H.; Stone, S.P. Successful use of feedback to improve antibiotic prescribing and reduce Clostridium difficile infection: A controlled interrupted time series. J. Antimicrob. Chemother. 2007, 59, 990–995. [Google Scholar] [CrossRef]
- Baysari, M.T.; Egan, K.O.B.; Li, L.; Richardson, K.; Sandaradura, I.; Westbrook, J.I.; Day, R.O. Audit and feedback of antibiotic use: Utilising electronic prescription data. Appl. Clin. Inform. 2013, 4, 583–595. [Google Scholar] [PubMed] [Green Version]
- Dodd, M.; Adolphe, A.; Parada, A.; Brett, M.; Culbreath, K.; Mercier, R.C. Clinical Impact of a Rapid Streptococcal Antigen Test on Antibiotic Use in Adult Patients. Diagn. Microbiol. Infect. Dis. 2018, 91, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Rogan, D.T.; Kochar, M.S.; Yang, S.; Quinn, J.V. Impact of Rapid Molecular Respiratory Virus Testing on Real-Time Decision Making in a Pediatric Emergency Department. J. Mol. Diagn. 2017, 19, 460–467. [Google Scholar] [CrossRef] [Green Version]
- Brotherton, A.L. Metrics of Antimicrobial Stewardship Programs. Med. Clin. North Am. 2018, 102, 965–976. [Google Scholar] [CrossRef]
- Acworth, J.; Babl, F.; Borland, M.; Ngo, P.; Krieser, D.; Schutz, J.; Pitt, R.; Cotterell, E.; Jamison, S.; Neutze, J.; et al. Patterns of presentation to the Australian and New Zealand Paediatric Emergency Research Network. Emerg. Med. Australas 2009, 21, 59–66. [Google Scholar] [CrossRef]
| Author Year; Country; Study Period; Setting | Study Design; Population and Sample Size | Objective | Intervention | Key Findings |
|---|---|---|---|---|
| Ambroggio et al., 2013 [24]; USA; 1 May 2011–21 July 2012; Cincinnati Children’s Hospital Medical Centre (CCHMC) | Retrospective Cohort Study; 3 months–19 years, discharge diagnosis code of pneumonia (noncomplicated or pneumonia-related sx n = 217 | Evaluate quality improvement in a setting without a formal ASP |
| Improvement in appropriate Abx prescribing in the ED following the guideline seminar (0% to 82%) |
| Forrest et al., 2020 [30]; USA; 90 days; Urgent Care Centre | Cohort Study; Adults and children with URIs and/or head, ears, nose, throat viral illnesses presenting to urgent care n = 279 | Improve patient-centred right care for patients of 65 years and younger with URIs and/or head, ears, nose, throat viral illnesses presenting to ED from 36.2% to 80% within 90 days | Rapid-cycle Quality Improvement (QI) project with 4 × 2-weekly Plan-Do-Study-Act (PDSA) cycles:
|
|
| Huang et al., 2020 [32]; Taiwan; January 2008–December 2017; Taichung Veterans General Hospital | Retrospective noncontrolled before-and-after study; Three age groups (<3 years, 3–6 years, 7–18 years) Nasopharynx, throat swab, and sputum culture from children <18 years n = 914 | Evaluate the impact of the implementation of the national PCY13 vaccination program and the 2013–2015 antimicrobial management project on antimicrobial drug susceptibility or respiratory tract bacteria in children | Three Temporal Stages:
|
|
| May et al., 2019 [27]; USA; December 2016–April 2018, Over 2 winter seasons and 1 intervening non-respiratory season; Level 1 ED | Prospective Pilot RCT; >12 months old, had symptoms of URTI or influenza-like illness and not on Abx n = 191 | Evaluate whether having rapid, multipathogen test results available during the ED visit would have a significant impact on management and outcomes in patients with clinical signs and symptoms of ARTI | Rapid, multipathogen respiratory panel (RP) test | Rapid RP testing associated with a trend towards decreased Abx use (–12% difference; p = 0.06/0.08, chi-square/Fisher exact test) that was larger in paediatric patients (−19% difference; p = 0.047/0.07) in an age-stratified post hoc analysis |
| McDaniel et al., 2018 [33]; USA; Preintervention: January–December 2015, Intervention: January–Feb 2016, Postintervention: March 2016–February 2017; Freestanding, tertiary children’s hospital | Noncontrolled before-and-after study; 2 months–18 y.o at ED with primary or secondary diagnosis of uncomplicated CAP. n = 544 (preintervention) n = 321 (postintervention) n = 290 (postintervention in freestanding hospital) | Examine whether implementation of a CAP pathway within 3 community hospital EDs and inpatient units improved process measures related to appropriate laboratory testing and antibiotic prescribing, and to compare performance on these measures between the community hospitals and a freestanding children’s hospital | Clinical decision tool (CDT) as a diagnostic aid for paediatric patients presenting with respiratory distress | Adherence to process measures increased postintervention for: appropriate lab testing, narrow-spectrum Abx stewardship and macrolide stewardship by 10.8% (95% CI = 4.7% to 16.9%), 8.3% (95% CI = 1.5% to 15.2%), and 3.1% (95% CI = −4.3% to 10.4%), respectively |
| Ouldali et al., 2017 [13]; France; November 2009–October 2014; 7 PEDS of Parisian university hospitals | Multicentric noncontrolled interrupted time series analysis; Paediatric patients visiting ED and diagnosed with ARTI. n = 242,534 | Assess the impact of implementing the 2011 national guidelines on antibiotic prescriptions for ARTI in PEDs | Implementation of 2011 French guidelines through:
|
|
| Pernica et al., 2021 [25]; Canada; Data analysed 1 March–8 July 2020; EDs of McMaster Children’s Hospital and the Children’s Hospital of Eastern Ontario | Two-centre parallel group noninferiority RCT; 6 months–10 years having fever within 48 h, respiratory symptoms, chest radiography and a primary diagnosis of pneumonia. n = 281 | Determine whether 5 days of high-dose amoxicillin for CAP was associated with noninferior rates of clinical cure compared with 10-days of high-dose amoxicillin | 5 days of high-dose amoxicillin therapy followed by 5 days of placebo (intervention) vs. 5 days of high-dose amoxicillin followed by a different formulation of 5 days of high-dose amoxicillin (control) |
|
| Rutman et al., 2017 [31]; USA; 1 August 2011–31 August 2013; Seattle Children’s Hospital, Tertiary, university-affiliated 350-bed freestanding | Retrospective cohort study; 2 months–18 years, assigned a primary ICD-9 diagnosis code associated with CAP | Determine the relationship between standardising ED and inpatient care for CAP and antimicrobial stewardship, clinical testing, and cost | CAP pathway implementation by the ED and inpatient pathway through multiple strategies:
|
|
| Shishido et al., 2021 [26], Japan; April 2014–September 2019; Kobe Children’s Primary Emergency Medical Centre | Retrospective noncontrolled before-and-after study; Most common diagnosis upper RTI, followed by gastroenteritis and bronchitis; 129,156 and 28,834 patients in the pre- and postintervention periods | Evaluate the effects of a nudge-based ASP in reducing unnecessary third-generation cephalosporin (3GC) prescriptions in paediatric primary emergency care centre | The implemented ASP utilizes monthly newsletters that report current antimicrobial use patterns and prescribing targets |
|
| Van de Maat et al., 2020 [28]; The Netherlands; 1 January 2016–27 August 2017 (baseline period), 28 August 2017–12 March 2018 (rollout period), intervention phase every 4 weeks, data collected until 30 September 2018 when target sample size achieved; Eight EDs in the Netherlands | Stepped-wedge randomised trial; 1–60 months presenting with fever and cough or dyspnoea Control n = 572 Intervention n = 340 | Safely reduce antibiotic prescription in children under 5 years with suspected lower RTI at the ED, by withholding antibiotics in children at low or intermediate risk of bacterial pneumonia, as predicted by the Feverkidstool | Antibiotics withheld in children with low or intermediate predicted risk of bacterial pneumonia, antibiotics prescribed in children with a high predicted risk (Validated clinical prediction model of Feverkidstool) |
|
| Weddle et al., 2017 [34]; USA; Chart review at 2 preintervention time points (3 m, 1 m before educational sessions) and 3 postintervention time points (1 m, 3 m, 9 m after educational sessions); 4 UCCs affiliated with a free-standing children’s hospital, UCC sites include both urban and suburban locations | Noncontrolled before-and-after study; Patients had one of these conditions: UTI, pharyngitis, SSTI, URI, AOM or ABS, most common diagnosis was URI, at 74% (2576/3496 patients) N = 26 | To determine if educational sessions would reduce inappropriate antibiotic usage. | Members of the institution’s antimicrobial stewardship program team provided 30 min educational sessions for each of the selected diagnoses |
|
| Yadav et al., 2019 [29]; USA; July 2017–February 2018 at UC Davis and Harbor-UCLA, November 2017–February 2018 at CHCO, a 12-month baseline period for statistical analysis; five EDs and four UCCs | Pragmatic, cluster RCT, Licensed clinicians at the participating sites eligible, diagnoses (primary and secondary) from the ICD-10-CM codes consistent with antibiotic-nonresponsive ARI diagnoses | Compare the effectiveness of an antibiotic stewardship intervention adapted for acute ambulatory care settings to a stewardship intervention that additionally incorporates behavioural nudges in reducing inappropriate prescriptions. | Two interventions are compared:
|
|
| Zhu et al., 2019 [35]; USA; 16 December 2013–15 December, 2015; ProMedica Toledo Children’s Hospital | Retrospective Noncontrolled before-and-after study; 1 month−18 years with uncomplicated ARTI admitted into the hospital or ED (those in the ED, had to be discharged from the ED for inclusion) ED group: n = 939 | Assess whether respiratory pathogen panel (RPP) testing decreases antibiotic days of therapy and length of hospital stay for paediatric patients with ARTI | Samples for RPP testing were collected via nasopharyngeal swabs. RPP was performed through PCR detection by BioFire FilmArray Assay which identifies common viral pathogens, as well as common bacterial pathogens | ED group:
|
| Outcomes | ||||||
|---|---|---|---|---|---|---|
| Authors | Intervention | Reduction in Inappropriate Antibiotic Prescription | Reduction in prescription of Broad-Spectrum Antibiotics | Reduction in Duration of Antibiotic Therapy | Patient Clinical Outcomes | Reduction of AMR |
| Ambroggio et al. [24] | Multifaceted education-based intervention | ND | Appropriate first-line Abx prescription: 0% to 82% | ND | ND | ND |
| Forrest et al. [30] | Multifaceted education-based intervention | Appropriate Abx prescription increased from 63% to 91% | ND | ND | Increased from 36% to 78% | ND |
| Huang et al. [32] | Multifaceted education-based intervention | ND | ND | ND | ND | p < 0.05 |
| May et al. [27] | Rapid, multipathogen respiratory panel test | −12%; 95% CI [−25% to 0.4%]; p = 0.06/0.08 | ND | ND | ND | ND |
| McDaniel et al. [33] | Multifaceted education-based intervention | ND | −10.8%; 95%CI [−4.7% to −16.9%]; p < 0.001 | ND | ND | ND |
| Ouldali et al. [13] | Multifaceted education-based intervention | −0.4% per 15-day period; p = 0.04 | ND | ND | ND | ND |
| Pernica et al. [25] | Reduced antibiotic therapy duration | ND | ND | ND | RD, −0.016; 97.5% CI −0.087 | ND |
| Rutman et al. [31] | Multifaceted education-based intervention | ND | −10% | ND | ND | ND |
| Shishido et al. [26] | Feedback, peer-comparison and nudge-based intervention | −67.2% Regression coefficient −0.58; p < 0.001 | ND | ND | ND | ND |
| Van de Maat et al. [28] | Multifaceted education-based intervention | [aOR] 1.07; 95% CI 0.57 to 2.01; p = 0.75 | ND | ND | [aOR] 0.53; 95% CI 0.32 to 0.88; p = 0.01 | ND |
| Weddle et al. [34] | Multifaceted education-based intervention | −2% p = 0.02 | ND | ND | ND | ND |
| Yadav et al. [29] | Feedback, peer-comparison and nudge-based intervention | OR = 0.67; 95% CI = 0.54 to 0.82 | ND | ND | ND | ND |
| Zhu et al. [36] | Rapid respiratory pathogen testing | 78.9% vs. 7.3%; p < 0.001 | ND | ND | ND | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weragama, K.; Mudgil, P.; Whitehall, J. Paediatric Antimicrobial Stewardship for Respiratory Infections in the Emergency Setting: A Systematic Review. Antibiotics 2021, 10, 1366. https://doi.org/10.3390/antibiotics10111366
Weragama K, Mudgil P, Whitehall J. Paediatric Antimicrobial Stewardship for Respiratory Infections in the Emergency Setting: A Systematic Review. Antibiotics. 2021; 10(11):1366. https://doi.org/10.3390/antibiotics10111366
Chicago/Turabian StyleWeragama, Keshani, Poonam Mudgil, and John Whitehall. 2021. "Paediatric Antimicrobial Stewardship for Respiratory Infections in the Emergency Setting: A Systematic Review" Antibiotics 10, no. 11: 1366. https://doi.org/10.3390/antibiotics10111366
APA StyleWeragama, K., Mudgil, P., & Whitehall, J. (2021). Paediatric Antimicrobial Stewardship for Respiratory Infections in the Emergency Setting: A Systematic Review. Antibiotics, 10(11), 1366. https://doi.org/10.3390/antibiotics10111366






