Susceptibility Testing of Colistin for Acinetobacter baumannii: How Far Are We from the Truth?
Abstract
1. Introduction
2. Results
2.1. Characteristics of A. baumannii Isolates
2.2. Antimicrobial Susceptibility
2.3. Identification of International Clonal Lineages and Association with Carbapenemase Genes
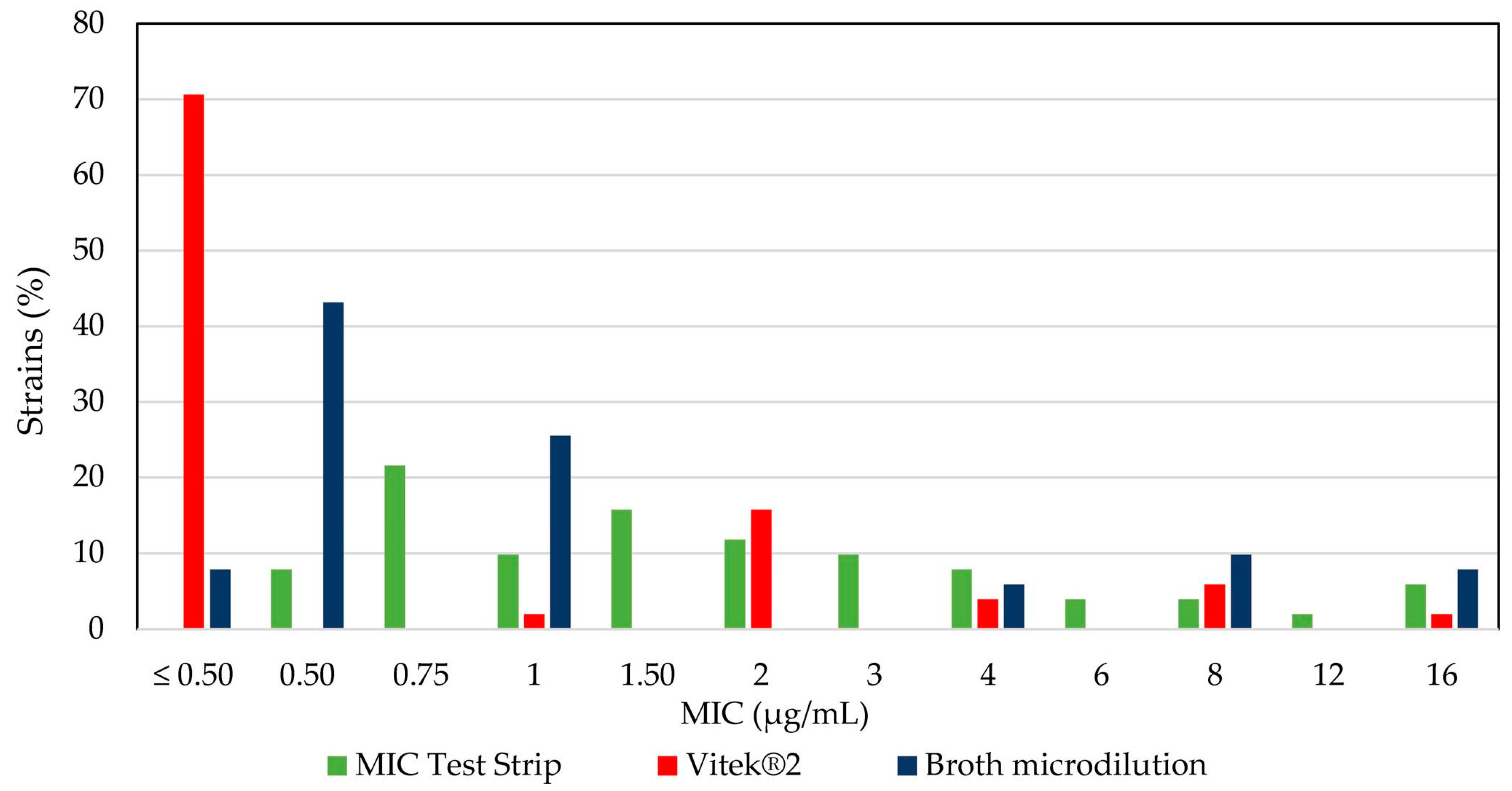
2.4. Comparative Analysis of Colistin Susceptibility Using Different Tests
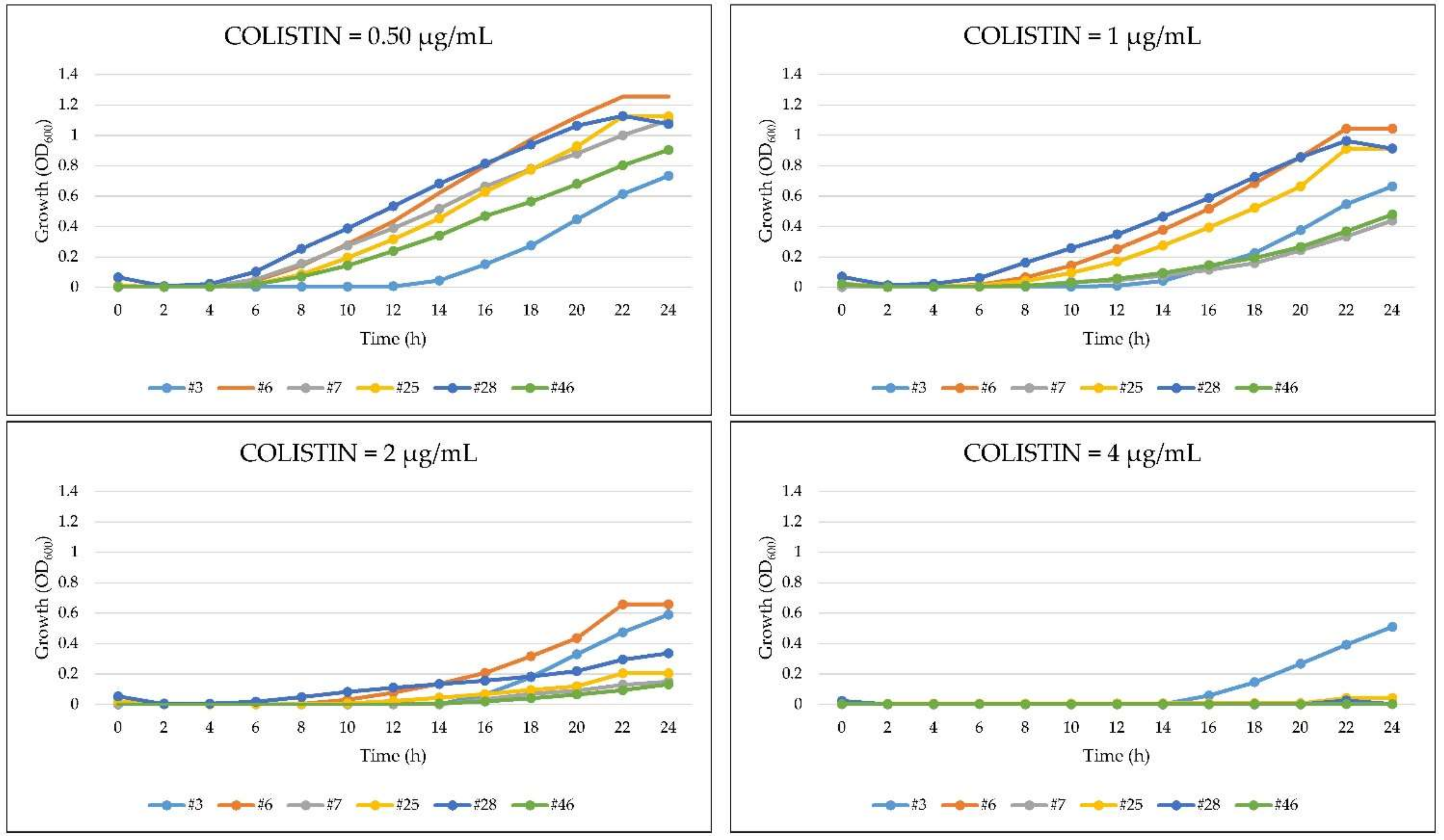
2.5. Growth Kinetics of A. baumannii in the Presence of Colistin
2.6. Heteroresistance to Colistin in A. baumannii
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- García-Patiño, M.G.; García-Contreras, R.; Licona-Limón, P. The Immune Response against Acinetobacter baumannii, an Emerging Pathogen in Nosocomial Infections. Front Immunol. 2017, 12, 8–441. [Google Scholar] [CrossRef] [PubMed]
- Gkrania-Klotsas, E.; Hershow, R.C. Colonization or infection with multidrug-resistant Acinetobacter baumannii may be an independent risk factor for increased mortality. Clin. Infect Dis. 2006, 43, 1224–1225. [Google Scholar] [CrossRef]
- Lin, M.F.; Lan, C.Y. Antimicrobial resistance in Acinetobacter baumannii: From bench to bedside. World J. Clin. Cases 2014, 2, 787–814. [Google Scholar] [CrossRef] [PubMed]
- Doi, Y. Treatment options for carbapenem-resistant Gram-negative bacterial infections. Clin. Infect. Dis. 2019, 69, S565–S575. [Google Scholar] [CrossRef] [PubMed]
- Docquier, J.D.; Mangani, S. Structure-function relationships of class D carbapenemases. Curr. Drug Targets 2016, 17, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Girlich, D.; Poirel, L.; Nordmann, P. First isolation of the blaOXA-23 carbapenemase gene from an environmental Acinetobacter baumannii isolate. Antimicrob. Agents Chemother. 2010, 54, 578–579. [Google Scholar] [CrossRef] [PubMed]
- Zarrilli, R.; Pournaras, S.; Giannouli, M.; Tsakris, A. Global evolution of multidrug-resistant Acinetobacter baumannii clonal lineages. Int. J. Antimicrob. Agents 2013, 41, 11–19. [Google Scholar] [CrossRef]
- Vasoo, S. Susceptibility Testing for the Polymyxins: Two Steps Back, Three Steps Forward? J Clin. Microbiol. 2019, 55, 2573–2582. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing. Available online: http://www.eucast.org/clinical_breakpoints (accessed on 1 June 2017).
- Dafopoulou, K.; Zarkotou, O.; Dimitroulia, E.; Hadjichristodoulou, C.; Gennimata, V.; Pournaras, S.; Tsakrisa, A. Comparative Evaluation of Colistin Susceptibility Testing Methods among carbapenem-nonsusceptible Klebsiella pneumoniae and Acinetobacter baumannii clinical isolates. Antimicrob. Agents Chemother. 2015, 59, 4625–4630. [Google Scholar] [CrossRef]
- Singhal, L.; Sharma, M.; Verma, S.; Kaur, R.; Basil Britto, X.; Kumar, S.M.; Ray, P.; Gautam, V. Comparative evaluation of broth microdilution with polystyrene and glass-coated plates, agar dilution, E-test, Vitek, and disk diffusion for susceptibility testing of colistin and polymyxin B on carbapenem-resistant clinical isolates of Acinetobacter baumannii. Microb. Drug Resist. 2018, 24, 1082–1088. [Google Scholar] [CrossRef]
- Bakthavatchalam, Y.D.; Pragasam, A.K.; Biswas, I.; Veeraraghavan, B. Polymyxin susceptibility testing, interpretative breakpoints and resistance mechanism: An update. J Glob. Antimicrob. Resist. 2018, 12, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Olaitan, A.O.; Morand, S.; Rolain, J.M. Mechanisms of polymyxin resistance: Acquired and intrinsic resistance in bacteria. Front. Microbiol. 2014, 5, 643. [Google Scholar] [CrossRef] [PubMed]
- Moffatt, J.H.; Harper, M.; Harrison, P.; Hale, J.D.; Vinogradov, E.; Seeman, T.; Henry, R.; Crane, B.; St Michael, F.; Cox, A.D.; et al. Colistin resistance in Acinetobacter baumannii is mediated by complete loss of lipopolysaccharide production. Antimicrob. Agents Chemother. 2010, 54, 4971–4977. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Rayner, C.R.; Nation, R.L.; Owen, R.J.; Spelman, D.; Tan, K.E.; Liolios, L. Heteroresistance to colistin in multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2006, 50, 2946–2950. [Google Scholar] [CrossRef]
- Hawley, J.S.; Murray, C.K.; Jorgensen, J.H. Colistin heteroresistance in Acinetobacter and its association with previous colistin therapy. Antimicrob. Agents Chemother. 2008, 51, 351–352. [Google Scholar] [CrossRef]
- Karakonstantis, S.; Saridakis, I. Colistin heteroresistance in Acinetobacter spp.: Systematic review and meta-analysis of the prevalence and discussion of the mechanisms and potential therapeutic implications. Int. J. Antimicrob. Agents. 2020, 56, 106065. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Turton, J.F.; Gabriel, S.N.; Valderrey, C. Use of sequence-based typing and multiplex PCR to identify clonal lineages of outbreak strains of Acinetobacter baumannii. Clin. Microbiol. Infect. 2007, 13, 807–815. [Google Scholar] [CrossRef]
- Dijkshoorn, L.; Aucken, H.; Gerner-Smidt, P. Comparison of outbreak and nonoutbreak Acinetobacter baumannii strains by genotypic and phenotypic methods. J. Clin. Microbiol. 1996, 34, 1519–1525. [Google Scholar] [CrossRef]
- Jean, S.S.; Hsueh, P.R. Current review of antimicrobial treatment of nosocomial pneumonia caused by multidrug-resistant pathogens. Expert Opin. Pharmacother. 2011, 12, 2145–2148. [Google Scholar] [CrossRef]
- Cai, Y.; Chai, D.; Wang, R.; Liang, B.; Bai, N. Colistin resistance of Acinetobacter baumannii: Clinical reports, mechanisms and antimicrobial strategies. J. Antimicrob. Chemother. 2012, 7, 1607–1615. [Google Scholar] [CrossRef] [PubMed]
- Gordon, N.C.; Wareham, D.W. A review of clinical and microbiological outcomes following treatment of infections involving multidrug-resistant Acinetobacter baumannii with tigecycline. J. Antimicrob. Chemother. 2009, 63, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Gur, D.; Korten, V.; Unal, S.; Deshpande, L.M.; Castanheira, M. Increasing carbapenem resistance due to the clonal dissemination of oxacillinase (OXA-23 and OXA-58)-producing Acinetobacter baumannii: Report from the Turkish SENTRY. J. Med. Microbiol. 2008, 57, 1529–1532. [Google Scholar] [CrossRef] [PubMed]
- Djahmi, N.; Dunyach-Remy, C.; Pantel-Dekhil, M.; Sotto, A.; Lavigne, J.P. Epidemiology of carbapenemase-producing Enterobacteriaceae and Acinetobacter baumannii in Mediterranean countries. Biomed. Res. Int. 2014, 2014, 305784. [Google Scholar] [CrossRef]
- D’Arezzo, S.; Capone, A.; Petrosillo, N.; Visca, P.; Ballardini, M.; Bartolini, S.; Bordi, E.; Di Stefano, A.; Galiè, M.; GRAB; et al. Epidemic multidrug-resistant Acinetobacter baumannii related to European clonal types I and II in Rome (Italy). Clin. Microbiol. Infect. 2009, 15, 347–357. [Google Scholar] [CrossRef]
- D’Arezzo, S.; Principe, L.; Capone, A.; Petrosillo, N.; Petrucca, A.; Visca, P. Changing carbapenemase gene pattern in an epidemic multidrug-resistant Acinetobacter baumannii lineage causing multiple outbreaks in central Italy. J. Antimicrob. Chemother. 2011, 66, 54–61. [Google Scholar] [CrossRef]
- Giannouli, M.; Cuccurullo, S.; Crivaro, V.; Di Popolo, A.; Bernardo, M.; Tomasone, F.; Amato, G.; Brisse, S.; Triassi, M.; Utili, R.; et al. Molecular epidemiology of multidrug-resistant Acinetobacter baumannii in a tertiary care hospital in Naples, Italy, shows the emergence of a novel epidemic clone. J. Clin. Microbiol. 2010, 48, 1223–1230. [Google Scholar] [CrossRef]
- D’Andrea, M.M.; Giani, T.; D’Arezzo, S.; Capone, A.; Petrosillo, N.; Visca, P.; Luzzaro, F.; Rossolini, G.M. Characterization of pABVA01, a plasmid encoding the OXA-24 carbapenemase from Italian isolates of Acinetobacter baumannii. Antimicrob. Agents Chemother. 2009, 53, 3528–3533. [Google Scholar] [CrossRef]
- Girardello, R.; Cury, A.P.; Franco, M.R.G.; Di Gióia, T.R.; De Almeida, J.N., Jr.; De Araújo, M.R.E.; Da Silva Duarte, A.J.; Rossi, F. Colistin susceptibility testing and Vitek-2™: Is it really useless? Diagn. Microbiol. Infect. Dis. 2018, 91, 309–311. [Google Scholar] [CrossRef]
- Sherman, E.X.; Wozniak, J.E.; Weiss, D.S. Methods to evaluate colistin heteroresistance in Acinetobacter baumannii. Methods Mol. Biol. 2019, 1946, 39–50. [Google Scholar] [CrossRef]
- MALDI BIOTYPER. Available online: https://www.bruker.com/products/mass-spectrometry-and-separations/maldi-biotyper-systems.html (accessed on 1 February 2017).
- BioEdit Software. Available online: http://www.mbio.ncsu.edu/BioEdit/bioedit.html (accessed on 1 September 2017).
- Woodford, N.; Ellington, M.J.; Coelho, J.M.; Turton, J.F.; Ward, M.E.; Brown, S.; Amyes, S.G.; Livermore, D.M. Multiplex PCR for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int. J. Antimicrob. Agents. 2006, 27, 351–353. [Google Scholar] [CrossRef]
- Higgins, P.G.; Lehmann, M.; Seifert, H. Inclusion of OXA-143 primers in a multiplex polymerase chain reaction (PCR) for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int. J. Antimicrob. Agents. 2010, 35, 305. [Google Scholar] [CrossRef]
- Karah, N.; Sundsfjord, A.; Towner, K.; Samuelsen, Ø. Insights into the global molecular epidemiology of carbapenem non-susceptible clones of Acinetobacter baumannii. Drug Resist Updat. 2012, 15, 237–247. [Google Scholar] [CrossRef]

| N. Patient | Id. Isolate | Source | N. Patient | Id. Isolate | Source |
|---|---|---|---|---|---|
| 1 | #1 | Respiratory Secretion | 16 | #26 | Respiratory Secretion |
| #2 | Blood Colture | 17 | #27 | Wound Swab | |
| 2 | #3 | Blood Colture | 18 | #28 | Respiratory Secretion |
| 3 | #4 | Respiratory Secretion | 19 | #29 | Respiratory Secretion |
| 4 | #5 | Respiratory Secretion | 20 | #30 | Sputum |
| 5 | #6 | Blood Colture | 21 | #31 | Nasal Swab |
| #7 | Respiratory Secretion | #32 | Blood Colture | ||
| 6 | #8 | Respiratory Secretion | #33 | Respiratory Secretion | |
| #9 | Blood Colture | 22 | #34 | Respiratory Secretion | |
| 7 | #10 | Blood Colture | #35 | Blood Colture | |
| #11 | Respiratory Secretion | 23 | #36 | Respiratory Secretion | |
| #12 | Central Venous Catheter | 24 | #37 | Blood Colture | |
| 8 | #13 | Blood Colture | 25 | #38 | Respiratory Secretion |
| 9 | #14 | Respiratory Secretion | 26 | #39 | Respiratory Secretion |
| 10 | #15 | Blood Colture | 27 | #40 | Blood Colture |
| #16 | Wound Swab | 28 | #41 | Respiratory Secretion | |
| 11 | #17 | Respiratory Secretion | 29 | #42 | Blood Colture |
| 12 | #18 | Respiratory Secretion | 30 | #43 | Cerebrospinal fluid |
| 13 | #19 | Urine | #44 | Blood Colture | |
| #20 | Respiratory Secretion | #45 | Central Venous Catheter | ||
| #21 | Blood Colture | #46 | Respiratory Secretion | ||
| 14 | #22 | Blood Colture | 31 | #47 | Blood Colture |
| #23 | Respiratory Secretion | 32 | #48 | Ascessive Liquid | |
| 15 | #24 | Respiratory Secretion | #49 | Urine | |
| #25 | Central Venous Catheter | #50 | Blood Colture | ||
| #51 | Respiratory Secretion |
| Primer | Sequence (5′–3′) | Target | Amplicon Size (bp) |
|---|---|---|---|
| blaOXA-51 FW | TAA TGC TTT GAT CGG CCT TG | blaOXA-51-like | 353 |
| blaOXA-51 RV | TGG ATT GCA CTT CAT CTT GG | ||
| blaOXA-23 FW | GAT CGG ATT GGA GAA CCA GA | blaOXA-23-like | 501 |
| blaOXA-23 RV | ATT TCT GAC CGC ATT TCC AT | ||
| blaOXA-24 FW | GGT TAG TTG GCC CCC TTA AA | blaOXA-24-like | 246 |
| blaOXA-24 RV | AGT TGA GCG AAA AGG GGA TT | ||
| blaOXA-58 FW | AAG TAT TGG GGC TTG TGC TG | blaOXA-58-like | 599 |
| blaOXA-58 RV | CCC CTC TGC GCT CTA CAT AC | ||
| blaOXA-143 FW | TGG CAC TTT CAG CAG TTC CT | blaOXA-143-like | 149 |
| blaOXA-143 RV | TAA TCT TGA GGG GGC CAA CC |
| Primer | Sequence (5’–3’) | Target | Amplicon Size (bp) |
|---|---|---|---|
| Group1ompAF306 | GAT GGC GTA AAT CGT GGT A | ompA | 355 |
| Group1and2ompAR660 | CAA CTT TAG CGA TTT CTG G | ||
| Group1csuEF | CTT TAG CAA ACA TGA CCT ACC | csuE | 702 |
| Group1csuER | TAC ACC CGG GTT AAT CGT | ||
| Gp1OXA66F89 | GCG CTT CAA AAT CTG ATG TA | blaOXA-51-like | 559 |
| Gp1OXA66R647 | GCG TAT ATT TTG TTT CCA TTC | ||
| Group2ompAF378 | GAC CTT TCT TAT CAC AAC GA | ompA | 343 |
| Group1and2ompAR660 | CAA CTT TAG CGA TTT CTG G | ||
| Group2csuEF | GGC GAA CAT GAC CTA TTT | csuE | 580 |
| Group2csuER | CTT CAT GGC TCG TTG GTT | ||
| Gp2OXA69F169 | CAT CAA GGT CAA ACT CAA | blaOXA-51-like | 162 |
| Gp2OXA69R330 | TAG CCT TTT TTC CCC ATC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sacco, F.; Visca, P.; Runci, F.; Antonelli, G.; Raponi, G. Susceptibility Testing of Colistin for Acinetobacter baumannii: How Far Are We from the Truth? Antibiotics 2021, 10, 48. https://doi.org/10.3390/antibiotics10010048
Sacco F, Visca P, Runci F, Antonelli G, Raponi G. Susceptibility Testing of Colistin for Acinetobacter baumannii: How Far Are We from the Truth? Antibiotics. 2021; 10(1):48. https://doi.org/10.3390/antibiotics10010048
Chicago/Turabian StyleSacco, Federica, Paolo Visca, Federica Runci, Guido Antonelli, and Giammarco Raponi. 2021. "Susceptibility Testing of Colistin for Acinetobacter baumannii: How Far Are We from the Truth?" Antibiotics 10, no. 1: 48. https://doi.org/10.3390/antibiotics10010048
APA StyleSacco, F., Visca, P., Runci, F., Antonelli, G., & Raponi, G. (2021). Susceptibility Testing of Colistin for Acinetobacter baumannii: How Far Are We from the Truth? Antibiotics, 10(1), 48. https://doi.org/10.3390/antibiotics10010048






