Synergistic Effect of Abietic Acid with Oxacillin against Methicillin-Resistant Staphylococcus pseudintermedius
Abstract
1. Introduction
2. Results
2.1. Identification and Antimicrobial Susceptibility of S. pseudintermedius Strains
2.2. Antimicrobial Activity of Abietic Acid
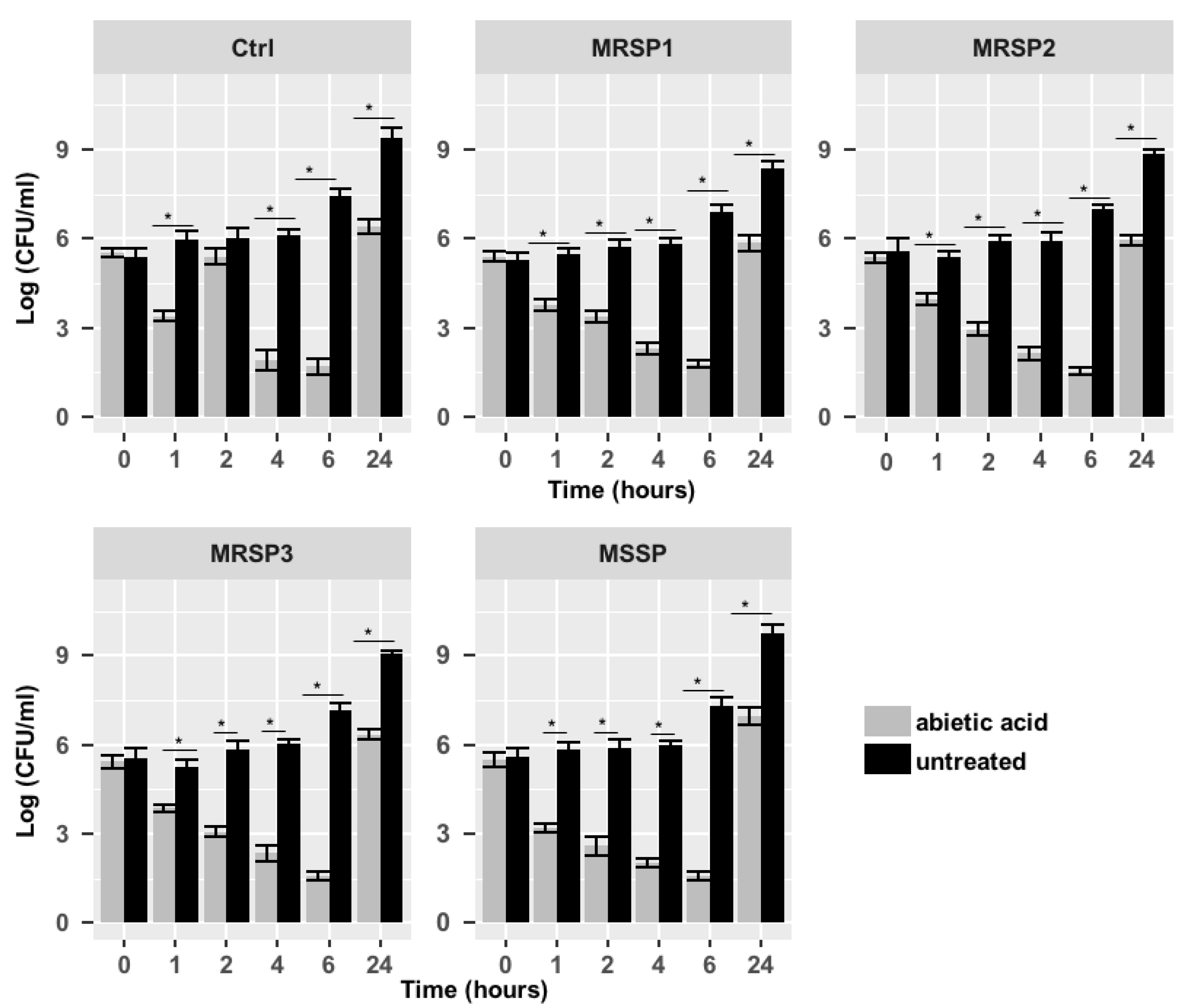
2.3. Synergistic Study
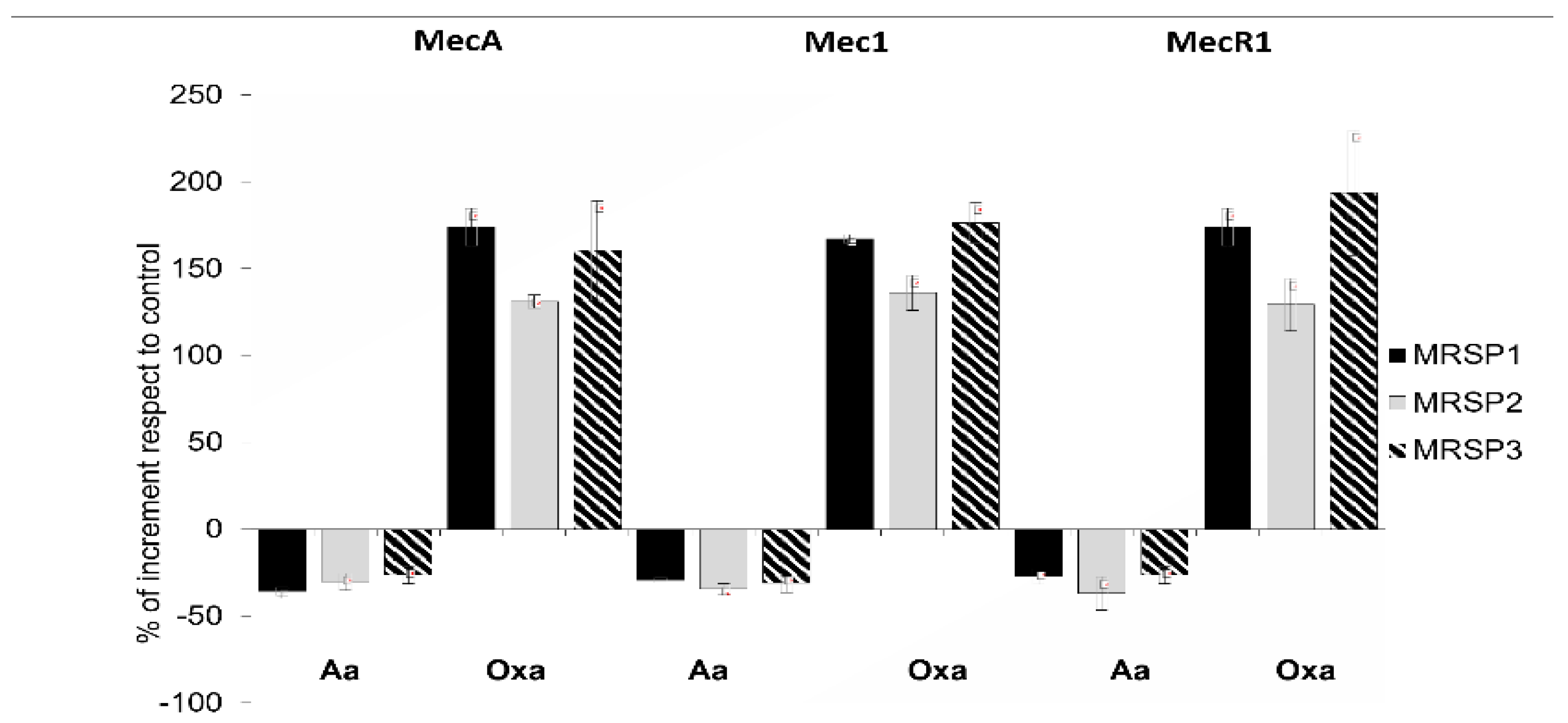
2.4. Molecular Analysis
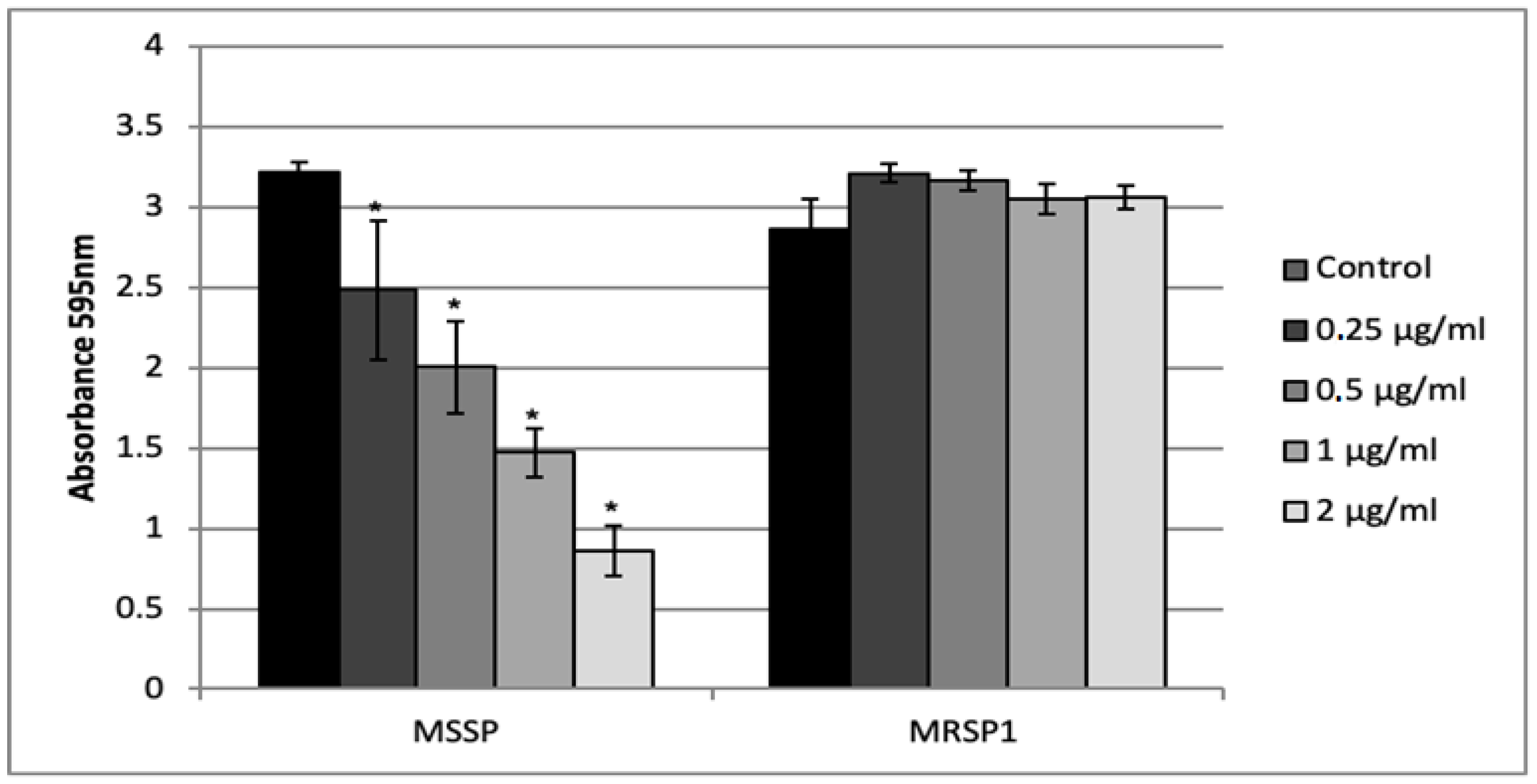
2.5. Effect of Abietic Acid on Biofilm Formation
2.6. Effect of Abietic Acid on Mature Biofilm
3. Discussion
4. Materials and Methods
4.1. Chemical Used
4.2. Bacterial Strains and Culture
4.3. Molecular Analysis
4.4. Antimicrobial Susceptibility Testing
4.5. Killing Rate
4.6. Checkerboard Method
4.7. RNA Isolation and RT-PCR
4.8. Effect of Abietic Acid on Biofilm Formation
4.9. Effect of Abietic Acid against Preformed Biofilm
4.10. Quantitation of Metabolic Activity of Mature Biofilm
4.11. Confocal Laser Scanning Microscopy
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bannoehr, J.; Guardabassi, L. Staphylococcus pseudintermedius in the dog: Taxonomy, diagnostics, ecology, epidemiology and pathogenicity. Vet. Dermatol. 2012, 23. [Google Scholar] [CrossRef] [PubMed]
- Paul, N.C.; Moodley, A.; Ghibaudo, G.; Guardabassi, L. Carriage of methicillin-resistant Staphylococcus pseudintermedius in small animal veterinarians: Indirect evidence of zoonotic transmission. Zoonoses Public Health 2011, 58, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Frank, L.A.; Kania, S.A.; Kirzeder, E.M.; Eberlein, L.C.; Bemis, D.A. Risk of colonization or gene transfer to owners of dogs with meticillin-resistant Staphylococcus pseudintermedius. Vet. Dermatol. 2009, 20, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, D.; Peters, B.M.; Li, L.; Li, B.; Xu, Z.; Shirliff, M.E. Staphylococcal chromosomal cassettes mec (SCCmec): A mobile genetic element in methicillin-resistant Staphylococcus aureus. Microb. Pathog. 2016, 101, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Arciola, C.R.; Campoccia, D.; Gamberini, S.; Donati, M.E.; Pirini, V.; Visai, L.; Speziale, P.; Montanaro, L. Antibiotic resistance in exopolysaccharide-forming Staphylococcus epidermidis clinical isolates from orthopaedic implant infections. Biomaterials 2005, 26, 6530–6535. [Google Scholar] [CrossRef] [PubMed]
- Stefanetti, V.; Bietta, A.; Pascucci, L.; Marenzoni, M.L.; Coletti, M.; Franciosini, M.P.; Passamonti, F.; Proietti, P.C. Investigation of the antibiotic resistance and biofilm formation of staphylococcus pseudintermedius strains isolated from canine pyoderma. Vet. Ital. 2017, 53, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.; Singh, A.; Nazarali, A.; Gibson, T.W.G.; Rousseau, J.; Weese, J.S. Evaluation of the Impact of Methicillin-Resistant Staphylococcus pseudintermedius Biofilm Formation on Antimicrobial Susceptibility. Vet. Surg. 2016, 45, 968–971. [Google Scholar] [CrossRef] [PubMed]
- Meroni, G.; Filipe, J.F.S.; Drago, L.; Martino, P.A. Investigation on antibiotic-resistance, biofilm formation and virulence factors in multi drug resistant and non multi drug resistant Staphylococcus pseudintermedius. Microorganisms 2019, 7, 702. [Google Scholar] [CrossRef] [PubMed]
- Twarog, N.R.; Connelly, M.; Shelat, A.A. A critical evaluation of methods to interpret drug combinations. Sci. Rep. 2020, 10, 5144. [Google Scholar] [CrossRef]
- Crawford, J.M.; Clardy, J. Bacterial symbionts and natural products. Chem. Commun. 2011, 47, 7559–7566. [Google Scholar] [CrossRef]
- Guzzo, F.; Scognamiglio, M.; Fiorentino, A.; Buommino, E.; D’Abrosca, B. Plant Derived Natural Products against Pseudomonas aeruginosa and Staphylococcus aureus: Antibiofilm Activity and Molecular Mechanisms. Molecules 2020, 25, 5024. [Google Scholar] [CrossRef] [PubMed]
- Roscetto, E.; Masi, M.; Esposito, M.; Di Lecce, R.; Delicato, A.; Maddau, L.; Calabrò, V.; Evidente, A.; Catania, M.R. Anti-Biofilm Activity of the Fungal Phytotoxin Sphaeropsidin A against Clinical Isolates of Antibiotic-Resistant Bacteria. Toxins 2020, 12, 444. [Google Scholar] [CrossRef] [PubMed]
- Bérdy, J. Thoughts and facts about antibiotics: Where we are now and where we are heading. J. Antibiot. 2012, 65, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Gigante, B.; Santos, C.; Silva, A.M.; Curto, M.J.M.; Nascimento, M.S.J.; Pinto, E.; Pedro, M.; Cerqueira, F.; Pinto, M.M.; Duarte, M.P.; et al. Catechols from abietic acid: Synthesis and evaluation as bioactive compounds. Bioorg. Med. Chem. 2003, 11, 1631–1638. [Google Scholar] [CrossRef]
- Gu, W.; Wang, S. Synthesis and antimicrobial activities of novel 1H-dibenzo[a,c]carbazoles from dehydroabietic acid. Eur. J. Med. Chem. 2010, 45, 4692–4696. [Google Scholar] [CrossRef] [PubMed]
- Leandro, L.F.; Cardoso, M.J.O.; Silva, S.D.C.; Souza, M.G.M.; Veneziani, R.C.S.; Ambrosio, S.R.; Martins, C.H.G. Antibacterial activity of Pinus elliottii and its major compound, dehydroabietic acid, against multidrug-resistant strains. J. Med. Microbiol. 2014, 63, 1649–1653. [Google Scholar] [CrossRef]
- Caetano da Silva, S.D.; Mendes de Souza, M.G.; Cardoso, M.J.O.; da Silva Moraes, T.; Ambrósio, S.R.; Veneziani, R.C.S.; Martins, C.H.G. Antibacterial activity of Pinus elliottii against anaerobic bacteria present in primary endodontic infections. Anaerobe 2014, 30, 146–152. [Google Scholar] [CrossRef]
- Buommino, E.; Carotenuto, A.; Antignano, I.; Bellavita, R.; Casciaro, B.; Loffredo, M.R.; Merlino, F.; Novellino, E.; Mangoni, M.L.; Nocera, F.P.; et al. The Outcomes of Decorated Prolines in the Discovery of Antimicrobial Peptides from Temporin-L. ChemMedChem 2019, 14, 1283–1290. [Google Scholar] [CrossRef]
- Bellavita, R.; Vollaro, A.; Catania, M.R.; Merlino, F.; De Martino, L.; Nocera, F.P.; DellaGreca, M.; Lembo, F.; Grieco, P.; Buommino, E. Novel Antimicrobial Peptide from Temporin L in The Treatment of Staphylococcus pseudintermedius and Malassezia pachydermatis in Polymicrobial Inter-Kingdom Infection. Antibiotics 2020, 9, 530. [Google Scholar] [CrossRef]
- Weese, J.S.; van Duijkeren, E. Methicillin-resistant Staphylococcus aureus and Staphylococcus pseudintermedius in veterinary medicine. Vet. Microbiol. 2010, 140, 418–429. [Google Scholar] [CrossRef]
- Rodrigues, A.C.; Belas, A.; Marques, C.; Cruz, L.; Gama, L.T.; Pomba, C. Risk Factors for Nasal Colonization by Methicillin-Resistant Staphylococci in Healthy Humans in Professional Daily Contact with Companion Animals in Portugal. Microb. Drug Resist. 2018, 24, 434–446. [Google Scholar] [CrossRef] [PubMed]
- Helfenstein, A.; Vahermo, M.; Nawrot, D.A.; Demirci, F.; İşcan, G.; Krogerus, S.; Yli-Kauhaluoma, J.; Moreira, V.M.; Tammela, P. Antibacterial profiling of abietane-type diterpenoids. Bioorg. Med. Chem. 2017, 25, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Cuzzucoli Crucitti, V.; Migneco, L.M.; Piozzi, A.; Taresco, V.; Garnett, M.; Argent, R.H.; Francolini, I. Intermolecular interaction and solid state characterization of abietic acid/chitosan solid dispersions possessing antimicrobial and antioxidant properties. Eur. J. Pharm. Biopharm. 2018, 125, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Laws, M.; Shaaban, A.; Rahman, K.M. Antibiotic resistance breakers: Current approaches and future directions. FEMS Microbiol. Rev. 2019, 43, 490–516. [Google Scholar] [CrossRef] [PubMed]
- Oluwatuyi, M.; Kaatz, G.W.; Gibbons, S. Antibacterial and resistance modifying activity of Rosmarinus officinalis. Phytochemistry 2004, 65, 3249–3254. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.K.; Moellering, R.C.; Eliopoulos, G.M. Antimicrobial combinations. In Antibiotics in Laboratory Medicine, 5th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005; pp. 365–440. [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals VET01-S2; Second Information Supplement; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2013. [Google Scholar]
- Rudkin, J.K.; Laabei, M.; Edwards, A.M.; Joo, H.S.; Otto, M.; Lennon, K.L.; O’Gara, J.P.; Waterfield, N.R.; Massey, R.C. Oxacillin alters the toxin expression profile of community-associated methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2014, 58, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Shiota, S.; Mizushima, T.; Ito, H.; Hatano, T.; Yoshida, T.; Tsuchiya, T. Marked potentiation of activity of β-lactams against methicillin-resistant Staphylococcus aureus by corilagin. Antimicrob. Agents Chemother. 2001, 45, 3198–3201. [Google Scholar] [CrossRef] [PubMed]
- Santiago, C.; Pang, E.L.; Lim, K.H.; Loh, H.S.; Ting, K.N. Inhibition of penicillin-binding protein 2a (PBP2a) in methicillin resistant Staphylococcus aureus (MRSA) by combination of ampicillin and a bioactive fraction from Duabanga grandiflora. BMC Complement. Altern. Med. 2015, 15. [Google Scholar] [CrossRef]
- Aranda, F.J.; Villalaín, J. The interaction of abietic acid with phospholipid membranes. Biochim. Biophys. Acta Biomembr. 1997, 1327, 171–180. [Google Scholar] [CrossRef]
- Little, S.V.; Bryan, L.K.; Hillhouse, A.E.; Cohen, N.D.; Lawhon, S.D. Characterization of agr Groups of Staphylococcus pseudintermedius Isolates from Dogs in Texas. mSphere 2019, 4. [Google Scholar] [CrossRef]
- Ito, Y.; Ito, T.; Yamashiro, K.; Mineshiba, F.; Hirai, K.; Omori, K.; Yamamoto, T.; Takashiba, S. Antimicrobial and antibiofilm effects of abietic acid on cariogenic Streptococcus mutans. Odontology 2020, 108, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Pompilio, A.; De Nicola, S.; Crocetta, V.; Guarnieri, S.; Savini, V.; Carretto, E.; Di Bonaventura, G. New insights in Staphylococcus pseudintermedius pathogenicity: Antibiotic-resistant biofilm formation by a human wound-associated strain. BMC Microbiol. 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Sadashiva, M.P.; Gowda, R.; Wu, X.; Inamdar, G.S.; Kuzu, O.F.; Rangappa, K.S.; Robertson, G.P.; Gowda, D.C. A non-cytotoxic N-dehydroabietylamine derivative with potent antimalarial activity. Exp. Parasitol. 2015, 155, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Tsubakishita, S.; Tanaka, Y.; Sakusabe, A.; Ohtsuka, M.; Hirotaki, S.; Kawakami, T.; Fukata, T.; Hiramatsu, K. Multiplex-PCR method for species identification of coagulase-positive staphylococci. J. Clin. Microbiol. 2010, 48, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Olajuyigbe, O.O.; Afolayan, A.J. In Vitro antibacterial and time-kill evaluation of the Erythrina caffra thunb. Extract against bacteria associated with diarrhoea. Sci. World J. 2012, 2012, 738314. [Google Scholar] [CrossRef] [PubMed]
- Vollaro, A.; Catania, M.R.; Iesce, M.R.; Sferruzza, R.; D’abrosca, B.; Donnarumma, G.; De Filippis, A.; Cermola, F.; DellaGreca, M.; Buommino, E. Antimicrobial and anti-biofilm properties of novel synthetic lignan-like compounds. New Microbiol. 2019, 42, 21–28. [Google Scholar] [PubMed]


| Strains | P | AM | AMC | OX | CAZ | IPM | C | CIP | GM | S | CM | E | VA | TE | LZD | SXT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ctrl | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S |
| MSSP | R | R | S | S | S | S | S | S | S | S | S | S | S | R | S | S |
| MRSP1 | R | R | R | R | R | R | S | R | R | R | S | R | S | R | S | R |
| MRSP2 | R | R | R | R | R | R | S | S | S | S | S | R | S | R | S | R |
| MRSP3 | R | R | R | R | R | R | S | S | R | R | S | S | S | R | S | S |
| Strains | MIC (μg/mL) | MBC (μg/mL) | Oxacillin (μg/mL) | Vancomicin (μg/mL) | FICindex |
|---|---|---|---|---|---|
| Ctrl | 8 | 16 | <0.25 | 1 | n.d. |
| MSSP | 8 | 32 | <0.25 | 2 | n.d. |
| MRSP1 | 32 | 64 | 10 | 2 | 0.375 |
| MRSP2 | 64 | 64 | 10 | 2 | 0.375 |
| MRSP3 | 32 | 64 | 10 | 2 | 0.375 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buommino, E.; Vollaro, A.; Nocera, F.P.; Lembo, F.; DellaGreca, M.; De Martino, L.; Catania, M.R. Synergistic Effect of Abietic Acid with Oxacillin against Methicillin-Resistant Staphylococcus pseudintermedius. Antibiotics 2021, 10, 80. https://doi.org/10.3390/antibiotics10010080
Buommino E, Vollaro A, Nocera FP, Lembo F, DellaGreca M, De Martino L, Catania MR. Synergistic Effect of Abietic Acid with Oxacillin against Methicillin-Resistant Staphylococcus pseudintermedius. Antibiotics. 2021; 10(1):80. https://doi.org/10.3390/antibiotics10010080
Chicago/Turabian StyleBuommino, Elisabetta, Adriana Vollaro, Francesca P. Nocera, Francesca Lembo, Marina DellaGreca, Luisa De Martino, and Maria R. Catania. 2021. "Synergistic Effect of Abietic Acid with Oxacillin against Methicillin-Resistant Staphylococcus pseudintermedius" Antibiotics 10, no. 1: 80. https://doi.org/10.3390/antibiotics10010080
APA StyleBuommino, E., Vollaro, A., Nocera, F. P., Lembo, F., DellaGreca, M., De Martino, L., & Catania, M. R. (2021). Synergistic Effect of Abietic Acid with Oxacillin against Methicillin-Resistant Staphylococcus pseudintermedius. Antibiotics, 10(1), 80. https://doi.org/10.3390/antibiotics10010080







