Influence of Glutathione and Ascorbic Acid Treatments during Vinification of Feteasca Regala Variety and Their Antioxidant Effect on Volatile Profile
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
4.1. Glutathione and Ascorbic Acid Influence on the Aroma Profile of Feteasca Regala Wines
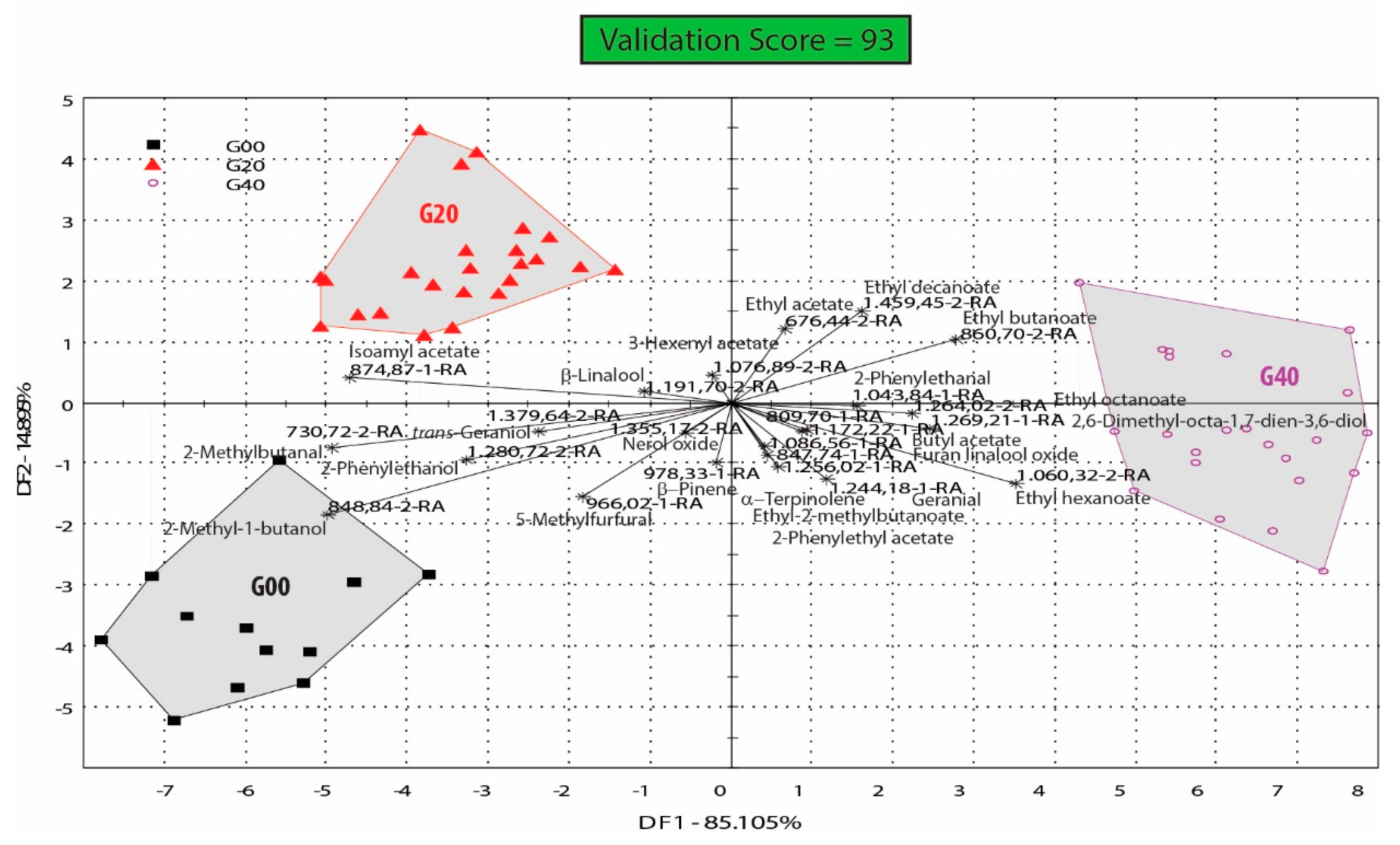
4.2. Glutathione Influence on the Aroma Profile of Feteasca Regala Wines
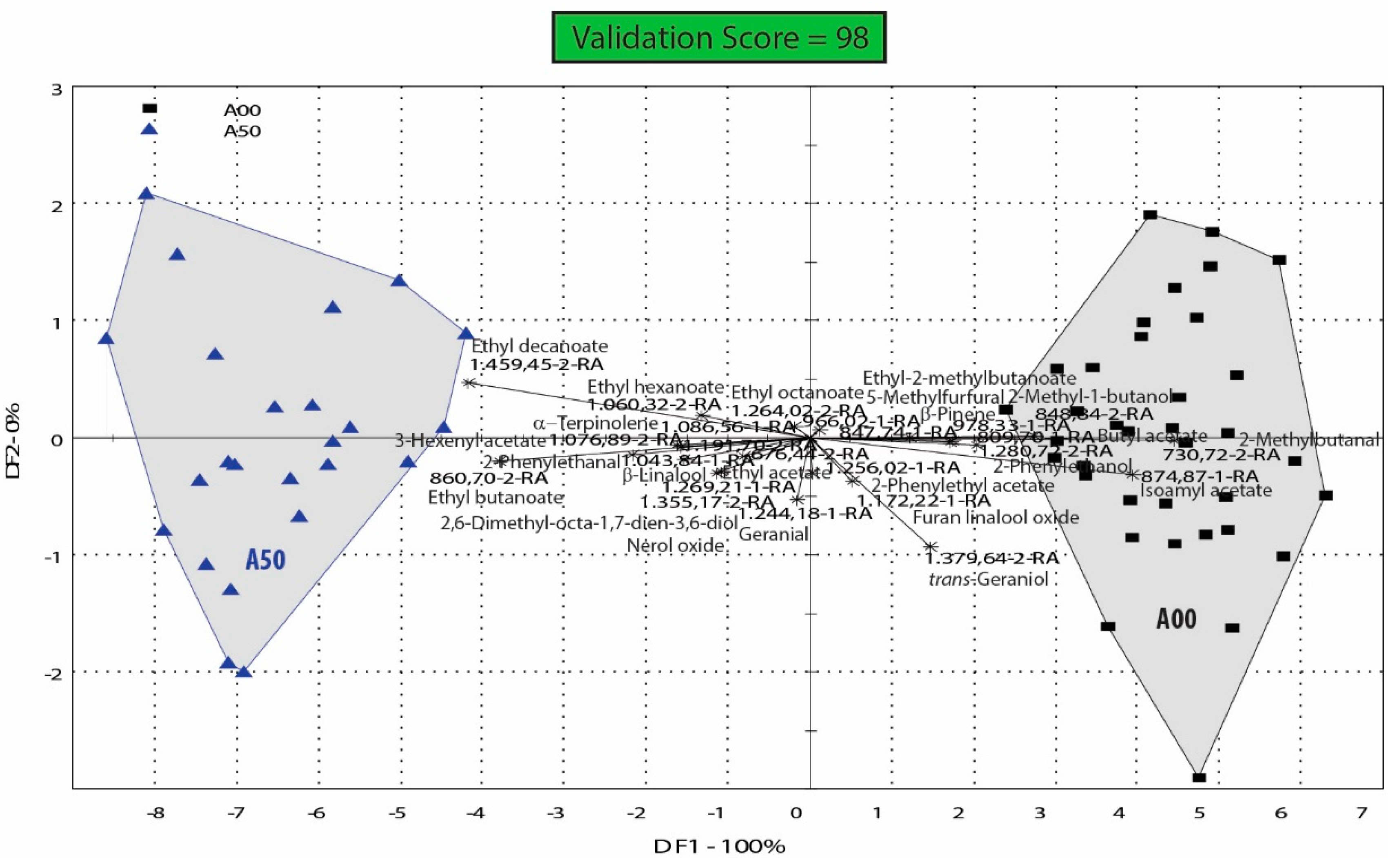
4.3. Ascorbic Acid Influence on the Aroma Profile of Feteasca Regala Wines
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cáceres-Mella, A.; Peña-Neira, Á.; Parraguez, J.; López-Solís, R.; Laurie, V.F.; Canals, J.M. Effect of inert gas and prefermentative treatment with polyvinylpolypyrrolidone on the phenolic composition of Chilean Sauvignon blanc wines. J. Sci. Food Agric. 2013, 93, 1928–1934. [Google Scholar] [CrossRef]
- Corona, O. Wine-making with Protection of Must against Oxidation in a Warm, Semi-arid Terroir. S. Afr. J. Enol. Vitic. 2010, 31, 58–63. [Google Scholar] [CrossRef]
- Wilson, D.L. Sparging with inert gas to remove oxygen and carbon dioxide. Aust. Grapegrow. Winemak. 1985, 256, 112–114. [Google Scholar]
- Wilson, D.L. Storage of wine using inert gas for prevention of oxidation. Grapegrow. Winemak. 1985, 256, 122–127. [Google Scholar]
- Wilson, D.L. Wine transfer using inert gas for prevention of oxidation. Aust. Grapegrow. Winemak. 1985, 256, 110–111. [Google Scholar]
- Wilson, D.L. Use of inert gases for wine quality maintenance—recent advances. In Proceedings of the 2nd International Cool Climate Viticulture and Oenology Symposium, Auckland, New Zealand, 11–15 January 1988; pp. 251–253. [Google Scholar]
- Gabrielli, M.; Aleixandre-Tudo, J.L.; Kilmartin, P.A.; Sieczkowski, N.; du Toit, W.J. Additions of Glutathione or Specific Glutathione-rich Dry Inactivated Yeast Preparation (DYP) to Sauvignon blanc Must: Effect on Wine Chemical and Sensory Composition. S. Afr. J. Enol. Vitic. 2017, 38, 18–28. [Google Scholar] [CrossRef]
- Comuzzo, P.; Battistutta, F.; Vendrame, M.; Páez, M.S.; Luisi, G.; Zironi, R. Antioxidant properties of different products and additives in white wine. Food Chem. 2016, 168, 107–114. [Google Scholar] [CrossRef]
- Makhotkina, O.; Herbst-Johnstone, M.; Logan, G.; Du Toit, W.; Kilmartin, P.A. Influence of sulfur dioxide additions at harvest on polyphenols, C6-compounds and varietal thiols in Sauvignon blanc. Am. J. Enol. Vitic. 2013, 64, 203–213. [Google Scholar] [CrossRef]
- Makhotkina, O.; Araujo, L.D.; Olejar, K.; Herbst-Johnstone, M.; Fedrizzi, B.; Kilmartin, P.A. Aroma impact of ascorbic acid and glutathione additions to Sauvignon blanc at harvest to supplement sulfur dioxide. Am. J. Enol. Vitic 2014, 65, 388–393. [Google Scholar] [CrossRef]
- Coetzee, C.; Lisjak, K.; Nicolau, L.; Kilmartin, P.; Toit, W.J. Oxygen and sulfur dioxide additions to Sauvignon blanc must: Effect on must and wine composition. Flavour Fragr. J. 2013, 28, 155–167. [Google Scholar] [CrossRef]
- Patel, P.; Herbst-Johnstone, M.; Lee, S.A.; Gardner, R.C.; Weaver, R.; Nicolau, L.; Kilmartin, P.A. Influence of juice pressing conditions on polyphenols, antioxidants, and varietal aromas of Sauvignon blanc microferments. J. Agric. Food Chem. 2010, 58, 7280–7288. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. Handbook of Enology Vol. 1—The Microbiology of Wine and Vinifications, 2nd ed.; John Wiley & Sons, Ltd: Chichester, England, 2006. [Google Scholar]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology Vol. 2—The Chemistry of Wine Stabilization and Treatments, 2nd ed.; John Wiley & Sons, Ltd: Chichester, England, 2006. [Google Scholar]
- Barril, C.; Clark, A.C.; Scollary, G.R. Chemistry of ascorbic acid and sulfur dioxide as an antioxidant system relevant to white wine. Anal. Chim. Acta 2012, 732, 186–193. [Google Scholar] [CrossRef]
- Barril, C.; Rutledge, D.N.; Scollary, G.R.; Clark, A.C. Ascorbic acid and white wine production: A review of beneficial versus detrimental impacts. Aust. J. Grape Wine Res. 2016, 22, 169–181. [Google Scholar] [CrossRef]
- Vignault, A.; González-Centeno, M.R.; Pascual, O.; Gombau, J.; Teissedre, P.-L. Chemical characterization, antioxidant properties and oxygen consumption rate of 36 commercial oenological tannins in a model wine solution. Food Chem. 2018, 268, 210–219. [Google Scholar] [CrossRef]
- Harbertson, J.F.; Parpinello, G.P.; Heymann, H.; Downey, M.O. Impact of exogenous tannin additions on wine chemistry and wine sensory character. Food Chem. 2012, 131, 999–1008. [Google Scholar] [CrossRef]
- Sonni, F.; Chinnici, F.; Natali, N.; Riponi, C. Pre-fermentative replacement of sulphur dioxide by lysozyme and oenological tannins: Effect on the formation and evolution of volatile compounds during the bottle storage of white wines. Food Chem. 2011, 129, 1193–1200. [Google Scholar] [CrossRef]
- Kritzinger, E.C.; Bauer, F.F.; du Toit, W.J. Role of glutathione in winemaking: A review. J. Agric. Food Chem. 2013, 61, 269–277. [Google Scholar] [CrossRef]
- Rodríguez-Bencomo, J.J.; Andújar-Ortiz, I.; Moreno-Arribas, M.V.; Simo, C.; Gonzalez, J.; Chana, A.; Dávalos, J.; Pozo-Bayón, A.M. Impact of glutathione-enriched inactive dry yeast preparations on the stability of terpenes during model wine aging. J. Agric. Food Chem. 2014, 62, 1373–1383. [Google Scholar] [CrossRef]
- Tomasevic, M.; Gracin, L.; Curko, N.; Kovacevic Ganic, K. Impact of pre-fermentative maceration and yeast strain along with glutathione and SO2 additions on the aroma of Vitis vinifera L. Posip wine and its evaluation during bottle aging. LWT-Food Sci. Technol. 2017, 81, 67–76. [Google Scholar] [CrossRef]
- OIV. Resolution OIV-OENO 445: Treatment of Must with Glutathione; International Organisation of Vine and Wine: Paris, France, 2015. [Google Scholar]
- OIV. Resolution OIV-OENO 446: Treatment of Wine with Glutathione; International Organisation of Vine and Wine: Paris, France, 2015. [Google Scholar]
- Antoce, O.A.; Cojocaru, G.A. Sensory profile changes induced by the antioxidant treatments of white wines–the case of glutathione, ascorbic acid and tannin treatments on Feteasca regala wines produced in normal cellar conditions. AgroLife Sci. J. 2017, 6, 19–30. [Google Scholar]
- Antoce, O.A.; Cojocaru, G.A. Detection with flash gas chromatography electronic nose of the general influences of glutathione, ascorbic acid, tannin and carbon dioxide treatments on the volatile profiles of white wines of Feteasca Regala. In Proceedings of the BIO Web of Conferences 9, 02003, 40th World Congress of Vine and Wine, Sofia, Bulgaria, 29 May–2 June 2017; pp. 1–6. [Google Scholar]
- Recamales, A.F.; Gallo, V.; Hernanz, D.; González-Miret, M.L.; Heredia, F.J. Effect of time and storage conditions on major volatile compounds of Zalema white wine. J. Food Qual. 2011, 34, 100–110. [Google Scholar] [CrossRef]
- Del Caro, A.; Piombino, P.; Genoves, A.; Moio, L.; Fanara, C.; Piga, A. Effect of Bottle Storage on Colour, Phenolics and Volatile Composition of Malvasia and Moscato White Wines. S. Afr. J. Enol. Vitic. 2014, 35, 128–138. [Google Scholar] [CrossRef]
- Cojocaru, G.A.; Antoce, O.A. Optimization of the alcoholic fermentation by correlating the initial sugar concentration with the inoculum size of yeasts and assimilable nitrogen requirements, Agriculture for Life, Scientific Papers, Series B. Horticulture 2014, 58, 171–180. [Google Scholar]
- OIV. Compendium of International Methods of Wine and must Analysis; Vol 1 and 2; OIV: Paris, France, 2019; ISBN 978-2-85038-003-7. [Google Scholar]
- Antoce, O.A.; Stroe, M.V.; Cojocaru, G.A. Tentative Application of An Electronic Nose to the Study of the Parentage of Romanian Grape Varieties Sarba and Alb Aromat, Agriculture and Agricultural Science Procedia; Elsevier: Amsterdam, The Netherlands, 2015; Volume 6, pp. 110–117. [Google Scholar]
- Antoce, O.A. Influence of Maceration Enzyme Treatment on the Colour and Volatile Profile of Two Red Romanian Wines. Rev. Chim. 2012, 63, 859–864. [Google Scholar]
- Antoce, O.A.; Namolosanu, C.I. Rapid and precise discrimination of wines by means of an electronic nose based on gas-chromatography. Rev. Chim. 2011, 62, 593–595. [Google Scholar]
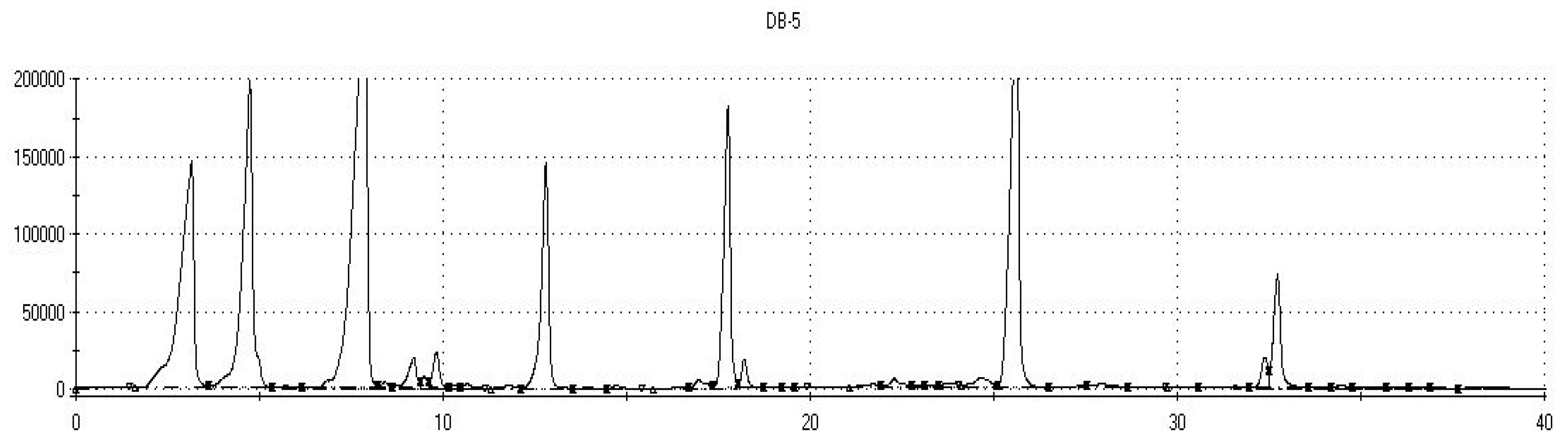
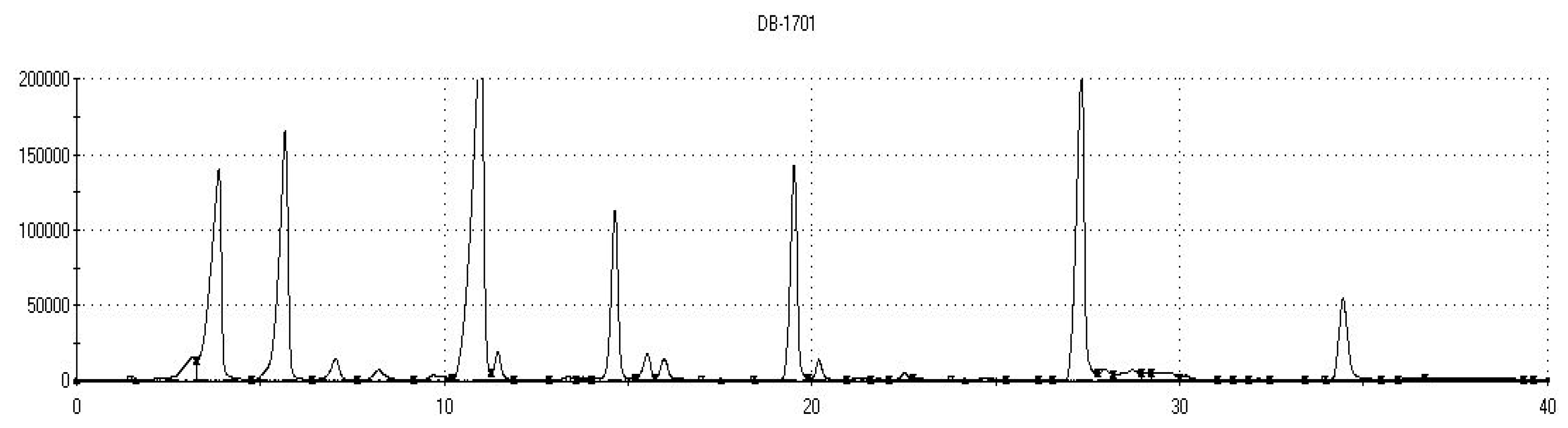

| Sample No. | Sample Code | Glutathione (mg/L) | Ascorbic Acid (mg/L) | Catechinic Tannin (mg/L) | Carbon Dioxide | Classification of Analyzed Groups | ||
|---|---|---|---|---|---|---|---|---|
| Additive Coding | G | A | T | CO2 | Groupe | Groupe | Groupe | |
| Time of Addition | Winemaking | Bottling | I | II | III | |||
| 1 | G00_A00_T00 | 0 | 0 | 0 | no | G00A00 | G00 | A00 |
| 2 | G00_A00_T20 | 0 | 0 | 20 | no | |||
| 3 | G00_A00_T00_CO2 | 0 | 0 | 0 | yes | |||
| 4 | G00_A00_T20_CO2 | 0 | 0 | 20 | yes | |||
| 5 | G20_A00_T00 | 20 | 0 | 0 | no | G20A00 | G20 | A00 |
| 6 | G20_A00_T20 | 20 | 0 | 20 | no | |||
| 7 | G20_A00_T00_CO2 | 20 | 0 | 0 | yes | |||
| 8 | G20_A00_T20_CO2 | 20 | 0 | 20 | yes | |||
| 9 | G20_A50_T00 | 20 | 50 | 0 | no | G20A50 | G20 | A50 |
| 10 | G20_A50_T20 | 20 | 50 | 20 | no | |||
| 11 | G20_A50_T00_CO2 | 20 | 50 | 0 | yes | |||
| 12 | G20_A50_T20_CO2 | 20 | 50 | 20 | yes | |||
| 13 | G40_A00_T00 | 40 | 0 | 0 | no | G40A00 | G40 | A00 |
| 14 | G40_A00_T20 | 40 | 0 | 20 | no | |||
| 15 | G40_A00_T00_CO2 | 40 | 0 | 0 | yes | |||
| 16 | G40_A00_T20_CO2 | 40 | 0 | 20 | yes | |||
| 17 | G40_A50_T00 | 40 | 50 | 0 | no | G40A50 | G40 | A50 |
| 18 | G40_A50_T20 | 40 | 50 | 20 | no | |||
| 19 | G40_A50_T00_CO2 | 40 | 50 | 0 | yes | |||
| 20 | G40_A50_T20_CO2 | 40 | 50 | 20 | yes | |||
| GC Run Conditions | Description |
|---|---|
| Analytical columns | DB5 (non-polar): 5% diphenyl, 95% dimethylpolysiloxane; DB1701 (low/mid polarity): 14% cyanopropylphenyl, 86% dimethylpolysiloxane. |
| Injection mode | 2.5 mL HS syringe, extraction of volatiles from the head-space of vials |
| Injector temperature | 250 °C |
| Temperature program | Initial column temperature 40 °C, increase rate of a 5 °C/s up to 200 °C |
| Carrier gas | Hydrogen in constant pressure mode, 16 psi |
| Oven temperature | 10 min at 60 °C and 500 rpm |
| Acquisition time | 46 s per sample; 5 min break between two samples |
| Tenax trap conditions | |
| Sampling temperature | 40 °C |
| Desorption temperature | 250 °C |
| Purge time | 50 s |
| Bake-out time | 50 s |
| FID run conditions | |
| FID temperature | 220 °C |
| FID fuel pressure | 35 psi |
| * Retention Time | GC Column | * Sample Kovats Indices | Database Kovats Indices | ** Sensor Label | Identified Volatile Organic Compounds | *** Sensory Descriptors |
|---|---|---|---|---|---|---|
| Aldehydes | ||||||
| 7.12 | DB1701 | 730.72 | 729 | 730.72-2 | 2-Methylbutanal | nutty, caramel, sweet |
| 16.70 | DB5 | 966.02 | 967 | 966.02-1 | 5-Methylfurfural | sweet, caramel, almond |
| 19.84 | DB5 | 1.043.84 | 1043 | 1.043.84-1 | 2-Phenylethanal | honey-like, apple, vegetable |
| Higher alcohols | ||||||
| 11.13 | DB1701 | 848.84 | 852 | 848.84-2 | 2-Methyl-1-butanol | fruity, onion |
| 28.14 | DB1701 | 1.280.72 | 1282 | 1.280.72-2 | 2-Phenylethanol | roses, honey, sweet |
| Ethyl esters of fatty acids | ||||||
| 5.74 | DB1701 | 676.44 | 673 | 676.44-2 | Ethyl acetate | ethereal, anise, pineapple |
| 11.88 | DB5 | 847.74 | 850 | 847.74-1 | Ethyl 2-methylbutanoate | apple, green, plum |
| 11.59 | DB1701 | 860.70 | 860 | 860.70-2 | Ethyl butanoate | banana, ethereal, pineapple |
| 19.68 | DB1701 | 1.060.32 | 1061 | 1.060.32-2 | Ethyl hexanoate | apple, banana, pineapple |
| 27.53 | DB1701 | 1.264.02 | 1260 | 1.264.02-2 | Ethyl octanoate | pear, fruity, fresh |
| 34.69 | DB1701 | 1.459.45 | − | 1.459.45-2 | Ethyl decanoate | grape, pear, oily |
| Acetate esters | ||||||
| 10.37 | DB5 | 809.70 | 810 | 809.70-1 | Butyl acetate | fruity, tropical, ethereal |
| 12.98 | DB5 | 874.87 | 874 | 874.87-1 | Isoamyl acetate | banana, fruity, sweet |
| 20.35 | DB1701 | 1.076.89 | 1080 | 1.076.89-2 | 3-Hexenyl acetate | fresh, green, apple |
| 28.01 | DB5 | 1.256.02 | 1257 | 1.256.02-1 | 2-Phenylethyl acetate | floral, pollen |
| Monoterpenes | ||||||
| 17.25 | DB5 | 978.33 | 978 | 978.33-1 | β-Pinene | pine, turpentine, resin |
| 21.54 | DB5 | 1.086.56 | 1087 | 1.086.56-1 | a-Terpinolene | herbal, pine, lemon, sweet |
| 24.80 | DB1701 | 1.191.70 | 1195 | 1.191.70-2 | β -Linalool | citrus, floral, sweet |
| 24.82 | DB5 | 1.172.22 | 1166 | 1.172.22-1 | Furan linalool oxide | earthy, floral, sweet |
| 28.47 | DB5 | 1.269.21 | 1272 | 1.269.21-1 | 2,6-Dimethyl-octa-1,7-dien-3,6-diol | sweet, floral, citrus |
| 27.53 | DB5 | 1.244.18 | 1243 | 1.244.18-1 | Geranial | lemon, sweet |
| 30.90 | DB1701 | 1.355.17 | 1353 | 1.355.17-2 | Nerol oxide | sweet, fruity, floral, rose |
| 31.79 | DB1701 | 1.379.64 | 1376 | 1.379.64-2 | trans-Geraniol | sweet, apple, apricot, rose |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cojocaru, G.A.; Antoce, A.O. Influence of Glutathione and Ascorbic Acid Treatments during Vinification of Feteasca Regala Variety and Their Antioxidant Effect on Volatile Profile. Biosensors 2019, 9, 140. https://doi.org/10.3390/bios9040140
Cojocaru GA, Antoce AO. Influence of Glutathione and Ascorbic Acid Treatments during Vinification of Feteasca Regala Variety and Their Antioxidant Effect on Volatile Profile. Biosensors. 2019; 9(4):140. https://doi.org/10.3390/bios9040140
Chicago/Turabian StyleCojocaru, George Adrian, and Arina Oana Antoce. 2019. "Influence of Glutathione and Ascorbic Acid Treatments during Vinification of Feteasca Regala Variety and Their Antioxidant Effect on Volatile Profile" Biosensors 9, no. 4: 140. https://doi.org/10.3390/bios9040140
APA StyleCojocaru, G. A., & Antoce, A. O. (2019). Influence of Glutathione and Ascorbic Acid Treatments during Vinification of Feteasca Regala Variety and Their Antioxidant Effect on Volatile Profile. Biosensors, 9(4), 140. https://doi.org/10.3390/bios9040140





