Antimicrobial Effect of Isotretinoin Therapy on Periodontal Pathogens: A Case-Control Study
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Sample
4.2. Subjects
4.3. Microbiological Sample Collection and Preparation
4.4. Q-PCR Analysis
4.5. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eick, S.; Nydegger, J.; Bürgin, W.; Salvi, G.E.; Sculean, A.; Ramseier, C. Microbiological analysis and the outcomes of periodontal treatment with or without adjunctive systemic antibiotics-a retrospective study. Clin. Oral Investig. 2018, 22, 3031–3041. [Google Scholar] [CrossRef]
- Aas, J.A.; Paster, B.J.; Stokes, L.N.; Olsen, I.; Dewhirst, F.E. Defining the normal bacterial flora of the oral cavity. J. Clin. Microbiol. 2005, 43, 5721–5732. [Google Scholar] [CrossRef] [PubMed]
- The Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Signat, B.; Roques, C.; Poulet, P.; Duffaut, D. Fusobacterium nucleatum in periodontal health and disease. Curr. Issues Mol. Biol. 2011, 13, 25–36. [Google Scholar]
- Mombelli, A. Microbial colonization of the periodontal pocket and its significance for periodontal therapy. Periodontology 2000 2018, 76, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Newman, M.G.; Carranza, F.A. Carranza’s Clinical Periodontology; Elsevier/Saunders: St. Louis, MI, USA, 2015. [Google Scholar]
- Cosgarea, R.; Eick, S.; Jepsen, S.; Arweiler, N.B.; Juncar, R.; Tristiu, R.; Salvi, G.E.; Heumann, C.; Sculean, A. Microbiological and host-derived biomarker evaluation following non-surgical periodontal therapy with short-term administration of systemic antimicrobials: Secondary outcomes of an RCT. Sci. Rep. 2020, 10, 16322. [Google Scholar] [CrossRef]
- Zaura, E.; ten Cate, J.M. Towards understanding oral health. Caries Res. 2015, 49, 55–61. [Google Scholar] [CrossRef]
- Baldwin, H.; Tan, J. Effects of diet on acne and its response to treatment. Am. J. Clin. Dermatol. 2021, 22, 55–65. [Google Scholar] [CrossRef]
- Bhatia, A.; Maisonneuve, J.F.; Persing, D.H. Propionibacterium Acnes and Chronic Diseases; National Academies Press: Washington, DC, USA, 2004. [Google Scholar]
- Bettoli, V.; Guerra-Tapia, A.; Herane, M.I.; Piquero-Martín, J. Challenges and Solutions in Oral Isotretinoin in Acne: Reflections on 35 Years of Experience. Clin. Cosmet. Investig. Dermatol. 2019, 12, 943–951. [Google Scholar] [CrossRef]
- (INN) Innfps. Proposed INN: List 118. 2017. Available online: https://www.who.int/medicines/publications/druginformation/innlists/PL118.pdf?ua=1 (accessed on 19 January 2018).
- Papakonstantinou, E.; Aletras, A.J.; Glass, E.; Tsogas, P.; Dionyssopoulos, A.; Adjaye, J.; Fimmel, S.; Gouvousis, P.; Herwig, R.; Lehrach, H.; et al. Matrix metalloproteinases of epithelial origin in facial sebum of patients with acne and their regulation by isotretinoin. J. Investig. Dermatol. 2005, 125, 673–684. [Google Scholar] [CrossRef]
- Piquero-Martin, J.; Misticone, S.; Piquero-Casals, V.; Piquero-Casals, J. Topic therapy-mini isotretinoin doses vs topic therapy-systemic anti- biotics in the moderate acne patients. Ann. Dermatol. Venereol. 2002, 129, S382. [Google Scholar]
- Nast, A.; Dréno, B.; Bettoli, V.; Bukvic Mokos, Z.; Degitz, K.; Dressler, C.; Finlay, A.Y.; Haedersdal, M.; Lambert, J.; Layton, A.; et al. European Evidence-based (S3) guideline for the treatment of acne—Update 2016. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- AlJasser, R.; Alaqeely, R.; Al-Hoqail, I.; Al-Haddab, M.; AlQahtani, S.; AlKenani, M.; AlZahrani, A.M.; AlOraini, S. Association between isotretinoin (Roaccutanne) use and changes in periodontal clinical parameters and MMP-8 and MMP-9 salivary levels. Front. Biosci. 2021, 26, 191–197. [Google Scholar]
- Al Jasser, R.; Al Aqeely, R.; Al Kenani, M.; Al Qahtani, S.; Al Zahrani, A.; Lambarte, R. The effect of systemic Isotretinoin on salivary tissue inhibitors of metalloproteinases 1 and 2 and salivary flow rate in periodontal disease. Saudi J. Biol. Sci. 2021, 48, 2464. [Google Scholar]
- Papaioannou, W.; Gizani, S.; Haffajee, A.D.; Quirynen, M.; Mamai-Homata, E.; Papagiannoulis, L. The microbiota on different oral surfaces in healthy children. Oral Microbiol. Immunol. 2009, 24, 183–189. [Google Scholar] [CrossRef]
- Holt, R.; Roberts, G.; Scully, C. ABC of oral health. Dental damage, sequelae, and prevention. BMJ 2000, 320, 1717–1719. [Google Scholar] [CrossRef][Green Version]
- Haffajee, A.D.; Socransky, S.S. Microbial etiological agents of destructive periodontal diseases. Periodontology 2000 1994, 5, 78–111. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D. The bacterial etiology of destructive periodontal disease: Current concepts. J. Periodontol. 1992, 63, 322–331. [Google Scholar] [CrossRef]
- Ji, S.; Kim, Y.; Min, B.-M.; Han, S.H.; Choi, Y. Innate immune responses of gingival epithelial cells to nonperiodontopathic and periodontopathic bacteria. J. Periodontal. Res. 2007, 42, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Gollnick, H.; Cunliffe, W.; Berson, D.; Dreno, B.; Finlay, A.; Leyden, J.J.; Shalita, A.R.; Thiboutot, D.; Global Alliance to Improve Outcomes in Acne. Global Alliance to Improve Outcomes in Acne Management of acne: A report from a Global Alliance to Improve Outcomes in Acne. J. Am. Acad. Dermatol. 2003, 49, S1–S37. [Google Scholar] [CrossRef] [PubMed]
- Leyden, J.J.; McGinley, K.J.; Foglia, A.N. Qualitative and quantitative changes in cutaneous bacteria associated with systemic isotretinoin therapy for acne conglobata. J. Investig. Dermatol. 1986, 86, 390–393. [Google Scholar] [CrossRef] [PubMed]
- King, K.; Jones, D.H.; Daltrey, D.C.; Cunliffe, W.J. A double-blind study of the effects of 13-cis-retinoic acid on acne, sebum excretion rate and microbial population. Br. J. Dermatol. 1982, 107, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Erdemir, U.; Okan, G.; Gungor, S.; Tekin, B.; Yildiz, S.O.; Yildiz, E. The oral adverse effects of isotretinoin treatment in acne vulgaris patients: A prospective, case-control study. Niger. J. Clin. Pract. 2017, 20, 860–866. [Google Scholar] [CrossRef]
- Lupi-Pégurier, L.; Muller-Bolla, M.; Fontas, E.; Ortonne, J.P. Reduced salivary flow induced by systemic isotretinoin may lead to dental decay. A prospective clinical study. Dermatology 2007, 214, 221–226. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: London, UK, 2013. [Google Scholar]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.C.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions—Introduction and key changes from the 1999 classification. J. Clin. Periodontol. 2018, 45, S1–S8. [Google Scholar] [CrossRef]
- Snoek-van Beurden, P.A.M.; Von den Hoff, J.W. Zymographic techniques for the analysis of matrix metalloproteinases and their inhibitors. Biotechniques 2005, 38, 73–83. [Google Scholar] [CrossRef]

| Species | Sequence (5′-3′) | Product Size (16S rRNA) |
|---|---|---|
| Porphyromonas gingivalis | ||
| Forward primer | 5′-TAC CCA TCG CCT TGG T-3′ | 19 bp |
| Reverse primer | 5′-CGG ACT AAA ACC GCA TAC ACT TG-3′ | 23 bp |
| Fusobacterium nucleatum | ||
| Forward primer | 5′-CGC AGA AGG TGA AAG TCC TGT AT-3′ | 23 bp |
| Reverse primer | 5′-TGG TCC TCA CTG ATT CAC ACA GA-3′ | 23 bp |
| Tannerella forsythia | ||
| Forward primer | 5′-ATC CTG GCT CAG GAT GAA CG-3′ | 20 bp |
| Reverse primer | 5′-TAC GCA TAC CCA TCC GCA A-3′ | 19 bp |
| Treponema denticola | ||
| Forward primer | 5′-AGA GCA AGC TCT CCC TTA CCG T-3′ | 22 bp |
| Reverse primer | 5′-TAA GGG CGG CTT GAA ATA ATG A-3′ | 22 bp |
| Variables | HC | HINN | GC | GINN | PC | PINN | p-Value |
|---|---|---|---|---|---|---|---|
| Polymerase chain reaction (%) | |||||||
| Porphyromonas gingivalis Mean (SD) | 0.13 (0.22) | 1.3 (2.4) | 10.5 (12.9) | 3.9 (6.4) | 25.4 (11.0) | 5.9 (5.9) | <0.001 * |
| Tannerella forsythia Mean (SD) | 3.5 (6.2) | 1.8 (4.5) | 1.9 (2.1) | 0.78 (1.2) | 21.2 (9.3) | 5.7 (5.5) | <0.001 * |
| Treponema denticola Mean (SD) | 6.6 (9.6) | 10.1 (9.3) | 9.3 (17.9) | 3.9 (9.3) | 24.9 (10.7) | 18.5 (11.5) | <0.001 * |
| Fusobacterium nucleatum Mean (SD) | 8.9 (15.9) | 8.6 (6.7) | 1.7 (3.7) | 3.2 (5.6) | 0.01 (0.02) | 8.7 (16.2) | <0.001 * |
| Variables | GINN | GC | p-Value | ||
|---|---|---|---|---|---|
| Median | Mean | Median | Mean | ||
| (IQR) | Ranks | (IQR) | Ranks | ||
| Polymerase chain reaction (%) | |||||
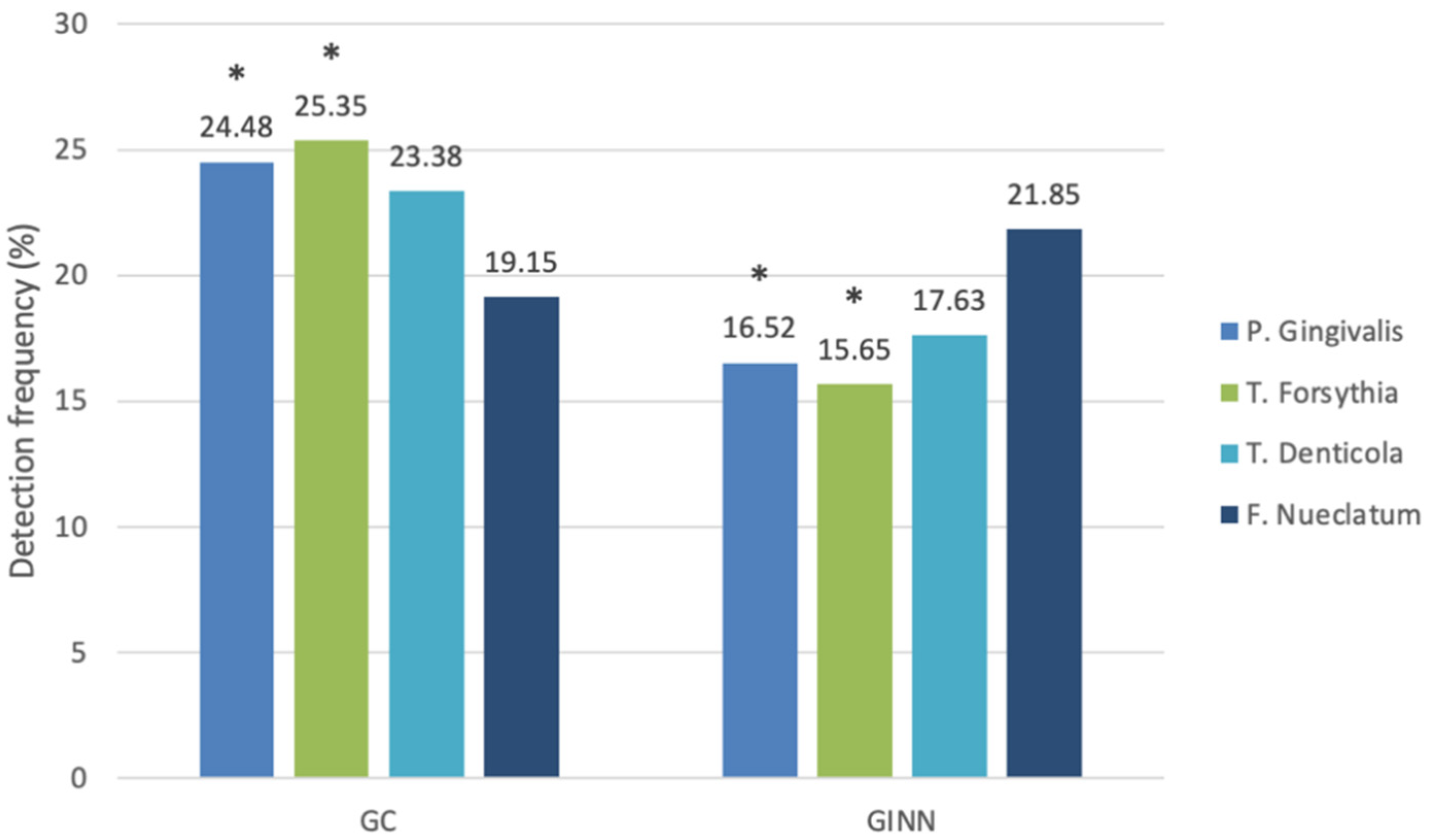
| Porphyromonas gingivalis | 1 (4.6) | 16.52 | 3.6 (22.4) | 24.48 | 0.032 * |
| Tannerella forsythia | 0.3 (0.6) | 15.65 | 1.3 (1.9) | 25.35 | 0.009 * |
| Treponema denticola | 0.8 (3.6) | 17.63 | 1.1 (9.9) | 23.38 | 0.12 |
| Fusobacterium nucleatum | 0.8 (2.2) | 21.85 | 0.5 (1.4) | 19.15 | 0.465 |
| Variables | PC | PINN | p-Value | ||
|---|---|---|---|---|---|
| Mean Ranks | Median | Mean Ranks | Median | ||
| (IQR) | (IQR) | ||||
| Polymerase chain reaction (%) | |||||
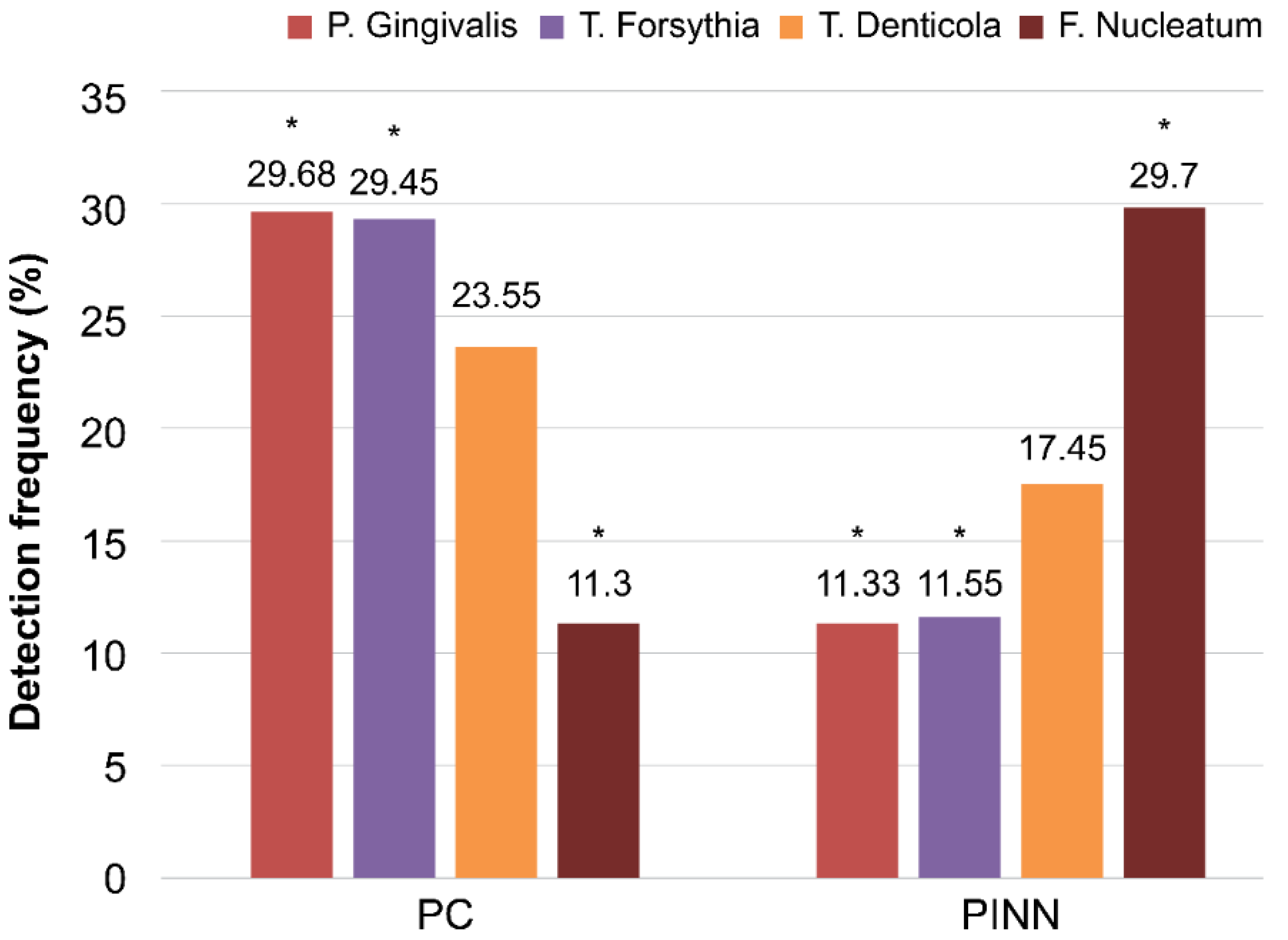
| Porphyromonas gingivalis | 29.68 | 23.1 (17.9) | 11.33 | 3.8 (8.2) | <0.0001 * |
| Tannerella forsythia | 29.45 | 18.8 (7) | 11.55 | 3.2 (7.6) | <0.0001 * |
| Treponema denticola | 23.55 | 23.1 (15.8) | 17.45 | 18.2 (16.6) | 0.098 |
| Fusobacterium nucleatum | 11.3 | 0.01 (0.03) | 29.7 | 2.3 (2.9) | <0.0001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlJasser, R.; AlAqeely, R.; AlZahrani, A.; AlKenani, M.; AlQahtani, S.; AlSarhan, M.; AlOtaibi, D.; Lambarte, R. Antimicrobial Effect of Isotretinoin Therapy on Periodontal Pathogens: A Case-Control Study. Antibiotics 2021, 10, 1286. https://doi.org/10.3390/antibiotics10111286
AlJasser R, AlAqeely R, AlZahrani A, AlKenani M, AlQahtani S, AlSarhan M, AlOtaibi D, Lambarte R. Antimicrobial Effect of Isotretinoin Therapy on Periodontal Pathogens: A Case-Control Study. Antibiotics. 2021; 10(11):1286. https://doi.org/10.3390/antibiotics10111286
Chicago/Turabian StyleAlJasser, Reham, Razan AlAqeely, Afnan AlZahrani, Manal AlKenani, Sadeem AlQahtani, Mohammed AlSarhan, Dalal AlOtaibi, and Rhodanne Lambarte. 2021. "Antimicrobial Effect of Isotretinoin Therapy on Periodontal Pathogens: A Case-Control Study" Antibiotics 10, no. 11: 1286. https://doi.org/10.3390/antibiotics10111286
APA StyleAlJasser, R., AlAqeely, R., AlZahrani, A., AlKenani, M., AlQahtani, S., AlSarhan, M., AlOtaibi, D., & Lambarte, R. (2021). Antimicrobial Effect of Isotretinoin Therapy on Periodontal Pathogens: A Case-Control Study. Antibiotics, 10(11), 1286. https://doi.org/10.3390/antibiotics10111286






