Innovative Sensor Approach to Follow Campylobacter jejuni Development
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Preparation
2.2. GC-MS Analysis
2.3. S3 Analysis
2.4. S3 Data Analysis
3. Results and Discussion
3.1. GC-MS Results
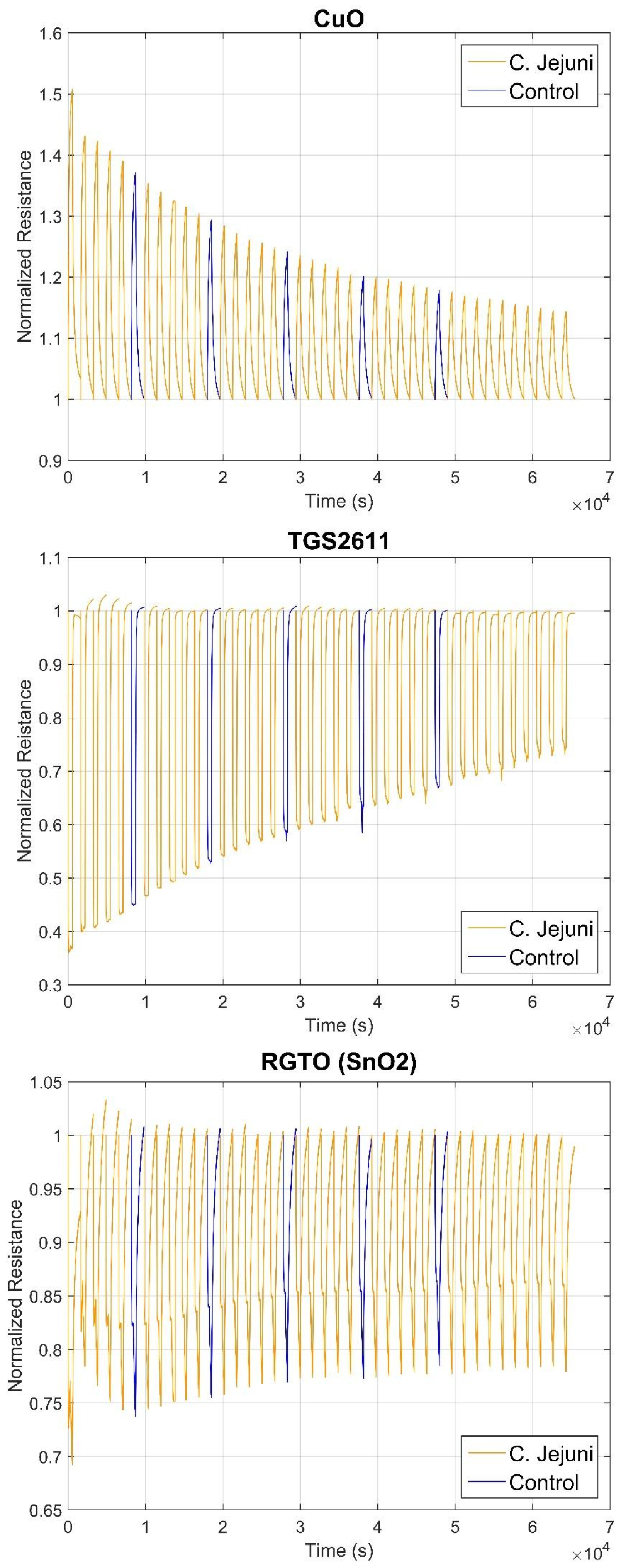
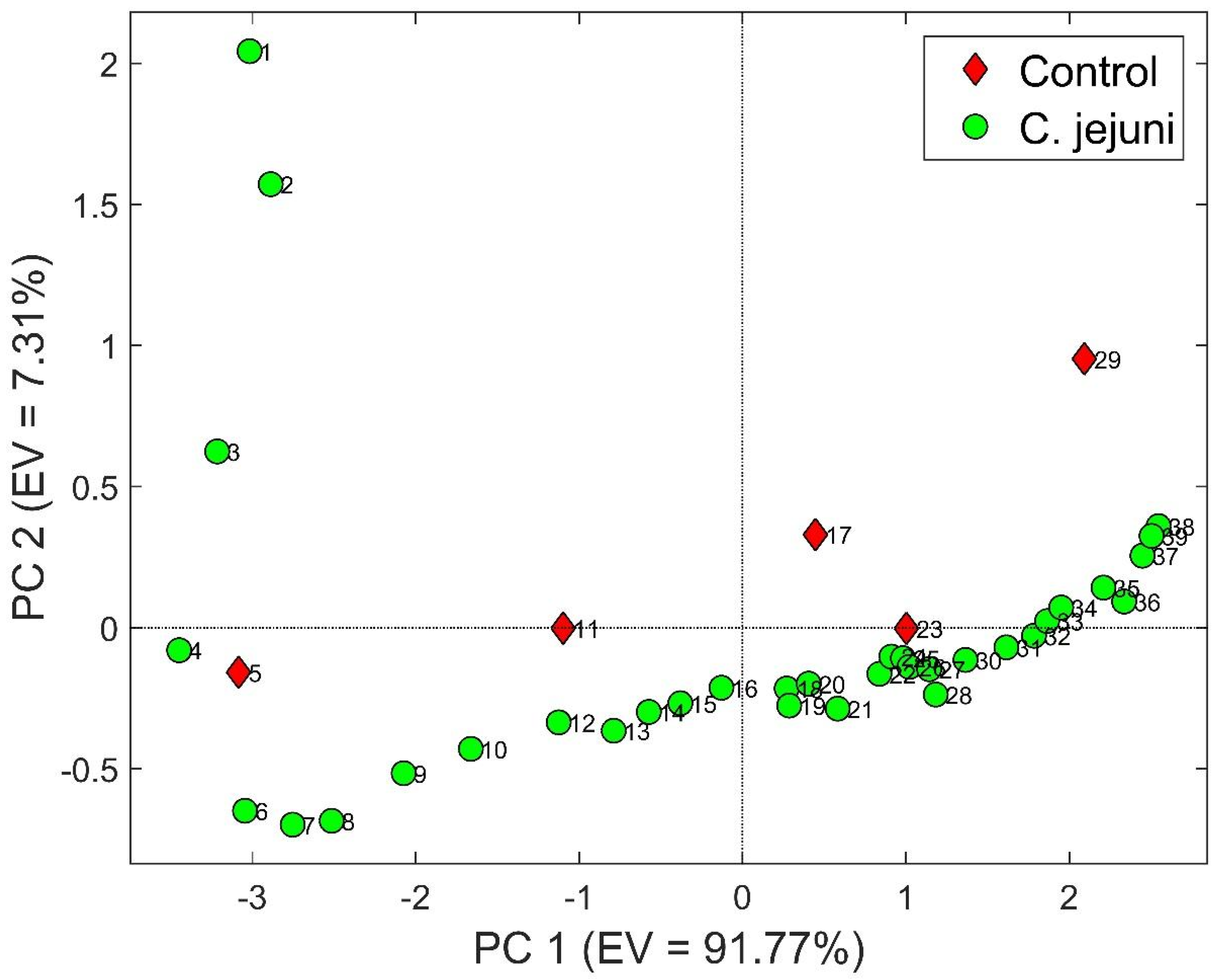
3.2. S3 Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yeung, R.M.W.; Morris, J. Food safety risk—Consumer perception and purchase behavior. Br. Food J. 2001, 103, 170–187. [Google Scholar] [CrossRef]
- Yu, H.; Neal, J.A.; Sirsat, S.A. Consumers’ food safety risk perceptions and willingness to pay for fresh-cut produce with lower risk of foodborne illness. Food Control 2018, 86, 83–89. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention, Foodborne Illnesses and Germs. Available online: https://www.cdc.gov/foodsafety/foodborne-germs.html (accessed on 31 October 2018).
- European Centers for Disease Prevention and Control, Campylobacteriosis—Annual Epidemiological Report 2016. Available online: https://ecdc.europa.eu/en/publications-data/campylobacteriosis-annual-epidemiological-report-2016-2014-data (accessed on 31 October 2018).
- Açik, M.N.; Çetinkaya, B. Heterogeneity of Campylobacter jejuni and Campylobacter coli strains from healthy sheep. Vet. Microbiol. 2006, 115, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.E.; Christensen, O.F.; Clough, H.E.; Diggle, P.J.; Hart, C.A.; Hazel, S.; Kemp, R.; Leatherbarrow, A.J.H.; Moore, A.; Sutherst, J.; et al. Frequency and spatial distribution of environmental Campylobacter spp. Appl. Environ. Microbiol. 2004, 70, 6501–6511. [Google Scholar] [CrossRef]
- Klipstein, F.A.; Engert, R.F. Properties of Crude Campylobacter jejuni Heat-Labile Enterotoxin. Infect. Immun. 1984, 45, 314–319. [Google Scholar] [PubMed]
- Jay, J.M.; Loessner, M.J.; Golden, D.A. Foodborne Gastroenteritis Caused by Vibrio, Yersinia and Campylobacter. In ModernFood Microbiology, 7th ed.; Heldman, D.R., Ed.; Heldman Associates: San Marcos, CA, USA, 2005; Volume 1, pp. 657–678. ISBN 0-387-23180-3. [Google Scholar]
- The National Antimicrobial Resistance Monitoring System: Enteric Bacteria. Available online: https://www.google.com/url?q=https://www.fda.gov/downloads/AnimalVeterinary/SafetyHealth/AntimicrobialResistance/NationalAntimicrobialResistanceMonitoringSystem/UCM528861.pdf&sa=D&ust=1543574350805000&usg=AFQjCNEMaE6VwxnVNmUYIOqrV7HnmBpcNQ (accessed on 30 November 2018).
- Artursson, K.; Schelin, J.; Lambertz, S.T.; Hansson, I.; Engvall, E.O. Foodborne pathogens in unpasteurized milk in Sweden. Int. J. Food Microbiol. 2018, 284, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Verhoeff-Bakkenesa, L.; Jansenb, H.A.P.M.; In’t Veldb, P.H.; Beumerc, R.R.; Zwieteringc, M.H.; van Leusden, F.M. Consumption of raw vegetables and fruits: A risk factor for Campylobacter infections. Int. J. Food Microbiol. 2011, 144, 406–412. [Google Scholar] [CrossRef]
- Cecchini, F.; Manzano, M.; Mandabi, Y.; Perelman, E.; Marks, R.S. Chemiluminescent DNA optical fibre sensor for Brettanomyces bruxellensis detection. J. Biotechnol. 2012, 157, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Jia, K.; Toury, T.; Ionescu, R.E. Fabrication of an atrazine acoustic immunosensor based on a drop-deposition procedure. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2012, 59, 2015–2021. [Google Scholar]
- Kubičárová, T.; Fojta, M.; Vidic, J.; Tomschik, M.; Suznjevic, D.; Paleček, E. Voltammetric and chronopotentiometric measurements with nucleic acid-modified mercury film on a glassy carbon electrode. Electroanalysis 2000, 12, 1390–1396. [Google Scholar] [CrossRef]
- Vidic, J.; Manzano, M.; Chang, C.M.; Jaffrezic-Renault, N. Advanced biosensors for detection of pathogens related to livestock and poultry. Vet. Res. 2017, 48, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Masdor, N.A.; Altintas, Z.; Tothilla, I.E. Sensitive detection of Campylobacter jejuni using nanoparticles enhanced QCM sensor. Biosens. Bioelectron. 2016, 78, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kim, H.S.; Chon, J.W.; Kim, D.H.; Hyeon, J.Y.; Seo, K.H. New colorimetric aptasensor for rapid on-site detection of Campylobacter jejuni and Campylobacter coli in chicken carcass samples. Anal. Chim. Acta 2018, 1029, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Sberveglieri, V.; Núñez-Carmona, E.; Ponzoni, A.; Comini, E.; Galstyan, V.; Zappa, D.; Pulvirenti, A. Skin microbiota monitoring by nanowire MOS sensors. In Proceedings of the 29th European Conference on Solid-State Transducers, EUROSENSORS 2015, Freiburg, Germany, 6–9 September 2015; Volume 120, pp. 756–759. [Google Scholar]
- Núñez-Carmona, E.; Soprani, M.; Sberveglieri, V. Nanowire (S3) Device for the quality control of drinking water. In Smart Sensors, Measurement and Instrumentation; Mukhopadhyay, S.C., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 23, pp. 179–203. [Google Scholar]
- Núñez-Carmona, E.; Sberveglieri, V.; Ponzoni, A.; Zappa, D.; Pulvirenti, A. Small Sensor Sistem S3 device to control the microbial contamination in water. In Proceedings of the 9th International Conference on Sensing Technology (ICST), Auckland, New Zealand, 8–11 December 2015; pp. 246–250. [Google Scholar]
- Núñez-Carmona, E.; Sberveglieri, V.; Ponzoni, A.; Galstyan, V.; Zappa, D.; Pulvirenti, A.; Comini, E. Detection of food and skin pathogen microbiota by means of an electronic nose based on metal oxide chemiresistors. Sens. Actuators B-Chem. 2017, 238, 1224–1230. [Google Scholar] [CrossRef]
- Zambotti, G.; Sberveglieri, V.; Gobbi, E.; Falasconi, M.; Núñez-Carmona, E.; Pulvirenti, A. Fast identification of microbiological contamination in vegetable soup by electronic nose. In Proceedings of the 28th European Conference on Solid-State Transducers, EUROSENSORS 2014, Brescia, Italy, 7–10 September 2014; pp. 246–250. [Google Scholar]
- Ponzoni, A.; Baratto, C.; Cattabiani, N.; Falasconi, M.; Galstyan, V.; Núñez-Carmona, E.; Rigoni, F.; Sberveglieri, V.; Zambotti, G.; Zappa, D. Smetal oxide gas sensors, a survey of selectivity issues addressed at the SENSOR lab, Brescia (Italy). Sensors 2017, 17, 714. [Google Scholar] [CrossRef]
- McFarland Standards. Available online: http://www.dalynn.com/dyn/ck_assets/files/tech/TM53.pdf (accessed on 30 November 2018).
- Abbatangelo, M.; Núñez-Carmona, E.; Sberveglieri, V.; Zappa, D.; Comini, E.; Sberveglieri, G. Application of a Novel S3 Nanowire Gas Sensor Device in Parallel with GC-MS for the Identification of Rind Percentage of Grated Parmigiano Reggiano. Sensors 2018, 18, 617. [Google Scholar] [CrossRef] [PubMed]
- Abbatangelo, M.; Núñez-Carmona, E.; Sberveglieri, V. Application of a novel S3 nanowire gas sensor device in parallel with GC-MS for the identification of Parmigiano Reggiano from US and European competitors. J. Food Eng. 2018, 236, 36–43. [Google Scholar] [CrossRef]
- Sberveglieri, V. Validation of Parmigiano Reggiano Cheese Aroma Authenticity, Categorized through the Use of an Array of Semiconductors Nanowire Device (S3). Materials 2016, 9, 81. [Google Scholar] [CrossRef]
- Sberveglieri, V.; Bhandari, M.P.; Núñez-Carmona, E.; Betto, G.; Sberveglieri, G. A novel MOS nanowire gas sensor device (S3) and GC-MS-based approach for the characterization of grated Parmigiano Reggiano cheese. Biosensors 2016, 6, 60. [Google Scholar] [CrossRef]
- Ponzoni, A.; Zappa, D.; Comini, E.; Sberveglieri, V.; Faglia, G.; Sberveglieri, G. Metal oxide nanowire gas sensors: Application of conductometric and surface ionization architectures. Chem. Eng. Trans. 2012, 30, 31–36. [Google Scholar] [CrossRef]
- Sberveglieri, G.; Concina, I.; Comini, E.; Falasconi, M.; Ferroni, M.; Sberveglieri, V. Synthesis and integration of tin oxide nanowires into an electronic nose. Vacuum 2012, 86, 532–535. [Google Scholar] [CrossRef]
- Wagner, R.S.; Ellis, W.C. Vapor-Liquid-Solid Mechanism of Single Crystal Growth. Appl. Phys. Lett. 1964, 4, 89–90. [Google Scholar] [CrossRef]
- Sberveglieri, G. Recent developments in semiconducting thin-film gas sensors. Sens. Actuators B-Chem. 1995, 23, 103–109. [Google Scholar] [CrossRef]
- Battersby, T.; Walsh, D.; Whyte, P.; Bolton, D.J. Campylobacter growth rates in four different matrices: Broiler caecal material, live birds, Bolton broth, and brain heart infusion broth. Infect. Ecol. Epidemiol. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
| Materials (Type) | Composition | Morphology | Operating Temperature (°C) | Response to 5 ppm of Ethanol | Selectivity | Limit of Detection (LOD) of Ethanol (ppm) |
|---|---|---|---|---|---|---|
| SnO2Au (n) | SnO2 functionalized with Au clusters | RGTO | 400 °C | 6.5 | 3 | 0.5 |
| SnO2 (n) | SnO2 | RGTO | 300 °C | 3.5 | 2.5 | 1 |
| SnO2 (n) | SnO2 | RGTO | 400 °C | 4 | 2 | 0.8 |
| SnO2Au + Au (n) | SnO2 grown with Au and functionalized with gold clusters | Nanowire | 350 °C | 7 | 2.5 | 0.5 |
| SnO2Au (n) | SnO2 grown with Au | Nanowire | 350 °C | 5 | 2.1 | 1 |
| CuO (p) | CuO | Nanowire | 400 °C | 1.5 | 1.5 | 1 |
| RT | VOC | Abundance | |
|---|---|---|---|
| Campylobacter | Control | ||
| 1.552 | 3-Butynol | 5,488,041 | 5,289,186 |
| 2.674 | Isovaleraldehyde | 28,336,125 | 25,535,856 |
| 5.386 | Dimethyl Disulfide | 5,401,037 | 6,048,406 |
| 8.666 | 3-O-Methyl-d-fructose | 912,105 | 904,062 |
| 9.281 | 1-Hydroperoxyheptane | 533,178 | 418,838 |
| 12.332 | 2,5-Dimethylpyrazine | 623,139 | 560,597 |
| 14.432 | Nonanal | 512,867 | 227,918 |
| 14.624 | 6-Methyloctadecane | 549,617 | 794,739 |
| 15.419 | 4-Methyl-2-oxovaleric acid | 457,664 | 412,957 |
| 15.805 | 2-Acetylamino-3-hydroxy-propionic acid | 27,861 | 51,993 |
| 16.017 | 1-(2-Methoxy-1-methylethoxy)-2-propanol | 519,216 | 240,149 |
| 16.372 | Ethylhexanol | 404,819 | 389,367 |
| 16.866 | Benzaldehyde | 12,509,648 | 13,515,034 |
| 17.430 | 3-Trifluoroacetoxydodecane | 92,701 | 168,299 |
| 18.455 | 3-Hydroxycyclohexanone | 145,875 | 206,891 |
| 18.695 | Acetophenone | 2,201,980 | 1,987,623 |
| 19.545 | [(2-Ethylhexyl)methyl]oxirane | 93,105 | 281,168 |
| 19.950 | Methoxy-phenyl-oxime | 1,545,714 | 1,301,333 |
| 20.871 | Heptanoic acid | 268,343 | 304,130 |
| 21.205 | Benzyl alcohol | 280,516 | 246,860 |
| 21.555 | 2-[2-(Benzyloxy)-1-(1-methoxy-1-methylethoxy)ethyl]oxirane | 226,967 | 202,114 |
| 22.021 | 1-Dodecanol | 222,887 | 482,480 |
| 22.465 | Phenyl carbamate | 51,364 | 93,104 |
| 23.580 | Octanal | 121,544 | 130,790 |
| 24.480 | Octadecanal | 131,841 | 76,150 |
| 25.078 | 2,6-Bis(tert-butyl)phenol | 691,529 | 597,086 |
| 27.565 | N,N-Dimethylformamide ethylene acetal | 53,073 | 24,830 |
| RT | VOC | Abundance | |
|---|---|---|---|
| Campylobacter | Control | ||
| 1.540 | 3-Butynol | 8,834,835 | 2,135,579 |
| 2.266 | Isovaleraldehyde | 221,889,809 | 3,852,176 |
| 4.142 | Dimethyl Disulfide | 42,167,678 | 0 |
| 8.377 | 1-Pentanol | 236,112,172 | 0 |
| 9.229 | Isoamyl Alcohol | 0 | 737,186 |
| 10.673 | Acetoin | 1,974,910 | 0 |
| 11.125 | 2-Methylbutyl isovalerate | 1,684,776 | 0 |
| 12.299 | 2,5-Dimethylpyrazine | 0 | 693,770 |
| 14.400 | Nonanal | 0 | 123,487 |
| 14.483 | Trimethylpyrazine | 0 | 258,896 |
| 14.600 | 6-Methyloctadecane | 0 | 60,501 |
| 15.275 | 2-Ethyl-3,6-dimethylpyrazine | 243,733 | 0 |
| 15.392 | 4-Methyl-2-oxovaleric acid | 454,870 | 273,645 |
| 15.702 | Ammonium acetate | 812,172 | 572,057 |
| 15.903 | 2,7-Dimethyl-4,5-octanediol | 945,696 | 0 |
| 16.186 | 1-(2-Methoxy-1-methylethoxy)-2-propanol | 0 | 79,930 |
| 16.295 | 2-Propyl-1-pentanol | 308,880 | 0 |
| 16.340 | Ethylhexanol | 267,747 | 301,354 |
| 16.845 | Benzaldehyde | 0 | 14,760,479 |
| 17.366 | 1-Octanol | 972,272 | 256,675 |
| 17.550 | Bicyclo[3.2.1]octan-6-ol | 164,638 | 0 |
| 17.946 | 2-Undecanone | 103,647 | 0 |
| 18.419 | 3-Hydroxycyclohexanone | 0 | 79,109 |
| 18.581 | Benzeneacetaldehyde | 5,087,169 | 0 |
| 18.668 | Acetophenone | 0 | 669,190 |
| 18.740 | 1-Nonanol | 1,331,499 | 0 |
| 18.914 | Methyl 4-hydroxybutanoate | 0 | 204,876 |
| 19.446 | γ-Methylmercaptopropyl alcohol | 690,884 | 0 |
| 19.923 | E-11,13-Tetradecadien-1-ol | 2,393,731 | 532,815 |
| 20.575 | β-Phenethyl acetate | 131,503 | 0 |
| 20.844 | Heptanoic acid | 488,771 | 215,935 |
| 21.187 | Benzyl alcohol | 342,619 | 146,035 |
| 21.540 | Phenylethyl Alcohol | 26,577,900 | 1,429,647 |
| 21.750 | m-Tolunitrile | 0 | 60,783 |
| 21.996 | 1-Dodecanol | 492,208 | 270,053 |
| 22.316 | Tropone | 165,612 | 56,068 |
| 22.443 | 4-Hydroxybenzenephosphonic acid | 0 | 77,574 |
| 22.684 | Nerolidyl acetate | 0 | 116,357 |
| 22.876 | Octanoic acid | 178,530 | 73,913 |
| 23.555 | 1,3,2-Dioxaborolane, 2-ethyl-4-(3-oxiranylpropyl)- | 0 | 48,973 |
| 23.822 | (9E)-9-Hexadecen-1-ol | 188,001 | 0 |
| 25.047 | 2,4-Di-tert-butylphenol | 204,650 | 200,860 |
| 26.632 | Pyrindan | 103,007,890 | 14,885,449 |
| 27.555 | N,N-Dimethylformamide ethylene acetal | 40,116 | 40,546 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Núñez-Carmona, E.; Abbatangelo, M.; Sberveglieri, V. Innovative Sensor Approach to Follow Campylobacter jejuni Development. Biosensors 2019, 9, 8. https://doi.org/10.3390/bios9010008
Núñez-Carmona E, Abbatangelo M, Sberveglieri V. Innovative Sensor Approach to Follow Campylobacter jejuni Development. Biosensors. 2019; 9(1):8. https://doi.org/10.3390/bios9010008
Chicago/Turabian StyleNúñez-Carmona, Estefanía, Marco Abbatangelo, and Veronica Sberveglieri. 2019. "Innovative Sensor Approach to Follow Campylobacter jejuni Development" Biosensors 9, no. 1: 8. https://doi.org/10.3390/bios9010008
APA StyleNúñez-Carmona, E., Abbatangelo, M., & Sberveglieri, V. (2019). Innovative Sensor Approach to Follow Campylobacter jejuni Development. Biosensors, 9(1), 8. https://doi.org/10.3390/bios9010008







