Abstract
In this work, we report on the development of a newly synthesized photoactive reversible azobenzene derived surfactant polymer, which enables active and fast control of the merging of microdroplets in microfluidic chambers, driven by a pulsed UV laser optical stimulus and the well known cis-trans photo-isomerisation of azobenzene groups. We show for the first time that merging of microdroplets can be achieved optically based on a photo-isomerization process with a high spatio-temporal resolution. Our results show that the physical process lying behind the merging of microdroplets is not driven by a change in surface activity of the droplet stabilizing surfactant under UV illumination (as originally expected), and they suggest an original mechanism for the merging of droplets based on the well-known opto-mechanical motion of azobenzene molecules triggered by light irradiation.
1. Introduction
Many lab-on-a-chip (LoC) applications have become possible thanks to the ability to control mixing of different droplet contents, which enabled the sequencing of many complex bio-chemical and biological reactions with a high level of control and flexibility over the last decade; see for a review [1,2,3,4,5,6,7]. Hence, among all manipulation schemes allowed by droplet-based microfluidics technology [8,9,10,11,12,13,14,15,16], active merging of microdroplets (AMD) is probably one of the most important. It is generally achieved using a high alternating current (AC) voltage [17], or using a direct current (DC) voltage [18]. Nevertheless, light-driven merging of droplets is a more attractive approach since light provides not only high temporal and spatial resolutions but also wavelength and intensity tunability [19,20,21].
Recently, Dunkel et al. [22] showed that active merging of microdroplets can be achieved optically and very selectively using the photolysis process of photolabile surfactants [22]. In the present study, we consider a new strategy based on the photo-isomerization process (Figure 1) of a newly synthesized azobenzene derivative surfactant polymer, whose structure is given in Figure 2 and which is named in this study KryAz600, to achieve an active merging of water-in-oil (W/O) microdroplets using a picosecond (ps) pulsed UV laser.
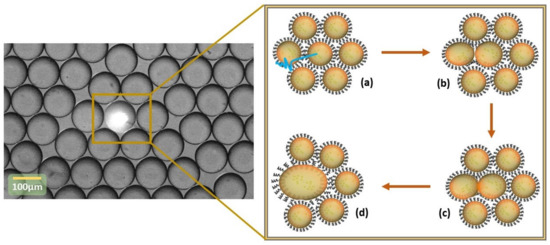
Figure 1.
Light-driven merging process principle. (a) Targeting droplets and change of surface activity of the surfactant molecules at the droplet surface under laser irradiation, (b) depletion of the surfactant molecules at the the droplet interface, (c) merging induced following UV laser irradiation and the trans to cis photo-conversion, (d) targeted droplets after merging.
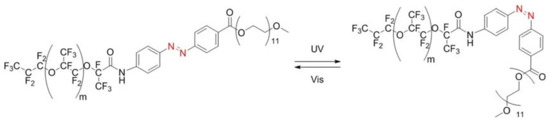
Figure 2.
Stable trans form (left) and metastable cis form (right) of the synthesized KryAz600 molecule; trans to cis transition occurs under UV irradiation and back to trans occurs under visible light.
This study was inspired initially by a previous work carried out by Takahashi et al. [23], who reported that a light-induced destabilization of an overall emulsion based on the photo-isomerization process of azobenzene-derived photosensitive surfactants, through the light induced interfacial activity change of gemini-like azobenzene derived surfactants and the conversion between a higher surface activity trans isomer and a lower surface activity cis isomer. This resulted in a destabilisation of the overall emulsion without the aim of achieving spatial differentiation and requesting several minutes of irradiation.
Our approach is different and is far from trivial. In fact, the dynamics of the change of azobenzene surfactants surface activity at the microscale, at which new interfaces are produced involve both the change of surface tension driven by the photo-isomerisation process and the diffusion of the new surfactant molecules to the interface, as well as the adsorption on the droplet surface, each partial step adding a typical time and length-scale [24]. Our approach is also different from the recent study reported by Dunkel et al. [22] as the mechanisms lying behind the two merging processes involved in the two studies are completely different. Moreover, photo-isomerization of azobenzene is fully reversible, which makes this new approach particularly suitable for the reuse of the photo-sensitive surfactant, which is generally produced in a small quantity.
2. Materials and Methods
2.1. Chemicals
Azobenzene derived molecules possess two stable geometric isomers: an energetically stable trans form and a meta-stable cis form. For most azobenzenes, molecules can be optically isomerized from trans to cis using light in the near UV and Visible: upon absorption of a photon with a wavelength in the near UV (around 330 nm), molecules convert, with high efficiency, from the trans isomer into the cis isomer. A second photon with a wavelength in the visible range (around 440 nm) can induce the back-conversion. UV illumination can also enable conversion of azobenzene molecules from a cis form to a trans form as photons of UV light have higher energy than visible light, which is sufficient to induce cis to trans isomerization. Azobenzene photo-isomerization is completely reversible and both forward and reverse photoisomerizations typically exhibit picosecond timescales. The trans isomer is thermodynamically more stable than the cis isomer, by approximately 50–100 kJ/mol and the energy barrier for thermal isomerization is in the order of 100–150 kJ/mol. Hence, in the dark, the cis isomer thermally relaxes back to the trans isomer on a timescale ranging from milliseconds to hours, or even days, depending on the substitution pattern around the azobenzene group and the local environment of the molecules.
We synthesized a new fluorinated azobenzene derivative surfactant polymer, named in this study KryAz600. It consists of a triblock copolymer surfactant, composed of a perfluoro-polyether (PFPE) hydrophobic chain, linked to a polyethylene-glycol (PEG-600) hydrophilic chain (Sigma-Aldrich, Saint-Quentin Fallavier, France) through an azobenzene group, as shown in Figure 2. The PFPE hydrophobic chain was derived from a commercially available carboxy-terminated fluorinated polymer, namely Krytox 157-FSH (Dupont) and linked to the azobenzene group following a similar procedure as described in detail by Lee et al. [25], see also supporting information at (S1) for details.
2.2. Experimental Setup
Nemesys syringe pumps (Cetoni GmbH, Korbussen, Germany) were used to fabricate monodisperse drops of size range 50 m to 150 m depending upon the need. To study and sort droplets, the optics part consists of a ps-pulsed UV laser source, with a peak wavelength at 355 nm and delivering 15.4 J of energy per pulse. In order to make the laser spot size adequate, the laser beam was cleaned and band-limited for enhanced detection (20 nm filter F). Figure 3 illustrates the optical path for the laser source. After the filter, the source was staged up and reflected by dichroic mirror (DM) (Tx = 506 nm, Semrock, Rochester, NY, USA) to the sample where a microscope objective (2×, 4×, or 10× Olympus Inverted Microscope) targets and acquires the reflected/scattered signal from the droplet under observation. At the detection side we used a standard speed camera for recording and visual inspection of the droplet generation, manipulation, and merging. For detection, a photo-multiplier tube (Hamamatsu) was used to monitor droplet generation frequency. For optimized 100 m drop-size (500 L/h and 100 L/h for the continuous and dispersed phase), the droplet frequency was found to be about 250 drops/second (scheme for droplet frequency was derived from the method discussed in [15]).
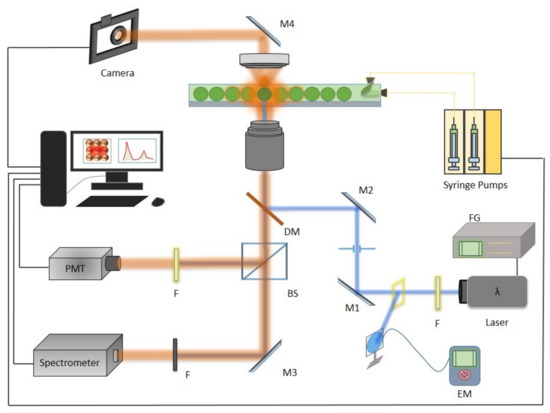
Figure 3.
The setup containing the droplet generation assembly, optics and detection unit. On the optical path, the ps-pulsed source (355 nm), F (filter), M1 and M2 mirrors for step-up, dichoric mirror (DM) (506 nm), camera with notch filter, BS (beam splitter) to reflect the acquired signal to detector (setup adapted from [15]).
2.3. Design and Microfabrication
Droplets were generated in a flow-focusing microfluidic device, which was fabricated using the standard soft-lithography technique, employing PDMS (Polydimethylsiloxane) for replica molding. The constructed microfluidic device had a square drive channel (guide length after the drop-maker) and a rectangular micro-analysis chamber constructed by stepper lithographic pattern. The advantage of the dual stage chamber is to facilitate the droplet in maintaining a spherical shape, which in turn reduced the pressure coalescence of microdroplets. Droplet stability can be increased by providing extra time to the surfactant molecule in order to localize around the droplet (exterior) wall. This attribute was achieved by increasing the drive channel length to almost three times the length of the drive channel mentioned in previous work [22].
The drive channel length was set to about 2100 m (Figure 4a), which is long enough to allow the oil soluble surfactant molecules to build a stabilizing monolayer around the droplets before the droplet come in contact with each other at the entrance, where they may merge. After the drive channel, droplets enter a rectangular chamber of the dimensions 4000 × 1200 m (Figure 4b) acting as droplet storage and an analysis chamber. At the end of the device assembly, a zig-zag channel leads to output for the droplet collection (Figure 4c). The advantage of droplet collection is to utilize the reversibility of the photo-active reversible compound and to perform off-chip micro-particle studies.
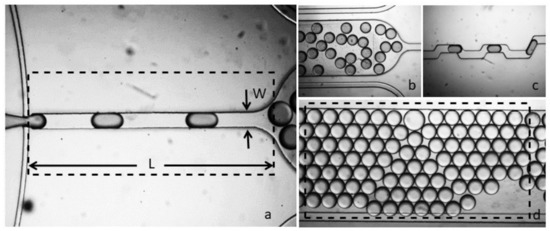
Figure 4.
The fabricated flow-focusing assembly, (a) drive channel with width W and length L, (b) droplet generation at 500 uL/h and 100 uL/h for drive and dispersed phase, (c) output channel for droplet frequency monitoring, (d) micro-analysis and storage chamber.
In order to make the device interior compatible with the oil-phase, the interior walls of the complete assembly was functionalized by commercially available surface coating agent, which consisted of a 2% solution of perfluoroctyl-dimethylsiloxane dissolved in HFE7100 fluorocarbon oil (3M). This coating enhanced the wettability of the channel walls and also reduced the risk of diffusion of the surfactant molecules to the PDMS. For the droplet generation, we used a fluorinated oil phase (HFE 7500, 3M, density 1.62 g/cm).
Photochromic reactions were induced in-situ by a continuous irradiation Hg/Xe lamp (Hamamatsu, LC6 Lightningcure, 200 W) equipped with narrow band interference filters of appropriate wavelengths. The irradiation power was measured using a photodiode from Ophir (PD300-UV). The photochromic quantum yields were determined by probing the sample with a Xenon lamp during the course of the light irradiation. Absorption changes were monitored by a charge coupled device (CCD) camera mounted on a spectrometer (Ocean Optics, Largo, FL, USA).
3. Results and Discussion
3.1. KryAz600 Surfactant Photokinetics
Likewise, most azobenzene derivatives, KryAz600 molecules switch under UV illumination from a stable trans form (t-KryAz600) to a metastable cis form (c-KryAz600). The trans form is characterized by a large absorption band with a maximum absorption around 335 nm (in HFE 7500 oil), while c-KryAz600 form has a weaker absorption band with a maximum absorption around 440 nm, as shown in Figure 5.
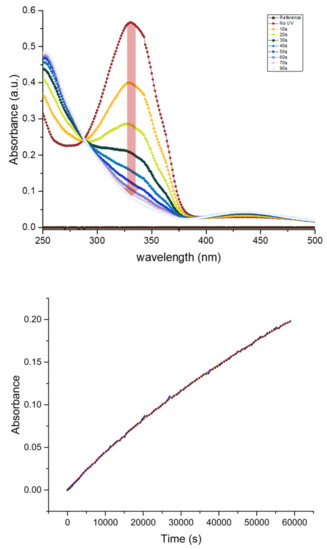
Figure 5.
(Upper) Conversion of the surfactant state under UV illumination, Top curve (red) being the first measurement with no UV treatment and subsequent state change transition of surfactant by 10 s of exposure to a UV lamp with power of 6.7 mW at 365 nm wavelength; (Bottom) slow exponential rise of the 335 nm absorption band intensity of the t-KryAz600 form in dark, this corresponds to a half-life h of the c-KryAz600 → t-KryAz600 transition.
Our results show that KryAz600 molecules transit spontaneously in the dark from the cis form to the trans form with a constant time as small as /s. This value was deduced from the exponential fit of the absorption curve of c-KryAz600 at 330 nm versus time, as shown in Figure 5. It corresponds to a half-life h. It is worth noting that the relatively long half-life of KryAz600 molecules is of great importance in our study since the cis form relaxes back very slowly to the trans form, if no UV illumination is used.
3.2. Microdroplets Stability Versus Surfactant Conformation and Concentration
Monodisperse microdroplets with a size of about 100 m were prepared using different surfactant concentrations dissolved in HFE7500 oil, ranging from 1 mM down to 15 M.
In order to quantify the effect of the cis and trans conformations on the stability of droplets, a control experiment was first conducted. Two samples of equal concentration of 1 mM () were prepared, one was left in the dark (overnight) in order to allow for all surfactant molecules present in the solution to transit to the thermodynamically stable trans state, and the other sample was illuminated with a 235 nm UV lamp for 1 h.
Droplets that were prepared using the non irradiated surfactant solution exhibited long term stability (Figure 6a) where as the droplets prepared using UV illuminated surfactant solution merged immediately in the observation chamber (Figure 6b). This shows that cis conformation of KryAz600 molecules is not suitable to ensure droplet stabilization. A quick glance at the molecule structure of Figure 2 shows that the cis conformation is less suitable to achieve a close packing of surfactant molecules at the droplet interface, than could be done using the trans isomer. In other words, cis isomer molecules lead to a lower surface density of surfactant molecules around the droplet and hence to a higher interfacial tension.
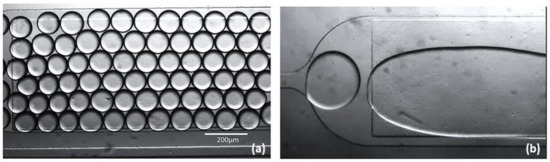
Figure 6.
Surfactant KryAz600, (a) mono-disperse (water-in-oil) droplets of stable trans state, (b) effect of UV exposure, unstable cis state causing coalescence of micro-droplets.
It is worth noting, that UV illuminated surfactant solution, when left in the dark overnight, enables us again to produce stable droplets. This demonstrates the reversibility of the process and the possibility to reuse surfactant solution for further experiments. Our observations show also that the merging of microdroplets under laser illumination is not the result of the thermo-capillary effect of the laser beam, since thermal effects are present for both droplets, prepared with the two types of isomers.
Also, because at the micrometer scale, the diffusion of surfactant molecules cannot be neglected, we found it necessary to investigate the relation between the merging time and the concentration of the surfactant molecules in the carrier fluorocarbon oil. This is particularly important in our study since a focused pulsed laser with a micrometer sized footprint is used for photo-isomerization.
Water-in-oil (W/O)droplets were produced and collected in an on-chip micro-analysis chamber with different surfactant concentrations, decreasing from 1 mM () to 15.6 M. This process depends on the kinetics of the depletion of surfactants under laser illumination. The first concentration, mM of the surfactant, was found to lead to very stable microdroplets with no merging process with irradiation times smaller than 10 min, whereas a fast merging (∼1 s) could be achieved with a concentration value of 25 M. It is worth noting that for smaller concentration values of surfactant (c < 25 M), a spontaneous merging of droplets is observed, which indicates that there are not enough surfactant molecules in the carrier oil solution for droplets stabilization. Successive dilutions of the surfactant solution resulted in a decrease of the merging time from about 10 min for (1 mM) to 1 s for 25 M. For lower concentrations, droplets were observed to be highly unstable. These results clearly indicate a decrease in merging time as the concentration of surfactant molecules decreases. Merging reduced sufficiently from almost 6 s for 62.5 M to 1 s for 15.6 M. This result may be interpreted as follows. As the concentration reduces, the number of idle-molecules in bulk drive phase take more time to replace the targeted molecules. Also, since the surfactant molecules are not oriented in a specific order, so the lower the concentration, the higher the diffusion time for the idle molecules to reach the depleted area.
To better understand these results, let us first calculate the flow time, , that each droplet takes to reach the observation micorchamber; corresponds to the flight time of droplets along the drive channel, from the nozzle to the end of the main channel, above which droplets start to collide with each other. Considering the length m of the drive channel and the mean velocity of microdroplets in this region, mm/s, one finds:
To ensure droplet stability, should be greater than the diffusion time, , which is approximately the necessary time lapse for building a stabilizing surfactant layer around the droplets to prevent their coalescence. Indeed, during , a given number of surfactant molecules diffuse from the bulk phase and adsorb arround the flowing droplet interface to form a stabilizing surfactant layer. Considering the total area of a microdroplet with a radius R ( m), the maximum packing of surfactant molecules, with a typical lateral dimension nm, i.e., with a molecular area , at the interface of the droplet is achieved with n molecules when . Droplets will be stable when the number of molecules at the interface is larger than a fraction of the maximum packing; therefore, the stability condition reads
According to Baret et al., [26] microdroplets become stable when the number of molecules at the interface becomes larger than a fraction of the maximum packing; therefore, the stability condition for microdroplets of radius R can be expressed as follows:
The number of free surfactant molecules required for the stabilization of the droplet interface, n, can be assumed to be dispersed in a volume surrounding the droplet over a distance from the interface (). Hence, n can be estimated as , where is Avogadro’s number. In a diffusion limited process, surfactant molecules confined in the volume will reach the droplet interface within a time given by the diffusion law: . It is possible thus to estimate an experimental value for the diffusion time according to the following equation:
Hence, for M, one finds ms (, whereas for c = 15 M, one finds ms (). These results are in good agreement with the observed surfactant concentration threshold for droplet stability, which was found to lay in the 25–15 M range.
To further analyze the merging behavior of microdroplets under irradiation, different sweeps of the laser beam over the whole micro-chamber were performed and first and second merging of the one drop to its neighbor and later (second merging) with the two times big merged drop to its neighboring droplet. Results presented in Figure 7 show a non-uniform but correlated first and second merging times. The rationale behind two merging observations relates to the change in volume and a slight increase in the merging time. Consider Figure 7, on left, two drops selected for merging and on right the big merged drop. For two separate drops of 100 m, the calculated volume was 0.5 nL each, while after merging the the big drop had a volume of 4 nL, thus eight times increase in volume. From the classical inverse relation of concentration with volume, the overall change in concentration of big merged drop was 0.125 times the concentration of the drive phase. Upon targeted merging of that droplet with the usual droplet caused a slight increase in merging time, depicted as a green curve in Figure 7.
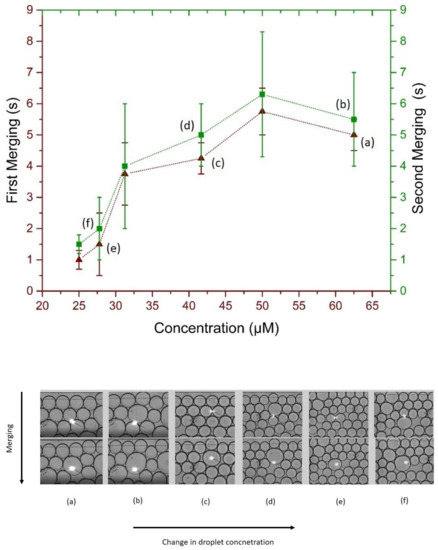
Figure 7.
(Upper) Merging time scale of irradiated droplets, maroon (triangles) for first merging and green (squares) for second merging, (Bottom) optical micro-graphs of the first and second merging and resulting change in targeted droplet volume.
3.3. Suggested Opto-Mechanical Model for Droplet Merging Mechanism
Unlike in the work reported by Takahashi et al. [23], where a broad UV illumination during several minutes of the overall emulsion resulted in a destabilisation of the overall emulsion, photo-isomerization is achieved in our study using a ps UV laser which is focused on a very tiny fraction of the emulsion. It lasts only a few seconds before droplet merging is achieved. At such time and space scales, the change in the activity of surfactant molecules involves both a change of surface tension driven by the photo-isomerisation process and the diffusion of new surfactant molecules from the bulk solution to the interface, as well as the adsorption on the droplet surface. If one considers a diffusion limited merging process mechanism, to achieve droplet interface destabilization and merging, the rate of photoisomerized surfactant molecules under pulsed UV laser illumination should be higher than the diffusion rate of non irradiated surfactant molecules from the bulk solution to the target droplet interface. Nevertheless, in the particular case of photo-isomerization, one should take account of the fact that the higher energy UV illumination can induce not only isomerization from trans to cis form but it can also induce the reverse transformation on cis molecules present under the UV light spot. Hence, when a pulsed UV laser is used, if one illuminates locally, for instance, a population of A isomers during a first pulse to B isomers, then the transformed B isomers would go back to their initial conformation (A) under illumination during the following pulse, unless diffusion is fast enough to deplete the illuminated region of B isomers during the first pulse of the UV laser.
Though UV light can drive both cis to trans and trans to cis photo-isomerizations, one should emphasize that a longer term UV illumination of azobenzene molecules would not lead in average to a mixture of 50% of molecules in each state. Indeed, the forward (trans to cis) and backward (cis to trans) photo-reactions of azobenzene molecules do not show the same quantum yield, i.e., the probability of inducing the photo-reaction after absorption of a photon. Therefore, the ratio of trans and cis isomers upon long term UV irradiation can be much higher than 50%. In the present case, this ratio is higher than 85% under 365 nm illumination as can be deduced from the absorbance curves of KryAz600 molecules around 335 nm after 90 s of illumination vs. their absorbance before irradiation (0 s), as shown in Figure 5.
Let us calculate the diffusion distance of surfactant molecules in the oil solution during the time lapse of a first single pulse, which is equal to 1 ms in our experiments (and which should not be confused with the duration of a pulse ≃ 400 ps). Using diffusion law, and considering a typical value of the diffusion coefficient of surfactant molecules in HFE7500 oil, about m/s [26,27], one finds a mean diffusion distance of
During the time lapse of one pulse. Even if considering a diffusion coefficient which is two orders of magnitude higher than the previous one, as reported by Dunkel et al. [22], one would find a mean diffusion distance of about m during each pulse. It is interesting to note that such values are much smaller than the size of the illuminated area with the focused laser beam, which is, ∼30 m in our experiments. This rough estimation of the diffusion of surfactant molecules during the time lapse between two successive illuminations shows that a large proportion of isomerized molecules during the first pulse will be still present in the illuminated area when the following light pulse triggers and hence cis isomers will recover their initial conformation (trans) under this second UV laser pulse.
Consequently, one should conclude that the observed merging process of droplets cannot be attributed to a change of surface activity of surfactant molecules under illumination, as surfactant molecules switch continuously from trans to cis and from cis to trans under successive UV laser pulses. The merging process should be based on opto-mechanical effects due to the well-known change of the length of the cis and trans azobenzene groups under illumination. Photo-isomerization reduces the distance between the 4 and 4’ positions of azobenzene groups from 0.99 nm in the trans state to 0.55 nm for the cis state [28]. Consequently, UV laser illumination produces geometric changes in the azobenzene group, which should translate into larger-scale motions extending to the overall surfactant droplet interface layer. After approximately one second of illumination, the oscillation of the droplet interface should lead to the merging of the droplets.
4. Conclusions
Active merging of microdroplets is one of the most important manipulations enabled by droplet-microfluidics, as it enables for on demand droplet targeting and sequencing of complex chemical and biological reactions. Nevertheless, the standard voltage-based methods are generally limited to spatially isolated pairs of droplets, as the long-range effect of the applied electric field does not have the potential to differentiate between flowing or confined droplets. We developed an optofluidic approach, which overcomes such limitations and allows for a rapid controlled coalescence of targeted microdroplets thanks to the application of a ps UV pulsed laser. Our approach is based on the photo-isomerization process of an azobenzene-derived surfactant. The success of such controlled coalescence is far from trivial. In fact, the dynamics of the change of surface tension at the microscale driven by photo-isomerisation processes; the diffusion of the new surfactant molecules to the interface; as well as the adsorption on the droplet surface, are complex and not well understood. By incorporating light activated switching in the surfactant, release or mixing of droplets contents could be programmed. In this study, we present the fast and dynamic switching of azobenzene derived surfactant for controlled coalescence of spatially selectable microdroplets as proof of concept.
Supplementary Materials
The following are available online at https://www.mdpi.com/2079-6374/9/4/129/s1, Figure S1: Chemical synthesis procedure of KryAz600 surfactant molecules.
Author Contributions
Conceptualization, M.B. and A.I.E.A.; Data curation, Z.H., N.B. (Nizar Bchellaoui), R.M. and A.I.E.A.; Formal analysis, Z.H. and A.I.E.A.; Funding acquisition, M.B. and A.I.E.A.; Investigation, Z.H., R.M. and A.I.E.A.; Methodology, C.D., Juan Xie, M.B. and A.I.E.A.; Project administration, M.B. and A.I.E.A.; Resources, C.D., Remi Metivier, N.B. (Nicolas Bogliotti), J.X., M.B. and A.I.E.A.; Software, Z.H. and A.I.E.A.; Supervision, N.B. (Nicolas Bogliotti), M.B. and A.I.E.A.; Validation, Z.H. and A.I.E.A.; Visualization, Z.H. and A.I.E.A.; Writing–original draft, Z.H. and A.I.E.A.; Writing–review editing, A.I.E.A.
Funding
This research received no external funding.
Acknowledgments
We thank Emile Auria for his contribution to the synthesis of the azobenzene-derived surfactant.
Conflicts of Interest
The authors declare no conflict of interests.
References
- Gu, H.; Duits, M.H.G.; Mugele, F. Droplets Formation and Merging in Two-Phase Flow Microfluidics. Int. J. Mol. Sci. 2011, 12, 2572–2597. [Google Scholar] [CrossRef] [PubMed]
- Agresti, J.J.; Antipov, E.; Abate, A.R.; Ahn, K.; Rowat, A.C.; Baret, J.C.; Marquez, M.; Klibanov, A.M.; Griffiths, A.D.; Weitz, D.A. Ultra-high-throughput screening in drop-based microfluidics for directed evolution. Proc. Natl. Acad. Sci. USA 2010, 107, 4004–4009. [Google Scholar] [CrossRef] [PubMed]
- Taly, V.; Pekin, D.; Abed, A.E.; Laurent-Puig, P. Detecting biomarkers with microdroplet technology. Trends Mol. Med. 2012, 18, 405–416. [Google Scholar] [CrossRef] [PubMed]
- deMello, A.J. Control and detection of chemical reactions in microfluidic systems. Nature 2006, 442, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Chen, D.L.; Ismagilov, R.F. Reactions in Droplets in Microfluidic Channels. Angew. Chem. Int. Ed. 2006, 45, 7336–7356. [Google Scholar] [CrossRef]
- Witters, D.; Sun, B.; Begolo, S.; Rodriguez-Manzano, J.; Robles, W.; Ismagilov, R.F. Digital biology and chemistry. Lab Chip 2014, 14, 3225–3232. [Google Scholar] [CrossRef]
- Kumacheva, E.; Garstecki, P. Microfluidic Reactors for Polymer Particles; Wiley: Chichester, UK, 2011. [Google Scholar]
- Thorsen, T.; Robert, R.W.; Arnold, F.H.; Quake, S.R. Dynamic Pattern Formation in a Vesicle-Generating Microfluidic Device. Phys. Rev. Lett. 2001, 86, 4163–4166. [Google Scholar] [CrossRef]
- Thorsen, T.; Maerkl, S.J.; Quake, S.R. Microfluidic Large Scale Integration. Science 2002, 298, 580–584. [Google Scholar] [CrossRef]
- Teh, S.Y.; Lin, R.; Hung, L.H.; Lee, A.P. Droplet microfluidics. Lab Chip 2008, 8, 198–220. [Google Scholar] [CrossRef]
- Guo, M.T.; Rotem, A.; Heyman, J.A.; Weitz, D.A. Droplet microfluidics for high-throughput biological assays. Lab Chip 2012, 12, 2146–2155. [Google Scholar] [CrossRef]
- Dak, P.; Ebrahimi, A.; Swaminathan, V.; Duarte-Guevara, C.; Bashir, R.; Alam, M. Droplet-based biosensing for lab-on-a-chip, open microfluidics platforms. Biosensors 2016, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Wang, X.; Wang, J.; Tian, H.; Zhao, P.; Tian, Y.; Gu, Y.; Wang, L.; Wang, C. Droplet microfluidics for the production of microparticles and nanoparticles. Micromachines 2017, 8, 22. [Google Scholar] [CrossRef]
- Bchellaoui, N.; Hayat, Z.; Mami, M.; Dorbez-Sridi, R.; El Abed, A.I. Microfluidic-assisted formation of highly monodisperse and mesoporous silica soft microcapsules. Sci. Rep. 2017, 7, 16326. [Google Scholar] [CrossRef]
- Hayat, Z.; El Abed, A. High-Throughput Optofluidic Acquisition of Microdroplets in Microfluidic Systems. Micromachines 2018, 9, 183. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Barea, J.; Lee, J.; Kang, D.K. Recent Advances in Droplet-based Microfluidic Technologies for Biochemistry and Molecular Biology. Micromachines 2019, 10, 412. [Google Scholar] [CrossRef]
- Chabert, M.; Dorfman, K.; Viovy, J.L. Droplet fusion by alternating current (AC) field electro-coalescence in microchannels. Electrophoresis 2005, 26, 3706–3715. [Google Scholar] [CrossRef]
- Link, D.R.; Grasland-Mongrain, E.; Duri, A.; Sarrazin, F.; Cheng, Z.; Cristobal, G.; Marquez, M.; Weitz, D.A. Electric control of droplets in microfluidic devices. Angew. Chem. Int. Ed. 2006, 45, 2556–2560. [Google Scholar] [CrossRef]
- Mayer, G.; Heckel, A. Biologically Active Molecules with a “Light Switch”. Angew. Chem. Int. Ed. 2006, 45, 4900–4921. [Google Scholar] [CrossRef]
- Gorostiza, P.; Isacoff, E.Y. Optical Switches for Remote and Noninvasive Control of Cell Signaling. Science 2008, 322, 395–399. [Google Scholar] [CrossRef]
- Baigl, D. Photo-actuation of liquids for light-driven microfluidics: state of the art and perspectives. Lab Chip 2012, 12, 3637–3653. [Google Scholar] [CrossRef]
- Dunkel, P.; Hayat, Z.; Barosi, A.; Bchellaoui, N.; Dhimane, H.; Dalko, P.I.; El Abed, A.I. Photolysis-driven merging of microdroplets in microfluidic chambers. Lab Chip 2016, 16, 1484–1491. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Fukuyasu, K.; Horiuchi, T.; Kondo, Y.; Stroeve, P. Photoinduced Demulsification of Emulsions Using a Photoresponsive Gemini Surfactant. Langmuir 2014, 30, 41–47. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brosseau, Q.; Vrignon, J.; Baret, J.C. Microfluidic Dynamic Interfacial Tensiometry ([small mu ]DIT). Soft Matter 2014, 10, 3066–3076. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Collins, J.W.; Aubrecht, D.M.; Sperling, R.A.; Solomon, L.; Ha, J.W.; Yi, G.R.; Weitz, D.A.; Manoharan, V.N. Synchronized reinjection and coalescence of droplets in microfluidics. Lab Chip 2014, 14, 509–513. [Google Scholar] [CrossRef]
- Baret, J.C.; Kleinschmidt, F.; Harrak, A.E.; Griffiths, A.D. Kinetic Aspects of Emulsion Stabilization by Surfactants: A Microfluidic Analysis. Langmuir 2009, 25, 6088–6093. [Google Scholar] [CrossRef]
- Rosen, M.J. Surfactants and Interfacial Phenomena, 3rd ed.; Wiley: Hoboken, NJ, USA, 2004. [Google Scholar]
- Mahimwalla, Z.; Yager, K.G.; Mamiya, J.I.; Shishido, A.; Priimagi, A.; Barrett, C.J. Azobenzene photomechanics: prospects and potential applications. Polym. Bull. 2012, 69, 967–1006. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).