Prevalence, Patterns, Association with Biofilm Formation, Effects on Milk Quality and Risk Factors for Antibiotic Resistance of Staphylococci from Bulk-Tank Milk of Goat Herds
Abstract
:1. Introduction
2. Results
2.1. Staphylococcal Recovery and Presence of Antibiotic Resistance
2.2. Biofilm Formation
2.3. Associations with Milk Quality
2.4. Variables Associated with Isolation of Resistant or Multi-Resistant Staphylococcal Isolates from Bulk-Tank Milk
2.4.1. Isolation of Oxacillin-Resistant Staphylococcal Isolates
2.4.2. Isolation of Staphylococcal Isolates Resistant to at Least One Antibiotic
2.4.3. Isolation of Multi-Resistant Staphylococcal Isolates
3. Discussion
3.1. Presence of Antibiotic Resistance in Staphylococcal Isolates
3.2. Predictors for Antibiotic-Resistant Staphylococcal Isolates
4. Materials and Methods
4.1. Goat Herds and Sampling
4.2. Laboratory Examinations
4.3. Data Management and Analysis
4.3.1. Data Management
4.3.2. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Management system applied in the herd (description according to EFSA classification) [EFSA 2014] |
| Month into the lactation period at sampling (month) |
| Machine- or hand-milking (description) |
| No. of does in the herd (no.) |
| Total milk quantity per doe obtained during the preceding milking period (litres) |
| Average number of kids born per doe (no.) |
| Collaboration with a veterinarian (yes/no) |
| Total visits made annually by veterinarians to the herd during the preceding season (no.) |
| Clinical mastitis annual incidence risk in the herd (%) |
| Age of kid removal from their dams (days) |
| Daily number of milking sessions (no.) |
| Duration of the dry period (months) |
| Means of calculating live bodyweight for the administration of pharmaceutical products (weighing/estimation) |
| Routine overdosing (compared to the dose prescribed) of pharmaceuticals (yes/no) |
| Annual frequency of systemic disinfections in the farm (no. of occasions) |
| Routine administration of antimicrobials in newborns (yes/no) |
| Vaccination against mastitis (yes/no) |
| Administration of ‘dry-ewe’ treatment at the end of the lactation period (yes/no) |
| Use of teat disinfection after milking (yes/no) |
| Age of the farmer (years) |
| Length of previous animal farming experience of the farmer (years) |
| Education of the farmer (description) |
| Farmer by profession (yes/no) |
| Family tradition in farming (yes/no) |
| Presence of working staff in the herd (yes/no) |
References
- Hellenic Agricultural Organisation—Demeter (2020). Deliveries of Ovine and Caprine Milk by Region and Regional Authority and Average Milk Price—Calendar Year 2019. Cumulative Data Updated. Available online: https://www.elgo.gr/images/ELOGAK_files/Statistics/2020/AIGO_Παραδόσεις_Πρόβειου_και_Γίδινου_Γάλακτος_2019.pdf (accessed on 20 December 2020).
- Pulina, G.; Milan, M.J.; Lavin, M.P.; Theodoridis, A.; Morin, E.; Capote, J.; Thomas, D.L.; Francesconi, A.H.D.; Caja, G. Current production trends, farm structures, and economics of the dairy sheep and goat sector. J. Dairy Sci. 2018, 101, 6715–6729. [Google Scholar] [CrossRef] [Green Version]
- Muehlherr, J.E.; Zweifel, C.; Corti, S.; Blanco, J.E.; Stephan, R. Microbiological quality of raw goat’s and ewe’s bulk-tank milk in Switzerland. J. Dairy Sci. 2003, 86, 3849–3856. [Google Scholar] [CrossRef]
- Rola, J.G.; Sosnowski, M.; Ostrowska, M.; Osek, J. Prevalence and antimicrobial resistance of coagulase-positive staphylococci isolated from raw goat milk. Small Rumin. Res. 2015, 123, 124–128. [Google Scholar] [CrossRef]
- Lianou, D.T.; Michael, C.K.; Vasileiou, N.G.C.; Petinaki, E.; Cripps, P.J.; Tsilipounidaki, K.; Katsafadou, A.I.; Politis, A.P.; Kordalis, N.G.; Ioannidi, K.S.; et al. Extensive countrywide field investigation of somatic cell counts and total bacterial counts in bulk tank raw milk in goat herds in Greece. J. Dairy Res. 2021, 88, 307–313. [Google Scholar] [CrossRef]
- Altaf, M.; Ijaz, M.; Iqbal, M.K.; Rehman, A.; Avais, M.; Ghaffar, A.; Ayyub, R.M. Molecular characterization of methicillin resistant Staphylococcus aureus (MRSA) and associated risk factors with the occurrence of goat mastitis. Pak. Vet. J. 2020, 40, 1–6. [Google Scholar]
- Caruso, M.; Latorre, L.; Santagada, G.; Fraccalvieri, R.; Miccolupo, A.; Sottili, R.; Palazzo, L.; Parisi, A. Methicillin-resistant Staphylococcus aureus (MRSA) in sheep and goat bulk tank milk from Southern Italy. Small Rumin. Res. 2016, 135, 26–31. [Google Scholar] [CrossRef]
- Lira, M.C.; Givisiez, P.E.N.; de Sousa, F.G.C.; Magnani, M.; de Souza, E.L.; Spricigo, D.A.; Gebreyes, W.A.; Wondwossen, A.; de Oliveira, C.J.B. Biofilm-forming and antimicrobial resistance traits of staphylococci isolated from goat dairy plants. J. Inf. Dev. Ctries 2016, 10, 932–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obaidat, M.M.; Roess, A.A.; Mahasneh, A.A.; Al-Hakimi, R.A. Antibiotic-resistance, enterotoxin gene profiles and farm-level prevalence of Staphylococcus aureus in cow, sheep and goat bulk tank milk in Jordan. Int. Dairy J. 2018, 81, 28–34. [Google Scholar] [CrossRef]
- European Union. Regulation (EC) No. 853/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules foron the hygiene of foodstuffs. Off. J. Eur. Union 2004, L 139/55. [Google Scholar]
- Andreoletti, O.; Baggesen, D.L.; Bolton, D.; Butaye, P.; Cook, P.; Davies, R.; Escamez, P.S.F.; Griffin, J.; Hald, T.; Havellar, A.; et al. Scientific opinion on the public health risks related to the consumption of raw drinking milk. EFSA J 2015, 13, 3940. [Google Scholar]
- Virdis, S.; Scarano, C.; Cossu, F.; Spanu, V.; Spanu, C.; De Santis, E.P.L. Antibiotic Resistance in Staphylococcus aureus and coagulase negative staphylococci isolated from goats with subclinical mastitis. Vet. Med. Int. 2010, 2010, 517060. [Google Scholar] [CrossRef] [Green Version]
- Koop, G.; De Visscher, A.; Collar, C.A.; Bacon, D.A.C.; Maga, E.A.; Murray, J.D.; Supre, K.; De Vliegher, S.; Haesebrouck, F.; Rowe, J.D.; et al. Identification of coagulase-negative staphylococcus species from goat milk with the API Staph identification test and with transfer RNA-intergenic spacer PCR combined with capillary electrophoresis. J. Dairy Sci. 2012, 95, 7200–7205. [Google Scholar] [CrossRef]
- Michael, C.K.; Lianou, D.T.; Vasileiou, N.G.C.; Tsilipounidaki, K.; Katsafadou, A.I.; Politis, A.I.; Kordalis, N.G.; Ioannidi, K.S.; Gougoulis, D.A.; Trikalinou, C.; et al. Association of staphylococcal populations on teatcups of milking rarlours with vaccination against staphylococcal mastitis in sheep and goat farms. Pathogens 2021, 10, 385. [Google Scholar] [CrossRef]
- Anderson, K.L.; Kearns, R.; Lyman, R.; Correa, M.T. Staphylococci in dairy goats and human milkers, and the relationship with herd management practices. Small Rumin. Res. 2019, 171, 13–22. [Google Scholar] [CrossRef]
- Verhoeven, P.O.; Gagnaire, J.; Botelho-Nevers, E.; Grattard, F.; Carricajo, A.; Lucht, F.; Pozzetto, B.; Berthelot, P. Detection and clinical relevance of Staphylococcus aureus nasal carriage: An update. Expert Rev. Anti-Infect. Ther. 2014, 12, 75–89. [Google Scholar] [CrossRef]
- Vasileiou, N.G.C.; Sarrou, S.; Papagiannitsis, C.; Chatzopoulos, D.C.; Malli, E.; Mavrogianni, V.S.; Petinaki, E.; Fthenakis, G.C. Antimicrobial agent susceptibility and typing of staphylococcal isolates from subclinical mastitis in ewes. Microb. Drug. Res. 2019, 25, 1099–1110. [Google Scholar] [CrossRef]
- Mavrogianni, V.S.; Menzies, P.I.; Fragkou, I.A.; Fthenakis, G.C. Principles of mastitis treatment in sheep and goats. Vet. Clin. N. Am. Food Anim. Pract. 2011, 27, 115–120. [Google Scholar] [CrossRef]
- Koulenti, D.; Fragkou, P.C.; Tsiodras, S. Editorial for Special Issue ‘Multidrug-Resistant Pathogens’. Microorganisms 2020, 8, 1383. [Google Scholar] [CrossRef]
- Hellenic Veterinary Association. European Day for Information and Awareness for the Use of Antibiotics. Press release (ref. 1631, 15 Nov. 2018), Athens, 2018.
- Wassenaar, T.M.; Ussery, D.; Nielsen, L.N.; Ingmer, H. Review and phylogenetic analysis of qac genes that reduce susceptibility to quaternary ammonium compounds in Staphylococcus species. Eur. J. Microbiol. Immunol. 2015, 5, 44–61. [Google Scholar] [CrossRef] [Green Version]
- Slifierz, M.J.; Friendship, R.M.; Weese, J.S. Methicillin-resistant Staphylococcus aureus in commercial swine herds is associated with disinfectant and zinc usage. Appl. Environ. Microbiol. 2015, 81, 2690–2695. [Google Scholar] [CrossRef] [Green Version]
- Templeton, M.R.; Oddy, F.; Leung, W.K.; Rogers, M. Chlorine and UV disinfection of ampicillin-resistant and trimetoprim-resistant Escherichia coli. Can. J. Civ. Eng. 2009, 36, 889–894. [Google Scholar] [CrossRef]
- Khan, S.; Beattie, T.; Knapp, C. Relationship between antibiotic- and disinfectant-resistance profiles in bacteria harvested from tap water. Chemosphere 2016, 152, 132–141. [Google Scholar] [CrossRef] [Green Version]
- Sidhu, M.S.; Heir, E.; Leegaard, T.; Wiger, K.; Holck, A. Frequency of disinfectant resistance genes and genetic linkage with beta-lactamase transposon Tn552 among clinical staphylococci. Antimicrobal Agents Chemother. 2002, 46, 2797–2803. [Google Scholar] [CrossRef] [Green Version]
- Russell, A.D. Do biocides select for antibiotic resistance? J. Pharm. Pharmacol. 2000, 52, 227–233. [Google Scholar] [CrossRef]
- Deng, W.; Quan, Y.; Yang, S.Z.; Guo, L.J.; Zhang, X.L.; Liu, S.L.; Chen, S.J.; Zhou, K.; He, L.; Li, B.; et al. Antibiotic resistance in salmonella from retail foods of animal origin and its association with disinfectant and heavy metal resistance. Microb. Drug Res. 2018, 24, 782–791. [Google Scholar] [CrossRef] [Green Version]
- Yarwood, J.M.; McCormick, J.K.; Paustian, M.L.; Kapur, V.; Schlievert, P.M. Repression of the Staphylococcus aureus accessory gene regulator in serum and in vivo. J. Bacteriol. 2002, 184, 1095–1101. [Google Scholar] [CrossRef] [Green Version]
- Malachowa, N.; DeLeo, F.R. Mobile genetic elements of Staphylococcus aureus. Cell. Mol. Life Sci. 2010, 18, 3057–3071. [Google Scholar] [CrossRef] [Green Version]
- Shearer, J.E.S.; Wireman, J.; Hostetler, J.; Forberger, H.; Borman, J.; Gill, J.; Sanchez, S.; Mankin, A.; LaMarre, J.; Lindsay, J.A.; et al. Major families of multiresistant plasmids from geographically and epidemiologically diverse staphylococci. Gen. Genom. Genet. 2011, 1, 581–591. [Google Scholar] [CrossRef] [Green Version]
- Allen, H.K.; Trachsel, J.; Looft, T.; Casey, T.A. Finding alternatives to antibiotics. Antimicrob. Ther. Rev. Inf. Dis. Curr. Emerg. Conc. 2014, 1323, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Heinz, D. Farm workers contracting drug-resistant bacteria, study shows. Healthline 2013. Available online: https://www.healthline.com/health-news/public-farmhands-develop-antibiotic-resistance-070613 (accessed on 26 August 2021).
- Lianou, D.T.; Chatziprodromidou, I.P.; Vasileiou, N.G.C.; Michael, C.K.; Mavrogianni, V.S.; Politis, A.P.; Kordalis, N.G.; Billinis, C.; Giannakopoulos, A.; Papadopoulos, E.; et al. A detailed questionnaire for the evaluation of health management in dairy sheep and goats. Animals 2020, 10, 1489. [Google Scholar] [CrossRef] [PubMed]
- Laird, D.T.; Gambrel-Lenarz, S.A.; Scher, F.M.; Graham, T.E.; Reddy, R. Microbiological Count Methods. In Standard Methods for the Examination of Dairy Products, 17th ed.; Wehr, H.M., Frank, J.F., Eds.; APHA Press: Washington, DC, USA, 2004; pp. 153–186. [Google Scholar]
- Barrow, G.I.; Feltham, R.K.A. Manual for the Identification of Medical Bacteria, 3rd ed.; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Euzeby, J.P. List of bacterial names with standing in nomenclature: A folder available on the Internet. Int. J. Syst. Bacteriol. 1997, 47, 590–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasileiou, N.G.C.; Chatzopoulos, D.C.; Gougoulis, D.A.; Sarrou, S.; Katsafadou, A.I.; Spyrou, V.; Mavrogianni, V.S.; Petinaki, E.; Fthenakis, G.C. Slime-producing staphylococci as causal agents of subclinical mastitis in sheep. Vet. Microbiol. 2018, 224, 93–99. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiggans, G.R.; Shook, G.E. A lactation measure of somatic cell count. J. Dairy Sci. 1987, 70 (Suppl. 13), 2666–2672. [Google Scholar] [CrossRef]
- Franzoi, M.; Manuelian, C.L.; Penasa, M.; De Marchi, M. Effects of somatic cell score on milk yield and mid-infrared predicted composition and technological traits of Brown Swiss, Holstein Friesian, and Simmental cattle breeds. J. Dairy Sci. 2020, 103, 791–804. [Google Scholar] [CrossRef]
- Wang, H.; McEntire, J.C.; Zhang, L.; Li, X.; Doyle, M. The transfer of antibiotic resistance from food to humans: Facts, implications and future directions. Rev. Sci. Tech. (Int. Off. Epizoot.) 2012, 31, 249–260. [Google Scholar] [CrossRef]
- Schwarz, S.; Loeffler, A.; Kadlec, K. Bacterial resistance to antimicrobial agents and its impact on veterinary and human medicine. Vet. Dermatol. 2017, 28, 82-e19. [Google Scholar] [CrossRef] [Green Version]

| Staphylococcal Species | Frequency of Staphylococcal Isolates | |||
|---|---|---|---|---|
| All Isolates 1 | Resistant Isolates 2,3 | Multi-Resistant Isolates 3 | Biofilm-Forming Isolates 3 | |
| Staphylococcus aureus | 21 (0.263) | 5 (0.238) | 0 (0.000) | 17 (0.810) |
| Staphylococcus equorum | 11 (0.138) | 9 (0.818) | 8 (0.727) | 8 (0.727) |
| Staphylococcus simulans | 9 (0.113) | 1 (0.111) | 0 (0.000) | 6 (0.667) |
| Staphylococcus capitis | 6 (0.075) | 5 (0.833) | 2 (0.333) | 5 (0.833) |
| Staphylococcus lentus | 5 (0.063) | 2 (0.400) | 2 (0.400) | 1 (0.200) |
| Staphylcoccus haemolyticus | 4 (0.050) | 1 (0.250) | 0 (0.000) | 2 (0.500) |
| Staphylococcus vitulinus | 4 (0.050) | 4 (1.000) | 4 (1.000) | 3 ().750) |
| Staphylococcus kloosii | 3 (0.038) | 2 (0.667) | 2 (0.667) | 2 (0.667) |
| Staphylococcus pettenkoferi | 3 (0.038) | 3 (1.000) | 0 (0.000) | 3 (1.000) |
| Staphylococcus cohnii subsp. urealyticum | 2 (0.025) | 1 (0.500) | 1 (0.500) | 1 (0.500) |
| Staphylococcus lugdunensis | 2 (0.025) | 2 (1.000) | 0 (0.000) | 1 (0.500) |
| Staphylococcus warneri | 2 (0.025) | 2 (1.000) | 1 (0.500) | 2 (1.000) |
| Staphylococcus xylosus | 2 (0.025) | 1 (0.500) | 1 (0.500) | 2 (1.000) |
| Staphylococcus auricularis | 1 (0.012) | 0 (0.000) | 0 (0.000) | 1 (1.000) |
| Staphylococcus chromogenes | 1 (0.012) | 0 (0.000) | 0 (0.000) | 1 (1.000) |
| Staphylococcus cohnii subsp. cohnii | 1 (0.012) | 1 (1.000) | 1 (1.000) | 0 (0.000) |
| Staphylococcus epidermidis | 1 (0.012) | 1 (1.000) | 0 (0.000) | 1 (1.000) |
| Staphylococcus hominis | 1 (0.012) | 0 (0.000) | 0 (0.000) | 1 (1.000) |
| Staphylococcus intermedius | 1 (0.012) | 0 (0.000) | 0 (0.000) | 1 (1.000) |
| Total | 80 | 40 (0.500) | 22 (0.275) | 58 (0.725) |
| Location of Herds (Part of the Country) | Herds (n) | Herds in Which Resistant Staphylococcal Isolates Were Recovered (n) 1 |
|---|---|---|
| Central part | 36 | 13 (0.361) |
| Islands | 16 | 2 (0.125) |
| Northern part | 36 | 11 (0.306) |
| Southern part | 31 | 10 (0.323) |
| Variable (n = 1) | Odds Ratio 1 (95% Confidence Intervals) | p |
|---|---|---|
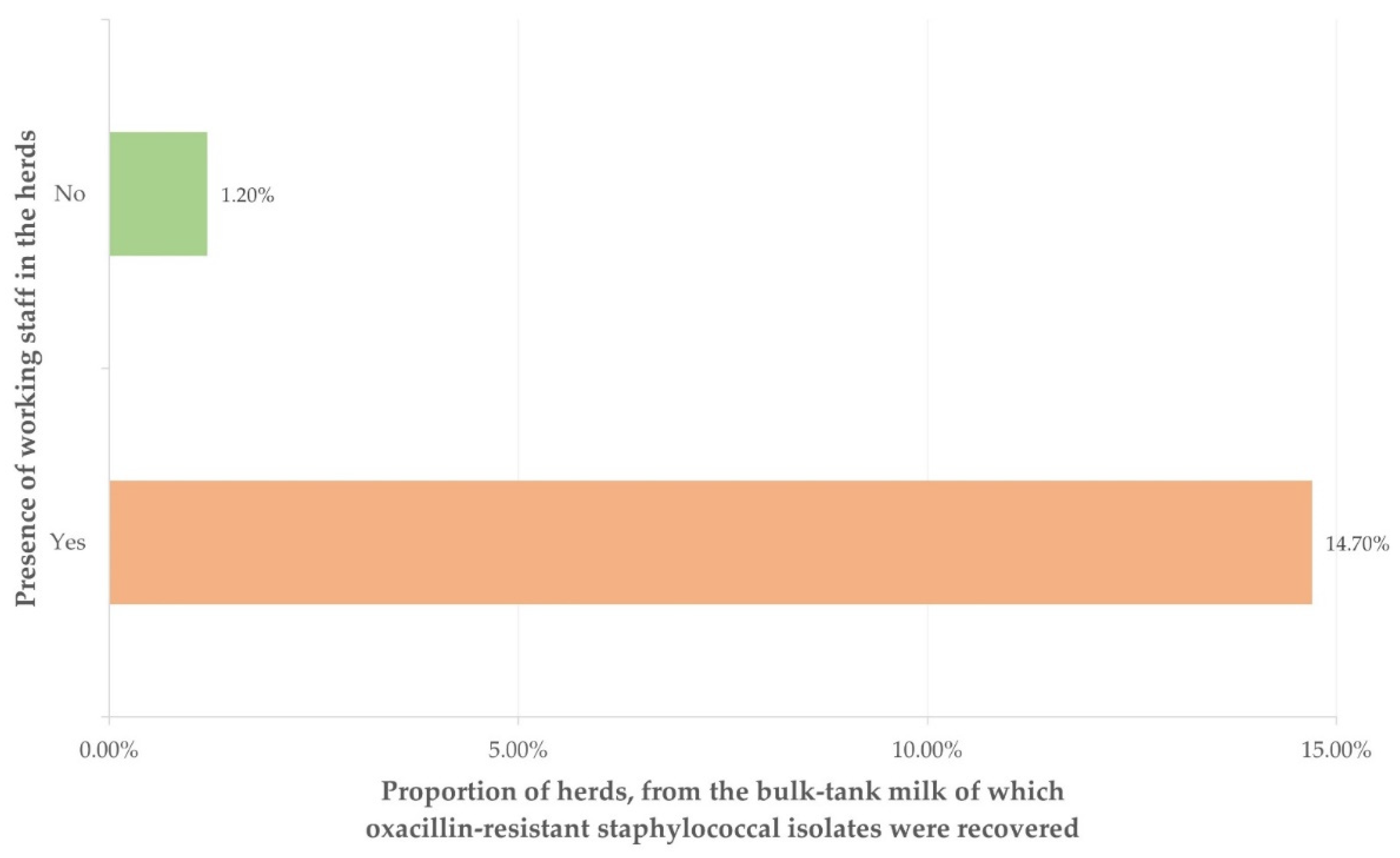
| Presence of working staff in the herd | 0.005 | |
| Yes (n = 34) | 14.483 (1.624–129.171) | 0.017 |
| No (n = 85) | reference | - |
| Variable (n = 2) | Odds Ratios 1 (95% Confidence Intervals) | p |
|---|---|---|
| Farmer by profession | 0.001 | |
| Full-time (n = 105) | reference | - |
| Part-time (n = 14) | 5.200 (1.602–16.882) | 0.006 |
| Annual frequency of systemic disinfectionsin the farm | 0.018 | |
| 0–1 occasion (n = 33) | reference | - |
| 2–10 occasions (n = 76) | 1.327 (0.499–3.529) | 0.57 |
| >10 occasions (n = 10) | 33.429 (3.601–310.331) | 0.002 |
| Variable (n = 3) | Odds Ratio 1 (95% Confidence Intervals) | p |
|---|---|---|
| Annual frequency of systemic disinfectionsin the farm | 0.002 | |
| 0–1 occasion (n = 33) | reference | - |
| 2–10 occasions (n = 76) | 1.343 (0.339–5.317) | 0.67 |
| >10 occasions (n = 10) | 23.333 (3.859–141.077) | 0.0006 |
| Presence of working staff in the herd | 0.016 | |
| Yes (n = 34) | 3.519 (1.281–9.668) | 0.015 |
| No (n = 85) | reference | - |
| Farmer by profession | 0.022 | |
| Yes (n = 105) | reference | - |
| No (n = 14) | 3.611 (1.056–12.349) | 0.041 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lianou, D.T.; Petinaki, E.; Cripps, P.J.; Gougoulis, D.A.; Michael, C.K.; Tsilipounidaki, K.; Skoulakis, A.; Katsafadou, A.I.; Vasileiou, N.G.C.; Giannoulis, T.; et al. Prevalence, Patterns, Association with Biofilm Formation, Effects on Milk Quality and Risk Factors for Antibiotic Resistance of Staphylococci from Bulk-Tank Milk of Goat Herds. Antibiotics 2021, 10, 1225. https://doi.org/10.3390/antibiotics10101225
Lianou DT, Petinaki E, Cripps PJ, Gougoulis DA, Michael CK, Tsilipounidaki K, Skoulakis A, Katsafadou AI, Vasileiou NGC, Giannoulis T, et al. Prevalence, Patterns, Association with Biofilm Formation, Effects on Milk Quality and Risk Factors for Antibiotic Resistance of Staphylococci from Bulk-Tank Milk of Goat Herds. Antibiotics. 2021; 10(10):1225. https://doi.org/10.3390/antibiotics10101225
Chicago/Turabian StyleLianou, Daphne T., Efthymia Petinaki, Peter J. Cripps, Dimitris A. Gougoulis, Charalambia K. Michael, Katerina Tsilipounidaki, Anargyros Skoulakis, Angeliki I. Katsafadou, Natalia G. C. Vasileiou, Themis Giannoulis, and et al. 2021. "Prevalence, Patterns, Association with Biofilm Formation, Effects on Milk Quality and Risk Factors for Antibiotic Resistance of Staphylococci from Bulk-Tank Milk of Goat Herds" Antibiotics 10, no. 10: 1225. https://doi.org/10.3390/antibiotics10101225
APA StyleLianou, D. T., Petinaki, E., Cripps, P. J., Gougoulis, D. A., Michael, C. K., Tsilipounidaki, K., Skoulakis, A., Katsafadou, A. I., Vasileiou, N. G. C., Giannoulis, T., Katsarou, E. I., Voidarou, C., Mavrogianni, V. S., Caroprese, M., & Fthenakis, G. C. (2021). Prevalence, Patterns, Association with Biofilm Formation, Effects on Milk Quality and Risk Factors for Antibiotic Resistance of Staphylococci from Bulk-Tank Milk of Goat Herds. Antibiotics, 10(10), 1225. https://doi.org/10.3390/antibiotics10101225












