A New Promising Anti-Infective Agent Inhibits Biofilm Growth by Targeting Simultaneously a Conserved RNA Function That Controls Multiple Genes
Abstract
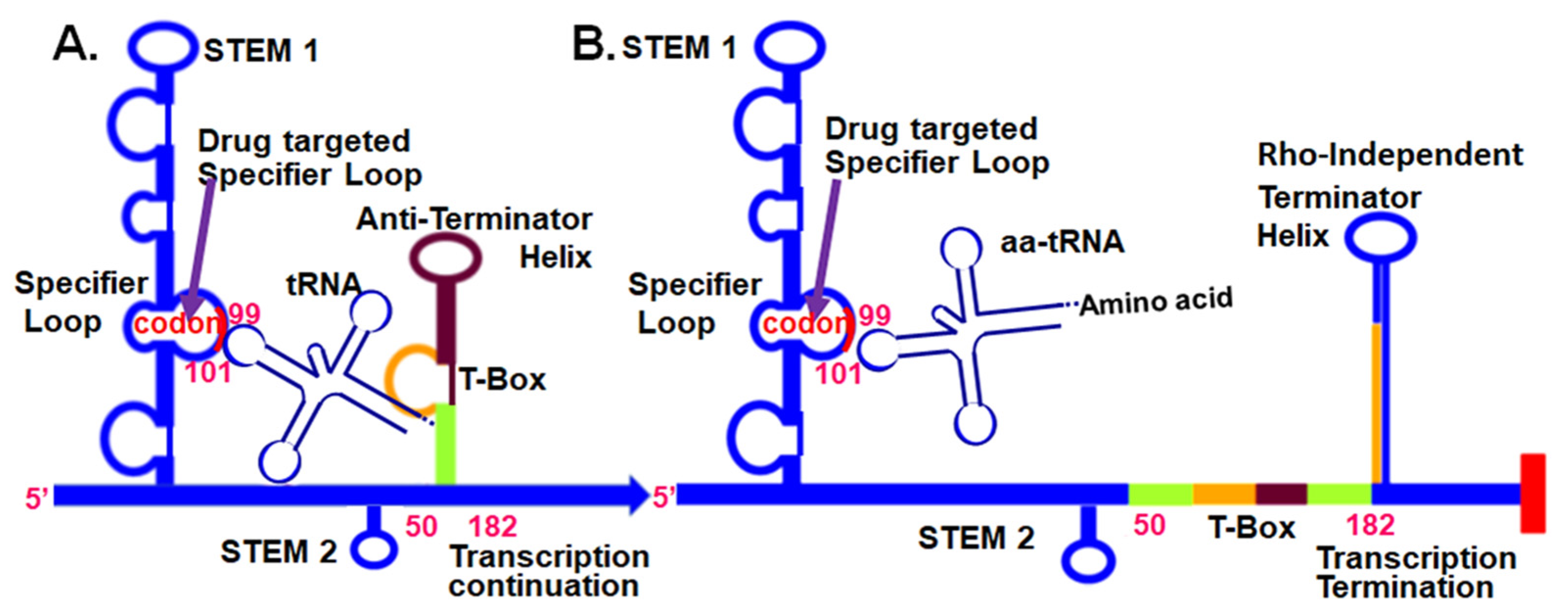
1. Introduction
2. Results
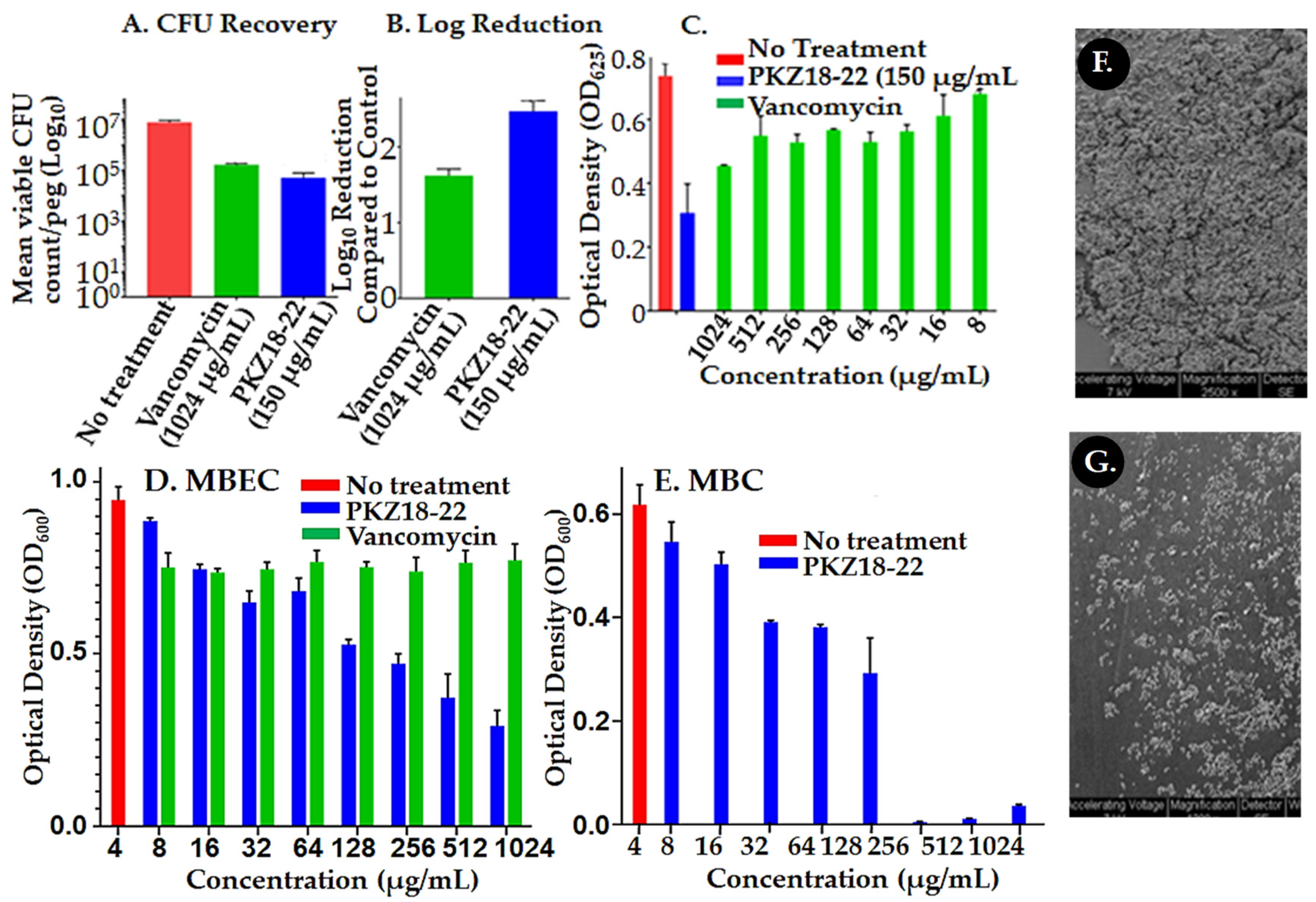
2.1. Biofilm Growth Is Retarded by PKZ18 Analogs
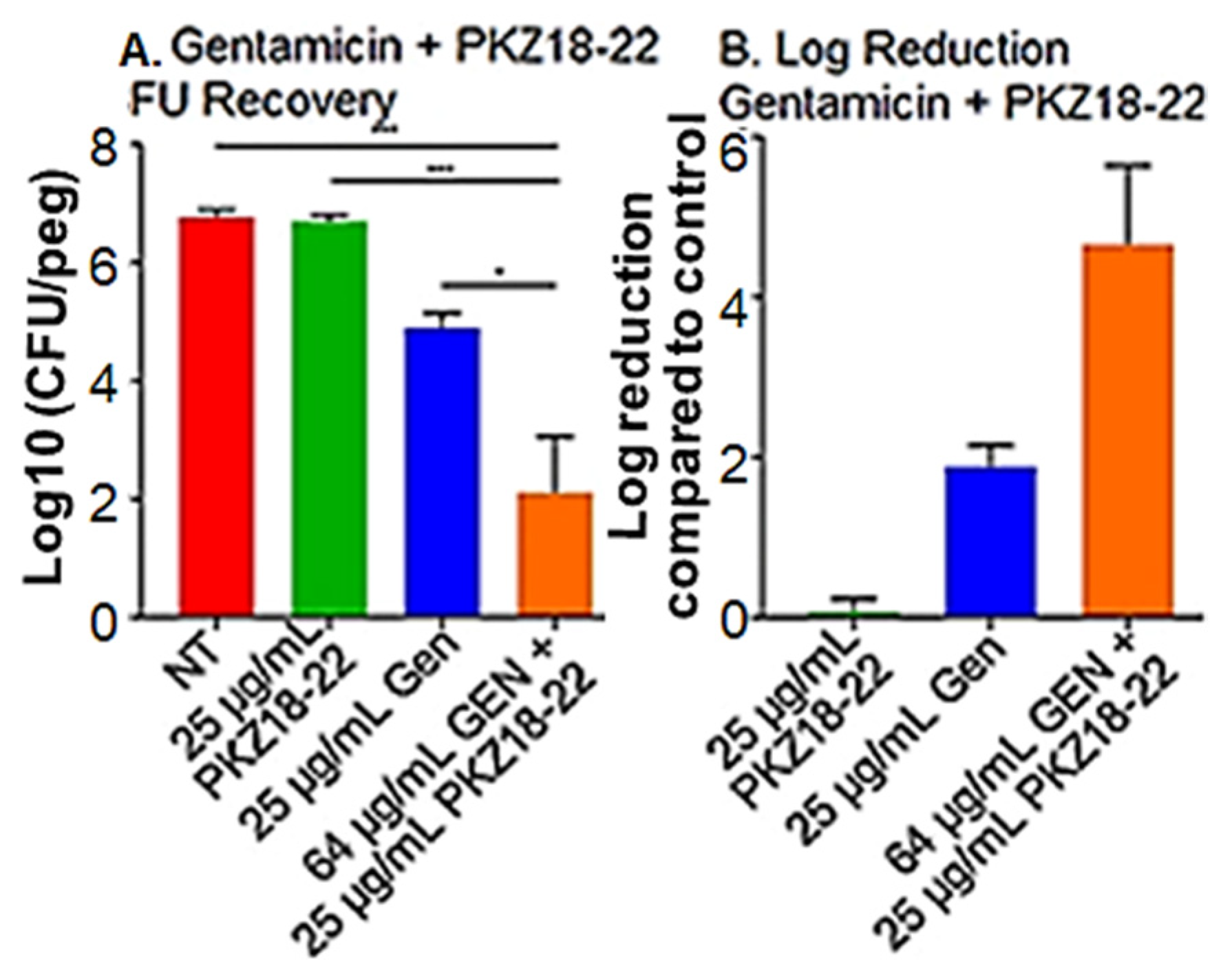
2.2. Synergistic Activity of PKZ18-22 with Common Clinical Antibiotics
2.3. Synergy of PKZ18 and Common Antibiotics in Planktonic Cultures
3. Discussion
4. Materials and Methods
4.1. Determining if Biofilm Formation Is Susceptible to PKZ18 Analogs In Vitro
4.2. Determine the Synergy of Different PKZ18 Analogs and Antibiotic Combinations to Treat Established Staphylococcus Biofilms In Vitro
4.3. CFU Recovery
4.4. Scanning Electron Microscopy
4.5. Confocal Laser Scanning Imaging
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barsoumian, A.E.; Mende, K.; Sanchez, C.J.; Beckius, M.L.; Wenke, J.C.; Murray, C.K.; Akers, K.S. Clinical infectious outcomes associated with biofilm-related bacterial infections: A retrospective chart review. BMC Infect. Dis. 2015, 15, 1–7. [Google Scholar] [CrossRef]
- Luther, M.K.; Parente, D.M.; Caffrey, A.R.; Daffinee, K.E.; Lopes, V.V.; Martin, E.T.; Laplante, K.L. Clinical and Genetic Risk Factors for Biofilm-FormingStaphylococcus aureus. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial Biofilm and its Role in the Pathogenesis of Disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.-F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Mi, G.; Wang, M.; Webster, T.J. In vitro and ex vivo systems at the forefront of infection modeling and drug discovery. Biomaterials 2018, 198, 228–249. [Google Scholar] [CrossRef] [PubMed]
- Billings, N.; Birjiniuk, A.; Samad, T.S.; Doyle, P.S.; Ribbeck, K. Material properties of biofilms—A review of methods for understanding permeability and mechanics. Rep. Prog. Phys. 2015, 78, 036601. [Google Scholar] [CrossRef]
- Remy, B.; Mion, S.; Plener, L.; Elias, M.; Chabriere, E.; Daude, D. Interference in bacterial quorum sensing: A biophar-maceutical perspective. Front. Pharm. 2018, 9, 203. [Google Scholar] [CrossRef]
- Stewart, P.S. Mechanisms of antibiotic resistance in bacterial biofilms. Int. J. Med. Microbiol. 2002, 292, 107–113. [Google Scholar] [CrossRef]
- González, J.F.; Hahn, M.M.; Gunn, J.S. Chronic biofilm-based infections: Skewing of the immune response. Pathog. Dis. 2018, 76. [Google Scholar] [CrossRef]
- Mah, T.-F.C.; O’Toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J. Bone Jt. Surg. Am. 2007, 89, 780–785. [Google Scholar] [CrossRef]
- Holleyman, R.J.; Baker, P.N.; Charlett, A.; Gould, K.; Deehan, D.J. Analysis of Causative Microorganism in 248 Primary Hip Arthroplasties Revised for Infection: A Study Using the NJR Dataset. HIP Int. 2016, 26, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Corró, S.; Vicente, M.; Rodríguez-Pardo, D.; Pigrau, C.; Lung, M.; Corona, P.S.; Curró, S.; Carrera, L. Vancomycin-Gentamicin Prefabricated Spacers in 2-Stage Revision Arthroplasty for Chronic Hip and Knee Periprosthetic Joint Infection: Insights Into Reimplantation Microbiology and Outcomes. J. Arthroplast. 2019, 35, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-L.; Hou, Y.; Zhang, B.-Q.; Chen, Y.-F.; Wang, Q.; Wang, K.; Chen, Z.-Y.; Li, X.-W.; Lin, J.-H. Identifying Common Pathogens in Periprosthetic Joint Infection and Testing Drug-resistance Rate for Different Antibiotics: A Prospective, Single Center Study in Beijing. Orthop. Surg. 2018, 10, 235–240. [Google Scholar] [CrossRef]
- Rosteius, T.; Jansen, O.; Fehmer, T.; Baecker, H.; Citak, M.; Schildhauer, T.A.; Geßmann, J. Evaluating the microbial pat-tern of periprosthetic joint infections of the hip and knee. J. Med. Microbiol. 2018, 67, 1608–1613. [Google Scholar] [CrossRef]
- Knecht, C.S.; Moley, J.P.; McGrath, M.S.; Granger, J.F.; Stoodley, P.; Dusane, D.H. Antibiotic loaded calcium sulfate bead and pulse lavage eradicates biofilms on metal implant materials in vitro. J. Orthop. Res. 2018, 36, 2349–2354. [Google Scholar] [CrossRef]
- Eades, C.; Hughes, S.; Heard, K.; Moore, L.S.P. Antimicrobial therapies for Gram-positive infections. Clin. Pharm. 2017, 15, 28. [Google Scholar]
- Connelly, C.M.; Moon, M.H.; Schneekloth, J.S., Jr. The Emerging Role of RNA as a Therapeutic Target for Small Molecules. Cell Chem. Biol. 2016, 23, 1077–1090. [Google Scholar] [CrossRef]
- Rizvi, N.F.; Smith, G.F. RNA as a small molecule druggable target. Bioorg. Med. Chem. Lett. 2017, 27, 5083–5088. [Google Scholar] [CrossRef]
- Murata, A.; Nakamori, M.; Nakatani, K. Modulating RNA secondary and tertiary structures by mismatch binding ligands. Methods 2019, 167, 78–91. [Google Scholar] [CrossRef]
- Lünse, C.E.; Schüller, A.; Mayer, G. The promise of riboswitches as potential antibacterial drug targets. Int. J. Med. Microbiol. 2014, 304, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Sudarsan, N.; Cohen-Chalamish, S.; Nakamura, S.; Emilsson, G.M.; Breaker, R.R. Thiamine Pyrophosphate Riboswitches Are Targets for the Antimicrobial Compound Pyrithiamine. Chem. Biol. 2005, 12, 1325–1335. [Google Scholar] [CrossRef]
- Wang, H.; Mann, P.A.; Xiao, L.; Gill, C.; Galgoci, A.M.; Howe, J.A.; Villafania, A.; Barbieri, C.M.; Malinverni, J.C.; Sher, X.; et al. Dual-Targeting Small-Molecule Inhibitors of the Staphylococcus aureus FMN Riboswitch Disrupt Riboflavin Homeostasis in an Infectious Setting. Cell Chem. Biol. 2017, 24, 576–588.e6. [Google Scholar] [CrossRef] [PubMed]
- Howe, J.A.; Wang, H.; Fischmann, T.O.; Balibar, C.J.; Xiao, L.; Galgoci, A.M.; Malinverni, J.C.; Mayhood, T.W.; Villafania, A.; Nahvi, A.; et al. Selective small-molecule inhibition of an RNA structural element. Nat. Cell Biol. 2015, 526, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Pedrolli, D.B.; Matern, A.; Wang, J.; Ester, M.; Siedler, K.; Breaker, R.; Mack, M. A highly specialized Flavin mononucle-otide riboswitch responds differently to similar ligands and confers roseoflavin resistance to Streptomyces davawen-sis. Nucleic Acids Res. 2012, 40, 8662–8673. [Google Scholar] [CrossRef]
- Podnecky, N.L.; Rhodes, K.A.; Mima, T.; Drew, H.R.; Chirakul, S.; Wuthiekanun, V.; Schupp, J.M.; Sarovich, D.S.; Currie, B.J.; Keim, P.; et al. Mechanisms of Resistance to Folate Pathway Inhibitors inBurkholderia pseudomallei: Deviation from the Norm. mBio 2017, 8, e01357-17. [Google Scholar] [CrossRef]
- Leiman, S.A.; Richardson, C.; Foulston, L.; Elsholz, A.K.W.; First, E.A.; Losick, R. Identification and Characterization of Mutations Conferring Resistance to d-Amino Acids in Bacillus subtilis. J. Bacteriol. 2015, 197, 1632–1639. [Google Scholar] [CrossRef]
- Ataide, S.F.; Wilson, S.N.; Dang, S.; Rogers, T.; Roy, B.; Banerjee, R.; Henkin, T.M.; Ibba, M. Mechanisms of Resistance to an Amino Acid Antibiotic That Targets Translation. ACS Chem. Biol. 2007, 2, 819–827. [Google Scholar] [CrossRef]
- Blount, K.F.; Megyola, C.; Plummer, M.; Osterman, D.; O’Connell, T.; Aristoff, P.; Quinn, C.; Chrusciel, R.A.; Poel, T.J.; Schostarez, H.J.; et al. Novel Riboswitch-Binding Flavin Analog That Protects Mice against Clostridium difficile Infection without Inhibiting Cecal Flora. Antimicrob. Agents Chemother. 2015, 59, 5736–5746. [Google Scholar] [CrossRef]
- Perkins, K.R.; Atilho, R.M.; Moon, M.H.; Breaker, R.R. Employing a ZTP riboswitch to detect bacterial folate biosyn-thesis inhibitors in a small molecule high-throughput screen. ACS Chem. Biol. 2019, 14, 2841–2850. [Google Scholar] [CrossRef]
- Francklyn, C.S.; Mullen, P. Progress and challenges in aminoacyl-tRNA synthetase-based therapeutics. J. Biol. Chem. 2019, 294, 5365–5385. [Google Scholar] [CrossRef]
- Lowy, F.D. Antimicrobial resistance: Example of Staphylococcus aureus. J. Clin. Investig. 2003, 111, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.R.; Henkin, T.M.; Agris, P.F. tRNA regulation of gene expression: Interactions of an mRNA 5’-UTR with a regulatory tRNA. RNA 2006, 12, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Pantosti, A.; Sanchini, A.; Monaco, M. Mechanisms of antibiotic resistance in Staphylococcus aureus. Future Microbiol. 2007, 2, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Moser, C.; Wang, H.-Z.; Høiby, N.; Song, Z.-J. Strategies for combating bacterial biofilm infections. Int. J. Oral Sci. 2015, 7, 1–7. [Google Scholar] [CrossRef]
- Wood, T.K.; Knabel, S.J.; Kwan, B.W. Bacterial Persister Cell Formation and Dormancy. Appl. Environ. Microbiol. 2013, 79, 7116–7121. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Antibiotic Resistance Threats in the United States, 2019; US Department of Health and Human Services, CDC: Atlanta, GA, USA, 2019. Available online: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf (accessed on 13 November 2019).
- World Health Organization. Antibiotic Resistance. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance (accessed on 24 August 2020).
- Chen, M.; Yu, Q.; Sun, H. Novel Strategies for the Prevention and Treatment of Biofilm Related Infections. Int. J. Mol. Sci. 2013, 14, 18488–18501. [Google Scholar] [CrossRef]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Hall-Stoodley, L.; Stoodley, P. Targeting microbial biofilms: Current and prospec-tive therapeutic strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [CrossRef]
- Frohlich, K.M.; Weintraub, S.F.; Bell, J.T.; Todd, G.C.; Väre, V.Y.P.; Schneider, R.; Kloos, Z.A.; Tabe, E.S.; Cantara, W.A.; Stark, C.J.; et al. Discovery of Small-Molecule Antibiotics against a Unique tRNA-Mediated Regulation of Transcription in Gram-Positive Bacteria. ChemMedChem 2019, 14, 758–769. [Google Scholar] [CrossRef]
- Väre, V.Y.P.; Schneider, R.F.; Kim, H.; Lasek-Nesselquist, E.; McDonough, K.A.; Agris, P.F. Small-molecule antibiotics inhibit tRNA regulated 1 gene expression in Staphylococcus aureus and work synergistically with aminoglycoside antibiotics. Antimicrob. Agents Chemother. 2020, in press. [Google Scholar]
- Green, N.J.; Grundy, F.J.; Henkin, T.M. The T box mechanism: tRNA as a regulatory molecule. FEBS Lett. 2010, 584, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Fuchs, R.T.; Grundy, F.J.; Henkin, T.M. Riboswitch RNAs: Regulation of gene expression by direct moni-toring of a physiological signal. RNA Biol. 2010, 7, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Grigg, J.C.; Chen, Y.; Grundy, F.J.; Henkin, T.M.; Pollack, L.; Ke, A. T box RNA decodes both the information content and geometry of tRNA to affect gene expression. Proc. Natl. Acad. Sci. USA 2013, 110, 7240–7245. [Google Scholar] [CrossRef] [PubMed]
- Henkin, T.M. Riboswitch RNAs: Using RNA to sense cellular metabolism. Genes Dev. 2008, 22, 3383–3390. [Google Scholar] [CrossRef] [PubMed]
- Coraça-Huber, D.; Fille, M.; Hausdorfer, J.; Pfaller, K.; Nogler, M. Evaluation of MBEC™-HTP biofilm model for studies of implant associated infections. J. Orthop. Res. 2012, 30, 1176–1180. [Google Scholar] [CrossRef]
- Blanchard, C.; Brooks, L.; Beckley, A.; Colquhoun, J.; Dewhurst, S.; Dunman, P.M. Neomycin Sulfate improves the an-timicrobial activity of mupirocin-based antibacterial ointments. Antimicrob Agents Chemother. 2015, 60, 862–872. [Google Scholar] [CrossRef]
- Mgbeahuruike, E.E.; Stålnacke, M.; Vuorela, H.; Holm, Y. Antimicrobial and synergistic effects of commercial pip-erine and piperlongumine in combination with conventional antimicrobials. Antibiotics 2019, 8, 55. [Google Scholar] [CrossRef]
- Xu, X.; Xu, L.; Yuan, G.; Wang, Y.; Qu, Y.; Zhou, M. Synergistic combination of two antimicrobial agents closing each other’s mutant selection windows to prevent antimicrobial resistance. Sci. Rep. 2018, 8, 1–7. [Google Scholar] [CrossRef]
- Cheesman, M.; Ilanko, A.; Blonk, B.; Cock, I.E. Developing new antimicrobial therapies: Are synergistic combina-tions of plant extracts/compounds with conventional antibiotics the solution? Pharm. Rev. 2017, 11, 57–72. [Google Scholar]
- Vitreschak, A.G.; Mironov, A.A.; Lyubetsky, V.A.; Gelfand, M.S. Comparative genomic analysis of T-box regulatory systems in bacteria. RNA 2008, 14, 717–735. [Google Scholar] [CrossRef]
- Gutiérrez-Preciado, A.; Henkin, T.M.; Grundy, F.J.; Yanofsky, C.; Merino, E. Biochemical features and functional im-plications of the RNA-based T-box regulatory mechanism. Microbiol. Mol. Biol. Rev. 2009, 73, 36–61. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Means, M.J.; Acquaah-Harrison, G.; Bergmeier, S.C.; Hines, J.V. Characterization of a 1,4-disubstituted 1,2,3-triazole binding to T box antiterminator RNA. Bioorg. Med. Chem. 2012, 20, 1298–1302. [Google Scholar] [CrossRef] [PubMed]
- Means, J.A.; Katz, S.; Nayek, A.; Anupam, R.; Hines, J.V.; Bergmeier, S.C. Structure–activity studies of oxazolidinone ana-logs as RNA-binding agents. Bioorg. Med. Chem. Lett. 2006, 16, 3600–3604. [Google Scholar] [CrossRef] [PubMed]
- Anupam, R.; Nayek, A.; Green, N.J.; Grundy, F.J.; Henkin, T.M.; Means, J.A.; Bergmeier, S.C.; Hines, J.V. 4,5-Disubstituted oxa-zolidinones: High affinity molecular effectors of RNA function. Bioorg. Med. Chem. Lett. 2008, 18, 3541–3544. [Google Scholar] [CrossRef] [PubMed]
- Maciagiewicz, I.; Zhou, S.; Bergmeier, S.C.; Hines, J.V. Structure activity studies of RNA binding oxazolidinone deriva-tives. Bioorg. Med. Chem. Lett. 2011, 21, 4524–4527. [Google Scholar] [CrossRef]
- Zhou, S.; Acquaah-Harrison, G.; Jack, K.D.; Bergmeier, S.C.; Hines, J.V. Ligand induced changes in T-box antiterminator RNA sta-bility. Chem. Biol. Drug Discov. 2012, 79, 202–208. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martinez-Irujo, J.J.; Villahermosa, M.L.; Alberdi, E.; Santiago, E. A checkerboard method to evaluate interactions between drugs. Biochem. Pharm. 1996, 51, 635–644. [Google Scholar] [CrossRef]
- Meletiadis, J.; Pournaras, S.; Roilides, E.; Walsh, T.J. Defining Fractional Inhibitory Concentration Index Cutoffs for Additive Interactions Based on Self-Drug Additive Combinations, Monte Carlo Simulation Analysis, and In Vitro-In Vivo Correlation Data for Antifungal Drug Combinations against Aspergillus fumigatus. Antimicrob. Agents Chemother. 2009, 54, 602–609. [Google Scholar] [CrossRef]
- Orhan, G.; Bayram, A.; Zer, Y.; Balci, I. Synergy tests by E test and checkerboard methods of antimicrobial combina-tions against Brucella melitensis. J. Clin. Microbiol. 2005, 43, 140–143. [Google Scholar] [CrossRef]
- Di Veroli, G.Y.; Fornari, C.; Wang, D.; Mollard, S.; Bramhall, J.L.; Richards, F.M.; Jodrell, D.I. Combenefit: An interactive platform for the analysis and visualization of drug combinations. Bioinformatics. 2016, 32, 2866–2868. [Google Scholar] [CrossRef]
- Shortridge, M.D.; Varani, G. Structure based approaches for targeting non-coding RNAs with small molecules. Curr. Opin. Struct. Biol. 2015, 30, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.-M.; Choi, Y.H.; Tu, M.-J. RNA Drugs and RNA Targets for Small Molecules: Principles, Progress, and Challenges. Pharm. Rev. 2020, 72, 862–898. [Google Scholar] [CrossRef] [PubMed]
- Seethaler, M.; Hertlein, T.; Wecklein, B.; Ymeraj, A.; Ohlsen, K.; Lalk, M.; Hilgeroth, A. Novel small-molecule antibacte-rials against Gram-positive pathogens of Staphylococcus and Enterococcus species. Antibiotics 2019, 8, 210. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seyler, T.M.; Moore, C.; Kim, H.; Ramachandran, S.; Agris, P.F. A New Promising Anti-Infective Agent Inhibits Biofilm Growth by Targeting Simultaneously a Conserved RNA Function That Controls Multiple Genes. Antibiotics 2021, 10, 41. https://doi.org/10.3390/antibiotics10010041
Seyler TM, Moore C, Kim H, Ramachandran S, Agris PF. A New Promising Anti-Infective Agent Inhibits Biofilm Growth by Targeting Simultaneously a Conserved RNA Function That Controls Multiple Genes. Antibiotics. 2021; 10(1):41. https://doi.org/10.3390/antibiotics10010041
Chicago/Turabian StyleSeyler, Thorsten M., Christina Moore, Haein Kim, Sheetal Ramachandran, and Paul F. Agris. 2021. "A New Promising Anti-Infective Agent Inhibits Biofilm Growth by Targeting Simultaneously a Conserved RNA Function That Controls Multiple Genes" Antibiotics 10, no. 1: 41. https://doi.org/10.3390/antibiotics10010041
APA StyleSeyler, T. M., Moore, C., Kim, H., Ramachandran, S., & Agris, P. F. (2021). A New Promising Anti-Infective Agent Inhibits Biofilm Growth by Targeting Simultaneously a Conserved RNA Function That Controls Multiple Genes. Antibiotics, 10(1), 41. https://doi.org/10.3390/antibiotics10010041





