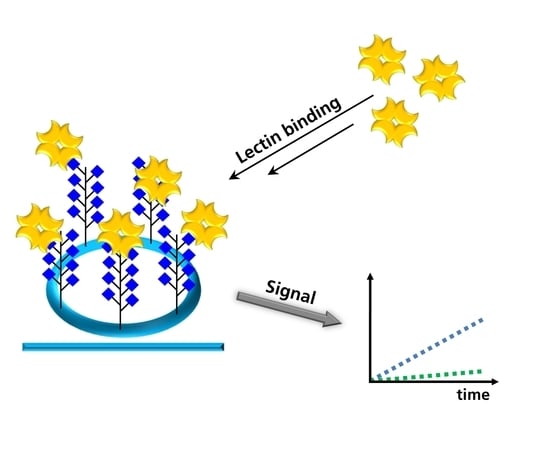
Rapid Drop-Test for Lectin Binding with Glycopolymer-Coated Optical Ring Resonators
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analytics
2.3. Production of Ring Resonators
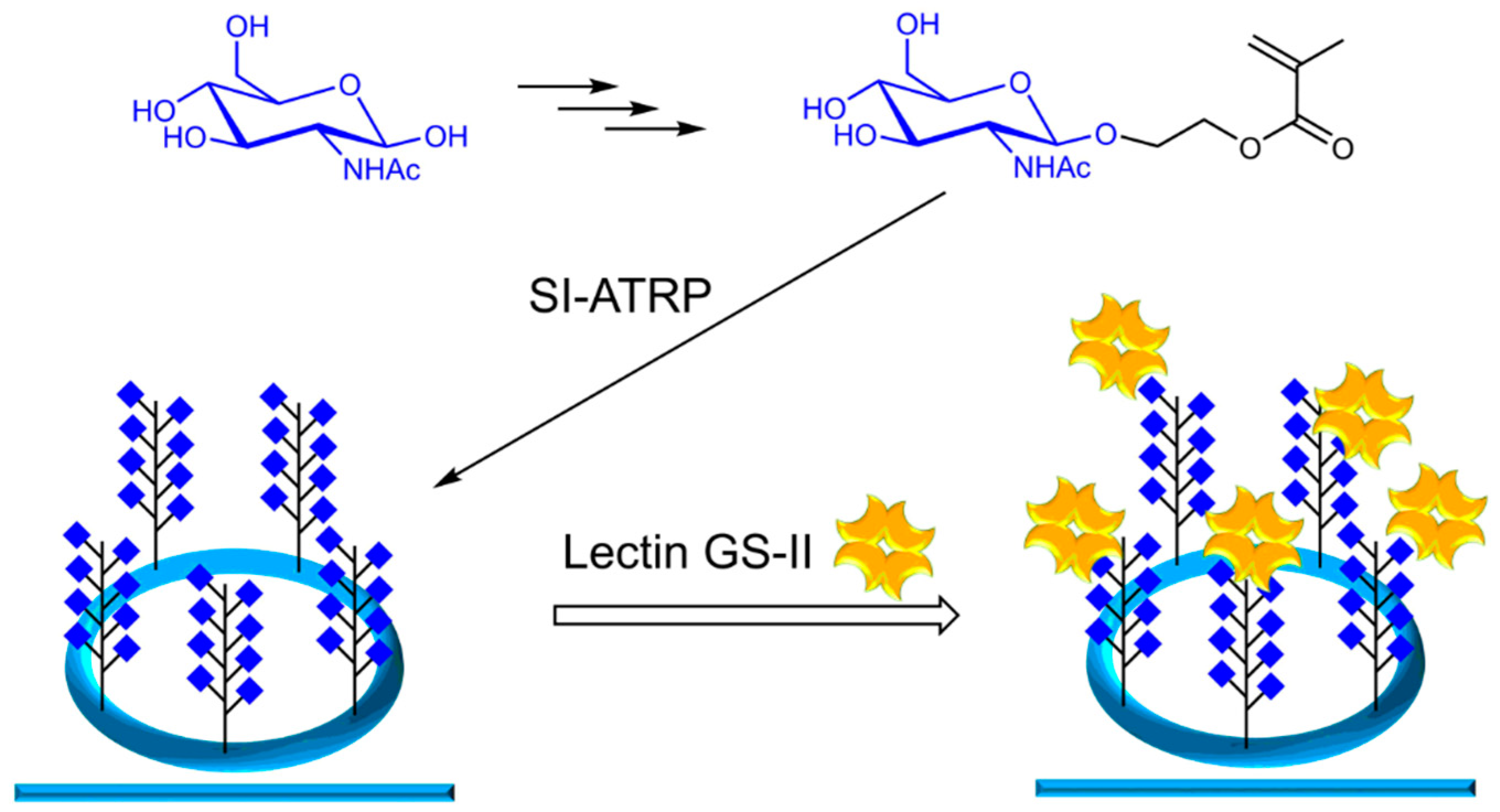
2.4. Synthesis Glycomonomer GlcNAcEMA
2.5. Microcontact-Printing
2.6. Synthesis of Glycopolymers on Ring Resonators
2.7. Binding Analysis Using Optical Ring-Resonators
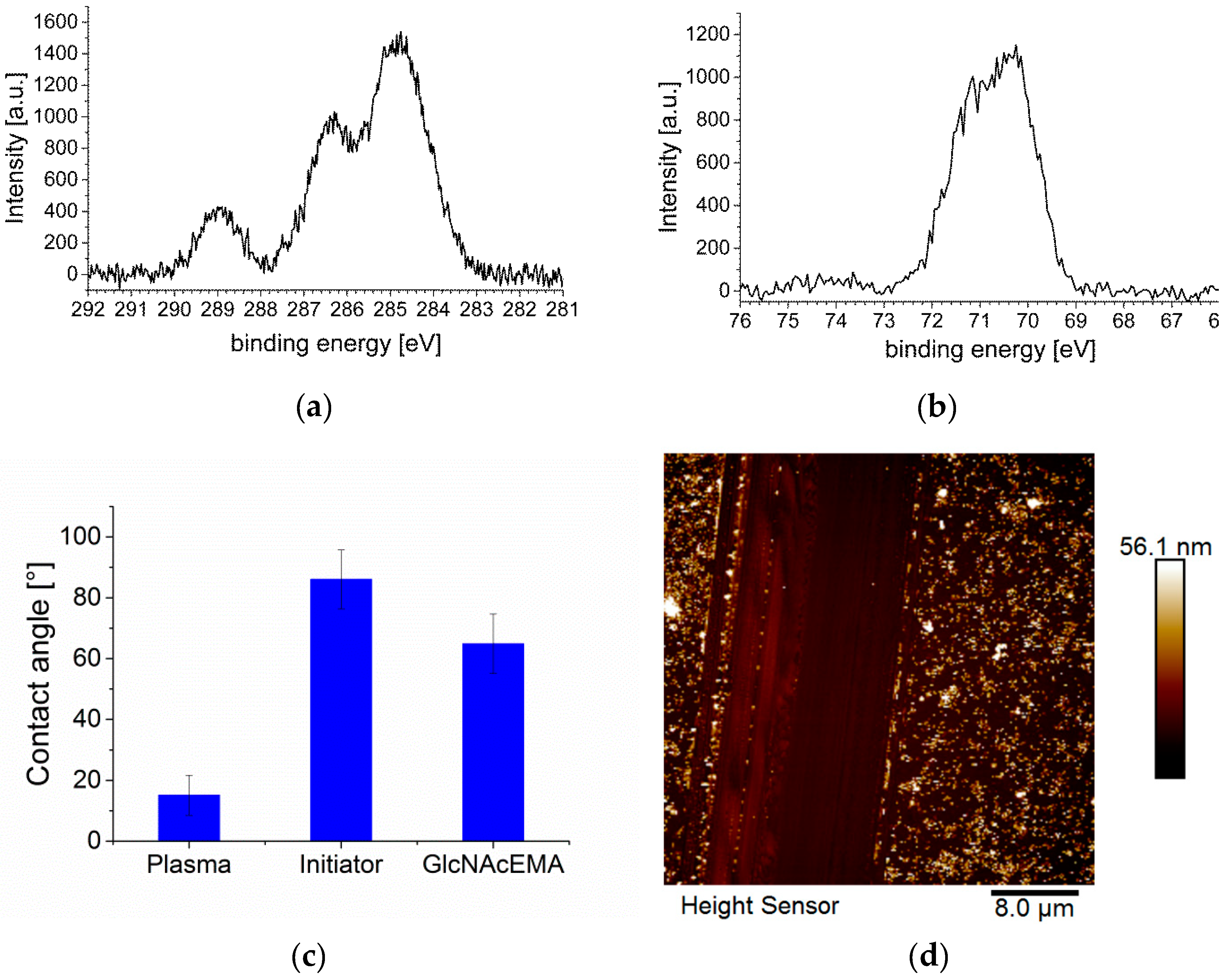
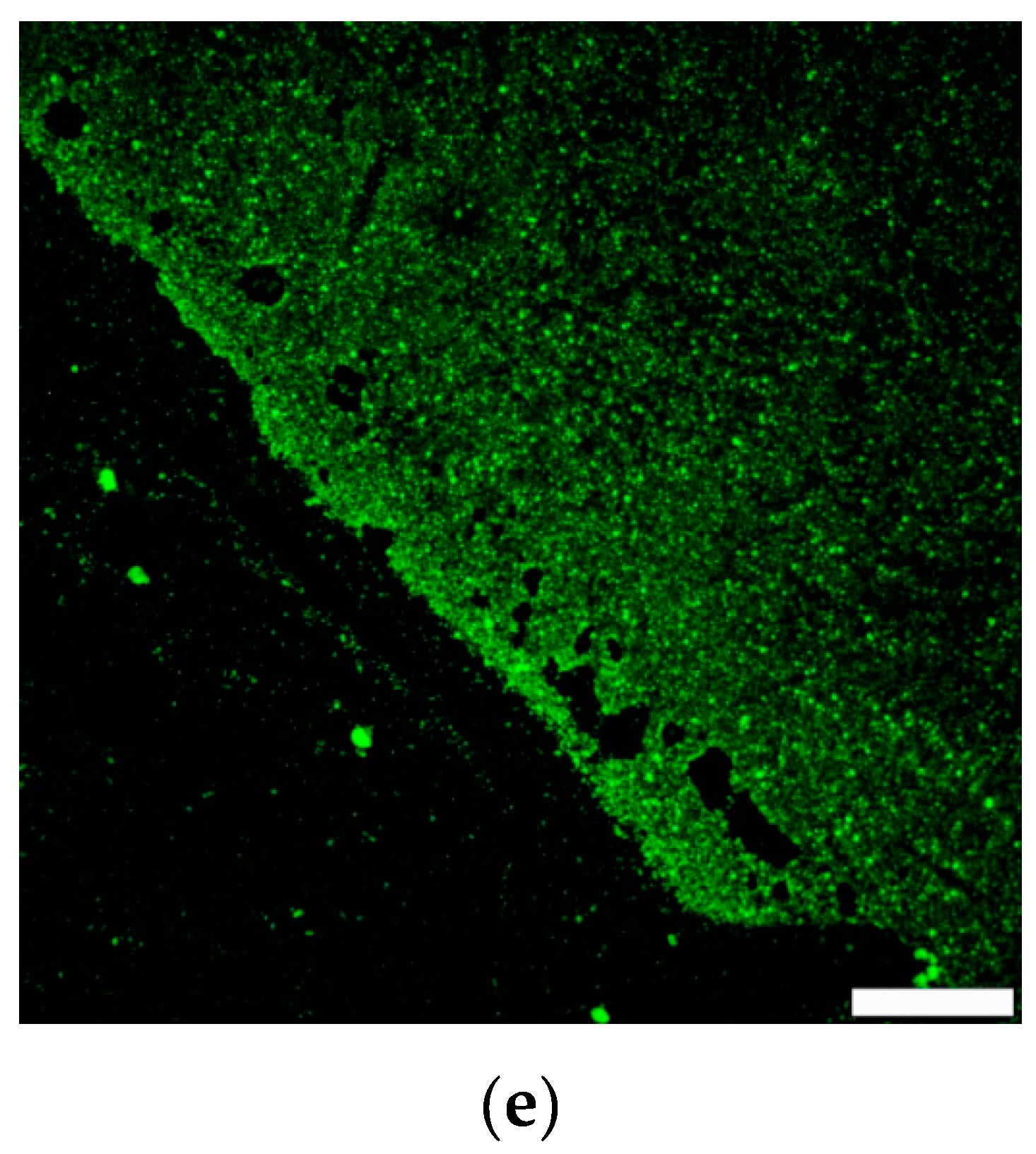
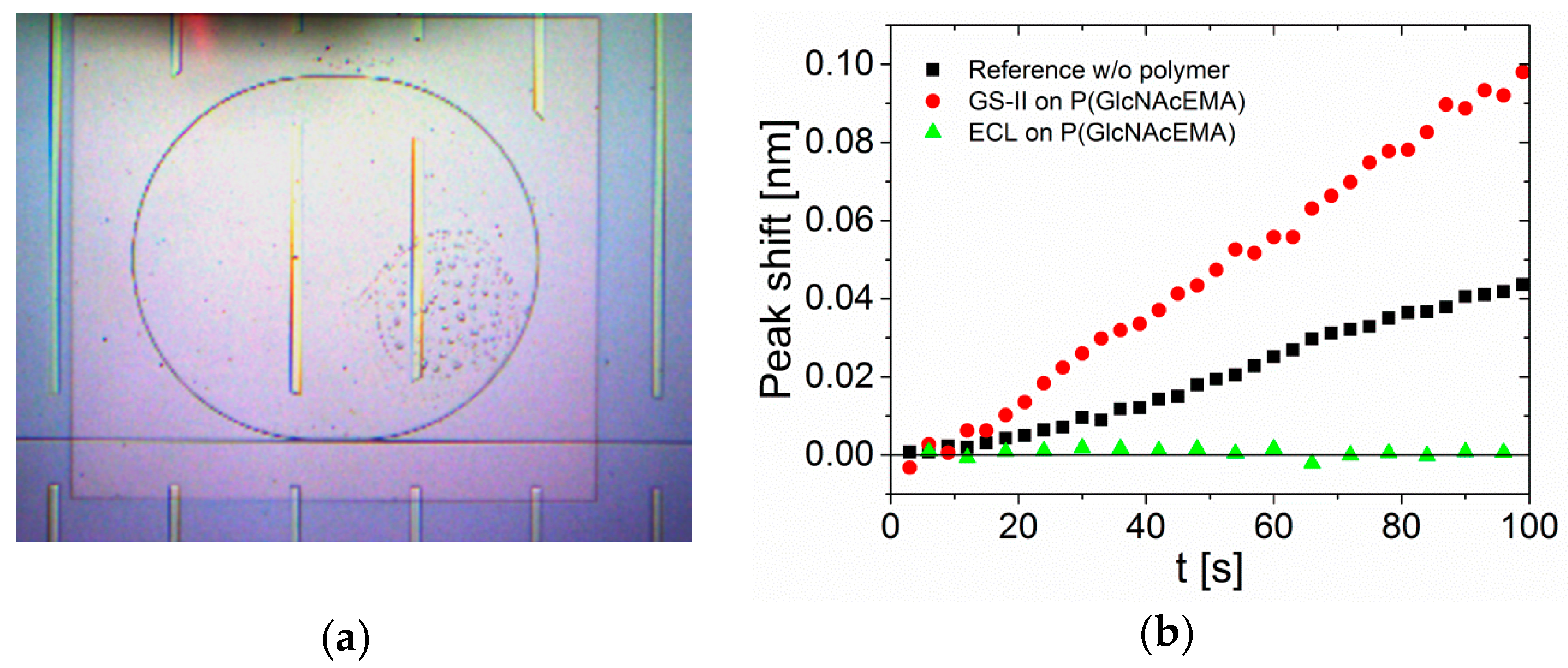
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mody, R.; Joshi, S.; Chaney, W. Use of lectins as diagnostic and therapeutic tools for cancer. J. Pharmacol. Toxicol. Methods 1995, 33, 1–10. [Google Scholar] [CrossRef]
- Slifkin, M.; Doyle, R.J. Lectins and their application to clinical microbiology. Clin. Microbiol. Rev. 1990, 3, 197–218. [Google Scholar] [CrossRef] [PubMed]
- Poole, J.; Day, C.J.; Itzstein, M.; Paton, J.C.; Jennings, M.P. Glycointeractions in bacterial pathogenesis. Nat. Rev. Microbiol. 2018, 16, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Ghazarian, H.; Idoni, B.; Oppenheimer, S.B. A glycobiology review: Carbohydrates, lectins and implications in cancer therapeutics. Acta Histochem. 2011, 113, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.-H.; Wu, C.-Y.; Greenberg, W.A.; Wong, C.-H. Glycan arrays: Biological and medical applications. Curr. Opin. Chem. Biol. 2008, 12, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Oyelaran, O.; Gildersleeve, J.C. Glycan arrays: Recent advances and future challenges. Curr. Opin. Chem. Biol. 2009, 13, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Lazar, J.; Park, H.; Rosencrantz, R.R.; Böker, A.; Elling, L.; Schnakenberg, U. Evaluating the Thickness of Multivalent Glycopolymer Brushes for Lectin Binding. Macromol. Rapid Commun. 2015, 36, 1472–1478. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zuo, P.; Ye, B.-C. Label-free electrochemical impedance spectroscopy biosensor for direct detection of cancer cells based on the interaction between carbohydrate and lectin. Biosens. Bioelectron. 2013, 43, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Rosencrantz, R.R.; Nguyen, V.H.; Park, H.; Schulte, C.; Böker, A.; Schnakenberg, U.; Elling, L. Lectin binding studies on a glycopolymer brush flow-through biosensor by localized surface plasmon resonance. Anal. Bioanal. Chem. 2016, 408, 5633–5640. [Google Scholar] [CrossRef] [PubMed]
- Vornholt, W.; Hartmann, M.; Keusgen, M. SPR studies of carbohydrate–lectin interactions as useful tool for screening on lectin sources. Biosens. Bioelectron. 2007, 22, 2983–2988. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Rosencrantz, R.R.; Elling, L.; Böker, A. Glycopolymer brushes for specific lectin binding by controlled multivalent presentation of N-acetyllactosamine glycan oligomers. Macromol. Rapid Commun. 2015, 36, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Fan, X. Optical ring resonators for biochemical and chemical sensing. Anal. Bioanal. Chem. 2011, 399, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; White, I.M.; Shopova, S.I.; Zhu, H.; Suter, J.D.; Sun, Y. Sensitive optical biosensors for unlabeled targets: A review. Anal. Chim. Acta 2008, 620, 8–26. [Google Scholar] [CrossRef] [PubMed]
- Orghici, R.; Lützow, P.; Burgmeier, J.; Koch, J.; Heidrich, H.; Schade, W.; Welschoff, N.; Waldvogel, S. A microring resonator sensor for sensitive detection of 1,3,5-trinitrotoluene (TNT). Sensors 2010, 10, 6788–6795. [Google Scholar] [CrossRef] [PubMed]
- Ksendzov, A.; Lin, Y. Integrated optics ring-resonator sensors for protein detection. Opt. Lett. 2005, 30, 3344. [Google Scholar] [CrossRef] [PubMed]
- Luchansky, M.S.; Washburn, A.L.; McClellan, M.S.; Bailey, R.C. Sensitive on-chip detection of a protein biomarker in human serum and plasma over an extended dynamic range using silicon photonic microring resonators and sub-micron beads. Lab Chip 2011, 11, 2042–2044. [Google Scholar] [CrossRef] [PubMed]
- Washburn, A.L.; Luchansky, M.S.; Bowman, A.L.; Bailey, R.C. Quantitative, label-free detection of five protein biomarkers using multiplexed arrays of silicon photonic microring resonators. Anal. Chem. 2010, 82, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, F.; Hosseini, E.S.; Song, X.; Gottfried, D.S.; Chamanzar, M.; Raeiszadeh, M.; Cummings, R.D.; Eftekhar, A.A.; Adibi, A. Multiplexed detection of lectins using integrated glycan-coated microring resonators. Biosens. Bioelectron. 2016, 80, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Horton, D. 2-Acetamido-3,4,6-tri-O-acetyl-2-deoxy-α-d-glucopyranosyl Chloride. Gen. Carbohydr. Method 1972, 282–285. [Google Scholar] [CrossRef]
- Koenigs, W.; Knorr, E. Ueber einige Derivate des Traubenzuckers und der Galactose. Ber. Dtsch. Chem. Ges. 1901, 34, 957–981. [Google Scholar] [CrossRef]
- Schmidt, R.R. Neue Methoden zur Glycosid—und Oligosaccharidsynthese—gibt es Alternativen zur Koenigs-Knorr-Methode? Angew. Chem. 1986, 98, 213–236. [Google Scholar] [CrossRef]
- Zhu-Salzman, K.; Shade, R.E.; Koiwa, H.; Salzman, R.A.; Narasimhan, M.; Bressan, R.A.; Hasegawa, P.M.; Murdock, L.L. Carbohydrate binding and resistance to proteolysis control insecticidal activity of Griffonia simplicifolia lectin II. Proc. Nat. Acad. Sci. USA 1998, 95, 15123–15128. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, J.L.; Lis, H.; Sharon, N. Purification and Properties of a d-Galactose/N-Acetyl-d-galactosamine-Specific Lectin from Erythrina cristagalli. Eur. J. Biochem. 1982, 123, 247–252. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schulte-Osseili, C.; Kleinert, M.; Keil, N.; Rosencrantz, R.R. Rapid Drop-Test for Lectin Binding with Glycopolymer-Coated Optical Ring Resonators. Biosensors 2019, 9, 24. https://doi.org/10.3390/bios9010024
Schulte-Osseili C, Kleinert M, Keil N, Rosencrantz RR. Rapid Drop-Test for Lectin Binding with Glycopolymer-Coated Optical Ring Resonators. Biosensors. 2019; 9(1):24. https://doi.org/10.3390/bios9010024
Chicago/Turabian StyleSchulte-Osseili, Christine, Moritz Kleinert, Norbert Keil, and Ruben R. Rosencrantz. 2019. "Rapid Drop-Test for Lectin Binding with Glycopolymer-Coated Optical Ring Resonators" Biosensors 9, no. 1: 24. https://doi.org/10.3390/bios9010024
APA StyleSchulte-Osseili, C., Kleinert, M., Keil, N., & Rosencrantz, R. R. (2019). Rapid Drop-Test for Lectin Binding with Glycopolymer-Coated Optical Ring Resonators. Biosensors, 9(1), 24. https://doi.org/10.3390/bios9010024