Which Gait Parameters and Walking Patterns Show the Significant Differences Between Parkinson’s Disease and Healthy Participants?
Abstract
1. Introduction
2. Materials and Methods
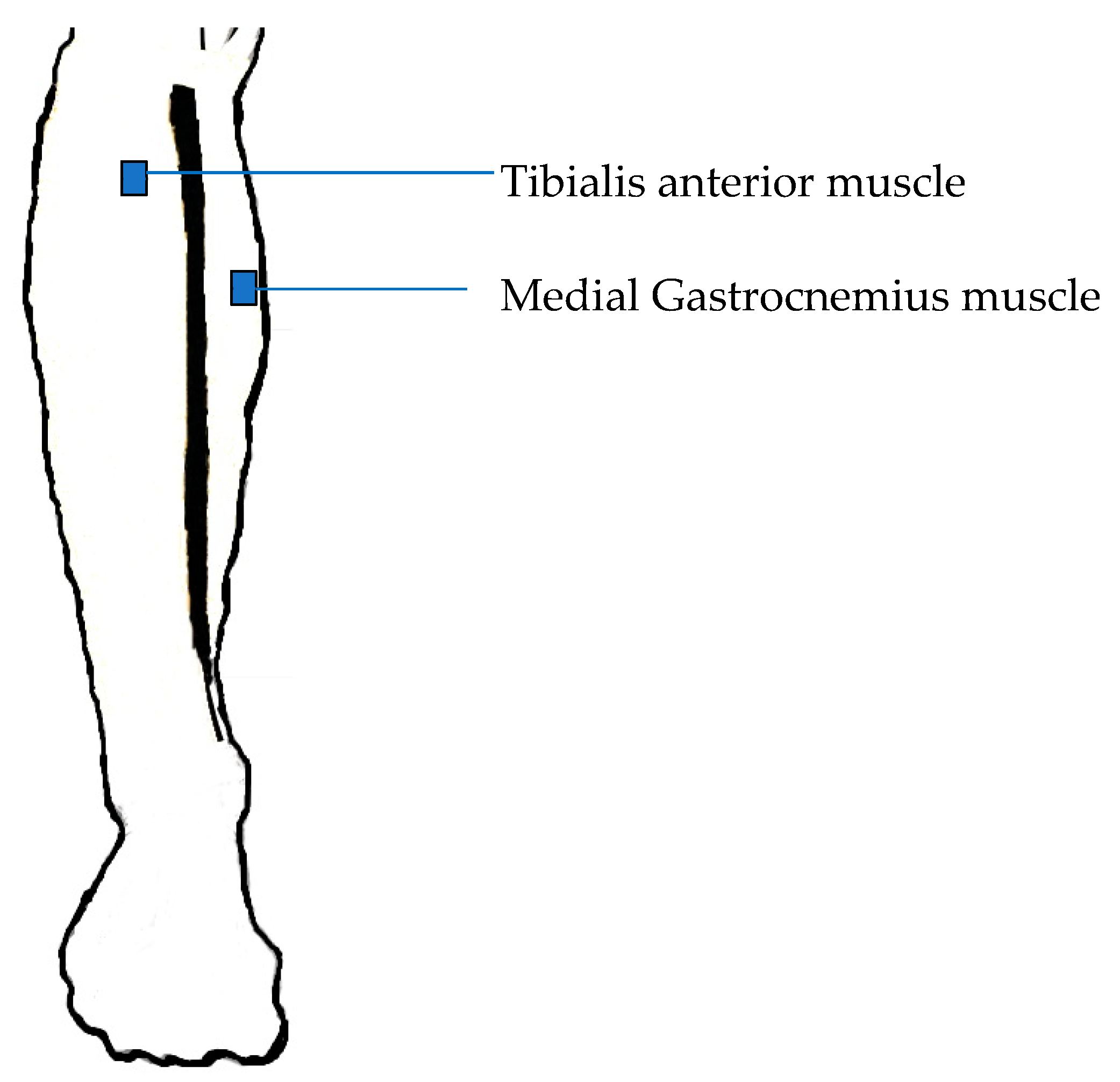
2.1. Data Recording
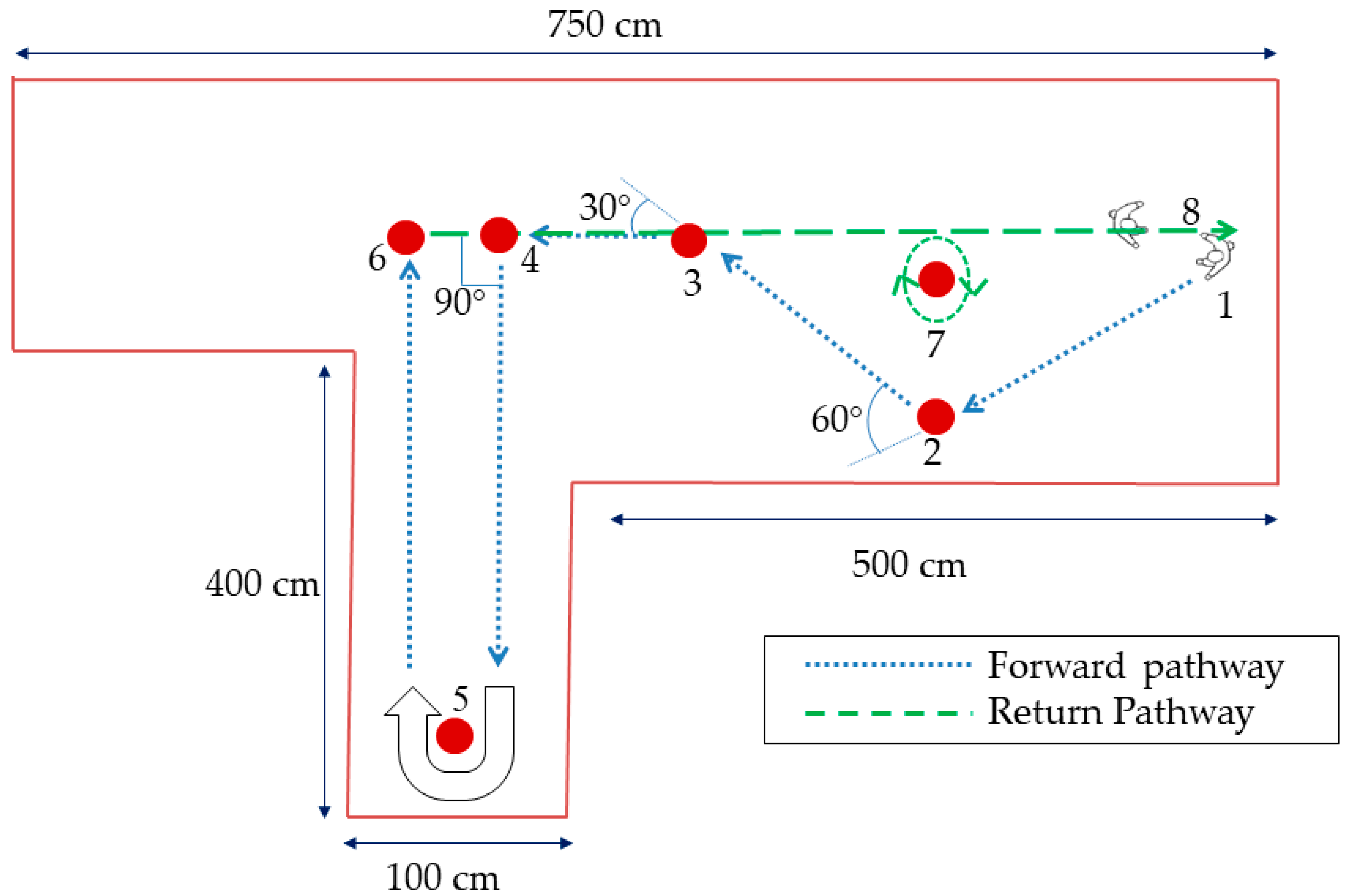
2.2. Experimental Protocol
2.3. Pre-processing of the Signal
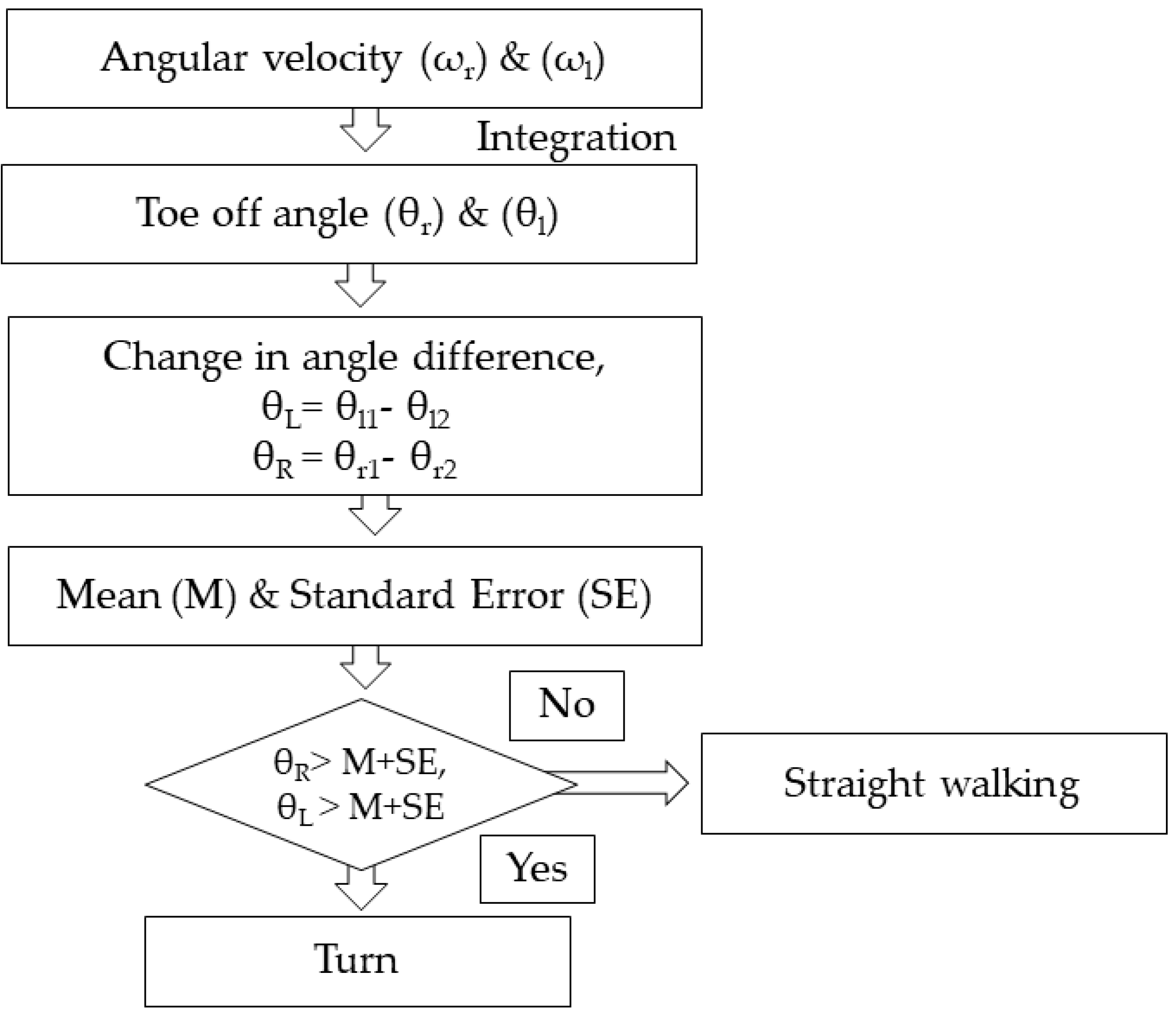
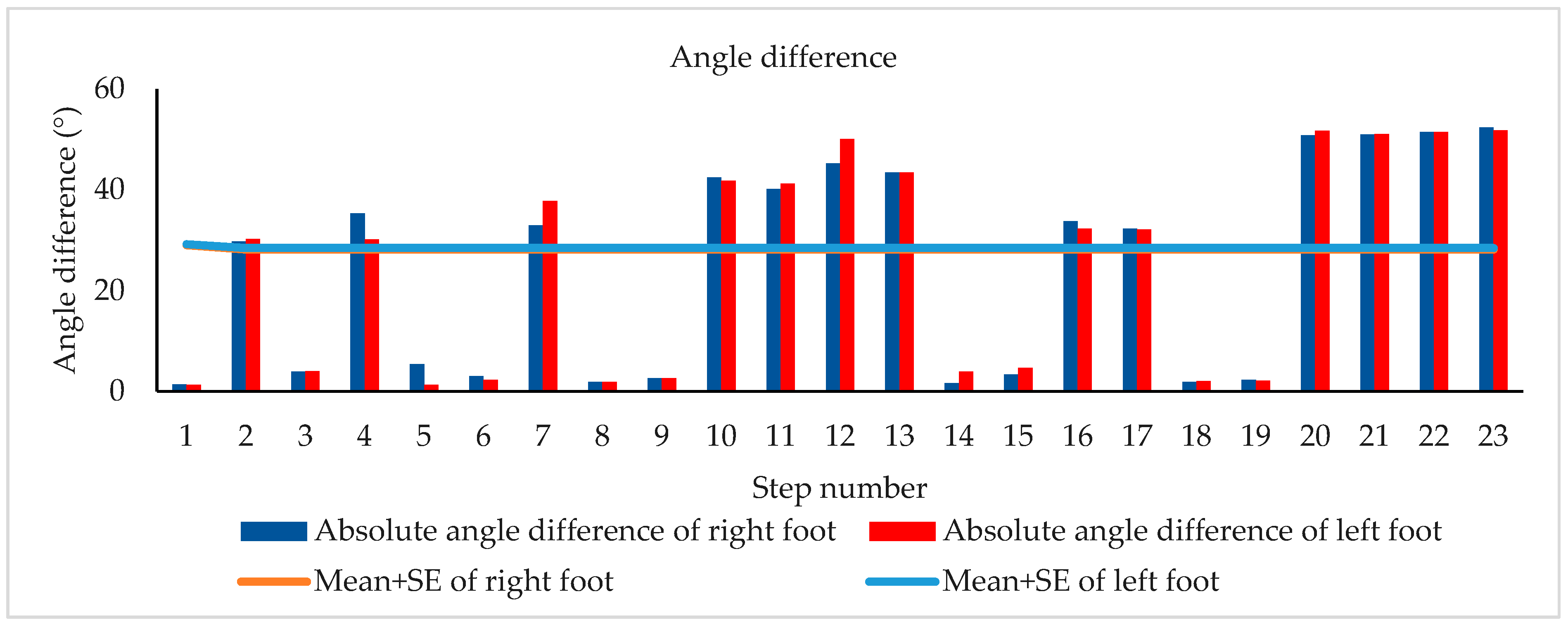
2.4. Turn Identification
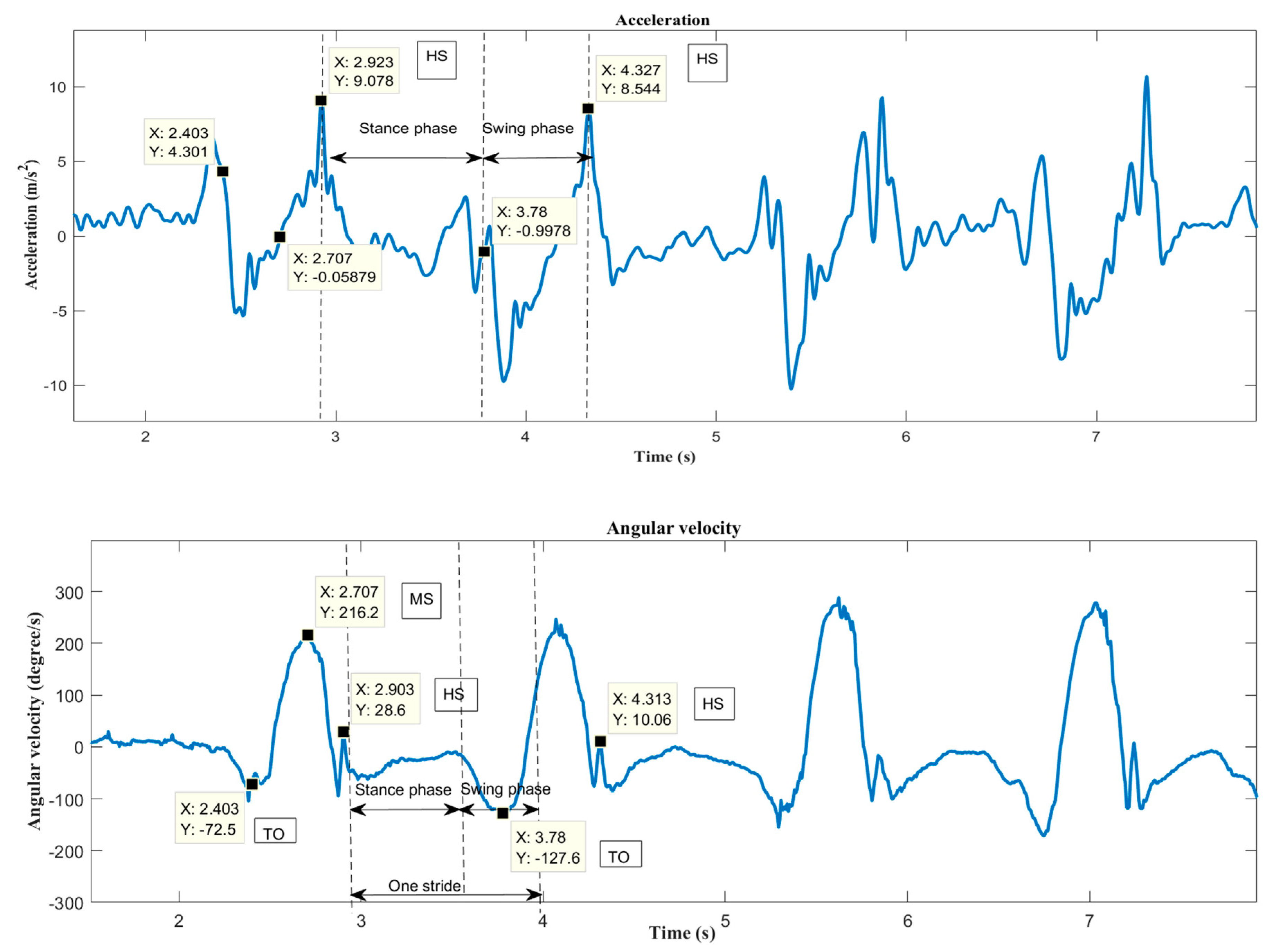
2.5. Gait Phase Identification
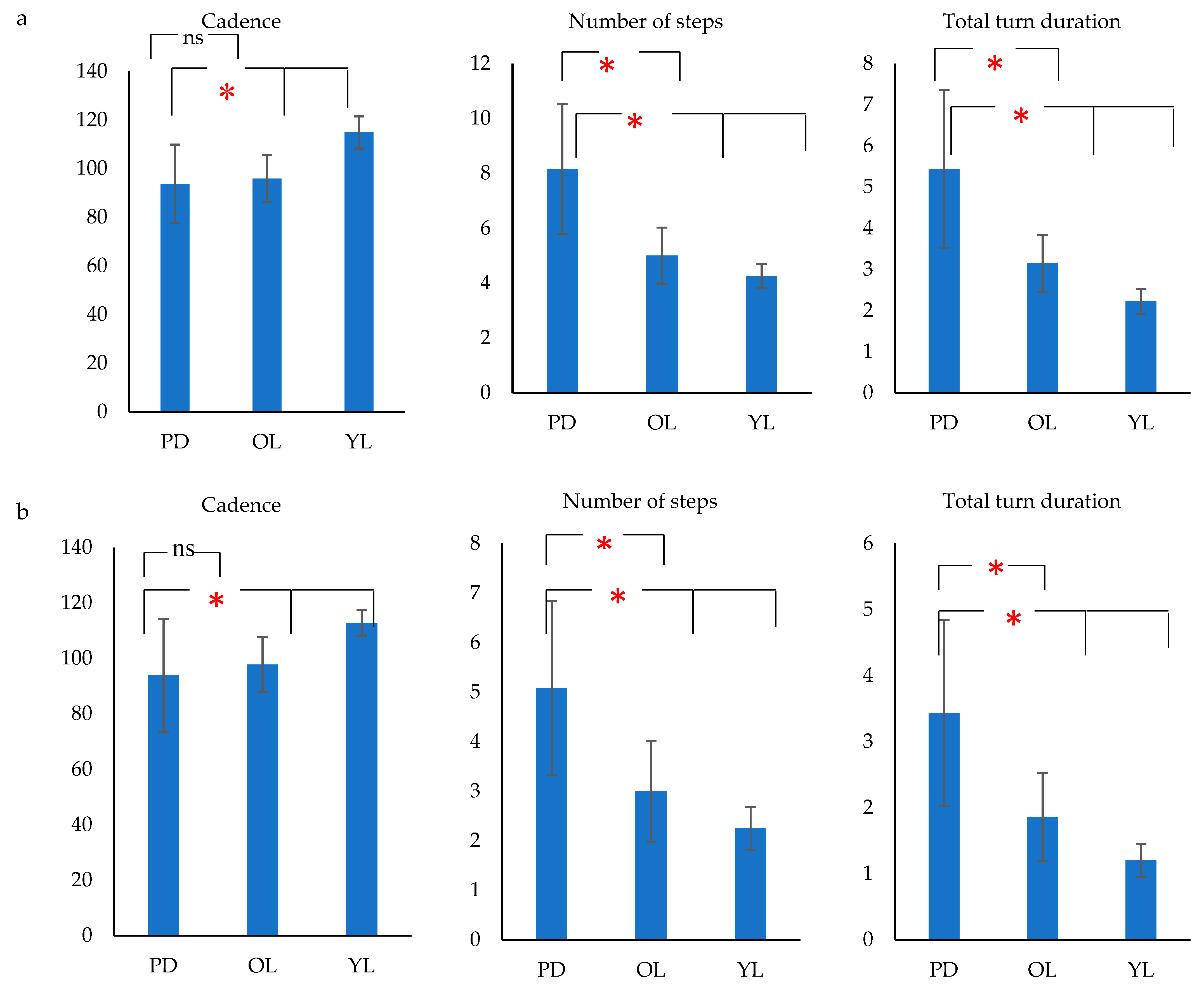
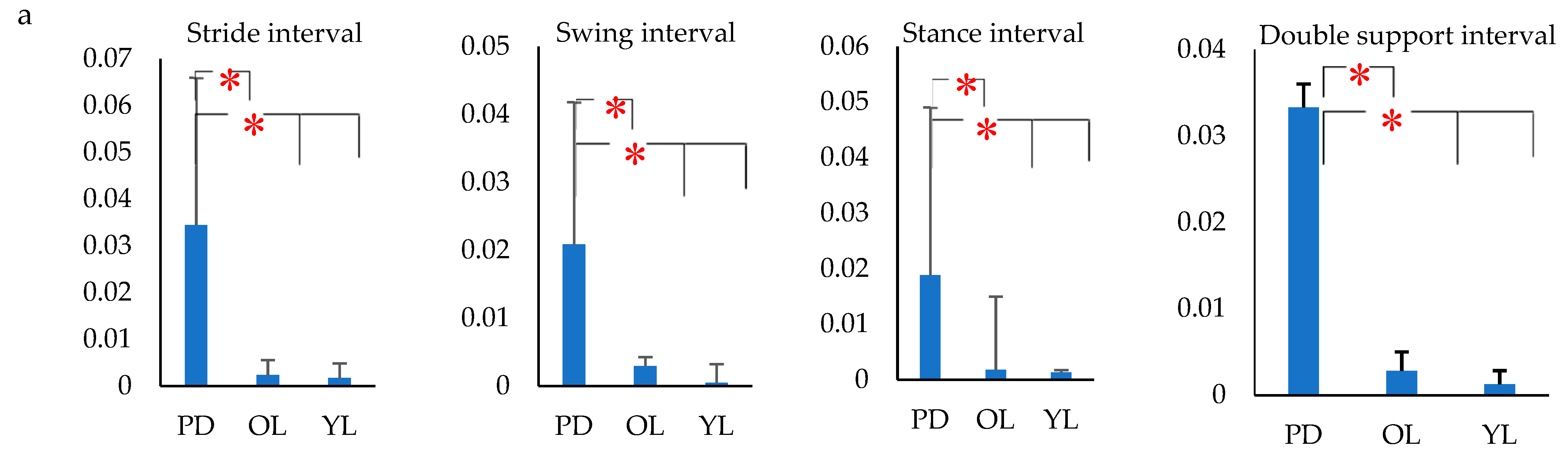
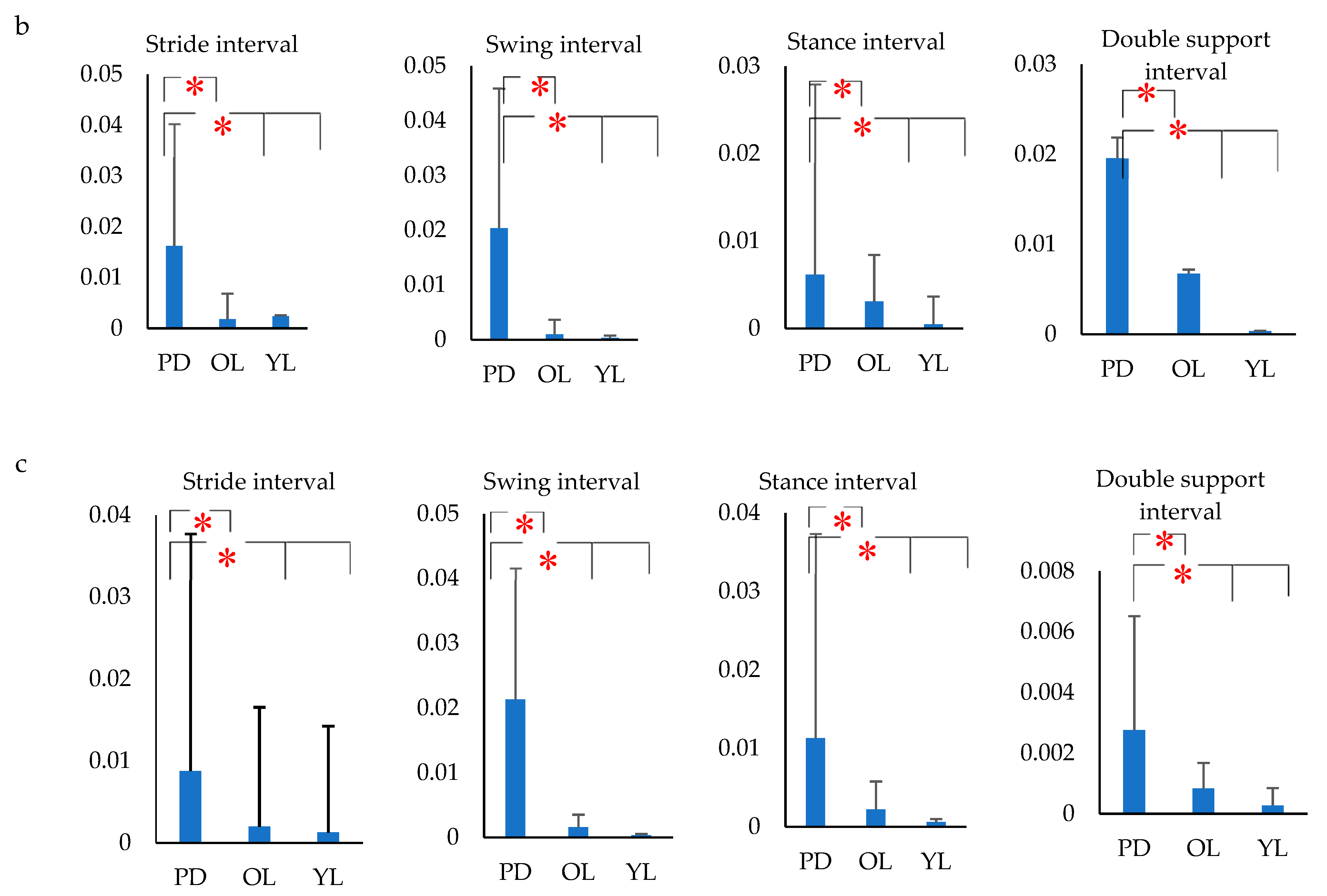
2.6. Gait Feature Extraction
- Number of steps during turn (steps).
- Total turn duration (s).
- Cadence = total number of steps/total turn duration (steps/min) for turns and for straight walking the total turn duration was the total duration of straight walking.
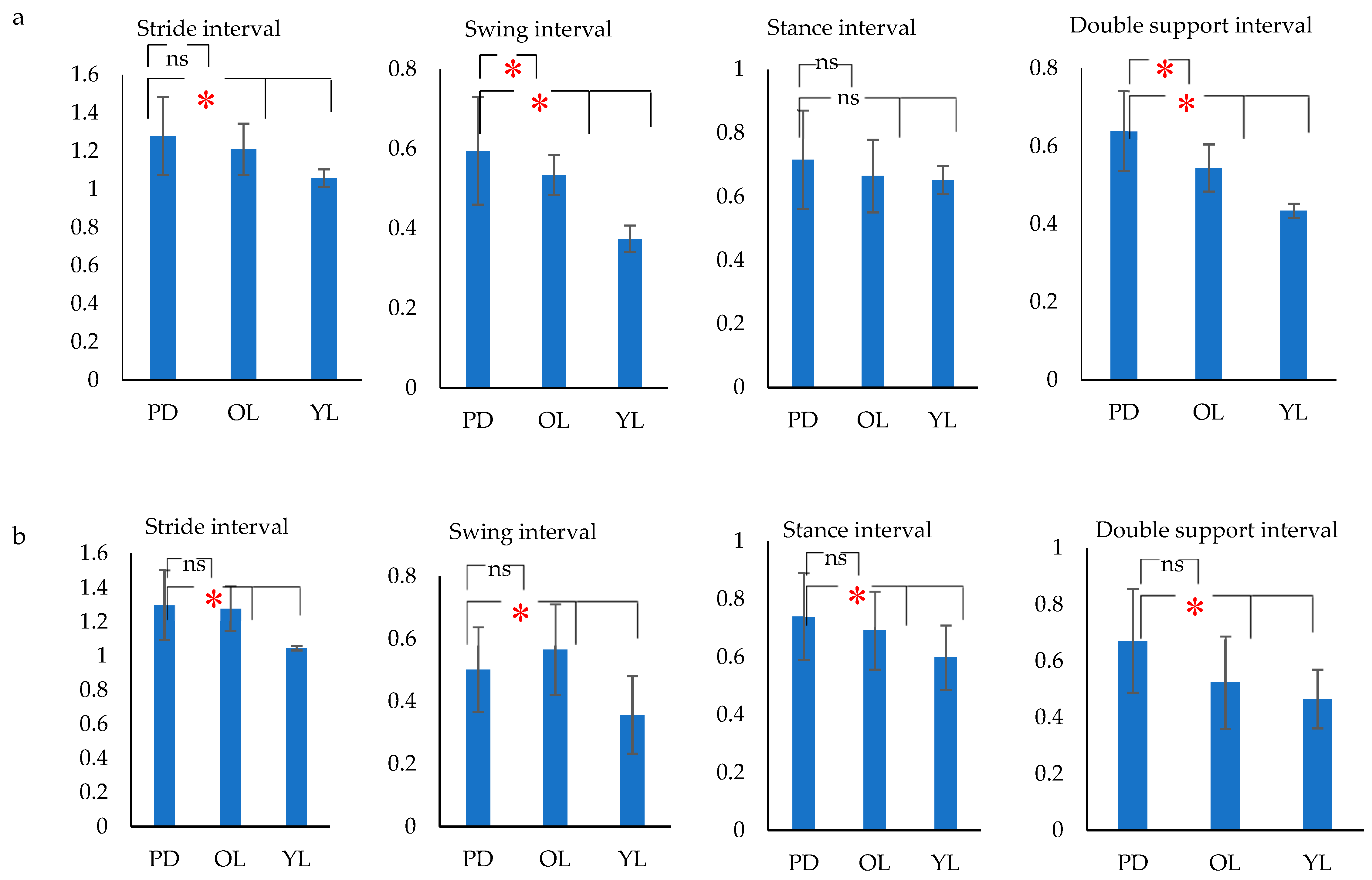
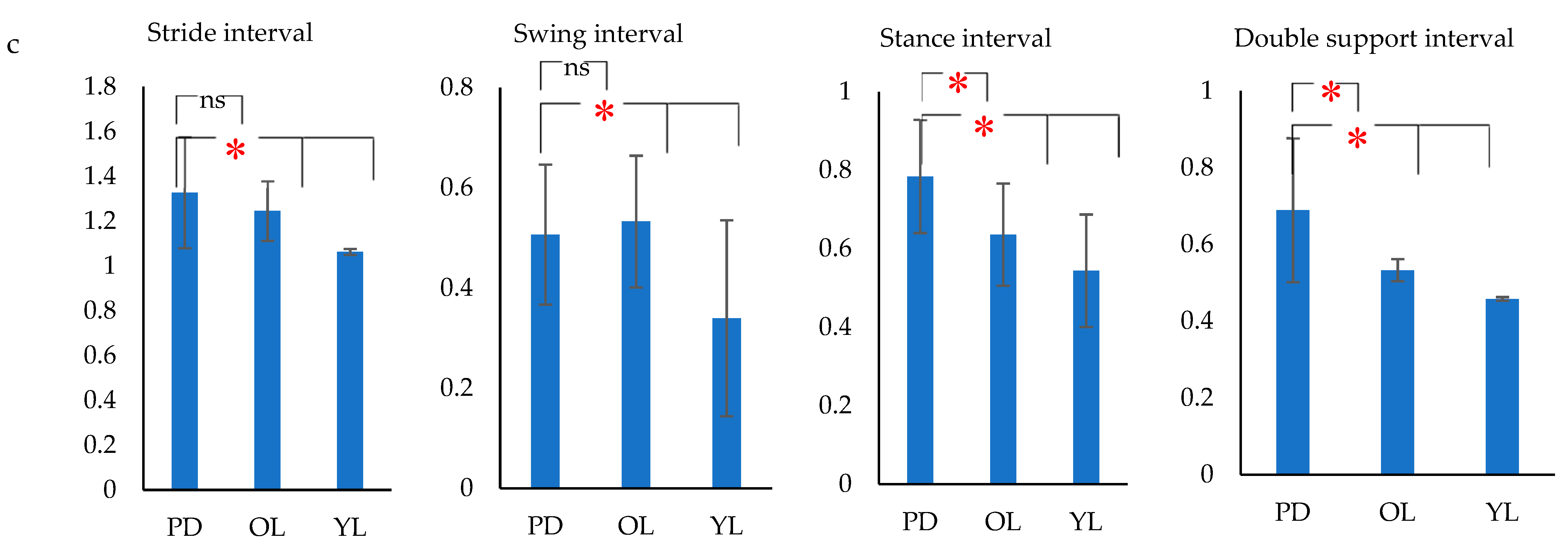
- Stride duration– Time from HS to HS of same foot (s).
- Stance duration–Time from HS to TO of same foot (s).
- Swing duration–Time from TO to HS of same foot (s).
- Double support duration–Time from right HS to left TO + Time from left HS to right TO (s)
- The variance of gait intervals was computed using the coefficient of variance (σ), as it was found to be the most common method in analyzing the gait fluctuation [53]. The σ for each gait interval was calculated as the ratio of standard deviation of the gait parameter to the mean of the gait parameter. The variance of the stride interval, swing interval, stance interval and double support interval were represented as σst, σsw, σsta and σds respectively. Similarly, the mean of the stride interval, swing interval, stance interval and double support interval were represented as µst, µsw, µsta and µds, respectively.
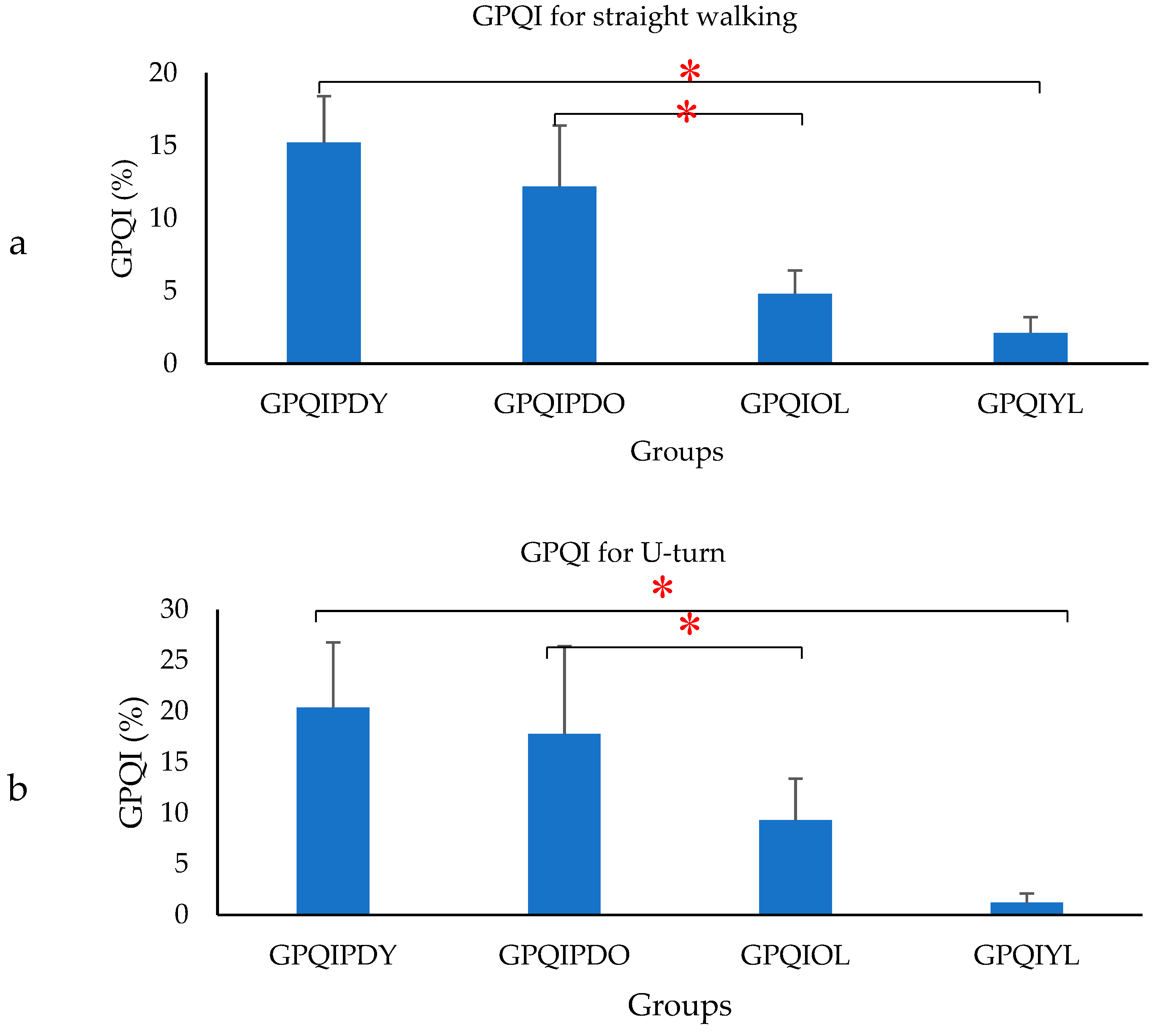
- Gait Phase Quality Index (GPQI) was calculated using the following formula [35]:where FDSPD, SSPD, SDSPD, SWPD represented the percentage gait phase of PD, mFDSPD, mSSPD, mSDSPD, mSWPD represented the average value of percentage gait phase of OL. The GPQI calculation was computed for PD, to access the effect of gait phase distribution and represented by GPQIPDO and for OL computed as the gait phase distribution of OL, which differed from the OL average and represented by GPQIOL. Similarly, a calculation was performed with respect to the average value of the percentage gait phase of YL. The corresponding GPQI value for PD was represented by GPQIPDY and compared with GPQIYL. The GPQI was calculated for each subject and the average values were plotted.
2.7. Statistical Analysis
3. Results
4. Discussion
5. Limitations of This Study
Author Contributions
Funding
Conflicts of Interest
References
- Allcock, L.M.; Rowan, E.N.; Steen, I.N.; Wesnes, K.; Kenny, R.A.; Burn, D.J. Impaired attention predicts falling in Parkinson’s disease. Parkinsonism Relat. Disord. 2009, 15, 110–115. [Google Scholar] [CrossRef]
- Contreras, A.; Grandas, F. Risk of falls in Parkinson’s disease: A cross-sectional study of 160 patients. Parkinson’s Dis 2012, 2012, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Latt, M.D.; Lord, S.R.; Morris, J.G.; Fung, V.S. Clinical and physiological assessments for elucidating falls risk in Parkinson’s disease. Mov Disord 2009, 24, 1280–1289. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Xiong, W.X.; Liu, F.T.; Sun, Y.M.; Luo, S.; Ding, Z.T.; Wu, J.J.; Wang, J. Objective and quantitative assessment of motor function in Parkinson’s disease-from the perspective of practical applications. Ann. Transl Med. 2016, 4, 90. [Google Scholar] [CrossRef]
- Hausdorff, J.M.; Lowenthal, J.; Herman, T.; Gruendlinger, L.; Peretz, C.; Giladi, N. Rhythmic auditory stimulation modulates gait variability in Parkinson’s disease. Eur J. Neurosci 2007, 26, 2369–2375. [Google Scholar] [CrossRef]
- Hausdorff, J.M.; Balash, J.; Giladi, N. Effects of cognitive challenge on gait variability in patients with Parkinson’s disease. J. Geriatr Psychiatry Neurol 2003, 16, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Rios, D.A.; Hausdorff, J.M.; Edelberg, H.K. Gait variability and fall risk in community-living older adults: A 1-year prospective study. Arch. Phys. Med. Rehabil 2001, 82, 1050–1060. [Google Scholar]
- Solomont, J.; Kowall, N.; Hausdorff, J.M. Influence of executive function on locomotor function: Divided attention increases gait variability in Alzheimer’s disease. J. Am. Geriatr Soc. 2003, 51, 1633–1637. [Google Scholar]
- Hausdorff, J.M.; Schaafsma, J.D.; Balash, Y.; Bartels, A.L.; Gurevich, T.; Giladi Nagai, K. Impaired regulation of stride variability in Parkinson’s disease subjects with freezing of gait. Exp. Brain Res. 2003, 149, 187–194. [Google Scholar] [CrossRef]
- Frenkel-Toledo, S.; Giladi, N.; Peretz, C.; Herman, T.; Gruendlinger, L.; Hausdorff, J.M. Effect of gait speed on gait rhythmicity in Parkinson’s disease: Variability of stride time and swing time respond differently. J. Neuroeng Rehabil 2005, 2, 23. [Google Scholar] [CrossRef]
- Osamu, H.; Yoshitaka, S.; Toyokazu, S.; Harukazu, T. Spectral analysis of gait variability of stride interval time seires: Comparison of young, elderly and Parkinson’s disease patients. J. Phys. Ther. Sci 2009, 21, 105–111. [Google Scholar]
- Krishnan, S.; Wu, Y. Statistical Analysis of Gait Rhythm in Patients with Parkinson’s Disease. IEEE Trans. Neural Syst Rehabil Eng. 2010, 18, 150–158. [Google Scholar]
- Morris, M.E.; Morris, S.; Iansek, R. Reliability of measurements obtained with the Timed “Up & Go” test in people with Parkinson disease. Phys. Ther 2001, 81, 810–818. [Google Scholar]
- Blin, O.; Ferrandez, A.M.; Serratrice, G. Quantitative analysis of gait in Parkinson patients: Increased variability of stride length. J. Neurol Sci 1990, 98, 91–97. [Google Scholar] [CrossRef]
- Kirchner, M.; Schubert, P.; Liebherr, M.; Haas, C.T. Detrended fluctuation analysis and adaptive fractal analysis of stride time data in Parkinson’s disease: Stitching together short gait trials. Plos one 2014, 9, e85787. [Google Scholar] [CrossRef]
- Hausdorff, J.M.; Schweiger, A.; Herman, T.; Yogev-Seligmann, G.; Giladi, N. Dual-task decrements in gait: Contributing factors among healthy older adults. J. Gerontol A Biol Sci Med. Sci 2008, 63, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Hagovska, M.; Olekszyova, Z. Relationships between balance control and cognitive functions, gait speed, and activities of daily living. Z Gerontol Geriatr 2016, 49, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Park, J.H. The effects of dual-task gait training on foot pressure in elderly women. J. Phys. Ther. Sci 2015, 27, 143–144. [Google Scholar] [CrossRef]
- Seung, K.; Jerome, G.J.; Simonsick, E.M.; Studenski, S.; Hausdorff, J.M.; Ferrucci, L. Differential associations between dual-task walking abilities and usual gait patterns in healthy older adults—Results from the Baltimore Longitudinal Study of Aging. Gait Posture 2018, 63, 63–67. [Google Scholar]
- Emmanuel, S.; Kunzler, M.R.; Bobbert, M.F.; Duysens, J.; Carpes, F.P. 30 min of treadmill walking at self-selected speed does not increase gait variability in independent elderly. J. S Sci 2018, 36, 1305–1311. [Google Scholar]
- Seung, K.; Hausdorff, J.M.; Ferrucci, L. Age-associated differences in the gait pattern changes of older adults during fast-speed and fatigue conditions: Results from the Baltimore longitudinal study of ageing. Age ageing 2010, 39, 688–694. [Google Scholar]
- Almarwani, M.; VanSwearingen, J.M.; Perera, S.; Sparto, P.J.; Brach, J.S. Challenging the motor control of walking: Gait variability during slower and faster pace walking conditions in younger and older adults. Arch. Gerontol Geriatr. 2016, 66, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Hausdorff, J.M. Gait dynamics in Parkinson’s disease: Common and distinct behavior among stride length, gait variability, and fractal-like scaling. Chaos 2009, 19, 026113. [Google Scholar] [CrossRef] [PubMed]
- Turcato, A.M.; Godi, M.; Giardini, M.; Arcolin, I.; Nardone, A.; Giordano, A.; Schieppati, M. Abnormal gait pattern emerges during curved trajectories in high-functioning Parkinsonian patients walking in line at normal speed. Plos one 2018, 13, e0197264. [Google Scholar] [CrossRef] [PubMed]
- Haertner, L.; Elshehabi, M.; Zaunbrecher, L.; Pham, M.H.; Maetzler, C.; van Uem, J.M.; Hobert, M.A.; Hucker, S.; Nussbaum, S.; Berg, D. Effect of Fear of Falling on Turning Performance in Parkinson’s Disease in the Lab and at Home. Front. Aging Neurosci. 2018, 10, 78. [Google Scholar] [CrossRef] [PubMed]
- Bengevoord, A.; Vervoort, G.; Spildooren, J.; Heremans, E.; Vandenberghe, W.; Bloem, B.R.; Nieuwboer, A. Center of mass trajectories during turning in patients with Parkinson’s disease with and without freezing of gait. Gait Posture 2016, 43, 54–59. [Google Scholar] [CrossRef]
- Huxham, F.; Baker, R.; Morris, M.E.; Iansek, R. Head and trunk rotation during walking turns in Parkinson’s disease. Mov Disord 2008, 23, 1391–1397. [Google Scholar] [CrossRef] [PubMed]
- Spildooren, J.; Vercruysse, S.; Heremans, E.; Galna, B.; Desloovere, K. Head-pelvis coupling is increased during turning in patients with Parkinson’s disease and freezing of gait. Mov Disord 2013, 28, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Crenna, P.; Carpinella, I.; Rabuffetti, M.; Calabrese, E.; Mazzoleni, P.; Nemni, R.; Ferrarin, M. The association between impaired turning and normal straight walking in Parkinson’s disease. Gait Posture 2007, 26, 172–178. [Google Scholar] [CrossRef]
- King, L.; Mancini, M.; Priest, K.; Salarian, A.; Rodrigues-de-Paula, F.; Horak, F. Do Clinical Scales of Balance Reflect Turning Abnormalities in People With Parkinson’s Disease? JNPT 2012, 36, 25. [Google Scholar]
- Herman, T.; Giladi, N.; Hausdorff, J.M. Properties of the ‘timed up and go’ test: More than meets the eye. Gerontology 2011, 57, 203–210. [Google Scholar] [CrossRef]
- Maidan, I.; Bernad-Elazari, H.; Giladi, N.; Hausdorff, J.M.; Mirelman, A. When is Higher Level Cognitive Control Needed for Locomotor Tasks Among Patients with Parkinson’s Disease? Brain Topogr 2017, 30, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Johannesdottir, F.; Thrall, E.; Muller, J.; Keaveny, T.M.; Kopperdahl, D.L.; Bouxsein, M.L. Comparison of non-invasive assessments of strength of the proximal femur. Bone 2017, 105, 93–102. [Google Scholar] [CrossRef]
- Mellone, S.; Mancini, M.; King, L.A.; Horak, F.B.; Chiari, L. The quality of turning in Parkinson’s disease: A compensatory strategy to prevent postural instability? J. NeuroEng Rehabil 2016, 13, 39. [Google Scholar] [CrossRef]
- Mileti, I.; Germanotta, M.; Di Sipio, E.; Imbimbo, I.; Pacilli, A.; Erra, C.; Petracca, M.; Rossi, S.; Del Prete, Z.; Bentivoglio, A.R.; et al. Measuring Gait Quality in Parkinson’s Disease through Real-Time Gait Phase Recognition. Sensors 2018, 18, 919. [Google Scholar] [CrossRef] [PubMed]
- Guzik, A.; Druzbicki, M.; Przysada, G.; Szczepanik, M.; Bazarnik-Mucha, K.; Kwolek, A. The use of the Gait Variability Index for the evaluation of individuals after a stroke. Acta Bioeng Biomech 2018, 20, 171–177. [Google Scholar]
- Miller Koop, M.; Ozinga, S.J.; Rosenfeldt, A.B.; Alberts, J.L. Quantifying turning behavior and gait in Parkinson’s disease using mobile technology. IBRO 2018, 5, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.; Smulders, K.; Harker, G.; Stuart, S.; Nutt, J.G. Assessment of the ability of open- and closed-loop cueing to improve turning and freezing in people with Parkinson’s disease. Sci. Rep. 2018, 8, 12773. [Google Scholar] [CrossRef] [PubMed]
- On-Yee, L.; Halko, M.A.; Zhou, J.; Harrison, R.; Lipsitz, L.A.; Manor, B. Gait Speed and Gait Variability Are Associated with Different Functional Brain Networks. Front. Aging Neurosci. 2017, 9, 390. [Google Scholar]
- Estep, A.; Morrison, S.; Caswell, S.; Ambegaonkar, J.; Cortes, N. Differences in pattern of variability for lower extremity kinematics between walking and running. Gait Posture 2018, 60, 111–115. [Google Scholar] [CrossRef]
- Warlop, T.; Detrembleur, C.; Stoquart, G.; Lejeune, T.; Jeanjean, A. Gait Complexity and Regularity Are Differently Modulated by Treadmill Walking in Parkinson’s Disease and Healthy Population. Front. Physiol 2018, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Nayak, B.K. Understanding the relevance of sample size calculation. Indian J. Ophthalmol 2010, 58, 469–470. [Google Scholar] [CrossRef]
- Fong, D.; Tik, P.; Chan, Y.-Y. The Use of Wearable Inertial Motion Sensors in Human Lower Limb Biomechanics Studies: A Systematic Review. Sensors 2010, 10, 11556–11565. [Google Scholar] [CrossRef] [PubMed]
- Sijobert, B.; Benoussaad, M.; Denys, J.; Gibollet, R.; Geny, C.; Coste, C. Implementation and Validation of a Stride Length Estimation Algorithm, using a Single Basic Inertial Sensor on Healthy Subjects and Patients Suffering from Parkinson’s Disease. Health 2015, 7, 704–714. [Google Scholar] [CrossRef]
- Siddiqi, A.; Arjunan, S.P.; Kumar, D. Improvement of isometric dorsiflexion protocol for assessment of tibialis anterior muscle strength. MethodsX 2015, 2, 107–111. [Google Scholar] [CrossRef]
- Rueterbories, J.; Spaich, E.G.; Larsen, B.; Andersen, O.K. Methods for gait event detection and analysis in ambulatory systems. Med. Eng. Phys. 2010, 32, 545–552. [Google Scholar] [CrossRef]
- Mitschke, C.; Kiesewetter, P.; Milani, T. The Effect of the Accelerometer Operating Range on Biomechanical Parameters: Stride Length, Velocity, and Peak Tibial Acceleration during Running. Sensors 2018, 18, 130. [Google Scholar] [CrossRef] [PubMed]
- Moses, S. Family Practice Notebook. 1995. Available online: https://fpnotebook.com/legacy/ (accessed on 3 January 2018).
- England, S.E.; Verghese, J.; Mahoney, J.R.; Tranntzas, C.; Holtzer, R. Three-level rating of turns while walking. Gait Posture 2015, 41, 300–303. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grech, C.; Formosa, C.; Gatt, A. Shock attenuation properties at heel strike: Implications for the clinical management of the cavus foot. J. Orthop 2016, 13, 148–151. [Google Scholar] [CrossRef]
- Ginis, P.; Pirani, R.; Basaia, S.; Ferrari, A.; Chiari, L.; Heremans, E.; Canning, C.G.; Nieuwboer, A. Focusing on heel strike improves toe clearance in people with Parkinson’s disease: An observational pilot study. Physiotherapy 2017, 103, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Ferster, M.; Mazilu, S.; Troester, G. Gait Parameters Change Prior to Freezing in Parkinson’s Disease: A Data-Driven Study with Wearable Inertial Units. Bodynets 2015, 3, 159–166. [Google Scholar]
- Chau, T.; Young, S.; Redekop, S. Managing variability in the summary and comparison of gait data. J. Neuroeng Rehabil 2005, 2, 22. [Google Scholar] [CrossRef]
- Ghasemi, A.; Zahediasl, S. Normality Tests for Statistical Analysis: A Guide for Non-Statisticians. Int J. Endocrinol Metab 2012, 10, 486–489. [Google Scholar] [CrossRef]
- Siegel, C. Nonparametric Statistics for the Behavioral Sciences, 2nd ed.; McGraw-Hill: New York, NY, USA, 1988. [Google Scholar]
- Schwartz, M.H.; Trost, J.P.; Wervey, R.A. Measurement and management of errors in quantitative gait data. Gait Posture 2004, 20, 196–203. [Google Scholar] [CrossRef]
- Menz, H.B.; Lord, S.R.; Fitzpatrick, R.C. Acceleration patterns of the head and pelvis when walking on level and irregular surfaces. Gait Posture 2003, 18, 35–46. [Google Scholar] [CrossRef]
- Richardson, J.K.; Thies, S.B.; Demott, T.K.; Ashton-Miller, J.A. Interventions Improve Gait Regularity in Patients with Peripheral Neuropathy While Walking on an Irregular Surface Under Low Light. J. Am. Geriatr Soc. 2004, 52, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Stacoff, A.; Diezi, C.; Luder, G.; Stüssi, E.; Kramers-de Quervain, I.A. Ground reaction forces on stairs: Effects of stair inclination and age. Gait Posture 2005, 21, 24–38. [Google Scholar] [CrossRef]
- Griffin, L.; West, D.; West, B. Random Stride Intervals with Memory. J. Biol Phys. 2000, 26, 185–202. [Google Scholar] [CrossRef]
- Van Emmerik, R.E.A.; Ducharme, S.W.; Amado, A.C.; Hamill, J. Comparing dynamical systems concepts and techniques for biomechanical analysis. J. Sport Health Sci. 2016, 5, 3–13. [Google Scholar] [CrossRef]
- Roberts, M.; Mongeon, D.; Prince, F. Biomechanical parameters for gait analysis: A systematic review of healthy human gait. Phys. Med. Rehabil 2017, 4, 6. [Google Scholar] [CrossRef]
- Yang, A.C.; Tsai, S.J.; Lin, C.P.; Peng, C.K.; Huang, N.E. Frequency and amplitude modulation of resting-state fMRI signals and their functional relevance in normal aging. Neuro Aging 2018, 70, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Owings, T.M.; Grabiner, M.D. Variability of step kinematics in young and older adults. Gait Posture 2004, 20, 26–29. [Google Scholar] [CrossRef]
- Romero, V.; Fitzpatrick, P.; Roulier, S.; Duncan, A.; Richardson, M.J.; Schmidt, R. Evidence of embodied social competence during conversation in high functioning children with autism spectrum disorder. Plos one 2018, 13, e0193906. [Google Scholar]
- Spildooren, J.; Vercruysse, S.; Desloovere, K.; Vandenberghe, W.; Kerckhofs, E.; Nieuwboer, A. Freezing of gait in Parkinson’s disease: The impact of dual-tasking and turning. Mov Disord 2010, 25, 2563–2570. [Google Scholar] [CrossRef]
- Spildooren, J.; Vercruysse, S.; Meyns, P.; Vandenbossche, J.; Heremans, E.; Desloovere, K.; Vandenberghe, W.; Nieuwboer, A. Turning and unilateral cueing in Parkinson’s disease patients with and without freezing of gait. J. Neurosci 2012, 207, 298–306. [Google Scholar] [CrossRef]
- Chien, S.L.; Lin, S.Z.; Liang, C.C.; Soong, Y.S.; Lin, S.H.; Hsin, Y.L.; Lee, C.W.; Chen, S.Y. The efficacy of quantitative gait analysis by the GAITRite system in evaluation of parkinsonian bradykinesia. Parkinsonism Relat Disord 2006, 12, 438–442. [Google Scholar] [CrossRef]
- Morris, M.; Iansek, R.; Matyas, T.; Summers, J. The pathogenesis of gait hypokinesia in Parkinson’s disease. Brain 1994, 117, 1169. [Google Scholar] [CrossRef]
- Ringeval, F.; Eyben, F.; Kroupi, E.; Yuce, A.; Thiran, J.-P.; Ebrahimi, T.; Lalanne, D.; Schuller, B. Prediction of asynchronous dimensional emotion ratings from audiovisual and physiological data. Pattern Recognit Lett. 2015, 66, 22–30. [Google Scholar] [CrossRef]
- Lin, C.C.; Creath, R.A.; Rogers, M.W. Variability of Anticipatory Postural Adjustments During Gait Initiation in Individuals with Parkinson Disease. JNPT 2016, 40, 40–46. [Google Scholar] [CrossRef]
- Roemmich, R.T.; Nocera, J.R.; Vallabhajosula, S.; Amano, S.; Naugle, K.M.; Stegemoller, E.L.; Hass, C.J. Spatiotemporal variability during gait initiation in Parkinson’s disease. Gait Posture 2012, 36, 340–343. [Google Scholar] [CrossRef]
- Hausdorff, J.M. Gait variability: Methods, modeling and meaning. J. Neuroeng Rehabil 2005, 2, 19. [Google Scholar] [CrossRef]
- Skjaeret, N.; Nawaz, A.; Morat, T.; Schoene, D.; Helbostad, J.L.; Vereijken, B. Exercise and rehabilitation delivered through exergames in older adults: An integrative review of technologies, safety and efficacy. Int J. Med. Inform. 2016, 85, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Redgrave, P.; Rodriguez, M.; Smith, Y.; Rodriguez-Oroz, M.; Lehericy, S.; Bergman, H.; Agid, Y.; Delong, M.; Obeso, J. Goal-directed and habitual control in the basal ganglia: Implications for Parkinson’s disease. Nat. Rev. Neurosci. 2010, 11, 760. [Google Scholar] [CrossRef] [PubMed]
- Brach, J.S.; Studenski, S.; Perera, S.; VanSwearingen, J.M.; Newman, A.B. Stance time and step width variability have unique contributing impairments in older persons. Gait Posture 2008, 27, 431–439. [Google Scholar] [CrossRef]
- Snijders, A.H.; van de Warrenburg, B.P.; Giladi, N.; Bloem, B.R. Neurological gait disorders in elderly people: Clinical approach and classification. Lancet Neurol 2007, 6, 63–74. [Google Scholar] [CrossRef]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov Disord 2008, 23, 2129–2170. [Google Scholar] [CrossRef] [PubMed]
- Earhart, G.M. Dynamic control of posture across locomotor tasks. Mov Disord 2013, 28, 1501–1508. [Google Scholar] [CrossRef]
- Mancini, M.; Weiss, A.; Herman, T.; Hausdorff, J.M. Turn Around Freezing: Community-Living Turning Behavior in People with Parkinson’s Disease. Front. Neurol 2018, 9, 1–9. [Google Scholar] [CrossRef]
- Salarian, A.; Zampieri, C.; Horak, F.B.; Carlson-Kuhta, P.; Nutt, J.G.; Aminian, K. Analyzing 180 degrees turns using an inertial system reveals early signs of progression of Parkinson’s disease. Conf Proc. IEEE Eng. Med. Biol Soc. 2009, 2009, 224–227. [Google Scholar] [PubMed]
- Spildooren, J.; Vinken, C.; Van Baekel, L.; Nieuwboer, A. Turning problems and freezing of gait in Parkinson’s disease: A systematic review and meta-analysis. Disabil Rehabil 2018, 1, 1–11. [Google Scholar] [CrossRef]
- Ancillao, A.; van der Krogt, M.M.; Buizer, A.I.; Witbreuk, M.M.; Cappa, P.; Harlaar, J. Analysis of gait patterns pre- and post- Single Event Multilevel Surgery in children with Cerebral Palsy by means of Offset-Wise Movement Analysis Profile and Linear Fit Method. Hum. Mov Sci 2017, 55, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, J.D.; Giladi, N.; Balash, Y.; Bartels, A.L.; Gurevich, T.; Hausdorff, J.M. Gait dynamics in Parkinson’s disease: Relationship to Parkinsonian features, falls and response to levodopa. J. Neurol Sci 2003, 212, 47–53. [Google Scholar] [CrossRef]
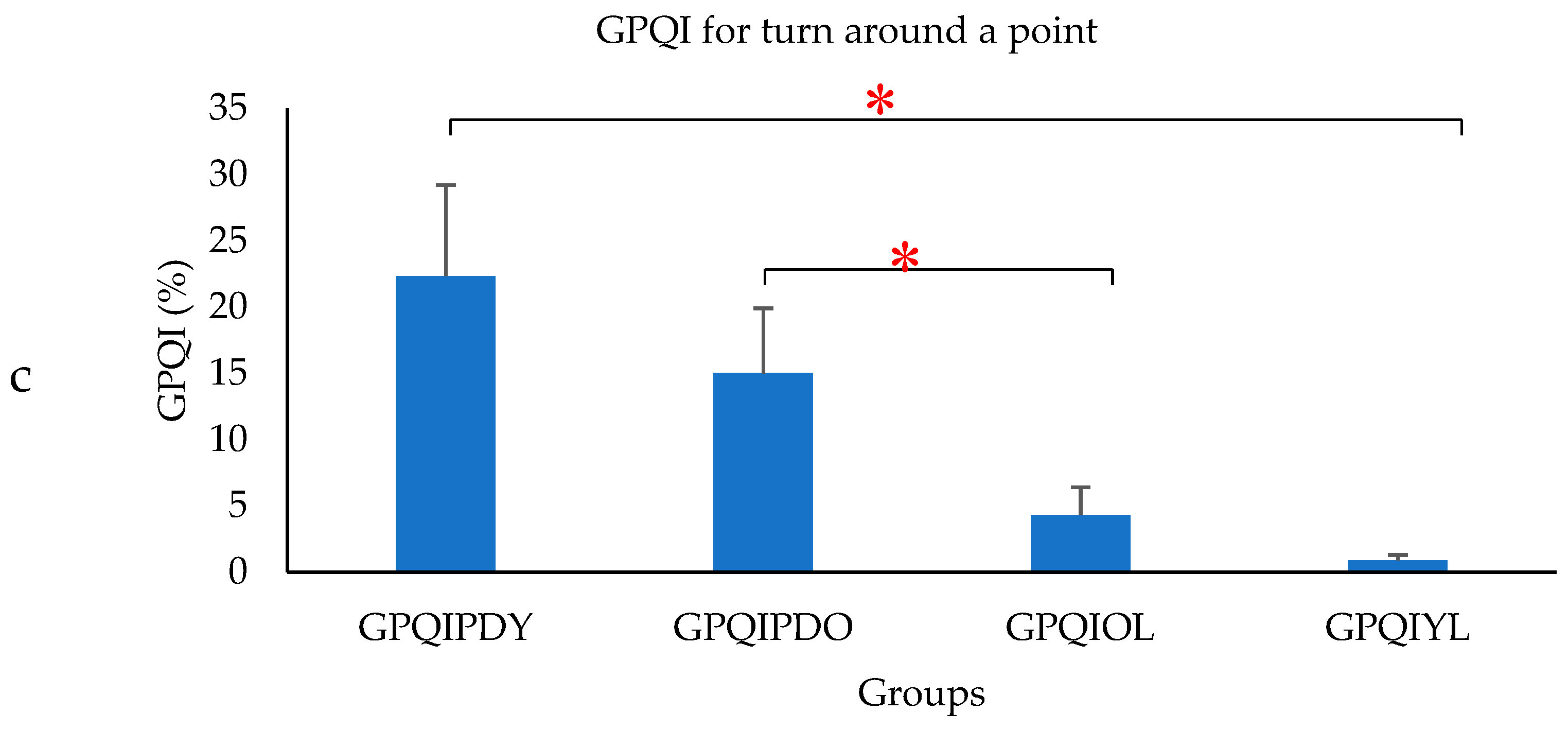
| PD | OL | YL | |
|---|---|---|---|
| Demographic variables | |||
| Age (Years) | 71.91 ± 8.64 | 67.25 ± 3.77 | 27.91 ± 2.43 |
| Gender (male/female) | 17/7 | 17/7 | 18/6 |
| Height (cm) | 169.26 ± 8.89 | 166.54 ± 8.20 | 161.33 ± 4.26 |
| Weight (kg) | 81.25 ± 15.86 | 73.58 ± 12.46 | 60.29 ± 8.07 |
| Clinical variables | |||
| Disease duration (Years) | 4.27 ± 3.15 | | |
| UPDRS III | 25.69 ± 10.95 | | |
| UDysRS | 0.79 ± 1.35 | - | - |
| H &Y | 2.27 ± 0.94 | | |
| Cognitive variables | |||
| Total MOCA score | 23.33 ± 5.30 | 27.33 ± 3.10 | 28.75 ± 1.35 |
| Visuospatial/executive function | 3.5 ± 1.74 | 4.41 ± 1.13 | 4.95 ± 0.20 |
| Attention | 4.70 ± 1.33 | 6 | 6 |
| Delayed recall | 2.41 ± 1.97 | 3.62 ± 1.55 | 4.16 ± 1.00 |
| Orientation | 5.56 ± 0.57 | 5.95 ± 0.20 | 5.62 ± 0.71 |
| Subject | Walking pattern | µst | µsw | µsta | µds | σst | σsw | σsta | σds |
|---|---|---|---|---|---|---|---|---|---|
| PD | Straight walking compared with U-turn | ns | ns | ns | ns | 0.001* | ns | 0.006* | 0.000* |
| Straight walking compared with turn around a point | ns | ns | ns | ns | 0.002* | ns | 0.005* | 0.005* | |
| OL | Straight walking compared with U-turn | ns | ns | 0.04* | ns | ns | ns | ns | 0.021* |
| Straight walking compared with turn around a point | ns | 0.045* | ns | ns | ns | ns | ns | 0.031* | |
| YL | Straight walking compared with U-turn | ns | ns | 0.047* | ns | ns | ns | ns | ns |
| Straight walking compared with turn around a point | ns | ns | ns | ns | ns | ns | ns | ns |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keloth, S.M.; Viswanathan, R.; Jelfs, B.; Arjunan, S.; Raghav, S.; Kumar, D. Which Gait Parameters and Walking Patterns Show the Significant Differences Between Parkinson’s Disease and Healthy Participants? Biosensors 2019, 9, 59. https://doi.org/10.3390/bios9020059
Keloth SM, Viswanathan R, Jelfs B, Arjunan S, Raghav S, Kumar D. Which Gait Parameters and Walking Patterns Show the Significant Differences Between Parkinson’s Disease and Healthy Participants? Biosensors. 2019; 9(2):59. https://doi.org/10.3390/bios9020059
Chicago/Turabian StyleKeloth, Sana M, Rekha Viswanathan, Beth Jelfs, Sridhar Arjunan, Sanjay Raghav, and Dinesh Kumar. 2019. "Which Gait Parameters and Walking Patterns Show the Significant Differences Between Parkinson’s Disease and Healthy Participants?" Biosensors 9, no. 2: 59. https://doi.org/10.3390/bios9020059
APA StyleKeloth, S. M., Viswanathan, R., Jelfs, B., Arjunan, S., Raghav, S., & Kumar, D. (2019). Which Gait Parameters and Walking Patterns Show the Significant Differences Between Parkinson’s Disease and Healthy Participants? Biosensors, 9(2), 59. https://doi.org/10.3390/bios9020059







