Preparation and Characterization of a Pectin Membrane-Based Optical pH Sensor for Fish Freshness Monitoring
Abstract
1. Introduction
2. Experimental
2.1. Chemicals and Instruments
2.2. Preparation of Reagents and Solutions
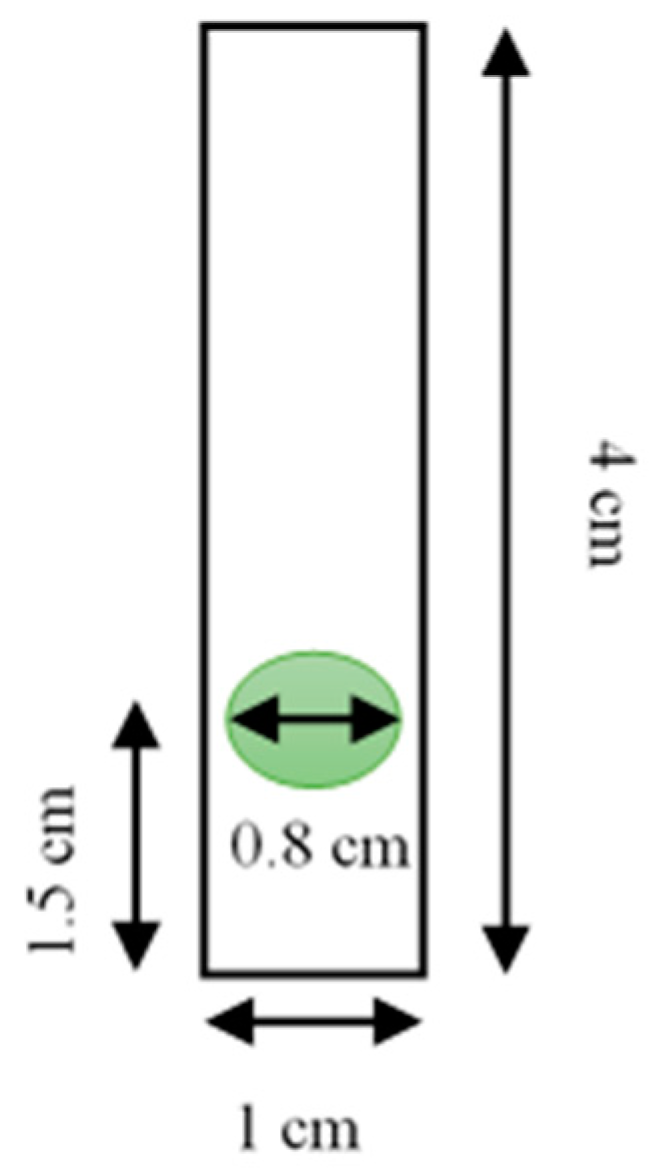
2.3. Preparation of the Pe/CI Optical pH Sensor
2.4. Response Optimization of the Pe/CI Optical pH Sensor
2.5. Determination of Fish Freshness
3. Results and Discussion
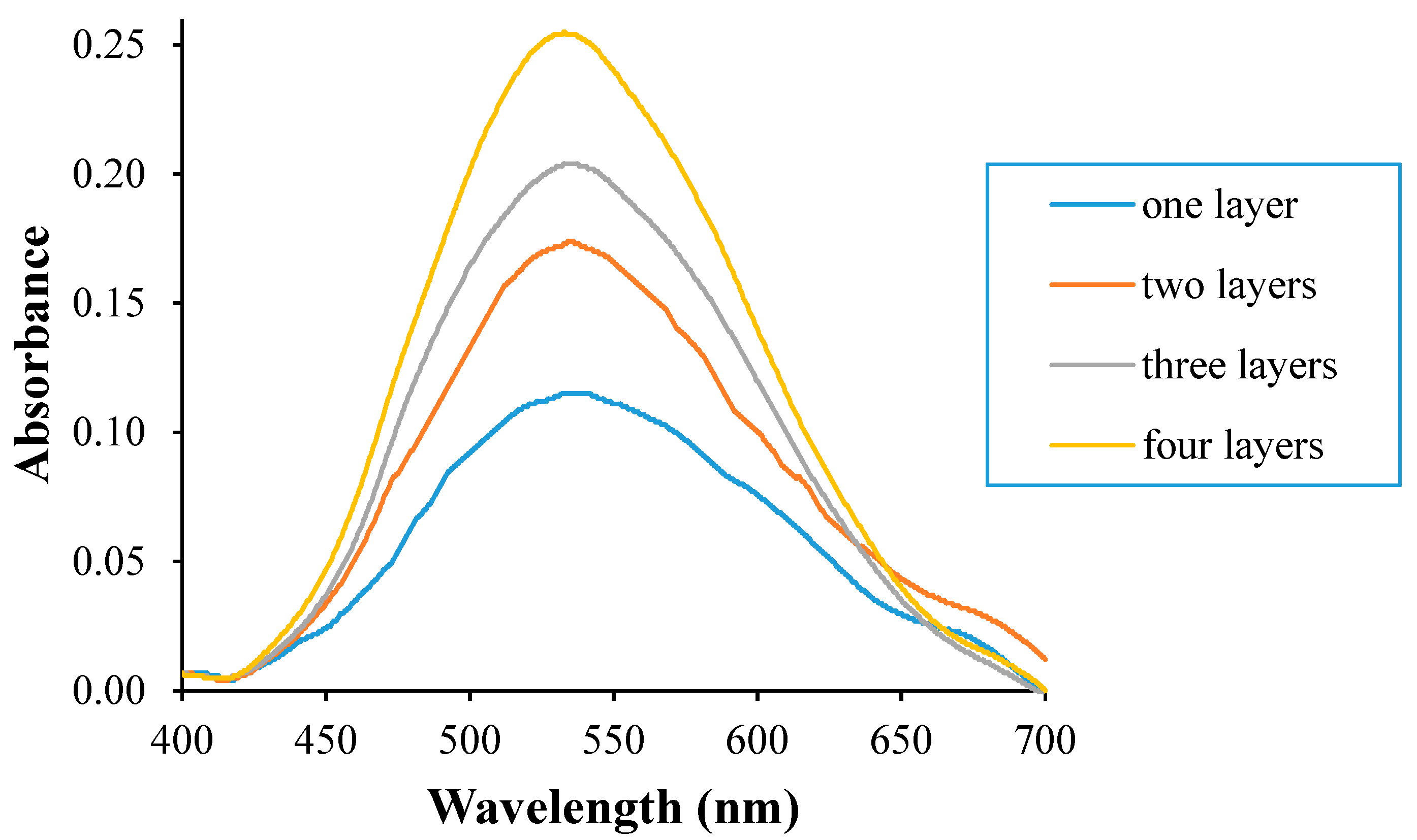
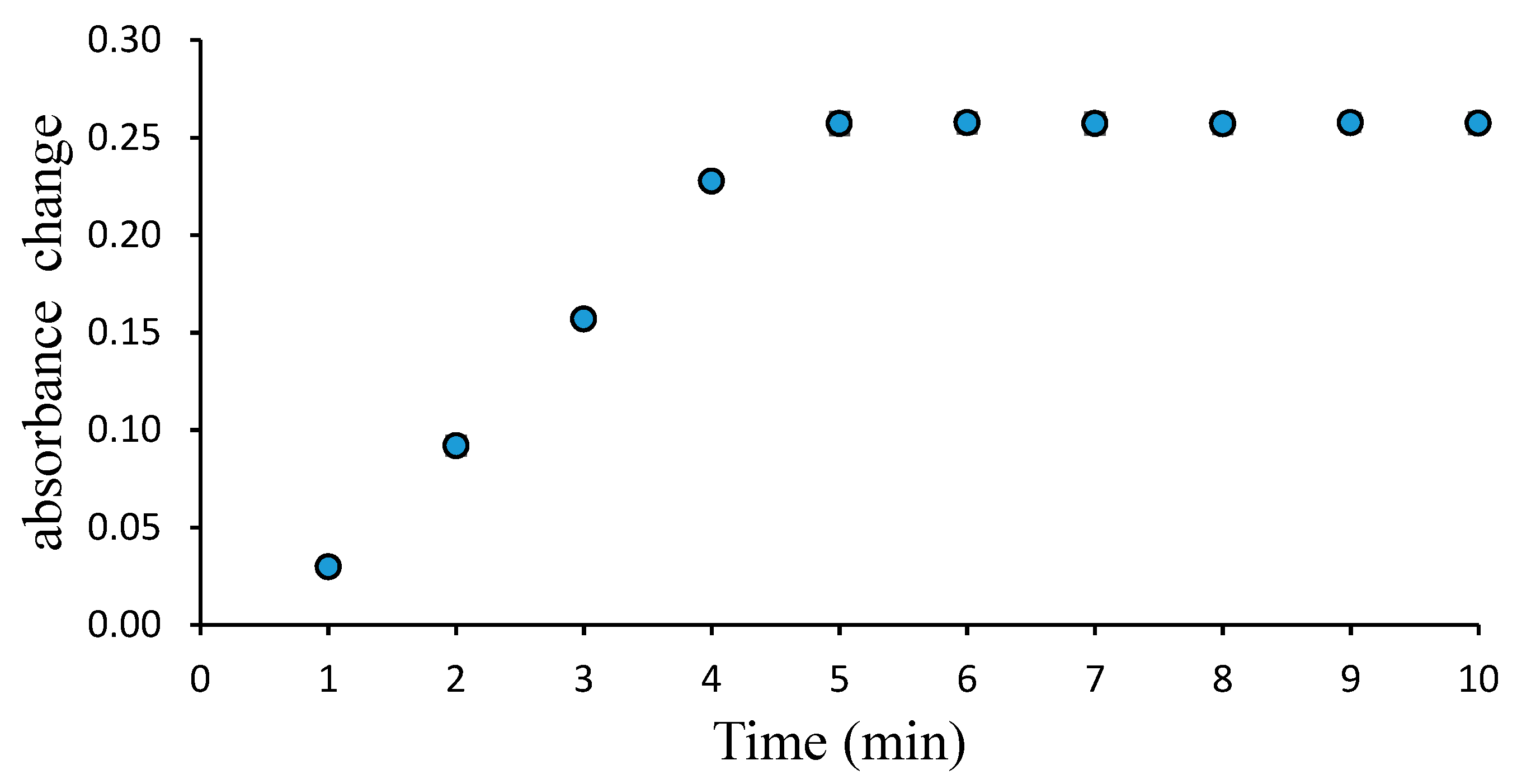
3.1. Absorbance Response of the Pe/CI Optical pH Sensor
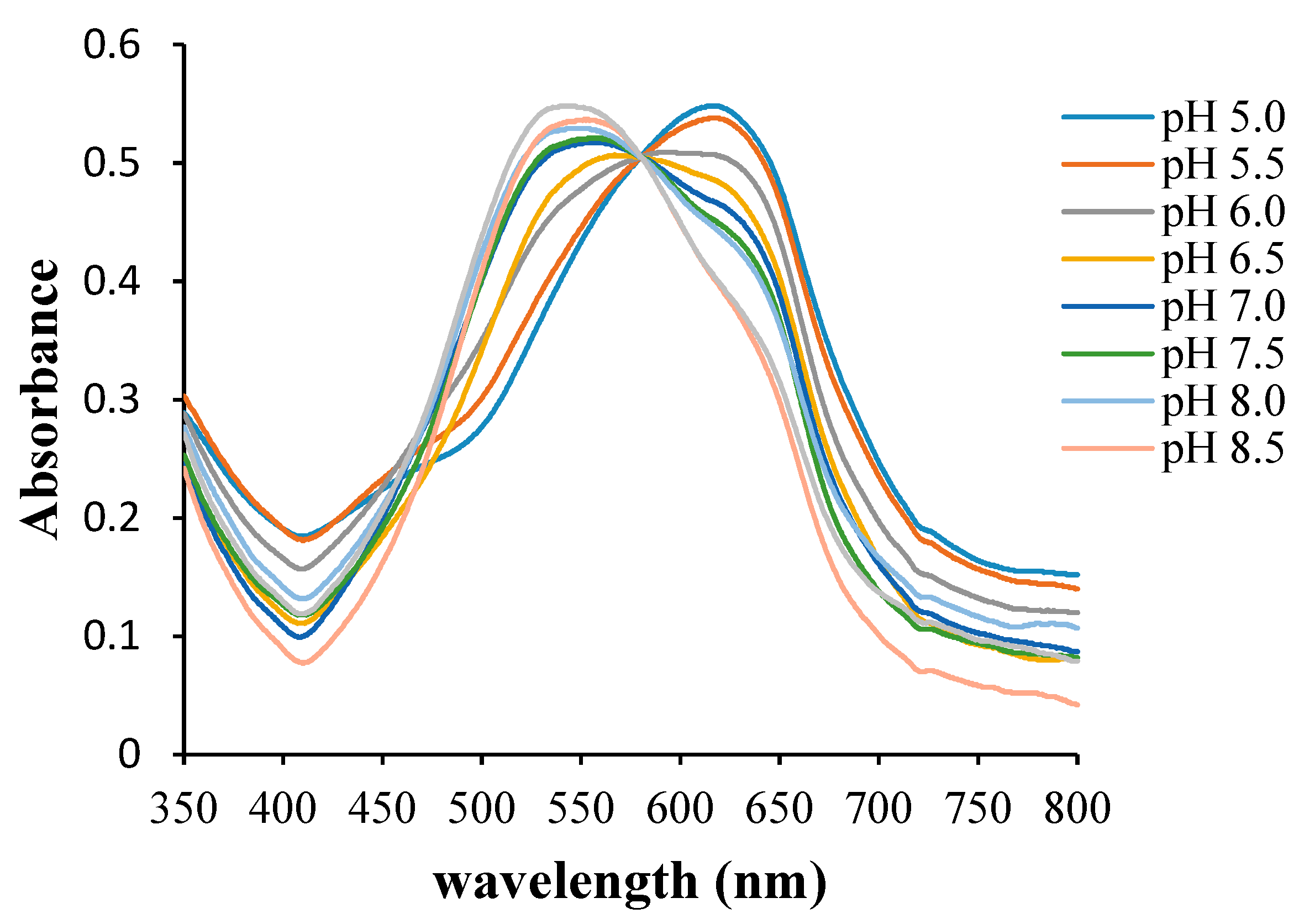
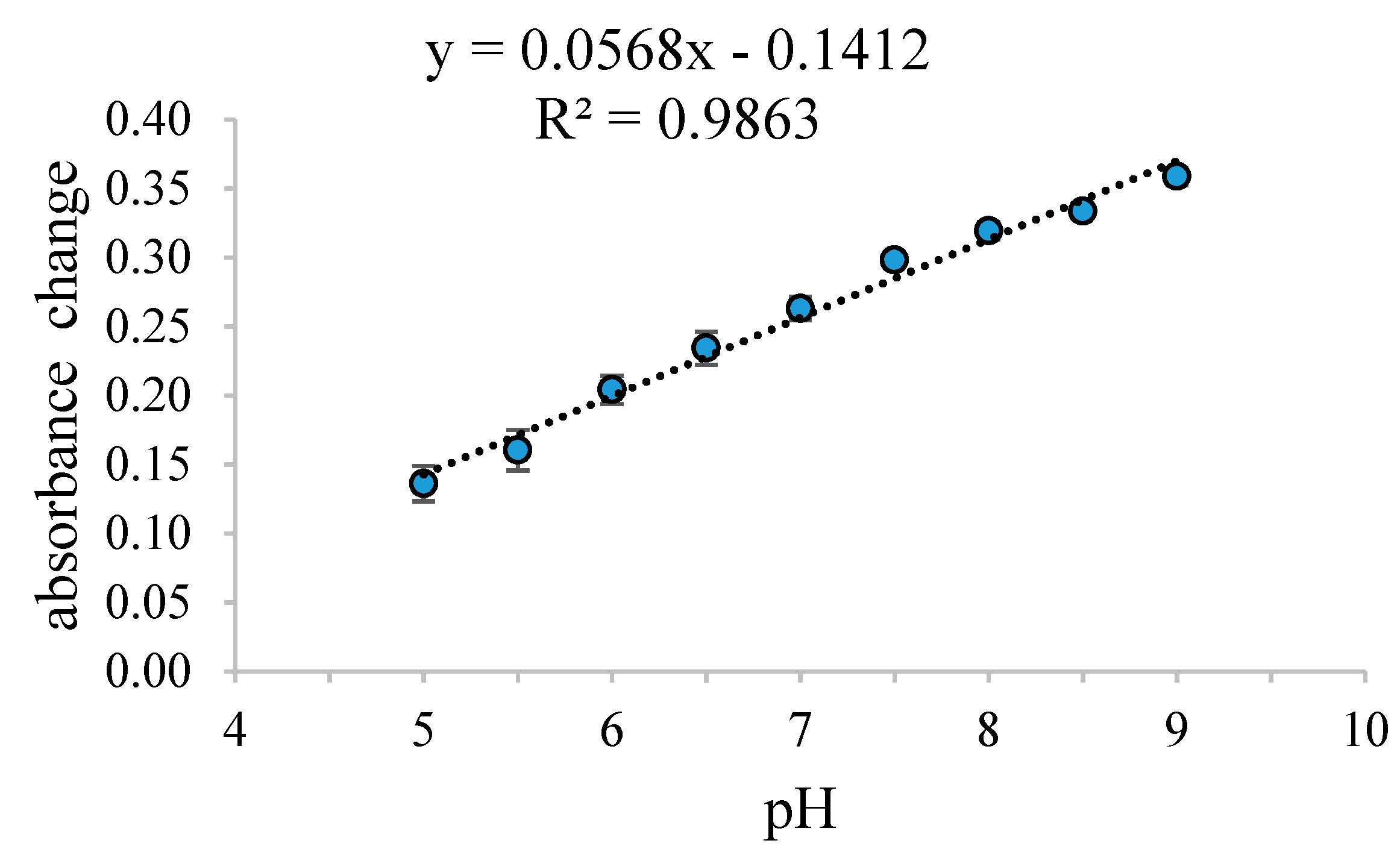
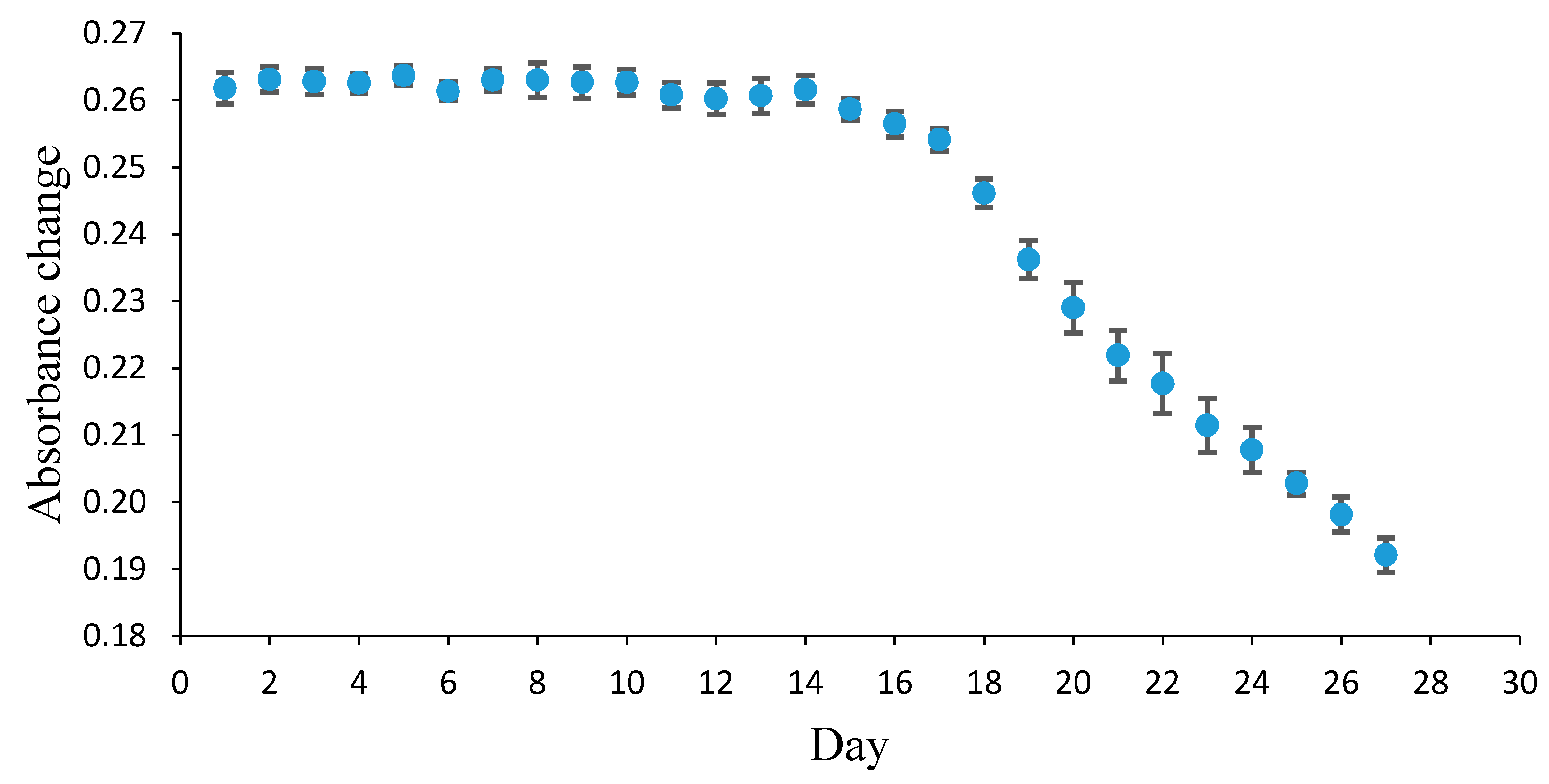
3.2. Effect of pH on the Response Time, Reproducibility, and Lifetime of the Pe/CI Optical pH Sensor
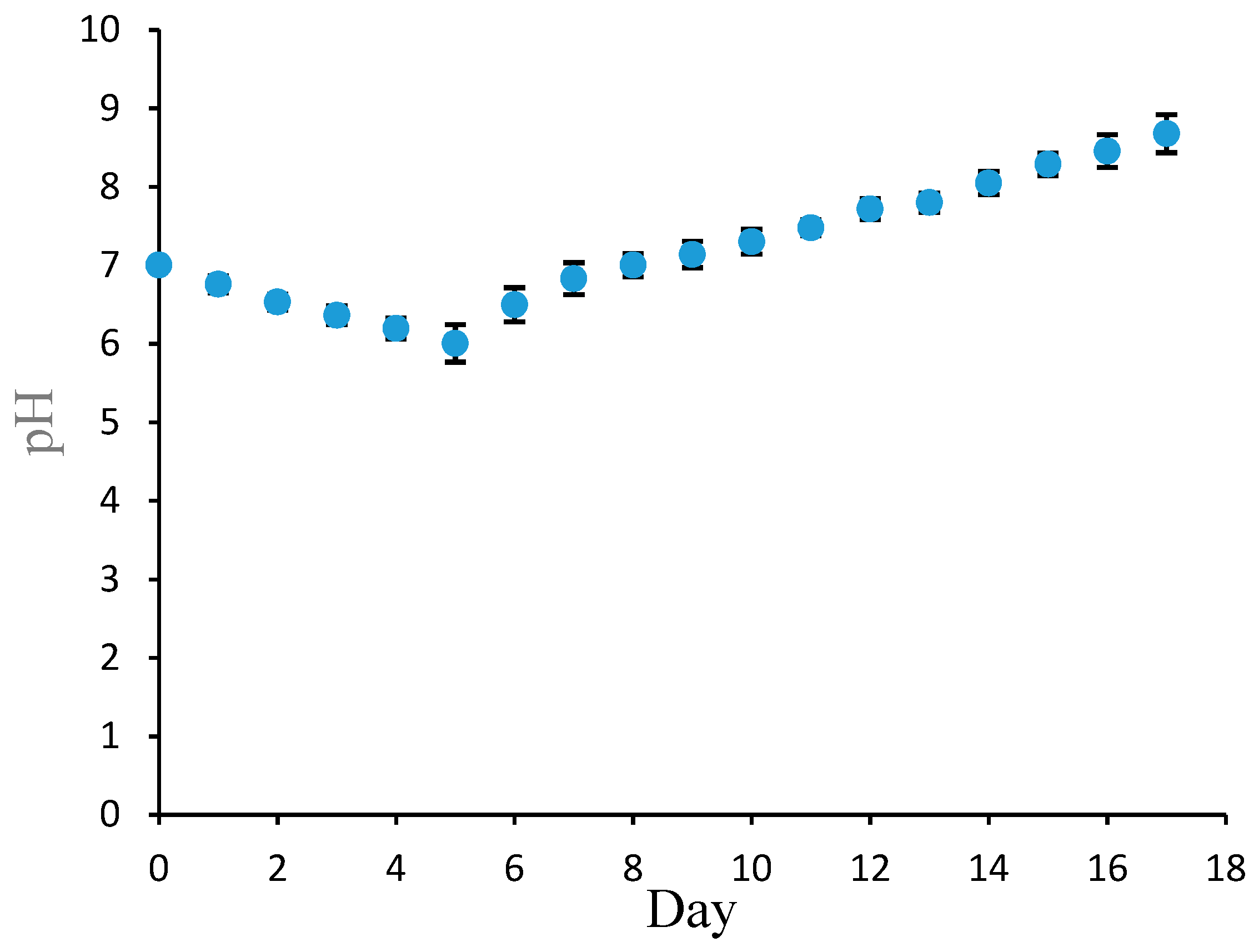
3.3. The Determination of Fish Freshness Using the Pe/CI Optical pH Sensor
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ashie, I.N.A.; Smith, J.P.; Simpson, B.K.; Haard, N.F. Spoilage and shelf-life extension of fresh fish and shellfish. Crit. Rev. Food Sci. Nutr. 1996, 36, 87–121. [Google Scholar]
- Fernandes, P. Enzymes in Fish and Seafood Processing. Front. Bioeng. Biotechnol. 2016, 4, 1–14. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, G.; Xiao, X.; Liu, Y.; Zheng, X. Application of microbial TTIs as smart label for food quality: Response mechanism, application and research trends. Trends Food Sci. Technol. 2016, 51, 12–23. [Google Scholar] [CrossRef]
- Olafsdottir, G.; Nesvadba, P.; Di Natale, C.; Careche, M.; Oehlenschläger, J.; Tryggvadóttir, S.V.; Schubring, R.; Kroeger, M.; Heia, K.; Esaiassen, M.; et al. Multisensor for fish quality determination. Trends Food Sci. Technol. 2004, 15, 86–93. [Google Scholar] [CrossRef]
- Byrne, L.; Lau, K.T.; Diamond, D. Monitoring of headspace total volatile basic nitrogen from selected fish species using reflectance spectroscopic measurements of pH sensitive films. Analyst 2002, 127, 1338–1341. [Google Scholar] [CrossRef]
- Park, J.S.; Lee, C.H.; Kwon, E.Y.; Lee, H.J.; Kim, J.Y.; Kim, S.H. Monitoring the contents of biogenic amines in fish and fish products consumed in Korea. Food Control 2010, 21, 1219–1226. [Google Scholar] [CrossRef]
- Pacquit, A.; Lau, K.; Mclaughlin, H.; Frisby, J.; Quilty, B.; Diamond, D. Development of a volatile amine sensor for the monitoring of fish spoilage. Talanta 2006, 69, 515–520. [Google Scholar] [CrossRef]
- Chan, S.T.; Yao, M.W.Y.; Wong, Y.C.; Wong, T.; Mok, C.S.; Sin, D.W.M. Evaluation of chemical indicators for monitoring freshness of food and determination of volatile amines in fish by headspace solid-phase microextraction and gas chromatography-mass spectrometry. Eur. Food Res. Technol. 2006, 224, 67–74. [Google Scholar] [CrossRef]
- Kuswandi, B.; Jayus; Restyana, A.; Abdullah, A.; Heng, L.Y.; Ahmad, M. A novel colorimetric food package label for fish spoilage based on polyaniline film. Food Control 2012, 25, 184–189. [Google Scholar] [CrossRef]
- Chun, H.; Kim, B.; Shin, H. Evaluation of a freshness indicator for quality of fish products during storage. Food Sci. Biotechnol. 2014, 23, 1719–1725. [Google Scholar] [CrossRef]
- Deng, S.; Doherty, W.; McAuliffe, M.A.; Salaj-Kosla, U.; Lewis, L.; Huyet, G. A low-cost, portable optical sensing system with wireless communication compatible of real-time and remote detection of dissolved ammonia. Photonic Sens. 2016, 6, 107–114. [Google Scholar] [CrossRef]
- Omar, A.F.; Matjafri, M.Z. Specialized optical fiber sensor for nondestructive intrinsic quality measurement of Averrhoa Carambola. Photonic Sens. 2013, 3, 272–282. [Google Scholar] [CrossRef]
- Hedwig, R.; Lahna, K.; Idroes, R.; Karnadi, I.; Tanra, I.; Iqbal, J.; Kwaria, D.; Kurniawan, D.P.; Kurniawan, K.H.; Tjia, M.O.; et al. Food analysis employing high energy nanosecond laser and low pressure He ambient gas. Microchem. J. 2019, 147, 356–364. [Google Scholar] [CrossRef]
- Devasenathipathy, R.; Mani, V.; Chen, S.; Arulraj, D.; Vasantha, V.S. Highly stable and sensitive amperometric sensor for the determination of trace level hydrazine at cross linked pectin stabilized gold nanoparticles decorated graphene nanosheets. Electrochim. Acta 2014, 135, 260–269. [Google Scholar] [CrossRef]
- Bakker, E.; Lerchi, M.; Rosatzin, T.; Rusterholz, B.; Simon, W. Synthesis and characterization of neutral hydrogen ion-selective chromoionophores for use in bulk optodes. Anal. Chim. Acta 1993, 278, 211–225. [Google Scholar] [CrossRef]
- Wong, F.; Ahmad, M.; Heng, L.; Peng, L. An optical biosensor for dichlovos using stacked sol–gel films containing acetylcholinesterase and a lipophilic chromoionophore. Talanta 2006, 69, 888–893. [Google Scholar] [CrossRef]
- Liang, X.; Chen, S.; Huang, H.; Liu, W. Study on sensitivity improving of fiber Bragg grating based pH sensor. Photonic Sens. 2014, 4, 28–33. [Google Scholar] [CrossRef]
- Spichiger, U.; Simon, W.; Bakker, E.; Lerchi, M.; Bühlmann, P.; Haug, J.; Kuratli, M.; Ozawa, S.; West, S. Optical sensors based on neutral carriers. Sens. Actuators B Chem. 1993, 11, 1–8. [Google Scholar] [CrossRef]
- Bakker, E.; Simon, W. Selectivity of ion-sensitive bulk optodes. Anal. Chem. 1992, 64, 1805–1812. [Google Scholar] [CrossRef]
- Ozawa, S.; Hauser, P.C.; Seiler, K.; Tan, S.S.S.; Morf, W.E.; Simon, W. Ammonia-gas-selective optical sensors based on neutral ionophores. Anal. Chem. 1991, 63, 640–644. [Google Scholar] [CrossRef]
- Heng, L.; Fang, T.; Chern, L.; Ahmad, M. Influence of Methacrylic-Acrylic Copolymer Composition on Plasticiser-free Optode Films for pH Sensors. Sensors 2003, 3, 83–90. [Google Scholar] [CrossRef]
- Long, R.; Bakker, E. Optical determination of ionophore diffusion coefficients in plasticized poly (vinyl chloride) sensing films. Anal. Chim. Acta 2004, 511, 91–95. [Google Scholar] [CrossRef]
- Eskin, N.A.; Shahidi, F. Biochemistry of Foods; Academic Press, Inc.: San Diego, CA, USA, 2012; ISBN 9780080918099. [Google Scholar]
- Mountney, G.J.; Gould, W.A. Practical Food Macrobiology and Technology; Van Nostrand Reinhold, Co.: New York, NY, USA, 1988. [Google Scholar]
- Wheaton, F.W.; Lawson, T.B. Processing Aquatic Food Product; John Wiley and Sons, Inc.: Hoboken, NY, USA, 1985. [Google Scholar]
| Number of Sensors | Absorbance before Adding Buffer Solution | Absorbance after Adding PBS pH 7 | Absorbance Change |
|---|---|---|---|
| 1 | 0.206 | 0.446 | 0.240 |
| 2 | 0.204 | 0.441 | 0.237 |
| 3 | 0.208 | 0.454 | 0.246 |
| 4 | 0.205 | 0.451 | 0.246 |
| 5 | 0.204 | 0.447 | 0.243 |
| 6 | 0.205 | 0.451 | 0.246 |
| 7 | 0.203 | 0.448 | 0.245 |
| 8 | 0.204 | 0.444 | 0.240 |
| 9 | 0.201 | 0.45 | 0.249 |
| 10 | 0.202 | 0.447 | 0.245 |
| Average | 0.2437 | ||
| STDV | 0.003653 | ||
| RSD (%) | 1.498976 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasanah, U.; Setyowati, M.; Efendi, R.; Muslem, M.; Md Sani, N.D.; Safitri, E.; Yook Heng, L.; Idroes, R. Preparation and Characterization of a Pectin Membrane-Based Optical pH Sensor for Fish Freshness Monitoring. Biosensors 2019, 9, 60. https://doi.org/10.3390/bios9020060
Hasanah U, Setyowati M, Efendi R, Muslem M, Md Sani ND, Safitri E, Yook Heng L, Idroes R. Preparation and Characterization of a Pectin Membrane-Based Optical pH Sensor for Fish Freshness Monitoring. Biosensors. 2019; 9(2):60. https://doi.org/10.3390/bios9020060
Chicago/Turabian StyleHasanah, Uswatun, Mita Setyowati, Rustam Efendi, Muslem Muslem, Nor Diyana Md Sani, Eka Safitri, Lee Yook Heng, and Rinaldi Idroes. 2019. "Preparation and Characterization of a Pectin Membrane-Based Optical pH Sensor for Fish Freshness Monitoring" Biosensors 9, no. 2: 60. https://doi.org/10.3390/bios9020060
APA StyleHasanah, U., Setyowati, M., Efendi, R., Muslem, M., Md Sani, N. D., Safitri, E., Yook Heng, L., & Idroes, R. (2019). Preparation and Characterization of a Pectin Membrane-Based Optical pH Sensor for Fish Freshness Monitoring. Biosensors, 9(2), 60. https://doi.org/10.3390/bios9020060






