Reflectance-Based Organic Pulse Meter Sensor for Wireless Monitoring of Photoplethysmogram Signal
Abstract
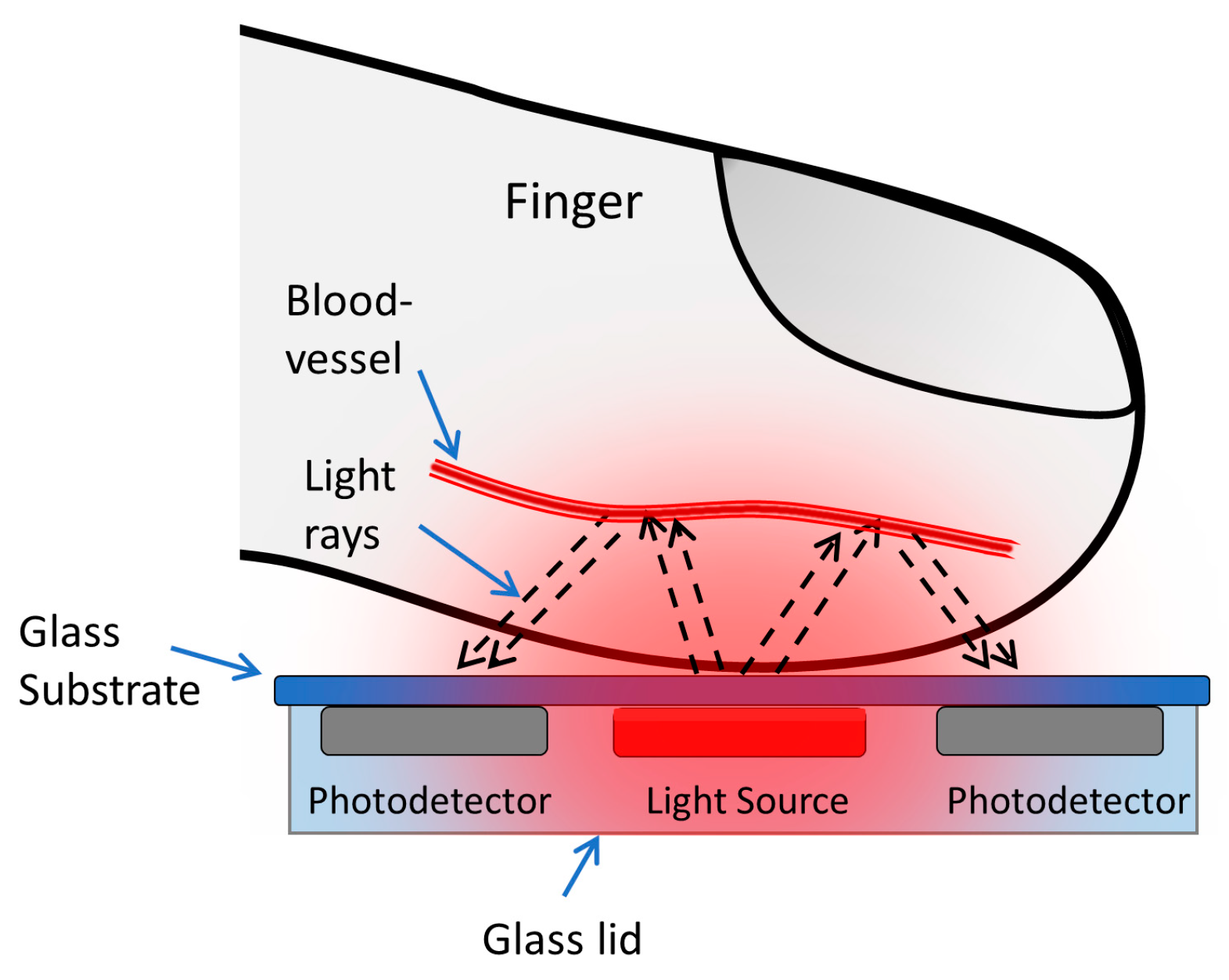
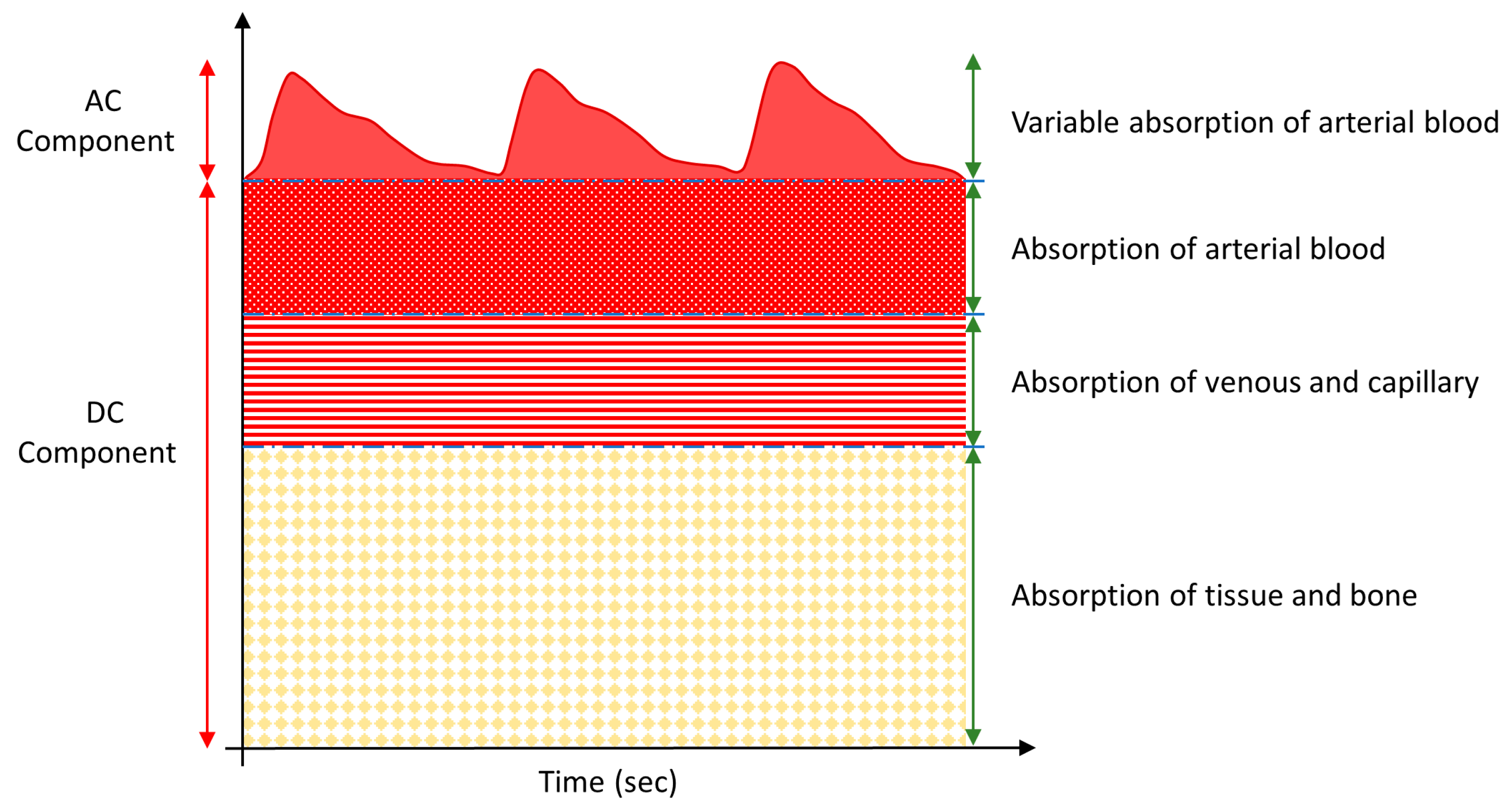
1. Introduction
2. Materials and Methods
2.1. Optical Simulation
2.2. The Organic Optoelectronic Device
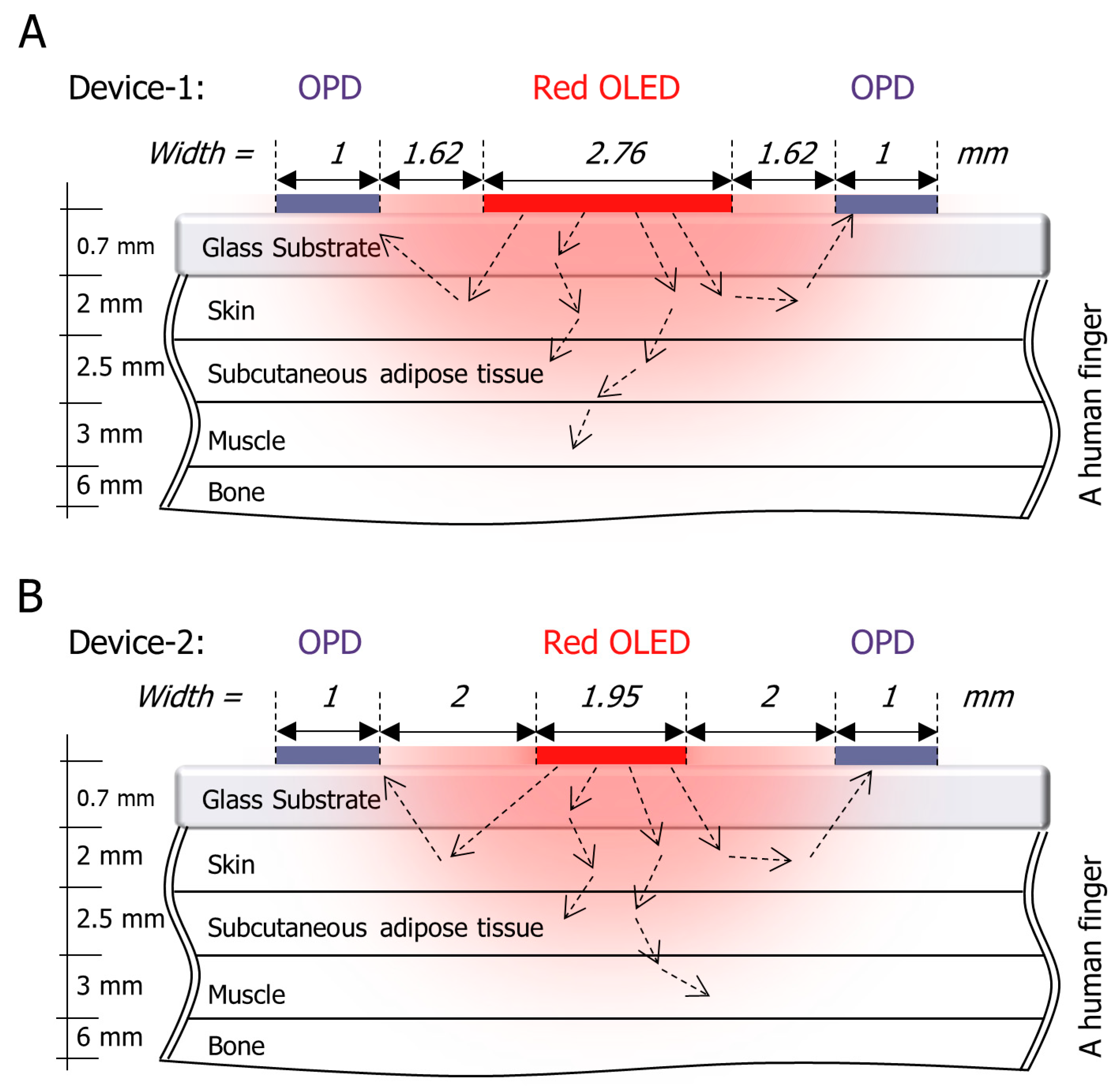
2.3. The Device Structure
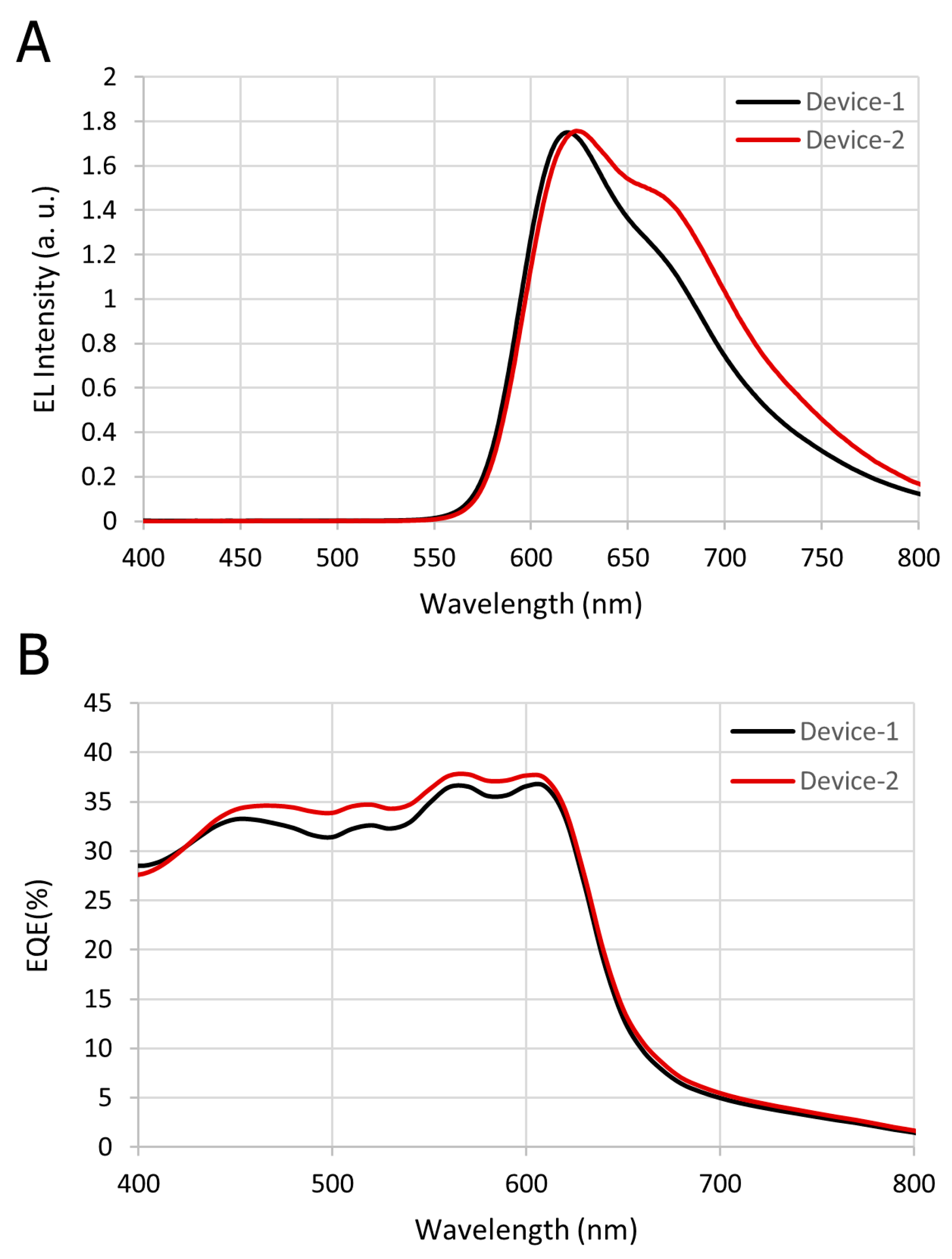
3. Results and Discussion
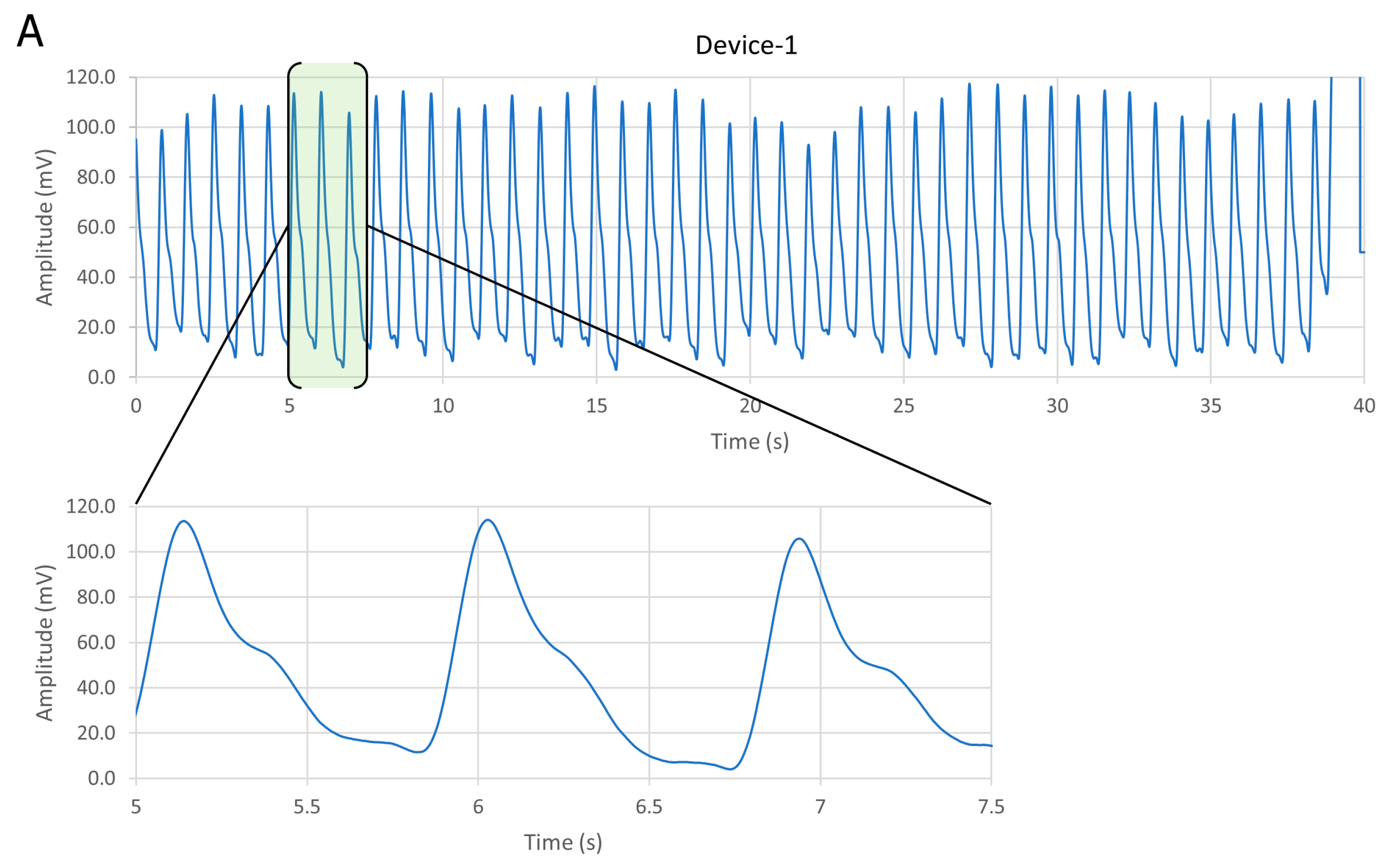
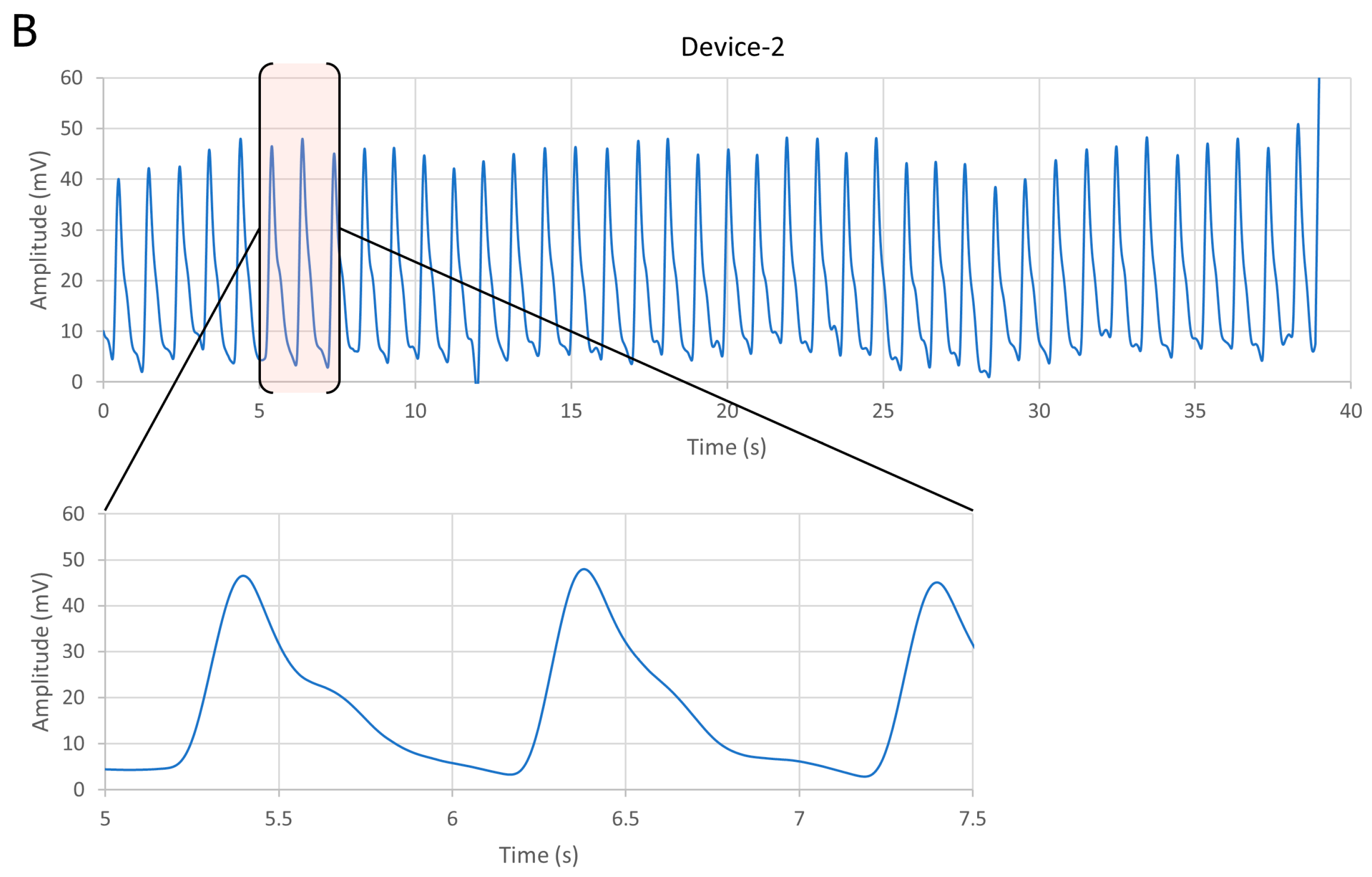
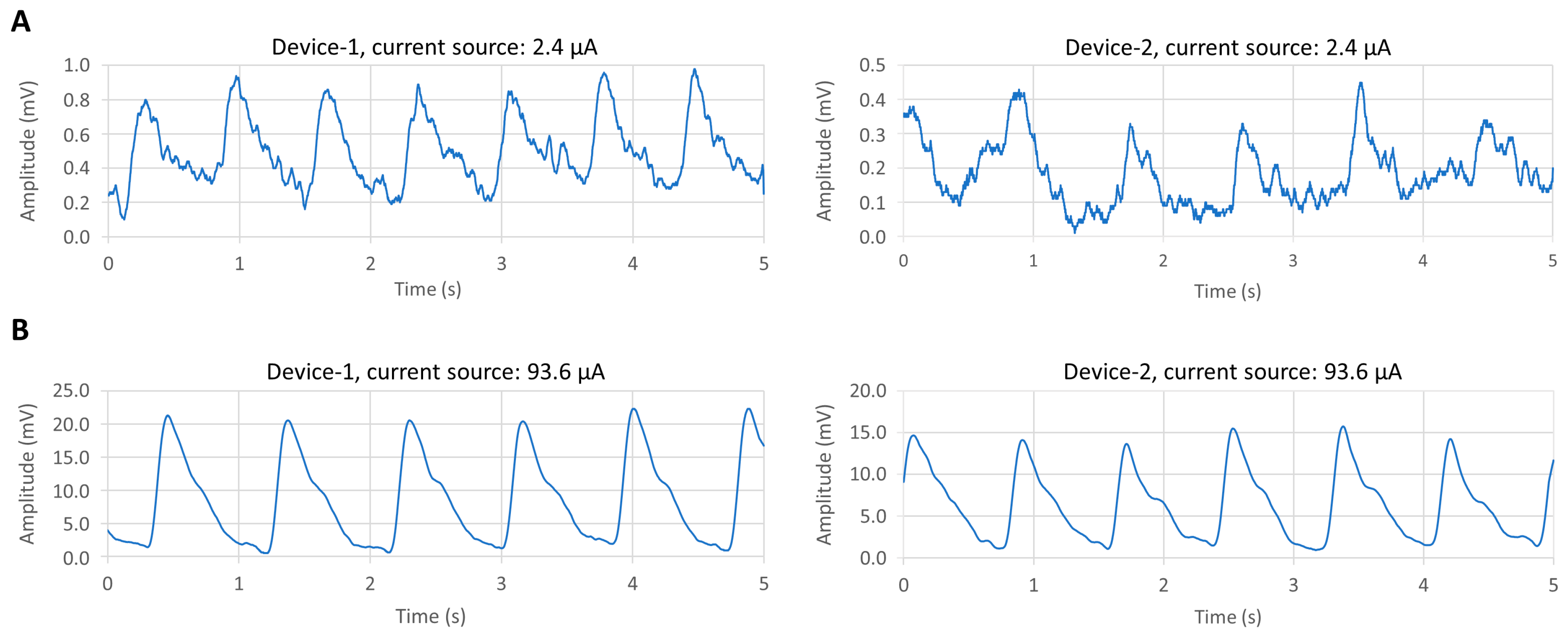
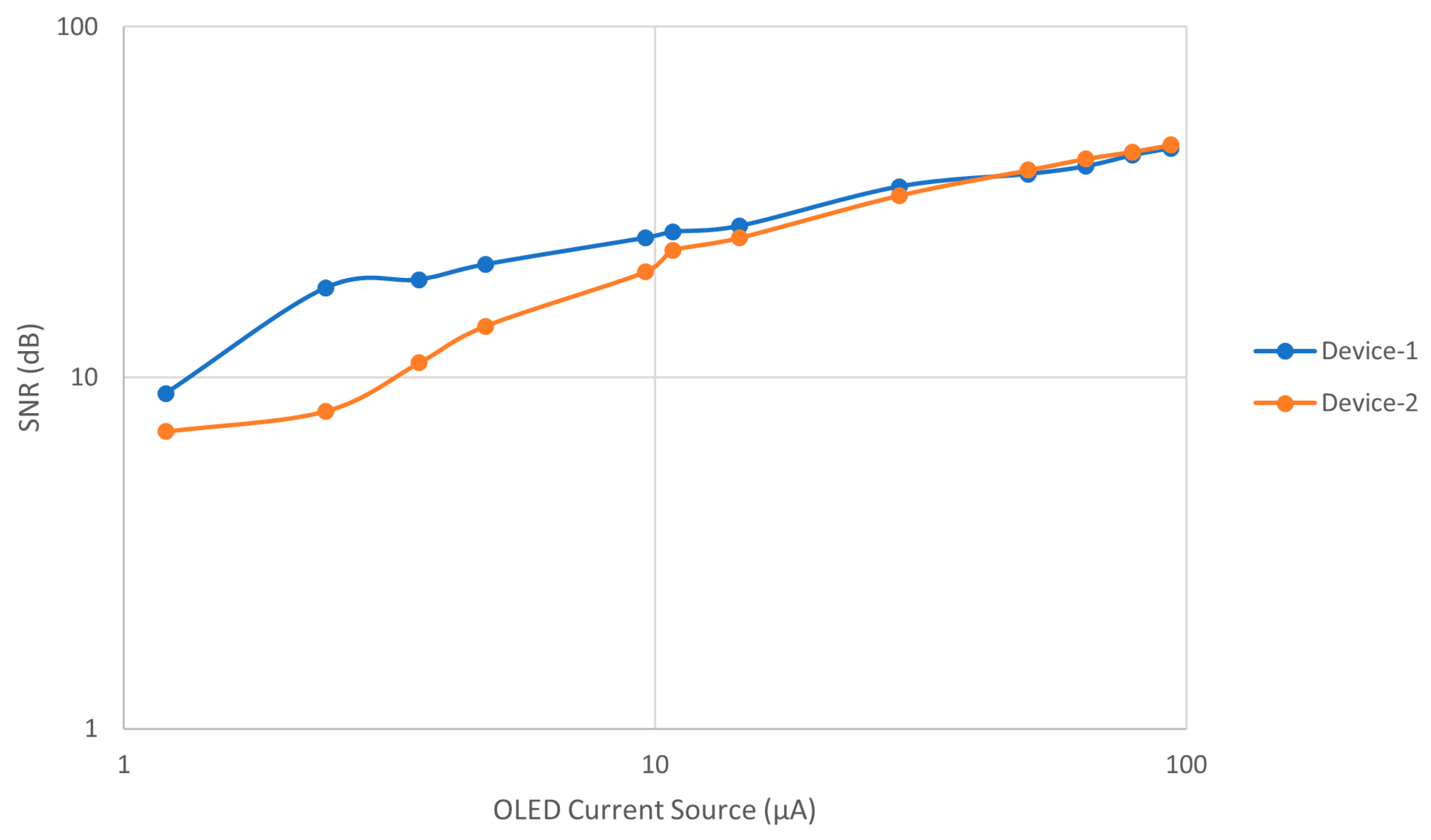
3.1. Comparative Results for Device-1 and Device-2
3.2. Results of BLE PPG Signal from Device-1
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Webster, J.G. Design of Pulse Oximeters; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Jubran, A. Pulse oximetry. In Applied Physiology in Intensive Care Medicine 1, 3rd ed.; Physiological Notes—Technical Notes—Seminal Studies in Intensive Care; Springer-Verlag: Berlin/Heidelberg, Germany, 2012; pp. 51–54. [Google Scholar]
- Guk, K.; Han, G.; Lim, J.; Jeong, K.; Kang, T. Evolution of Wearable Devices with Real-Time Disease Monitoring for Personalized Healthcare. Nanomaterials 2019, 9, 813. [Google Scholar] [CrossRef] [PubMed]
- de Kock, J.P.; Tarassenko, L. Pulse oximetry: theoretical and experimental models. Med. Biol. Eng. Comput. 1993, 31, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.T. Evolution of red organic light-emitting diodes: Materials and devices. Chem. Mater. 2004, 16, 4389–4400. [Google Scholar] [CrossRef]
- Thejo Kalyani, N.; Dhoble, S.J. Organic light emitting diodes: Energy saving lighting technology–A review. Renew. Sustain. Energy Rev. 2012, 16, 2696–2723. [Google Scholar] [CrossRef]
- Geffroy, B.; le Roy, P.; Prat, C. Organic light-emitting diode (OLED) technology: Materials, devices and display technologies. Polym. Int. 2006, 55, 572–582. [Google Scholar] [CrossRef]
- Mendelson, Y.; Pujary, C. Measurement site and photodetector size considerations in optimizing power consumption of a wearable reflectance pulse oximeter. In Proceedings of the 25th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Cancun, Mexico, 17–21 September 2003; pp. 3016–3019. [Google Scholar]
- Lochner, C.M.; Khan, Y.; Pierre, A.; Arias, A.C. All-organic optoelectronic sensor for pulse oximetry. Nat. Commun. 2014, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, Y.; Kaltenbrunner, M.; Koizumi, M.; Matsuhisa, N.; Yukita, W.; Zalar, P.; Jinno, H.; Someya, T.; Kitanosako, H.; Yokota, T. Ultraflexible organic photonic skin. Sci. Adv. 2016, 2, e1501856. [Google Scholar]
- Kim, H.; Lee, H.; Yoo, S.; Kim, M.; Kim, E.; Lee, J.; Yoo, H.-J.; Lee, Y. Toward all-day wearable health monitoring: An ultralow-power, reflective organic pulse oximetry sensing patch. Sci. Adv. 2018, 4, eaas9530. [Google Scholar]
- Li, K.; Warren, S. A wireless reflectance pulse oximeter with digital baseline control for unfiltered photoplethysmograms. IEEE Trans. Biomed. Circuits Syst. 2012, 6, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Ayance, T.; Trevi, C.G. Wireless heart rate and oxygen saturation monitor Wireless Heart Rate and Oxygen Saturation Monitor. AIP Conf. Proc. 2019, 2090, 1–5. [Google Scholar]
- Huang, C.; Chan, M.; Chen, C.; Lin, B. Novel Wearable and Wireless Ring-Type Pulse Oximeter with Multi-Detectors. Sensors 2014, 14, 17586–17599. [Google Scholar] [CrossRef] [PubMed]
- Spigulis, J.; Erts, R.; Nikiforovs, V.; Kviesis-kipge, E. Wearable wireless photoplethysmography sensors. In Proceedings of the Biophotonics: Photonic Solutions for Better Health Care, Strasbourg, France, 7–11 April 2008; Volume 6991, pp. 1–7. [Google Scholar]
- Ha, M.; Lim, S.; Ko, H. Wearable and flexible sensors for user-interactive health-monitoring devices. J. Mater. Chem. B 2018, 6, 4043–4064. [Google Scholar] [CrossRef]
- Elsamnah, F.; Bilgaiyan, A.; Affiq, M.; Shim, C.-H.; Ishidai, H.; Hattori, R. Comparative Design Study for Power Reduction in Organic Optoelectronic Pulse Meter Sensor. Biosensors 2019, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Bilgaiyan, A.; Sugawara, R.; Elsamnah, F.; Shim, C.; Affiq, M.; Hattori, R. Optimizing performance of reflectance-based organic Photoplethysmogram (PPG) sensor. In Proceedings of the Organic and Hybrid Sensors and Bioelectronics XI, San Diego, CA, USA, 19–23 August 2018; Volume 10738, p. 1073808. [Google Scholar]
- Elsamnah, F.; Hattori, R.; Shim, C.-H.; Bilgaiyan, A.; Sugawara, R.; Affiq, M. Reflectance-based Monolithic Organic Pulsemeter Device for Measuring Photoplethysmogram Signal. In Proceedings of the 2018 IEEE International Instrumentation and Measurement Technology Conference (I2MTC), Houston, TX, USA, 14–17 May 2018; pp. 1–5. [Google Scholar]
- Sommer, J.R.; Farley, R.T.; Graham, K.R.; Yang, Y.; Reynolds, J.R.; Xue, J.; Schanze, K.S. Efficient Near-Infrared Polymer and Organic Light-Emitting Diodes Based on Electrophosphorescence from. ACS Appl. Mater. Interfaces 2009, 1, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Li, C.; Xin, L.; Duan, L.; Qiao, J. High-efficiency and low efficiency roll-off near-infrared fluorescent OLEDs through triplet fusion. Chem. Sci. 2016, 7, 2888–2895. [Google Scholar] [CrossRef] [PubMed]
- Jacques, S.L. Optical properties of biological tissues: a review. Phys. Med. Biol. 2013, 58, R37. [Google Scholar] [CrossRef] [PubMed]
- Zamburlini, M.; Pejović-Milić, A.; Chettle, D.R.; Webber, C.E.; Gyorffy, J. In vivo study of an x-ray fluorescence system to detect bone strontium non-invasively. Phys. Med. Biol. 2007, 52, 2107–2122. [Google Scholar] [CrossRef] [PubMed]
- Akkus, O.; Uzunlulu, M.; Kizilgul, M. Evaluation of Skin and Subcutaneous Adipose Tissue Thickness for Optimal Insulin Injection. J. Diabetes Metab. 2012, 3. [Google Scholar] [CrossRef]
- Drahansky, M.; Kanich, O.; Brezinová, E.; Shinoda, K. Experiments with Optical Properties of Skin on Fingers. Int. J. Opt. Appl. 2016, 6, 37–46. [Google Scholar]
- Luthra, G. PROJECT #024: BLE Throughput—Pushing the Limits. Available online: http://www.cypress.com/blog/100-projects-100-days/project-024-ble-throughput-pushing-limits (accessed on 10 June 2019).


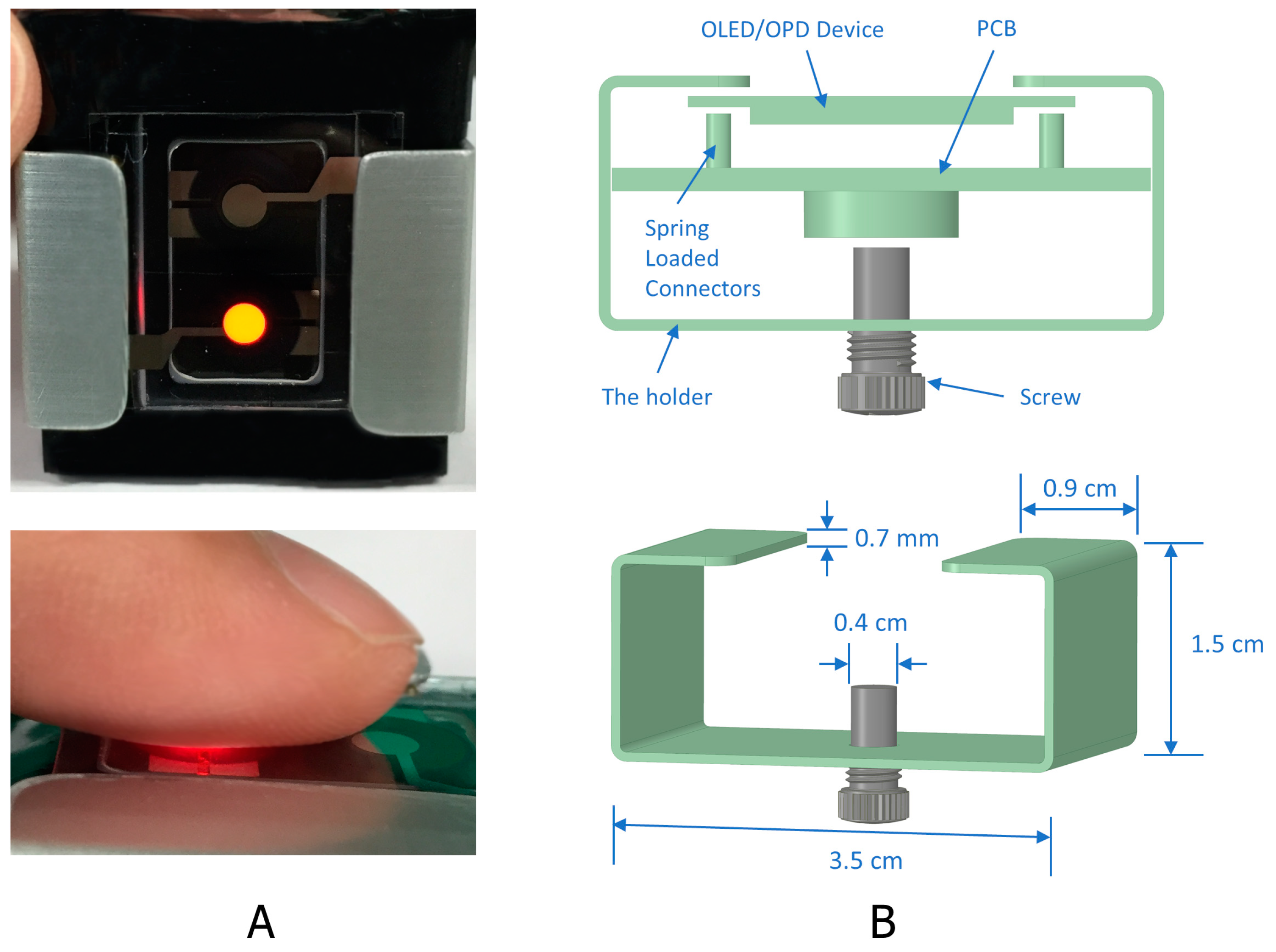

| Tissue | Wave-length (nm) | Index of Refraction (n) | Henyey–Greenstein (g) | Absorption Coefficient (Ua) in mm−1 | Scatter Coefficient (Us) in mm−1 | Thickness (mm) |
|---|---|---|---|---|---|---|
| Human Skin | 625 | 1.55 | 0.81 | 0.27 | 18.7 | 2 |
| Subcutaneous Fat | 625 | 1.44 | 0.9 | 1.14 | 12.8 | 2.5 |
| Muscle | 625 | 1.37 | 0.9 | 0.56 | 64.7 | 3 |
| Bone | 625 | 1.37 | 0.9 | 0.04 | 19.5 | 6 |
| Device No. | Average Vpp (mV) | SNR (dB) | Current Source (μA) |
|---|---|---|---|
| Device-1 | 20 | 45 | 93.6 |
| Device-2 | 13 | 46 | 93.6 |
| Device-1 | 0.7 | 18 | 2.4 |
| Device-2 | 0.3 | 8 | 2.4 |
| This Work | Reference [11] | Reference [17] | Reference [10] | Reference [9] | |
|---|---|---|---|---|---|
| OLED Type | Red OLED | Red OLED | Red OLED | Red PLED | Red OLED |
| Device Flexibility | Rigid | Flexible | Rigid | Flexible | Rigid |
| Voltage Supply (V) | 3.3 | 3.3 | 5 | 5 | 9 |
| OLED Driving Current (μA) | 2.4 | 21 | 20 | 1000 | 20000 |
| OLED Area (mm2) | 6 | 0.5 | 3 | N.C. | 4 |
| Power Consumption (μW) | 8 | 24 | 100 | N.C. | N.C. |
| PPG Signal-to-Noise Ratio (dB) | 18 | N.C. | 45 | N.C. | N.C. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsamnah, F.; Bilgaiyan, A.; Affiq, M.; Shim, C.-H.; Ishidai, H.; Hattori, R. Reflectance-Based Organic Pulse Meter Sensor for Wireless Monitoring of Photoplethysmogram Signal. Biosensors 2019, 9, 87. https://doi.org/10.3390/bios9030087
Elsamnah F, Bilgaiyan A, Affiq M, Shim C-H, Ishidai H, Hattori R. Reflectance-Based Organic Pulse Meter Sensor for Wireless Monitoring of Photoplethysmogram Signal. Biosensors. 2019; 9(3):87. https://doi.org/10.3390/bios9030087
Chicago/Turabian StyleElsamnah, Fahed, Anubha Bilgaiyan, Muhamad Affiq, Chang-Hoon Shim, Hiroshi Ishidai, and Reiji Hattori. 2019. "Reflectance-Based Organic Pulse Meter Sensor for Wireless Monitoring of Photoplethysmogram Signal" Biosensors 9, no. 3: 87. https://doi.org/10.3390/bios9030087
APA StyleElsamnah, F., Bilgaiyan, A., Affiq, M., Shim, C.-H., Ishidai, H., & Hattori, R. (2019). Reflectance-Based Organic Pulse Meter Sensor for Wireless Monitoring of Photoplethysmogram Signal. Biosensors, 9(3), 87. https://doi.org/10.3390/bios9030087






