Energy Transfer between Tm-Doped Upconverting Nanoparticles and a Small Organic Dye with Large Stokes Shift
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of NaYF4 Based Core and Core-Shell UCNPs with Different Mole Ratios of Lanthanide(III) Ions
2.2.1. Synthesis of NaYF4 Core-Only UCNPs
2.2.2. Preparation of the Shell Material
2.2.3. Synthesis of Core-Shell-UCNPs
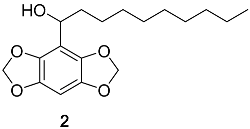
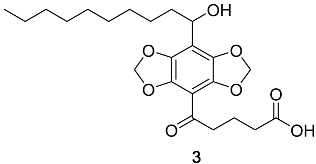
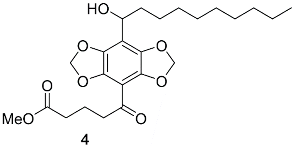
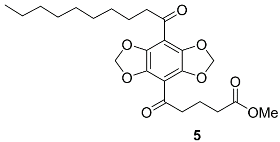
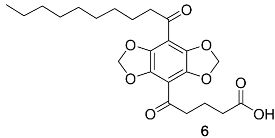
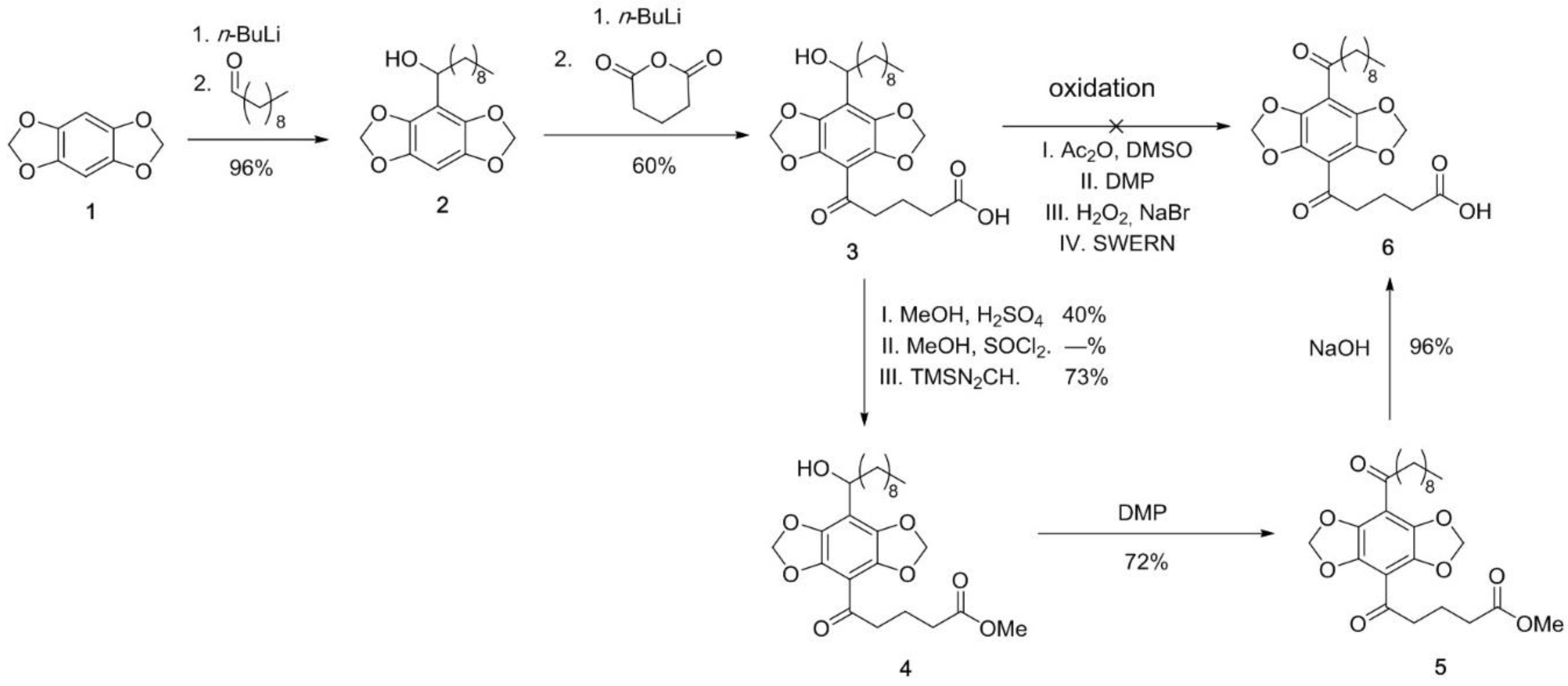
2.3. Synthesis of DBD-Labelled Fatty Acid (DBD-6)
2.4. Ligand Exchange with DBD-6
2.5. Steady-State and Time-Resolved Upconversion Luminescence Measurements at Room Temperature
2.6. UV/Vis Absorption Measurements
2.7. Calculation of the Number of DBD-6 Molecules per Single UCNP
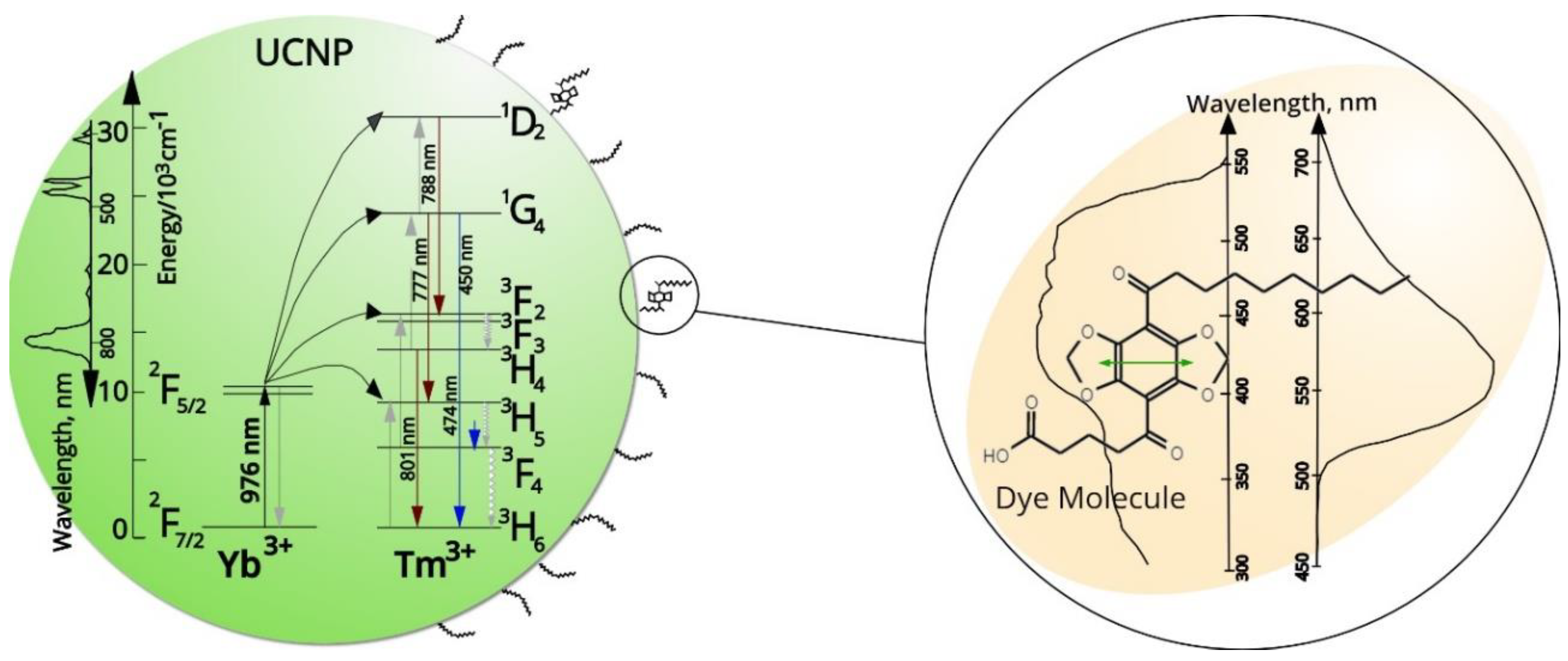
2.8. Resonance Energy Transfer (RET)
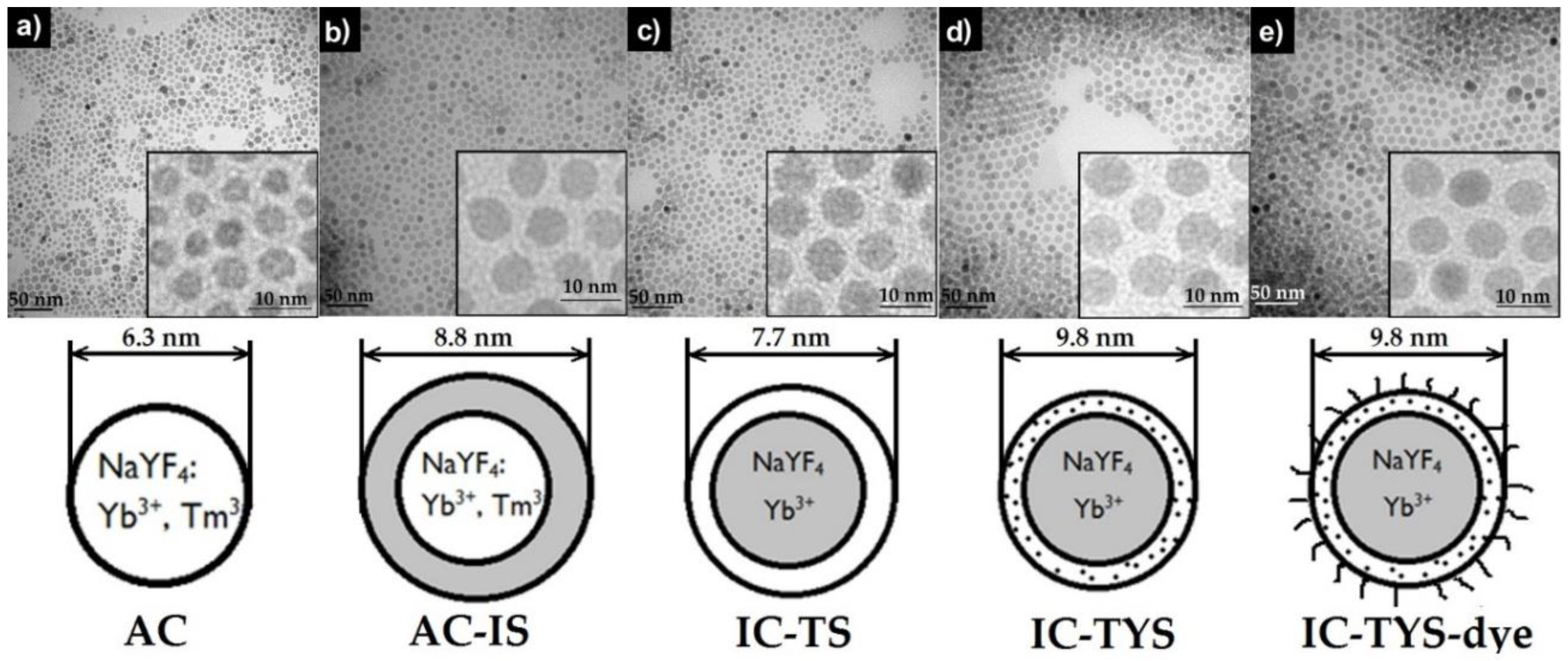
2.9. Structural and Size Investigations
3. Results
3.1. Synthesis and Characterisation of the DBD-6 Dye
3.2. Design and Synthesis of Dye-Labeled UCNP
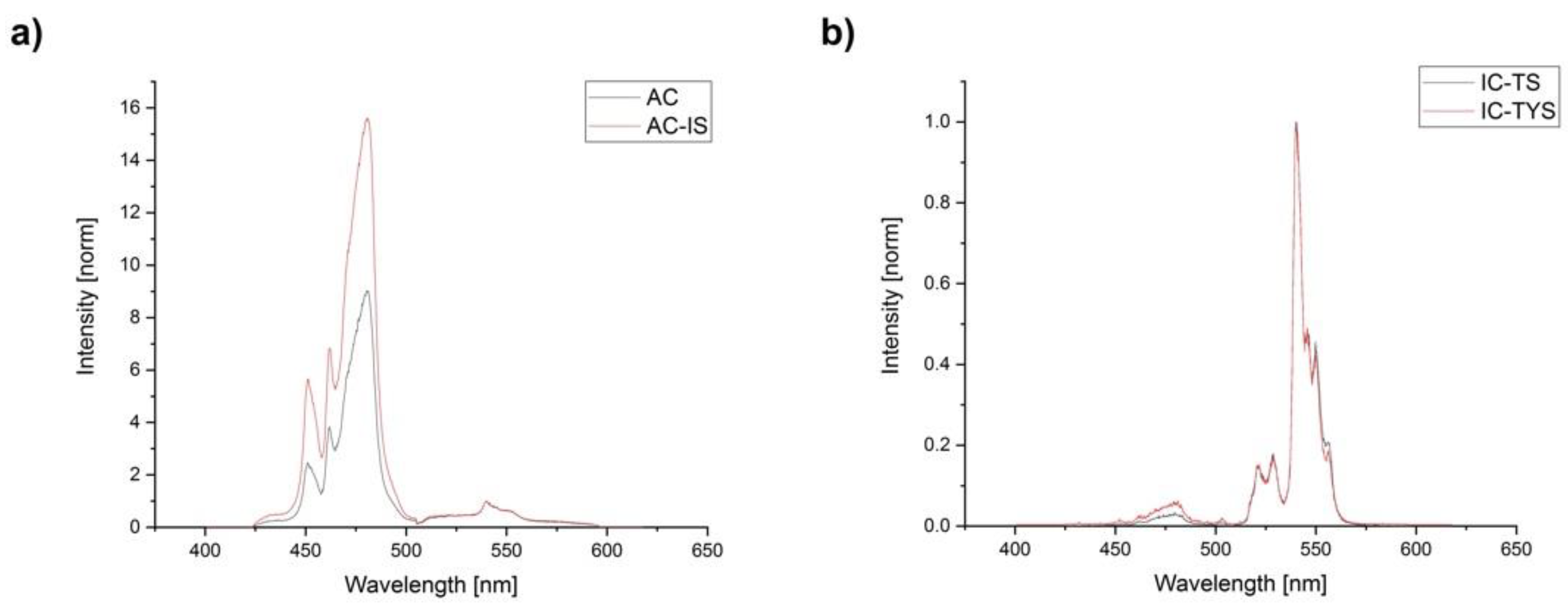
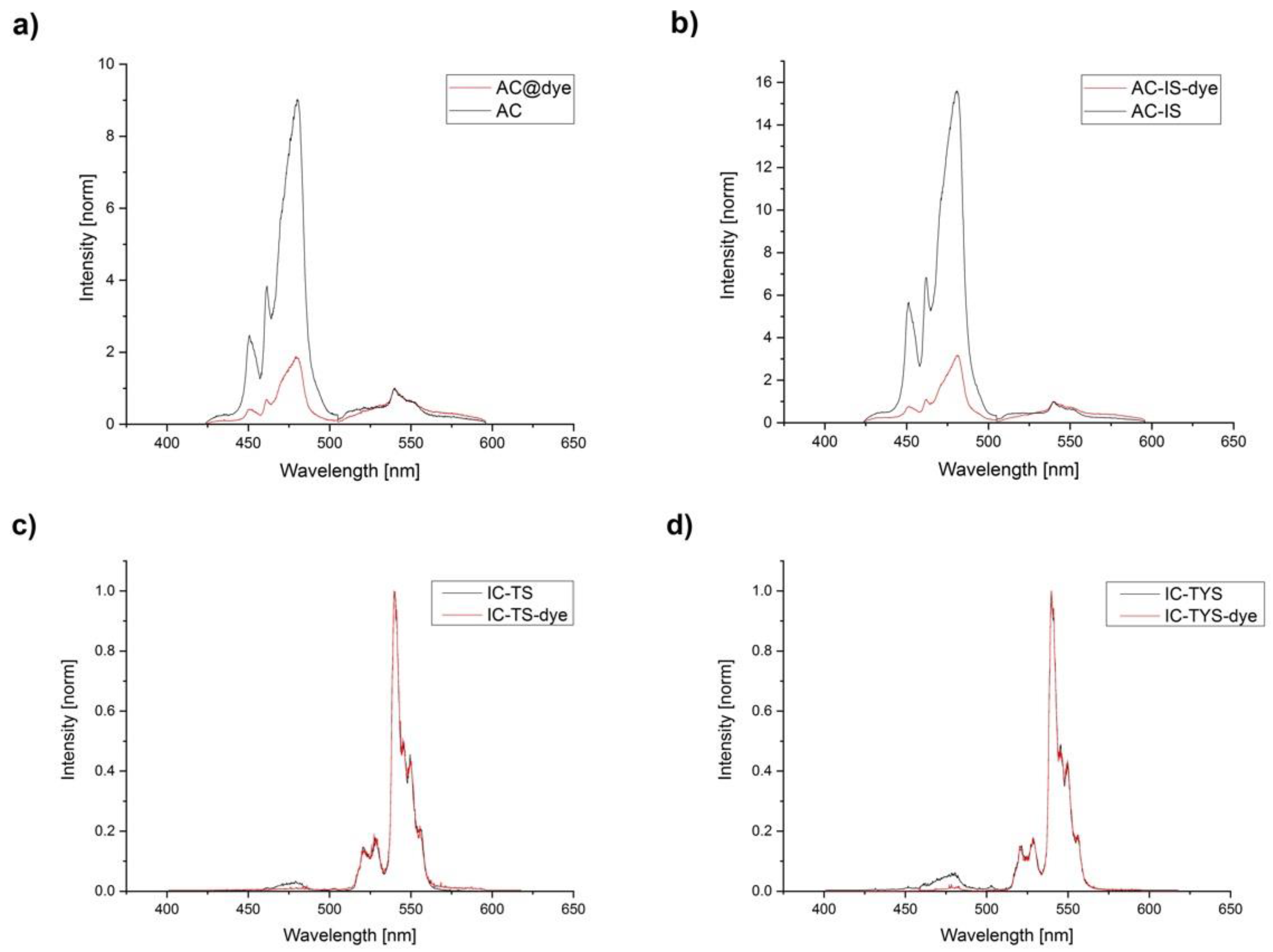
3.3. Luminescence Emission Spectra
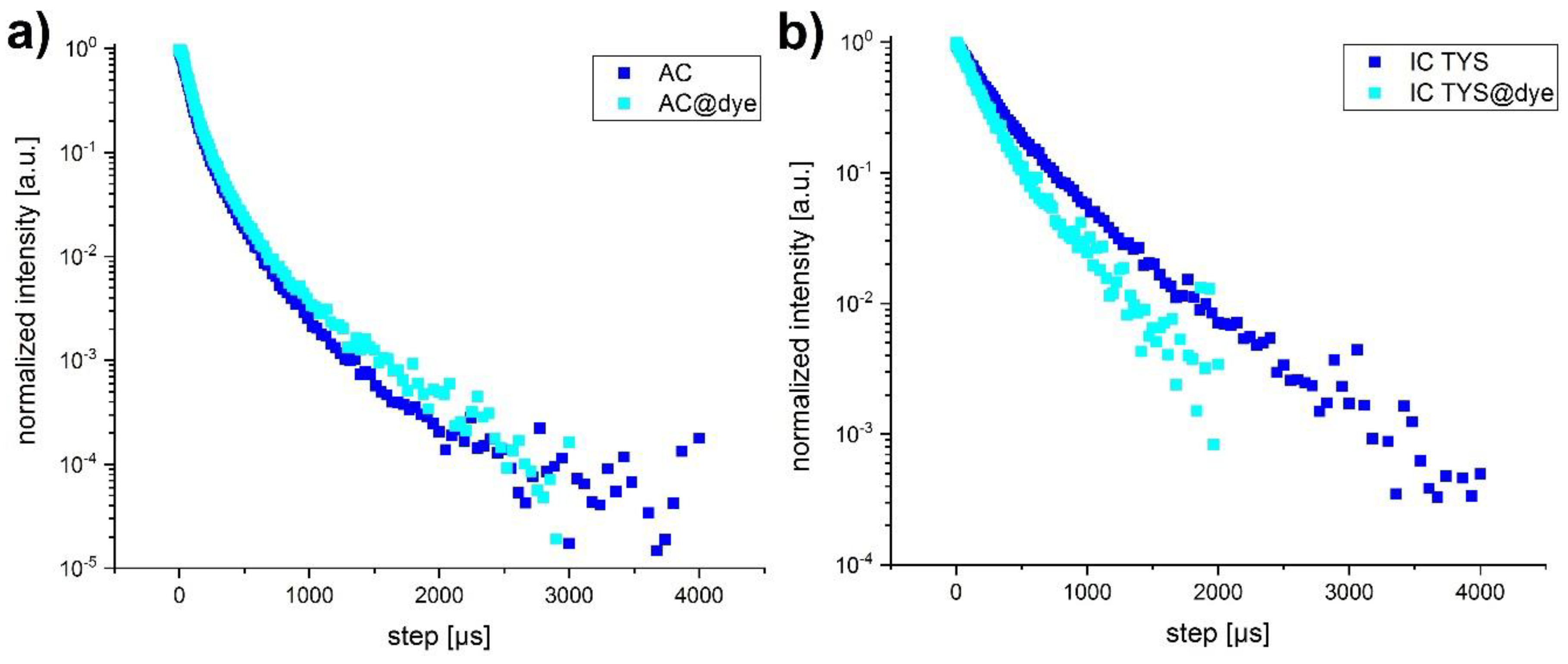
3.4. Luminescence Decay Kinetics of the Dye-Labelled UCNP
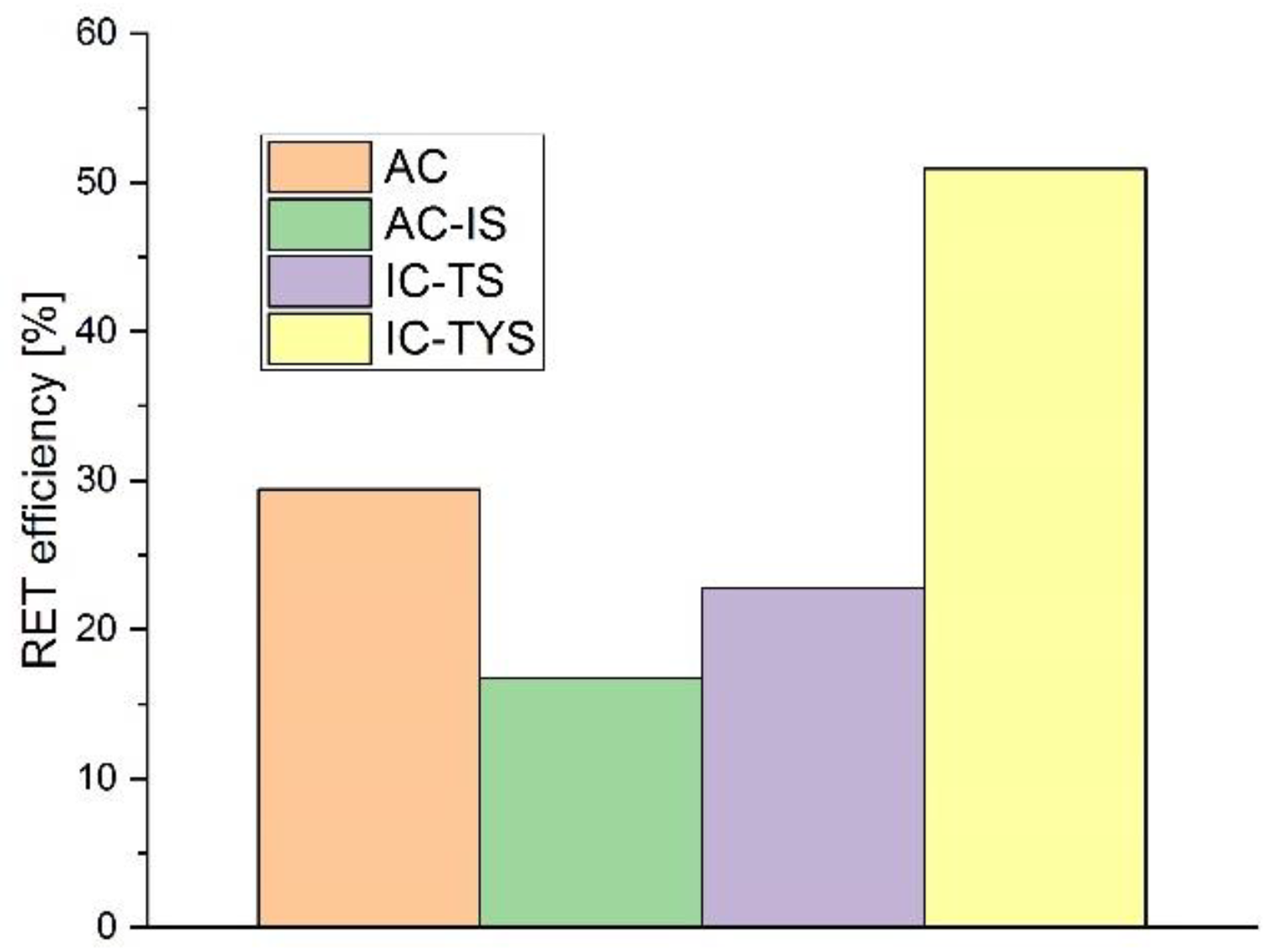
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. XRD Spectrum

Appendix A.2. DBD-6 Synthesis Intermediates

References
- Yuan, L.; Lin, W.; Zheng, K.; Zhu, S. FRET-based small-molecule fluorescent probes: Rational design and bioimaging applications. Acc. Chem. Res. 2013, 46, 1462–1473. [Google Scholar] [CrossRef]
- Fan, J.; Hu, M.; Zhan, P.; Peng, X. Energy transfer cassettes based on organic fluorophores: Construction and applications in ratiometric sensing. Chem. Soc. Rev. 2013, 42, 29–43. [Google Scholar] [CrossRef]
- Stein, I.H.; Steinhauer, C.; Tinnefeld, P. Single-Molecule Four-Color FRET Visualizes Energy-Transfer Paths on DNA Origami. J. Am. Chem. Soc. 2011, 133, 4193–4195. [Google Scholar] [CrossRef]
- Medintz, I.L.; Hildebrandt, N. FRET–Förster Resonance Energy Transfer, from Theory to Applications; Wiley-VCH: Weinheim, Germany, 2014; Chapter 5; pp. 105–164. ISBN 978-3-527-32816-1. [Google Scholar]
- Geißler, D.; Stufler, S.; Loehmannsroeben, H.-G.; Hildebrandt, N. Six-color time-resolved Förster resonance energy transfer for ultrasensitive multiplexed biosensing. J. Am. Chem. Soc. 2013, 135, 1102–1109. [Google Scholar] [CrossRef]
- Shi, J.; Tian, F.; Lyu, J.; Yang, M. Nanoparticle based fluorescence resonance energy transfer (FRET) for biosensing applications. J. Mater. Chem. B 2015, 3, 6989–7005. [Google Scholar] [CrossRef]
- Sreenivasan, V.; Zvyagin, A.V.; Goldys, E.M. Luminescent nanoparticles and their applications in the life sciences. J. Phys. Condens. Matter 2013, 25, 194101–194124. [Google Scholar] [CrossRef]
- Dabbousi, B.O.; Rodriguez-Viejo, J.; Mikulec, F.V.; Heine, J.R.; Mattoussi, H.; Ober, R.; Jensen, K.F.; Bawendi, M.G. (CdSe)ZnS core–shell quantum dots: Synthesis and characterization of a size series of highly luminescent nanocrystallites. J. Phys. Chem. B 1997, 101, 9463–9475. [Google Scholar] [CrossRef]
- Hines, M.A.; Guyot-Sionnest, P. Synthesis and characterization of strongly luminescing ZnS-capped CdSe nanocrystals. J. Phys. Chem. 1996, 100, 468–471. [Google Scholar] [CrossRef]
- Haase, M.; Schӓfer, H. Upconverting nanoparticles. Angew. Chem. Int. Ed. 2011, 50, 5808–5829. [Google Scholar] [CrossRef]
- Zheng, W.; Huang, P.; Tu, D.; Ma, E.; Zhu, H.; Chen, X. Lanthanide-doped upconversion nano-bioprobes: Electronic structures, optical properties, and biodetection. Chem. Soc. Rev. 2015, 44, 1379–1415. [Google Scholar] [CrossRef]
- Auzel, F. Upconversion and Anti-Stokes Processes with f and d Ions in Solids. Chem. Rev. 2004, 104, 139–174. [Google Scholar] [CrossRef]
- Lahtinen, S.; Wang, Q.; Soukka, T. Long-Lifetime Luminescent Europium(III) Complex as an Acceptor in an Upconversion Resonance Energy Transfer Based Homogeneous Assay. Anal. Chem. 2016, 88, 653–658. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, K.; Liu, X.; Dohnalová, K.; Gregorkiewicz, T.; Kong, X.; Aalders, M.C.G.; Buma, W.J.; Zhang, H. Critical shell thickness of core/shell upconversion luminescence nanoplatform for FRET application. J. Phys. Chem. Lett. 2011, 2, 2083–2088. [Google Scholar] [CrossRef]
- Muhr, V.; Wuerth, C.; Kraft, M.; Buchner, M.; Baeumner, A.J.; Resch-Genger, U.; Hirsch, T. Particle-Size-Dependent Förster Resonance Energy Transfer from Upconversion Nanoparticles to Organic Dyes. Anal. Chem. 2017, 89, 4868–4874. [Google Scholar] [CrossRef]
- Bhuckory, S.; Hemmer, E.; Wu, Y.-T.; Yahia-Ammar, A.; Vetrone, F.; Hildebrand, N. Core or Shell? Er3+ FRET Donors in Upconversion Nanoparticles. Eur. J. Inorg. Chem. 2017, 5186–5195. [Google Scholar] [CrossRef]
- Dukhno, O.; Przybilla, F.; Collot, M.; Klymchenko, A.; Pivovarenko, V.; Buchner, M.; Muhr, V.; Hirsch, T.; Mély, Y. Quantitative assessment of energy transfer in upconverting nanoparticles grafted with organic dyes. Nanoscale 2017, 9, 11994–12004. [Google Scholar] [CrossRef]
- Hesse, J.; Klier, D.T.; Sgarzi, M.; Nsubuga, A.; Bauer, C.; Grenzer, J.; Hübner, R.; Wislicenus, M.; Joshi, T.; Kumke, M.U.; et al. Rapid Synthesis of Sub-10 nm Hexagonal NaYF4-Based Upconverting Nanoparticles using Therminol®66. ChemistryOpen 2018, 7, 159–168. [Google Scholar] [CrossRef]
- Geßner, A. Neuartige Lanthanoid-Dotierte Mikro- und Mesoporöse Feststoffe Charakterisierung von Ion-Wirt-Wechselwirkungen, Speziesverteilung und Lumineszenzeigenschaften Mittels Eitaufgelöster Lumineszenzspektroskopie. Ph.D. Thesis, University of Potsdam, Potsdam-Golm, Germany, 2010. [Google Scholar]
- Mackenzie, I.E.; Goode, J.A.; Vakurov, A.; Nampi, P.P.; Saha, S.; Jose, G.; Millner, P.A. The theoretical molecular weight of NaYF4:RE upconversion nanoparticles. Sci. Rep. 2018, 8, 1106. [Google Scholar] [CrossRef]
- Mattsson, L.; Wegner, K.D.; Hildebrandt, N.; Soukka, T. Upconverting nanoparticle to quantum dot FRET for homogeneous double-nano biosensors. RSC Adv. 2015, 5, 13270–13277. [Google Scholar] [CrossRef]
- Li, X.; Shen, D.; Yang, J.; Yao, C.; Che, R.; Zhang, F.; Zhao, D. Successive Layer-by-Layer Strategy for Multi-Shell Epitaxial Growth: Shell Thickness and Doping Position Dependence in Upconverting Optical Properties. Chem. Mater. 2013, 25, 106–112. [Google Scholar] [CrossRef]
- Klier, D.T.; Kumke, M.U. Analyzing the effect of the crystal structure on upconversion luminescence in Yb3+, Er3+ co-doped NaYF4 nanomaterials. J. Mater. Chem. C 2015, 3, 11228–11238. [Google Scholar] [CrossRef]
- Bednarkiewicz, A.; Nyk, M.; Samoc, M.; Strek, W. Up-conversion FRET from Er3+/Yb3+:NaYF4 Nanophosphor to CdSe Quantum Dots. J. Phys. Chem. C 2010, 114, 17535–17541. [Google Scholar] [CrossRef]
- Kaiser, M.; Würth, C.; Kraft, M.; Hyppänen, I.; Soukka, T.; Resch-Genger, U. Power-dependent upconversion quantum yield of NaYF4:Yb3+, Er3+ nano- and micrometer-sized particles–measurements and simulations. Nanoscale 2017, 9, 10051–10058. [Google Scholar] [CrossRef]
- Jin, L.M.; Chen, X.; Siu, C.K.; Wang, F.; Yu, S.F. Enhancing Multiphoton Upconversion from NaYF4:Yb/Tm@NaYF4 Core−Shell Nanoparticles via the Use of Laser Cavity. ACS Nano 2017, 11, 843–849. [Google Scholar] [CrossRef]
- Arppe, R.; Hyppänen, I.; Perälä, N.; Peltomaa, R.; Kaiser, M.; Würth, C.; Christ, S.; Resch-Genger, U.; Schäferling, M.; Soukka, T. Quenching of the upconversion luminescence of NaYF4:Yb3+, Er3+ and NaYF4:Yb3+,Tm3+ nanophosphors by water: The role of the sensitizer Yb3+ in non-radiative relaxation. Nanoscale 2015, 7, 11746–11757. [Google Scholar] [CrossRef]
- Eisold, U.; Behrends, N.; Wessig, P.; Kumke, M.U. Rigid Rod-Based FRET Probes for Membrane Sensing Applications. J. Phys. Chem. B 2016, 120, 9935–9943. [Google Scholar] [CrossRef]
- Wessig, P.; Behrends, N.; Kumke, M.U.; Eisold, U. FRET Pairs with Fixed Relative Orientation of Chromophores. Eur. J. Org. Chem. 2016, 26, 4476–4486. [Google Scholar] [CrossRef]
- Wessig, P.; John, L.; Mertens, M. Extending the Class of [1,3]-Dioxolo[4.5-f]benzodioxole (DBD) Fluorescent Dyes. Eur. J. Org. Chem. 2018, 14, 1674–1681. [Google Scholar] [CrossRef]
- Dess, D.B.; Martin, J.C. Readily accessible 12-I-5 oxidant for the conversion of primary and secondary alcohols to aldehydes and ketones. J. Org. Chem. 1983, 48, 4155–4156. [Google Scholar] [CrossRef]
| Solvent | λAbs [nm] | λEm [nm] | Δλ [nm] | τF [ns] | ε [M−1cm−1] | φF |
|---|---|---|---|---|---|---|
| DCM | 435 | 547 | 112 | 26.1 | 2970 | 0.66 |
| ACN | 422 | 560 | 138 | 21.9 | 2890 | 0.51 |
| methanol | 436 | 605 | 169 | 4.6 | 2550 | 0.08 |
| H2O | 463 | 557 | 94 | 8.1 a | 2530 | 0.09 |
| Sample | AC | AC-IS | IC-TS | IC-TYS | IC-TYS@dye |
|---|---|---|---|---|---|
| Diameter, nm | 6.3 ± 1.4 | 8.8 ± 1.7 | 7.7 ± 1.6 | 9.8 ± 1.5 | 9.8 ± 1.5 |
| UCNP Species | No Dye | With Dye |
|---|---|---|
| AC | 5.8 | 1.1 |
| AC-IS | 10.1 | 1.7 |
| IC-TS | 0.15 | 0.11 |
| IC-TYS | 0.13 | 0.04 |
| τ, µs/Fraction, % | AC | AC @dye | AC-IS | AC-IS @dye | IC-TS | IC-TS @dye | IC-TYS | IC-TYS @dye | |
|---|---|---|---|---|---|---|---|---|---|
| blue (470 nm) | τ1 | 119 (77%) | 84 (77%) | 173 (78%) | 144 (92%) | 228 (65%) | 176 (69%) | 161 (45%) | 79 (65%) |
| τ2 | 308 (23%) | 263 (23%) | 387 (22%) | 483 (8%) | 567 (35%) | 590 (31%) | 582 (55%) | 259 (35%) | |
| τav | 163 ± 26 | 125 ± 9 | 221 ± 30 | 171 ± 33 | 348 ± 71 | 305 ± 93 | 391 ± 44 | 141 ± 30 | |
| reference (550 nm) | τ1 | 68 (82%) | 69 (82%) | 132 (84%) | 116 (93%) | 64 (86%) | 61 (78%) | 92 (58%) | 57 (48%) |
| τ2 | 233 (18%) | 225 (18%) | 328 (16%) | 369 (7%) | 214 (14%) | 169 (22%) | 479 (42%) | 338 (52%) | |
| τav | 98 ± 8 | 97 ± 8 | 164 ± 23 | 135 ± 24 | 85 ± 4 | 85 ± 5 | 254 ± 12 | 204 ± 8 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López de Guereñu, A.; Bastian, P.; Wessig, P.; John, L.; Kumke, M.U. Energy Transfer between Tm-Doped Upconverting Nanoparticles and a Small Organic Dye with Large Stokes Shift. Biosensors 2019, 9, 9. https://doi.org/10.3390/bios9010009
López de Guereñu A, Bastian P, Wessig P, John L, Kumke MU. Energy Transfer between Tm-Doped Upconverting Nanoparticles and a Small Organic Dye with Large Stokes Shift. Biosensors. 2019; 9(1):9. https://doi.org/10.3390/bios9010009
Chicago/Turabian StyleLópez de Guereñu, Anna, Philipp Bastian, Pablo Wessig, Leonard John, and Michael U. Kumke. 2019. "Energy Transfer between Tm-Doped Upconverting Nanoparticles and a Small Organic Dye with Large Stokes Shift" Biosensors 9, no. 1: 9. https://doi.org/10.3390/bios9010009
APA StyleLópez de Guereñu, A., Bastian, P., Wessig, P., John, L., & Kumke, M. U. (2019). Energy Transfer between Tm-Doped Upconverting Nanoparticles and a Small Organic Dye with Large Stokes Shift. Biosensors, 9(1), 9. https://doi.org/10.3390/bios9010009





