Detailed Revision Risk Analysis after Single- vs. Two-Stage Revision Total Knee Arthroplasty in Periprosthetic Joint Infection: A Retrospective Tertiary Center Analysis
Abstract
:1. Introduction
2. Results
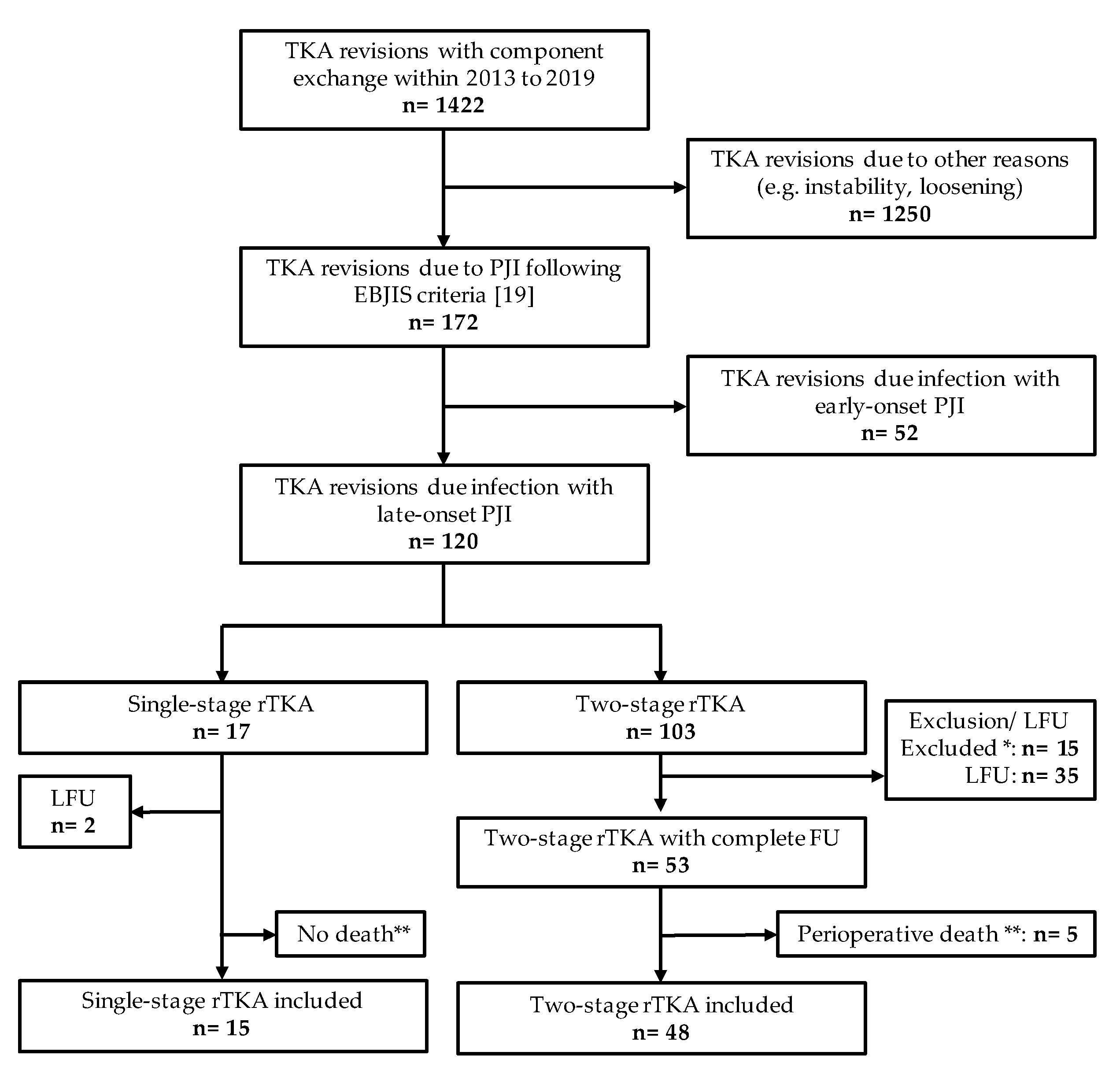
2.1. Group Sizes and Patient Inclusion
2.2. Demographic Data
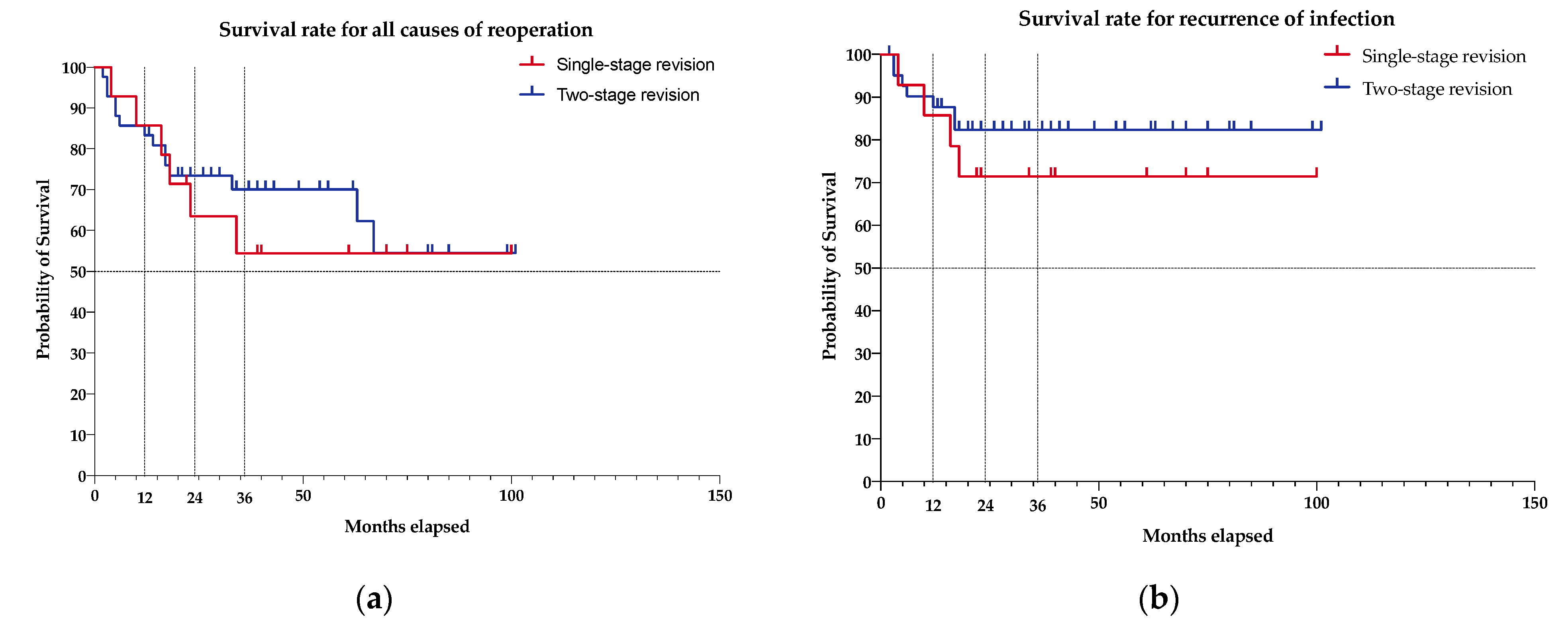
2.3. Survival Rate Analysis
2.4. Influence of Variables on Reinfection Rate
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bozic, K.J.; Kurtz, S.M.; Lau, E.; Ong, K.; Chiu, V.; Vail, T.P.; Rubash, H.E.; Berry, D.J. The Epidemiology of Revision Total Knee Arthroplasty in the United States. Clin. Orthop. Relat. Res. 2010, 468, 45–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiele, K.; Perka, C.; Matziolis, G.; Mayr, H.O.; Sostheim, M.; Hube, R. Current Failure Mechanisms after Knee Arthroplasty Have Changed: Polyethylene Wear Is Less Common in Revision Surgery. J. Bone Jt. Surg. Am. 2015, 97, 715–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suarez, J.; Griffin, W.; Springer, B.; Fehring, T.; Mason, J.B.; Odum, S. Why Do Revision Knee Arthroplasties Fail? J. Arthroplast. 2008, 23, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Klug, A.; Gramlich, Y.; Rudert, M.; Drees, P.; Hoffmann, R.; Weißenberger, M.; Kutzner, K.P. The Projected Volume of Primary and Revision Total Knee Arthroplasty Will Place an Immense Burden on Future Health Care Systems over the next 30 Years. Knee Surg. Sports Traumatol. Arthrosc. 2020. [Google Scholar] [CrossRef]
- Mortazavi, S.M.J.; Schwartzenberger, J.; Austin, M.S.; Purtill, J.J.; Parvizi, J. Revision Total Knee Arthroplasty Infection: Incidence and Predictors. Clin. Orthop. Relat. Res. 2010, 468, 2052–2059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bongers, J.; Jacobs, A.M.E.; Smulders, K.; van Hellemondt, G.G.; Goosen, J.H.M. Reinfection and Re-Revision Rates of 113 Two-Stage Revisions in Infected TKA. J. Bone Jt. Infect. 2020, 5, 137–144. [Google Scholar] [CrossRef]
- Gundtoft, P.H.; Pedersen, A.B.; Varnum, C.; Overgaard, S. Increased Mortality After Prosthetic Joint Infection in Primary THA. Clin. Orthop. Relat. Res. 2017, 475, 2623–2631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischbacher, A.; Borens, O. Prosthetic-Joint Infections: Mortality Over The Last 10 Years. J. Bone Jt. Infect. 2019, 4, 198–202. [Google Scholar] [CrossRef] [Green Version]
- Zahar, A.; Sarungi, M. Diagnosis and Management of the Infected Total Knee Replacement: A Practical Surgical Guide. J. EXP ORTOP 2021, 8, 14. [Google Scholar] [CrossRef]
- Oussedik, S.; Abdel, M.P.; Victor, J.; Pagnano, M.W.; Haddad, F.S. Alignment in Total Knee Arthroplasty: What’s in a Name? Bone Jt. J. 2020, 102-B, 276–279. [Google Scholar] [CrossRef]
- Moore, A.J.; Blom, A.W.; Whitehouse, M.R.; Gooberman-Hill, R. Deep Prosthetic Joint Infection: A Qualitative Study of the Impact on Patients and Their Experiences of Revision Surgery. BMJ Open 2015, 5, e009495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negus, J.J.; Gifford, P.B.; Haddad, F.S. Single-Stage Revision Arthroplasty for Infection-An Underutilized Treatment Strategy. J. Arthroplast. 2017, 32, 2051–2055. [Google Scholar] [CrossRef]
- Cochran, A.R.; Ong, K.L.; Lau, E.; Mont, M.A.; Malkani, A.L. Risk of Reinfection After Treatment of Infected Total Knee Arthroplasty. J. Arthroplast. 2016, 31, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Yaghmour, K.; Chisari, E.; Khan, W. Single-Stage Revision Surgery in Infected Total Knee Arthroplasty: A PRISMA Systematic Review. JCM 2019, 8, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van den Kieboom, J.; Tirumala, V.; Box, H.; Oganesyan, R.; Klemt, C.; Kwon, Y.-M. One-Stage Revision Is as Effective as Two-Stage Revision for Chronic Culture-Negative Periprosthetic Joint Infection after Total Hip and Knee Arthroplasty. Bone Jt. J. 2021, 103-B, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Matar, H.E.; Bloch, B.V.; Snape, S.E.; James, P.J. Outcomes of Single- and Two-Stage Revision Total Knee Arthroplasty for Chronic Periprosthetic Joint Infection: Long-Term Outcomes of Changing Clinical Practice in a Specialist Centre. Bone Jt. J. 2021, 103-B, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Thakrar, R.R.; Horriat, S.; Kayani, B.; Haddad, F.S. Indications for a Single-Stage Exchange Arthroplasty for Chronic Prosthetic Joint Infection: A Systematic Review. Bone Jt. J. 2019, 101-B, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Zahar, A.; Kendoff, D.O.; Klatte, T.O.; Gehrke, T.A. Can Good Infection Control Be Obtained in One-Stage Exchange of the Infected TKA to a Rotating Hinge Design? 10-Year Results. Clin. Orthop. Relat. Res. 2016, 474, 81–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wimmer, M.D.; Hischebeth, G.T.R.; Randau, T.M.; Gathen, M.; Schildberg, F.A.; Fröschen, F.S.; Kohlhof, H.; Gravius, S. Difficult-to-Treat Pathogens Significantly Reduce Infection Resolution in Periprosthetic Joint Infections. Diagn. Microbiol. Infect. Dis. 2020, 98, 115114. [Google Scholar] [CrossRef]
- Pangaud, C.; Ollivier, M.; Argenson, J.-N. Outcome of Single-Stage versus Two-Stage Exchange for Revision Knee Arthroplasty for Chronic Periprosthetic Infection. EFORT Open Rev. 2019, 4, 495–502. [Google Scholar] [CrossRef]
- Lazic, I.; Scheele, C.; Pohlig, F.; von Eisenhart-Rothe, R.; Suren, C. Treatment Options in PJI—Is Two-Stage Still Gold Standard? J. Orthop. 2021, 23, 180–184. [Google Scholar] [CrossRef]
- Thompson, O.; Rasmussen, M.; Stefánsdóttir, A.; Christensson, B.; Åkesson, P. A Population-Based Study on the Treatment and Outcome of Enterococcal Prosthetic Joint Infections. A Consecutive Series of 55 Cases. J. Bone Jt. Infect. 2019, 4, 285–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kheir, M.M.; Tan, T.L.; Higuera, C.; George, J.; Della Valle, C.J.; Shen, M.; Parvizi, J. Periprosthetic Joint Infections Caused by Enterococci Have Poor Outcomes. J. Arthroplast. 2017, 32, 933–947. [Google Scholar] [CrossRef] [PubMed]
- Vasso, M.; Schiavone Panni, A.; De Martino, I.; Gasparini, G. Prosthetic Knee Infection by Resistant Bacteria: The Worst-Case Scenario. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 3140–3146. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.M.A.; Lo, N.N.; Ab Rahman, S.; Chin, P.L.; Chia, S.-L.; Yeo, S.J. Two-Year Outcome of Early Deep MRSA Infections After Primary Total Knee Arthroplasty. J. Arthroplast. 2013, 28, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Mittal, Y.; Fehring, T.K.; Hanssen, A.; Marculescu, C.; Odum, S.M.; Osmon, D. Two-Stage Reimplantation for Periprosthetic Knee Infection Involving Resistant Organisms. J. Bone Jt. Surg. 2007, 89, 1227–1231. [Google Scholar] [CrossRef]
- Ohlmeier, M.; Alrustom, F.; Citak, M.; Salber, J.; Gehrke, T.; Frings, J. What Is the Mid-Term Survivorship of Infected Rotating-Hinge Implants Treated with One-Stage-Exchange? Clin. Orthop. Relat. Res. 2021. [Google Scholar] [CrossRef]
- Baochao, J.; Guoqing, L.; Xiaogang, Z.; Yang, W.; Wenbo, M.; Li, C. Midterm Survival of a Varus–Valgus Constrained Implant Following One-Stage Revision for Periprosthetic Joint Infection: A Single-Center Study. J. Knee Surg. 2021. [Google Scholar] [CrossRef]
- Izakovicova, P.; Borens, O.; Trampuz, A. Periprosthetic Joint Infection: Current Concepts and Outlook. EFORT Open Rev. 2019, 4, 482–494. [Google Scholar] [CrossRef]
- Razii, N.; Clutton, J.M.; Kakar, R.; Morgan-Jones, R. Single-Stage Revision for the Infected Total Knee Arthroplasty: The Cardiff Experience. Bone Jt. Open 2021, 2, 305–313. [Google Scholar] [CrossRef]
- Pelt, C.E.; Grijalva, R.; Anderson, L.; Anderson, M.B.; Erickson, J.; Peters, C.L. Two-Stage Revision TKA Is Associated with High Complication and Failure Rates. Adv. Orthop. 2014, 2014, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krenn, V.; Morawietz, L.; Perino, G.; Kienapfel, H.; Ascherl, R.; Hassenpflug, G.J.; Thomsen, M.; Thomas, P.; Huber, M.; Kendoff, D.; et al. Revised Histopathological Consensus Classification of Joint Implant Related Pathology. Pathol. Res. Pract. 2014, 210, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Reubsaet, L.L.; Ekkelenkamp, M.B. Pathogen-directed antibiotic therapy. In Management of Periprosthetic Joint Infections (PJIs); Elsevier: Amsterdam, The Netherlands, 2017; pp. 231–255. ISBN 978-0-08-100205-6. [Google Scholar]
| Demographic Variables | Single-Stage | Two-Stage | p Value | |
|---|---|---|---|---|
| Group size | n | 15 | 48 | |
| Age (years) | mean (±SD) | 65.0 (±10.2) | 69.3 (±11.1) | 0.315 |
| min | 49.0 | 51.0 | ||
| max | 84.0 | 93.0 | ||
| BMI (kg/ m2) | mean (±SD) min | 30.1 (±5.9) 20.2 | 29.9 (±7.2) 19.6 | 0.725 |
| max | 42.6 | 46.3 | ||
| Gender | female | 11 (73.3%) | 20 (41.7%) | 0.041 * |
| ASA score | I | 2 (13.3%) | 1 (2.1%) | 0.377 |
| II | 9 (60.0%) | 33 (68.8%) | ||
| III | 4 (26.7%) | 14 (29.2%) | ||
| IV | ||||
| Mc Pherson score | Host grade A | 7 (46.7%) | 8 (16.7%) | 0.150 |
| B | 4 (26.7%) | 27 (56.3%) | ||
| C | 4 (26.7%) | 13 (27.1%) | ||
| Local grade I | 4 (26.7%) | 2 (4.2%) | 0.015 * | |
| II | 7 (46.7%) | 18 (37.5%) | ||
| III | 4 (26.7%) | 28 (58.3%) | ||
| Number of preoperations | mean (± SD) | 2.1 (±1.2) | 3.3 (±2.3) | 0.081 |
| Follow up (months) | mean (± SD) | 47.3 (±19.2) | 40.7 (±23.1) | 0.982 |
| min | 22.0 | 18.0 | ||
| max | 75.0 | 92.0 | ||
| Implants after revision | CR/ PS | 3 (20.0%) | 6 (12.5%) | 0.048* |
| semi constrained | 8 (53.3%) | 13 (27.1%) | ||
| constrained | 4 (26.7%) | 27 (56.3%) | ||
| DFR | 1 (2.1%) | |||
| arthrodesis | 1 (2.1%) | |||
| Spacer type | mobile | 37 (77.1%) | - - - | |
| static | 11 (22.9%) | |||
| Organism characteristics | DTT | 4 (26.4%) | 18 (37.5%) | 0.544 |
| non DTT | 11 (73.3%) | 30 (62.5%) |
| Microbiological Organism | Resistance | Single-Stage | Two-Stage |
|---|---|---|---|
| Gram positive | |||
| Coagulase-negative staphylococcus | 5 (33.3%) | 13 (27.1%) | |
| of those: | Methicillin/Clindamycin | 2 (4.2%) | |
| Rifampicin | 2 (4.2%) | ||
| Staphylococcus aureus | 5 (10.4%) | ||
| of those: | Methicillin | 1 (2.1%) | |
| Divers Staphylococcus spp. | 2 (13.3) | 4 (8.33%) | |
| Streptococcus spp. | 2 (4.2%) | ||
| Bacillus spp. | 1 (2.1%) | ||
| Microccocus luteus | 1 (2.1%) | ||
| Enterococcus faecalis | 1 (6.7%) | 3 (6.3%) | |
| Propriobacterium acnes | 1 (6.7%) | ||
| Fungi | |||
| Candida spp. | 1 (6.7%) | 1 (2.1%) | |
| Others | |||
| Polymicrobial | 1 (6.7%) | 6 (12.5%) | |
| No growth | 4 (26.7%) | 11 (22.9%) |
| Cause of Revision | Single-Stage | Two-Stage | p Value ** | ||
|---|---|---|---|---|---|
| Revision rate | |||||
| Overall | n (% of all) | 7 (53.3%) | 15 (31.3%) | 0.684 | |
| Infection | n (% revision/% all) | 4 (57.1%/26.7%) | 7 (46.7%/14.6%) | 0.419 | |
| Loosening * | 2 (28.6%/13.3%) | 2 (13.3%/4.2%) | - - - | ||
| Fracture | 2 (13.3%/4.2%) | - - - | |||
| Instability | 1 (14.3%/6.7%) | 1 (6.7%/2.1%) | - - - | ||
| Unknown | 3 (20.0%/6.3%) | - - - | |||
| Time to revision | Overall | Months (± SD) | 15.0 (± 5.5) | 15.4 (± 16.3) | 0.331 |
| Infection | Months (± SD) | 12.0 (± 10.1) | 9.3 (± 5.7) | 0.497 |
| Variables | Single-Stage | Two-Stage | |||||
|---|---|---|---|---|---|---|---|
| Overall | Infection | p Value * | Overall | Infection | p Value * | ||
| Gender | female | 5 (71.4%) | 4 (100.0%) | 0.52 | 6 (40.0%) | 1 (14.3%) | 0.21 |
| male | 2 (28.6%) | 9 (60.0%) | 6 (85.7%) | ||||
| ASA score | I | 1 (14.3%) | 1 (25.0%) | 0.61 | 0.11 | ||
| II | 4 (57.1%) | 2 (50.0%) | 13 (86.7%) | 7 (100.0%) | |||
| III | 2 (28.6%) | 1 (25.0%) | 2 (13.3%) | ||||
| IV | |||||||
| Host grade | |||||||
| Mc Pherson score | A | 4 (57.1%) | 3 (75.0%) | 0.12 | 1 (6.7%) | 0.15 | |
| B | 2 (28.6%) | 1 (25.0%) | 8 (53.3%) | 4 (57.1%) | |||
| C | 1 (14.3%) | 6 (40.0%) | 3 (42.9%) | ||||
| Local grade | |||||||
| I | 2 (28.6%) | 1 (25.0%) | >0.99 | 0.88 | |||
| II | 3 (42.9%) | 2 (50.0%) | 8 (53.3%) | 3 (42.9%) | |||
| III | 2 (28.6%) | 1 (25.0%) | 7 (46.7%) | 4 (57.1%) | |||
| Number of preoperations | Mean (± SD) | 1.6 (±0.7) | 1.3 (± 0.4) | 0.13 | 2.9 (±1.6) | 2.4 (±1.0) | 0.41 |
| Implants after revision | CR/ PS | 2 (28.6%) | 1 (25.0%) | 0.52 | 2 (13.3%) | 2 (28.6%) | 0.10 |
| Semi-constrained | 3 (42.9%) | 3 (75.0%) | 6 (40.0%) | 4 (57.1%) | |||
| Constrained | 2 (28.6%) | 7 (46.7%) | 1 (14.3%) | ||||
| Spacer type | Mobile | 13 (86.7%) | 7 (100.0%) | 0.18 | |||
| Static | 2 (13.3%) | ||||||
| Organism characteristics | DTT | 2 (28.6%) | 0.52 | 9 (60.0%) | 5 (71.4%) | 0.09 | |
| non DTT | 5 (71.4%) | 4 (100.0%) | 6 (40.0%) | 2 (28.6%) | |||
| Authors | Year | Journal | Patients | Microbiological Organism | Follow-Up | Procedures | Implant Survival |
|---|---|---|---|---|---|---|---|
| Wimmer et al. [19] | 2020 | J Diagmicrobio | 45 | DTT | 2 yr | Two-stage | 68.9% |
| Thompson et al. [22] | 2019 | JBJI | 55 | Enterococcus | 5 yr | Single-/Two-stage | 67–80% |
| Kheir et al. [23] | 2017 | J Arthroplasty | 87 | Enterococcus | 4 yr | DAIR/Single-/Two-stage | 39.4%/45.5%/62.8% |
| Vasso et al. [24] | 2016 | KSSTA | 29 | MRSA, MRSE, MR-CoNS, MDR Pseudomonas, VRE, MDR Acinetobacter | 10 yr | Two-stage | 82.8% |
| Siddiqui et al. [25] | 2013 | J Arthopasty | 8 | MRSA | 2 yr | Two-stage | 88% |
| Mittal et al. [26] | 2007 | JBJS | 37 | MRSA, MRSE | 4.25 yr | Two-stage | 76% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuecking, L.-R.; Silligmann, J.; Savov, P.; Omar, M.; Windhagen, H.; Ettinger, M. Detailed Revision Risk Analysis after Single- vs. Two-Stage Revision Total Knee Arthroplasty in Periprosthetic Joint Infection: A Retrospective Tertiary Center Analysis. Antibiotics 2021, 10, 1177. https://doi.org/10.3390/antibiotics10101177
Tuecking L-R, Silligmann J, Savov P, Omar M, Windhagen H, Ettinger M. Detailed Revision Risk Analysis after Single- vs. Two-Stage Revision Total Knee Arthroplasty in Periprosthetic Joint Infection: A Retrospective Tertiary Center Analysis. Antibiotics. 2021; 10(10):1177. https://doi.org/10.3390/antibiotics10101177
Chicago/Turabian StyleTuecking, Lars-Rene, Julia Silligmann, Peter Savov, Mohamed Omar, Henning Windhagen, and Max Ettinger. 2021. "Detailed Revision Risk Analysis after Single- vs. Two-Stage Revision Total Knee Arthroplasty in Periprosthetic Joint Infection: A Retrospective Tertiary Center Analysis" Antibiotics 10, no. 10: 1177. https://doi.org/10.3390/antibiotics10101177
APA StyleTuecking, L.-R., Silligmann, J., Savov, P., Omar, M., Windhagen, H., & Ettinger, M. (2021). Detailed Revision Risk Analysis after Single- vs. Two-Stage Revision Total Knee Arthroplasty in Periprosthetic Joint Infection: A Retrospective Tertiary Center Analysis. Antibiotics, 10(10), 1177. https://doi.org/10.3390/antibiotics10101177







