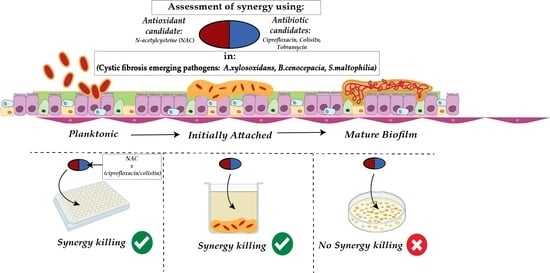
Effect of N-Acetylcysteine in Combination with Antibiotics on the Biofilms of Three Cystic Fibrosis Pathogens of Emerging Importance
Abstract
:1. Introduction
2. Results
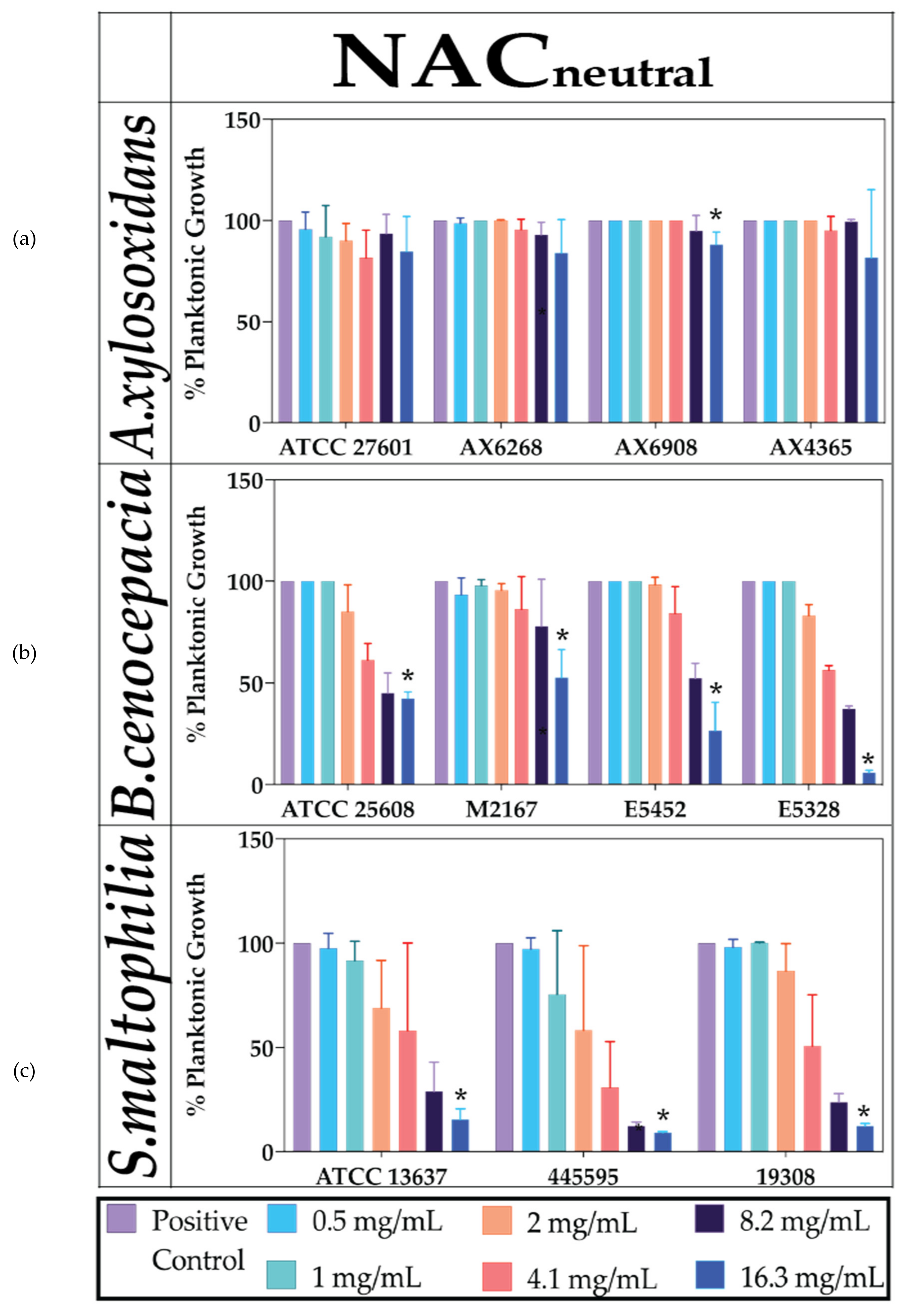
2.1. Neutralised NAC Is Ineffective against Planktonic Bacterial Growth
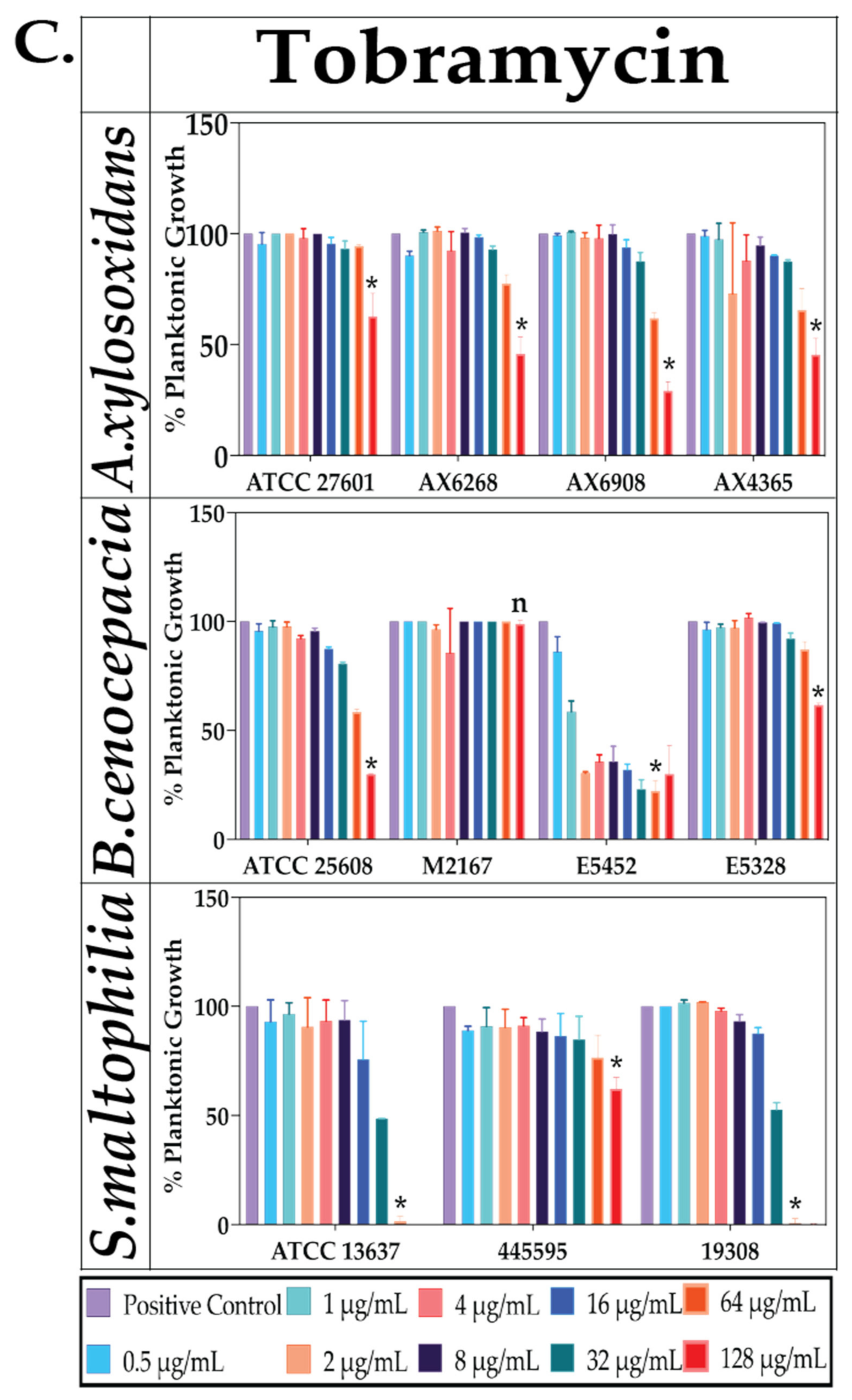
2.2. Effect of Antibiotics against Planktonic Bacterial Growth
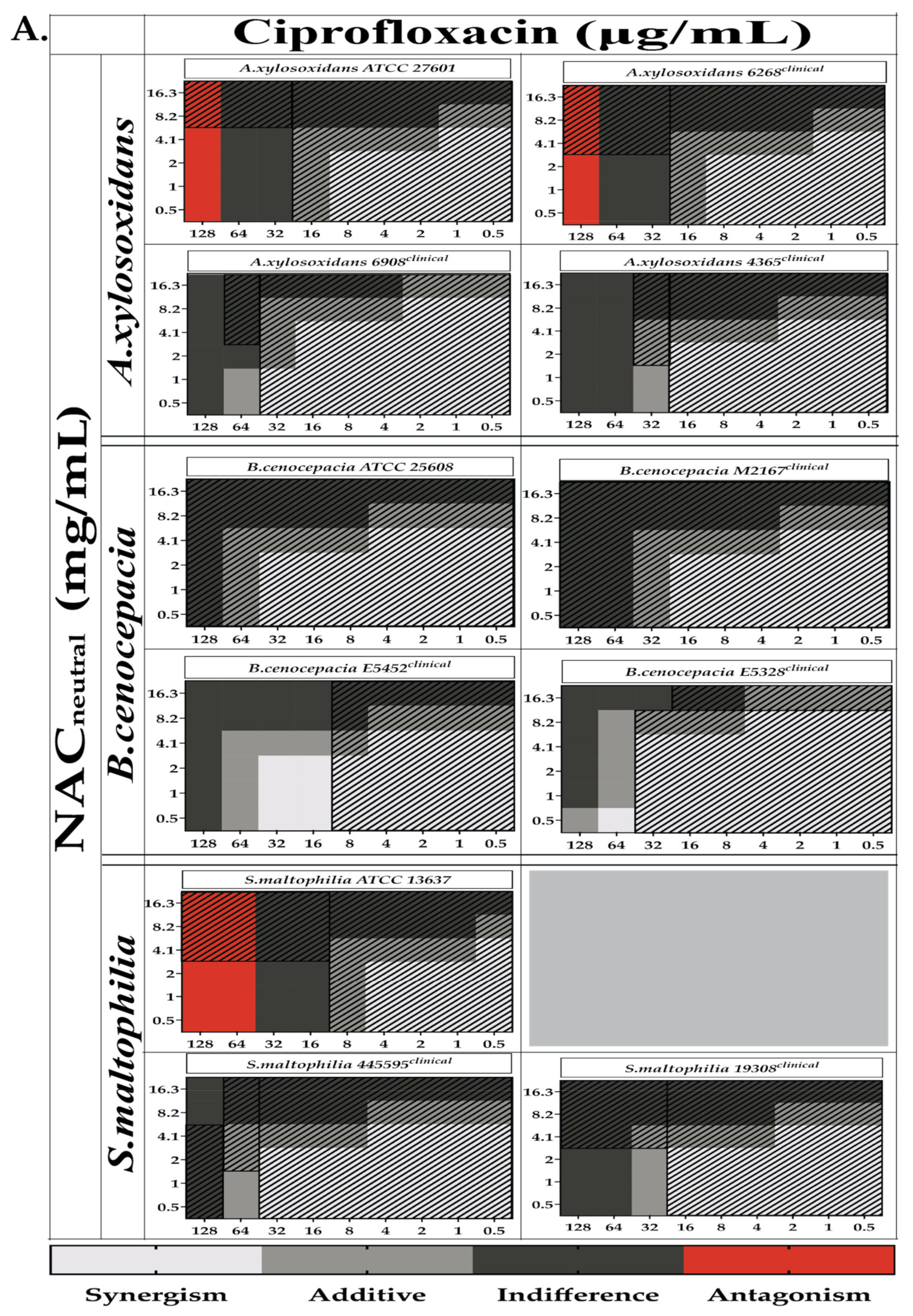
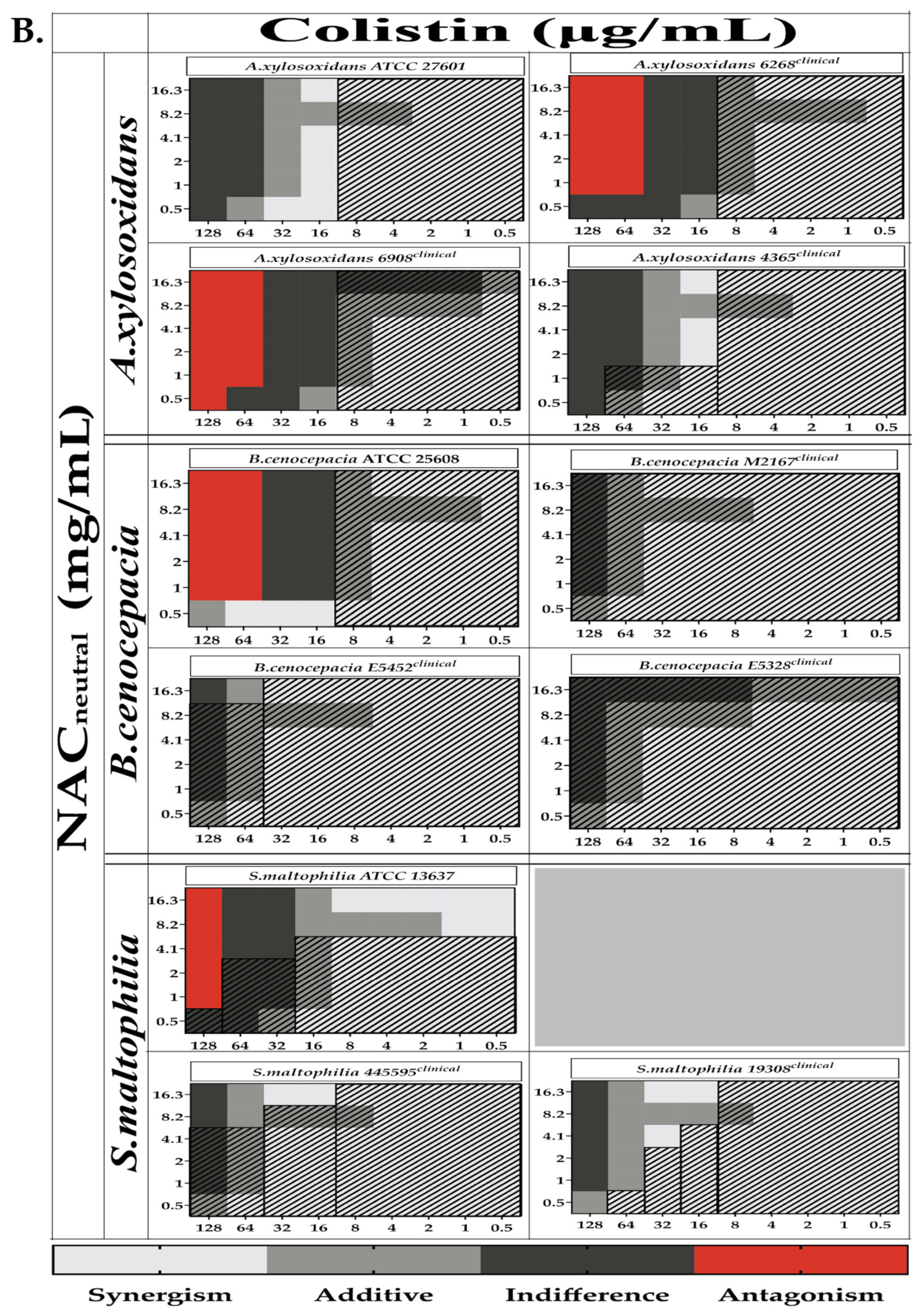
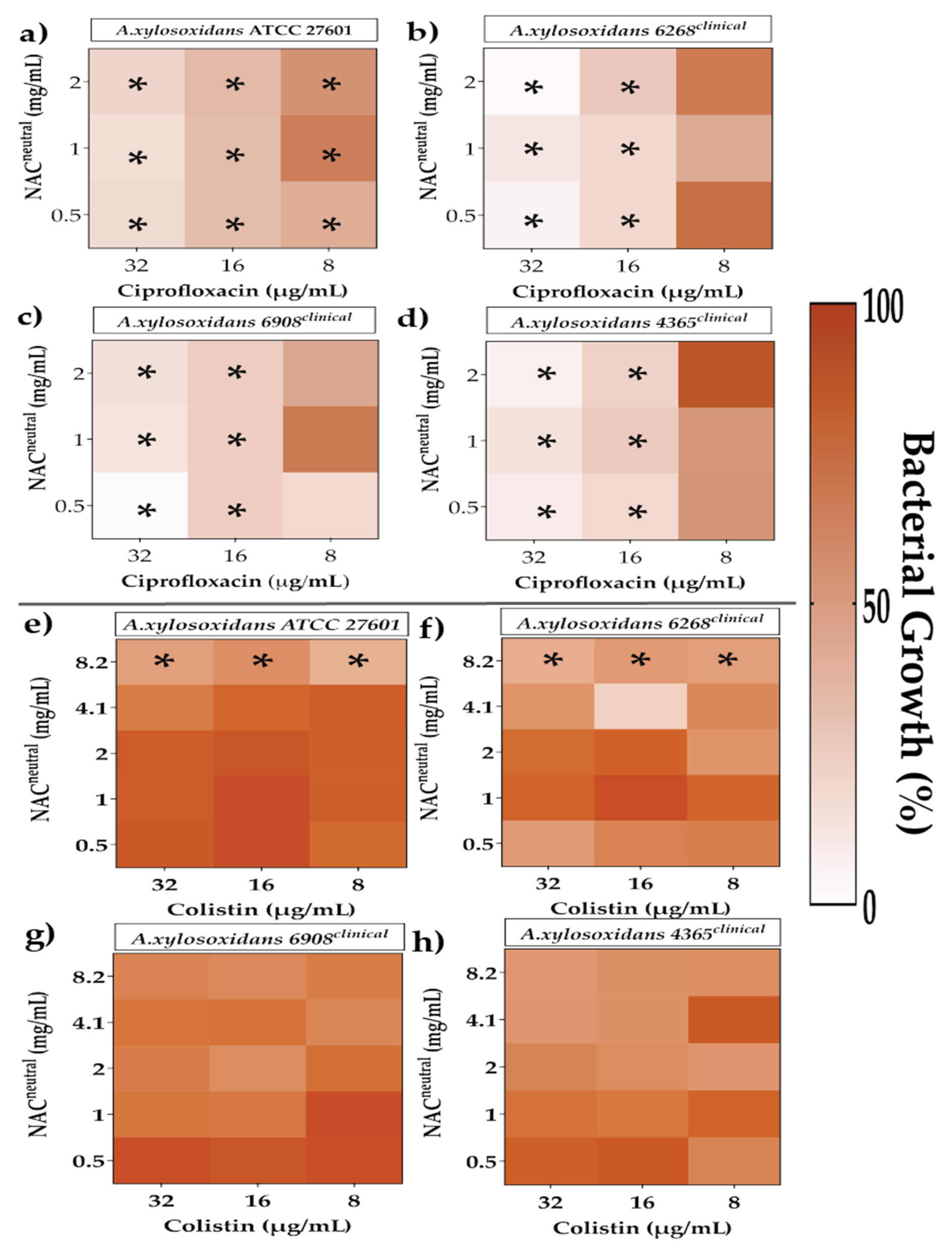
2.3. Combination Therapy Demonstrated Synergism and Additive Effect against Planktonic Bacteria in Checkerboard Assays
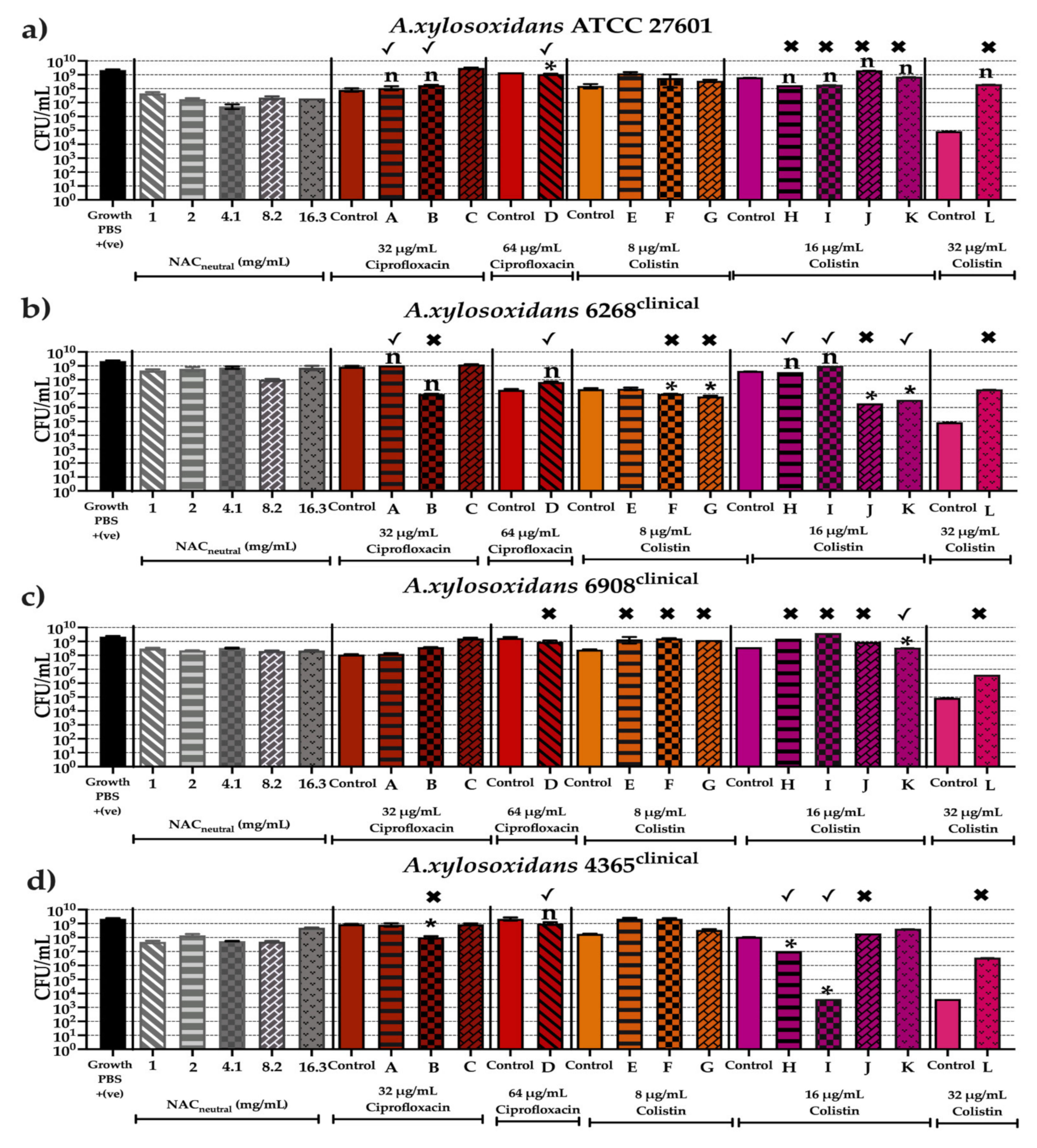
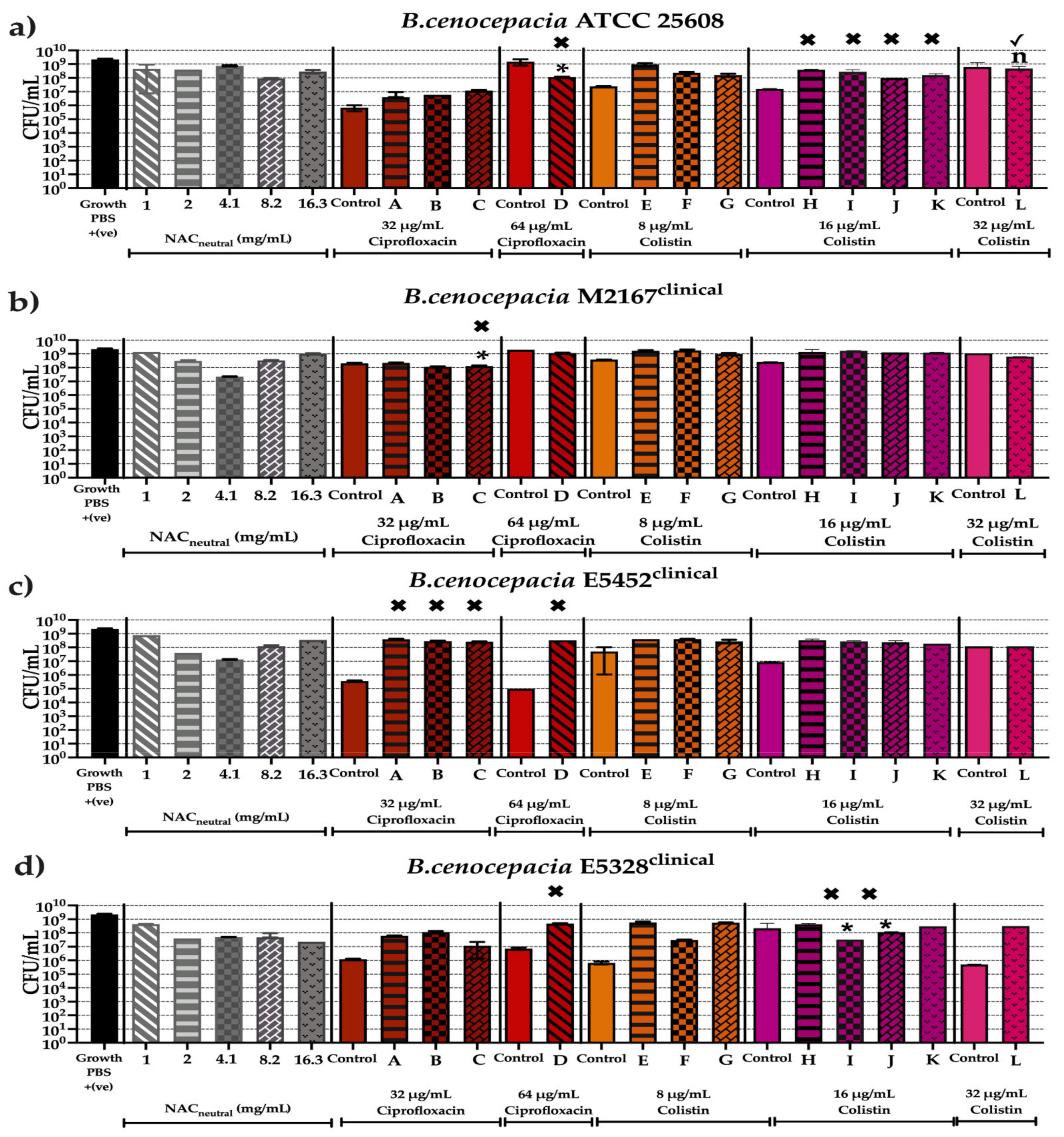
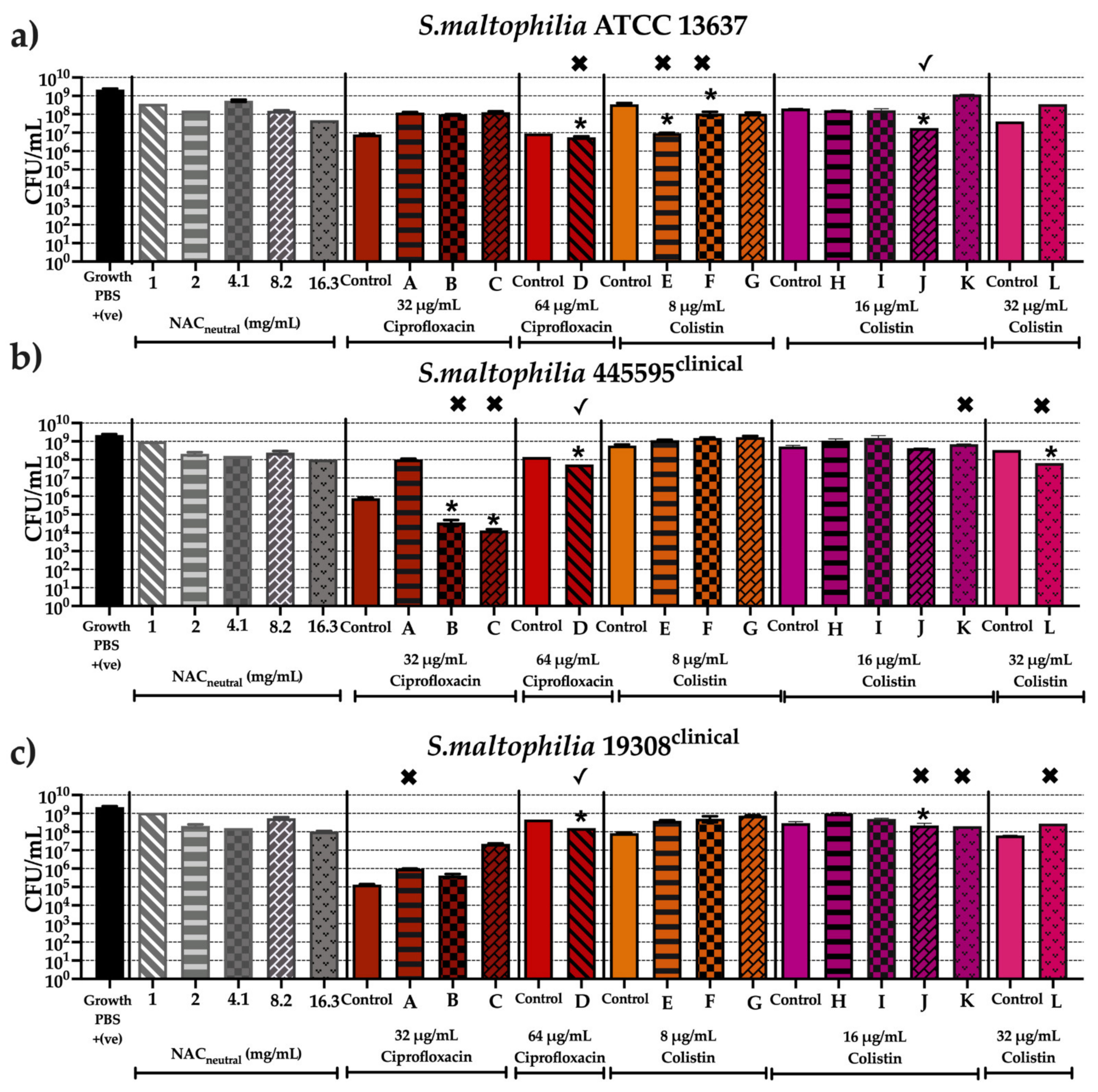
2.4. Effect of Synergistic and Additive Combination Therapies against Irreversibly Attached Bacteria
2.5. Time Course Bacteriostatic Effect of Synergistic and Additive Combination Therapies
2.6. Effect of Synergistic and Additive Combination Therapies against Mature Biofilms In Vitro
3. Discussion
4. Materials and Methods
4.1. Cystic Fibrosis Opportunist Strains Tested
4.2. Culture Conditions and Preparations of Treatments
4.3. Preparation of NAC Medium
4.4. Preparation of Antibiotics
4.5. Effect of Antioxidant and Antibiotics against PLANKTONIC Bacteria
4.6. Synergy Testing via Checkerboard Assays
4.7. Effect of Antioxidant and Antibiotics on Prevention of Biofilm Maturation Following Irreversible Bacterial Attachment
4.8. Time-Kill Assay of Single versus Combination Treatments against Planktonic Cultures
4.9. In Vitro Mature Biofilm Susceptibility Testing with Single and Combination Treatments Using Colony Forming Units (CFU/mL)
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Elborn, J.S. Cystic fibrosis. Lancet 2016, 388, 2519–2531. [Google Scholar] [CrossRef]
- Rubin, B.K. Cystic Fibrosis 2017-The Year in Review. Respir. Care 2018, 63, 238–241. [Google Scholar] [CrossRef]
- Gordon, V.; Bakhtiari, L.; Kovach, K. From molecules to multispecies ecosystems: The roles of structure in bacterial biofilms. Phys. Biol. 2019, 16, 041001. [Google Scholar] [CrossRef]
- Hatziagorou, E.; Orenti, A.; Drevinek, P.; Kashirskaya, N.; Mei-Zahav, M.; De Boeck, K. Changing epidemiology of the respiratory bacteriology of patients with cystic fibrosis-data from the European cystic fibrosis society patient registry. J. Cyst. Fibros 2020, 19, 376–383. [Google Scholar] [CrossRef]
- Salsgiver, E.L.; Fink, A.K.; Knapp, E.A.; LiPuma, J.J.; Olivier, K.N.; Marshall, B.C.; Saiman, L. Changing Epidemiology of the Respiratory Bacteriology of Patients With Cystic Fibrosis. Chest 2016, 149, 390–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parkins, M.D.; Floto, R.A. Emerging bacterial pathogens and changing concepts of bacterial pathogenesis in cystic fibrosis. J. Cyst. Fibros. 2015, 14, 293–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahern, S.; Dean, J.; Liman, J.; Ruseckaite, R.; Burke, N.; Gollan, M.; Keatley, L.; King, S.; Kotsimbos, T.; Middleton, P.G.; et al. Redesign of the Australian Cystic Fibrosis Data Registry: A multidisciplinary collaboration. Paediatr. Respir. Rev. 2021, 37, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Barsky, E.E.; Williams, K.A.; Priebe, G.P.; Sawicki, G.S. Incident Stenotrophomonas maltophilia infection and lung function decline in cystic fibrosis. Pediatr. Pulmonol. 2017, 52, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Cystic Fibrosis Foundation Patient Registry 2019 Annual Data Report; Cystic Fibrosis Foundation: Bethseda, ML, USA, 2020.
- Tetart, M.; Wallet, F.; Kyheng, M.; Leroy, S.; Perez, T.; Le Rouzic, O.; Wallaert, B.; Prevotat, A. Impact of Achromobacter xylosoxidans isolation on the respiratory function of adult patients with cystic fibrosis. ERJ Open Res. 2019, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godbert, B.; Briault, A. Cystic fibrosis: Achromobacter xylosoxidans colonized patients have more severe respiratory disease. Eur. Respir. J. 2013, 42, P1175. [Google Scholar]
- Somayaji, R.; Stanojevic, S.; Tullis, D.E.; Stephenson, A.L.; Ratjen, F.; Waters, V. Clinical Outcomes Associated with Achromobacter Species Infection in Patients with Cystic Fibrosis. Ann. Am. Thorac. Soc. 2017, 14, 1412–1418. [Google Scholar] [CrossRef] [PubMed]
- Waters, V.; Atenafu, E.G.; Salazar, J.G.; Lu, A.; Yau, Y.; Matukas, L.; Tullis, E.; Ratjen, F. Chronic Stenotrophomonas maltophilia infection and exacerbation outcomes in cystic fibrosis. J. Cyst. Fibros. 2012, 11, 8–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waters, V.; Yau, Y.; Prasad, S.; Lu, A.; Atenafu, E.; Crandall, I.; Tom, S.; Tullis, E.; Ratjen, F. Stenotrophomonas maltophilia in cystic fibrosis: Serologic response and effect on lung disease. Am. J. Respir. Crit. Care Med. 2011, 183, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M.; Dodd, M.E.; Govan, J.R.; Barcus, V.; Doherty, C.J.; Morris, J.; Webb, A.K. Burkholderia cenocepacia and Burkholderia multivorans: Influence on survival in cystic fibrosis. Thorax 2004, 59, 948–951. [Google Scholar] [CrossRef] [Green Version]
- Courtney, J.M.; Dunbar, K.E.; McDowell, A.; Moore, J.E.; Warke, T.J.; Stevenson, M.; Elborn, J.S. Clinical outcome of Burkholderia cepacia complex infection in cystic fibrosis adults. J. Cyst. Fibros. 2004, 3, 93–98. [Google Scholar] [CrossRef] [Green Version]
- De Soyza, A.; Meachery, G.; Hester, K.L.; Nicholson, A.; Parry, G.; Tocewicz, K.; Pillay, T.; Clark, S.; Lordan, J.L.; Schueler, S.; et al. Lung transplantation for patients with cystic fibrosis and Burkholderia cepacia complex infection: A single-center experience. J. Heart Lung Transpl. 2010, 29, 1395–1404. [Google Scholar] [CrossRef]
- Brooke, J.S.; Di Bonaventura, G.; Berg, G.; Martinez, J.L. Editorial: A Multidisciplinary Look at Stenotrophomonas maltophilia: An Emerging Multi-Drug-Resistant Global Opportunistic Pathogen. Front. Microbiol. 2017, 8, 1511. [Google Scholar] [CrossRef]
- Pompilio, A.; Savini, V.; Fiscarelli, E.; Gherardi, G.; Di Bonaventura, G. Clonal Diversity, Biofilm Formation, and Antimicrobial Resistance among Stenotrophomonas maltophilia Strains from Cystic Fibrosis and Non-Cystic Fibrosis Patients. Antibiotics 2020, 9, 15. [Google Scholar] [CrossRef] [Green Version]
- Lopes, S.P.; Ceri, H.; Azevedo, N.F.; Pereira, M.O. Antibiotic resistance of mixed biofilms in cystic fibrosis: Impact of emerging microorganisms on treatment of infection. Int. J. Antimicrob. Agents 2012, 40, 260–263. [Google Scholar] [CrossRef] [Green Version]
- Gurjar, M. Colistin for lung infection: An update. J. Intensive Care 2015, 3, 3. [Google Scholar] [CrossRef] [Green Version]
- Thai, T.; Salisbury, B.H.; Zito, P.M. Ciprofloxacin; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Rossi, S. Australian Medicines Handbook (Online). 2021. Available online: https://amhonline.amh.net.au/auth (accessed on 13 August 2021).
- Landini, G.; Di Maggio, T.; Sergio, F.; Docquier, J.D.; Rossolini, G.M.; Pallecchi, L. Effect of High N-Acetylcysteine Concentrations on Antibiotic Activity against a Large Collection of Respiratory Pathogens. Antimicrob. Agents Chemother. 2016, 60, 7513–7517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blasi, F.; Page, C.; Rossolini, G.M.; Pallecchi, L.; Matera, M.G.; Rogliani, P.; Cazzola, M. The effect of N-Acetylcysteine on biofilms: Implications for the treatment of respiratory tract infections. Respir. Med. 2016, 117, 190–197. [Google Scholar] [CrossRef] [Green Version]
- Pollini, S.; Di Pilato, V.; Landini, G.; Di Maggio, T.; Cannatelli, A.; Sottotetti, S.; Cariani, L.; Aliberti, S.; Blasi, F.; Sergio, F.; et al. In vitro activity of N-Acetylcysteine against Stenotrophomonas maltophilia and Burkholderia cepacia complex grown in planktonic phase and biofilm. PLoS ONE 2018, 13, e0203941. [Google Scholar] [CrossRef] [Green Version]
- Dinicola, S.; De Grazia, S.; Carlomagno, G.; Pintucci, J.P. N-Acetylcysteine as powerful molecule to destroy bacterial biofilms. A systematic review. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2942–2948. [Google Scholar]
- Zhao, T.; Liu, Y. N-Acetylcysteine inhibit biofilms produced by Pseudomonas aeruginosa. BMC Microbiol. 2010, 10, 140. [Google Scholar] [CrossRef] [Green Version]
- Ciacci, N.; Boncompagni, S.; Valzano, F.; Cariani, L.; Aliberti, S.; Blasi, F.; Pollini, S.; Rossolini, G.M.; Pallecchi, L. In Vitro Synergism of Colistin and N-Acetylcysteine against Stenotrophomonas maltophilia. Antibiotics 2019, 8, 101. [Google Scholar] [CrossRef] [Green Version]
- Aaron, S.D. Antibiotic synergy testing should not be routine for patients with cystic fibrosis who are infected with multiresistant bacterial organisms. Paediatr. Respir. Rev. 2007, 8, 256–261. [Google Scholar] [CrossRef]
- Burns, J.L.; Saiman, L. Burkholderia cepacia infections in cystic fibrosis. Pediatric Infect. Dis. J. 1999, 18, 155–156. [Google Scholar] [CrossRef] [PubMed]
- Tam, J.; Nash, E.F.; Ratjen, F.; Tullis, E.; Stephenson, A. Nebulized and oral thiol derivatives for pulmonary disease in cystic fibrosis. Cochrane Database Syst. Rev. 2013, 2013, CD007168. [Google Scholar] [CrossRef]
- Doern, C.D. When does 2 plus 2 equal 5? A review of antimicrobial synergy testing. J. Clin. Microbiol. 2014, 52, 4124–4128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodson, M.E.; Gallagher, C.G.; Govan, J.R. A randomised clinical trial of nebulised tobramycin or colistin in cystic fibrosis. Eur. Respir. J. 2002, 20, 658–664. [Google Scholar] [CrossRef] [Green Version]
- Akdag Cayli, Y.; Sahin, S.; Buttini, F.; Balducci, A.G.; Montanari, S.; Vural, I.; Oner, L. Dry powders for the inhalation of ciprofloxacin or levofloxacin combined with a mucolytic agent for cystic fibrosis patients. Drug. Dev. Ind. Pharm. 2017, 43, 1378–1389. [Google Scholar] [CrossRef]
- Aiyer, A.; Manoharan, A.; Paino, D.; Farrell, J.; Whiteley, G.S.; Kriel, F.H.; Glasbey, T.O.; Manos, J.; Das, T. Disruption of biofilms and killing of Burkholderia cenocepacia from cystic fibrosis lung using an antioxidant-antibiotic combination therapy. Int. J. Antimicrob. Agents 2021, 58, 106372. [Google Scholar] [CrossRef] [PubMed]
- Tenorio, M.; Graciliano, N.G.; Moura, F.A.; Oliveira, A.C.M.; Goulart, M.O.F. N-Acetylcysteine (NAC): Impacts on Human Health. Antioxidants 2021, 10, 967. [Google Scholar] [CrossRef] [PubMed]
- NPS MedicineWise. Omegapharm Acetylcysteine Solution: Consumer Medicine Information; National Prescribing Service MedicineWise: Sydney, Australia, 2018. [Google Scholar]
- Biswas, S.; Brunel, J.M.; Dubus, J.C.; Reynaud-Gaubert, M.; Rolain, J.M. Colistin: An update on the antibiotic of the 21st century. Expert Rev. Anti Infect Ther. 2012, 10, 917–934. [Google Scholar] [CrossRef] [PubMed]
- Yapa, S.W.S.; Li, J.; Patel, K.; Wilson, J.W.; Dooley, M.J.; George, J.; Clark, D.; Poole, S.; Williams, E.; Porter, C.J.; et al. Pulmonary and systemic pharmacokinetics of inhaled and intravenous colistin methanesulfonate in cystic fibrosis patients: Targeting advantage of inhalational administration. Antimicrob. Agents Chemother. 2014, 58, 2570–2579. [Google Scholar] [CrossRef] [Green Version]
- Levy, J.; Burns, J.L.; Mendelman, P.M.; Wong, K.; Mack, K.; Smith, A.L. Effect of tobramycin on protein synthesis in 2-deoxystreptamine aminoglycoside-resistant clinical isolates of Haemophilus influenzae. Antimicrob. Agents Chemother. 1986, 29, 474–481. [Google Scholar] [CrossRef] [Green Version]
- Prickett, M.H.; Hauser, A.R.; McColley, S.A.; Cullina, J.; Potter, E.; Powers, C.; Jain, M. Aminoglycoside resistance of Pseudomonas aeruginosa in cystic fibrosis results from convergent evolution in the mexZ gene. Thorax 2017, 72, 40–47. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, S.; Beaudoin, T.; Yau, Y.C.; Caraher, E.; Zlosnik, J.E.; Speert, D.P.; LiPuma, J.J.; Tullis, E.; Waters, V. Activity of Tobramycin against Cystic Fibrosis Isolates of Burkholderia cepacia Complex Grown as Biofilms. Antimicrob. Agents Chemother. 2016, 60, 348–355. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Jiménez, E.; del Campo, R.; Toledano, V.; Vallejo-Cremades, M.T.; Muñoz, A.; Largo, C.; Arnalich, F.; García-Rio, F.; Cubillos-Zapata, C.; López-Collazo, E. Biofilm vs. planktonic bacterial mode of growth: Which do human macrophages prefer? Biochem. Biophys. Res. Commun. 2013, 441, 947–952. [Google Scholar] [CrossRef]
- Sadowska, A.M. N-Acetylcysteine mucolysis in the management of chronic obstructive pulmonary disease. Ther. Adv. Respir. Dis. 2012, 6, 127–135. [Google Scholar] [CrossRef] [Green Version]
- Aldini, G.; Altomare, A.; Baron, G.; Vistoli, G.; Carini, M.; Borsani, L.; Sergio, F. N-Acetylcysteine as an antioxidant and disulphide breaking agent: The reasons why. Free Radic. Res. 2018, 52, 751–762. [Google Scholar] [CrossRef]
- Tirouvanziam, R.; Conrad, C.K.; Bottiglieri, T.; Herzenberg, L.A.; Moss, R.B.; Herzenberg, L.A. High-dose oral N-Acetylcysteine, a glutathione prodrug, modulates inflammation in cystic fibrosis. Proc. Natl. Acad. Sci. USA 2006, 103, 4628–4633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olofsson, A.C.; Hermansson, M.; Elwing, H. N-acetyl-L-cysteine affects growth, extracellular polysaccharide production, and bacterial biofilm formation on solid surfaces. Appl. Environ. Microbiol. 2003, 69, 4814–4822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, T.; Simone, M.; Ibugo, A.I.; Witting, P.K.; Manefield, M.; Manos, J. Glutathione Enhances Antibiotic Efficiency and Effectiveness of DNase I in Disrupting Pseudomonas aeruginosa Biofilms While Also Inhibiting Pyocyanin Activity, Thus Facilitating Restoration of Cell Enzymatic Activity, Confluence and Viability. Front. Microbiol. 2017, 8, 2429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonapace, C.R.; Bosso, J.A.; Friedrich, L.V.; White, R.L. Comparison of methods of interpretation of checkerboard synergy testing. Diagn. Microbiol. Infect. Dis. 2002, 44, 363–366. [Google Scholar] [CrossRef]
- den Hollander, J.G.; Horrevorts, A.M.; van Goor, M.L.; Verbrugh, H.A.; Mouton, J.W. Synergism between tobramycin and ceftazidime against a resistant Pseudomonas aeruginosa strain, tested in an in vitro pharmacokinetic model. Antimicrob. Agents Chemother. 1997, 41, 95–100. [Google Scholar] [CrossRef] [Green Version]
- Das, T.; Paino, D.; Manoharan, A.; Farrell, J.; Whiteley, G.; Kriel, F.H.; Glasbey, T.; Manos, J. Conditions Under Which Glutathione Disrupts the Biofilms and Improves Antibiotic Efficacy of Both ESKAPE and Non-ESKAPE Species. Front. Microbiol. 2019, 10, 2000. [Google Scholar] [CrossRef] [Green Version]





| Bacteria | Strain | Source | Antibiotic Profile | ||||
|---|---|---|---|---|---|---|---|
| TZP110 | CAZ30 | MEM10 | CIP5 | SXT25 | |||
| B. cenocepacia | ATCC 25608TM | ATCC (Manassas, Virginia) (Incision wound) | NA | S | S | NA | S |
| M2167 + | CF Sputum (+) | NA | R | R | NA | R | |
| E5452 + | CF Sputum (+) | NA | S | R | NA | R | |
| E5328 + | CF Sputum (+) | NA | S | S | NA | R | |
| S. maltophilia | ATCC 13637TM | ATCC (Oropharyngeal region) | NA | NA | NA | NA | S |
| 445595 ^ | CF Sputum ^ | NA | NA | NA | NA | S | |
| 19308 ^ | CF Sputum ^ | NA | NA | NA | NA | R | |
| A. xylosoxidans# | ATCC 27601TM | ATCC (Ear discharge) | S | I | S | R | R |
| 6268 ^ | CF Sputum ^ | S | I | S | R | R | |
| 6908 ^ | CF Sputum ^ | S | I | S | R | R | |
| 4365 ^ | CF Sputum ^ | NA | NA | NA | NA | R | |
| Bacteria | Neutral NAC (mg/mL) | Ciprofloxacin (μg/mL) | Colistin (μg/mL) | Tobramycin (μg/mL) | |
|---|---|---|---|---|---|
| A. xylosoxidans | ATCC 27601TM | >16.3 | 32 | >128 | >128 |
| 6268 | >16.3 | 32 | 32 | >128 | |
| 6908 | >16.3 | 32 | 8 | >128 | |
| 4365 | >16.3 | 32 | >128 | >128 | |
| B. cenocepacia | ATCC 25608 TM | >16.3 | 64 | 16 | >128 |
| M2167 | >16.3 | 64 | >128 | >128 | |
| E5452 | >16.3 | 64 | 64 | >128 | |
| E5328 | >16.3 | 64 | >128 | >128 | |
| S. maltophilia | ATCC 13637 TM | >16.3 | 16 | 32 | 64 |
| 445595 | >16.3 | 64 | 64 | >128 | |
| 19308 | >16.3 | 32 | 64 | 64 |
| Letter Reference | Neutral NAC (mg/mL) | Antibiotic (μg/mL) |
|---|---|---|
| A | 2 | 32 μg/mL Ciprofloxacin |
| B | 4.1 | |
| C | 8.2 | |
| D | 1 | 64 μg/mL Ciprofloxacin |
| E | 2 | 8 μg/mL Colistin |
| F | 4.1 | |
| G | 8.2 | |
| H | 2 | 16 μg/mL Colistin |
| I | 4.1 | |
| J | 8.2 | |
| K | 16.3 | |
| L | 16.3 | 32 μg/mL Colistin |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aiyer, A.; Visser, S.K.; Bye, P.; Britton, W.J.; Whiteley, G.S.; Glasbey, T.; Kriel, F.H.; Farrell, J.; Das, T.; Manos, J. Effect of N-Acetylcysteine in Combination with Antibiotics on the Biofilms of Three Cystic Fibrosis Pathogens of Emerging Importance. Antibiotics 2021, 10, 1176. https://doi.org/10.3390/antibiotics10101176
Aiyer A, Visser SK, Bye P, Britton WJ, Whiteley GS, Glasbey T, Kriel FH, Farrell J, Das T, Manos J. Effect of N-Acetylcysteine in Combination with Antibiotics on the Biofilms of Three Cystic Fibrosis Pathogens of Emerging Importance. Antibiotics. 2021; 10(10):1176. https://doi.org/10.3390/antibiotics10101176
Chicago/Turabian StyleAiyer, Aditi, Simone K. Visser, Peter Bye, Warwick J. Britton, Gregory S. Whiteley, Trevor Glasbey, Frederik H. Kriel, Jessica Farrell, Theerthankar Das, and Jim Manos. 2021. "Effect of N-Acetylcysteine in Combination with Antibiotics on the Biofilms of Three Cystic Fibrosis Pathogens of Emerging Importance" Antibiotics 10, no. 10: 1176. https://doi.org/10.3390/antibiotics10101176
APA StyleAiyer, A., Visser, S. K., Bye, P., Britton, W. J., Whiteley, G. S., Glasbey, T., Kriel, F. H., Farrell, J., Das, T., & Manos, J. (2021). Effect of N-Acetylcysteine in Combination with Antibiotics on the Biofilms of Three Cystic Fibrosis Pathogens of Emerging Importance. Antibiotics, 10(10), 1176. https://doi.org/10.3390/antibiotics10101176