Screening of an FDA-Approved Library for Novel Drugs against Y. pestis
Abstract
1. Introduction
2. Results and Discussion
2.1. Design of the Study
2.2. Identification of Novel Potential Antiplague Drugs
2.3. Characterization of the Anti-Y. pestis Inhibitory Activity of Novel Drugs
3. Materials and Methods
3.1. Bacterial Strains and Reagents
3.2. Compound Library and Screening
3.3. Statistics and Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perry, R.D.; Fetherston, J.D. Yersinia pestis--etiologic agent of plague. Clin. Microbiol. Rev. 1997, 10, 35–66. [Google Scholar] [CrossRef] [PubMed]
- Plague. Available online: https://www.cdc.gov/plague (accessed on 1 December 2020).
- Randremanana, R.; Andrianaivoarimanana, V.; Nikolay, B.; Ramasindrazana, B.; Paireau, J.; Ten Bosch, Q.A.; Rakotondramanga, J.M.; Rahajandraibe, S.; Rahelinirina, S.; Rakotomanana, F.; et al. Epidemiological characteristics of an urban plague epidemic in Madagascar, August-November, 2017: An outbreak report. Lancet Infect. Dis. 2019, 19, 537–545. [Google Scholar] [CrossRef]
- Kool, J.L. Risk of person-to-person transmission of pneumonic plague. Clin. Infect. Dis. 2005, 40, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Galimand, M.; Carniel, E.; Courvalin, P. Resistance of Yersinia pestis to antimicrobial agents. Antimicrob. Agents Chemother. 2006, 50, 3233–3236. [Google Scholar] [CrossRef] [PubMed]
- Cabanel, N.; Bouchier, C.; Rajerison, M.; Carniel, E. Plasmid-mediated doxycycline resistance in a Yersinia pestis strain isolated from a rat. Int. J. Antimicrob. Agents 2018, 51, 249–254. [Google Scholar] [CrossRef]
- Law, G.L.; Tisoncik-Go, J.; Korth, M.J.; Katze, M.G. Drug repurposing: A better approach for infectious disease drug discovery? Curr. Opin. Immunol. 2013, 25, 588–592. [Google Scholar] [CrossRef]
- Miro-Canturri, A.; Ayerbe-Algaba, R.; Smani, Y. Drug Repurposing for the Treatment of Bacterial and Fungal Infections. Front. Microbiol. 2019, 10, 41. [Google Scholar] [CrossRef]
- CLSI. M45 Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria; Clinical and Laboratories Standard Institute: Wayne, PA, USA, 2015. [Google Scholar]
- Hertwig, S.; Popp, A.; Freytag, B.; Lurz, R.; Appel, B. Generalized transduction of small Yersinia enterocolitica plasmids. Appl. Environ. Microbiol. 1999, 65, 3862–3866. [Google Scholar] [CrossRef]
- Popp, A.; Hertwig, S.; Lurz, R.; Appel, B. Comparative study of temperate bacteriophages isolated from Yersinia. Syst. Appl. Microbiol. 2000, 23, 469–478. [Google Scholar] [CrossRef]
- Andros, C.C.; Dubay, R.A.; Mitchell, K.D.; Chen, A.; Holmes, D.E.; Kennedy, D.R. A novel application of radiomimetic compounds as antibiotic drugs. J. Pharm. Pharmacol. 2015, 67, 1371–1379. [Google Scholar] [CrossRef]
- Vavra, J.J.; Deboer, C.; Dietz, A.; Hanka, L.J.; Sokolski, W.T. Streptozotocin, a new antibacterial antibiotic. Antibiot. Annu. 1959, 7, 230–235. [Google Scholar] [PubMed]
- Murray, V.; Chen, J.K.; Chung, L.H. The Interaction of the Metallo-Glycopeptide Anti-Tumour Drug Bleomycin with DNA. Int. J. Mol. Sci. 2018, 19, 1372. [Google Scholar] [CrossRef] [PubMed]
- Povirk, L.F.; Wubter, W.; Kohnlein, W.; Hutchinson, F. DNA double-strand breaks and alkali-labile bonds produced by bleomycin. Nucleic Acids Res. 1977, 4, 3573–3580. [Google Scholar] [CrossRef] [PubMed]
- Liston, D.R.; Davis, M. Clinically Relevant Concentrations of Anticancer Drugs: A Guide for Nonclinical Studies. Clin. Cancer Res. 2017, 23, 3489–3498. [Google Scholar] [CrossRef]
- Bolzan, A.D.; Bianchi, M.S. Genotoxicity of streptozotocin. Mutat. Res. 2002, 512, 121–134. [Google Scholar] [CrossRef]
- Shifman, O.; Steinberger-Levy, I.; Aloni-Grinstein, R.; Gur, D.; Aftalion, M.; Ron, I.; Mamroud, E.; Ber, R.; Rotem, S. A Rapid Antimicrobial Susceptibility Test for Determining Yersinia pestis Susceptibility to Doxycycline by RT-PCR Quantification of RNA Markers. Front. Microbiol. 2019, 10, 754. [Google Scholar] [CrossRef]
- Steinberger-Levy, I.; Shifman, O.; Zvi, A.; Ariel, N.; Beth-Din, A.; Israeli, O.; Gur, D.; Aftalion, M.; Maoz, S.; Ber, R. A Rapid Molecular Test for Determining Yersinia pestis Susceptibility to Ciprofloxacin by the Quantification of Differentially Expressed Marker Genes. Front. Microbiol. 2016, 7, 763. [Google Scholar] [CrossRef]
- Soo, V.W.; Kwan, B.W.; Quezada, H.; Castillo-Juarez, I.; Perez-Eretza, B.; Garcia-Contreras, S.J.; Martinez-Vazquez, M.; Wood, T.K.; Garcia-Contreras, R. Repurposing of Anticancer Drugs for the Treatment of Bacterial Infections. Curr. Top. Med. Chem. 2017, 17, 1157–1176. [Google Scholar] [CrossRef]
- Berman, J. Miltefosine, an FDA-approved drug for the ‘orphan disease’, leishmaniasis. Expert Opin. Orphan Drugs 2015, 3, 727–735. [Google Scholar] [CrossRef]
- A phase 2 IV gallium study for patients with cyctic fibrosis. Available online: http//clinicaltrials.gov/ct2/show/NCT02354859 (accessed on 1 December 2020).
- Antibacterial agent in clinical development. Available online: https://www.who.int/medicines/areas/rational_use/antibacterial_agents_clinical_development/en/ (accessed on 1 December 2020).
- Zaman, S.B.; Hussain, M.A.; Nye, R.; Mehta, V.; Mamun, K.T.; Hossain, N. A Review on Antibiotic Resistance: Alarm Bells are Ringing. Cureus 2017, 9, e1403. [Google Scholar] [CrossRef]
- Rawson, T.M.; Moore, L.S.P.; Zhu, N.; Ranganathan, N.; Skolimowska, K.; Gilchrist, M.; Satta, G.; Cooke, G.; Holmes, A. Bacterial and fungal co-infection in individuals with coronavirus: A rapid review to support COVID-19 antimicrobial prescribing. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yakovlieva, L.; de Haan, B.J.; de Vos, P.; Minnaard, A.J.; Witte, M.D.; Walvoort, M.T.C. Selective Modification of Streptozotocin at the C3 Position to Improve Its Bioactivity as Antibiotic and Reduce Its Cytotoxicity towards Insulin-Producing beta Cells. Antibiotics 2020, 9, 182. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Weingarten, R.A.; Xu, M.; Southall, N.; Dai, S.; Shinn, P.; Sanderson, P.E.; Williamson, P.R.; Frank, K.M.; Zheng, W. Rapid antimicrobial susceptibility test for identification of new therapeutics and drug combinations against multidrug-resistant bacteria. Emerg. Microbes Infect. 2016, 5, e116. [Google Scholar] [CrossRef] [PubMed]
- Zauberman, A.; Cohen, S.; Mamroud, E.; Flashner, Y.; Tidhar, A.; Ber, R.; Elhanany, E.; Shafferman, A.; Velan, B. Interaction of Yersinia pestis with macrophages: Limitations in YopJ-dependent apoptosis. Infect. Immun. 2006, 74, 3239–3250. [Google Scholar] [CrossRef] [PubMed]
- Tidhar, A.; Levy, Y.; Zauberman, A.; Vagima, Y.; Gur, D.; Aftalion, M.; Israeli, O.; Chitlaru, T.; Ariel, N.; Flashner, Y.; et al. Disruption of the NlpD lipoprotein of the plague pathogen Yersinia pestis affects iron acquisition and the activity of the twin-arginine translocation system. PLoS Negl. Trop. Dis. 2019, 13, e0007449. [Google Scholar] [CrossRef] [PubMed]
| No | CAS Number a | Drug Name | Primary Action b | % Growth c | |
|---|---|---|---|---|---|
| 24 h | 48 h | ||||
| 1 | 112811-59-3 | Gatifloxacin | Fluoroquinolone antibiotic | <0.1 | <0.1 |
| 2 | 364622-82-2 | Doripenem hydrate | Carbapenem antibiotic | <0.1 | <0.1 |
| 3 | 69-52-3 | Ampicillin | β-lactam antibiotic | <0.1 | <0.1 |
| 4 | 64-75-5 | Tetracycline hydrochloride | Antibiotic | <0.1 | <0.1 |
| 5 | 161715-24-8 | Tebipen pivoxil | Carbapenem antibiotic | <0.1 | 4 |
| 6 | 148016-81-3 | Doripenem | Carbapenem antibiotic | <0.1 | 7 |
| 7 | 119478-56-7 | Meropenem trihydrate | β-lactam antibiotic | 5 | 7 |
| 8 | 26787-78-0 | Amoxicillin | β-lactam antibiotic | <0.1 | 5 |
| 9 | 7177-48-2 | Ampicillin trihydrate | β-lactam antibiotic | 4 | 6 |
| 10 | 69-57-8 | Penicillin G sodium | β-lactam antibiotic | 3 | 6 |
| 11 | 104376-79-6 | Ceftriaxone sodium trihydrate | cephalosporin antibiotic | <0.1 | <0.1 |
| 12 | 57-09-0 | Cetrimonium bromide (CTAB) | antiseptic | 1 | 4 |
| 13 | 123-03-5 | Cetylpyridinium chloride | Anti-infection antiseptic | <0.1 | 2 |
| 14 | 72-80-0 | Chlorquinaldol | Antifungal and antibacterial | <0.1 | 16 |
| 15 | 57808-65-8 | Closantel | Gram-positive antibacterial activity inhibitor | 5 | 15 |
| 16 | 61438-64-0 | Closantel sodium | Gram-positive antibacterial activity inhibitor | 3 | 8 |
| 17 | 73231-34-2 | Florfenicol | Antibacterial agent | 4 | 6 |
| 18 | 98079-52-8 | Lomefloxacin HCl | Fluoroquinolone antibiotic | 7 | 11 |
| 19 | 56391-57-2 | Netilmicin sulfate | Active aminoglycoside antibiotic | 9 | 7 |
| 20 | 59703-84-3 | Piperacillin sodium | Semisynthetic, broad-spectrum, ampicillin derived ureidopenicillin antibiotic | <0.1 | 5 |
| 21 | 32986-56-4 | Tobramycin | Aminoglycoside antibiotic | 4 | 7 |
| 22 | 37091-65-9 | Azlocillin sodium salt | Semisynthetic penicillin and β-lactam antibiotic | 7 | 9 |
| 23 | 124858-35-1 | Nadifloxacin | Topical fluoroquinolone antibiotic | 4 | 10 |
| 24 | 70458-95-6 | Pefloxacin mesylate | Synthetic chemotherapeutic and antibacterial agent | 4 | 9 |
| 25 | 13292-46-1 | Rifampin | DNA-dependent RNA polymerase inhibitor antibiotic | 4 | 11 |
| 26 | 24390-14-5 | Doxycycline hyclate | tetracycline-class antibiotic | <0.1 | <0.1 |
| 27 | 1950-7-7 | Mitomycin C | Inhibits DNA synthesis, antibiotic, antitumor agent | <0.1 | <0.1 |
| 28 | 18883-66-4 | Streptozocin | Antibiotic, antitumor agent | <0.1 | 1.4 |
| 29 | 186826-86-8 | Moxifloxacin HCl | Fluoroquinolone antibiotic | <0.1 | <0.1 |
| 30 | 115550-35-1 | Marbofloxacin | Fluoroquinolone antibiotic for veterinary use | <0.1 | <0.1 |
| 31 | 9041-93-4 | Bleomycin Sulfate | Chemotherapy agent, induces DNA strand break | <0.1 | <0.1 |
| 32 | 91832-40-5 | Cefdinir | Third-generation cephalosporin antibiotic | <0.1 | 2 |
| 33 | 62893-19-0 | Cefoperazone | Third-generation cephalosporin antibiotic | <0.1 | 2 |
| 34 | 98106-17-3 | Difloxacin HCl | Quinolone antimicrobial antibiotic | <0.1 | 1 |
| 35 | 738-70-5 | Trimethoprim | Bacteriostatic antibiotic | 9 | 14 |
| 36 | 61379-65-5 | Rifapentine | Antibiotic drug used in the treatment of tuberculosis | 7 | 4 |
| 37 | 163253-35-8 | Sitafloxacin hydrate | Broad-spectrum oral fluoroquinolone antibiotic | 4 | 3 |
| 38 | 13463-41-7 | Zinc pyrithione | Proton pump inhibitor | 2 | 7 |
| 39 | 82419-36-1 | Ofloxacin | Fluoroquinolone antibiotic | 8 | 10 |
| 40 | 10592-13-9 | Doxycycline HCl | Tetracycline antibiotic | 5 | 7 |
| 41 | 93106-60-6 | Enrofloxacin | Fluoroquinolone antibiotic | 3 | 8 |
| 42 | 64485-93-4 | Cefotaxime (sodium salt) | Cephalosporin antibiotic | 7 | 9 |
| 43 | 70458-96-7 | Norfloxacin | Fluoroquinolone antibiotic | 9 | 9 |
| 44 | 3963-95-9 | Methacycline HCl | Tetracycline antibiotic | 0 | 7 |
| 45 | 64953-12-4 | Moxalactam (sodium salt) | β-lactam antibiotic | <0.1 | <0.1 |
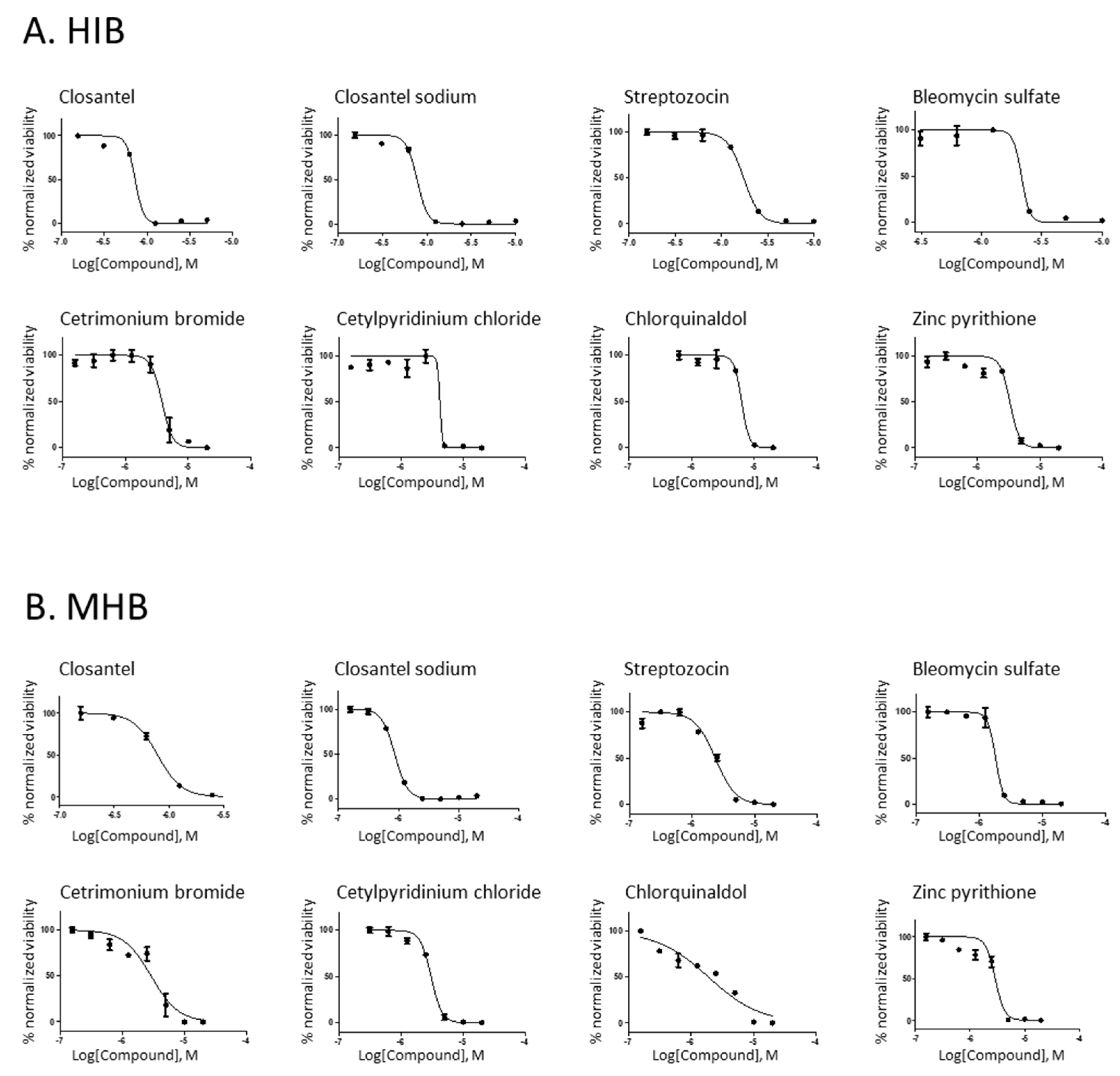
| Drug Name | Chemical Structure | Therapeutic Classification | IC50 (µM) (HIB 1) | IC90 (µM) (HIB) | IC50 (µM) (MHB 2) | IC90 (µM) (MHB) | MIC (µg/mL) (HIB) |
|---|---|---|---|---|---|---|---|
| Closantel | 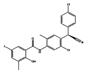 | vermifuge | 0.8 | 1.03 | 1.29 | 3 | 0.82 |
| Closantel sodium | 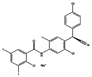 | vermifuge | 1.3 | 2.4 | 1.5 | 2.9 | 3.4 |
| Bleomycin sulfate |  | anticancer | 2.2 | 3.77 | 2 | 2.54 | 15 |
| Streptozocin |  | anticancer | 2.2 | 4.75 | 2.65 | 5.36 | 2.6 |
| Cetrimonium bromide |  | antiseptic | 4.78 | 6.3 | 4.3 | 8.3 | 3.6 |
| Cetylpyridinium chloride |  | antiseptic | 4.5 | 4.9 | 3.45 | 6.1 | 3.3 |
| Chlorquinaldol |  | antiseptic | 5.7 | 7 | 2.33 | 4 | 2.2 |
| Zincpyrithione |  | antifungal | 3.29 | 4.8 | 2.89 | 4.49 | 1.588 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gur, D.; Chitlaru, T.; Mamroud, E.; Zauberman, A. Screening of an FDA-Approved Library for Novel Drugs against Y. pestis. Antibiotics 2021, 10, 40. https://doi.org/10.3390/antibiotics10010040
Gur D, Chitlaru T, Mamroud E, Zauberman A. Screening of an FDA-Approved Library for Novel Drugs against Y. pestis. Antibiotics. 2021; 10(1):40. https://doi.org/10.3390/antibiotics10010040
Chicago/Turabian StyleGur, David, Theodor Chitlaru, Emanuelle Mamroud, and Ayelet Zauberman. 2021. "Screening of an FDA-Approved Library for Novel Drugs against Y. pestis" Antibiotics 10, no. 1: 40. https://doi.org/10.3390/antibiotics10010040
APA StyleGur, D., Chitlaru, T., Mamroud, E., & Zauberman, A. (2021). Screening of an FDA-Approved Library for Novel Drugs against Y. pestis. Antibiotics, 10(1), 40. https://doi.org/10.3390/antibiotics10010040





