1. Introduction
Arrhythmic disorders represent a common referral for cardiology consultations and have a strong impact on resource utilization of health care systems [
1]. As many arrhythmias are characterized by intermittent, short-lasting episodes, accurate rhythm documentation is often challenging and a common dilemma for the clinician.
Prolonged ambulatory rhythm monitoring is crucial for the diagnosis of intermittent arrhythmia and the correlation between rhythm and symptoms. Conventional technologies for noninvasive rhythm monitoring include Holter monitoring or external cardiac event recorders. Traditional Holter recording over 24–48 h provides excellent signal quality and high specificity, but is limited by low sensitivity and an overall low diagnostic yield in the vast majority of cases [
2]. Despite those obvious limitations, Holter monitoring has been traditionally considered to be the gold standard for ambulatory rhythm monitoring [
3]. External cardiac event recorders offer recording durations of 30–90 days and provide improved diagnostic yield compared to Holter monitoring, but are cumbersome and their utility is limited by poor patient compliance [
4]. Additional shortcomings include their dependence on appropriate patient-activated event recordings (non-looping external event recorders) and significant wearing discomfort and skin irritation (looping external event recorders).
At present, implantable cardiac monitors (ICMs) represent the only technology for continuous ambulatory long-term monitoring [
4]. However, the insertion of ICMs requires minor surgery and is associated with significant costs, and current battery longevities are limited to 3 years. Because of that, novel concepts of wearable ECG sensors have emerged in the past few years. However, most contemporary systems only report heart rates and no ECG tracings and none of it has provided data on the validation of signal quality during direct comparison with Holter monitoring [
3,
5,
6,
7,
8,
9].
Other forms of wearable ECG sensors are already commercially available, but have been typically designed for recreational purposes. At present, clinical experience with those products is limited to pure, numerical detection of the heart rate and respiratory rate [
5,
6,
7,
8,
9]. Those forms of wearable ECG sensors do not provide ECG rhythm strips and thus, could not be used for distinct rhythm analysis at this point [
5,
6,
7,
8,
9]. An additional drawback of other contemporary systems is the lack of direct signal validation against Holter recording or another established technology for ambulatory rhythm monitoring. To our knowledge, there is currently no study that has assessed the signal quality of wearable ECG sensors by medical experts (i.e., cardiac electrophysiologists). The utility and potential importance of wearable sensors for rhythm monitoring has been reported previously [
10] and the feasibility of cloud-based data transmission/management has been demonstrated by a recent study using a telemedicine surveillance system for atrial fibrillation (AF) screening [
11].
The OMsignal system represents a novel, alternative tool for non-invasive ambulatory rhythm monitoring with theoretically unlimited recording capacity and the potential of increased sensitivity to detect intermittent or subclinical arrhythmia. The aim of this study was to validate in a first step the signal quality of the OMsignal garments and signal coverage over 24 h against a standard Holter recording to demonstrate the general utility of the technology for advanced rhythm diagnosis.
2. Materials and Methods
2.1. Study Population
In this prospective phase-1 trial, healthy individuals underwent 24-h simultaneous rhythm monitoring wearing the OMsignal system together with a 3-lead standard Holter recording (Spiderflash, LivaNova, London, UK) with an ECG sampling rate of 200 samples per second. The study was approved by the local ethics and review board and written informed consent was obtained from all study subjects.
2.2. OMgarments–Wearable ECG Sensors
The OMsignal system (
Figure 1) consists of a garment (OMbra for women or OMshirt for men) with integrated sensors and an acquisition module for data recording and processing. The garment contains 3 silicone-based electrodes that are in contact with the skin, recording a single-lead ECG. Two electrodes are situated just below the major pectoral muscles, left and right, providing a modified V5-lead signature. The electrode located at the bottom of the left scapula acts as a right leg lead. A woven wire at the ribcage level records respiratory inductance plethysmography. OMsignal garments must be worn according to a sizing chart to ensure optimal signal quality and wearing comfort (
Table S1). The garments used in this project are offered in 4 sizes for both women and men and are machine washable. The ECG sensors are connected to the acquisition module through 5 snaps (
Figure 1C–D). The acquisition module has the capacity to save or process ECG signals for offline analysis or real-time monitoring. Data transfer from the acquisition module to a computer simply requires a USB cable. In addition, the acquisition module can also be configured for direct data transfer into a web-based cloud, offering theoretically unlimited recording/storage capacity and direct long-term remote monitoring (
Supplementary Figure S1). For this study, the recording time of the acquisition module was set to 24 h with a sampling rate of 125 samples per second.
2.3. Validation of Signal Coverage
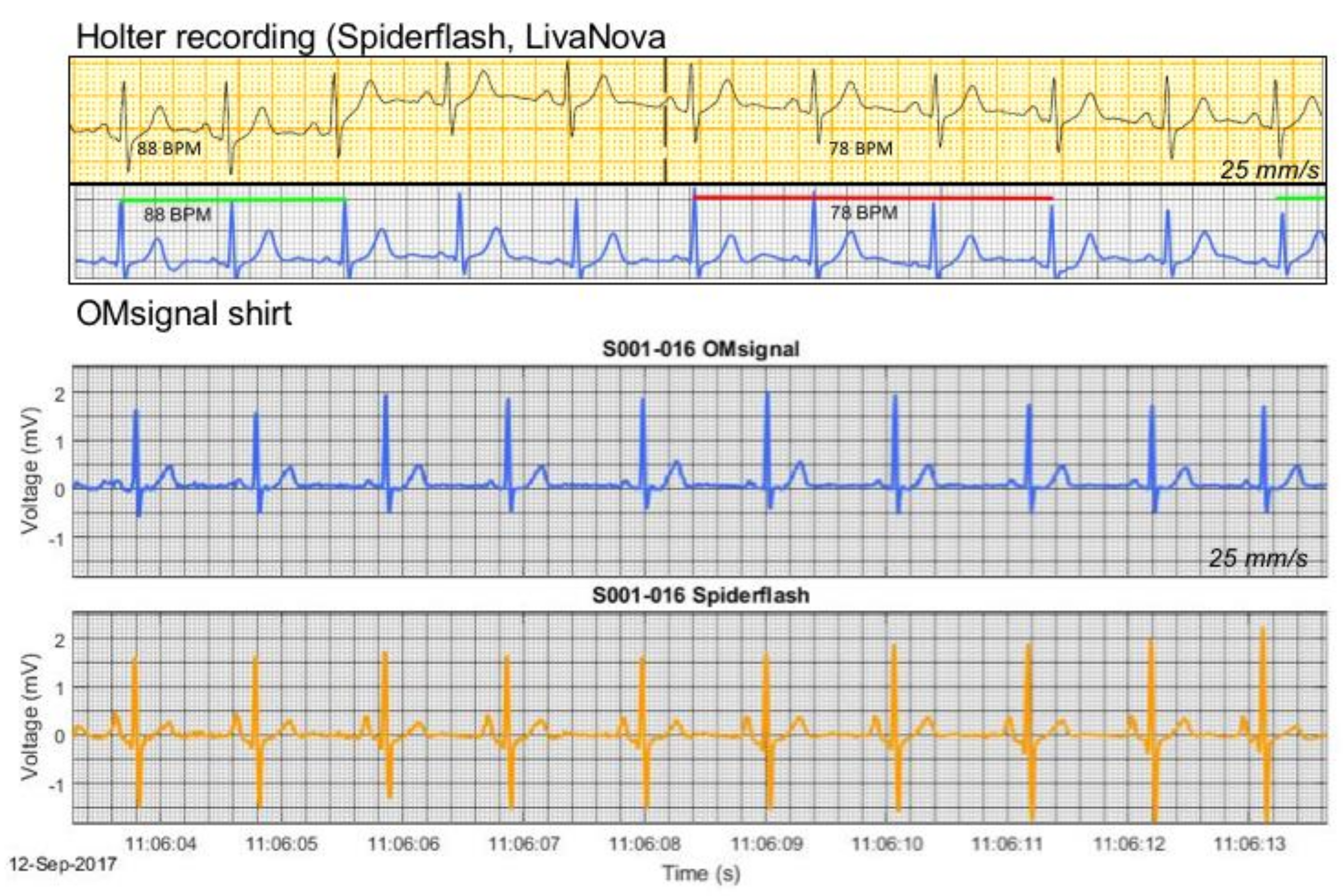
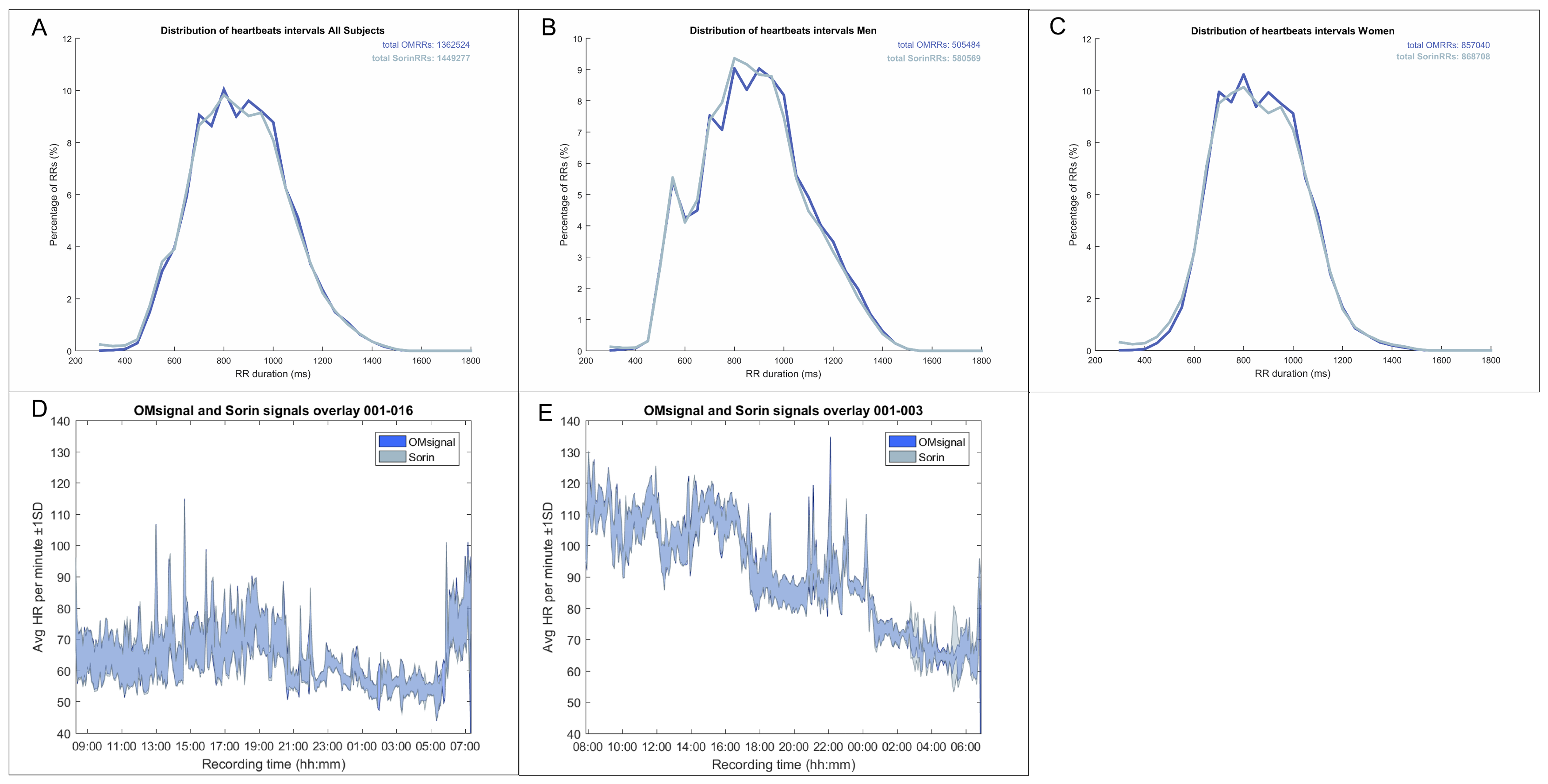
To validate the overall suitability of the OMsignal system for rhythm analysis and monitoring, we assessed the signal quality and completeness of signal coverage (R-R intervals) over a 24-h recording period. Signal coverage was assessed using automated algorithms and the accuracy compared to the Holter recording was verified using Bland Altman analysis (see below).
R-R intervals were calculated using the built-in algorithms of each device. The OMsignal module embeds a modified Pan-Tompkins real-time QRS detection method with heuristic filtering for misdetections. The comparison of heart rates evaluated the difference between the OMsignal and the Holter recording for every second. This resolution of comparison is crucial to validate the capacity to measure physiological phenomena, such as heart rate variability. In this extent, heart rate was calculated over each R-R and interpolated second by second, which was the smallest time interval allowing accurate comparison. Signal temporal alignment between the Holter and OMsignal consisted of three components: (a) An offset, (b) a constant drift component, and (c) a variable drift component. The offset is to align recordings since they do not start at the same time. It is corrected using the best fit of a cross-correlation of the heart rate between the two signals. The constant time drift is due to hardware clock limitations, where 1 s on one device might correspond to 0.98 or 1.02 s from the other device. The drift between the two signals was calculated through cross-correlation for each hour. The time drift was calculated as the average slope of the 24 offsets. Those changes in time precision are also known to be affected by temperature such that the drift might change if the device is at room vs. body temperature. This phenomenon was taken into consideration by correcting time with the overall drift, but to the hour specific offset. Thus, the nonlinearity was partially compensated by having a specific offset per hour. To ensure maximal accuracy of the planned Bland-Altman analysis, all R-R intervals longer than 2 s without detection of an R-R interval in one device or the other were rejected.
2.4. Validation of Signal Quality
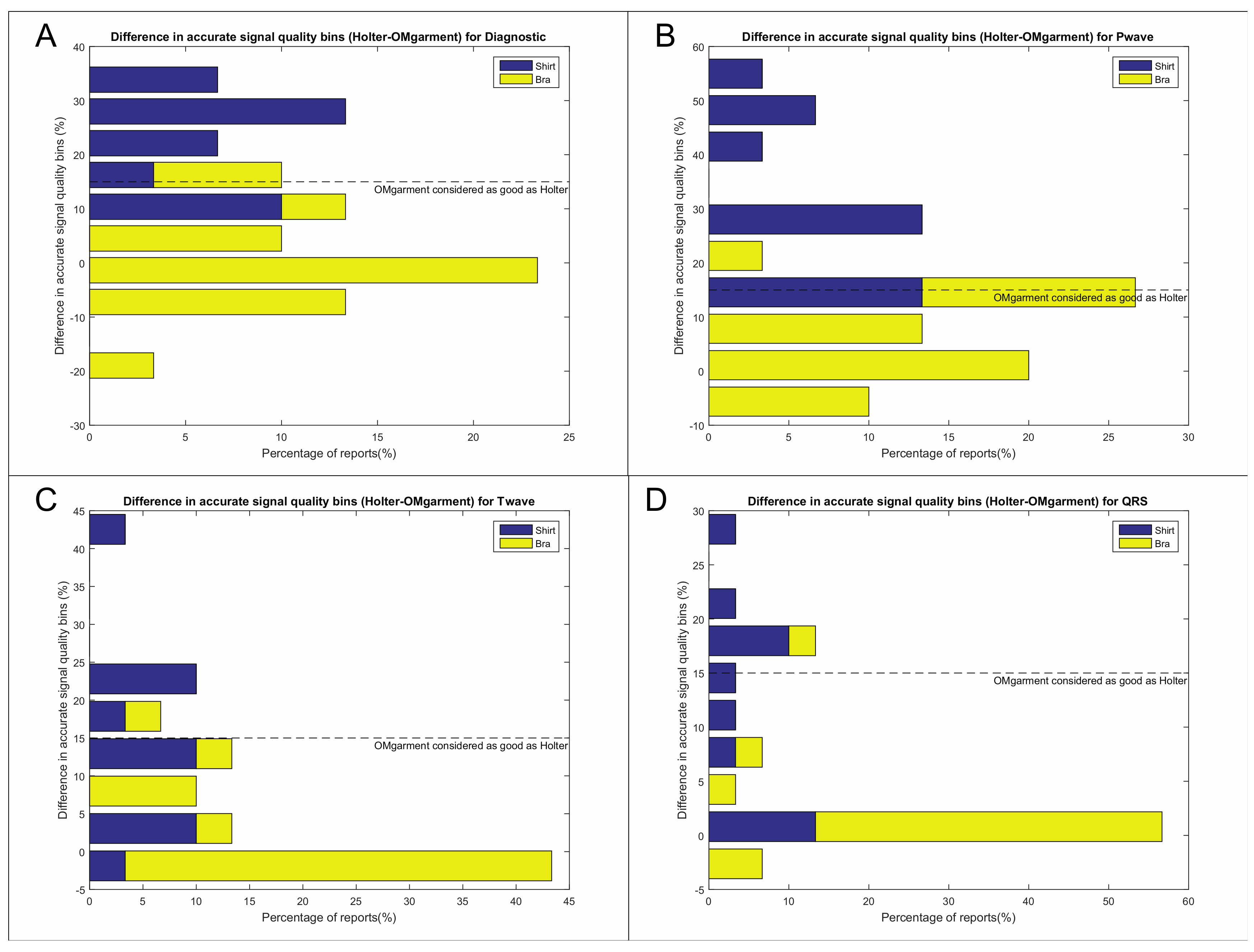
Signal quality of the OMgarments was qualitatively assessed regarding the adequate distinction of P-waves, QRS-complexes, T-waves, and noise levels (
Appendix A). Among the two ECG leads of the Spiderflash Holter monitoring, the lead with the highest signal quality and lowest rate of high-frequency artefacts was chosen for comparison to the OMshirt/OMbra. Assessment of signal quality was independently performed by 3 experienced electrophysiologists blinded to the recording technology (see
Appendix A). For analyses purposes, the ECG signal had its median removed, but no further filter was applied prior to presentation to electrophysiologists.
2.5. Statistical Analysis
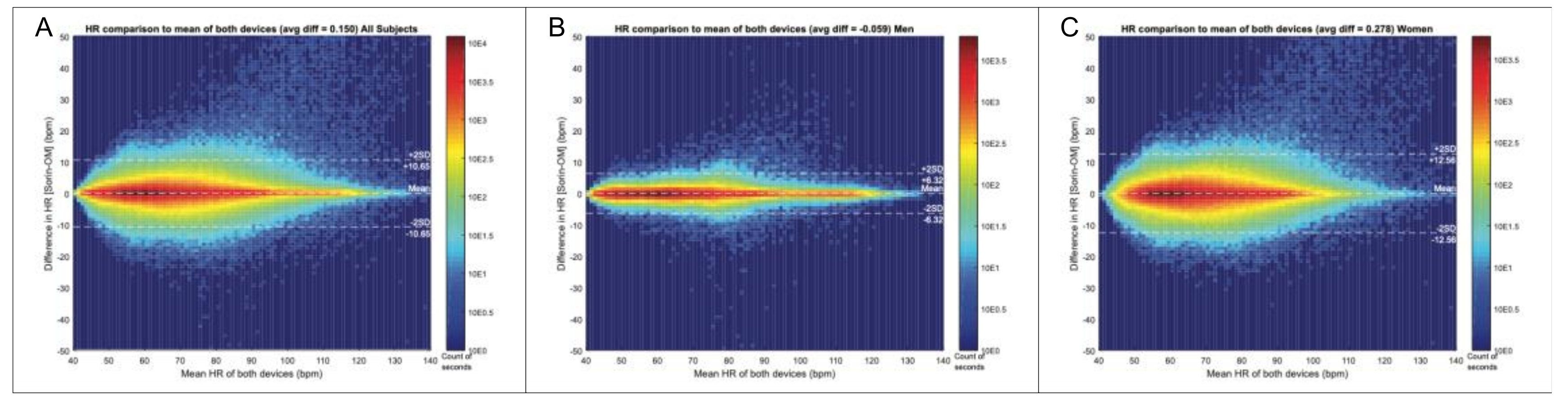
Qualitative and categorical variables were analyzed using the Fisher exact test or χ2-test where appropriate. Continuous variables were tested for normality and are displayed as mean ± standard deviation (SD) or median with interquartile range (IQR) where appropriate. Continuous variables were analyzed using the Student’s t-test or Mann-Whitney-U test where appropriate. McNemar’s testing was performed for noninferiority analysis and the comparison of signal quality and noise level assuming a noninferiority margin of 15%. Bland-Altman analysis was used to compare the signal coverage between Holter recordings and the OMsignal system. The Bland-Altman comparison method was selected since both devices were assumed to have inaccuracies to a limited degree at some point of the recording. This method allows the comparison of measurements from two devices without the obligation of one owning the “true” value. The comparison is done by evaluating the relationship between the heart rate differences and the average heart rates of both devices at every second of the recording. The comparison was calculated at the second interval resolution to demonstrate the close beat-to-beat match between the 2 devices to provide the resolution expected from medical ambulatory devices. This metric can only be computed when both devices detected RRs simultaneously, which represented 91% of the recordings’ duration. These values can be summarized in distribution plots and agreement metrics (e.g.,: ±2 bpm agreement when the average heart rate for both devices is 96 bpm). Interobserver agreement between the two blinded electrophysiologists was analyzed performing kappa and R statistics. A p value < 0.05 was considered statistically significant. All statistical analysis was conducted using Stata 14.1 Software (StataCorp, College Station, TX, USA).
4. Discussion
Extended ambulatory rhythm monitoring is a key element for the diagnosis and management of various rhythm disorders [
4,
12]. However, the diagnostic yield of ambulatory rhythm monitoring highly depends on the frequency of symptoms and the recording duration [
13,
14]. At present, long-term ambulatory rhythm monitoring is limited to external cardiac event recorders (up to 90 days) and implantable cardiac monitors (up to 3 years) [
12,
13,
14].
The OMgarments represent a novel form of wearable ECG sensors providing equivalent signal quality and may be an alternative tool for ambulatory rhythm monitoring. The garments include bras for women and shirts for men that contain integrated electrodes detecting a real-time single-lead surface ECG. In the present study, we demonstrated that the OMgarments provide excellent signal coverage equivalent to standard Holter recording combined with a high signal quality. A particular strength of this phase-1 validation study was the direct, real-time head-to-head recording with OMgarments and a standard 3-lead Holter in each study subject–an approach that has been rarely performed in previous studies introducing novel non-invasive monitoring devices. Using this synchronized recording approach, we showed a very close overlay of R-R coverage (within 2 bpm over 24 h) between the OMgarments and the standard Holter. To the best of our knowledge, no previous study comparing non-invasive monitoring devices has ever reported such a close signal coverage. Excellent R-R coverage is a crucial element for reliable rhythm diagnosis and our study has shown that the OMgarments do not miss heart beats that might have otherwise been recorded by looping event recorders or standard Holter monitoring.
The garments also provided high signal quality that was non-inferior compared to Holter recording. Accurate distinction of P-waves, QRS-complexes, and T-waves was present in most study subjects and based on a semi-quantitative evaluation by experienced electrophysiologists. The overall better signal quality in women is most likely related to a better skin contact of the bra electrode compared to the shirt electrode in men. Although the garment size was selected based on individual under-chest circumference and bust circumference, the overall fit was better in women. Those issues are currently being addressed in the design of the next generation of OMshirts. Changes of the electrode position and size as well as improved noise filter algorithms should overcome the observed differences in noise levels between bras and shirts in the future.
An additional advantage of the garment in this study is the high wearing comfort and minimal risk of skin irritation (7% in this study) compared to conventional electrodes of Holter/looping external event recorders (47% in this study) or adhesive cardiac monitoring patches (personal clinical observations). Moreover, conventional looping external event recorders are cumbersome and interfere with showering, sports, and other daily life activities, resulting in poor patient compliance and reduced diagnostic yield [
4]. Adhesive cardiac monitor patches are characterized by ease-of-use, increased wearing comfort, and allow showering [
15], but have limited recording durations (7–30 days) compared to conventional external loop recorders or implantable cardiac monitors [
4,
16,
17] This study was intentionally limited to a recording period of 24 h, but the built-in capacity of signal transmission from the garment into a web based cloud provides already the technical basis for long-term monitoring, which will be studied in an upcoming multicenter trial. Also, the signal quality of the garments remains stable over prolonged recording periods, including washing (unpublished results), whereas there are some concerns about decreased signal quality and loss of automated rhythm analysis in adhesive monitor patches [
18].
At present, implantable cardiac monitors (ICMs) represent the only device of ambulatory long-term monitoring beyond a period of 3 months. The utility and diagnostic accuracy of ICMs is well-established and the recent model of one manufacturer has been miniaturized to a volume of only 1.2 cm
3 [
4,
19]. However, ICMs are invasive with a small, but real risk of complications and the recording capacity is limited to 57 min, whereas the wearable ECG sensors, like the OMgarments, provide potentially unlimited recording (Steinberg et al., 2017). Moreover, the insertion of an ICM is associated with significant upfront costs compared to wearable ECG sensors, like the garments of this study.
The potential spectrum of clinical applications for wearable ECG sensors, like the OMgarments, is broad, including diagnosis of unexplained syncope or palpitations, screening for subclinical atrial fibrillation, monitoring of heart rate variability and antiarrhythmic treatment effects, or screening for recurrent arrhythmia after ablation procedures. From a population health perspective, the screening for subclinical atrial fibrillation (AF) has gained wide interest, since two recent large-scale prospective studies have demonstrated that occult AF is a major source of cryptogenic ischemic stroke [
20,
21]. In the pivotal CRYSTAL-AF and EMBRACE-AF study, subclinical AF was detected in 12–16% of study subjects over 3–12 months using an ICM (CRYSTAL-AF) or external non-looping event recorder (EMBRACE-AF) [
20,
21]. Extended monitoring up to 3 years through an ICM in the CRYSTAL-AF trial was even associated with an increase of subclinical AF up to 30% of all study subjects. Based on those results, long-term rhythm monitoring to search for AF has been recommended in all individuals with cryptogenic stroke [
22], making wearable ECG sensors, like the OMgarments, an attractive, easy-to-use, and less costly alternative. In a similar manner, wearable ECG sensors may enrich the arsenal of ambulatory long-term monitoring tools to assess medical and interventional treatment effects in various arrhythmia as well as risk prediction in structural heart disease or inherited arrhythmia [
4,
23,
24].
One of the main challenges of wearable ECG sensors is the use of dry contact electrodes on a recording surface subject to movement artifacts. The current study demonstrates that OMsignal garments have a similar performance to a Holter monitor. As previously shown, such garments may have great potential in clinical settings. The cost of an OMsignal unit is quite low (180$ US), allowing a wider distribution of ambulatory monitoring. Patient set up and follow up are also simplified. Electrode placement is standardized using a sizing chart and once a patient is properly equipped, garments provide recordings of reproducible signal quality. The follow up is simplified as wireless transmission allows remote data transfer to a secured cloud and faster data processing. Combined with the rechargeable module, it would allow prolonged monitoring periods although this has yet to be demonstrated. Another challenge of prolonged recordings is the interaction between skin and the dry contact electrodes, which is minimized by the integrated electrodes of the garment.
Wearable ECG sensors, like the OMgarments, are designed to integrate medical data acquisition into usual daily life activities. The emergence of artificial intelligence to monitor cardiac conditions opens the door for novel strategies of prolonged ambulatory monitoring [
25,
26]. Biointeractive garments, such as the presented one, could leverage these novel technologies.
Limitations
This is a single-center study for a proof-of-concept that was conducted in healthy individuals. The sample size of this study is very modest, however, the crossover design with simultaneous Holter recording assured a true head-to-head design transforming each study subject into its own control. Performing a beat-by-beat analysis for signal coverage resulted in a large amount of quantitative data for the overall analysis. Therefore, we do not believe that the small sample size may have influenced the overall message of this study. All study subjects had a normal body mass index and there may be some uncertainty about the recording accuracy in individuals with extreme body weights. Overall signal quality was better in women, which is related to the better wearing fit of the OMbra. Despite that difference, the overall signal quality in men was still sufficient to establish a rhythm diagnosis in the vast majority of cases and there was no difference between males and females with regards to the accurate detection of heart rate and heart rate variability over 24 h. The present study was limited to a recording period of 24-h and a consistent signal quality over longer monitoring periods has yet to be demonstrated. An upcoming multicenter trial will address the utility of the wearable ECG sensors for mid- and long-term monitoring.