A Strategy for Hospital Pharmacists to Control Antimicrobial Resistance (AMR) in Japan
Abstract
1. Introduction
2. Antimicrobial Stewardship with MRSA and Extended-Spectrum β-Lactamase (ESBL)-Producing Enterobacteriaceae
2.1. Epidemiology of MRSA in Japan
2.2. Antimicrobial Stewardship of MRSA by Pharmacists in Japan
2.3. Anti-Extended-Spectrum β-Lactamase (ESBL)-Producing Enterobacteriaceae Agents Stewardship
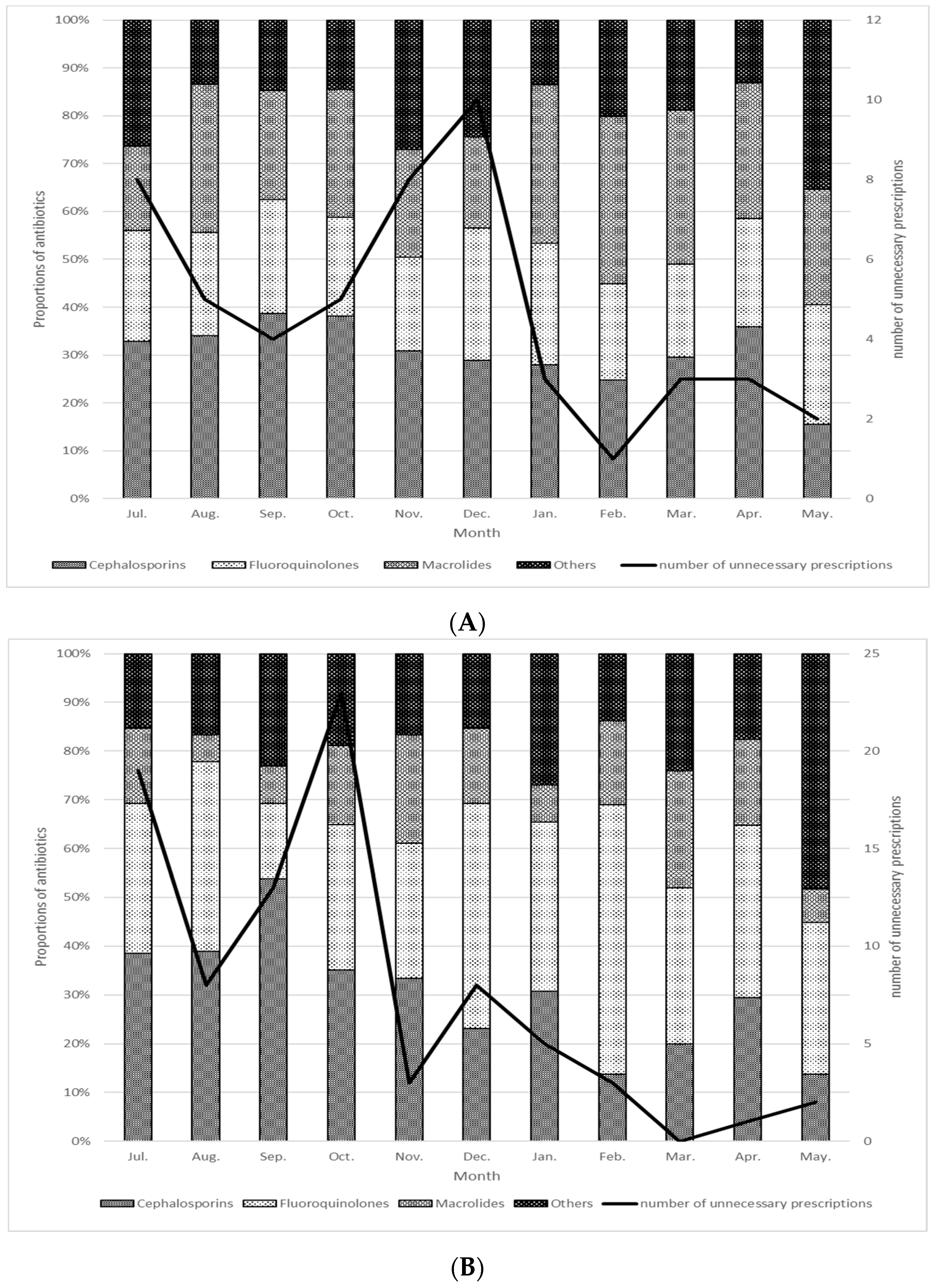
Epidemiology of ESBL in Japan
2.4. Antimicrobial Stewardship of ESBL by Pharmacists in Japan
3. Antifungal Stewardship
3.1. Epidemiology of Fungal Infections in Japan
3.2. Antifungal Stewardship by Pharmacists in Japan
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Action Plan on Antimicrobial Resistance. 2015. Available online: https://www.who.int/antimicrobial-resistance/global-action-plan/en/ (accessed on 11 August 2021).
- World Health Organization. Global Action Plan on Antimicrobial Resistance. 2020. Available online: https://www.who.int/antimicrobial-resistance/publications/global-action-plan/en/ (accessed on 11 August 2021).
- World Health Organization. Country Self Assessment in Global Database for Antimicrobial Resistance. 2020. Available online: https://amrcountryprogress.org/ (accessed on 11 August 2021).
- The Government of Japan. NAP on Antimicrobial Resistance (AMR) 2016–2020. 2020. Available online: https://www.mhlw.go.jp/file/06-Seisakujouhou-10900000-Kenkoukyoku/0000138942.pdf (accessed on 11 August 2021).
- Muraki, Y.; Yagi, T.; Tsuji, Y.; Nishimura, N.; Tanabe, M.; Niwa, T.; Watanabe, T.; Fujimoto, S.; Takayama, K.; Murakami, N.; et al. Japanese antimicrobial consumption surveillance: First report on oral and parenteral antimicrobial consumption in Japan (2009–2013). J. Glob. Antimicrob. Resist. 2016, 7, 19–23. [Google Scholar] [CrossRef]
- Ebihara, F.; Hamada, Y.; Maruyama, T.; Nakamura, S.; Takahashi, S.; Hasegawa, Y.; Konishi, T.; Mitsuda, T.; Kikuchi, K.; Kimura, T. Outpatient use of oral antibiotics and initiatives for appropriate use. Jpn. J. Chemother. 2021, 69, 392–397. [Google Scholar]
- Brody, H. Medicine’s ethical responsibility for health care reform—The Top Five list. N. Engl. J. Med. 2010, 362, 283–285. [Google Scholar] [CrossRef]
- AMR Alliance Japan. Recommendations toward the Next National Action Plan on Antimicrobial Resistance. Available online: https://hgpi.org/en/wp-content/uploads/sites/2/Action_Plan_Recommendations_ENG.pdf (accessed on 11 August 2021).
- Uematsu, H.; Yamashita, K.; Kunisawa, S.; Fushimi, K.; Imanaka, Y. The economic burden of methicillin-resistant Staphylococcus aureus in community-onset pneumonia inpatients. Am. J. Infect. Control 2016, 44, 1628–1633. [Google Scholar] [CrossRef]
- Ministry of Health, Labour and Welfare. Japan Nosocomial Infections Surveillance Clinical Laboratory Division [CLSI 2012 Version]. Available online: https://janis.mhlw.go.jp/english/report/open_report/2020/3/1/ken_Open_Report_Eng_202000_clsi2012.pdf (accessed on 11 August 2021).
- Tsuzuki, S.; Matsunaga, N.; Yahara, K.; Gu, Y.; Hayakawa, K.; Hirabayashi, A.; Kajihara, T.; Sugai, M.; Shibayama, K.; Ohmagari, N. National trend of blood-stream infection attributable deaths caused by Staphylococcus aureus and Escherichia coli in Japan. J. Infect. Chemother. 2020, 26, 367–371. [Google Scholar] [CrossRef]
- Tsuzuki, S.; Matsunaga, N.; Yahara, K.; Shibayama, K.; Sugai, M.; Ohmagari, N. Disease burden of bloodstream infections caused by antimicrobial-resistant bacteria: A population-level study, Japan, 2015–2018. Int. J. Infect. Dis. 2021, 108, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Lopez, A.D. The utility of DALYs for public health policy and research: A reply. Bull. World Health Organ. 1997, 75, 377–381. [Google Scholar] [PubMed]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis 2019, 19, 56–66. [Google Scholar] [CrossRef]
- Uematsu, H.; Yamashita, K.; Mizuno, S.; Kunisawa, S.; Shibayama, K.; Imanaka, Y. Effect of methicillin-resistant Staphylococcus aureus in Japan. Am. J. Infect. Control. 2018, 46, 1142–1147. [Google Scholar] [CrossRef]
- Japanese Society of Chemotherapy/Japanese Association for Infectious Diseases. Practical Guidelines for the Management and Treatment of Infections Caused by MRSA (In Japanese). 2019. Available online: http://www.chemotherapy.or.jp/guideline/guideline_mrsa_2019.html (accessed on 18 October 2021).
- Goto, R.; Inose, R.; Kusama, Y.; Kawabe, A.; Ishii, S.; Ebisui, A.; Ishikane, M.; Yagi, T.; Ohmagari, N.; Muraki, Y. Trends of the Use of Anti-methicillin-Resistant Staphylococcus aureus Agents in Japan Based on Sales Data from 2006 to 2015. Biol. Pharm. Bull. 2020, 43, 1906–1910. [Google Scholar] [CrossRef]
- Hansen, S.; Schwab, F.; Asensio, A.; Carsauw, H.; Heczko, P.; Klavs, I.; Lyytikäinen, O.; Palomar, M.; Riesenfeld-Orn, I.; Savey, A.; et al. Methicillin-resistant Staphylococcus aureus (MRSA) in Europe: Which infection control measures are taken? Infection 2010, 38, 159–164. [Google Scholar] [CrossRef]
- Kim, Y.C.; Kim, M.H.; Song, J.E.; Ahn, J.Y.; Oh, D.H.; Kweon, O.M.; Lee, D.; Kim, S.B.; Kim, H.W.; Jeong, S.J.; et al. Trend of methicillin-resistant Staphylococcus aureus (MRSA) bacteremia in an institution with a high rate of MRSA after the reinforcement of antibiotic stewardship and hand hygiene. Am. J. Infect. Control 2013, 41, e39–e43. [Google Scholar] [CrossRef]
- Johnson, P.D.; Martin, R.; Burrell, L.J.; Grabsch, E.A.; Kirsa, S.W.; O’Keeffe, J.; Mayall, B.C.; Edmonds, D.; Barr, W.; Bolger, C.; et al. Efficacy of an alcohol/chlorhexidine hand hygiene program in a hospital with high rates of nosocomial methicillin-resistant Staphylococcus aureus (MRSA) infection. Med. J. Aust. 2005, 183, 509–514. [Google Scholar] [CrossRef]
- MacDonald, A.; Dinah, F.; MacKenzie, D.; Wilson, A. Performance feedback of hand hygiene, using alcohol gel as the skin decontaminant, reduces the number of inpatients newly affected by MRSA and antibiotic costs. J. Hosp. Infect. 2004, 56, 56–63. [Google Scholar] [CrossRef]
- Aldeyab, M.A.; Scott, M.G.; Kearney, M.P.; Alahmadi, Y.M.; Magee, F.A.; Conlon, G.; McElnay, J.C. Impact of an enhanced antibiotic stewardship on reducing methicillin-resistant Staphylococcus aureus in primary and secondary healthcare settings. Epidemiol. Infect. 2014, 142, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Lawes, T.; López-Lozano, J.M.; Nebot, C.; Macartney, G.; Subbarao-Sharma, R.; Dare, C.R.; Edwards, G.F.; Gould, I.M. Turning the tide or riding the waves? Impacts of antibiotic stewardship and infection control on MRSA strain dynamics in a Scottish region over 16 years: Non-linear time series analysis. BMJ Open 2015, 5, e006596. [Google Scholar] [CrossRef] [PubMed]
- Niwa, T.; Shinoda, Y.; Suzuki, A.; Ohmori, T.; Yasuda, M.; Ohta, H.; Fukao, A.; Kitaichi, K.; Matsuura, K.; Sugiyama, T.; et al. Outcome measurement of extensive implementation of antimicrobial stewardship in patients receiving intravenous antibiotics in a Japanese university hospital. J. Clin. Pract. 2012, 66, 999–1008. [Google Scholar] [CrossRef]
- Ohashi, K.; Matsuoka, T.; Shinoda, Y.; Fukami, Y.; Shindoh, J.; Yagi, T.; Yoshimura, T.; Sugiyama, T. Evaluation of treatment outcomes of patients with MRSA bacteremia following antimicrobial stewardship programs with pharmacist intervention. Int. J. Clin. Pract. 2018, 72, e13065. [Google Scholar] [CrossRef] [PubMed]
- Rybak, M.J.; Le, J.; Lodise, T.P.; Levine, D.P.; Bradley, J.S.; Liu, C.; Mueller, B.A.; Pai, M.P.; Wong-Beringer, A.; Rotschafer, J.C.; et al. Therapeutic Monitoring of Vancomycin for Serious Methicillin-resistant Staphylococcus aureus Infections: A Revised Consensus Guideline and Review by the American Society of Health-system Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Clin. Infect. Dis. 2020, 71, 1361–1364. [Google Scholar]
- Suzuki, A.; Hamada, Y.; Ikeda, H.; Tanaka, H.; Yanagihara, M.; Namiki, M.; Watanabe, T.; Sasaki, T. Comparison of trough concentration and area under the curve of vancomycin associated with the incidence of nephrotoxicity and predictors of a high trough level. J. Infect. Chemother. 2021, 27, 455–460. [Google Scholar] [CrossRef]
- Hashimoto, N.; Kimura, T.; Hamada, Y.; Niwa, T.; Hanai, Y.; Chuma, M.; Fujii, S.; Matsumoto, K.; Shigemi, A.; Kawamura, H.; et al. Candidates for area under the time-concentration curve-guided dosing and risk reduction based on analyses of risk factors associated with nephrotoxicity in vancomycin-treated patients. J. Glob. Antimicrob. Resist. 2021, 27, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Ferri, M.; Ranucci, E.; Romagnoli, P.; Giaccone, V. Antimicrobial resistance: A global emerging threat to public health systems. Crit. Rev. Food Sci. Nutr. 2017, 57, 2857–2876. [Google Scholar] [CrossRef] [PubMed]
- Willyard, C. The drug-resistant bacteria that pose the greatest health threats. Nature 2017, 543, 15. [Google Scholar] [CrossRef] [PubMed]
- Woerther, P.L.; Burdet, C.; Chachaty, E.; Andremont, A. Trends in human fecal carriage of extended-spectrum β-lactamases in the community: Toward the globalization of CTX-M. Clin. Microbiol. Rev. 2013, 26, 744–758. [Google Scholar] [CrossRef]
- Chong, Y.; Shimoda, S.; Shimono, N. Current epidemiology, genetic evolution and clinical impact of extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae. Infect. Genet. Evol. 2018, 61, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, M.; Yamada, Y.; Matsuo, K.; Komiya, Y.; Uchiyama, M.; Nagata, N.; Takata, T.; Jimi, S.; Imakyure, O. Change in the Antimicrobial Resistance Profile of Extended-Spectrum β-Lactamase-Producing Escherichia coli. J. Clin. Med. Res. 2019, 11, 635–641. [Google Scholar] [CrossRef]
- Amann, S.; Neef, K.; Kohl, S. Antimicrobial resistance (AMR). Eur. J. Hosp. Pharm. 2019, 26, 175–177. [Google Scholar] [CrossRef]
- FAO. The FAO Action Plan on Antimicrobial Resistance 2016–2020. Available online: http://www.fao.org/3/a-i5996e.pdf (accessed on 11 August 2021).
- Nishiyama, M.; Praise, S.; Tsurumaki, K.; Baba, H.; Kanamori, H.; Watanabe, T. Prevalence of Antibiotic-Resistant Bacteria ESKAPE among Healthy People Estimated by Monitoring of Municipal Wastewater. Antibiotics 2021, 10, 495. [Google Scholar] [CrossRef]
- Harris, P.N.A.; Tambyah, P.A.; Lye, D.C.; Mo, Y.; Lee, T.H.; Yilmaz, M.; Alenazi, T.H.; Arabi, Y.; Falcone, M.; Bassetti, M.; et al. Effect of piperacillin-tazobactam vs. meropenem on 30-day mortality for patients with E. coli or Klebsiella pneumoniae bloodstream infection and ceftriaxone resistance: A randomized clinical trial. JAMA 2018, 320, 984–994. [Google Scholar] [CrossRef]
- Doi, Y. Treatment Options for Carbapenem-resistant Gram-negative Bacterial Infections. Clin. Infect. Dis. 2019, 69, S565–s575. [Google Scholar] [CrossRef]
- Wada, K.; Yokoyama, T.; Uno, S.; Araki, M.; Sadahira, T.; Maruyama, Y.; Acosta, H.; Nakajima, H.; Hiyama, Y.; Kunishima, Y.; et al. Nationwide surveillance of bacterial pathogens isolated from patients with acute uncomplicated cystitis in 2018: Conducted by the Japanese Research Group for Urinary Tract Infections (JRGU). J. Infect. Chemother. 2021, 27, 1169–1180. [Google Scholar] [CrossRef]
- Nakai, H.; Hagihara, M.; Kato, H.; Hirai, J.; Nishiyama, N.; Koizumi, Y.; Sakanashi, D.; Suematsu, H.; Yamagishi, Y.; Mikamo, H. Prevalence and risk factors of infections caused by extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae. J. Infect. Chemother. 2016, 22, 319–326. [Google Scholar] [CrossRef]
- Tulara, N.K. Nitrofurantoin and Fosfomycin for Extended Spectrum Beta-lactamases Producing Escherichia coli and Klebsiella pneumoniae. J. Glob. Infect. Dis. 2018, 10, 19–21. [Google Scholar] [CrossRef]
- Fleming-Dutra, K.E.; Hersh, A.L.; Shapiro, D.; Bartoces, M.; Enns, E.A.; File, T.M., Jr.; Finkelstein, J.A.; Gerber, J.S.; Hyun, D.Y.; Linder, J.A.; et al. Prevalence of Inappropriate Antibiotic Prescriptions among US Ambulatory Care Visits, 2010–2011. JAMA 2016, 315, 1864–1873. [Google Scholar] [CrossRef]
- Doron, S.; Davidson, L.E. Antimicrobial stewardship. Mayo Clin. Proc. 2011, 86, 1113–1123. [Google Scholar] [CrossRef]
- Kusama, Y.; Tsuzuki, S.; Muraki, Y.; Koizumi, R.; Ishikane, M.; Ohmagari, N. The effects of Japan’s National Action Plan on Antimicrobial Resistance on antimicrobial use. Int. J. Infect. Dis. 2021, 103, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Tanaka, I.; Seyama, Y.; Sekikawa, R.; Suzuki, S.; Nagasawa, M.; Hino, S. The effectiveness of prescription support and treatment reporting system on the appropriate usage of oral third-generation cephalosporins. J. Infect. Chemother. 2021, 27, 419–423. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. Available online: https://www.who.int/news-room/detail/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 1 August 2021).
- Matsumura, Y.; Yamamoto, M.; Nagao, M.; Tanaka, M.; Takakura, S.; Ichiyama, S. In vitro activities and detection performances of cefmetazole and flomoxef for extended-spectrum β-lactamase and plasmid-mediated AmpC β-lactamase-producing Enterobacteriaceae. Diagn. Microbiol. Infect. Dis. 2016, 84, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Yamamoto, M.; Nagao, M.; Komori, T.; Fujita, N.; Hayashi, A.; Shimizu, T.; Watanabe, H.; Doi, S.; Tanaka, M.; et al. Multicenter retrospective study of cefmetazole and flomoxef for treatment of extended-spectrum-beta-lactamase-producing Escherichia coli bacteremia. Antimicrob. Agents Chemother. 2015, 59, 5107–5113. [Google Scholar] [CrossRef]
- Tashiro, S.; Hayashi, M.; Takemura, W.; Igarashi, Y.; Liu, X.; Mizukami, Y.; Kojima, N.; Enoki, Y.; Taguchi, K.; Yokoyama, Y.; et al. Pharmacokinetics/Pharmacodynamics Evaluation of Flomoxef against Extended-Spectrum Beta-Lactamase-Producing Escherichia coli In Vitro and In Vivo in a Murine Thigh Infection Model. Pharm. Res. 2021, 38, 27–35. [Google Scholar] [CrossRef]
- Doi, A.; Shimada, T.; Harada, S.; Iwata, K.; Kamiya, T. The efficacy of cefmetazole against pyelonephritis caused by extended-spectrum beta-lactamase-producing Enterobacteriaceae. Int. J. Infect. Dis. 2013, 17, e159–e163. [Google Scholar] [CrossRef] [PubMed]
- Fukuchi, T.; Iwata, K.; Kobayashi, S.; Nakamura, T.; Ohji, G. Cefmetazole for bacteremia caused by ESBL-producing enterobacteriaceae comparing with carbapenems. BMC Infect. Dis. 2016, 16, 427. [Google Scholar] [CrossRef]
- Hamada, Y.; Matsumura, Y.; Nagashima, M.; Akazawa, T.; Doi, Y.; Hayakawa, K. Retrospective evaluation of appropriate dosing of cefmetazole for invasive urinary tract infection due to extended-spectrum β-lactamase-producing Escherichia coli. J. Infect. Chemother. 2021, 27, 1602–1606. [Google Scholar] [CrossRef] [PubMed]
- Wisplinghoff, H.; Bischoff, T.; Tallent, S.M.; Seifert, H.; Wenzel, R.P.; Edmond, M.B. Nosocomial bloodstream infections in US hospitals: Analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 2004, 39, 309–317. [Google Scholar] [CrossRef]
- Kakeya, H.; Yamada, K.; Kaneko, Y.; Yanagihara, K.; Tateda, K.; Maesaki, S.; Takesue, Y.; Tomono, K.; Kadota, J.I.; Kaku, M.; et al. National Trends in the Distribution of Candida Species Causing Candidemia in Japan from 2003 to 2014. Med. Mycol. J. 2018, 59, E19–E22. [Google Scholar] [CrossRef][Green Version]
- Kishimoto, K.; Kasai, M.; Kawamura, N.; Ito, Y.; Yoshida, M.; Hasegawa, D.; Kawasaki, K.; Kosaka, Y. Clinical features in proven and probable invasive fungal disease in children and adolescents at a pediatric referral center: A 5-year experience. World J. Pediatr. 2019, 15, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Kawabe, A.; Muraki, Y.; Inose, R.; Kusama, Y.; Goto, R.; Ebisui, A.; Ishii, S.; Ishikane, M.; Ohge, H.; Ohmagari, N.; et al. Trends of Antifungal Use Based on Sales Data in Japan from 2006 to 2015. Biol. Pharm. Bull. 2020, 43, 1248–1252. [Google Scholar] [CrossRef]
- Agrawal, S.; Barnes, R.; Brüggemann, R.J.; Rautemaa-Richardson, R.; Warris, A. The role of the multidisciplinary team in antifungal stewardship. J. Antimicrob. Chemother. 2016, 71, ii37–ii42. [Google Scholar] [CrossRef]
- Hamdy, R.F.; Zaoutis, T.E.; Seo, S.K. Antifungal stewardship considerations for adults and pediatrics. Virulence 2017, 8, 658–672. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, H.; Yamada, K.; Imoto, W.; Yamairi, K.; Shibata, W.; Namikawa, H.; Yoshii, N.; Nakaie, K.; Okada, Y.; Fujita, A.; et al. The effects of antifungal stewardship programs at a tertiary-care teaching hospital in Japan. J. Infect. Chemother. 2019, 25, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Samura, M.; Hirose, N.; Kurata, T.; Ishii, J.; Nagumo, F.; Takada, K.; Koshioka, S.; Uchida, M.; Yamamoto, S.; Inoue, J.; et al. Support for fungal infection treatment mediated by pharmacist-led antifungal stewardship activities. J. Infect. Chemother. 2020, 26, 272–279. [Google Scholar] [CrossRef]
- Hamada, Y.; Ebihara, F.; Kimura, T.; Kikuchi, K. In Proceedings of the 63th Annual Meeting of the Japanese Society for Medical Mycology, Chiba, Japan, 29 October 2019; Available online: https://www.mycology-jp.org/en/ (accessed on 11 August 2021).
- Walsh, T.J.; Anaissie, E.J.; Denning, D.W.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Segal, B.H.; Steinbach, W.J.; Stevens, D.A.; et al. Treatment of aspergillosis: Clinical practice guidelines of the Infectious Diseases Society of America. Clin. Infect. Dis. 2008, 46, 327–360. [Google Scholar] [CrossRef]
- Ullmann, A.J.; Akova, M.; Herbrecht, R.; Viscoli, C.; Arendrup, M.C.; Arikan-Akdagli, S.; Bassetti, M.; Bille, J.; Calandra, T.; Castagnola, E.; et al. ESCMID guideline for the diagno-sis and management of Candida diseases 2012: Adults with haematological malignancies and after haematopoietic stem cell transplantation (HCT). Clin. Microbiol. Infect. 2012, 18 (Suppl. 7), 53–67. [Google Scholar] [CrossRef]
- Saito, T.; Fujiuchi, S.; Tao, Y.; Sasaki, Y.; Ogawa, K.; Suzuki, K.; Tada, A.; Kuba, M.; Kato, T.; Kawabata, M.; et al. Efficacy and safety of voriconazole in the treatment of chronic pulmonary aspergillosis: Experience in Japan. Infection 2012, 40, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Luong, M.L.; Hosseini-Moghaddam, S.M.; Singer, L.G.; Chaparro, C.; Azad, S.; Lazar, N.; Boutros, P.C.; Keshavjee, S.; Rotstein, C.; Husain, S. Risk factors for voriconazole hepatotoxicity at 12 weeks in lung transplant recipients. Am. J. Transpl. 2012, 12, 1929–1935. [Google Scholar] [CrossRef] [PubMed]
- Zonios, D.I.; Gea-Banacloche, J.; Childs, R.; Bennett, J.E. Hallucinations during voriconazole therapy. Clin. Infect. Dis. 2008, 47, e7–e10. [Google Scholar] [CrossRef] [PubMed]
- Imhof, A.; Schaer, D.J.; Schanz, U.; Schwarz, U. Neurological adverse events to voriconazole: Evidence for therapeutic drug monitoring. Swiss Med. Wkly. 2006, 136, 739–742. [Google Scholar] [CrossRef]
- Boyd, A.E.; Modi, S.; Howard, S.J.; Moore, C.B.; Keevil, B.G.; Denning, D.W. Adverse reactions to voriconazole. Clin. Infect. Dis. 2004, 39, 1241–1244. [Google Scholar] [CrossRef]
- Walsh, T.J.; Pappas, P.; Winston, D.J.; Lazarus, H.M.; Petersen, F.; Raffalli, J.; Yanovich, S.; Stiff, P.; Greenberg, R.; Donowitz, G.; et al. Voriconazole compared with liposomal amphotericin B for empirical antifungal therapy in patients with neutropenia and persistent fever. N. Engl. J. Med. 2002, 346, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Hamada, Y.; Seto, Y.; Yago, K.; Kuroyama, M. Investigation and threshold of optimum blood concentration of voriconazole: A descriptive statistical meta-analysis. J. Infect. Chemother. 2012, 18, 501–507. [Google Scholar] [CrossRef]
- Hamada, Y.; Tokimatsu, I.; Mikamo, H.; Kimura, M.; Seki, M.; Takakura, S.; Ohmagari, N.; Takahashi, Y.; Kasahara, K.; Matsumoto, K.; et al. Practice guidelines for therapeutic drug monitoring of voriconazole: A consensus review of the Japanese Society of Chemotherapy and the Japanese Society of Therapeutic Drug Monitoring. J. Infect. Chemother. 2013, 19, 381–392. [Google Scholar] [CrossRef] [PubMed]
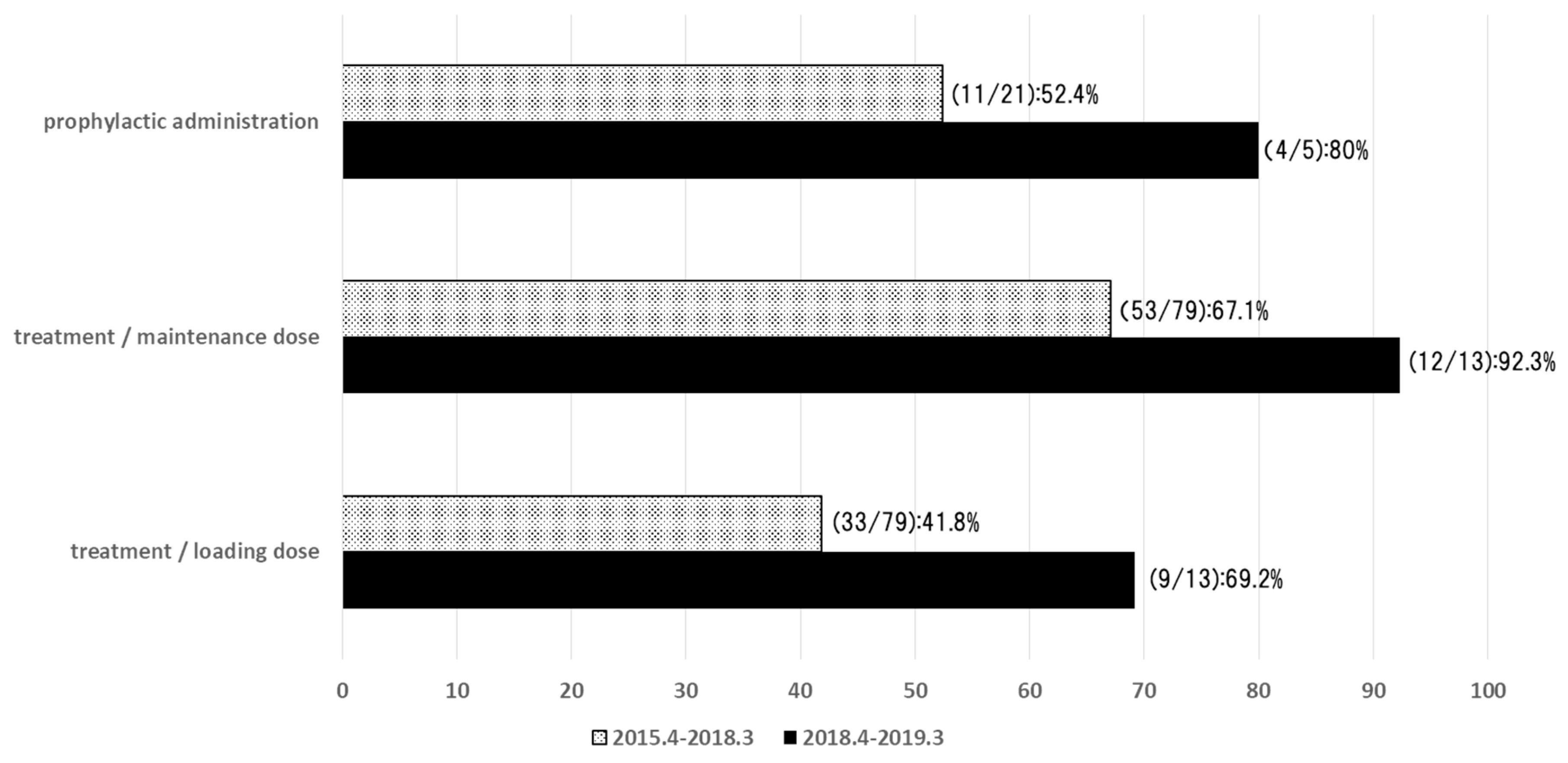
- Hamada, Y.; Ueda, T.; Miyazaki, Y.; Nakajima, K.; Fukunaga, K.; Miyazaki, T.; Nakada-Motokawa, N.; Nagao, M.; Kawamura, H.; Shigemi, A.; et al. Effects of antifungal stewardship using therapeutic drug monitoring in voriconazole therapy on the prevention and control of hepatotoxicity and visual symptoms: A multicentre study con-ducted in Japan. Mycoses 2020, 63, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Hanai, Y.; Hamada, Y.; Kimura, T.; Matsumoto, K.; Takahashi, Y.; Fujii, S.; Nishizawa, K.; Miyazaki, Y.; Takesue, Y. Favorable Effects of Voriconazole Trough Concentrations Exceeding 1 μg/mL on Treatment Success and All-Cause Mortality: A Systematic Review and Meta-Analysis. J. Fungi 2021, 7, 306. [Google Scholar] [CrossRef] [PubMed]
- Hanai, Y.; Hamada, Y.; Kimura, T.; Matsumoto, K.; Takahashi, Y.; Fujii, S.; Nishizawa, K.; Takesue, Y. Optimal trough concentration of voriconazole with therapeutic drug monitoring in children: A systematic re-view and meta-analysis. J. Infect. Chemother. 2020, 27, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.; Déméautis, T.; Dupont, D.; Kramer, R.; Garnier, H.; Durieu, I.; Sénéchal, A.; Reix, P.; Couraud, S.; Devouassoux, G.; et al. Azole resistance in Aspergillus fumigatus isolates from respiratory specimens in Lyon University Hospitals, France: Prevalence and mechanisms involved. Int. J. Antimicrob. Agents 2021. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, M.; Izumikawa, K.; Hirano, K.; Ide, S.; Mihara, T.; Hosogaya, N.; Takazono, T.; Morinaga, Y.; Nakamura, S.; Kurihara, S.; et al. Correlation between triazole treatment history and susceptibility in clinically isolated Aspergillus fumigatus. Antimicrob. Agents Chemother. 2012, 56, 4870–4875. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.Y.; Chuang, Y.C.; Wu, U.I.; Sun, H.Y.; Wang, J.T.; Sheng, W.H.; Chen, Y.C.; Chang, S.C. Mechanisms of Azole Resistance and Trailing in Candida tropicalis Bloodstream Isolates. J. Fungi 2021, 7, 612. [Google Scholar] [CrossRef] [PubMed]
- Finazzi, S.; Paci, G.; Antiga, L.; Brissy, O.; Carrara, G.; Crespi, D.; Csato, G.; Csomos, A.; Duek, O.; Facchinetti, S.; et al. PROSAFE: A European endeavor to improve quality of critical care medicine in seven countries. Minerva Anestesiol 2020, 86, 1305–1320. [Google Scholar] [CrossRef] [PubMed]
| Process Index | Examples of Index |
|---|---|
| Antifungal drug consumption | Days of therapy per 1000 patient-days, defined daily doses per 1000 patient-days, or individual patients treated with antifungal drugs (excluded prophylaxis) |
| Compliance with institutional guidelines | Proportion of compliance using template of each facility for the following items and confirmation |
| Choice of drug | Proportion of patients treated with drug of choice for indication |
| Dose | Approved indications and dosages of each country |
| Administration period | For fungaemia, administration for at least 14 days after negative confirmation of blood culture |
| Therapeutic drug monitoring | Proportion of patients on azole and voriconazole/posaconazole for whom serum level was checked appropriately from time of initiation |
| Drug–drug interaction (DDI) | Proportion of patients on azole for whom DDI was checked appropriately from time of initiation |
| Step-down | Proportion of patients with fluconazole-sensitive Candida for whom therapy was switched from broad-spectrum agent, polyene, or echinocandin to fluconazole and intravenous to oral formulation |
| Use of diagnostic tests | Proportion of compliance with guideline recommendations for monitoring serum galactomannan or (1,3)-β-d-glucan or novel approaches |
| Source control | Proportion of patients with candidemia with catheter removal |
| Outcome index | Examples of metric |
| Treatment of invasive fungal infection | Proportion of patients with clinical cure or proportion of patients with candidemia with recurrent infection |
| Resistance | Proportion of Candida isolates caused by fluconazole-resistant strains |
| Cost | Total cost of prescriptions per year, stratified by antifungal drug |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamada, Y.; Ebihara, F.; Kikuchi, K. A Strategy for Hospital Pharmacists to Control Antimicrobial Resistance (AMR) in Japan. Antibiotics 2021, 10, 1284. https://doi.org/10.3390/antibiotics10111284
Hamada Y, Ebihara F, Kikuchi K. A Strategy for Hospital Pharmacists to Control Antimicrobial Resistance (AMR) in Japan. Antibiotics. 2021; 10(11):1284. https://doi.org/10.3390/antibiotics10111284
Chicago/Turabian StyleHamada, Yukihiro, Fumiya Ebihara, and Ken Kikuchi. 2021. "A Strategy for Hospital Pharmacists to Control Antimicrobial Resistance (AMR) in Japan" Antibiotics 10, no. 11: 1284. https://doi.org/10.3390/antibiotics10111284
APA StyleHamada, Y., Ebihara, F., & Kikuchi, K. (2021). A Strategy for Hospital Pharmacists to Control Antimicrobial Resistance (AMR) in Japan. Antibiotics, 10(11), 1284. https://doi.org/10.3390/antibiotics10111284







