Bacteriophage Cocktail-Mediated Inhibition of Pseudomonas aeruginosa Biofilm on Endotracheal Tube Surface
Abstract
1. Introduction
2. Results
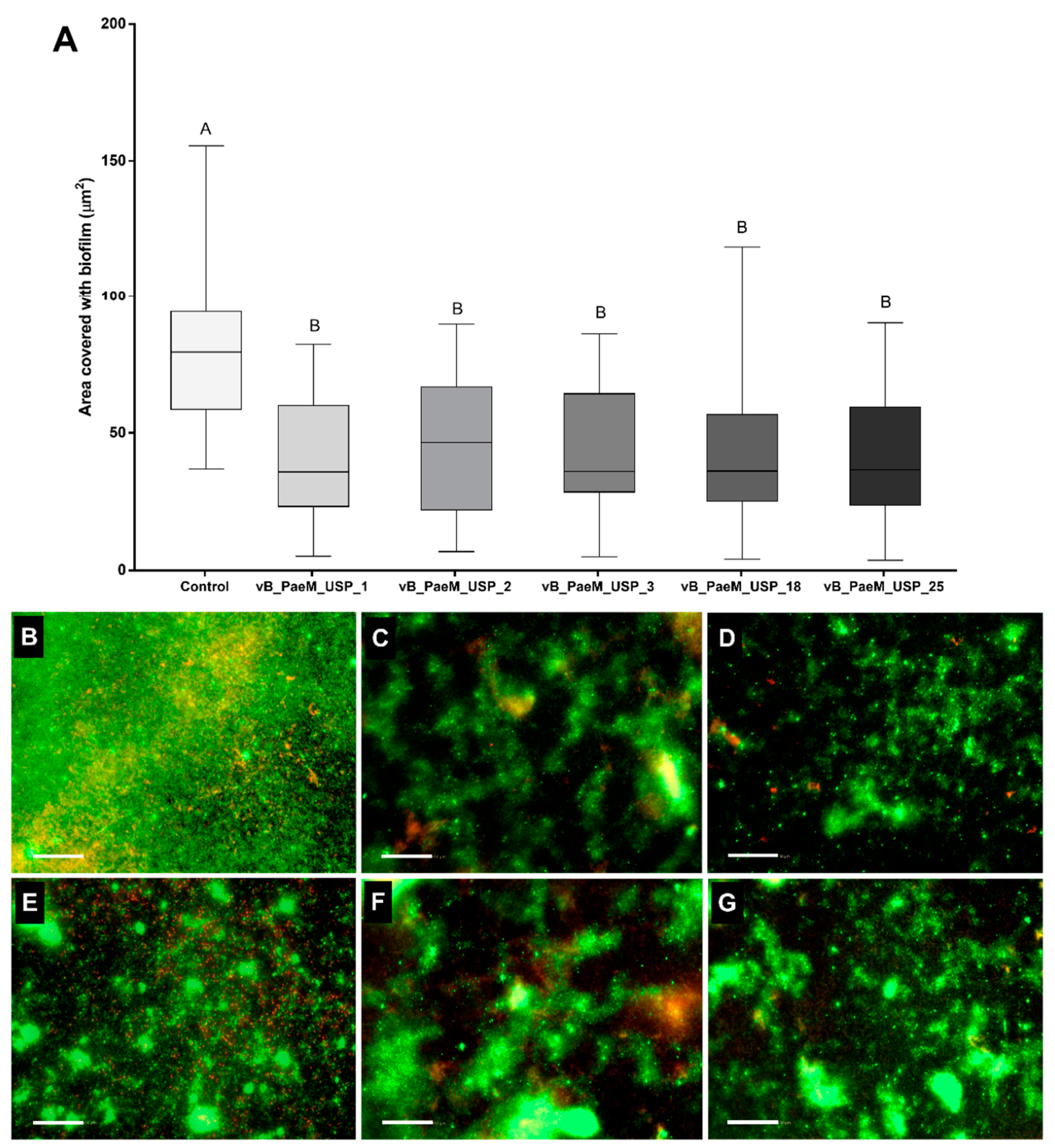
2.1. Screening Phages for Anti-Biofilm Activity
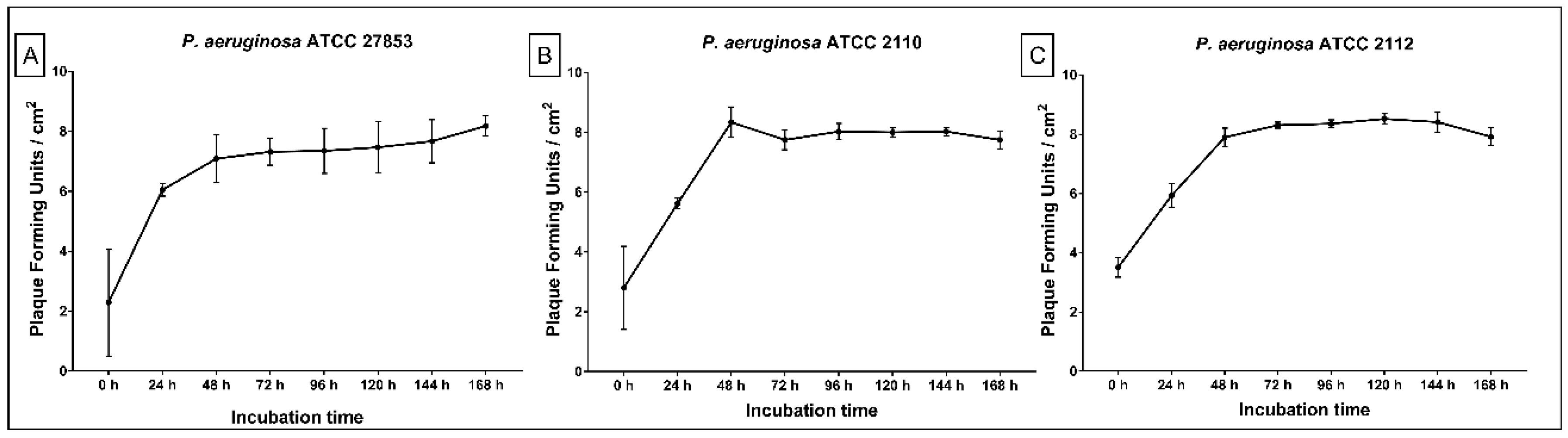
2.2. Replication of ET Adsorbed Phage During Biofilm Growth
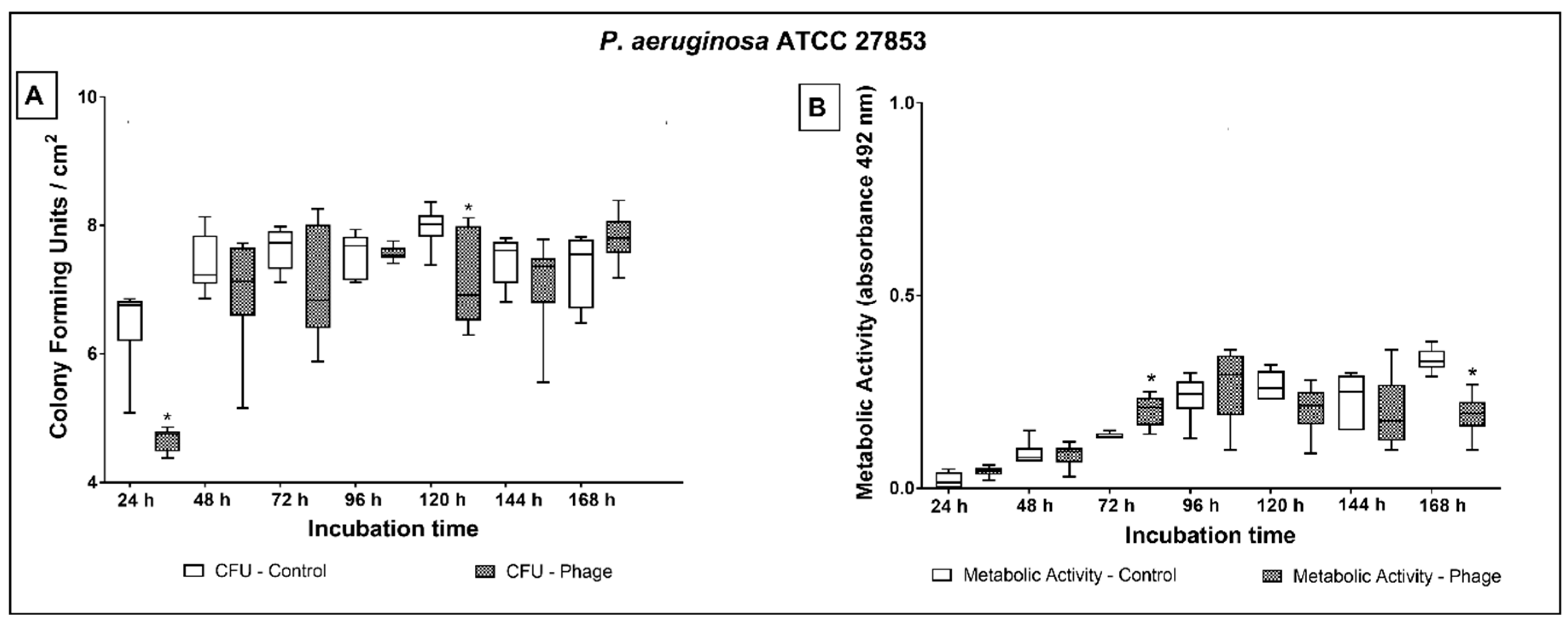
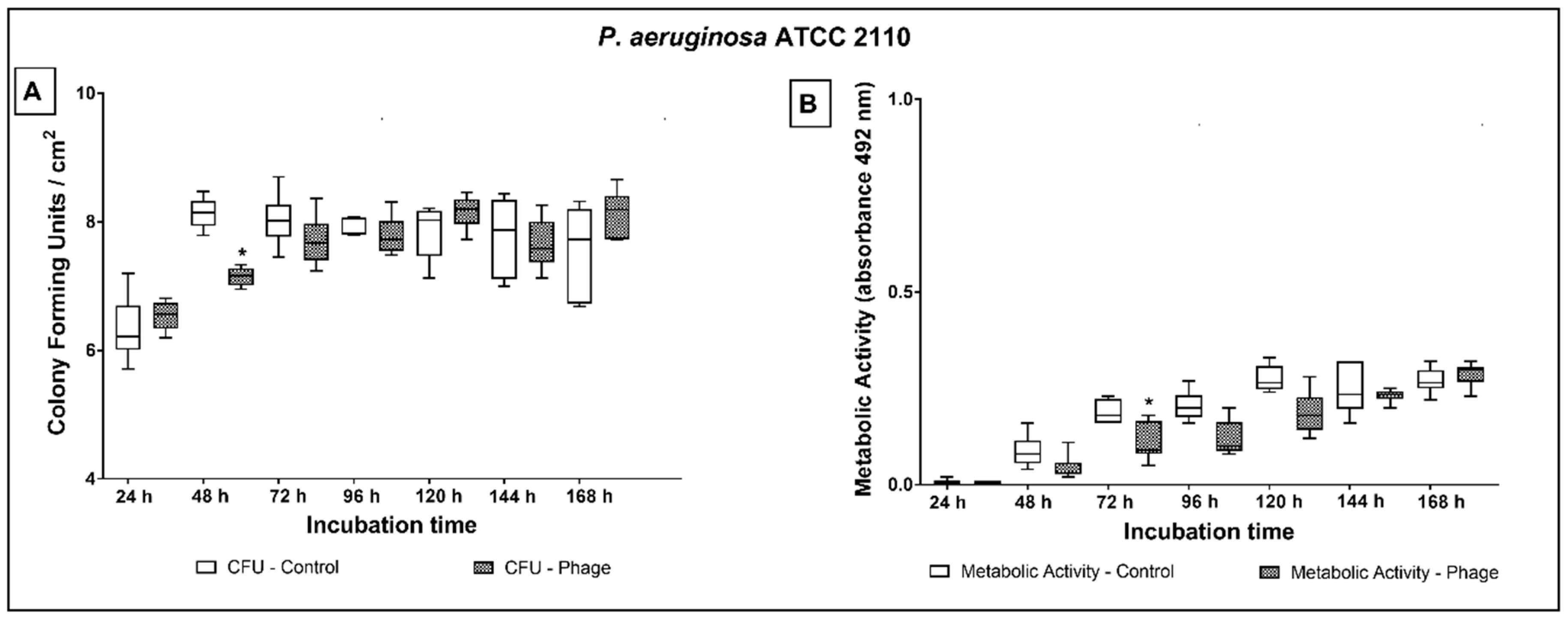
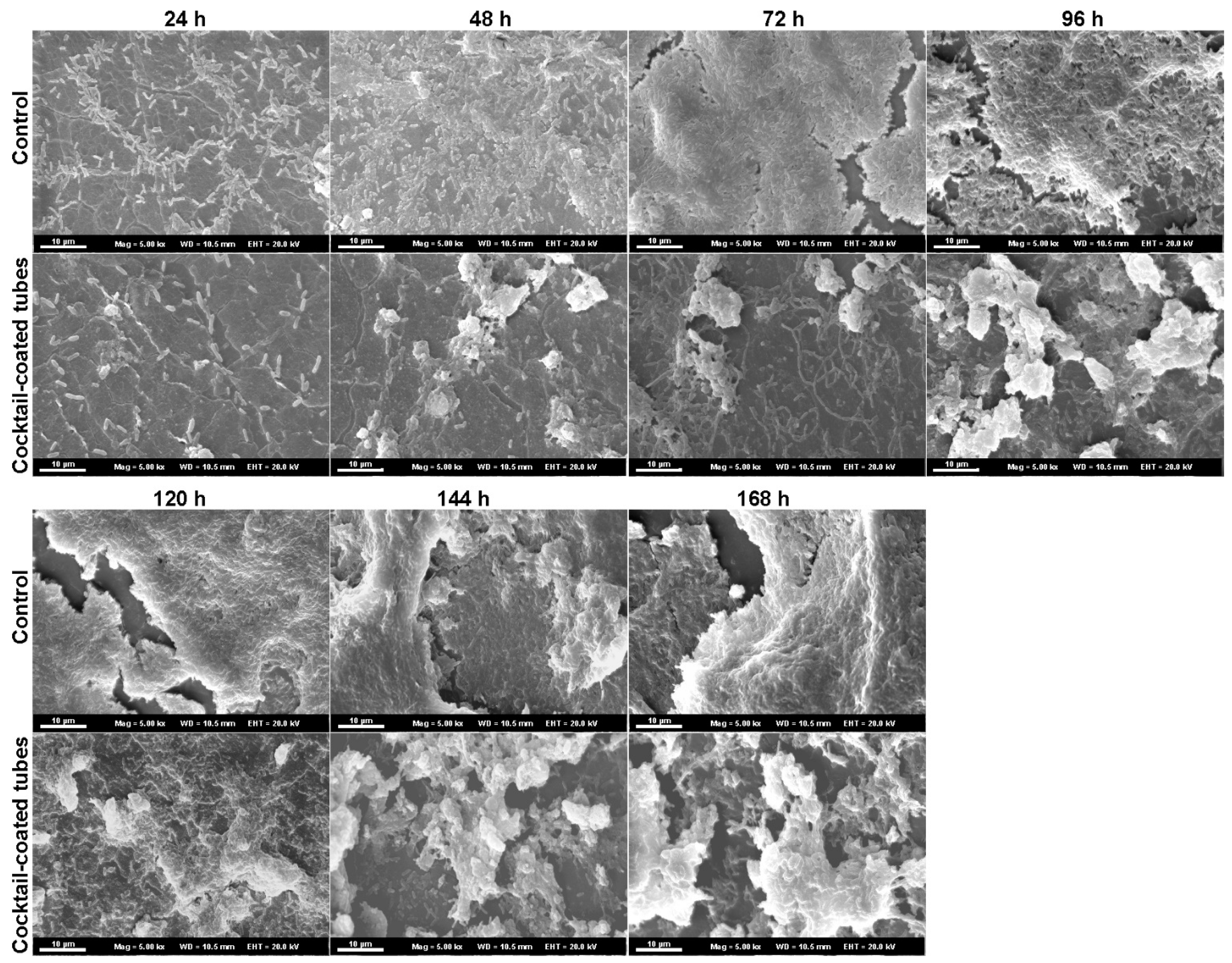
2.3. Phage Cocktail Effect on P. aeruginosa Biofilms
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains, Growth Conditions, and Bacteriophages
4.2. Screening Phages for Anti-Biofilm Activity
4.3. Phage Cocktail Pretreatment of Endotracheal Tube Surfaces
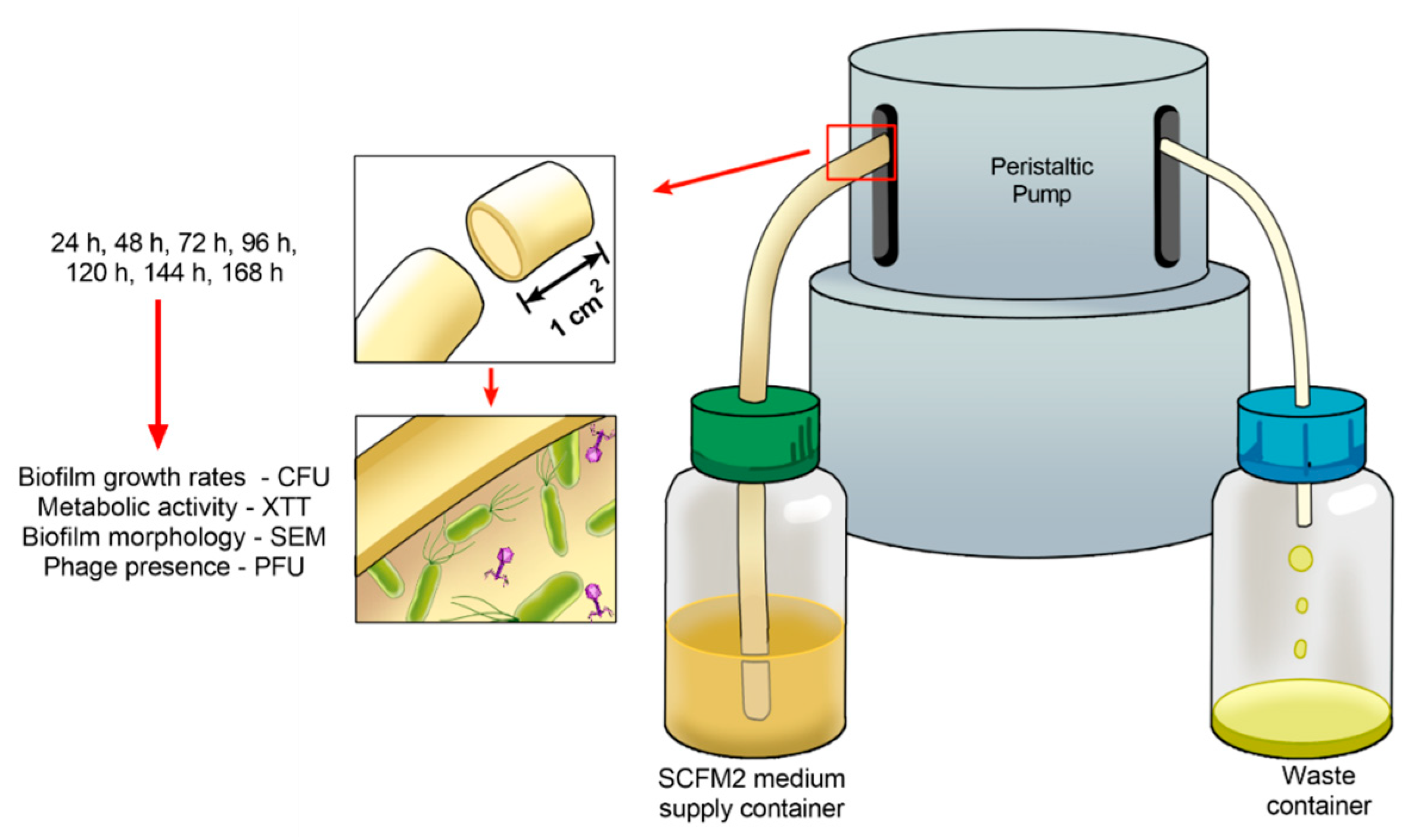
4.4. Developing Biofilms on Endotracheal Tube Pretreated with the Phage Cocktail
4.5. Analysis of the Phage Cocktail Effect on P. aeruginosa Biofilms
4.6. Replication of ET Adsorbed Phage During Biofilm Growth
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Francois, B.; Laterre, P.-F.; Luyt, C.-E.; Chastre, J. The challenge of ventilator-associated pneumonia diagnosis in COVID-19 patients. Crit. Care 2020, 24, 289. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Barat, L.; Torres, A. Biofilms in ventilator-associated pneumonia. Futur. Microbiol. 2016, 11, 1599–1610. [Google Scholar] [CrossRef] [PubMed]
- Diaconu, O.; Siriopol, I.; Poloșanu, L.I.; Grigoraș, I. Endotracheal Tube Biofilm and its Impact on the Pathogenesis of Ventilator-Associated Pneumonia. J. Crit. Care Med. 2018, 4, 50–55. [Google Scholar] [CrossRef] [PubMed]
- De Souza, P.R.; De Andrade, D.; Cabral, D.B.; Watanabe, E. Endotracheal tube biofilm and ventilator-associated pneumonia with mechanical ventilation. Microsc. Res. Tech. 2014, 77, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Danin, P.-É.; Girou, E.; Legrand, P.; Louis, B.; Fodil, R.; Christov, C.; Devaquet, J.; Isabey, D.; Brochard, L. Description and Microbiology of Endotracheal Tube Biofilm in Mechanically Ventilated Subjects. Respir. Care 2014, 60, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Bassi, G.L.; Fernandez-Barat, L.; Saucedo, L.; Giunta, V.; Martí, J.D.; Ranzani, O.T.; Xiol, E.A.; Rigol, M.; Roca, I.; Muñoz, L.; et al. Endotracheal tube biofilm translocation in the lateral Trendelenburg position. Crit. Care 2015, 19, 12–59. [Google Scholar] [CrossRef] [PubMed]
- Coppadoro, A.; Bellani, G.; Bronco, A.; Lucchini, A.; Bramati, S.; Zambelli, V.; Marcolin, R.; Pesenti, A. The use of a novel cleaning closed suction system reduces the volume of secretions within the endotracheal tube as assessed by micro-computed tomography: A randomized clinical trial. Ann. Intensiv. Care 2015, 5, 1–8. [Google Scholar] [CrossRef]
- Berra, L.; Coppadoro, A.; Bittner, E.A.; Kolobow, T.; Laquerriere, P.; Pohlmann, J.R.; Bramati, S.; Moss, J.; Pesenti, A. A clinical assessment of the Mucus Shaver: A device to keep the endotracheal tube free from secretions. Crit. Care Med. 2012, 40, 119–124. [Google Scholar] [CrossRef]
- Berra, L.; De Marchi, L.; Yu, Z.-X.; Laquerriere, P.; Baccarelli, A.; Kolobow, T. Endotracheal Tubes Coated with Antiseptics Decrease Bacterial Colonization of the Ventilator Circuits, Lungs, and Endotracheal Tube. Anesthesiology 2004, 100, 1446–1456. [Google Scholar] [CrossRef]
- Kollef, M.H.; Afessa, B.; Anzueto, A.; Veremakis, C.; Kerr, K.M.; Margolis, B.D.; Craven, D.E.; Roberts, P.R.; Arroliga, A.C.; Hubmayr, R.D.; et al. Silver-Coated Endotracheal Tubes and Incidence of Ventilator-Associated Pneumonia: The NASCENT randomized trial. JAMA 2008, 300, 805–813. [Google Scholar] [CrossRef]
- Tokmaji, G.; Vermeulen, H.; Müller, M.C.A.; Kwakman, P.H.S.; Schultz, M.J.; Zaat, S.A.J. Silver-coated endotracheal tubes for prevention of ventilator-associated pneumonia in critically ill patients. Cochrane Database Syst. Rev. 2015, 8, CD009201. [Google Scholar] [CrossRef] [PubMed]
- Ozcelik, B.; Pasic, P.; Sangwan, P.; Be, C.L.; Glattauer, V.; Thissen, H.; Boulos, R.A. Evaluation of the Novel Antimicrobial BCP3 in a Coating for Endotracheal Tubes. ACS Omega 2020, 5, 10288–10296. [Google Scholar] [CrossRef] [PubMed]
- Adair, C.; Gorman, S.P.; Byers, L.; Jones, D.; Feron, B.; Crowe, M.; Webb, H.; McCarthy, G.; Milligan, K. Eradication of endotracheal tube biofilm by nebulised gentamicin. Intensiv. Care Med. 2002, 28, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Guillon, A.; Fouquenet, D.; Morello, E.; Henry, C.O.; Georgeault, S.; Si-Tahar, M.; Hervé, V. Treatment ofPseudomonas aeruginosaBiofilm Present in Endotracheal Tubes by Poly-L-Lysine. Antimicrob. Agents Chemother. 2018, 62, 00564-18. [Google Scholar] [CrossRef] [PubMed]
- Ibis, F.; Ercan, U.K. Inactivation of biofilms in endotracheal tube by cold atmospheric plasma treatment for control and prevention of ventilator-associated pneumonia. Plasma Process. Polym. 2020, 17, e2000065. [Google Scholar] [CrossRef]
- Vandecandelaere, I.; Matthijs, N.; Van Nieuwerburgh, F.; Deforce, D.; Vosters, P.; De Bus, L.; Nelis, H.J.; Depuydt, P.; Coenye, T. Assessment of Microbial Diversity in Biofilms Recovered from Endotracheal Tubes Using Culture Dependent and Independent Approaches. PLoS ONE 2012, 7, e38401. [Google Scholar] [CrossRef] [PubMed]
- Bardes, J.M.; Waters, C.; Motlagh, H.; Wilson, A. The prevalence of oral flora in the biofilm microbiota of the endotracheal tube. Am. Surg. 2016, 82, 403–406. [Google Scholar] [CrossRef]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad Bugs, No Drugs: No ESKAPE! An Update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef]
- Moradali, M.F.; Ghods, S.; Rehm, B.H. Pseudomonas aeruginosa Lifestyle: A Paradigm for Adaptation, Survival, and Persistence. Front. Cell. Infect. Microbiol. 2017, 7, 39. [Google Scholar] [CrossRef]
- Otterbeck, A.; Hanslin, K.; Lantz, E.L.; Larsson, A.; Stålberg, J.; Lipcsey, M. Inhalation of specific anti-Pseudomonas aeruginosa IgY antibodies transiently decreases P. aeruginosa colonization of the airway in mechanically ventilated piglets. Intensiv. Care Med. Exp. 2019, 7, 21. [Google Scholar] [CrossRef]
- Dexter, A.M.; Scott, J.B. Airway Management and Ventilator-Associated Events. Respir. Care 2019, 64, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Forti, F.; Roach, D.R.; Cafora, M.; Pasini, M.E.; Horner, D.S.; Fiscarelli, E.V.; Rossitto, M.; Cariani, L.; Briani, F.; Debarbieux, L.; et al. Design of a Broad-Range Bacteriophage Cocktail That ReducesPseudomonas aeruginosaBiofilms and Treats Acute Infections in Two Animal Models. Antimicrob. Agents Chemother. 2018, 62, 02573-17. [Google Scholar] [CrossRef] [PubMed]
- Loc-Carrillo, C.; Abedon, S.T. Pros and cons of phage therapy. Bacteriophage 2011, 1, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Aleshkin, A.V.; Ershova, O.N.; Volozhantsev, N.V.; Svetoch, E.A.; Popova, A.V.; Rubalskii, E.O.; Borzilov, A.I.; Aleshkin, V.A.; Afanas’Ev, S.S.; Karaulov, A.V.; et al. Phagebiotics in treatment and prophylaxis of healthcare-associated infections. Bacteriophage 2016, 6, e1251379. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.C.; Bim, F.L.; Monteiro, R.M.; Macedo, A.P.; Santos, E.S.; Silva-Lovato, C.H.; Paranhos, H.F.O.; Melo, L.D.R.; Santos, S.B.; Watanabe, E. Identification and Characterization of New Bacteriophages to Control Multidrug-Resistant Pseudomonas aeruginosa Biofilm on Endotracheal Tubes. Front. Microbiol. 2020, 11, 580779. [Google Scholar] [CrossRef]
- Labrie, S.J.; Samson, J.E.; Moineau, S. Bacteriophage resistance mechanisms. Nat. Rev. Genet. 2010, 8, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Seed, K.D. Battling Phages: How Bacteria Defend against Viral Attack. PLOS Pathog. 2015, 11, e1004847. [Google Scholar] [CrossRef]
- Abedon, S.T. Bacteriophage exploitation of bacterial biofilms: Phage preference for less mature targets? FEMS Microbiol. Lett. 2016, 363, fnv246. [Google Scholar] [CrossRef]
- Azeredo, J.; Azevedo, N.F.; Briandet, R.; Cerca, N.; Coenye, T.; Costa, A.R.; Desvaux, M.; Di Bonaventura, G.; Hébraud, M.; Jaglic, Z.; et al. Critical review on biofilm methods. Crit. Rev. Microbiol. 2017, 43, 313–351. [Google Scholar] [CrossRef]
- Magana, M.; Sereti, C.; Ioannidis, A.; Mitchell, C.A.; Ball, A.R.; Magiorkinis, E.; Chatzipanagiotou, M.S.; Hamblin, M.R.; Hadjifrangiskou, M.; Tegos, G.P. Options and Limitations in Clinical Investigation of Bacterial Biofilms. Clin. Microbiol. Rev. 2018, 31, e00084-16. [Google Scholar] [CrossRef]
- Chan, B.K.; Abedon, S.T.; Loc-Carrillo, C. Phage cocktails and the future of phage therapy. Futur. Microbiol. 2013, 8, 769–783. [Google Scholar] [CrossRef] [PubMed]
- Cademartiri, R.; Anany, H.; Gross, I.; Bhayani, R.; Griffiths, M.; Brook, M.A. Immobilization of bacteriophages on modified silica particles. Biomaterials 2010, 31, 1904–1910. [Google Scholar] [CrossRef]
- Melo, L.D.R.; Veiga, P.; Cerca, N.; Kropinski, A.M.; Almeida, C.; Azeredo, J.; Sillankorva, S. Development of a Phage Cocktail to Control Proteus mirabilis Catheter-associated Urinary Tract Infections. Front. Microbiol. 2016, 7, 1024. [Google Scholar] [CrossRef]
- Milo, S.; Hathaway, H.; Nzakizwanayo, J.; Alves, D.R.; Esteban, P.P.; Jones, B.V.; Jenkins, A.T.A. Prevention of encrustation and blockage of urinary catheters by Proteus mirabilis via pH-triggered release of bacteriophage. J. Mater. Chem. B 2017, 5, 5403–5411. [Google Scholar] [CrossRef] [PubMed]
- Leppänen, M.; Maasilta, I.J.; Sundberg, L.-R. Antibacterial Efficiency of Surface-Immobilized Flavobacterium-Infecting Bacteriophage. ACS Appl. Bio Mater. 2019, 2, 4720–4727. [Google Scholar] [CrossRef]
- Hosseinidoust, Z.; Van De Ven, T.G.M.; Tufenkji, N. Bacterial Capture Efficiency and Antimicrobial Activity of Phage-Functionalized Model Surfaces. Langmuir 2011, 27, 5472–5480. [Google Scholar] [CrossRef]
- Fu, W.; Forster, T.; Mayer, O.; Curtin, J.J.; Lehman, S.M.; Donlan, R.M. Bacteriophage Cocktail for the Prevention of Biofilm Formation by Pseudomonas aeruginosa on Catheters in an In Vitro Model System. Antimicrob. Agents Chemother. 2009, 54, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Lehman, S.M.; Donlan, R. Bacteriophage-Mediated Control of a Two-Species Biofilm Formed by Microorganisms Causing Catheter-Associated Urinary Tract Infections in anIn VitroUrinary Catheter Model. Antimicrob. Agents Chemother. 2014, 59, 1127–1137. [Google Scholar] [CrossRef]
- Wang, C.; Sauvageau, D.; Elias, A.L. Immobilization of Active Bacteriophages on Polyhydroxyalkanoate Surfaces. ACS Appl. Mater. Interfaces 2016, 8, 1128–1138. [Google Scholar] [CrossRef]
- Nilsson, A.S. Pharmacological limitations of phage therapy. Upsala J. Med Sci. 2019, 124, 218–227. [Google Scholar] [CrossRef]
- Pearl, S.; Gabay, C.; Kishony, R.; Oppenheim, A.; Balaban, N.Q. Nongenetic Individuality in the Host–Phage Interaction. PLoS Biol. 2008, 6, e120. [Google Scholar] [CrossRef] [PubMed]
- Darch, S.E.; Kragh, K.N.; Abbott, E.A.; Bjarnsholt, T.; Bull, J.J.; Whiteley, M. Phage Inhibit Pathogen Dissemination by Targeting Bacterial Migrants in a Chronic Infection Model. mBio 2017, 8, e00240-17. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, K.; Rørbo, N.; Rybtke, M.L.; Martinet, M.G.; Tolker-Nielsen, T.; Høiby, N.; Middelboe, M.; Ciofu, O.P. aeruginosa flow-cell biofilms are enhanced by repeated phage treatments but can be eradicated by phage–ciprofloxacin combination. Pathog. Dis. 2019, 77, ftz011. [Google Scholar] [CrossRef] [PubMed]
- Połaska, M.; Sokołowska, B. Bacteriophages—a new hope or a huge problem in the food industry. AIMS Microbiol. 2019, 5, 324–346. [Google Scholar] [CrossRef]
- Melo, L.D.R.; Oliveira, H.; Pires, D.; Dąbrowska, K.; Azeredo, J. Phage therapy efficacy: A review of the last 10 years of preclinical studies. Crit. Rev. Microbiol. 2020, 46, 78–99. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2001. [Google Scholar]

| Isolates | Source | Antibiotic Resistance * | Reference |
|---|---|---|---|
| P. aeruginosa_Mi_1 † | Blood | S | [25] |
| P. aeruginosa_Mi_2 † | Sputum | S | [25] |
| P. aeruginosa_Mi_6 † | Urine | S | [25] |
| P. aeruginosa_Mi_7 † | Sputum | S | [25] |
| P. aeruginosa_Ba_164 † | Prosthetic biofilm | S | [25] |
| P. aeruginosa_Ba_168 † | Prosthetic biofilm | S | [25] |
| P. aeruginosa_Ba_169 † | Prosthetic biofilm | S | [25] |
| P. aeruginosa_Trac_20 † | Tracheal secretion | S | [25] |
| P. aeruginosa_Trac_23 † | Tracheal secretion | S | [25] |
| P. aeruginosa_Ren_1 † | Saliva | S | [25] |
| P. aeruginosa_ATCC 27853 ‡ | Blood | S | [25] |
| P. aeruginosa_ATCC 2108 ‡ | Sputum | AMK, CFZ, CTX, GEN, IMP, TGC | [25] |
| P. aeruginosa_ATCC 2110 ‡ | Sputum | AMP, CFZ, CTX, FOX, NIT, TGC, SXT | [25] |
| P. aeruginosa_ATCC 2112 ‡ | Sputum | AMC, AMP, CFZ, CPD, CRO, CTX, CXM, FOX, NIT, SXT, TET, TGC, | [25] |
| P. aeruginosa_ATCC 2113 ‡ | Sputum | AMP, AMC, CFZ, CTX, NIT, SAM, SXT | [25] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, V.C.; Macedo, A.P.; Melo, L.D.R.; Santos, S.B.; Hermann, P.R.S.; Silva-Lovato, C.H.; Paranhos, H.F.O.; Andrade, D.; Watanabe, E. Bacteriophage Cocktail-Mediated Inhibition of Pseudomonas aeruginosa Biofilm on Endotracheal Tube Surface. Antibiotics 2021, 10, 78. https://doi.org/10.3390/antibiotics10010078
Oliveira VC, Macedo AP, Melo LDR, Santos SB, Hermann PRS, Silva-Lovato CH, Paranhos HFO, Andrade D, Watanabe E. Bacteriophage Cocktail-Mediated Inhibition of Pseudomonas aeruginosa Biofilm on Endotracheal Tube Surface. Antibiotics. 2021; 10(1):78. https://doi.org/10.3390/antibiotics10010078
Chicago/Turabian StyleOliveira, Viviane C., Ana P. Macedo, Luís D. R. Melo, Sílvio B. Santos, Paula R. S. Hermann, Cláudia H. Silva-Lovato, Helena F. O. Paranhos, Denise Andrade, and Evandro Watanabe. 2021. "Bacteriophage Cocktail-Mediated Inhibition of Pseudomonas aeruginosa Biofilm on Endotracheal Tube Surface" Antibiotics 10, no. 1: 78. https://doi.org/10.3390/antibiotics10010078
APA StyleOliveira, V. C., Macedo, A. P., Melo, L. D. R., Santos, S. B., Hermann, P. R. S., Silva-Lovato, C. H., Paranhos, H. F. O., Andrade, D., & Watanabe, E. (2021). Bacteriophage Cocktail-Mediated Inhibition of Pseudomonas aeruginosa Biofilm on Endotracheal Tube Surface. Antibiotics, 10(1), 78. https://doi.org/10.3390/antibiotics10010078











