Electrochemical Detection and Characterization of Nanoparticles with Printed Devices
Abstract
:1. Introduction
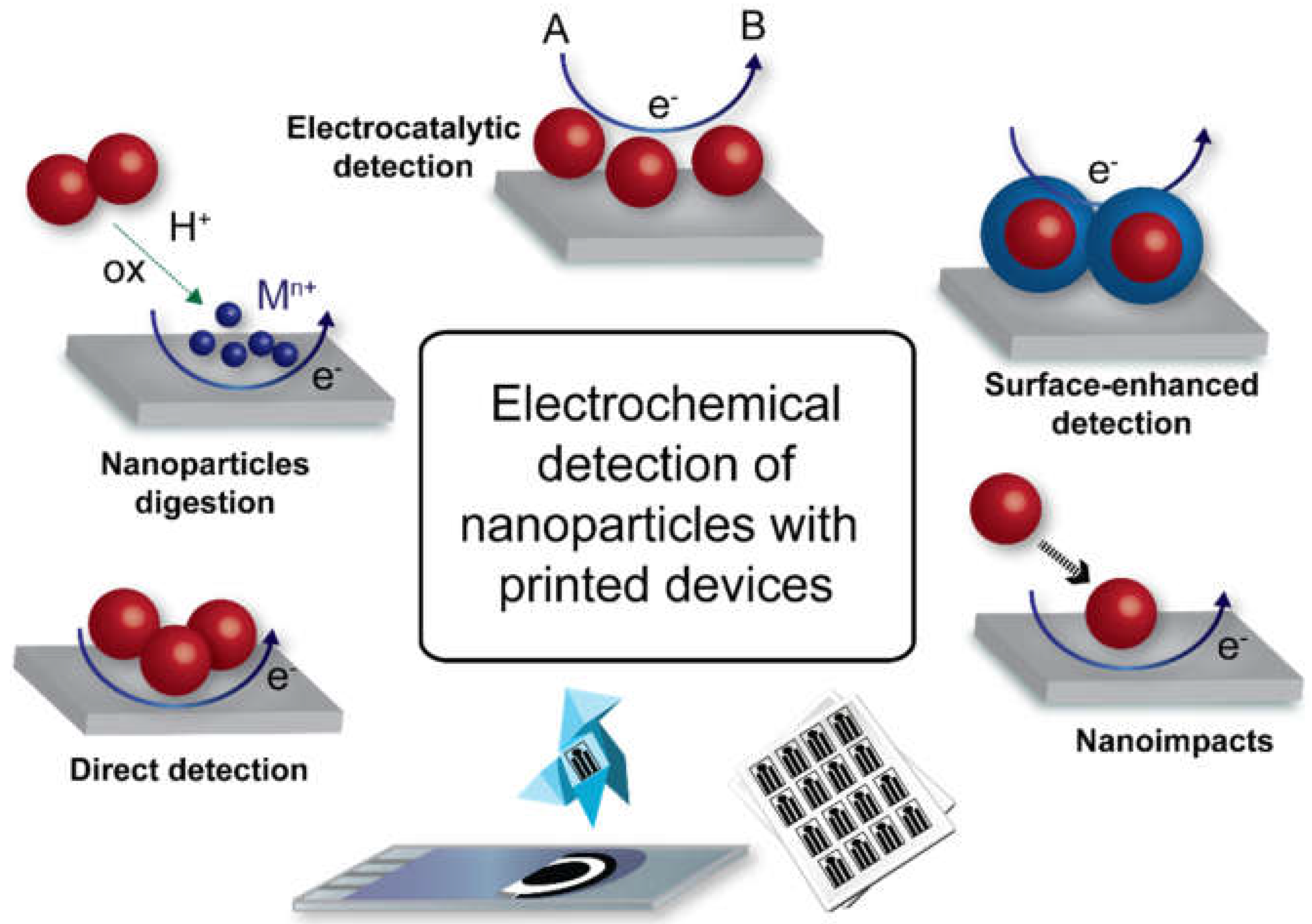
2. Strategies for Electrochemical Detection of Nanoparticles Using Printed Devices
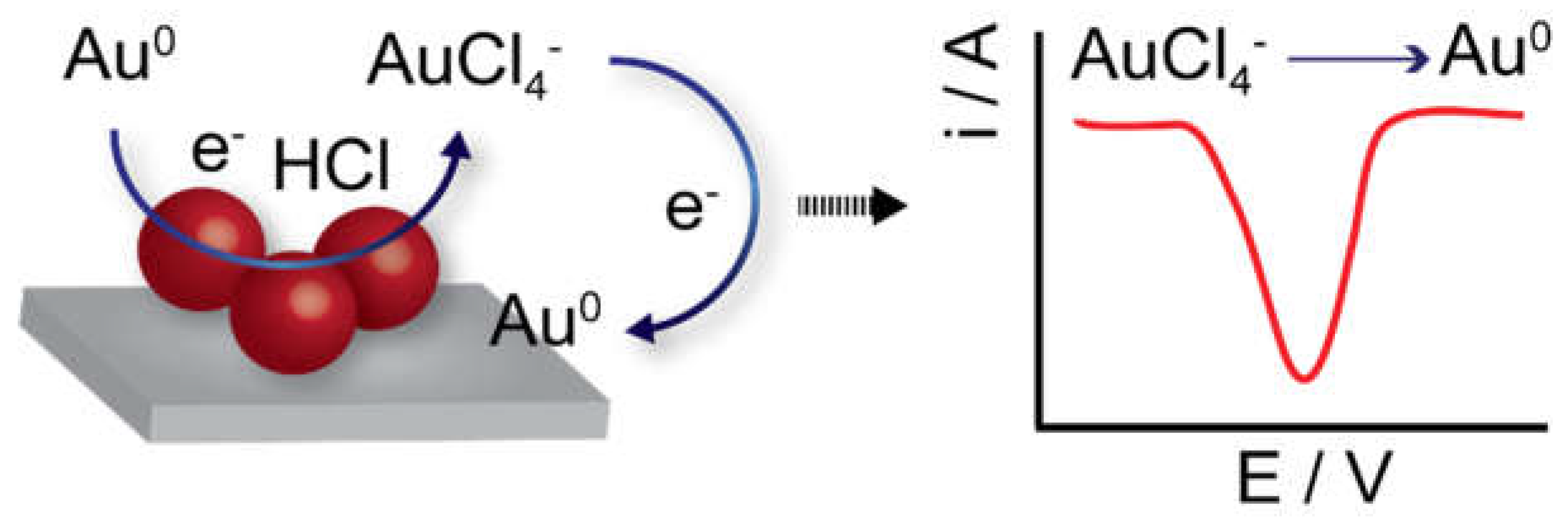
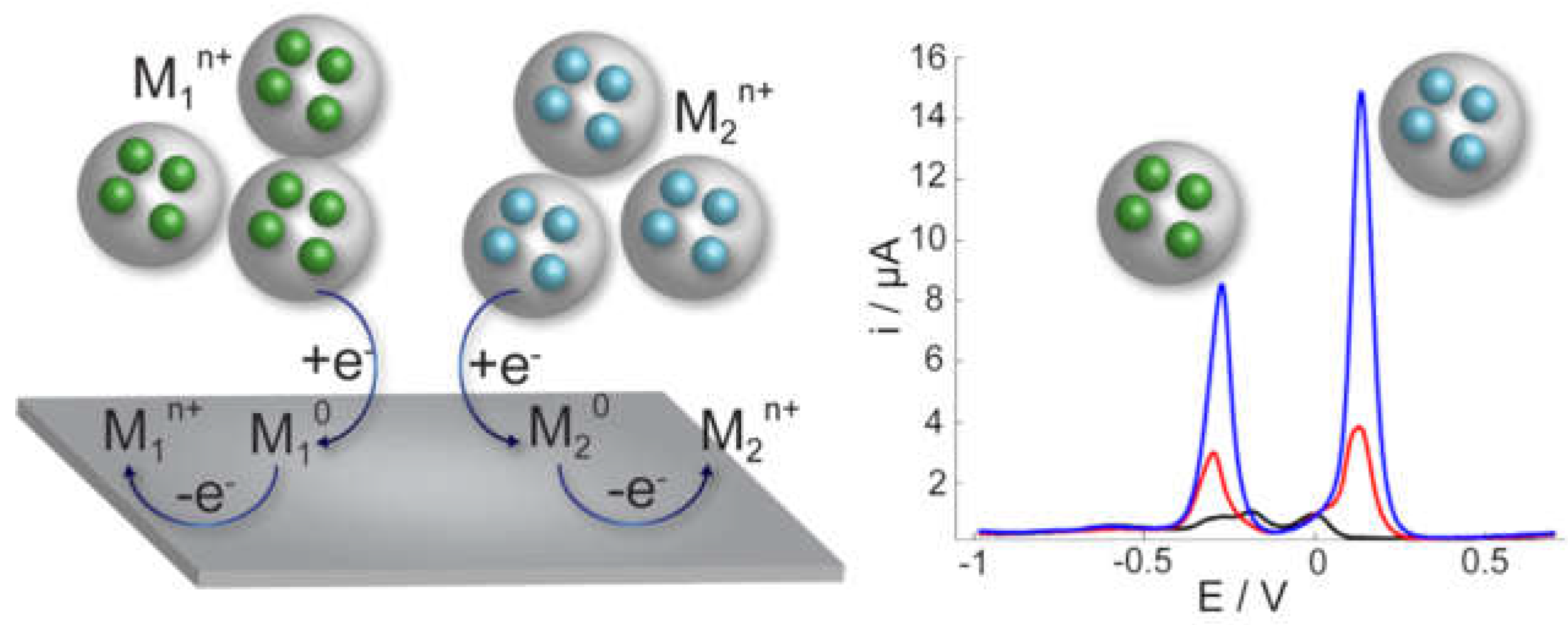
2.1. Direct Detection Methods
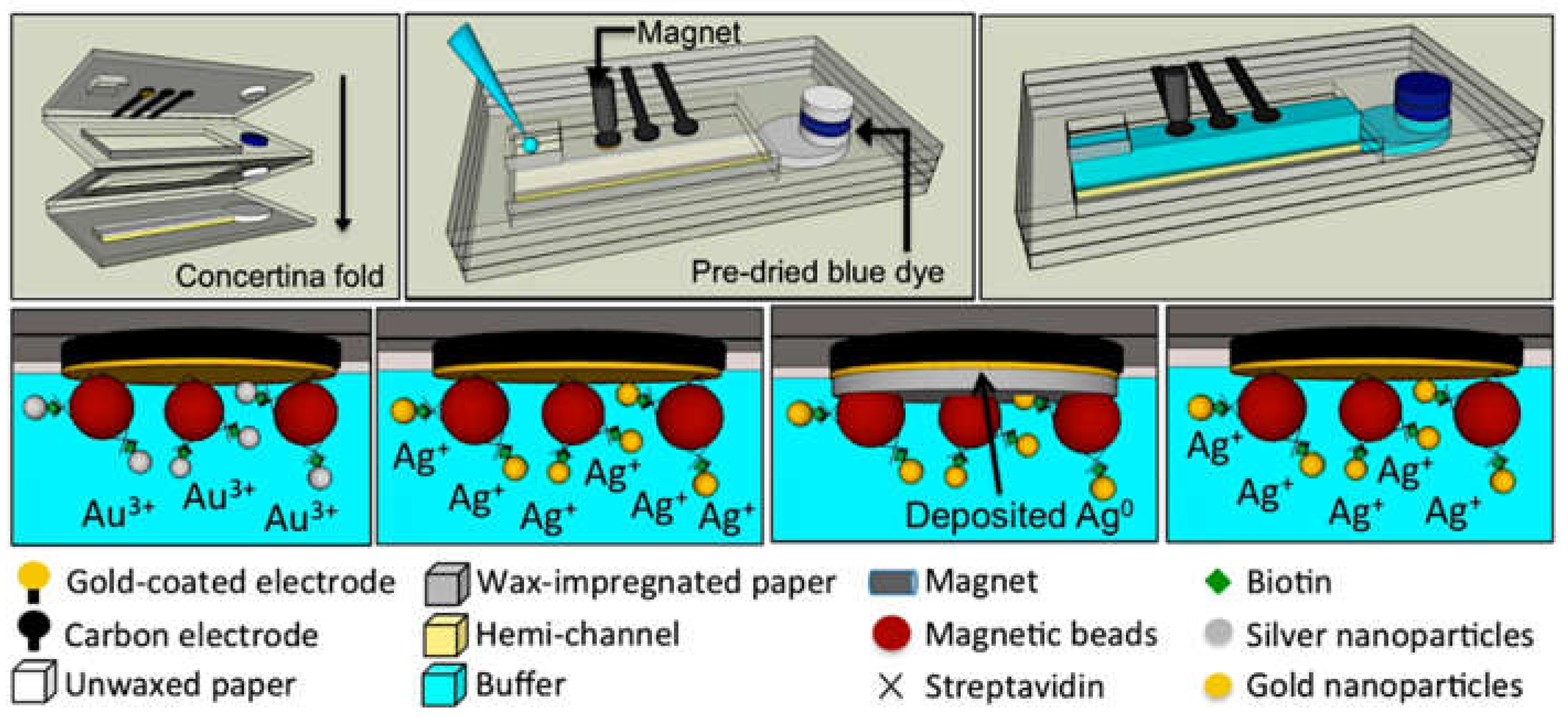
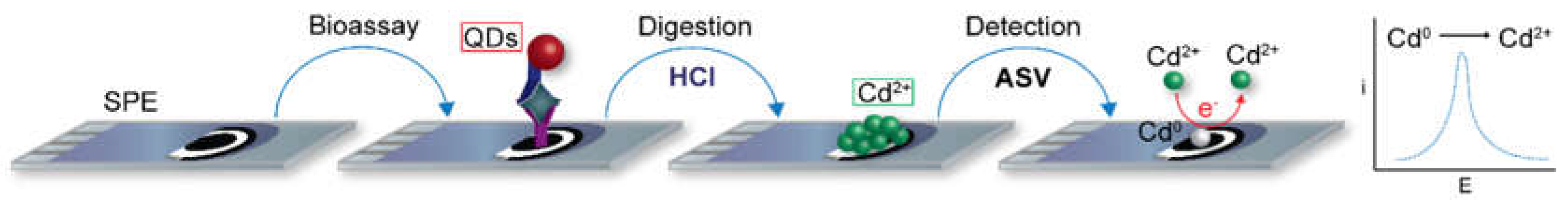
2.2. Detection with a Preceding Digestion Step
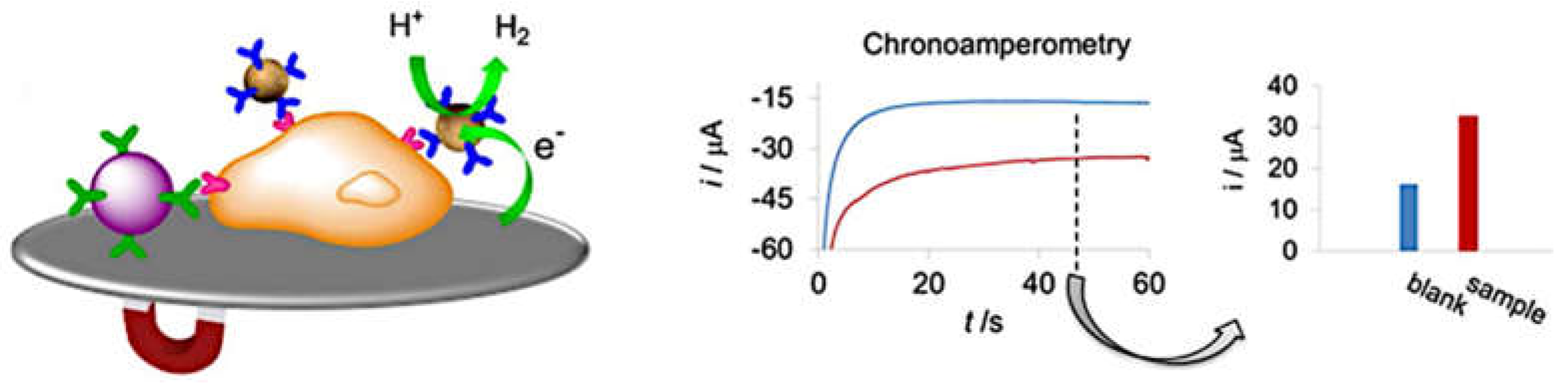
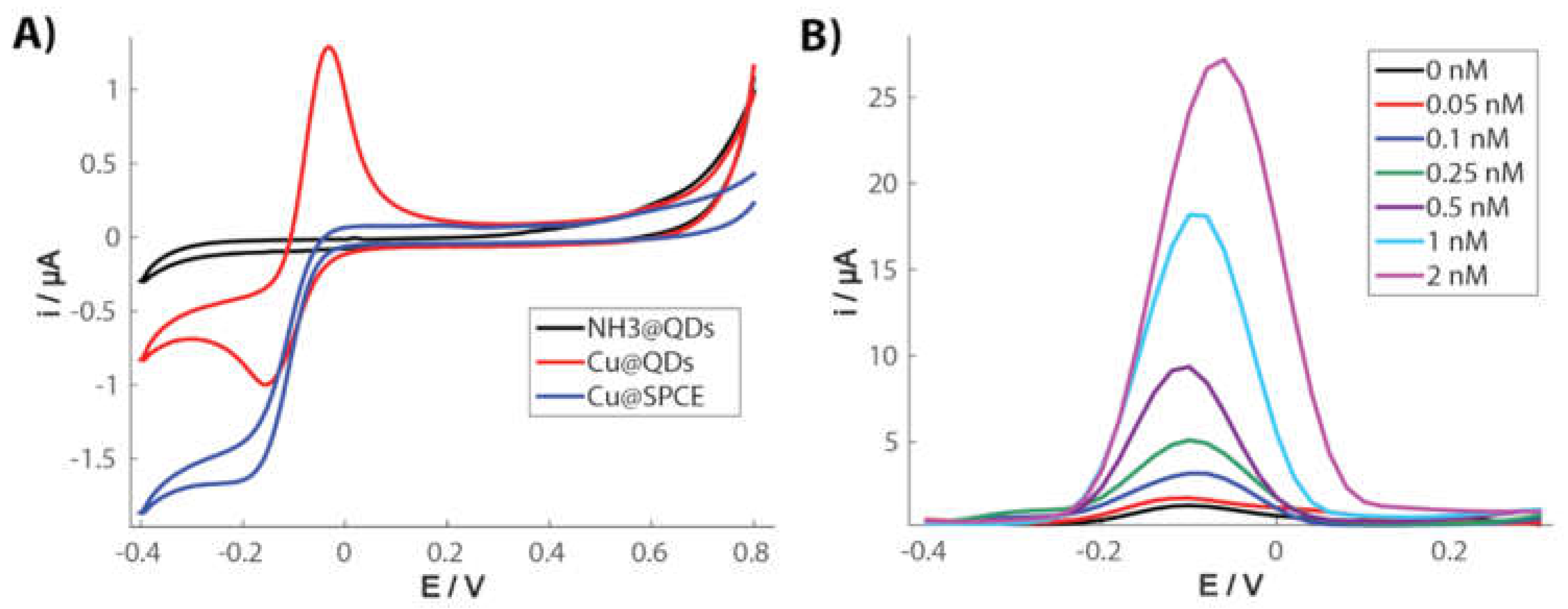
2.3. Electrocatalytic Detection Methods
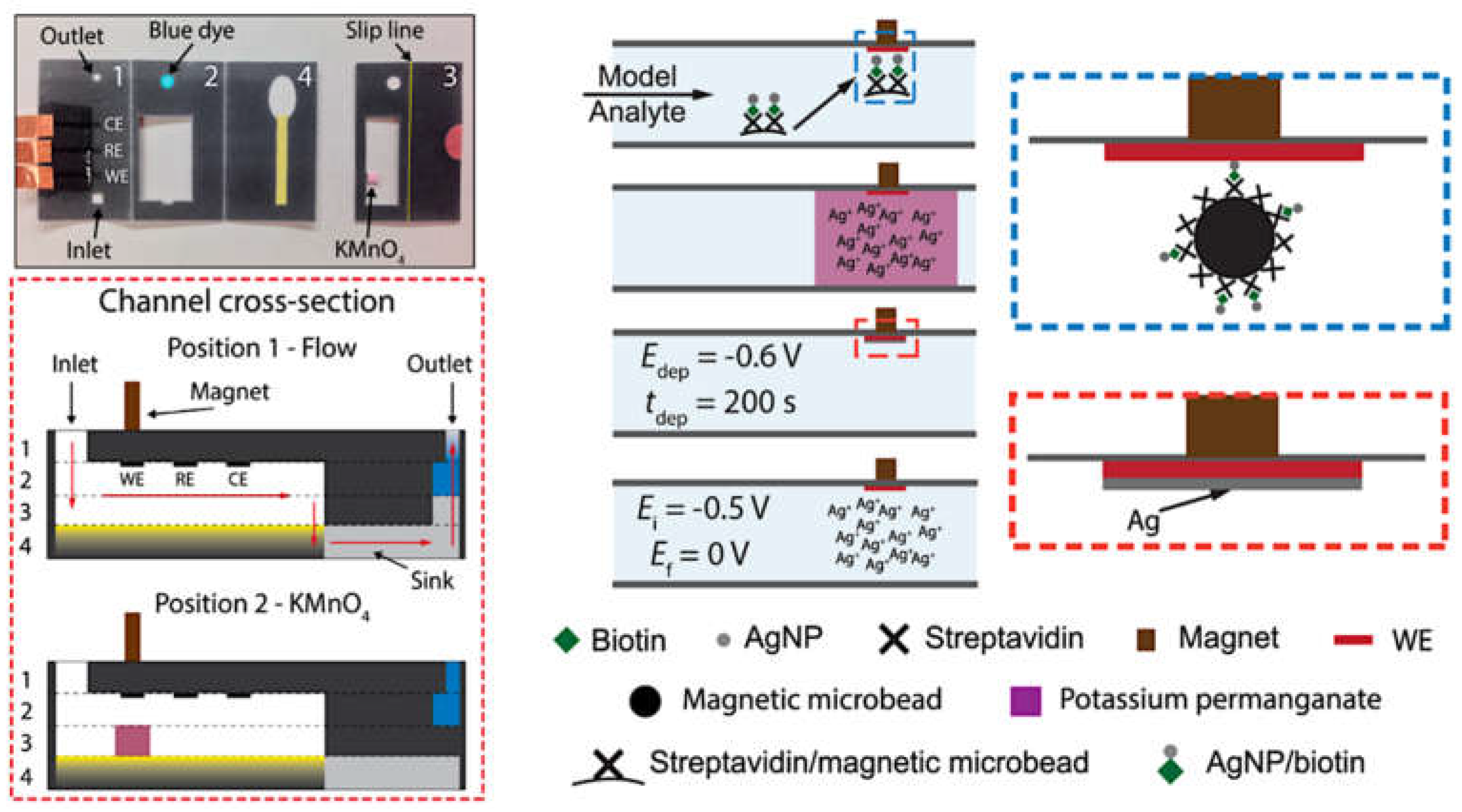
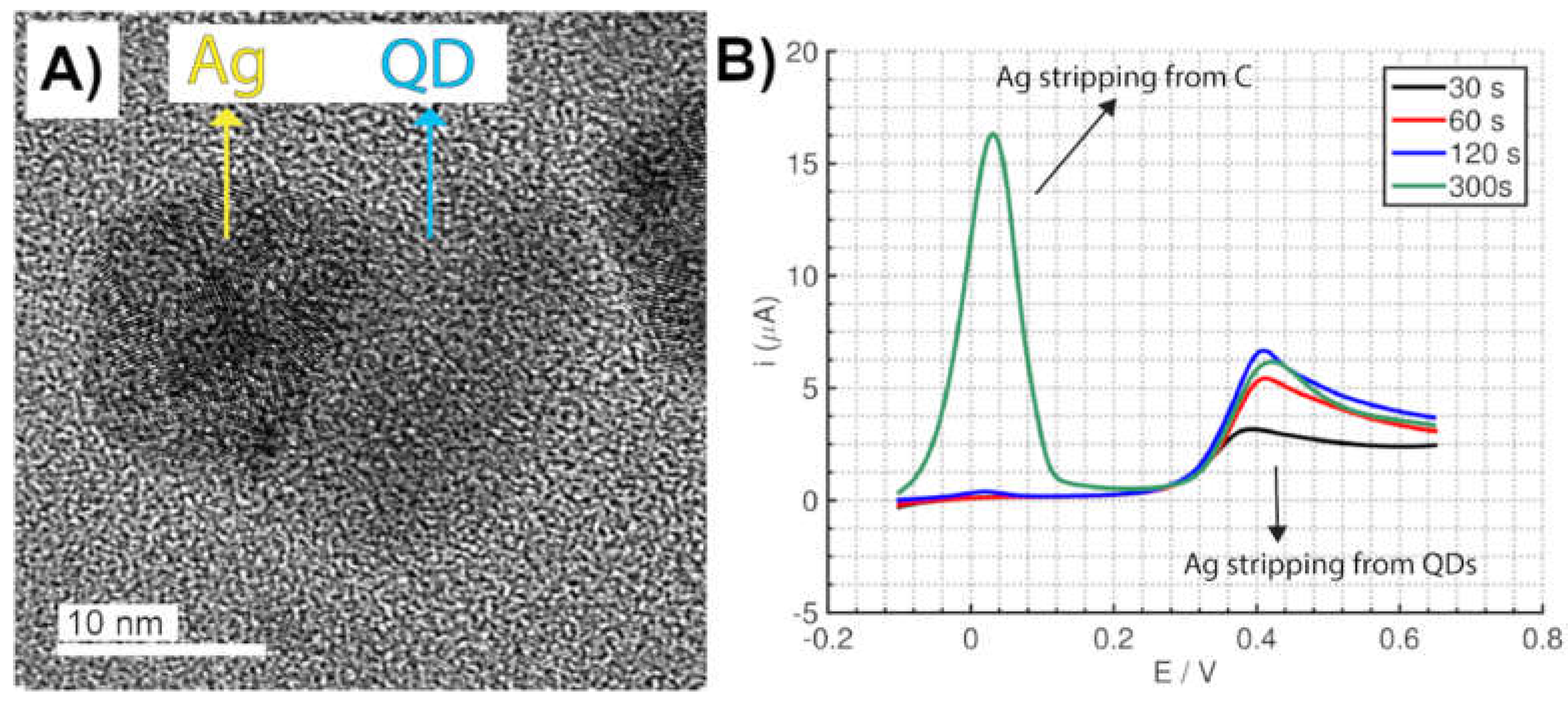
2.4. Surface-Enhanced Detection Methods
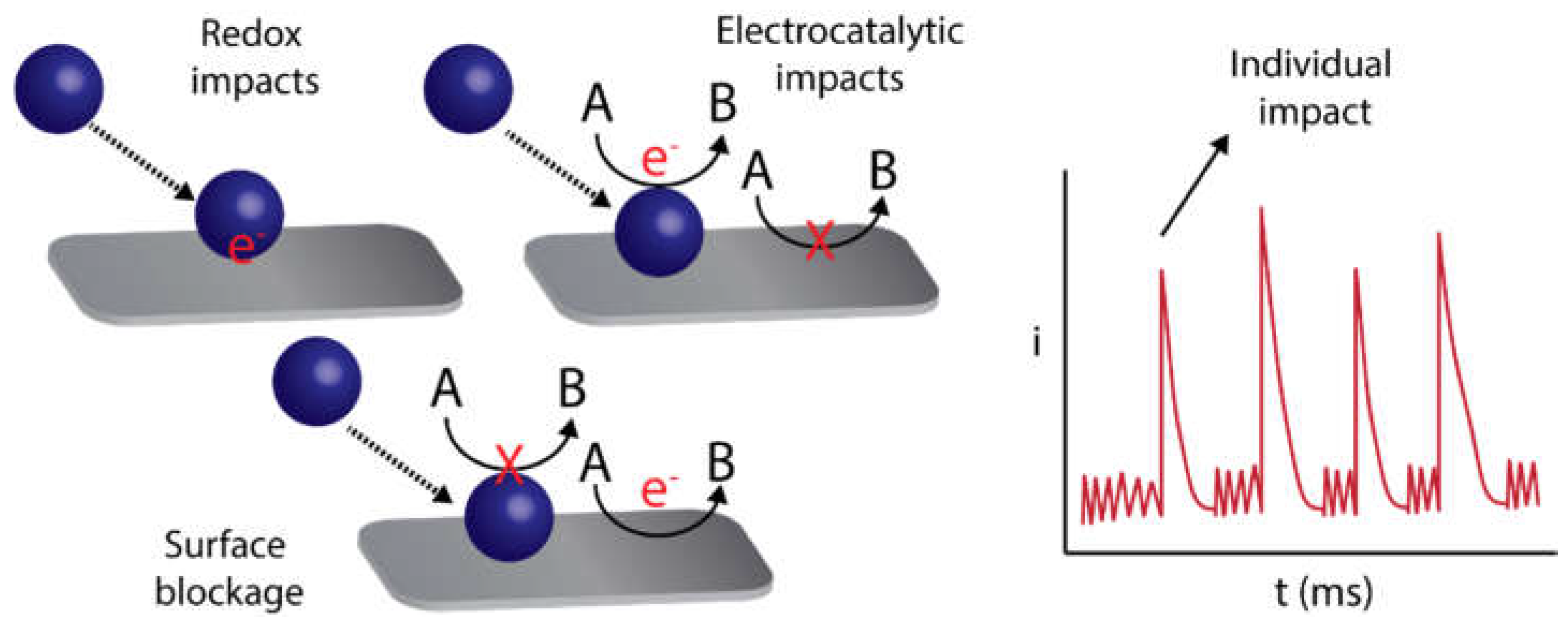
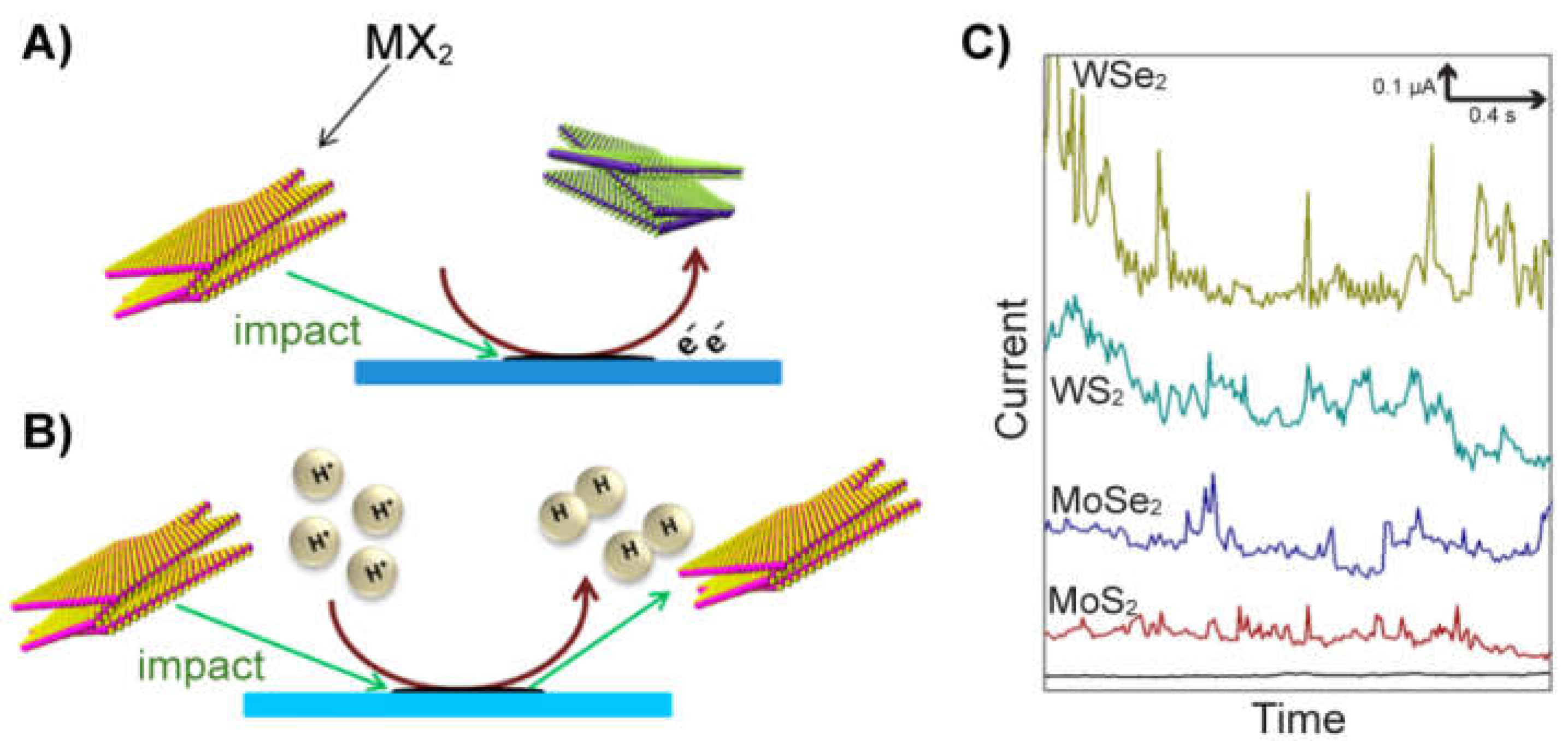
2.5. Detection by Nanoimpacts
3. Conclusions and Outlook
Funding
Conflicts of Interest
References
- Rao, C.N.R.; Müller, A.; Cheetham, A.K. The Chemistry of Nanomaterials: Synthesis, Properties and Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2004; ISBN 9783527602476. [Google Scholar]
- Geckeler, K.E.; Nishide, H. Advanced Nanomaterials; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2009; ISBN 9783527628940. [Google Scholar]
- Rogers, K.R.; Navratilova, J.; Stefaniak, A.; Bowers, L.; Knepp, A.K.; Al-Abed, S.R.; Potter, P.; Gitipour, A.; Radwan, I.; Nelson, C.; et al. Characterization of engineered nanoparticles in commercially available spray disinfectant products advertised to contain colloidal silver. Sci. Total Environ. 2018, 619–620, 1375–1384. [Google Scholar] [CrossRef] [PubMed]
- Wood, V.; Bulović, V. Colloidal quantum dot light-emitting devices. Nano Rev. 2010, 1, 5202. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.-J.; Yang, J.-H.; Kang, J.; Yan, Y.; Wei, S.-H. Halide perovskite materials for solar cells: A theoretical review. J. Mater. Chem. A 2015, 3, 8926–8942. [Google Scholar] [CrossRef]
- Vallés, C.; Jiménez, P.; Muñoz, E.; Benito, A.M.; Maser, W.K. Graphene: 2D-Building Block for Functional Nanocomposites; Springer: Dordrecht, The Netherlands, 2011; pp. 143–148. [Google Scholar]
- Klaine, S.J.; Alvarez, P.J.J.; Batley, G.E.; Fernandes, T.F.; Handy, R.D.; Lyon, D.Y.; Mahendra, S.; McLaughlin, M.J.; Lead, J.R. Nanomaterials in the environment: Behavior, fate, bioavailability, and effects. Environ. Toxicol. Chem. 2008, 27, 1825–1851. [Google Scholar] [CrossRef] [PubMed]
- Contado, C. Nanomaterials in consumer products: A challenging analytical problem. Front. Chem. 2015, 3, 48. [Google Scholar] [CrossRef]
- Laborda, F.; Bolea, E.; Cepriá, G.; Gómez, M.T.; Jiménez, M.S.; Pérez-Arantegui, J.; Castillo, J.R. Detection, characterization and quantification of inorganic engineered nanomaterials: A review of techniques and methodological approaches for the analysis of complex samples. Anal. Chim. Acta 2016, 904, 10–32. [Google Scholar] [CrossRef]
- Carlson, C.; Hussain, S.M.; Schrand, A.M.; Braydich-Stolle, L.K.; Hess, K.L.; Jones, R.L.; Schlager, J.J. Unique cellular interaction of silver nanoparticles: Size-dependent generation of reactive oxygen species. J. Phys. Chem. B 2008, 112, 13608–13619. [Google Scholar] [CrossRef] [PubMed]
- Koutilellis, G.D.; Economou, A.; Efstathiou, C.E. A potentiostat featuring an integrator transimpedance amplifier for the measurement of very low currents—Proof-of-principle application in microfluidic separations and voltammetry. Rev. Sci. Instrum. 2016, 87, 034101. [Google Scholar] [CrossRef] [PubMed]
- Jeanneret, S.; Crespo, G.A.; Afshar, M.G.; Bakker, E. GalvaPot, a custom-made combination galvanostat/potentiostat and high impedance potentiometer for decentralized measurements of ionophore-based electrodes. Sens. Actuators B Chem. 2015, 207, 631–639. [Google Scholar] [CrossRef]
- Meloni, G.N. Building a microcontroller based potentiostat: A inexpensive and versatile platform for teaching electrochemistry and instrumentation. J. Chem. Educ. 2016, 93, 1320–1322. [Google Scholar] [CrossRef]
- Grattieri, M.; Minteer, S.D. Self-powered biosensors. ACS Sens. 2018, 3, 44–53. [Google Scholar] [CrossRef]
- Li, M.; Li, D.; Xiu, G.; Long, Y. Applications of screen-printed electrodes in current environmental analysis. Curr. Opin. Electrochem. 2017, 3, 137–143. [Google Scholar] [CrossRef]
- Rama, E.C.; Costa-García, A. Screen-printed electrochemical Immunosensors for the detection of cancer and cardiovascular biomarkers. Electroanalysis 2016, 28, 1700–1715. [Google Scholar] [CrossRef]
- Martín-Yerga, D.; Pérez-Junquera, A.; Hernández-Santos, D.; Fanjul-Bolado, P. Time-resolved luminescence spectroelectrochemistry at screen-printed electrodes: Following the Redox-dependent fluorescence of [Ru(bpy)3]2+. Anal. Chem. 2017, 89, 10649–10654. [Google Scholar] [CrossRef]
- Martín-Yerga, D.; Pérez-Junquera, A.; González-García, M.B.; Hernández-Santos, D.; Fanjul-Bolado, P. In situ spectroelectrochemical monitoring of dye bleaching after electrogeneration of chlorine-based species: Application to chloride detection. Anal. Chem. 2018, 90, 7442–7449. [Google Scholar] [CrossRef]
- Tonello, S.; Abate, G.; Borghetti, M.; Marziano, M.; Serpelloni, M.; Uberti, D.L.; Lopomo, N.F.; Memo, M.; Sardini, E. Wireless point-of-care platform with screen-printed sensors for biomarkers detection. IEEE Trans. Instrum. Meas. 2017, 66, 2448–2455. [Google Scholar] [CrossRef]
- Martín-Yerga, D.; Álvarez-Martos, I.; Blanco-López, M.C.; Henry, C.S.; Fernández-Abedul, M.T. Point-of-need simultaneous electrochemical detection of lead and cadmium using low-cost stencil-printed transparency electrodes. Anal. Chim. Acta 2017, 981, 24–33. [Google Scholar] [CrossRef]
- Dungchai, W.; Chailapakul, O.; Henry, C.S. Electrochemical detection for paper-based microfluidics. Anal. Chem. 2009, 81, 5821–5826. [Google Scholar] [CrossRef]
- Cunningham, J.C.; DeGregory, P.R.; Crooks, R.M. New functionalities for paper-based sensors lead to simplified user operation, lower limits of detection, and New applications. Annu. Rev. Anal. Chem. 2016, 9, 183–202. [Google Scholar] [CrossRef]
- Taleat, Z.; Khoshroo, A.; Mazloum-Ardakani, M. Screen-printed electrodes for biosensing: A review (2008–2013). Microchim. Acta 2014, 181, 865–891. [Google Scholar] [CrossRef]
- Neves, M.M.P.S.; González-García, M.B.; Hernández-Santos, D.; Fanjul-Bolado, P. Screen-printed electrochemical 96-well plate: A high-throughput platform for multiple analytical applications. Electroanalysis 2014, 26, 2764–2772. [Google Scholar] [CrossRef]
- Desmet, C.; Marquette, C.A.; Blum, L.J.; Doumèche, B. Paper electrodes for bioelectrochemistry: Biosensors and biofuel cells. Biosens. Bioelectron. 2016, 76, 145–163. [Google Scholar] [CrossRef]
- Smith, S.; Korvink, J.G.; Mager, D.; Land, K. The potential of paper-based diagnostics to meet the ASSURED criteria. RSC Adv. 2018, 8, 34012–34034. [Google Scholar] [CrossRef]
- Gong, M.M.; Sinton, D. Turning the page: Advancing paper-based microfluidics for broad diagnostic application. Chem. Rev. 2017, 117, 8447–8480. [Google Scholar] [CrossRef]
- Economou, A. Screen-printed electrodes modified with “Green” metals for electrochemical stripping analysis of toxic elements. Sensors 2018, 18, 1032. [Google Scholar] [CrossRef]
- Pérez-Fernández, B.; Martín-Yerga, D.; Costa-García, A. Electrodeposition of nickel nanoflowers on screen-printed electrodes and their application to non-enzymatic determination of sugars. RSC Adv. 2016, 6, 83748–83757. [Google Scholar] [CrossRef]
- Pérez-Fernández, B.; Martín-Yerga, D.; Costa-García, A. Galvanostatic electrodeposition of copper nanoparticles on screen-printed carbon electrodes and their application for reducing sugars determination. Talanta 2017, 175, 108–113. [Google Scholar] [CrossRef]
- Kim, J.; Jeerapan, I.; Sempionatto, J.R.; Barfidokht, A.; Mishra, R.K.; Campbell, A.S.; Hubble, L.J.; Wang, J. Wearable bioelectronics: enzyme-based body-worn electronic devices. Acc. Chem. Res. 2018, 51, 2820–2828. [Google Scholar] [CrossRef]
- Kim, J.; Sempionatto, J.R.; Imani, S.; Hartel, M.C.; Barfidokht, A.; Tang, G.; Campbell, A.S.; Mercier, P.P.; Wang, J. Simultaneous monitoring of sweat and interstitial fluid using a single Wearable biosensor platform. Adv. Sci. 2018, 5, 1800880. [Google Scholar] [CrossRef]
- Ray, T.R.; Choi, J.; Bandodkar, A.J.; Krishnan, S.; Gutruf, P.; Tian, L.; Ghaffari, R.; Rogers, J.A. Bio-integrated wearable systems: A comprehensive review. Chem. Rev. 2019. [Google Scholar] [CrossRef]
- Mishra, R.K.; Barfidokht, A.; Karajic, A.; Sempionatto, J.R.; Wang, J.; Wang, J. Wearable potentiometric tattoo biosensor for on-body detection of G-type nerve agents simulants. Sens. Actuators B Chem. 2018, 273, 966–972. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Martin, A.; García-Carmona, L.; Barfidokht, A.; Kurniawan, J.F.; Moreto, J.R.; Tang, G.; Shin, A.; Liu, X.; Escarpa, A.; et al. Skin-worn soft microfluidic potentiometric detection system. Electroanalysis 2019, 31, 239–245. [Google Scholar] [CrossRef]
- Lei, J.; Ju, H. Signal amplification using functional nanomaterials for biosensing. Chem. Soc. Rev. 2012, 41, 2122–2134. [Google Scholar] [CrossRef]
- Ruiz-Valdepeñas Montiel, V.; Gutiérrez, M.L.; Torrente-Rodríguez, R.M.; Povedano, E.; Vargas, E.; Reviejo, Á.J.; Linacero, R.; Gallego, F.J.; Campuzano, S.; Pingarrón, J.M. Disposable amperometric polymerase chain Reaction-free biosensor for direct detection of adulteration with horsemeat in raw lysates targeting mitochondrial DNA. Anal. Chem. 2017, 89, 9474–9482. [Google Scholar] [CrossRef]
- Ruiz-Valdepeñas Montiel, V.; Povedano, E.; Vargas, E.; Torrente-Rodríguez, R.M.; Pedrero, M.; Reviejo, A.J.; Campuzano, S.; Pingarrón, J.M. Comparison of different strategies for the Development of highly sensitive electrochemical nucleic acid biosensors using neither nanomaterials nor nucleic acid amplification. ACS Sens. 2018, 3, 211–221. [Google Scholar] [CrossRef]
- Kokkinos, C.; Economou, A. Emerging trends in biosensing using stripping voltammetric detection of metal-containing nanolabels—A review. Anal. Chim. Acta 2017, 961, 12–32. [Google Scholar] [CrossRef]
- Kokkinos, C.; Economou, A.; Prodromidis, M.I. Electrochemical immunosensors: Critical survey of different architectures and transduction strategies. TrAc Trends Anal. Chem. 2016, 79, 88–105. [Google Scholar] [CrossRef]
- Ju, H.; Zhang, X.; Wang, J. Biofunctionalization of nanomaterials. In NanoBiosensing. Biological and Medical Physics, Biomedical Engineering; Springer: New York, NY, USA, 2011; pp. 1–38. [Google Scholar]
- De la Escosura-Muñiz, A.; Ambrosi, A.; Merkoçi, A. Electrochemical analysis with nanoparticle-based biosystems. TrAc Trends Anal. Chem. 2008, 27, 568–584. [Google Scholar] [CrossRef]
- Valera, E.; Hernández-Albors, A.; Marco, M.-P. Electrochemical coding strategies using metallic nanoprobes for biosensing applications. TrAc Trends Anal. Chem. 2016, 79, 9–22. [Google Scholar] [CrossRef]
- Wang, J. Electrochemical biosensing based on noble metal nanoparticles. Microchim. Acta 2012, 177, 245–270. [Google Scholar] [CrossRef]
- Huo, X.; Liu, X.; Liu, J.; Sukumaran, P.; Alwarappan, S.; Wong, D.K.Y. Strategic applications of nanomaterials as sensing platforms and signal amplification markers at electrochemical immunosensors. Electroanalysis 2016, 28, 1730–1749. [Google Scholar] [CrossRef]
- Pedrero, M.; Campuzano, S.; Pingarrón, J.M. Electrochemical (bio)sensing of clinical markers using quantum dots. Electroanalysis 2017, 29, 24–37. [Google Scholar] [CrossRef]
- Barfidokht, A.; Ciampi, S.; Luais, E.; Darwish, N.; Gooding, J.J. Distance-dependent electron transfer at passivated electrodes decorated by gold nanoparticles. Anal. Chem. 2013, 85, 1073–1080. [Google Scholar] [CrossRef]
- Barfidokht, A.; Ciampi, S.; Luais, E.; Darwish, N.; Gooding, J.J. The influence of organic-film morphology on the efficient electron transfer at passivated polymer-modified electrodes to which nanoparticles are attached. ChemPhysChem 2013, 14, 2190–2197. [Google Scholar] [CrossRef]
- Taufik, S.; Barfidokht, A.; Alam, M.T.; Jiang, C.; Parker, S.G.; Gooding, J.J. An antifouling electrode based on electrode–organic layer–nanoparticle constructs: Electrodeposited organic layers versus self-assembled monolayers. J. Electroanal. Chem. 2016, 779, 229–235. [Google Scholar] [CrossRef]
- Lim, S.A.; Yoshikawa, H.; Tamiya, E.; Yasin, H.M.; Ahmed, M.U. A highly sensitive gold nanoparticle bioprobe based electrochemical immunosensor using screen printed graphene biochip. RSC Adv. 2014, 4, 58460–58466. [Google Scholar] [CrossRef]
- Xu, Q.; Yan, F.; Lei, J.; Leng, C.; Ju, H. Disposable electrochemical Immunosensor by using carbon sphere/gold nanoparticle composites as labels for signal amplification. Chem. Eur. J. 2012, 18, 4994–4998. [Google Scholar] [CrossRef]
- Omidfar, K.; Zarei, H.; Gholizadeh, F.; Larijani, B. A high-sensitivity electrochemical immunosensor based on mobile crystalline material-41–polyvinyl alcohol nanocomposite and colloidal gold nanoparticles. Anal. Biochem. 2012, 421, 649–656. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, T.R.; Martucci, D.H.; Faria, R.C. Simple disposable microfluidic device for Salmonella typhimurium detection by magneto-immunoassay. Sens. Actuators B Chem. 2018, 255, 684–691. [Google Scholar] [CrossRef]
- Parolo, C.; Medina-Sánchez, M.; Montón, H.; de la Escosura-Muñiz, A.; Merkoçi, A. Paper-based electrodes for nanoparticles detection. Part. Part. Syst. Charact. 2013, 30, 662–666. [Google Scholar] [CrossRef]
- De la Escosura-Muñiz, A.; Parolo, C.; Maran, F.; Mekoçi, A. Size-dependent direct electrochemical detection of gold nanoparticles: Application in magnetoimmunoassays. Nanoscale 2011, 3, 3350–3356. [Google Scholar] [CrossRef] [PubMed]
- López-Marzo, A.M.; Hoyos-de-la-Torre, R.; Baldrich, E. NaNO3/NaCl oxidant and polyethylene glycol (PEG) capped gold nanoparticles (AuNPs) as a novel green route for AuNPs detection in electrochemical biosensors. Anal. Chem. 2018, 90, 4010–4018. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Xu, Y.; Zhu, N.; He, P.; Fang, Y. An electrochemical DNA hybridization detection assay based on a silver nanoparticle label. Analyst 2002, 127, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Toh, H.S.; Batchelor-McAuley, C.; Tschulik, K.; Compton, R.G. Electrochemical detection of chloride levels in sweat using silver nanoparticles: A basis for the preliminary screening for cystic fibrosis. Analyst 2013, 138, 4292–4297. [Google Scholar] [CrossRef] [PubMed]
- Navolotskaya, D.V.; Toh, H.S.; Batchelor-McAuley, C.; Compton, R.G. Voltammetric study of the influence of various phosphate anions on silver nanoparticle oxidation. ChemistryOpen 2015, 4, 595–599. [Google Scholar] [CrossRef]
- Cepriá, G.; Córdova, W.R.; Jiménez-Lamana, J.; Laborda, F.; Castillo, J.R. Silver nanoparticle detection and characterization in silver colloidal products using screen printed electrodes. Anal. Methods 2014, 6, 3072–3078. [Google Scholar] [CrossRef]
- Cheng, W.; Stuart, E.J.E.; Tschulik, K.; Cullen, J.T.; Compton, R.G. A disposable sticky electrode for the detection of commercial silver NPs in seawater. Nanotechnology 2013, 24, 505501. [Google Scholar] [CrossRef]
- Hao, N.; Li, H.; Long, Y.; Zhang, L.; Zhao, X.; Xu, D.; Chen, H. An electrochemical immunosensing method based on silver nanoparticles. J. Electroanal. Chem. 2011, 656, 50–54. [Google Scholar] [CrossRef]
- Song, W.; Li, H.; Liang, H.; Qiang, W.; Xu, D. Disposable electrochemical aptasensor array by using in situ DNA Hybridization inducing silver nanoparticles aggregate for signal amplification. Anal. Chem. 2014, 86, 2775–2783. [Google Scholar] [CrossRef]
- Hori, N.; Chikae, M.; Kirimura, H.; Takamura, Y. Highly sensitive detection using dual working electrode and concentration process in electrochemical metalloimmunoassay. Electrochim. Acta 2015, 174, 799–805. [Google Scholar] [CrossRef]
- Cunningham, J.C.; Kogan, M.R.; Tsai, Y.; Luo, L.; Richards, I.; Crooks, R.M. Paper-based sensor for electrochemical detection of silver nanoparticle labels by galvanic exchange. ACS Sens. 2016, 1, 40–47. [Google Scholar] [CrossRef]
- Scida, K.; Cunningham, J.C.; Renault, C.; Richards, I.; Crooks, R.M. Simple, sensitive, and quantitative electrochemical detection method for paper analytical devices. Anal. Chem. 2014, 86, 6501–6507. [Google Scholar] [CrossRef]
- Russo, L.; Puntes, V.; Merkoçi, A. Tunable electrochemistry of gold-silver alloy nanoshells. Nano Res. 2018, 11, 6336–6345. [Google Scholar] [CrossRef]
- Russo, L.; Leva Bueno, J.; Bergua, J.F.; Costantini, M.; Giannetto, M.; Puntes, V.; de la Escosura-Muñiz, A.; Merkoçi, A. Low-cost strategy for the development of a rapid electrochemical assay for bacteria detection based on AuAg nanoshells. ACS Omega 2018, 3, 18849–18856. [Google Scholar] [CrossRef]
- Merkoçi, A.; Marcolino-Junior, L.H.; Marín, S.; Fatibello-Filho, O.; Alegret, S. Detection of cadmium sulphide nanoparticles by using screen-printed electrodes and a handheld device. Nanotechnology 2007, 18, 035502. [Google Scholar] [CrossRef]
- Marin, S.; Merkoçi, A. Direct electrochemical stripping detection of cystic-fibrosis-related DNA linked through cadmium sulfide quantum dots. Nanotechnology 2009, 20, 055101. [Google Scholar] [CrossRef]
- Medina-Sánchez, M.; Miserere, S.; Marín, S.; Aragay, G.; Merkoçi, A. On-chip electrochemical detection of CdS quantum dots using normal and multiple recycling flow through modes. Lab Chip 2012, 12, 2000. [Google Scholar] [CrossRef]
- Freitas, M.; Viswanathan, S.; Nouws, H.P.A.; Oliveira, M.B.P.P.; Delerue-Matos, C. Iron oxide/gold core/shell nanomagnetic probes and CdS biolabels for amplified electrochemical immunosensing of Salmonella typhimurium. Biosens. Bioelectron. 2014, 51, 195–200. [Google Scholar] [CrossRef]
- Lu, J.; Ge, S.; Ge, L.; Yan, M.; Yu, J. Electrochemical DNA sensor based on three-dimensional folding paper device for specific and sensitive point-of-care testing. Electrochim. Acta 2012, 80, 334–341. [Google Scholar] [CrossRef]
- Li, L.; Kong, Q.; Zhang, Y.; Dong, C.; Ge, S.; Yu, J. A 3D electrochemical immunodevice based on a porous Pt-paper electrode and metal ion functionalized flower-like Au nanoparticles. J. Mater. Chem. B 2015, 3, 2764–2769. [Google Scholar] [CrossRef]
- Carrasco-Rodríguez, J.; Martín-Yerga, D.; Garrido, L.; Costa-García, A.; García Alonso, F.J. Sequential incorporation of metallic cations (Cd2+ and Hg2+) and N-octylamine into titanium phosphate nanoparticles and their subsequent release in acid media. Dalt. Trans. 2017, 46, 7061–7073. [Google Scholar] [CrossRef]
- Martín-Yerga, D.; Carrasco-Rodríguez, J.; González-García, M.B.; García Alonso, F.J.; Costa-García, A. Determination of silver-modified titanium phosphate nanoparticles by voltammetric and electrocatalytic methods. Electroanalysis 2014, 26, 2574–2579. [Google Scholar] [CrossRef]
- Martín-Yerga, D.; Carrasco-Rodríguez, J.; García Alonso, F.J.; Costa-García, A. Competitive electrochemical biosensing of biotin using cadmium-modified titanium phosphate nanoparticles and 8-channel screen-printed disposable electrodes. Anal. Methods 2017, 9, 3983–3991. [Google Scholar] [CrossRef]
- Carrasco-Rodríguez, J.; García Alonso, F.J.; Costa-García, A.; Martín-Yerga, D. Tuning the incorporation of electroactive metals into titanium phosphate nanoparticles and the reverse metal extraction process: Application as electrochemical labels in multiplex biosensing. Electrochem. Commun. 2017, 83, 1–5. [Google Scholar] [CrossRef]
- Authier, L.; Grossiord, C.; Brossier, P.; Limoges, B. Gold nanoparticle-based quantitative electrochemical detection of amplified human cytomegalovirus DNA using disposable microband electrodes. Anal. Chem. 2001, 73, 4450–4456. [Google Scholar] [CrossRef]
- Szymanski, M.S.; Porter, R.A. Preparation and quality control of silver nanoparticle–antibody conjugate for use in electrochemical immunoassays. J. Immunol. Methods 2013, 387, 262–269. [Google Scholar] [CrossRef]
- Szymanski, M.; Turner, A.; Porter, R. Electrochemical dissolution of silver nanoparticles and its application in metalloimmunoassay. Electroanalysis 2010, 22, 191–198. [Google Scholar] [CrossRef]
- Li, X.; Scida, K.; Crooks, R.M. Detection of hepatitis B virus DNA with a paper electrochemical sensor. Anal. Chem. 2015, 87, 9009–9015. [Google Scholar] [CrossRef]
- Cunningham, J.C.; Scida, K.; Kogan, M.R.; Wang, B.; Ellington, A.D.; Crooks, R.M. Paper diagnostic device for quantitative electrochemical detection of ricin at picomolar levels. Lab Chip 2015, 15, 3707–3715. [Google Scholar] [CrossRef]
- Wang, J.; Liu, G.; Merkoçi, A. Electrochemical coding technology for simultaneous detection of multiple DNA targets. J. Am. Chem. Soc. 2003, 125, 3214–3215. [Google Scholar] [CrossRef]
- Liu, G.; Wang, J.; Kim, J.; Jan, M.R.; Collins, G.E. Electrochemical coding for multiplexed immunoassays of proteins. Anal. Chem. 2004, 76, 7126–7130. [Google Scholar] [CrossRef]
- Pinwattana, K.; Wang, J.; Lin, C.-T.; Wu, H.; Du, D.; Lin, Y.; Chailapakul, O. CdSe/ZnS quantum dots based electrochemical immunoassay for the detection of phosphorylated bovine serum albumin. Biosens. Bioelectron. 2010, 26, 1109–1113. [Google Scholar] [CrossRef] [PubMed]
- Nian, H.; Wang, J.; Wu, H.; Lo, J.; Chiu, K.; Pounds, J.G.; Lin, Y. Electrochemical immunoassay of cotinine in serum based on nanoparticle probe and immunochromatographic strip. Anal. Chim. Acta 2012, 713, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Martín-Yerga, D.; González-García, M.B.; Costa-García, A. Biosensor array based on the in situ detection of quantum dots as electrochemical label. Sens. Actuators B Chem. 2013, 182, 184–189. [Google Scholar] [CrossRef]
- Martín-Yerga, D.; González-García, M.B.; Costa-García, A. Electrochemical immunosensor for anti-tissue transglutaminase antibodies based on the in situ detection of quantum dots. Talanta 2014, 130, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Martín-Yerga, D.; Costa-García, A. Towards a blocking-free electrochemical immunosensing strategy for anti-transglutaminase antibodies using screen-printed electrodes. Bioelectrochemistry 2015, 105, 88–94. [Google Scholar] [CrossRef]
- Martín-Yerga, D.; Bouzas-Ramos, D.; Menéndez-Miranda, M.; Bustos, A.R.M.; Encinar, J.R.; Costa-Fernández, J.M.; Sanz-Medel, A.; Costa-García, A. Voltammetric determination of size and particle concentration of cd-based quantum dots. Electrochim. Acta 2015, 166, 100–106. [Google Scholar] [CrossRef]
- Kokkinos, C.; Prodromidis, M.; Economou, A.; Petrou, P.; Kakabakos, S. Quantum dot-based electrochemical DNA biosensor using a screen-printed graphite surface with embedded bismuth precursor. Electrochem. Commun. 2015, 60, 47–51. [Google Scholar] [CrossRef]
- Kokkinos, C.; Prodromidis, M.; Economou, A.; Petrou, P.; Kakabakos, S. Disposable integrated bismuth citrate-modified screen-printed immunosensor for ultrasensitive quantum dot-based electrochemical assay of C-reactive protein in human serum. Anal. Chim. Acta 2015, 886, 29–36. [Google Scholar] [CrossRef]
- Medina-Sánchez, M.; Miserere, S.; Cadevall, M.; Merkoçi, A. Enhanced detection of quantum dots labeled protein by simultaneous bismuth electrodeposition into microfluidic channel. Electrophoresis 2016, 37, 432–437. [Google Scholar] [CrossRef]
- Medina-Sánchez, M.; Miserere, S.; Morales-Narváez, E.; Merkoçi, A. On-chip magneto-immunoassay for Alzheimer’s biomarker electrochemical detection by using quantum dots as labels. Biosens. Bioelectron. 2014, 54, 279–284. [Google Scholar] [CrossRef]
- Kokkinos, C.; Angelopoulou, M.; Economou, A.; Prodromidis, M.; Florou, A.; Haasnoot, W.; Petrou, P.; Kakabakos, S. Lab-on-a-membrane foldable devices for duplex drop-volume electrochemical biosensing using quantum dot tags. Anal. Chem. 2016, 88, 6897–6904. [Google Scholar] [CrossRef]
- Kokkinos, C.T.; Giokas, D.L.; Economou, A.S.; Petrou, P.S.; Kakabakos, S.E. Paper-based microfluidic device with integrated sputtered electrodes for stripping voltammetric determination of DNA via quantum dot labeling. Anal. Chem. 2018, 90, 1092–1097. [Google Scholar] [CrossRef]
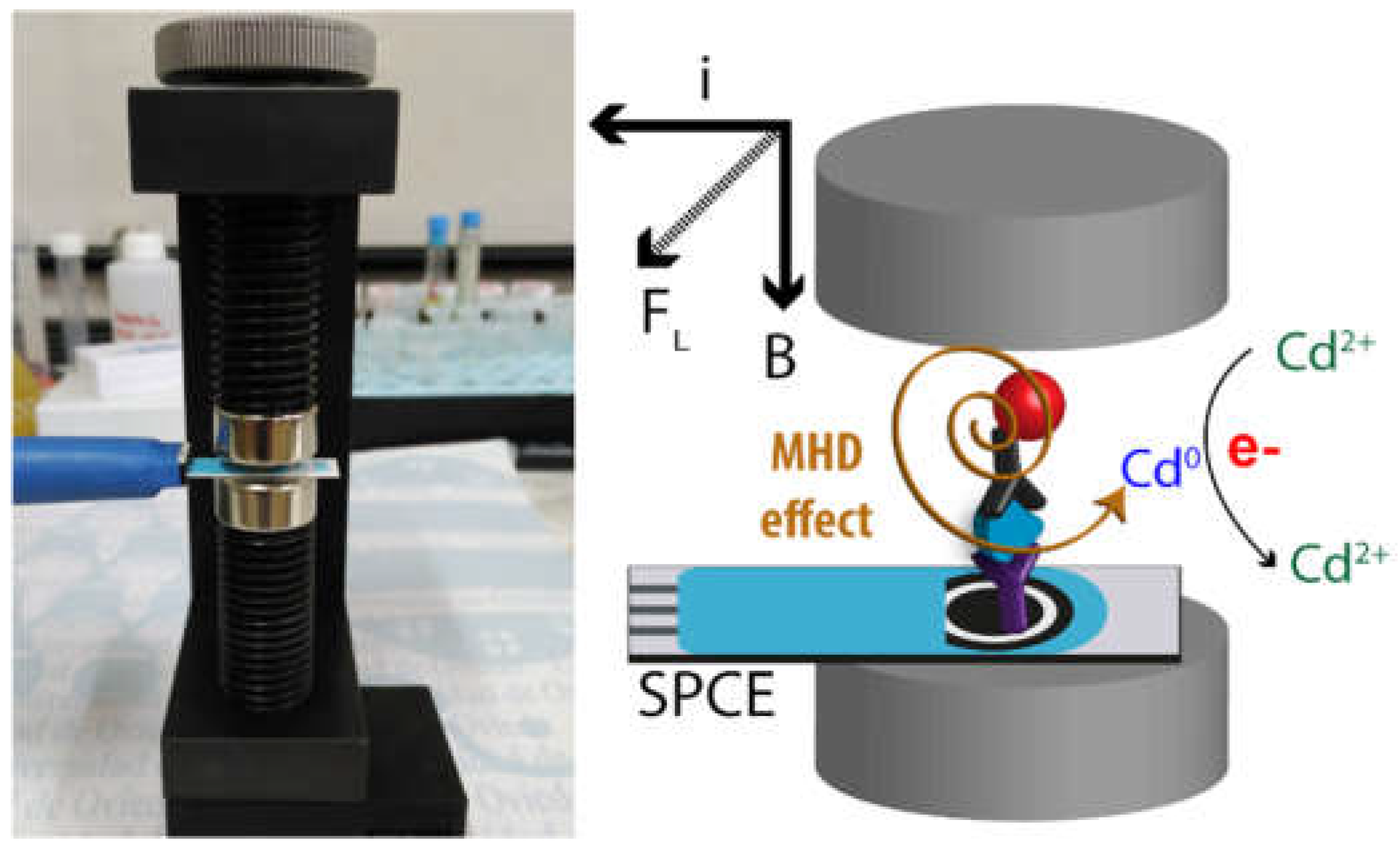
- Martín-Yerga, D.; Fanjul-Bolado, P.; Hernández-Santos, D.; Costa-García, A. Enhanced detection of quantum dots by the magnetohydrodynamic effect for electrochemical biosensing. Analyst 2017, 142, 1591–1600. [Google Scholar] [CrossRef]
- Pereira Silva Neves, M.M.; González-García, M.B.; Bobes-Limenes, P.; Pérez-Junquera, A.; Hernández-Santos, D.; Vidal-Iglesias, F.J.; Solla-Gullón, J.; Fanjul-Bolado, P. A non-enzymatic ethanol sensor based on a nanostructured catalytic disposable electrode. Anal. Methods 2017, 9, 5108–5114. [Google Scholar] [CrossRef]
- Higgins, D.; Landers, A.T.; Ji, Y.; Nitopi, S.; Morales-Guio, C.G.; Wang, L.; Chan, K.; Hahn, C.; Jaramillo, T.F. Guiding electrochemical carbon dioxide reduction toward carbonyls using copper silver thin films with interphase miscibility. ACS Energy Lett. 2018, 3, 2947–2955. [Google Scholar] [CrossRef]
- Maltez-da Costa, M.; de la Escosura-Muñiz, A.; Nogués, C.; Barrios, L.; Ibáñez, E.; Merkoçi, A. Detection of circulating cancer cells using electrocatalytic gold nanoparticles. Small 2012, 8, 3605–3612. [Google Scholar] [CrossRef]
- Maltez-da Costa, M.; de la Escosura-Muñiz, A.; Nogués, C.; Barrios, L.; Ibáñez, E.; Merkoçi, A. Simple monitoring of cancer cells using nanoparticles. Nano Lett. 2012, 12, 4164–4171. [Google Scholar] [CrossRef]
- De la Escosura-Muñiz, A.; Sánchez-Espinel, C.; Díaz-Freitas, B.; González-Fernández, A.; Maltez-da Costa, M.; Merkoçi, A. Rapid identification and quantification of tumor cells using an electrocatalytic method based on gold nanoparticles. Anal. Chem. 2009, 81, 10268–10274. [Google Scholar] [CrossRef]
- Espinoza-Castañeda, M.; de la Escosura-Muñiz, A.; González-Ortiz, G.; Martín-Orúe, S.M.; Pérez, J.F.; Merkoçi, A. Casein modified gold nanoparticles for future theranostic applications. Biosens. Bioelectron. 2013, 40, 271–276. [Google Scholar] [CrossRef]
- Sun, G.; Liu, H.; Zhang, Y.; Yu, J.; Yan, M.; Song, X.; He, W. Gold nanorods-paper electrode based enzyme-free electrochemical immunoassay for prostate specific antigen using porous zinc oxide spheres–silver nanoparticles nanocomposites as labels. New J. Chem. 2015, 39, 6062–6067. [Google Scholar] [CrossRef]
- Rivas, L.; de la Escosura-Muñiz, A.; Pons, J.; Merkoçi, A. Alzheimer disease biomarker detection through Electrocatalytic water oxidation induced by iridium oxide nanoparticles. Electroanalysis 2014, 26, 1287–1294. [Google Scholar] [CrossRef]
- Abbott, D.F.; Lebedev, D.; Waltar, K.; Povia, M.; Nachtegaal, M.; Fabbri, E.; Copéret, C.; Schmidt, T.J. Iridium oxide for the oxygen evolution reaction: Correlation between particle size, morphology, and the surface hydroxo layer from operando XAS. Chem. Mater. 2016, 28, 6591–6604. [Google Scholar] [CrossRef]
- Leng, C.; Wu, J.; Xu, Q.; Lai, G.; Ju, H.; Yan, F. A highly sensitive disposable immunosensor through direct electro-reduction of oxygen catalyzed by palladium nanoparticle decorated carbon nanotube label. Biosens. Bioelectron. 2011, 27, 71–76. [Google Scholar] [CrossRef]
- Charoenkitamorn, K.; Tue, P.; Kawai, K.; Chailapakul, O.; Takamura, Y. Electrochemical immunoassay using open circuit potential detection labeled by platinum nanoparticles. Sensors 2018, 18, 444. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, D.; Alocilja, E.C.; Chakrabartty, S. Biomolecules detection using a silver-enhanced gold nanoparticle-based biochip. Nanoscale Res. Lett. 2010, 5, 533–538. [Google Scholar] [CrossRef]
- Hernández-Santos, D.; González-García, M.; Costa-García, A. Electrochemical determination of gold nanoparticles in colloidal solutions. Electrochim. Acta 2000, 46, 607–615. [Google Scholar] [CrossRef]
- Mao, X.; Jiang, J.; Luo, Y.; Shen, G.; Yu, R. Copper-enhanced gold nanoparticle tags for electrochemical stripping detection of human IgG. Talanta 2007, 73, 420–424. [Google Scholar] [CrossRef]
- De la Escosura-Muñiz, A.; Maltez-da Costa, M.; Merkoçi, A. Controlling the electrochemical deposition of silver onto gold nanoparticles: Reducing interferences and increasing the sensitivity of magnetoimmuno assays. Biosens. Bioelectron. 2009, 24, 2475–2482. [Google Scholar] [CrossRef]
- Lai, G.; Wang, L.; Wu, J.; Ju, H.; Yan, F. Electrochemical stripping analysis of nanogold label-induced silver deposition for ultrasensitive multiplexed detection of tumor markers. Anal. Chim. Acta 2012, 721, 1–6. [Google Scholar] [CrossRef]
- Lai, G.; Yan, F.; Wu, J.; Leng, C.; Ju, H. Ultrasensitive multiplexed immunoassay with electrochemical stripping analysis of silver nanoparticles catalytically deposited by gold nanoparticles and enzymatic reaction. Anal. Chem. 2011, 83, 2726–2732. [Google Scholar] [CrossRef]
- Duangkaew, P.; Wutikhun, T.; Laocharoensuk, R. Triple signal amplification strategy based on size and shape transformation of ultrasmall sub-10 nm gold nanoparticles tag towards sensitivity improvement of electrochemical immunosensors. Sens. Actuators B Chem. 2017, 239, 430–437. [Google Scholar] [CrossRef]
- Martín-Yerga, D.; Rama, E.C.; Costa-García, A. Electrochemical study and applications of selective electrodeposition of silver on quantum dots. Anal. Chem. 2016, 88, 3739–3746. [Google Scholar] [CrossRef]
- Martín-Yerga, D.; Costa-García, A. Stabilization of electrogenerated copper species on electrodes modified with quantum dots. Phys. Chem. Chem. Phys. 2017, 19, 5018–5027. [Google Scholar] [CrossRef]
- Martín-Yerga, D.; Costa-García, A. Electrochemical detection of quantum dots by stabilization of electrogenerated copper species. Electrochem. Commun. 2017, 74, 53–56. [Google Scholar] [CrossRef]
- Sokolov, S.V.; Eloul, S.; Kätelhön, E.; Batchelor-McAuley, C.; Compton, R.G. Electrode–particle impacts: A users guide. Phys. Chem. Chem. Phys. 2017, 19, 28–43. [Google Scholar] [CrossRef]
- Stevenson, K.J.; Tschulik, K. A materials driven approach for understanding single entity nano impact electrochemistry. Curr. Opin. Electrochem. 2017, 6, 38–45. [Google Scholar] [CrossRef]
- Neves, M.; Martín-Yerga, D. Advanced nanoscale approaches to single-(bio)entity sensing and imaging. Biosensors 2018, 8, 100. [Google Scholar] [CrossRef]
- Zhou, Y.-G.; Rees, N.V.; Compton, R.G. The electrochemical detection and characterization of silver nanoparticles in aqueous solution. Angew. Chem. Int. Ed. 2011, 50, 4219–4221. [Google Scholar] [CrossRef]
- Bentley, C.L.; Kang, M.; Unwin, P.R. Time-resolved detection of surface oxide formation at individual gold nanoparticles: Role in electrocatalysis and new approach for sizing by electrochemical impacts. J. Am. Chem. Soc. 2016, 138, 12755–12758. [Google Scholar] [CrossRef]
- Dick, J.E.; Hilterbrand, A.T.; Boika, A.; Upton, J.W.; Bard, A.J. Electrochemical detection of a single cytomegalovirus at an ultramicroelectrode and its antibody anchoring. Proc. Natl. Acad. Sci. USA 2015, 112, 5303–5308. [Google Scholar] [CrossRef]
- Nasir, M.Z.M.; Pumera, M. Impact electrochemistry on screen-printed electrodes for the detection of monodispersed silver nanoparticles of sizes 10–107 nm. Phys. Chem. Chem. Phys. 2016, 18, 28183–28188. [Google Scholar] [CrossRef]
- Lim, C.S.; Pumera, M. Impact electrochemistry: Colloidal metal sulfide detection by cathodic particle coulometry. Phys. Chem. Chem. Phys. 2015, 17, 26997–27000. [Google Scholar] [CrossRef]
- Giovanni, M.; Ambrosi, A.; Sofer, Z.; Pumera, M. Impact electrochemistry of individual molybdenum nanoparticles. Electrochem. Commun. 2015, 56, 16–19. [Google Scholar] [CrossRef]
- Mayorga-Martinez, C.C.; Mohamad Latiff, N.; Eng, A.Y.S.; Sofer, Z.; Pumera, M. Black phosphorus nanoparticle labels for immunoassays via hydrogen evolution reaction mediation. Anal. Chem. 2016, 88, 10074–10079. [Google Scholar] [CrossRef]
- Lim, C.S.; Tan, S.M.; Sofer, Z.; Pumera, M. Impact electrochemistry of layered transition metal dichalcogenides. ACS Nano 2015, 9, 8474–8483. [Google Scholar] [CrossRef]
- Thearle, R.A.; Sofer, Z.; Bouša, D.; Pumera, M. Impact electrochemistry: Detection of graphene nanosheets labeled with metal nanoparticles through oxygen reduction mediation. ChemPhysChem 2016, 17, 2096–2099. [Google Scholar] [CrossRef]
- Dick, J.E.; Hilterbrand, A.T.; Strawsine, L.M.; Upton, J.W.; Bard, A.J. Enzymatically enhanced collisions on ultramicroelectrodes for specific and rapid detection of individual viruses. Proc. Natl. Acad. Sci. USA 2016, 113, 6403–6408. [Google Scholar] [CrossRef]

© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Yerga, D. Electrochemical Detection and Characterization of Nanoparticles with Printed Devices. Biosensors 2019, 9, 47. https://doi.org/10.3390/bios9020047
Martín-Yerga D. Electrochemical Detection and Characterization of Nanoparticles with Printed Devices. Biosensors. 2019; 9(2):47. https://doi.org/10.3390/bios9020047
Chicago/Turabian StyleMartín-Yerga, Daniel. 2019. "Electrochemical Detection and Characterization of Nanoparticles with Printed Devices" Biosensors 9, no. 2: 47. https://doi.org/10.3390/bios9020047
APA StyleMartín-Yerga, D. (2019). Electrochemical Detection and Characterization of Nanoparticles with Printed Devices. Biosensors, 9(2), 47. https://doi.org/10.3390/bios9020047




