An All-Glass Microfluidic Network with Integrated Amorphous Silicon Photosensors for on-Chip Monitoring of Enzymatic Biochemical Assay
Abstract
1. Introduction
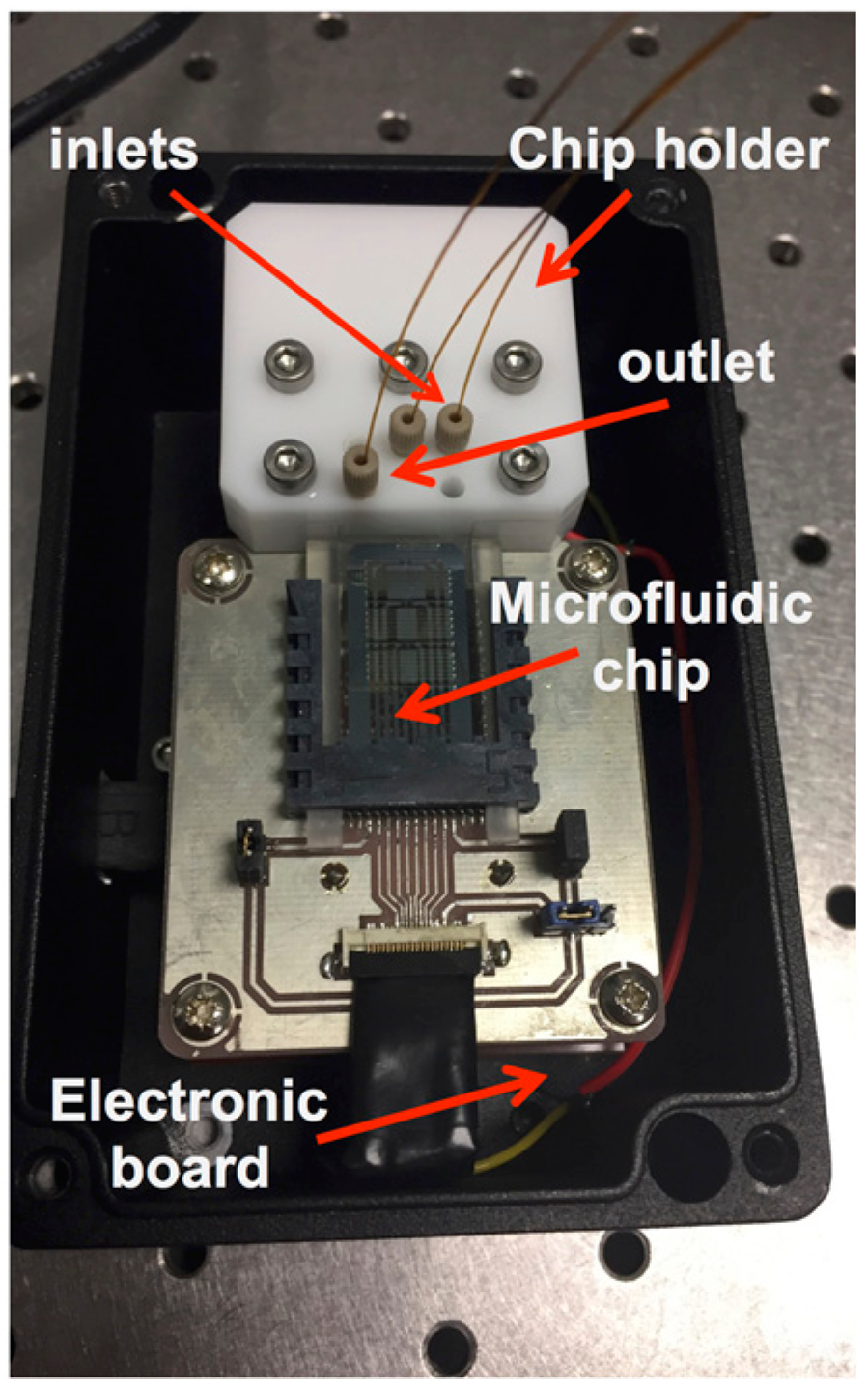
2. Materials and Methods
- the fabrication process of the thin-film amorphous-silicon photosensors should not affect the interior of the microfluidic glass network. More specifically, the chemical surface composition of the glass channels is important for the enzymatic functionalization protocol (which is based on silanol groups), and thus the materials (gases, liquids) involved in the procedure to realize photosensors should not enter the fluidic network.
- the chemical treatment and functionalization of the microfluidic interior should not affect the functionality and performance of the thin film amorphous silicon photosensors.
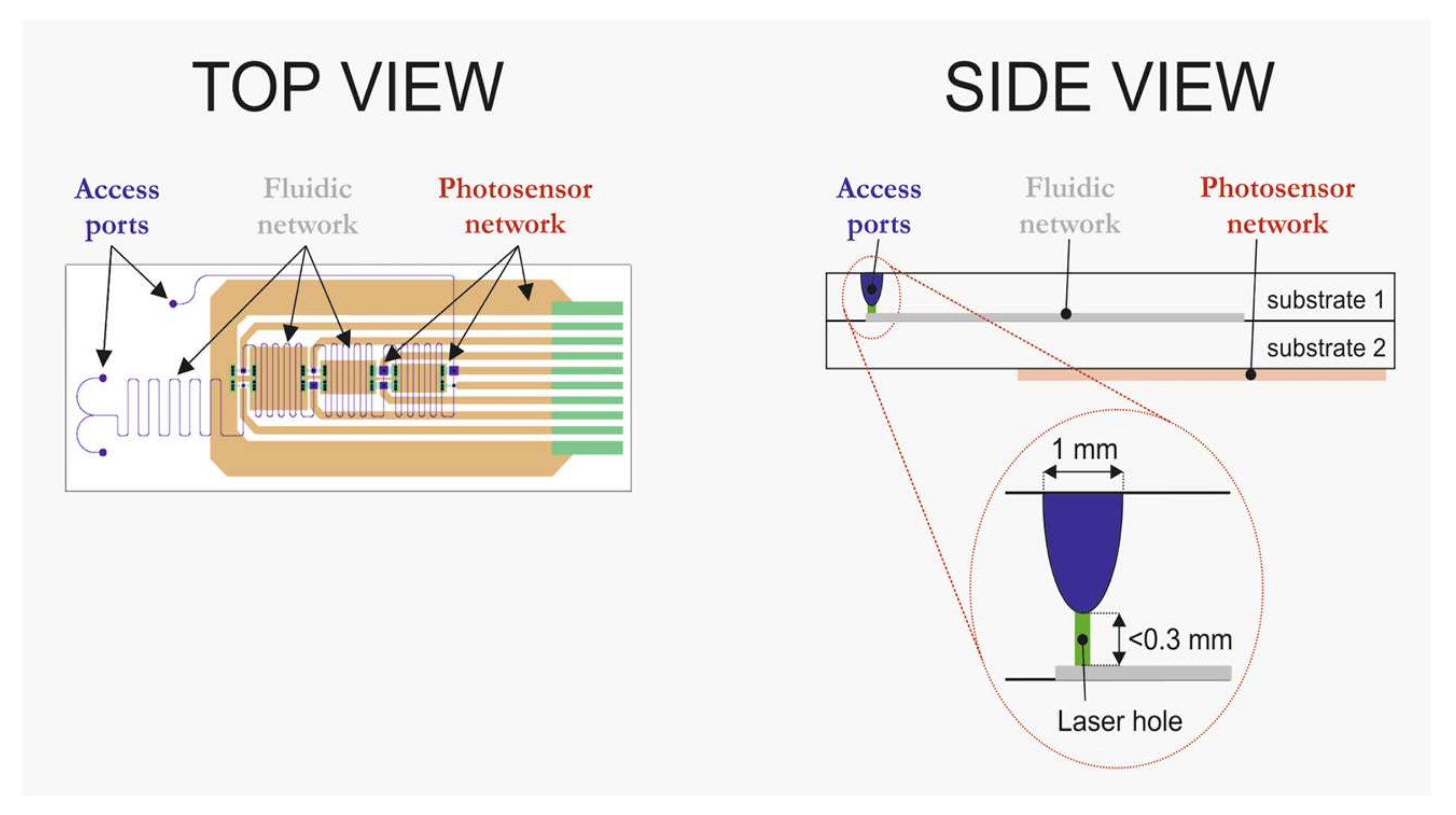
- realization of fluidic networks on the bondside of a 1.1 mm thick Borofloat 33 (BF33) substrate;
- realization of powderblasted pits in the 1.1 mm thick BF33 substrate (at the non-bond side). These powderblasted pits are the fluidic access ports (note: these pits are not yet in contact with the microfluidic channel network);
- realization of thin film amorphous silicon photosensors on the stack. Sensors are deposited onto the 0.5 mm thick substrate, so that the sensors are as close as possible to the fluidic network;
- realization of fluidic accesses to the microfluidic network, by means of laser drilling at the bottom of the predefined powderblasted pits;
- realization of individual chips via dicing of the BF33 stack.
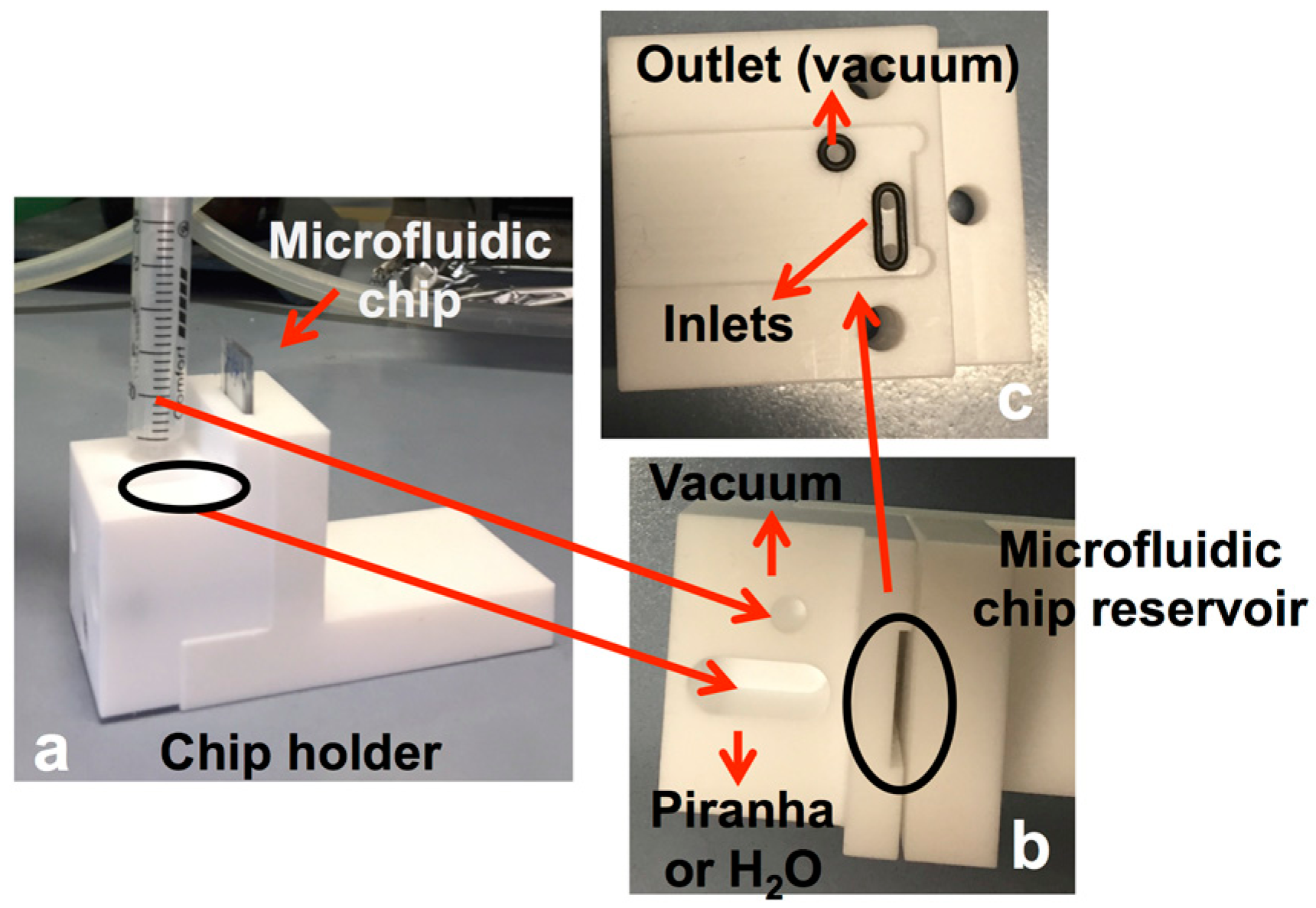
- cleaning of the outside of the stack by Piranha solution (H2SO4:H2O2 3:1) and ultrasonic treatment in deionized water;
- deposition (by magnetron sputtering) of a 100 nm-thick layer of Indium Tin Oxide (ITO) and subsequent patterning (by UV-photolithography). This transparent conductive oxide (TCO) is the front contact of the photodiode;
- PECVD-deposition of the a-Si:H layers;
- deposition (by evaporation) of a Cr/Al/Cr stacked layer, acting as bottom contact of the photosensor;
- patterning of the Cr/Al/Cr stack and a-Si:H layers by means of wet and dry etching, respectively. This step defines the geometry of the photodiodes (which are aligned with respect to the microfluidic channels);
- deposition and patterning of a 5 μm-thick layer of SU-8 3005 (MicroChem, Westborough, MA, USA), which acts as passivation and insulation layer on the lateral walls of the diodes. This patterning step also defines the via holes on top of the diodes; deposition and patterning, by magnetron sputtering, of a 200 nm-thick TiW layer for contacting the bottom contact of the diodes through the via holes and for the electrical connections to external pads.
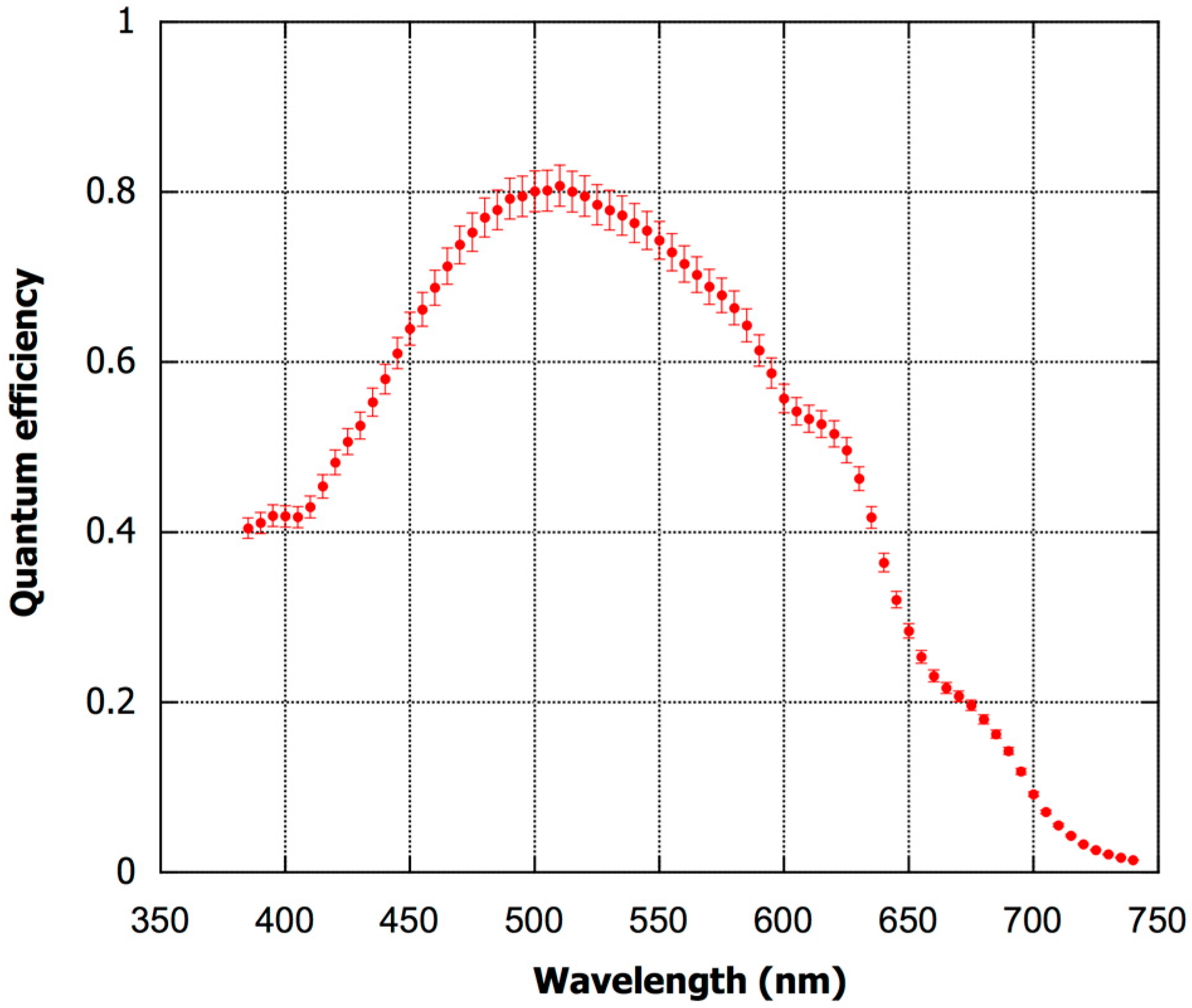
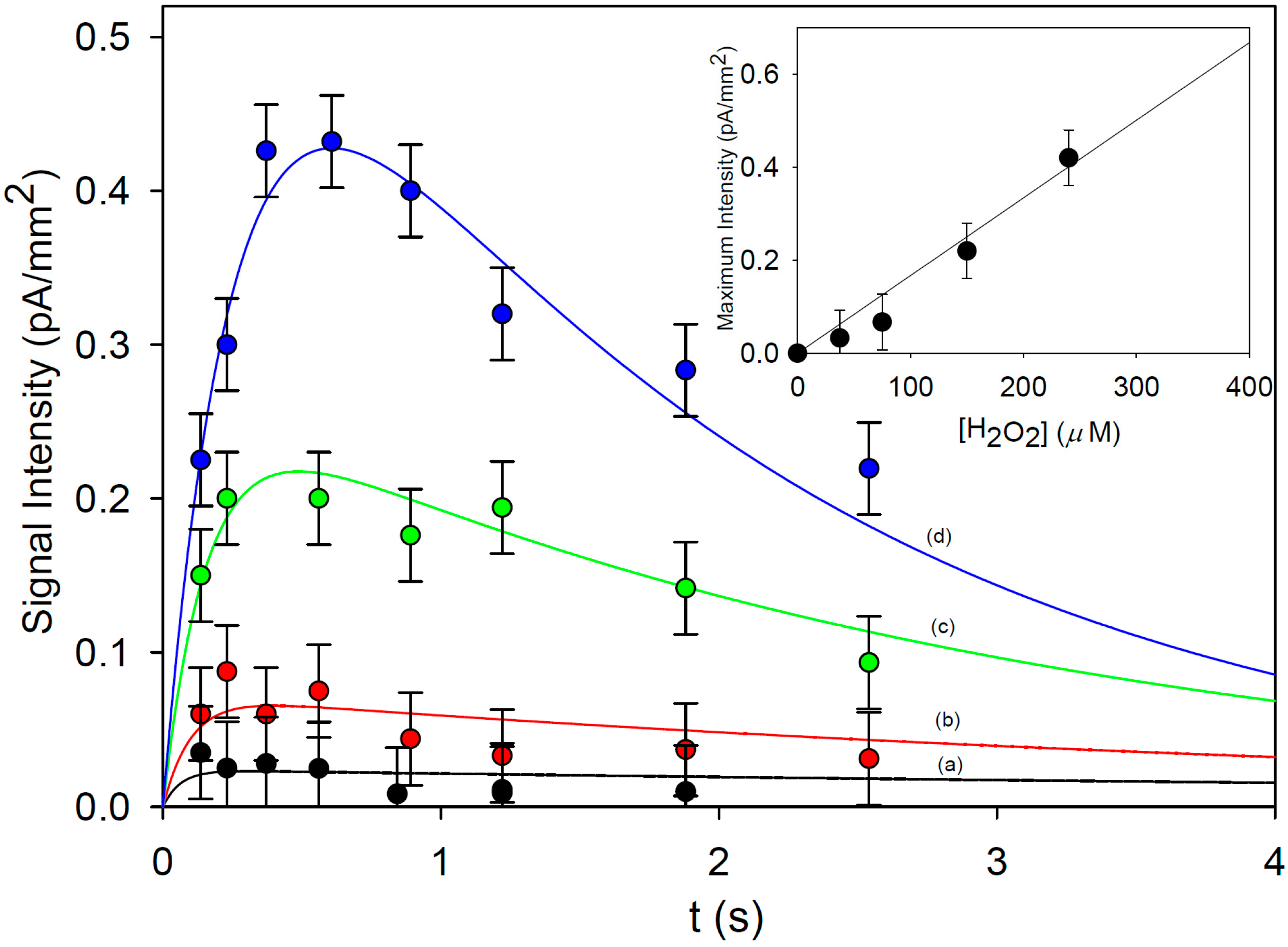
3. Results and Discussion
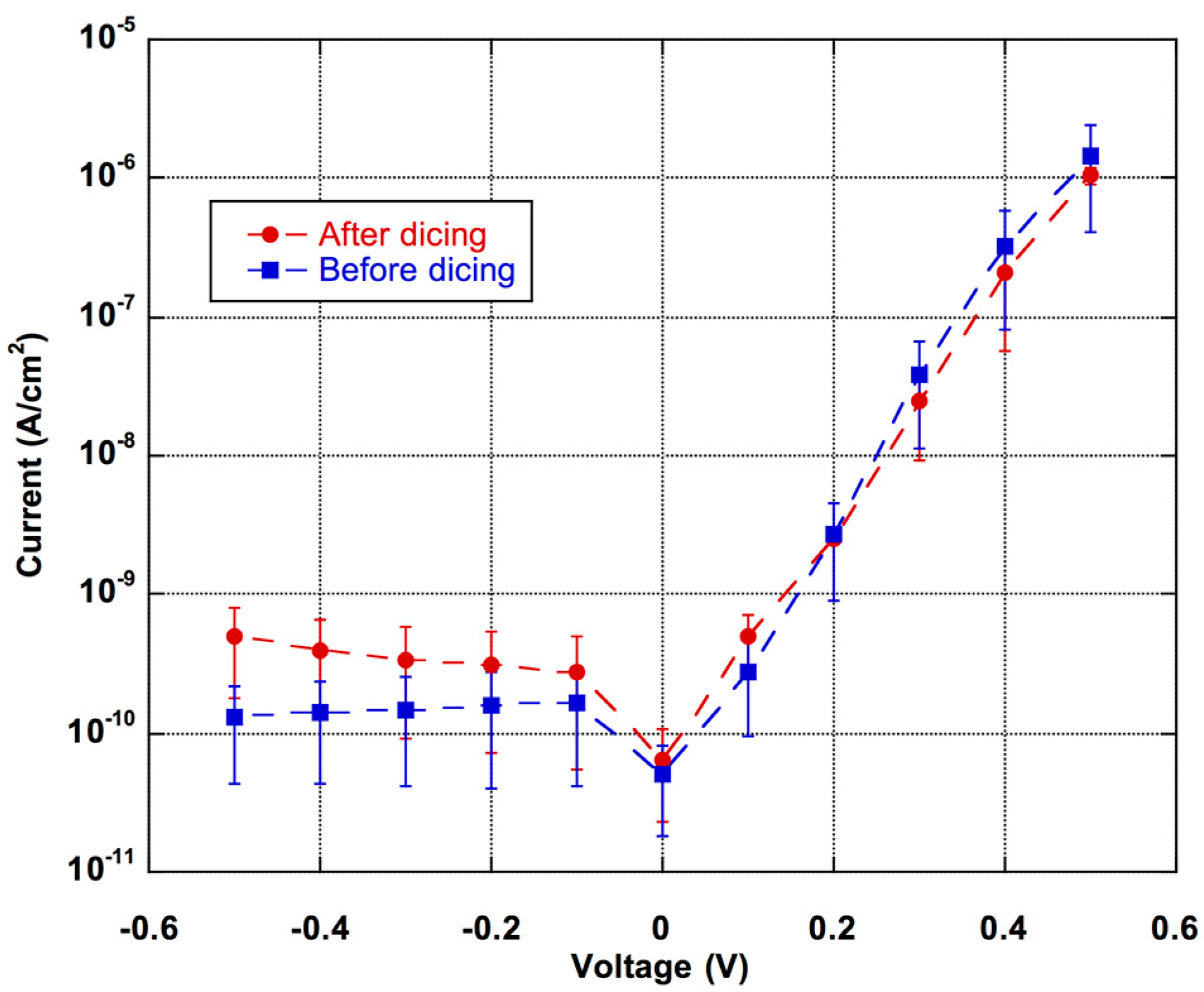
- fabrication of the microfluidic network should not affect the photosensor performance;
- the photodiodes have to be optically aligned with the microfluidic network and their deposition should leave the channel clean for further utilization;
- the surface chemistry treatment should not affect the functionality of both the photodiodes and the channel network.
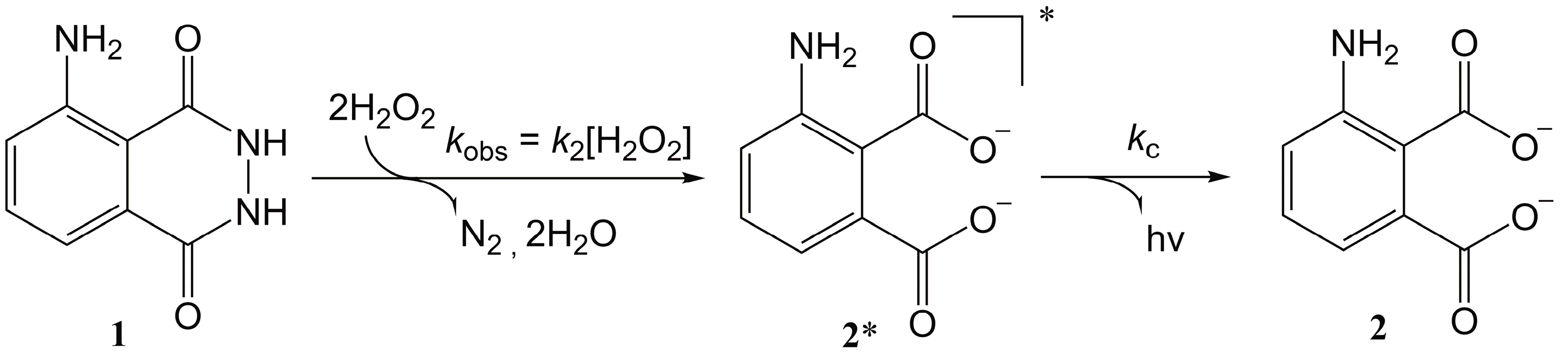
- the chemiluminescence approach, used in our work, does not need an excitation source, leading to a lower power consumption and a more compact system;
- the distance between the site where the chemiluminescence occurs and the detectors (the a-Si:H photosensors) is reduced to 700 microns (the thickness of the glass hosting the photosensors) which minimizes the optical loss to the light diffusion;
- the alignment between the radiation source (the inner of the channel) and the detectors is done automatically during the device fabrication;
- our system is completely re-usable (without any disposable part) just by cleaning with piranha the microfluidic channel. This is possible thanks to the use of the all-glass microfluidic approach. Therefore, such a cleaning process is not possible with a PDMS network.
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bange, A.; Halsall, H.; Heineman, W. Microfluidic immunosensor systems. Biosens. Bioelectron. 2005, 20, 2488–2503. [Google Scholar] [CrossRef] [PubMed]
- Luka, G.; Ahmadi, A.; Najjaran, H.; Alocilja, E.; DeRosa, M.; Wolthers, K.; Malki, A.; Aziz, H.; Althani, A.; Hoorfar, M. Microfluidics integrated biosensors: A leading technology towards lab-on-a-chip and sensing applications. Sensors 2015, 15, 30011–30031. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Zapatero-Rodriguez, J.; Estrela, P.; O’Kennedy, R. Point-of-care diagnostics in low resource settings: Present status and future role of microfluidics. Biosensors 2015, 5, 577–601. [Google Scholar] [CrossRef] [PubMed]
- Kovarik, M.; Gach, P.; Ornoff, D.; Wang, Y.; Balowski, J.; Farrag, L.; Allbritton, N. Micro total analysis systems for cell biology and biochemical assays. Anal. Chem. 2012, 84, 516–540. [Google Scholar] [CrossRef] [PubMed]
- Bilitewski, U.; Genrich, M.; Kadow, S.; Mersal, G. Biochemical analysis with microfluidic systems. Anal. Bioanal. Chem. 2003, 377, 556–569. [Google Scholar] [CrossRef] [PubMed]
- Bruijns, B.; van Asten, A.; Tiggelaar, R.; Gardeniers, H. Microfluidic devices for forensic DNA analysis: A review. Biosensors 2016, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.; Gaur, G.; Neethirajan, S. Rapid detection of food allergens by microfluidics elisa-based optical sensor. Biosensors 2016, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Caputo, D.; de Cesare, G.; Lo Vecchio, N.; Nascetti, A.; Parisi, E.; Scipinotti, R. Polydimethylsiloxane material as hydrophobic and insulating layer in electrowetting-on-dielectric systems. Microelectron. J. 2014, 45, 1684–1690. [Google Scholar] [CrossRef]
- Yang, Y.; Noviana, E.; Nguyen, M.P.; Geiss, B.J.; Dandy, D.S.; Henry, C.S. Paper-based microfluidic devices: Emerging themes and applications. Anal. Chem. 2017, 89, 71–91. [Google Scholar] [CrossRef] [PubMed]
- Costantini, F.; Benetti, E.M.; Reinhoudt, D.N.; Huskens, J.; Vancso, G.J.; Verboom, W. Enzyme-functionalized polymer brush films on the inner wall of silicon-glass microreactors with tunable biocatalytic activity. Lab Chip 2010, 10, 3407–3412. [Google Scholar] [CrossRef] [PubMed]
- Costantini, F.; Sberna, C.; Petrucci, G.; Manetti, C.; deCesare, G.; Nascetti, A.; Caputo, D. Lab-on-chip system combining a microfluidic-ELISA with an array of amorphous silicon photosensors for the detection of celiac disease epitopes. Sens. Biosens. Res. 2015, 6, 51–58. [Google Scholar] [CrossRef]
- Costantini, F.; Sberna, C.; Petrucci, G.; Reverberi, M.; Domenici, F.; Fanelli, C.; Manetti, C.; de Cesare, G.; DeRosa, M.; Nascetti, A.; et al. Aptamer-based sandwich assay for on chip detection of ochratoxin a by an array of amorphous silicon photosensors. Sens. Actuators B Chem. 2016, 230, 31–39. [Google Scholar] [CrossRef]
- Costantini, F.; Tiggelaar, R.; Sennato, S.; Mura, F.; Schlautmann, S.; Bordi, F.; Gardeniers, H.; Manetti, C. Glucose level determination with a multi-enzymatic cascade reaction in a functionalized glass chip. Analyst 2013, 138, 5019–5024. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, N.; Dutta, D. Kinetic elisa in microfluidic channels. Biosensors 2011, 1, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Ligler, F.S. Perspective on optical biosensors and integrated sensor systems. Anal. Chem. 2009, 81, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Lafleur, J.; Jonsson, A.; Senkbeil, S.; Kutter, J. Recent advances in lab-on-a-chip for biosensing applications. Biosens. Bioelectron. 2016, 76, 213–233. [Google Scholar] [CrossRef] [PubMed]
- Erickson, D.; Li, D. Integrated microfluidic devices. Anal. Chim. Acta 2004, 507, 11–26. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, S.; Zhang, X. Recent developments and applications of chemiluminescence sensors. Anal. Chim. Acta 2005, 541, 37–47. [Google Scholar] [CrossRef]
- Lok, K.; Kwok, Y.; Nguyen, N. Double spiral detection channel for on-chip chemiluminescence detection. Sens. Actuators B Chem. 2012, 169, 144–150. [Google Scholar] [CrossRef]
- Hofmann, O.; Miller, P.; Sullivan, P.; Jones, T.S.; deMello, J.C.; Bradley, D.D.C.; deMello, A.J. Thin-film organic photodiodes as integrated detectors for microscale chemiluminescence assays. Sen. Actuators B Chem. 2005, 106, 878–884. [Google Scholar]
- Jorgensen, A.M.; Mogensen, K.B.; Kutter, J.P.; Geschke, O. A biochemical microdevice with an integrated chemiluminescence detector. Sens. Actuators B Chem. 2003, 90, 15–21. [Google Scholar] [CrossRef]
- Novo, P.; Prazeres, D.M.; Chu, V.; Conde, J.P. Microspot-based elisa in microfluidics: Chemiluminescence and colorimetry detection using integrated thin-film hydrogenated amorphous silicon photodiodes. Lab Chip 2011, 11, 4063–4071. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Amatatongchai, M.; Nacapricha, D.; Hofmann, O.; de Mello, J.C.; Bradley, D.D.C.; de Mello, A.J. Thin-film organic photodiodes for integrated on-chip chemiluminescence detection—Application to antioxidant capacity screening. Sens. Actuators B Chem. 2009, 140, 643–648. [Google Scholar] [CrossRef]
- Wang, X.; Hofmann, O.; Das, R.; Barrett, E.; Demello, A.; Demello, J.; Bradley, D. Integrated thin-film polymer/fullerene photodetectors for on-chip microfluidic chemiluminescence detection. Lab Chip 2007, 7, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Caputo, D.; de Cesare, G.; Fanelli, C.; Nascetti, A.; Ricelli, A.; Scipinotti, R. Innovative detection system of ochratoxin a by thin film photodiodes. Sensors 2007, 7, 1317–1322. [Google Scholar] [CrossRef]
- Mirasoli, M.; Nascetti, A.; Caputo, D.; Zangheri, M.; Scipinotti, R.; Cevenini, L.; de Cesare, G.; Roda, A. Multiwell cartridge with integrated array of amorphous silicon photosensors for chemiluminescence detection: Development, characterization and comparison with cooled-ccd luminograph. Anal. Bioanal. Chem. 2014, 406, 5645–5656. [Google Scholar] [CrossRef] [PubMed]
- Costantini, F.; Bula, W.P.; Salvio, R.; Huskens, J.; Gardeniers, H.; Reinhoudt, D.N.; Verboom, W. Nanostructure based on polymer brushes for efficient heterogeneous catalysis in microreactors. J. Am. Chem. Soc. 2009, 131, 1650–1651. [Google Scholar] [CrossRef] [PubMed]
- Costantini, F.; Domenici, F.; Mura, F.; Scipinotti, R.; Sennato, S.; Manetti, C.; Bordi, F. A new nanostructured stationary phase for ultra-thin layer chromatography: A brush-gel polymer film. Nanosci. Nanotechnol. Lett. 2013, 5, 1155–1163. [Google Scholar] [CrossRef]
- Azzaroni, O. Polymer brushes here, there, and everywhere: Recent advances in their practical applications and emerging opportunities in multiple research fields. J. Polym. Sci. Polym. Chem. 2012, 50, 3225–3258. [Google Scholar] [CrossRef]
- Tahirovic, A.; Copra, A.; Omanovic-Miklicanin, E.; Kalcher, K. A chemiluminescence sensor for the determination of hydrogen peroxide. Talanta 2007, 72, 1378–1385. [Google Scholar] [CrossRef] [PubMed]
- Costantini, F.; Benetti, E.M.; Tiggelaar, R.M.; Gardeniers, H.; Reinhoudt, D.N.; Huskens, J.; Vancso, G.J.; Verboom, W. A brush-gel/metal-nanoparticle hybrid film as an efficient supported catalyst in glass microreactors. Chem. Eur. J. 2010, 16, 12406–12411. [Google Scholar] [CrossRef] [PubMed]
- Caputo, D.; de Cesare, G.; Scipinotti, R.; Stasio, N.; Costantini, F.; Manetti, C.; Nascetti, A. On-chip diagnosis of celiac disease by an amorphous silicon chemiluminescence detector. In Lecture Notes in Electrical Engineering, Proceedings of the 17th National Conference on Sensors and Microsystems, Brescia, Italy, 5–7 Feruray 2013; Springer: Cham, Switherland, 2014; Volume 268, pp. 183–187. [Google Scholar]
- Costantini, F.; Nascetti, A.; Scipinotti, R.; Domenici, F.; Sennato, S.; Gazza, L.; Bordi, F.; Pogna, N.; Manetti, C.; Caputo, D.; et al. On-chip detection of multiple serum antibodies against epitopes of celiac disease by an array of amorphous silicon sensors. Rsc Adv. 2014, 4, 2073–2080. [Google Scholar] [CrossRef]
- Savelli, C.; Salvio, R. Guanidine-based polymer brushes grafted onto silica nanoparticles as efficient artificial phosphodiesterases. Chem. Eur. J. 2015, 21, 5856–5863. [Google Scholar] [CrossRef] [PubMed]
- Fasolato, C.; Domenici, F.; Sennato, S.; Mura, F.; De Angelis, L.; Luongo, F.; Costantini, F.; Bordi, F.; Postorino, P. Dimensional scale effects on surface enhanced Raman scattering efficiency of self-assembled silver nanoparticle clusters. Appl. Phys. Lett. 2014, 105, 073105. [Google Scholar] [CrossRef]
- Nascetti, A.; Colonia, G.; Caputo, D.; de Cesare, G. Sophie: A general purpose sub-picoamps current readout electronics. In Lecture Notes in Electrical Engineering, Proceedings of the 2nd National Conference on Sensors, Rome, Italy, 19–21 Februray 2014; Springer: Cham, Switherland, 2014; Volume Voulme 319, pp. 285–289. [Google Scholar]
- Anslyn, E.V.; Dougherty, D.A. Modern Physical Organic Chemistry; University Science Books: Sausalito, CA, USA, 2006; ISBN 978-1-891389-31-3. [Google Scholar]
- Salvio, R. The guanidinium unit in the catalysis of phosphoryl transfer reactions: From molecular spacers to nanostructured supports. Chem. Eur. J. 2015, 21, 10960–10971. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ruiz, I.; Masvidal-Codina, E.; Ackermann, T.N.; Llobera, A. Photonic lab-on-chip (PhLOC) for enzyme-catalyzed reactions in continuous flow. Microfluid. Nanofluid. 2015, 18, 1277–1286. [Google Scholar] [CrossRef]
- Chabinyc, M.L.; Chiu, D.T.; McDonald, J.C.; Stroock, A.D.; Christian, J.F.; Karger, A.M.; Whitesides, G.M. An integrated fluorescence detection system in poly(dimethylsiloxane) for microfluidic applications. Anal. Chem. 2001, 73, 4491–4498. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costantini, F.; Tiggelaar, R.M.; Salvio, R.; Nardecchia, M.; Schlautmann, S.; Manetti, C.; Gardeniers, H.J.G.E.; De Cesare, G.; Caputo, D.; Nascetti, A. An All-Glass Microfluidic Network with Integrated Amorphous Silicon Photosensors for on-Chip Monitoring of Enzymatic Biochemical Assay. Biosensors 2017, 7, 58. https://doi.org/10.3390/bios7040058
Costantini F, Tiggelaar RM, Salvio R, Nardecchia M, Schlautmann S, Manetti C, Gardeniers HJGE, De Cesare G, Caputo D, Nascetti A. An All-Glass Microfluidic Network with Integrated Amorphous Silicon Photosensors for on-Chip Monitoring of Enzymatic Biochemical Assay. Biosensors. 2017; 7(4):58. https://doi.org/10.3390/bios7040058
Chicago/Turabian StyleCostantini, Francesca, Roald M. Tiggelaar, Riccardo Salvio, Marco Nardecchia, Stefan Schlautmann, Cesare Manetti, Han J. G. E. Gardeniers, Giampiero De Cesare, Domenico Caputo, and Augusto Nascetti. 2017. "An All-Glass Microfluidic Network with Integrated Amorphous Silicon Photosensors for on-Chip Monitoring of Enzymatic Biochemical Assay" Biosensors 7, no. 4: 58. https://doi.org/10.3390/bios7040058
APA StyleCostantini, F., Tiggelaar, R. M., Salvio, R., Nardecchia, M., Schlautmann, S., Manetti, C., Gardeniers, H. J. G. E., De Cesare, G., Caputo, D., & Nascetti, A. (2017). An All-Glass Microfluidic Network with Integrated Amorphous Silicon Photosensors for on-Chip Monitoring of Enzymatic Biochemical Assay. Biosensors, 7(4), 58. https://doi.org/10.3390/bios7040058









