Abstract
Physiological changes in animals exposed to elevated ambient temperature are characterized by the redistribution of blood toward the periphery to dissipate heat, with a consequent decline in blood flow and oxygen and nutrient supply to splanchnic tissues. Metabolic adaptations and gut dysfunction lead to oxidative stress, translocation of lumen contents, and release of proinflammatory mediators, activating a systemic inflammatory response. This review discusses the activation and development of the inflammatory response in heat-stressed models.
1. Introduction
Homeothermic organisms are sensitive to elevated environmental temperature, leading to hyperthermia, which can compromise the normal functioning of various organs [1]. Ex vivo and in vitro studies conducted to characterize the direct effects of heat have shown that heat directly affects intestinal permeability and function [2,3]. In mammals and birds at the onset of a hyperthermic event, activation of the autonomic nervous system mediated by endogenous catecholamines generates an increase in respiration and heart rate and recirculation of blood with a secondary restricted blood and nutrient flow to the gastrointestinal tract (GIT), consequently altering Ca+ and energy cellular metabolism in these tissues and lowering the adaptive capacity to a thermal load [4]. Moreover, a decrease in feed intake to lower the production of metabolic heat and maintain the thermal balance between the heat produced and dissipated is a hallmark of heat stress. Lower nutrient intake triggers adaptations of energy and protein metabolic pathways in addition to those elicited directly by the hyperthermic effect. Heat stress models in pigs [5,6,7], poultry [8,9,10], and ruminants [11,12,13] have been used to characterize changes in the metabolism of macronutrients, but conclusive results have not been reported. Therefore, the phenotype of heat stress includes losses in productivity due to the direct and indirect effects of heat on physiology and metabolism.
Depending on the severity of the heat load, limited nutrient supply and direct thermal effects lead to intestinal dysfunction and can compromise the structure and function of the intestinal mucosa, as shown in studies conducted in rodents and pigs [4,14,15,16,17]. The mechanism by which heat stress alters the intestine is not well understood. However, the pathophysiology of this condition includes loss of tight junction integrity [3,16], translocation of lumen bacteria and their products (i.e., endotoxins [4,16]), and an imbalance between the production of reactive oxygen species (ROS) and their elimination by scavenger systems [4,18]. Consequently, systemic and intestinal inflammation have been observed in livestock and rodents exposed to mild to severe heat [17,19,20,21,22,23] and may be responsible for part of the negative effects of heat stress. The inflammatory response has the potential to impair nutrient absorption, gut health, and the immunological status of the affected organism [18]. In this review, we discuss the cellular and molecular features of the immunophysiological response to heat stress and interventions that aim to prevent harmful effects and the associated inflammatory response.
2. How Does Heat Stress Affect the Structure and Function of the Intestinal Mucosa?
2.1. Structural Aspects
Exposure to high ambient temperatures may result in structural changes in the small intestine of rodents, poultry, and livestock [4,19,20,23,24,25,26,27,28,29]. The intestinal epithelium is composed of a single layer of cells that lines the inner surface of the small and large intestine, which, in addition to providing a solid protective barrier, functions as a very precise absorptive machinery. In mammals and birds, this cell line folds along its path through the GIT to increase its contact surface and thus its absorptive power, generating villi (raised portions) and invaginations between villi, which are termed crypts. In vivo models for studying gut physiology have shown hyperplastic crypts combined with reduced villus area, suggesting rapid tissue adaptation to nutrient shortages [30,31,32]. Heat stress may affect the intestinal structure by shortening villus height and increasing crypt depth, with a consequent decrease in the villus:crypt ratio, as shown in poultry [19,20,24,25,33], rodents [21,27], and pigs [22,23] (Table 1). The aforementioned effects have been found in different heat-stressed animal models (i.e., ambient temperatures from 33 to 39 °C for 1.5 to 24 h/day, and duration of insult from 1 to 30 days). Furthermore, heat stress has led to epithelial desquamation at the tips of villi and exposure of the lamina propria in the duodenum and jejunum of pigs (i.e., ambient temperature of 40 °C for 2–5 h) [28,29] and in the jejunum and ileum of rats (i.e., ambient temperature of 40 °C for 2 h/day for 10 days [26] or 3 days [34]). Although the direct effect of heat may result in epithelial loss [2], the shift in blood flow away from the gut with a concomitant shortage in nutrient supply to the intestine may contribute to the alterations in intestinal architecture observed in heat-stressed animals.

Table 1.
Structural changes in the intestinal epithelium of different animals subjected to hyperthermia.
Changes in the epithelial ultrastructure of the jejunum have been found in animals exposed to heat stress. Electron microscopy analysis revealed that a large amount of inflamed fibrous substances flow out of the hyperthermic rat jejunal epithelium [34]. In pigs and rats, heat stress (40 °C for 2–5 h/day for 10 days) affects epithelial cells of the jejunum, shortens microvillus height and increases the number of mitochondria with shortened internal cristae and secondary lysosomes compared with the jejunum in thermoneutral (TN) animals [26,28,29]. Vacuolization in the epithelium in the jejunum of rats exposed to heat stress has also been reported, possibly in association with the progressive loss of epithelial cells [26]. Although these changes in cellular structure and ultrastructure have been observed in all portions of the SI, it seems that the jejunum has greater susceptibility than other segments of the GIT [28,29,34].
In addition to loss of the epithelium of villi, the metabolic alterations generated by heat stress in the intestinal stem cells at the bottom of crypts may delay epithelial cell turnover and replenishment. Intestinal stem cells exhibit a high regenerative power that ensures the turnover of most mature epithelial cells in less than five days [35]; however, exposure to high temperatures can alter the proliferation and apoptosis of intestinal stem cells. As recently demonstrated by Zhou et al. [36] using in vitro models, continuous heat exposure at 41 °C for 72 h of undifferentiated porcine jejunal epithelial cells inhibits cell proliferation and increases apoptosis via inhibition of the Wnt/β-catenin pathway, the main signal that activates the proliferation of intestinal epithelial cells in the depth of intestinal crypts [37].
2.2. Functional Aspects
Stressful events such as exposure to heat can alter the permeability of the intestine to luminal contents (e.g., nutrients and markers) [2,17,38,39]. As alterations in intestinal permeability may reveal changes in absorptive mechanisms (e.g., paracellular pore and leak pathways), this approach is used to assess intestinal function. Gut permeability can be studied by measuring the passage of markers such as fluorescein isothiocyanate-dextran (FITC-D), creatinine, cobalt-EDTA, lactulose and mannitol in vivo and by measuring transepithelial electrical resistance (TER) and permeability to horseradish peroxidase ex vivo and in vitro. Studies have shown that FITC-D increases while TER decreases in the jejunum and/or ileum of pigs and poultry exposed to heat stress [16,19,25,38,40,41,42], indicating an increase in mucosal permeability. In pigs subjected to heat stress (31 ± 1 °C, 12 h/day for 7 days), it was determined that ileum and colon permeability increased, as indicated by a larger lactulose: mannitol ratio [43]. Furthermore, increased intestinal permeability was observed in mice [2,44] exposed to heat stress. In vitro studies have demonstrated increases in permeability or decreases in TER in cultures of epithelial cells, such as intestinal epithelial cell-6 (IEC-6) [44], porcine jejunal cell line (IEC-J2) [43], and human colon-derived crypt-like cells T84 [45] exposed to heat treatments.
Tight junctions (TJs) and adherent junction proteins play a key role in controlling the permeability of the intestinal epithelium [2,46] and in controlling the passage of nutrients via the paracellular space between adjacent cells [47]. Expression of TJ proteins (i.e., occludin and zonula occludens-1 (ZO-1) in the jejunum [19] of chickens and ZO-1 in the jejunum of dairy cows [17]) was reduced during heat stress, and heat stress reduced expression of occludin and claudin-3 in the ileum of pigs [16]. Such an increase in occludin expression was also reported in a study with Caco-2 cells exposed to heat [48]. Gene expression analyses showed higher mRNA expression of claudin-5 and ZO-1 in the jejunum and claudin-1 and -5 and ZO-1 in the ileum in heat-stressed broilers [49]. It was reported that in pigs, heat stress increases the mRNA abundance of occludin, ZO-1 and claudin genes (i.e., pig jejunum after exposure to constant 35 °C for 7 days [23]); in dairy cows, heat stress increases the mRNA abundance of the ZO-1 and claudin-3 genes (i.e., cow jejunum after constant 28 °C for 4 days [17]). However, mRNA levels of occludin, ZO-1, and claudin-1 genes decrease in the jejunum [19,25] of heat-stressed broilers. These contradictory results can be attributed in part to differences between species and studies concerning the duration and severity of heat exposure. It is possible that permeability changes associated with heat stress are mediated by altering expression of TJs in the intestinal epithelium. This relationship has been described in both in vivo and in vitro models of enteritis, aiming to better understand the role of cellular mechanisms to prevent and treat the clinical conditions of humans. Heat stress may lead to changes in TJ expression in the epithelium, but the role of these changes in intestinal permeability remains elusive.
3. What Components of the Immune System Are Activated during Heat Stress?
Innate immunity, and different components of adaptive immunity, can be affected by stress, such as exposure to heat [50]. Although the immunity of higher organisms has a complex framework in which various physical and chemical components participate at molecular, cellular, and tissue levels, innate immunity is the first line of defense against external insults. Adaptive immunity is mediated clonally by T and B lymphocytes and provides tissues with immunological specificity and reaction memory. Heat activates the hypothalamic–pituitary–adrenal (HPA) and the sympathetic–adrenal–medullary (SAM) axes to regulate the response to the stressors and, consequently, elicit changes in the immune response (Figure 1). The release of cortisol during periods of acute stress acts as a stimulus for the immune system; however, during chronic stress cortisol secretion can cause immune suppression. Koch et al. [17] reported a specific effect of chronic heat stress causing infiltration of cells from the adaptive immune system into the lamina propria of the jejunum of lactating dairy cattle.
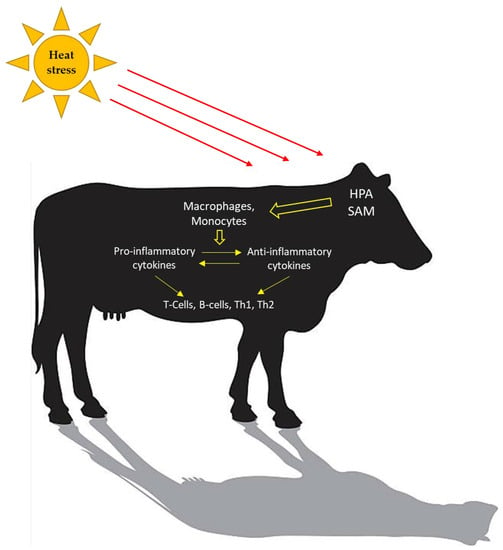
Figure 1.
Heat stress activates an immune response. Concentrations of pro-inflammatory and anti-inflammatory cytokines change to maintain homeostasis. The adaptive immune system is cell-mediated (T-lymphocytes; Th1 and Th2) and humoral-mediated (B-lymphocytes).
Studies have shown that the small intestine of broilers [25], pigs [22], and rodents [34] exposed to heat stress impairs innate immunity. In particular, goblet cells are related to mucin production (e.g., mucin-2) and antimicrobial peptides (e.g., protegrin 1–5), chemical components that play a fundamental role in maintaining the integrity of the intestinal mucosa [51]. A decrease in mRNA expression of Mucin-2 and PG 1–5 in the intestinal mucosa of heat-stressed pigs [22] and poultry [25] has been reported. Studies in calves born to cows exposed to heat stress during the last ~60 days of gestation report a low serum concentration and absorptive capacity of immunoglobulin G (IgG) during the first weeks of life [52].
Quinteiro-Filho et al. [53] also found that heat stress decreased the oxidative responses of peritoneal macrophages and the relative weight of the bursa of Fabricius when broilers were exposed to 31 °C (10 h/day for 7 days). Another group of broilers exposed to 36 °C (10 h/day for 7 days) presented a more marked reaction, with reduced thymus and spleen weights in addition to that of the bursa of Fabricius [53]. He et al. [54] found reductions in the growth index of lymphoid organs and an increase in mRNA abundance of several pro-inflammatory cytokines, i.e., interleukin 1 beta, 4, 6 (IL-1β, IL-4, IL-6), and tumor necrosis factor alpha (TNF-α) in the spleen of broilers exposed to 37 ± 2 °C (8 h/day for 14 days).
Sheep exposed to heat stress exhibit increased concentrations of plasma TNF-α, a key mediator of inflammation, and increased total white blood cell, monocyte, and granulocyte counts (40 °C for 12 h followed by 30 °C for 12 h for 30 days) [55]. Expression of proteins associated with the innate immune response, such as haptoglobin, heat shock protein 90-α (HSP90AA1), and the endoplasmic precursor (HSP90B1), is induced in heat-stressed pigs (constant 30 °C for 21 days) [56]. Several reports have shown an increase in the acute-phase protein haptoglobin, serum albumin A, and serum endotoxins in pigs exposed to heat stress for several hours or days [56,57,58]. In agreement with data from pigs, cattle exposed to elevated ambient temperature show greater plasma concentrations of acute-phase proteins (~34 °C 8 h/day for 7 to 21 days) [59,60]. Collectively, these results reveal activation of systemic inflammation during heat stress. Although the role of inflammation is not clear, inflammatory pathway activation may offer opportunities for interventions to treat and prevent the detrimental effects of heat stress [61].
3.1. Heat Shock Proteins
Heat shock proteins (HSPs) constitute the heat shock response, acting as signals of cell dysfunction in response to stress stimuli [62,63] (Figure 2). The association between the expression of several HSPs and heat stress accounts for the protective function of these proteins against cell damage [64]. This is because hyperthermia and oxidative stress have negative effects on protein folding, altering their specific biological functions. The heat shock response induces the expression of HSP, which helps prevent or reverse protein misfolding and provides an environment for proper folding (Figure 2). Heat shock proteins are divided into different families according to their molecular weight [65], and of these, HSP90, HSP70, and HSP27 are the most studied in heat-stressed animals [66] (Table 2).
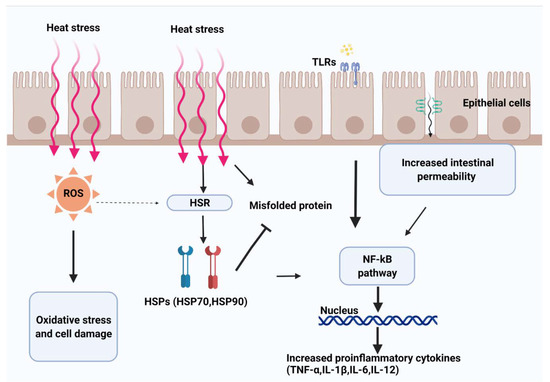
Figure 2.
Heat leads to splanchnic hypoxia and metabolic stress. Heat and hypoxia increase the production of oxidants (e.g., reactive oxygen species (ROS)), consequently activating the heat shock response pathway (HSR) to maintain homeostasis. Higher exposure to oxidants increases epithelial permeability by disrupting tight junction proteins. Increased permeability of the intestinal mucosa mediates the passage of luminal contents (e.g., endotoxins and bacteria), activating the immune response mediated by the Toll-like receptor (TLR) signaling pathway and contributing to the host defense system.

Table 2.
Characterization of heat shock proteins (HSP) and Toll-like receptors (TLR) activated during heat stress.
In rodents, HSP70 has protective power in the intestinal mucosa of rats exposed to heat stress. This effect was demonstrated experimentally by inhibiting production of this protein, which, in turn, resulted in augmented mucosal damage of the small intestine [67]. Elevated expression of genes that encode HSP70 and HSP90 was detected in the jejunum of chickens after 15 days of exposure to heat stress [33]. Varasteh et al. [49] also found higher mRNA expression of different HSPs in the intestine of chickens exposed to high ambient temperatures.
Overall, expression of HSPs varies among tissues. For example, the abundance of HSP70 in chicken liver under thermoneutral conditions is double that in muscle; however, under heat stress conditions, HSP70 levels increase at the same proportion in both organs [68]. Similarly, the brains of heat-stressed chickens show almost twice as much HSP70 mRNA than the muscle and liver tissues of the same chicken [68] or rabbit [69]. This tissue specificity characteristic of HSP70 mRNA expression may be associated with the greater sensitivity of essential organs (such as the brain) to heat exposure, especially during severe hyperthermia events [70]. Recent studies in heat-stressed chickens have shown that levels of HSF47, HSF60, and HSP70 increase earlier in the duodenum and jejunum (after 3–6 h of heat exposure) and later in the ileum (after 6–12 h) [71]. Moreover, gene expression of these HSPs after 3 h of acute exposure to elevated temperatures is greater than that of the control in the different portions of the small intestine [71]. Collectively, a better understanding of the role of the heat shock response is of great significance for developing preventive strategies against heat stress.
Heat shock proteins are capable of inducing immune cells, monocytes, macrophages, and dendritic cells to release proinflammatory cytokines such as TNF-α, IL-1β, IL-6, and interleukin 12 (IL-12) [72,73,74,75]. Moreover, studies have reported that HSP activation of immune cells and production of cytokines can be mediated by the NF-κB pathway [72]. Indeed, heat shock proteins may be related to activation of the NF-κB pathway because an increase in expression of NF-κB in the liver [76] and jejunum [33] occurs in birds subjected to hyperthermia (34–37 °C, 8 h/day for 15–32 days). The transcription factor NF-κB regulates multiple aspects of innate and adaptive immune functions: it induces expression of various proinflammatory genes, including those encoding cytokines and chemokines, serving as a key regulator of inflammatory responses [77] (Figure 2). In addition, NF-κB plays a critical role in regulating the survival, activation and differentiation of innate immune cells and inflammatory T cells [78]. Therefore, the role of the heat shock response and NF-κB pathway in the regulation of immune activities is key for characterizing the impact of heat stress on animals.
3.2. Toll-Like Receptors
Toll-like receptors (TLRs) are found in animal and plant cells and are typically expressed on the cell surface (TLR1–2, TLR4–6, and TLR10–13) or in endosomes (TLR3 and TLR7–9) of immune (e.g., macrophage, neutrophil, dendritic and natural killer cells) and nonimmune cells [79]. Toll-like receptors have been associated with innate defense against invading microorganisms because TLRs recognize structurally conserved molecules derived from microbes, e.g., bacterial lipopolysaccharides (LPS) [80,81]. In addition, the TLR pathway is activated in response to the binding of endogenous HSP or chromatin-associated protein high-mobility group Box 1 in the intestinal epithelium [82]. Activation of TLR pathways ultimately leads to upregulation or suppression of genes that coordinate the inflammatory response and other events (i.e., cell proliferation and survival and activation of adaptive immunity, Table 2).
Under heat stress conditions, microbial endotoxins may enter the mucosa of the gut and activate TLR pathways, triggering the release of cytokines and coordination of a proinflammatory response (Figure 2). To this end, activation of the TLR pathway has been observed in the GIT of goats [83,84], rodents [34,85], chickens [49], and pigs [86] exposed to heat stress, but the local mechanism and order of events have yet to be determined. Studies in goats exposed to heat stress have shown overexpression of TLR1, TLR3, TLR6, TLR7, TLR8, and TLR10 mRNA in the liver [87]. Furthermore, overexpression of TLR2, TLR4, TLR6, TLR9, and TLR10 mRNA was observed in peripheral blood mononuclear cells (PBMCs) of heat-stressed goats [83]. Although Varasteh et al. [49] found that TLR2 was not affected, mRNA expression of TLR4 was increased in the jejunum and ileum of chickens exposed to temperatures of 38 ± 1 °C for 8 h/day for 5 days. Heat-stressed pigs also exhibit elevated expression of TLR4 in PBMCs on days 1 and 7 at a constant ambient temperature of 35 °C [86]. Nonetheless, heat stress (40 °C 2 h/day for 3 days) reduces expression of TLR2 and TLR4 in the jejunum of rats [34]. In the ruminal epithelium of heat-stressed dairy cows (constant 28 °C for 4 days), activation of TLR4 or the downstream targets of TLR4 (such as IRAK4, p38MAPK, SAPK/JNK, and NF-κB) were not detected, suggesting that heat stress may not affect this segment of the GIT [88]. Collectively, these results suggest an immediate and direct effect of heat and possibly an indirect effect of lumen bacterial antigens on TLR pathway activation in the small intestine; however, conclusive results and the significance of these findings in the pathogenesis of heat-stressed animals have not been clearly defined.
3.3. Reactive Oxygen Species
Redox homeostasis can be defined as the capacity of an organism to adapt to and control imbalance between oxidants and antioxidants [89]. Oxidants may be derived from numerous sources, such as mitochondria, xanthine oxidases or other oxidases, and peroxidases. In general, an imbalance in favor of oxidants leads to disruption of redox signaling and control and may eventually lead to molecular and cellular dysfunction or damage. Indeed, imbalance between the production of reactive oxygen species (ROS) and cellular antioxidant defense systems may be one of the main consequences of acute heat exposure leading to oxidative stress [4,90] (Figure 2).
Excessive accumulation of NO, O2, and H2O2 occurs due to biochemical dysfunction of cellular respiration and metabolism of purines during cell turnover and to nitric oxide synthase activity in response to intestinal hypoxia caused by shifts in blood circulation from splanchnic tissues to the periphery [4]. Wang et al. [91] observed a rapid increase in ROS production in the mitochondria of duodenal, jejunal, and ileal epithelial cells in chickens exposed to 36 °C for 8 h/day. An in vitro study with intestinal epithelial cells demonstrated that exposure to 42 °C for 60 min increases the concentration of ROS and mitochondrial dysfunction and early apoptotic rates [92]. In addition to increased ROS production, heat stress decreases antioxidative enzyme activity in intestinal tissue in rodents [26]. Heat stress may reduce natural antioxidant capabilities, leading to oxidative stress, in the intestines of pigs [40]. In accordance with this, dietary supplementation with selenium and vitamin E alleviate oxidative stress in heat-stressed pigs [40].
Reactive oxygen species act as secondary messengers that mediate the upregulation of the heat shock response pathway during hyperthermia [93]. Reactive oxygen species activation of the heat shock response pathway leads to the breakdown of oxidized intracellular proteins, which appear unfolded or malformed. These signals function to a greater extent with cytoprotection and adaptation to the survival of cells with accumulation of nonnative oxidized proteins after a heat stress event [50].
Heat stress may lead to intestinal damage and translocation of microorganisms and bacterial antigens in pigs, goats, and rats [2,4,15,16,94]. Moreover, the release of ROS plays an important role as part of the innate immunity mediated by neutrophils in response to a stress event such as bacterial translocation. In fact, immune cells release ROS to reduce bacterial colonization and growth and activate the immune response.
In summary, heat stress seems to promote greater production of ROS as a consequence of circulatory system adaptations, but excessive production of ROS via mechanisms related to cell metabolism, division, and immune function may contribute to an imbalance in the oxidative state, leading to oxidative stress and cellular damage [26]. The role of ROS production in livestock exposed to heat stress warrants further investigation to improve our understanding of heat stress-mediated physiology.
4. Nutritional Interventions to Avoid or Lessen the Effects of Heat Stress
Dietary interventions have shown a direct beneficial effect on intestinal structure and functionality during heat stress. Some of the dietary compounds with the best prospects for use are detailed below.
4.1. Dietary Amino Acids
Dietary amino acids are critical for maintaining the integrity and function of the gut [95]. In recent years, various amino acids, such as L-arginine [22,96] and methionine [97], have been assessed for preventing the negative effects of heat stress on the normal functioning of the GIT. For example, L-arginine acts on numerous metabolic pathways, including protein synthesis and modulation of the immune response [98]. This amino acid can prevent impaired intestinal morphology, limiting villus atrophy in the jejunum and ileum after an inflammatory response [99], and it has been shown to improve the capacity of damaged intestinal hypoxia recovery [100]. The cellular protective activity of dietary L-arginine against a specific heat stress challenge has also been demonstrated [101]. Furthermore, increased intestinal permeability produced by heat stress events [45] are reduced in the presence of dietary L-arginine. This result was shown in heat-stressed mice supplemented with L-arginine, which presented a reduction in excessive intestinal permeability after 4 h of stimulation [102].
The beneficial effects of L-arginine in the development of the epithelial mucosa have been reported in growing piglets supplemented with this amino acid, which presented greater development of villus height throughout the small intestine and crypt depths in the duodenum and jejunum [103]. This response appears not only under physiological conditions but also when the epithelium undergoes a stressful event. With a prolonged thermal stimulus (i.e., 3 days), beneficial effects of L-arginine administration on the integrity of the intestinal structure have been reported, as revealed by a greater villus height with a consequent increase in the villus:crypt ratio in the jejunum of rats. Although the pathways by which L-arginine reduce the impairment generated by hyperthermia in the intestinal mucosa are still uncertain, upregulation of tight junction (ZO-1, occludin, and claudin-6) and adhesion junction (E-cadherin) proteins appears to be key to maintaining intestinal integrity when disrupted by hyperthermia [104] (Table 3). One of the pathways that plays a key role in the anchoring of TJ proteins is activation (through phosphorylation) of AMP-activated protein kinase (AMPK), a key protein involved in energy balance, which promotes expression of ZO-1 and occludin [105,106]. However, phosphorylation of AMPK can be altered by heat stress events [101]. The benefits of L-arginine supplementation in maintaining intestinal integrity after heat stress injury may be channeled by maintaining activation of the AMPK pathway by this amino acid.
Regarding the oxidative effects of heat stress, an increase in radical scavengers such as superoxide dismutase, catalase, and glutathione peroxidase has been observed in the liver and plasma of heat-stressed quails supplemented with methionine [107].

Table 3.
Effect of nutritional interventions on the structural and functional changes to small intestine during heat stress.
Table 3.
Effect of nutritional interventions on the structural and functional changes to small intestine during heat stress.
| Animal Model | Heat Stress Protocol 1 | Days of Sampling 2 | Nutritional Interventions | Intestinal Morphology | Intestinal Barrier Function | Ref. 14 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Type 3 | Product | Tissue | Item 10 | Change 11 | Tissue | AJ or TJ Protein 13 | Change | ||||
| Pigs | 35 ± 1.0 °C—12 h/day | 30 | EAA | L-arginine (1% of diet) | Jejunum | VH, V:C | ↑ | Jejunum | ZO-1 (mRNA) | = | [22] |
| CD | = | OCLD (mRNA) | ↑ | ||||||||
| Rat | 40 °C—3 h/day | 3 | EAA | L-arginine (250 mg/kg BW) | Jejunum | VH | ↑ | Jejunum | ZO-1, CLDN1 (mRNA) | ↑ | [101] |
| CD | ↓ | ||||||||||
| Rats | 40 °C—3 h/day | 3 | EAA | L-arginine (0.5% of diet) | Jejunum | VH, V:C | ↑ | Jejunum | ZO-1, OCLD, CLDN6, E-Cadherin (mRNA) | ↑ | [104] |
| CD | = | ZO-1, OCLD, CLDN6, E-Cadherin | ↑ | ||||||||
| Rats | 45 °C—25 min/day | 4 h | Prebiotic | Yeast culture 4 | SI12 | ZO-1, OCLD, CLDN, JAM-A | ↑ | [108] | |||
| Rats | 45 °C—25 min/day | 4 h | Prebiotic | Yeast culture | VH, MT | ↑ | [109] | ||||
| Broilers | 38 ± 1.0 °C—8 h/day | 5 | Prebiotic | GOS 5 (1% of diet) | Jejunum | E-Cadherin | ↓ | [49] | |||
| CLDN1, CLDN5, ZO-1 | = | ||||||||||
| Ileum | E-Cadherin, CLDN1, CLDN5, ZO-1 | = | |||||||||
| GOS (2.5% of diet) | Jejunum | E-cadherin, CLDN5, ZO-1 | ↓ | ||||||||
| CLDN1 | = | ||||||||||
| Ileum | E-Cadherin, CLDN 1, CLDN5, ZO-1 | = | |||||||||
| Broilers | 33 °C—10 h/day | 20 | Probiotic | Probiotic A 6 | Jejunum | VH | ↑ | OCLD | ↑ | [19] | |
| CD, V:C | = | ZO-1 | = | ||||||||
| Broilers | 35 ± 2 °C—24 h/day | 21 | Prebiotic | MOS 7 | Ileum | VH | ↓ | [110] | |||
| VW, VSA | = | ||||||||||
| CD | ↑ | ||||||||||
| Probiotic | Probiotic B 8 | VH | ↓ | ||||||||
| VW, CD, VSA | ↑ | ||||||||||
| Pre + Pro | Combination 9 | VH | ↓ | ||||||||
| VW, VSA | = | ||||||||||
| CD | ↑ | ||||||||||
| Broilers | 35 ± 2 °C—24 h/day | 42 | Prebiotic | MOS | VH, CD, VSA | = | [110] | ||||
| VW | ↑ | ||||||||||
| Probiotic | Probiotic B | VH, VW, CD, VSA | = | ||||||||
| Pre + Pro | Combination | VH, CD, VSA | ↑ | ||||||||
| VW | = | ||||||||||
| Rats | 40 °C—2 h/day | 3 | Antioxidant | Ferulic acid (50 mg/kg diet) | Jejunum | E-cadherin, OCLD, ZO-1 | ↑ | [44] | |||
1 Heat stress (HS) protocol, including maximum temperature (°C) and intensity (hours of max temperature per day). 2 Day when the animals of the experiment (or some of them) were sacrificed and intestine samples were taken to evaluate the structural and/or functional changes of the tissue. 3 EEA, essential amino acid. 4 Produced by fermentation of Saccharomyces cerevisiae, 7 mg/kg BW. 5 Galacto-oligosaccharides (Vivinal© GOS syrup, Borculo, The Netherlands). 6 Comprised Bacillus licheniformis, Bacillus subtilis, and Lactobacillus plantarum (Chinese Academy of Agricultural Science), 1.5% of diet. 7 Mannan-oligosaccharide, 0.5% of diet. 8 Comprised Lactobacillus plantarum, Lactobacillus delbrueckii ssp. Bulgaricus, Lactobacillus acidophilus, Lactobacillus rhamnosus, Bifidobacterium bifidum, and Streptococcus salivarius ssp. (Protexin©, Probiotics International Ltd., Somerset, UK.). 9 Combination of prebiotic and probiotic described as 7 and 8. 10 VH, villus height; CD, crypt depth; V:C, villus height to crypt depth ratio; MT, mucosa thickness; VW, villus width; VSA, villus surface area. 11 Change produced by nutritional interventions in an HS environment compared with groups in the same HS conditions but without the nutritional intervention (increase ↑ or decrease ↓ when p < 0.05, and without differences, = when p > 0.05). 12 Small inetstine. 13 Adherens junction (AJ) or tight junction (TJ) proteins; ZO-1, zonula Occludens-1; OCLD, occludin; CLDN, claudin; JAM-A, junctional adhesion molecule A. mRNA indicates relative mRNA expression levels of the protein of interest. 14 Reference.
4.2. Probiotic and Prebiotics
In recent years, probiotics and prebiotics such as yeast extracts or galacto-oligosaccharides (GOSs) have been promoted as feed additives to enhance immunity and GIT health [111] in heat-stressed cows [60,112,113], birds [49], and rats [108]. In most of these studies, the benefits in productive parameters, health, and welfare of the animals as a result of supplementation with pro- and prebiotics were examined.
For example, Liu et al. [114] observed that dietary addition of a yeast-derived β-glucan- and mannan-rich probiotic produced through an insoluble preparation of the cell wall from Saccharomyces cerevisiae reduced the expression of HSP70 mRNA in PBMCs from cattle exposed to heat stress. These findings were later supported by results from liver samples collected from heat-stressed cows [115] (Table 4). The authors attributed this downregulation of HSP70 to a decrease in free radical production due to an improvement in antioxidative protection in cows supplemented with the probiotic [114].

Table 4.
Effect of nutritional interventions on the expression of inflammatory-related genes in different tissues during heat stress.
Alteration of the intestinal morphology (e.g., decrease in villi height and mucosa thickness) in heat-stressed rats was alleviated by supplementing their diet with a prebiotic fermentative product of S. cerevisiae [109]. In addition, the magnitude of the loss of Paneth and goblet cells in the intestinal mucosa was reduced in heat-stressed rats consuming a prebiotic prepared from S. cerevisiae [108]. In rats subjected to heat stress, oral supplementation with an S. cerevisiae prebiotic protected the integrity of the intestinal barrier, apparently by promoting expression of TJ proteins, i.e., occludin, claudin, ZO-1, and junctional adhesion molecule A (JAM-A) in the intestine [108] (Table 3).
GOS-based prebiotics have been used to maintain intestinal homeostasis, improving the metabolism of the intestinal epithelium in a model of disease in rodents [117,118]. In heat-stressed chickens, oral supplementation with GOSs reversed the increase in mRNA expression of stress biomarkers in the jejunum, such as HSPs, HSFs, E-cadherin, TJ, and TLR4, but it had no effect on reducing elevated mRNA expression of these markers in the ileum of their heat-stressed counterparts [49]. Heat-stressed chickens fed mannan-oligosaccharide probiotic mixtures displayed reduced heat-induced changes in intestinal morphology and intestinal barrier function [19,110]. Protective effects of GOS-based prebiotics in CACO-2 cells exposed to 42 °C for 24 h have also been reported [119]. Treatment with GOSs protected cells against heat stress, as observed by a decrease in heat-induced HSP70 and HSP90 mRNA and protein levels and by suppression of the heat-induced oxidative stress response, which was assessed by mRNA expression of heme oxygenase-1. Furthermore, in this model, heat-induced disruption of the epithelial structure was particularly associated with derangement of E-cadherin, which was mitigated by pretreatment of cells with GOSs [119]. GOSs may have a macromolecule-stabilizing feature that protects cells against oxidative stress and protein carbonylation. Nondigestible oligosaccharides, such as GOSs, stabilize the structure of lipid bilayers and proteins and prevent protein aggregation and oxidative changes in large molecules [119].
4.3. Antioxidants
Considering that the oxidative balance is disturbed in heat-stressed animals, dietary supplementation with antioxidants seems to be a logical nutritional intervention. Two common antioxidants, vitamin E and selenium (Se), act synergistically to neutralize free radicals, thus improving preventive antioxidant systems in ruminants [120,121], pigs [40], and poultry [122] exposed to heat stress.
For instance, supplementation of vitamin E and Se can elicit positive physiological responses in heat-stressed animals. Reductions in heat-induced increases in rectal temperature and respiration have been observed in pigs [123] and broilers [124] and in respiration and heart rate in heat-stressed ewes [121]. The improvement in physiological parameters may be related to a reduction in the associated increase in the inflammatory tone and improvement in cellular metabolism [4,121].
Supplementation of vitamin E and Se causes a reduction in oxidative stress (subsequently reducing glutathione peroxidase activity) in the small intestine of pigs exposed to heat stress (35 °C, 8 h/day for 2 days) [40], and supplementation with Se reduces the negative effect of heat stress in growing pigs [116]. In vitro models show the protective effect of Se against exposure to high temperatures at the cellular and molecular levels in porcine small intestinal epithelial cells (i.e., IPCE-J2). Using IPCE-J2 cells, Tang et al. [125] described a heat-induced increase in expression of HSP70, which was reduced due to the presence of Se. In addition, deregulation of the gene and protein expression of claudin-1 and ZO-1 caused by heat stress was reversed in Se treatments [125]. Supplementation with Se reduced expression of proinflammatory cytokines and promoters of oxidative stress, such as IL-6, IL-8, interferon beta (IFN-β), nitric oxide synthase 2 (INOS-2), and monocyte chemoattractant protein-1 (MCP-1) [125].
Furthermore, supplementation with ferulic acid, a powerful antioxidant from the phenolic acid family that is present in numerous vegetables, showed preventive efficacy against thermal injury to the integrity of the intestinal epithelial barrier. This was confirmed in studies with IEC-6 cells, showing an improvement in heat stress-induced TER [44,126]. A decrease in FITC-D associated with the administration of dose-dependent ferulic acid was corroborated in vivo in heat stressed rats [44], reflecting a positive effect on the mucosal membrane integrity of the small intestine by ferulic acid under these conditions. Additionally, decreases in ROS generation were found in IEC-6 cells under heat conditions when increasing the dose of ferulic acids [126]. An improvement associated with ferulic acid with treatment was also found in the ultrastructure of TJ morphology and an increase in TJ proteins (i.e., occludin, ZO-1) and E-cadherin in this cell line [126] and in the jejunum of rats subjected to heat stress [44] (Table 3). Although it is not entirely clear how this compound protects the integrity of the intestinal mucosa, recent evidence supports that the protection against the loss of integrity of the intestinal barrier exerted by ferulic acids in heat stress conditions may be due to activate the PI3K/Akt-mediated Nrf2/HO-1 antioxidant signaling pathway [126].
5. Concluding Remarks and Future Perspectives
Physiological changes in animals exposed to elevated ambient temperature are characterized by redistribution of the blood toward the periphery to dissipate heat with a consequent decline in blood flow and oxygen and nutrient supply to the splanchnic tissues. Consequently, metabolic adaptations and gut dysfunction may lead to excessive accumulation of oxidants, translocation of lumen bacteria and endotoxins, and release of proinflammatory mediators. The heat stress phenotype includes activation of a systemic inflammatory response, which may be alleviated by nutritional interventions promoting the maintenance of intestinal homeostasis while reducing systemic inflammation. Future research should aim to elucidate the role of the immune inflammatory response in heat-stressed animals. Nutritional and therapeutic interventions may enhance thermal tolerance to heat by reducing the accumulation of oxidants while maintaining intestinal integrity, but additional data in support of this theory are required.
Author Contributions
Conceptualization, A.G.R., J.M.C. and Z.Y.; investigation, J.M.C., A.G.R. and Z.Y.; data curation, J.M.C. and Z.Y.; writing—original draft preparation, J.M.C.; writing—review and editing, A.G.R.; supervision, A.G.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors recognize Reshma N Mini Ravi for helping with this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fajardo, L.F. Pathological effects of hyperthermia in normal tissues. Cancer Res. 1984, 44, 4826–4836. [Google Scholar]
- Lambert, G.P.; Gisolfi, C.V.; Berg, D.J.; Moseley, P.L.; Oberley, L.W.; Kregel, K.C. Selected Contribution: Hyperthermia-induced intestinal permeability and the role of oxidative and nitrosative stress. J. Appl. Physiol. 2002, 92, 1750–1761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dokladny, K.; Moseley, P.L.; Ma, T.Y. Physiologically relevant increase in temperature causes an increase in intestinal epithelial tight junction permeability. Am. J. Physiol. Liver Physiol. 2006, 290, G204–G212. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.M.; Buettner, G.R.; Oberley, L.W.; Xu, L.; Matthes, R.D.; Gisolfi, C.V. Mechanisms of circulatory and intestinal barrier dysfunction during whole body hyperthermia. Am. J. Physiol.—Heart Circ. Physiol. 2001, 280, 509–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, J.W.; Hale, B.J.; Seibert, J.T.; Romoser, M.R.; Adur, M.K.; Keating, A.F.; Baumgard, L.H. Physiological mechanisms through which heat stress compromises reproduction in pigs. Mol. Reprod. Dev. 2017, 84, 934–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayorga, E.J.; Renaudeau, D.; Ramirez, B.C.; Ross, J.W.; Baumgard, L.H. Heat stress adaptations in pigs. Anim. Front. 2019, 9, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Bloemhof, S.; Van Der Waaij, E.H.; Merks, J.W.M.; Knol, E.F. Sow line differences in heat stress tolerance expressed in reproductive performance traits. J. Anim. Sci. 2008, 86, 3330–3337. [Google Scholar] [CrossRef]
- Lara, L.J.; Rostagno, M.H. Impact of heat stress on poultry production. Animals 2013, 3, 356–369. [Google Scholar] [CrossRef]
- Fouad, A.M.; Chen, W.; Ruan, D.; Wang, S.; Xia, W.G.; Zheng, C.T. Impact of heat stress on meat, egg quality, immunity and fertility in poultry and nutritional factors that overcome these effects: A review. Int. J. Poult. Sci. 2016, 15, 81–95. [Google Scholar] [CrossRef] [Green Version]
- Saeed, M.; Abbas, G.; Alagawany, M.; Kamboh, A.A.; Abd El-Hack, M.E.; Khafaga, A.F.; Chao, S. Heat stress management in poultry farms: A comprehensive overview. J. Therm. Biol. 2019, 84, 414–425. [Google Scholar] [CrossRef]
- West, J.W. Effects of heat-stress on production in dairy cattle. J. Dairy Sci. 2003, 86, 2131–2144. [Google Scholar] [CrossRef]
- Tao, S.; Dahl, G.E. Invited review: Heat stress effects during late gestation on dry cows and their calves. J. Dairy Sci. 2013, 96, 4079–4093. [Google Scholar] [CrossRef]
- Marai, I.F.M.; El-Darawany, A.A.; Fadiel, A.; Abdel-Hafez, M.A.M. Physiological traits as affected by heat stress in sheep-A review. Small Rumin. Res. 2007, 71, 1–12. [Google Scholar] [CrossRef]
- Oliver, S.R.R.; Phillips, N.A.A.; Novosad, V.L.L.; Bakos, M.P.P.; Talbert, E.E.E.; Clanton, T.L.L. Hyperthermia induces injury to the intestinal mucosa in the mouse: Evidence for an oxidative stress mechanism. Am. J. Physiol.—Regul. Integr. Comp. Physiol. 2012, 302, 845–853. [Google Scholar] [CrossRef] [Green Version]
- Pearce, S.C.; Sanz-Fernandez, M.V.; Hollis, J.H.; Baumgard, L.H.; Gabler, N.K. Short-term exposure to heat stress attenuates appetite and intestinal integrity in growing pigs. J. Anim. Sci. 2014, 92, 5444–5454. [Google Scholar] [CrossRef] [Green Version]
- Pearce, S.C.; Mani, V.; Boddicker, R.L.; Johnson, J.S.; Weber, T.E.; Ross, J.W.; Rhoads, R.P.; Baumgard, L.H.; Gabler, N.K. Heat stress reduces intestinal barrier integrity and favors intestinal glucose transport in growing pigs. PLoS ONE 2013, 8, e70215. [Google Scholar] [CrossRef]
- Koch, F.; Thom, U.; Albrecht, E.; Weikard, R.; Nolte, W.; Kuhla, B.; Kuehn, C. Heat stress directly impairs gut integrity and recruits distinct immune cell populations into the bovine intestine. Proc. Natl. Acad. Sci. USA 2019, 116, 10333–10338. [Google Scholar] [CrossRef] [Green Version]
- Sejian, V.; Bhatta, R.; Gaughan, J.B.; Dunshea, F.R.; Lacetera, N. Review: Adaptation of animals to heat stress. Animal 2018, 12, S431–S444. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Xiao, K.; Ke, Y.L.; Jiao, L.F.; Hu, C.H.; Diao, Q.Y.; Shi, B.; Zou, X.T. Effect of a probiotic mixture on intestinal microflora, morphology, and barrier integrity of broilers subjected to heat stress. Poult. Sci. 2014, 93, 581–588. [Google Scholar] [CrossRef]
- Santos, R.R.; Awati, A.; Roubos-van den Hil, P.J.; Tersteeg-Zijderveld, M.H.G.; Koolmees, P.A.; Fink-Gremmels, J. Quantitative histo-morphometric analysis of heat-stress-related damage in the small intestines of broiler chickens. Avian Pathol. 2015, 44, 19–22. [Google Scholar] [CrossRef]
- Wei, L.; Li, Y.; Chang, Q.; Guo, G.; Lan, R. Effects of chitosan oligosaccharides on intestinal oxidative stress and inflammation response in heat stressed rats. Exp. Anim. 2020, 70, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Xiong, Y.; Wu, Q.; Wang, M.; Liu, S.; Jiang, Z.; Wang, L. Effects of dietary supplementation with l-arginine on the intestinal barrier function in finishing pigs with heat stress. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1134–1143. [Google Scholar] [CrossRef] [PubMed]
- Pearce, S.C.; Mani, V.; Weber, T.E.; Rhoads, R.P.; Patience, J.F.; Baumgard, L.H.; Gabler, N.K. Heat stress and reduced plane of nutrition decreases intestinal integrity and function in pigs. J. Anim. Sci. 2013, 91, 5183–5193. [Google Scholar] [CrossRef] [PubMed]
- Wickramasuriya, S.S.; Kim, E.; Cho, H.M.; Shin, T.K.; Kim, B.; Lee, M.; Seo, S.; Heo, J.M.; Choi, H. Differential effects of dietary methionine isomers on broilers challenged with acute heat stress. J. Poult. Sci. 2019, 56, 195–203. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, X.H.; Yang, L.; Chen, X.Y.; Jiang, R.S.; Jin, S.H.; Geng, Z.Y. Resveratrol alleviates heat stress-induced impairment of intestinal morphology, microflora, and barrier integrity in broilers. Poult. Sci. 2017, 96, 4325–4332. [Google Scholar] [CrossRef]
- Yu, J.; Liu, F.; Yin, P.; Zhao, H.; Luan, W.; Hou, X.; Zhong, Y.; Jia, D.; Zan, J.; Ma, W.; et al. Involvement of oxidative stress and mitogen-activated protein kinase signaling pathways in heat stress-induced injury in the rat small intestine. Stress 2013, 16, 99–113. [Google Scholar] [CrossRef]
- Cheng, K.; Song, Z.; Li, S.; Yan, E.; Zhang, H.; Zhang, L.; Wang, C.; Wang, T. Effects of resveratrol on intestinal oxidative status and inflammation in heat-stressed rats. J. Therm. Biol. 2019, 85, 102415. [Google Scholar] [CrossRef]
- Liu, F.; Yin, J.; Du, M.; Yan, P.; Xu, J.; Zhu, X.; Yu, J. Heat-stress-induced damage to porcine small intestinal epithelium associated with downregulation of epithelial growth factor signaling. J. Anim. Sci. 2009, 87, 1941–1949. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Yin, P.; Liu, F.; Cheng, G.; Guo, K.; Lu, A.; Zhu, X.; Luan, W.; Xu, J. Effect of heat stress on the porcine small intestine: A morphological and gene expression study. Comp. Biochem. Physiol. Part A 2010, 156, 119–128. [Google Scholar] [CrossRef]
- Burrin, D.G.; Stoll, B.; Jiang, R.; Chang, X.; Hartmann, B.; Holst, J.J.; Greeley, G.H.; Reeds, P.J. Minimal enteral nutrient requirements for intestinal growth in neonatal piglets: How much is enough? Am. J. Clin. Nutr. 2000, 71, 1603–1610. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.S.; Begum, S.M.K.N.; Rahman, M.M.; Mazumder, R.N.; Parvez, M.; Gazi, M.A.; Hasan, M.M.; Fahim, S.M.; Das, S.; Mahfuz, M.; et al. Alterations in the histological features of the intestinal mucosa in malnourished adults of Bangladesh. Sci. Rep. 2021, 11, 2355. [Google Scholar] [CrossRef]
- Nilaweera, K.N.; Speakman, J.R. Regulation of intestinal growth in response to variations in energy supply and demand. Obes. Rev. 2018, 19, 61–72. [Google Scholar] [CrossRef]
- Liu, L.; Fu, C.; Yan, M.; Xie, H.; Li, S.; Yu, Q.; He, S.; He, J. Resveratrol modulates intestinal morphology and HSP70/90, NF-κB and EGF expression in the jejunal mucosa of black-boned chickens on exposure to circular heat stress. Food Funct. 2016, 7, 1329–1338. [Google Scholar] [CrossRef]
- Liu, X.; Li, H.; Lu, A.; Zhong, Y.; Hou, X.; Wang, N.; Jia, D.; Zan, J.; Zhao, H.; Xu, J.; et al. Reduction of intestinal mucosal immune function in heat-stressed rats and bacterial translocation. Int. J. Hyperth. 2012, 28, 756–765. [Google Scholar] [CrossRef]
- Darwich, A.S.; Aslam, U.; Ashcroft, D.M.; Rostami-Hodjegan, A. Meta-analysis of the turnover of intestinal epithelia in preclinical animal species and humans. Drug Metab. Dispos. 2014, 42, 2016–2022. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Huang, D.; Zhu, M.; Gao, C.; Yan, H.; Li, X.; Wang, X. Wnt/β-catenin-mediated heat exposure inhibits intestinal epithelial cell proliferation and stem cell expansion through endoplasmic reticulum stress. J. Cell. Physiol. 2020, 235, 5613–5627. [Google Scholar] [CrossRef]
- Van Der Flier, L.G.; Clevers, H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol. 2009, 71, 241–260. [Google Scholar] [CrossRef]
- Sanz Fernandez, M.V.; Pearce, S.C.; Gabler, N.K.; Patience, J.F.; Wilson, M.E.; Socha, M.T.; Torrison, J.L.; Rhoads, R.P.; Baumgard, L.H. Effects of supplemental zinc amino acid complex on gut integrity in heat-stressed growing pigs. Animal 2014, 8, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Moseley, P.L.; Gapen, C.; Wallen, E.S.; Walter, M.E.; Peterson, M.W. Thermal stress induces epithelial permeability. Am. J. Physiol.—Cell Physiol. 1994, 267, 425–434. [Google Scholar] [CrossRef]
- Liu, F.; Cottrell, J.J.; Furness, J.B.; Rivera, L.R.; Kelly, F.W.; Wijesiriwardana, U.; Pustovit, R.V.; Fothergill, L.J.; Bravo, D.M.; Celi, P.; et al. Selenium and vitamin E together improve intestinal epithelial barrier function and alleviate oxidative stress in heat-stressed pigs. Exp. Physiol. 2016, 101, 801–810. [Google Scholar] [CrossRef] [Green Version]
- Gabler, N.K.; Koltes, D.; Schaumberger, S.; Murugesan, G.R.; Reisinger, N. Diurnal heat stress reduces pig intestinal integrity and increases endotoxin translocation. Transl. Anim. Sci. 2018, 2, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Goo, D.; Kim, J.H.; Park, G.H.; Reyes, J.B.D.; Kil, D.Y. Effect of heat stress and stocking density on growth performance, breast meat quality, and intestinal barrier function in broiler chickens. Animals 2019, 9, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mani, V.; Rubach, J.K.; Sanders, D.J.; Pham, T.; Koltes, D.A.; Gabler, N.K.; Poss, M.J. Evaluation of the protective effects of zinc butyrate in IPEC-J2 cells and grower pigs under heat stress. Transl. Anim. Sci. 2019, 3, 842–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, S.; Liu, F.; Xu, L.; Yin, P.; Li, D.; Mei, C.; Jiang, L.; Ma, Y.; Xu, J. Protective effects of ferulic acid against heat stress-induced intestinal epithelial barrier dysfunction in vitro and in vivo. PLoS ONE 2016, 11, e0145236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, P.C.; He, S.H.; Zheng, P.Y. Investigation into the signal transduction pathway via which heat stress impairs intestinal epithelial barrier function. J. Gastroenterol. Hepatol. 2007, 22, 1823–1831. [Google Scholar] [CrossRef]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef]
- Nasu, Y.; Ido, A.; Tanoue, S.; Hashimoto, S.; Sasaki, F.; Kanmura, S.; Setoyama, H.; Numata, M.; Funakawa, K.; Moriuchi, A.; et al. Hepatocyte growth factor stimulates the migration of gastric epithelial cells by altering the subcellular localization of the tight junction protein ZO-1. J. Gastroenterol. 2013, 48, 193–202. [Google Scholar] [CrossRef]
- Dokladny, K.; Ye, D.; Kennedy, J.C.; Moseley, P.L.; Ma, T.Y. Cellular and molecular mechanisms of heat stress-induced up-regulation of occludin protein expression: Regulatory role of heat shock factor-1. Am. J. Pathol. 2008, 172, 659–670. [Google Scholar] [CrossRef] [Green Version]
- Varasteh, S.; Braber, S.; Akbari, P.; Garssen, J.; Fink-Gremmels, J. Differences in susceptibility to heat stress along the chicken intestine and the protective effects of galacto-oligosaccharides. PLoS ONE 2015, 10, e0138975. [Google Scholar] [CrossRef] [Green Version]
- Santoro, M.G. Heat shock factors and the control of the stress response. Biochem. Pharmacol. 2000, 59, 55–63. [Google Scholar] [CrossRef]
- Yi, H.; Hu, W.; Chen, S.; Lu, Z.; Wang, Y. Cathelicidin-WA Improves Intestinal Epithelial Barrier Function and Enhances Host Defense against Enterohemorrhagic Escherichia coli O157:H7 Infection. J. Immunol. 2017, 198, 1696–1705. [Google Scholar] [CrossRef] [Green Version]
- Strong, R.A.; Silva, E.B.; Cheng, H.W.; Eicher, S.D. Acute brief heat stress in late gestation alters neonatal calf innate immune functions1. J. Dairy Sci. 2015, 98, 7771–7783. [Google Scholar] [CrossRef]
- Quinteiro-Filho, W.M.; Ribeiro, A.; Ferraz-de-Paula, V.; Pinheiro, M.L.; Sakai, M.; Sá, L.R.M.; Ferreira, A.J.P.; Palermo-Neto, J. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci. 2010, 89, 1905–1914. [Google Scholar] [CrossRef]
- He, S.; Yu, Q.; He, Y.; Hu, R.; Xia, S.; He, J. Dietary resveratrol supplementation inhibits heat stress-induced high-activated innate immunity and inflammatory response in spleen of yellow-feather broilers. Poult. Sci. 2019, 98, 6378–6387. [Google Scholar] [CrossRef]
- Swanson, R.M.; Tait, R.G.; Galles, B.M.; Duffy, E.M.; Schmidt, T.B.; Petersen, J.L.; Yates, D.T. Heat stress-induced deficits in growth, metabolic efficiency, and cardiovascular function coincided with chronic systemic inflammation and hypercatecholaminemia in ractopamine-supplemented feedlot lambs. J. Anim. Sci. 2019, 98, skaa168. [Google Scholar] [CrossRef]
- Cui, Y.; Hao, Y.; Li, J.; Bao, W.; Li, G.; Gao, Y.; Gu, X. Chronic heat stress induces immune response, oxidative stress response, and apoptosis of finishing pig liver: A proteomic approach. Int. J. Mol. Sci. 2016, 17, 393. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Wang, C.; Hao, Y.; Gu, X.; Wang, H. Chronic heat stress induces acute phase responses and serum metabolome changes in finishing pigs. Animals 2019, 9, 395. [Google Scholar] [CrossRef] [Green Version]
- Song, R.; Foster, D.N.; Shurson, G.C. Effects of feeding diets containing bacitracin methylene disalicylate to heat-stressed finishing pigs. J. Anim. Sci. 2011, 89, 1830–1843. [Google Scholar] [CrossRef]
- Kaufman, J.D.; Bailey, H.R.; Kennedy, A.M.; Löffler, F.E.; Ríus, A.G. Cooling and dietary crude protein affected milk production on heat-stressed dairy cows. Livest. Sci. 2020, 240, 104111. [Google Scholar] [CrossRef]
- Kaufman, J.D.; Seidler, Y.; Bailey, H.R.; Whitacre, L.; Bargo, F.; Lüersen, K.; Rimbach, G.; Pighetti, G.M.; Ipharraguerre, I.R.; Ríus, A.G. A postbiotic from Aspergillus oryzae attenuates the impact of heat stress in ectothermic and endothermic organisms. Sci. Rep. 2021, 11, 6407. [Google Scholar] [CrossRef]
- Lim, C.L.; Wilson, G.; Brown, L.; Coombes, J.S.; Mackinnon, L.T. Pre-existing inflammatory state compromises heat tolerance in rats exposed to heat stress. Am. J. Physiol. Integr. Comp. Physiol. 2007, 292, R186–R194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ritossa, F. Discovery of the heat shock response. Cell Stress Chaperones 1996, 1, 97. [Google Scholar] [CrossRef] [Green Version]
- De Maio, A.; Gabriella Santoro, M.; Tanguay, R.M.; Hightower, L.E. Ferruccio Ritossa’s scientific legacy 50 years after his discovery of the heat shock response: A new view of biology, a new society, and a new journal. Cell Stress Chaperones 2012, 17, 139–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, A.; Chauhan, N.R.; Chowdhury, D.; Singh, A.; Meena, R.C.; Chakrabarti, A.; Singh, S.B. Heat stress modulated gastrointestinal barrier dysfunction: Role of tight junctions and heat shock proteins. Scand. J. Gastroenterol. 2017, 52, 1315–1319. [Google Scholar] [CrossRef] [PubMed]
- Feder, M.E.; Hofmann, G.E. Heat-shock proteins, molecular chaperones, and the stress response: Evolutionary and ecological physiology. Annu. Rev. Physiol. 1999, 61, 243–282. [Google Scholar] [CrossRef] [Green Version]
- Archana, P.R.; Aleena, J.; Pragna, P.; Vidya, M.K.; Abdul Niyas, P.A.; Bagath, M.; Krishnan, G.; Manimaran, A.; Beena, V.; Kurien, E.K.; et al. Role of Heat Shock Proteins in Livestock Adaptation to Heat Stress. J. Dairy Vet. Anim. Res. 2017, 5, 00127. [Google Scholar] [CrossRef]
- Hao, Y.; Feng, Y.; Li, J.; Gu, X. Role of MAPKs in HSP70’s Protection against Heat Stress-Induced Injury in Rat Small Intestine. Biomed Res. Int. 2018, 2018, 1571406. [Google Scholar] [CrossRef] [Green Version]
- Zhen, F.S.; Du, H.L.; Xu, H.P.; Luo, Q.B.; Zhang, X.Q. Tissue and allelic-specific expression of hsp70 gene in chickens: Basal and heat-stress-induced mRNA level quantified with real-time reverse transcriptase polymerase chain reaction. Br. Poult. Sci. 2006, 47, 449–455. [Google Scholar] [CrossRef]
- Manzerra, P.; Rush, S.J.; Brown, I.R. Tissue-Specific differences in heat shock protein hsc70 and hsp70 in the control and and hyperthermic rabbit. J. Cell. Physiol. 1997, 170, 130–137. [Google Scholar] [CrossRef]
- Leandro, N.S.M.; Gonzales, E.; Ferro, J.A.; Ferro, M.I.T.; Givisiez, P.E.N.; Macari, M. Expression of heat shock protein in broiler embryo tissues after acute cold or heat stress. Mol. Reprod. Dev. 2004, 67, 172–177. [Google Scholar] [CrossRef]
- Siddiqui, S.H.; Kang, D.; Park, J.; Choi, H.W.; Shim, K. Acute heat stress induces the differential expression of heat shock proteins in different sections of the small intestine of chickens based on exposure duration. Animals 2020, 10, 1234. [Google Scholar] [CrossRef]
- Basu, S.; Binder, R.J.; Suto, R.; Anderson, K.M.; Srivastava, P.K. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-κB pathway. Int. Immunol. 2000, 12, 1539–1546. [Google Scholar] [CrossRef]
- Asea, A.; Kraeft, S.K.; Kurt-Jones, E.A.; Stevenson, M.A.; Chen, L.B.; Finberg, R.W.; Koo, G.C.; Calderwood, S.K. HSP70 stimulates cytokine production through a CD 14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat. Med. 2000, 6, 435–442. [Google Scholar] [CrossRef]
- Swaroop, S.; Mahadevan, A.; Shankar, S.K.; Adlakha, Y.K.; Basu, A. HSP60 critically regulates endogenous IL-1β production in activated microglia by stimulating NLRP3 inflammasome pathway. J. Neuroinflamm. 2018, 15, 177. [Google Scholar] [CrossRef]
- Martine, P.; Rébé, C. Heat shock proteins and inflammasomes. Int. J. Mol. Sci. 2019, 20, 4508. [Google Scholar] [CrossRef] [Green Version]
- Orhan, C.; Akdemir, F.; Sahin, N.; Tuzcu, M.; Komorowski, J.R.; Hayirli, A.; Sahin, K. Chromium histidinate protects against heat stress by modulating the expression of hepatic nuclear transcription factors in quail. Br. Poult. Sci. 2012, 53, 828–835. [Google Scholar] [CrossRef]
- Li, Q.; Verma, I.M. NF-κB regulation in the immune system. Nat. Rev. Immunol. 2002, 2, 725–734. [Google Scholar] [CrossRef]
- Hatada, E.N.; Krappmann, D.; Scheidereit, C. NF-κB and the innate immune response. Curr. Opin. Immunol. 2000, 12, 52–58. [Google Scholar] [CrossRef]
- Sallustio, F.; Curci, C.; Stasi, A.; De Palma, G.; Divella, C.; Gramignoli, R.; Castellano, G.; Gallone, A.; Gesualdo, L. Role of toll-like receptors in actuating stem/progenitor cell repair mechanisms: Different functions in different cells. Stem Cells Int. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Medzhitov, R.; Preston-Hurlburt, P.; Janeway, C.A. A human homologue of the Drosophila toll protein signals activation of adaptive immunity. Nature 1997, 388, 394–397. [Google Scholar] [CrossRef]
- Hemmi, H.; Takeuchi, O.; Kawai, T.; Kaisho, T.; Sato, S.; Sanjo, H.; Matsumoto, M.; Hoshino, K.; Wagner, H.; Takeda, K.; et al. A Toll-like receptor recognizes bacterial DNA. Nature 2000, 408, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Scaffidi, P.; Misteli, T.; Bianchi, M.E. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 2002, 418, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Dangi, S.S.; Gupta, M.; Singh, J.; Thakur, N.; Naskar, S.; Nanda, P.K.; Mohanty, N.; Das, A.K.; Bandopadhayay, S.; et al. Expression of TLR genes in Black Bengal goat (Capra hircus) during different seasons. Small Rumin. Res. 2015, 124, 17–23. [Google Scholar] [CrossRef]
- Vandana, G.D.; Bagath, M.; Sejian, V.; Krishnan, G.; Beena, V.; Bhatta, R. Summer season induced heat stress impact on the expression patterns of different toll-like receptor genes in Malabari goats. Biol. Rhythm Res. 2019, 50, 466–482. [Google Scholar] [CrossRef]
- Dehbi, M.; Uzzaman, T.; Baturcam, E.; Eldali, A.; Ventura, W.; Bouchama, A. Toll-Like Receptor 4 and High-Mobility Group Box 1 Are Critical Mediators of Tissue Injury and Survival in a Mouse Model for Heatstroke. PLoS ONE 2012, 7, e44100. [Google Scholar] [CrossRef]
- Ju, X.-H.; Xu, H.-J.; Yong, Y.-H.; An, L.-L.; Jiao, P.-R.; Liao, M. Heat stress upregulation of Toll-like receptors 2/4 and acute inflammatory cytokines in peripheral blood mononuclear cell (PBMC) of Bama miniature pigs: An in vivo and in vitro study. Animal 2014, 8, 1462–1468. [Google Scholar] [CrossRef] [Green Version]
- Sophia, I.; Sejian, V.; Bagath, M.; Bhatta, R. Quantitative expression of hepatic toll-like receptors 1–10 mRNA in Osmanabadi goats during different climatic stresses. Small Rumin. Res. 2016, 141, 11–16. [Google Scholar] [CrossRef]
- Eslamizad, M.; Albrecht, D.; Kuhla, B. The effect of chronic, mild heat stress on metabolic changes of nutrition and adaptations in rumen papillae of lactating dairy cows. J. Dairy Sci. 2020, 103, 8601–8614. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Lin, H.; Decuypere, E.; Buyse, J. Acute heat stress induces oxidative stress in broiler chickens. Comp. Biochem. Physiol.—Mol. Integr. Physiol. 2006, 144, 11–17. [Google Scholar] [CrossRef]
- Wang, J.; Xue, X.; Liu, Q.; Zhang, S.; Peng, M.; Zhou, J.; Chen, L.; Fang, F. Effects of duration of thermal stress on growth performance, serum oxidative stress indices, the expression and localization of ABCG2 and mitochondria ROS production of skeletal muscle, small intestine and immune organs in broilers. J. Therm. Biol. 2019, 85, 102420. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Xie, W.; Gu, Z.; Xu, Q.; Su, L. Oxidative stress regulates mitogen-activated protein kinases and c-Jun activation involved in heat stress and lipopolysaccharide-induced intestinal epithelial cell apoptosis. Mol. Med. Rep. 2017, 16, 2579–2587. [Google Scholar] [CrossRef] [Green Version]
- Land, W. Allograft injury mediated by reactive oxygen species: From conserved proteins of Drosophila to acute and chronic rejection of human transplants. Part II: Role of reactive oxygen species in the induction of the heat shock response as a regulator of innate. Transplant. Rev. 2003, 17, 31–44. [Google Scholar] [CrossRef]
- Wang, L.; Xue, B.; Wang, K.; Li, S.; Li, Z. Effect of heat stress on endotoxin flux across mesenteric-drained and portal-drained viscera of dairy goat. J. Anim. Physiol. Anim. Nutr. 2011, 95, 468–477. [Google Scholar] [CrossRef]
- Wang, W.W.; Qiao, S.Y.; Li, D.F. Amino acids and gut function. Amino Acids 2009, 37, 105–110. [Google Scholar] [CrossRef]
- Zhu, W.; Jiang, W.; Wu, L.Y. Dietary L-arginine supplement alleviates hepatic heat stress and improves feed conversion ratio of Pekin ducks exposed to high environmental temperature. J. Anim. Physiol. Anim. Nutr. 2014, 98, 1124–1131. [Google Scholar] [CrossRef]
- Pate, R.T.; Luchini, D.; Murphy, M.R.; Cardoso, F.C. Effects of rumen-protected methionine on lactation performance and physiological variables during a heat stress challenge in lactating Holstein cows. J. Dairy Sci. 2020, 103, 2800–2813. [Google Scholar] [CrossRef]
- Ren, W.; Rajendran, R.; Zhao, Y.; Tan, B.; Wu, G.; Bazer, F.W.; Zhu, G.; Peng, Y.; Huang, X.; Deng, J.; et al. Amino acids as mediators of metabolic cross talk between host and pathogen. Front. Immunol. 2018, 9, 319. [Google Scholar] [CrossRef]
- Zhu, H.L.; Liu, Y.L.; Xie, X.L.; Huang, J.J.; Hou, Y.Q. Effect of l-arginine on intestinal mucosal immune barrier function in weaned pigs after Escherichia coli LPS challenge. Innate Immun. 2013, 19, 242–252. [Google Scholar] [CrossRef]
- Sukhotnik, I.; Helou, H.; Mogilner, J.; Lurie, M.; Bernsteyn, A.; Coran, A.G.; Shiloni, E. Oral arginine improves intestinal recovery following ischemia-reperfusion injury in rat. Pediatr. Surg. Int. 2005, 21, 191–196. [Google Scholar] [CrossRef] [Green Version]
- Xia, Z.; Huang, L.; Yin, P.; Liu, F.; Liu, Y.; Zhang, Z.; Lin, J.; Zou, W.; Li, C. l-Arginine alleviates heat stress-induced intestinal epithelial barrier damage by promoting expression of tight junction proteins via the AMPK pathway. Mol. Biol. Rep. 2019, 46, 6435–6451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, K.A.; Soares, A.D.N.; Wanner, S.P.; dos Santos, R.G.C.; Fernandes, S.O.A.; Martins, F.S.; Nicoli, J.R.; Coimbra, C.C.; Cardoso, V.N. L-arginine supplementation prevents increases in intestinal permeability and bacterial translocation in male swiss mice subjected to physical exercise under environmental heat stress. J. Nutr. 2014, 144, 218–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, K.; Guan, S.; Li, T.; Huang, R.; Wu, G.; Ruan, Z.; Yin, Y. Dietary L -arginine supplementation enhances intestinal development and expression of vascular endothelial growth factor in weanling piglets. Br. J. Nutr. 2011, 105, 703–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.; Yin, P.; Liu, F.; Liu, Y.Y.; Liu, Y.Y.; Xia, Z. Protective effects of L-arginine on the intestinal epithelial barrier under heat stress conditions in rats and IEC-6 cell line. J. Anim. Physiol. Anim. Nutr. 2020, 104, 385–396. [Google Scholar] [CrossRef]
- Rowart, P.; Wu, J.; Caplan, M.J.; Jouret, F. Implications of AMPK in the formation of epithelial tight junctions. Int. J. Mol. Sci. 2018, 19, 2040. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.J.; Sun, X.; Du, M. AMPK in regulation of apical junctions and barrier function of intestinal epithelium. Tissue Barriers 2018, 6, 1–13. [Google Scholar] [CrossRef]
- Kalvandi, O.; Sadeghi, A.; Karimi, A. Methionine supplementation improves reproductive performance, antioxidant status, immunity and maternal antibody transmission in breeder Japanese quail under heat stress conditions. Arch. Anim. Breed. 2019, 62, 275–286. [Google Scholar] [CrossRef]
- Ducray, H.A.G.; Globa, L.; Pustovyy, O.; Morrison, E.; Vodyanoy, V.; Sorokulova, I. Yeast fermentate prebiotic improves intestinal barrier integrity during heat stress by modulation of the gut microbiota in rats. J. Appl. Microbiol. 2019, 127, 1192–1206. [Google Scholar] [CrossRef]
- Ducray, H.A.G.; Globa, L.; Pustovyy, O.; Reeves, S.; Robinson, L.; Vodyanoy, V.; Sorokulova, I. Mitigation of heat stress-related complications by a yeast fermentate product. J. Therm. Biol. 2016, 60, 26–32. [Google Scholar] [CrossRef] [Green Version]
- Sohail, M.U.; Hume, M.E.; Byrd, J.A.; Nisbet, D.J.; Ijaz, A.; Sohail, A.; Shabbir, M.Z.; Rehman, H. Effect of supplementation of prebiotic mannan-oligosaccharides and probiotic mixture on growth performance of broilers subjected to chronic heat stress. Poult. Sci. 2012, 91, 2235–2240. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Zhang, B.X.; Yao, K.Y.; Yoon, I.; Chung, Y.H.; Wang, J.K.; Liu, J.X. Effects of supplemental levels of Saccharomyces cerevisiae fermentation product on lactation performance in dairy cows under heat stress. Asian Australas. J. Anim. Sci. 2016, 29, 801–806. [Google Scholar] [CrossRef] [Green Version]
- Al-Qaisi, M.; Horst, E.A.; Mayorga, E.J.; Goetz, B.M.; Abeyta, M.A.; Yoon, I.; Timms, L.L.; Appuhamy, J.A.; Baumgard, L.H. Effects of a Saccharomyces cerevisiae fermentation product on heat-stressed dairy cows. J. Dairy Sci. 2020, 103, 9634–9645. [Google Scholar] [CrossRef]
- Liu, J.; Ye, G.; Zhou, Y.; Liu, Y.; Zhao, L.; Liu, Y.; Chen, X.; Huang, D.; Liao, S.F.; Huang, K. Feeding glycerol-enriched yeast culture improves performance, energy status, and heat shock protein gene expression of lactating Holstein cows under heat stress. J. Anim. Sci. 2014, 92, 2494–2502. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, J.; Ye, G.; Gan, F.; Hamid, M.; Liao, S.; Huang, K. Protective effects of zymosan on heat stress-induced immunosuppression and apoptosis in dairy cows and peripheral blood mononuclear cells. Cell Stress Chaperones 2018, 23, 1069–1078. [Google Scholar] [CrossRef]
- Gan, F.; Ren, F.; Chen, X.; Lv, C.; Pan, C.; Ye, G.; Shi, J.; Shi, X.; Zhou, H.; Shituleni, S.A.; et al. Effects of selenium-enriched probiotics on heat shock protein mRNA levels in piglet under heat stress conditions. J. Agric. Food Chem. 2013, 61, 2385–2391. [Google Scholar] [CrossRef]
- Zhong, Y.; Cai, D.; Cai, W.; Geng, S.; Chen, L.; Han, T. Protective effect of galactooligosaccharide-supplemented enteral nutrition on intestinal barrier function in rats with severe acute pancreatitis. Clin. Nutr. 2009, 28, 575–580. [Google Scholar] [CrossRef]
- Akbari, P.; Braber, S.; Alizadeh, A.; Verheijden, K.A.T.; Schoterman, M.H.C.; Kraneveld, A.D.; Garssen, J.; Fink-Gremmels, J. Galacto-oligosaccharides protect the intestinal barrier by maintaining the tight junction network and modulating the inflammatory responses after a challenge with the mycotoxin deoxynivalenol in human Caco-2 cell monolayers and B6C3F1 mice. J. Nutr. 2015, 145, 1604–1613. [Google Scholar] [CrossRef] [Green Version]
- Varasteh, S.; Braber, S.; Garssen, J.; Fink-Gremmels, J. Galacto-oligosaccharides exert a protective effect against heat stress in a Caco-2 cell model. J. Funct. Foods 2015, 16, 265–277. [Google Scholar] [CrossRef]
- Calamari, L.; Petrera, F.; Abeni, F.; Bertin, G. Metabolic and hematological profiles in heat stressed lactating dairy cows fed diets supplemented with different selenium sources and doses. Livest. Sci. 2011, 142, 128–137. [Google Scholar] [CrossRef]
- Chauhan, S.S.; Celi, P.; Leury, B.J.; Clarke, I.J.; Dunshea, F.R. Dietary antioxidants at supranutritional doses improve oxidative status and reduce the negative effects of heat stress in sheep. J. Anim. Sci. 2014, 92, 3364–3374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habibian, M.; Sadeghi, G.; Ghazi, S.; Moeini, M.M. Selenium as a feed supplement for heat-stressed poultry: A review. Biol. Trace Elem. Res. 2015, 165, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Celi, P.; Cottrell, J.J.; Chauhan, S.S.; Leury, B.J.; Dunshea, F.R. Effects of a short-term supranutritional selenium supplementation on redox balance, physiology and insulin-related metabolism in heat-stressed pigs. J. Anim. Physiol. Anim. Nutr. 2018, 102, 276–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shakeri, M.; Cottrell, J.J.; Wilkinson, S.; Ringuet, M.; Furness, J.B.; Dunshea, F.R. Betaine and antioxidants improve growth performance, breast muscle development and ameliorate thermoregulatory responses to cyclic heat exposure in broiler chickens. Animals 2018, 8, 162. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Cao, L.; Jia, G.; Liu, G.; Chen, X.; Tian, G.; Cai, J.; Shang, H.; Zhao, H. The protective effect of selenium from heat stress-induced porcine small intestinal epithelial cell line (IPEC-J2) injury is associated with regulation expression of selenoproteins. Br. J. Nutr. 2019, 122, 1081–1090. [Google Scholar] [CrossRef]
- He, S.; Guo, Y.; Zhao, J.; Xu, X.; Song, J.; Wang, N.; Liu, Q. Ferulic acid protects against heat stress-induced intestinal epithelial barrier dysfunction in IEC-6 cells via the PI3K/Akt-mediated Nrf2/HO-1 signaling pathway. Int. J. Hyperth. 2018, 35, 112–121. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).