Pathophysiological Associations and Measurement Techniques of Red Blood Cell Deformability
Abstract
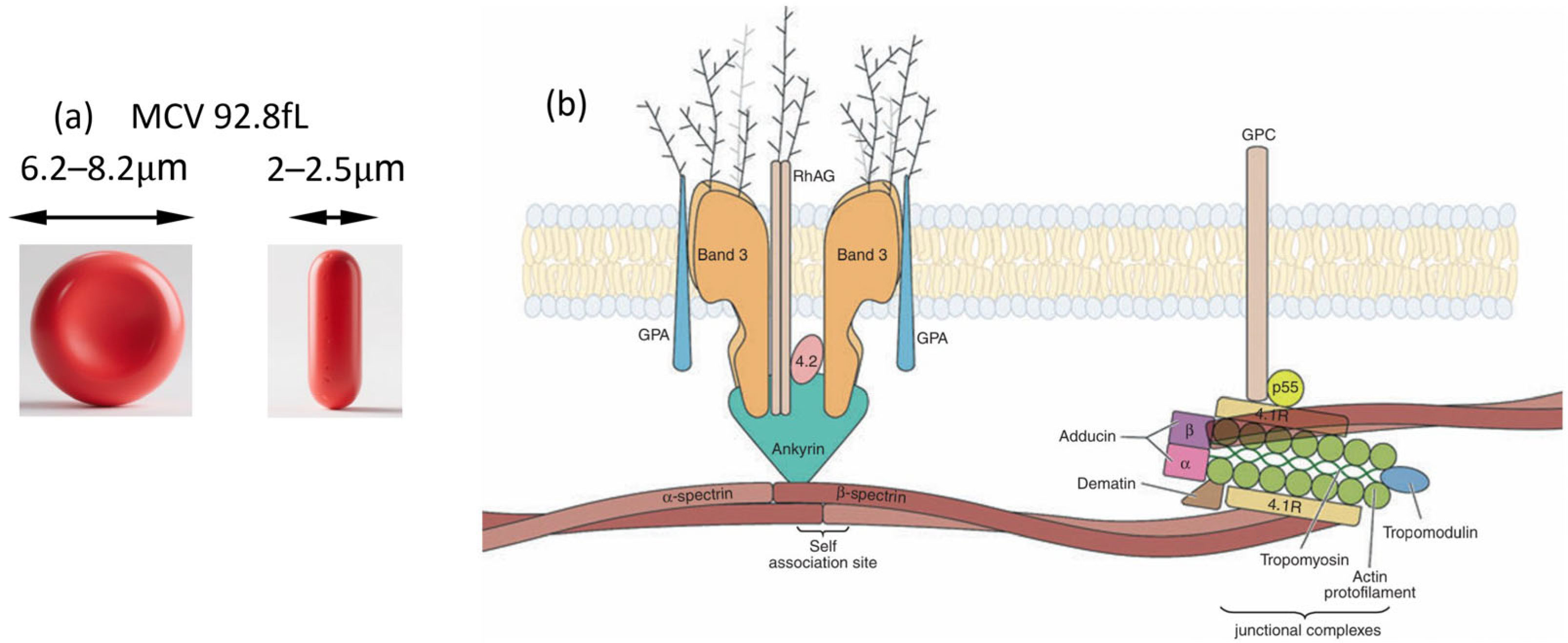
1. Introduction
2. RBC Deformability in Health and Disease
3. Mechanics Evaluation of RBC Deformability
3.1. Elasticity
- (1)
- Area expansion/compressibility modulus (): it is mainly domain by the RBC membrane [55]. Moreover, different from the other modulus, it corresponds to the surface dilation or so call expansion with shear or bending.
- (2)
- Shear elastic modulus (): elongation or shear of the surface area that contributes from the spectrin network [56].
- (3)
- Bending modulus (B): represents the energy needed to change the surface curvature [57].
3.2. Viscosity
- (1)
- Cytoplasm viscosity: normally determined by Hb concentration, which can be measured by dynamic fluctuations of RBC membrane, obtaining 2–5 mPa∙s [58].
- (2)
- Membrane viscosity, considering a 2D condition. , the coefficient of surface viscosity. The shear force can be expressed as,
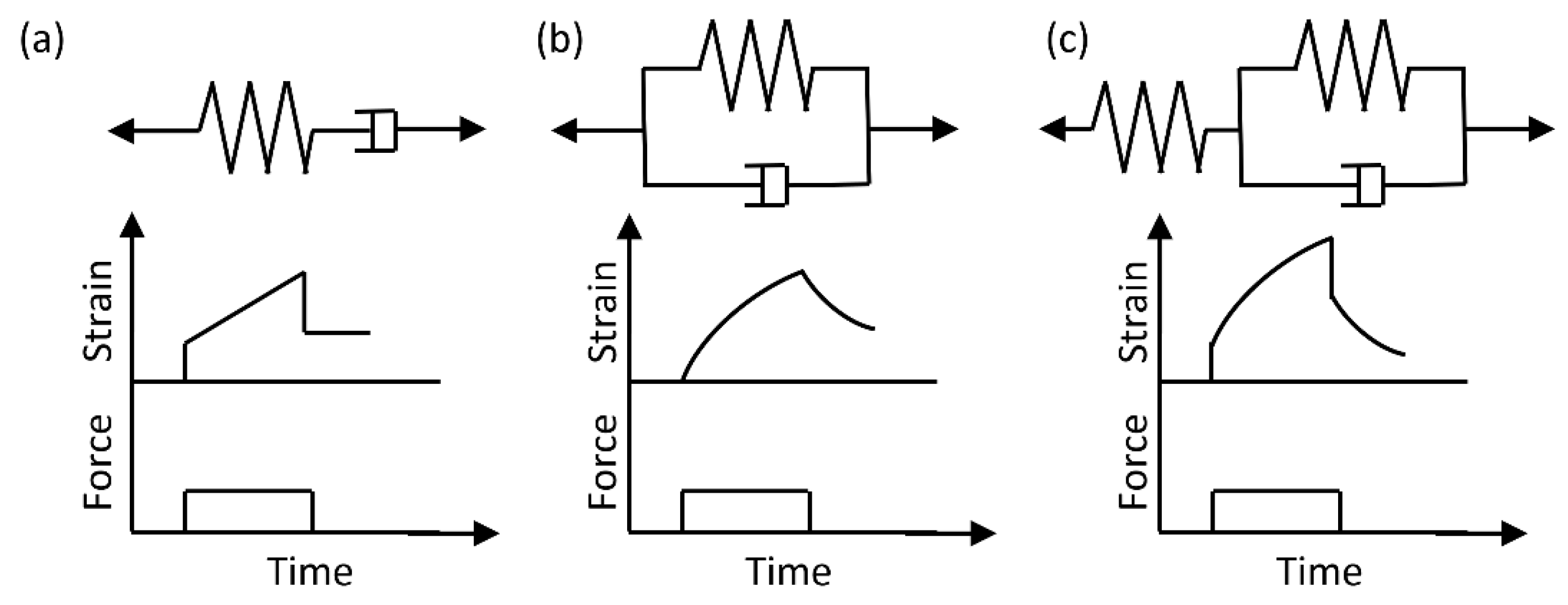
3.3. Viscoelastic Models and Experimental Methods
4. Techniques for Measuring RBC Deformability
4.1. Single Cell Measurement Techniques
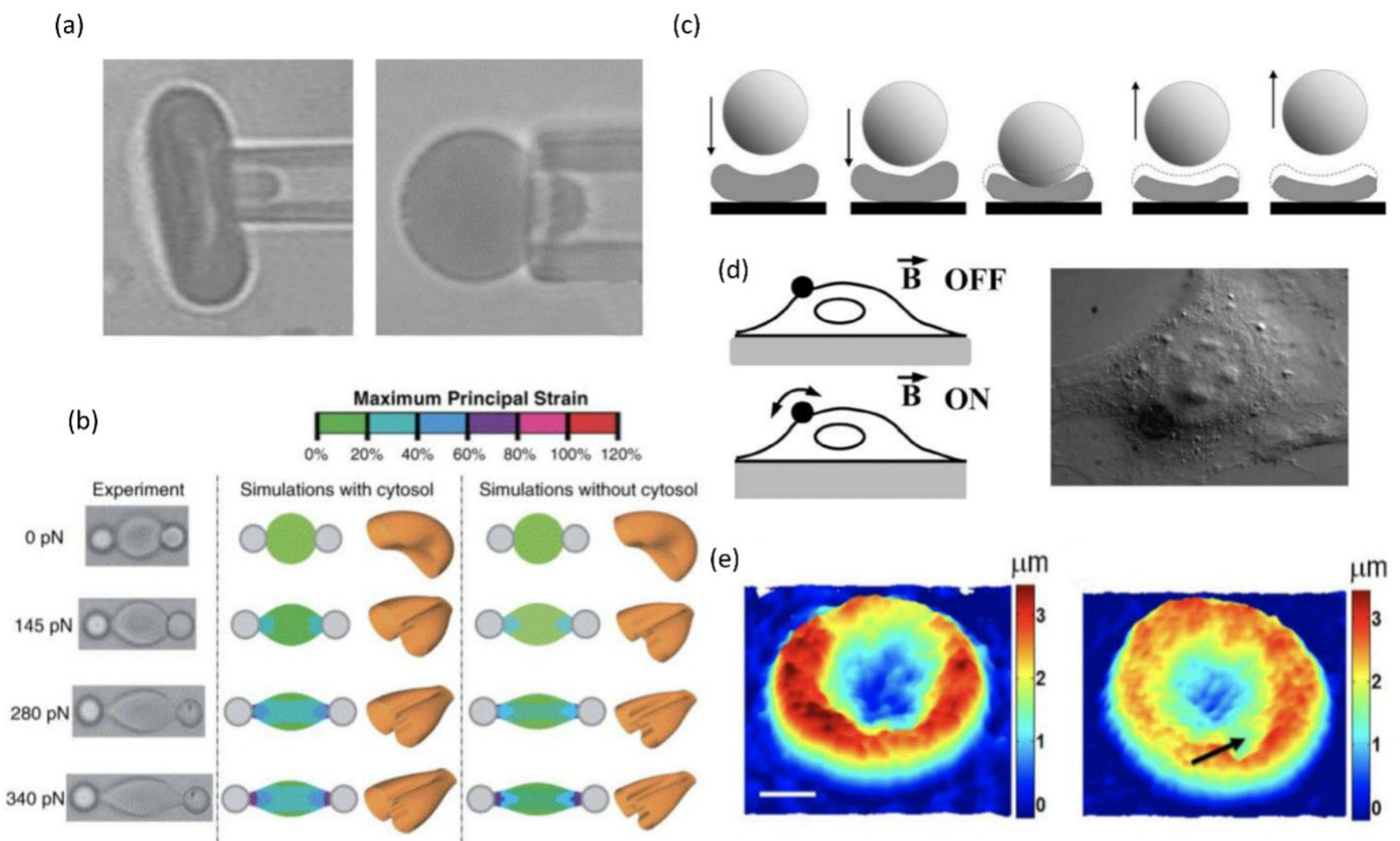
4.1.1. Micropipette Aspiration
4.1.2. Optical Tweezer
4.1.3. Atomic Force Microscopy
4.1.4. Magnetic Twisting Cytometry
4.1.5. Quantitative Phase Imaging
4.2. Bulk Measurement Techniques
4.2.1. Blood Viscometer and Ektacytometry
4.2.2. Filtration Test
4.2.3. Erythrocyte Sedimentation Rate
4.3. Microfluidic Methods
4.3.1. Hydrodynamic Stretching
4.3.2. Passing Through Constrictions
4.3.3. Motion and Shapes
4.3.4. Machine Learning
5. Discussion and Conclusions
Funding
Conflicts of Interest
Abbreviations
| RBC | Red blood cell |
| MCV | Mean Corpuscular Volume |
| MCHC | Mean Corpuscular Hemoglobin Concentration |
| ATP | Adenosine Triphosphate |
| HbS | Abnormal Sickle Hemoglobin |
| HbA | Normal Hemoglobin |
| ICU | Intensive Care Unit |
| AFM | AtomicForce Microscopy |
| CCD | Charge-Coupled Device |
| MTC | Magnetic Twisting Cytometry |
| QPI | Quantitative Phase Imaging |
| DLS | Dynamic Light Scattering |
| ESR | Erythrocyte Sedimentation Rate |
| AI | Artificial Intelligence |
| GDM | Gestational Diabetes Mellitus |
References
- Smith, M.M.; Renew, J.R.; Nelson, J.A.; Barbara, D.W. Red Blood Cell Disorders: Perioperative Considerations for Patients Undergoing Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2019, 33, 1393–1406. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, K.; Park, Y. Blood Cell—An Overview of Studies in Hematology; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Rand, R.P.; Burton, A.C. Mechanical Properties of the Red Cell Membrane I. Membrane Stiffness and Intracellular Pressure. Biophys. J. 1964, 4, 115–135. [Google Scholar] [CrossRef]
- Mohandas, N.; Clark, M.R.; Jacobs, M.S.; Shohet, S.B. Analysis of Factors Regulating Erythrocyte Deformability. J. Clin. Investig. 1980, 66, 563–573. [Google Scholar] [CrossRef]
- An, X.; Mohandas, N. Disorders of Red Cell Membrane. Br. J. Haematol. 2008, 141, 367–375. [Google Scholar] [CrossRef]
- Dobbe, J.G.G.; Hardeman, M.R.; Streekstra, G.J.; Strackee, J.; Ince, C.; Grimbergen, C.A. Analyzing Red Blood Cell-Deformability Distributions. Blood Cells Mol. Dis. 2002, 28, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Prudinnik, D.S.; Kussanova, A.; Vorobjev, I.A.; Tikhonov, A.; Ataullakhanov, F.I.; Barteneva, N.S. Deformability of Heterogeneous Red Blood Cells in Aging and Related Pathologies. Aging Dis. 2024, 16, 1242–1264. [Google Scholar] [CrossRef] [PubMed]
- Ebenuwa, I.; Violet, P.-C.; Tu, H.; Lee, C.; Munyan, N.; Wang, Y.; Niyyati, M.; Patra, K.; Wilkins, K.J.; Parrow, N.; et al. Altered RBC Deformability in Diabetes: Clinical Characteristics and RBC Pathophysiology. Cardiovasc. Diabetol. 2024, 23, 370. [Google Scholar] [CrossRef] [PubMed]
- Matthews, K.; Lamoureux, E.S.; Myrand-Lapierre, M.-E.; Duffy, S.P.; Ma, H. Technologies for Measuring Red Blood Cell Deformability. Lab. Chip 2022, 22, 1254–1274. [Google Scholar] [CrossRef]
- Hanzawa, K.; Kai, M.; Hiraga, A.; Watanabe, S. Fragility of Red Cells during Exercise Is Affected by Blood PH and Temperature. Equine Vet. J. 1999, 31, 610–611. [Google Scholar] [CrossRef]
- Kuzman, D.; Žnidarčič, T.; Gros, M.; Vrhovec, S.; Svetina, S.; Žekš, B. Effect of PH on Red Blood Cell Deformability. Pflügers Arch. Eur. J. Physiol. 2000, 440, R193–R194. [Google Scholar] [CrossRef]
- Matthews, K.; Myrand-Lapierre, M.-E.; Ang, R.R.; Duffy, S.P.; Scott, M.D.; Ma, H. Microfluidic Deformability Analysis of the Red Cell Storage Lesion. J. Biomech. 2015, 48, 4065–4072. [Google Scholar] [CrossRef]
- Stuart, M.J.; Nagel, R.L. Sickle-Cell Disease. Lancet 2004, 364, 1343–1360. [Google Scholar] [CrossRef]
- Wajer, S.D.; Taomoto, M.; McLeod, D.S.; McCally, R.L.; Nishiwaki, H.; Fabry, M.E.; Nagel, R.L.; Lutty, G.A. Velocity Measurements of Normal and Sickle Red Blood Cells in the Rat Retinal and Choroidal Vasculatures. Microvasc. Res. 2000, 60, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Ung, R.; Alapan, Y.; Hasan, M.N.; Romelfanger, M.; He, P.; Tam, A.; Rosanwo, T.; Akkus, A.; Cakar, M.A.; Icoz, K.; et al. Point-of-Care Screening for Sickle Cell Disease by a Mobile Micro-Electrophoresis Platform. Blood 2015, 126, 3379. [Google Scholar] [CrossRef]
- Mohandas, N.; An, X. Malaria and Human Red Blood Cells. Med. Microbiol. Immun. 2012, 201, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Tangpukdee, N.; Duangdee, C.; Wilairatana, P.; Krudsood, S. Malaria Diagnosis: A Brief Review. Korean J. Parasitol. 2009, 47, 93–102. [Google Scholar] [CrossRef]
- Cho, Y.I.; Mooney, M.P.; Cho, D.J. Hemorheological Disorders in Diabetes Mellitus. J. Diabetes Sci. Technol. 2008, 2, 1130–1138. [Google Scholar] [CrossRef]
- Bravo, N.; Torres, J.; González-Ortiz, M.; Staforelli-Vivanco, J.P. Flickering of Fetal Erythrocytes Membrane under Gestational Diabetes Observed with Dual Time Resolved Membrane Fluctuation Spec-troscopy. Biochem. Biophys. Rep. 2023, 36, 101556. [Google Scholar] [CrossRef]
- Jacobi, J. Pathophysiology of Sepsis. Am. J. Health-Syst. Pharmacy 2002, 59, S3–S8. [Google Scholar] [CrossRef]
- Hinshaw, L.B.P. Sepsis/Septic Shock. Crit. Care Med. 1996, 24, 1072–1078. [Google Scholar] [CrossRef]
- Yodice, P.C.; Astiz, M.E.; Kurian, B.M.; Lin, R.Y.; Rackow, E.C. Neutrophil Rheologic Changes in Septic Shock. Am. J. Resp. Crit. Care 1997, 155, 38–42. [Google Scholar] [CrossRef]
- Piper, R.D.; Pitt-Hyde, M.; Li, F.; Sibbald, W.J.; Potter, R.F. Microcirculatory Changes in Rat Skeletal Muscle in Sepsis. Am. J. Resp. Crit. Care 1996, 154, 931–937. [Google Scholar] [CrossRef]
- Vincent, J.-L. Update on Sepsis: Pathophysiology and Treatment. Acta Clin. Belg. 2016, 55, 79–87. [Google Scholar] [CrossRef]
- Begg, T.B.; Wade, I.M.; Bronte-Stewart, B. The Red Cell Electrophoretic Mobility in Atherosclerotic and Other Individuals. J. Atheroscler. Res. 1966, 6, 303–312. [Google Scholar] [CrossRef]
- Alapan, Y.; Matsuyama, Y.; Little, J.A.; Gurkan, U.A. Dynamic Deformability of Sickle Red Blood Cells in Microphysiological Flow. Technology 2016, 4, 71–79. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Liumbruno, G.; Grazzini, G.; Zolla, L. Red Blood Cell Storage: The Story so Far. Blood Transfus. Trasfus. Del. Sangue 2009, 8, 82–88. [Google Scholar] [CrossRef]
- Weed, R.I.; LaCelle, P.L.; Merrill, E.W. Metabolic Dependence of Red Cell Deformability. J. Clin. Investig. 1969, 48, 795–809. [Google Scholar] [CrossRef]
- Berezina, T.L.; Zaets, S.B.; Morgan, C.; Spillert, C.R.; Kamiyama, M.; Spolarics, Z.; Deitch, E.A.; Machiedo, G.W. Influence of Storage on Red Blood Cell Rheological Properties. J. Surg. Res. 2002, 102, 6–12. [Google Scholar] [CrossRef]
- Islamzada, E.; Matthews, K.; Lamoureux, E.S.; Duffy, S.P.; Scott, M.D.; Ma, H. Degradation of Red Blood Cell Deformability during Cold Storage in Blood Bags. Ejhaem 2022, 3, 63–71. [Google Scholar] [CrossRef]
- Cima, L.G.; Discher, D.E.; Tong, J.; Williams, M.C. A Hydrodynamic Interpretation of Crisis in Sickle Cell Anemia. Microvasc. Res. 1994, 47, 41–54. [Google Scholar] [CrossRef]
- Kucukal, E.; Man, Y.; Hill, A.; Liu, S.; Bode, A.; An, R.; Kadambi, J.; Little, J.A.; Gurkan, U.A. Whole Blood Viscosity and Red Blood Cell Adhesion: Potential Biomarkers for Targeted and Curative Therapies in Sickle Cell Disease. Am. J. Hematol. 2020, 95, 1246–1256. [Google Scholar] [CrossRef]
- Jiménez-Díaz, M.B.; Ebert, D.; Salinas, Y.; Pradhan, A.; Lehane, A.M.; Myrand-Lapierre, M.-E.; O’Loughlin, K.G.; Shackleford, D.M.; de Almeida, M.J.; Carrillo, A.K.; et al. (+)-SJ733, a Clinical Candidate for Malaria That Acts through ATP4 to Induce Rapid Host-Mediated Clearance of Plasmodium. Proc. Natl. Acad. Sci. USA 2014, 111, E5455–E5462. [Google Scholar] [CrossRef]
- Yedgar, S.; Koshkaryev, A.; Barshtein, G. The Red Blood Cell in Vascular Occlusion. Pathophysiol. Haemost. Thromb. 2002, 32, 263–268. [Google Scholar] [CrossRef]
- Ohiagu, F.O.; Chikezie, P.C.; Ahaneku, C.C.; Chikezie, C.M.; Law-Obi, F.C. Pathophysiology of Severe Malaria Infection. Asian J. Health Sci. 2021, 7, ID22. [Google Scholar] [CrossRef]
- Kim, C.C.; Wilson, E.B.; DeRisi, J.L. Improved Methods for Magnetic Purification of Malaria Parasites and Haemozoin. Malar. J. 2010, 9, 17. [Google Scholar] [CrossRef]
- Yadav, A.S.; Harris, N.R. Effect of Tempol on Diabetes-Induced Decreases in Retinal Blood Flow in the Mouse. Curr. Eye Res. 2011, 36, 456–461. [Google Scholar] [CrossRef]
- Brown, C.D.; Ghali, H.S.; Zhao, Z.; Thomas, L.L.; Friedman, E.A. Association of Reduced Red Blood Cell Deformability and Diabetic Nephropathy. Kidney Int. 2005, 67, 295–300. [Google Scholar] [CrossRef]
- Maeda, N.; Kon, K.; Imaizumi, K.; Sekiya, M.; Shiga, T. Alteration of Rheological Properties of Human Erythrocytes by Crosslinking of Membrane Proteins. Biochim. Et. Biophys. Acta BBA Biomembr. 1983, 735, 104–112. [Google Scholar] [CrossRef]
- Waitzman, M.B.; Colley, A.M.; Nardelli-Olkowska, K. Metabolie Approaches to Studies on Diabetic Microangiopathy. Diabetes 1977, 26, 510–519. [Google Scholar] [CrossRef]
- Moutzouri, A.G.; Athanassiou, G.A.; Dimitropoulou, D.; Skoutelis, A.T.; Gogos, C.A. Severe Sepsis and Diabetes Mellitus Have Additive Effects on Red Blood Cell Deformability. J. Infect. 2008, 57, 147–151. [Google Scholar] [CrossRef]
- Reggiori, G.; Occhipinti, G.; Gasperi, A.D.; Vincent, J.-L.; Piagnerelli, M. Early Alterations of Red Blood Cell Rheology in Critically Ill Patients. Crit. Care Med. 2009, 37, 3041–3046. [Google Scholar] [CrossRef]
- Nemeth, N.; Furka, I.; Miko, I. Hemorheological Changes in Ischemia-Reperfusion: An Overview on Our Experimental Surgical Data. Clin. Hemorheol. Micro 2014, 57, 215–225. [Google Scholar] [CrossRef]
- Braun, R.D.; Wienczewski, C.A.; Abbas, A. Erythrocyte Flow in Choriocapillaris of Normal and Diabetic Rats. Microvasc. Res. 2009, 77, 247–255. [Google Scholar] [CrossRef][Green Version]
- Arend, O.; Remky, A.; Jung, F.; Kiesewetter, H.; Reim, M.; Wolf, S. Role of Rheologic Factors in Patients with Acute Central Retinal Vein Occlusion. Ophthalmology 1996, 103, 80–86. [Google Scholar] [CrossRef]
- Bharadwaj, A.S.; Appukuttan, B.; Wilmarth, P.A.; Pan, Y.; Stempel, A.J.; Chipps, T.J.; Benedetti, E.E.; Zamora, D.O.; Choi, D.; David, L.L.; et al. Role of the Retinal Vascular Endothelial Cell in Ocular Disease. Prog. Retin. Eye Res. 2013, 32, 102–180. [Google Scholar] [CrossRef]
- Agrawal, R.; Sherwood, J.; Chhablani, J.; Ricchariya, A.; Kim, S.; Jones, P.H.; Balabani, S.; Shima, D. Red Blood Cells in Retinal Vascular Disorders. Blood Cells Mol. Dis. 2016, 56, 53–61. [Google Scholar] [CrossRef][Green Version]
- Noda, K.; Nakao, S.; Ishida, S.; Ishibashi, T. Leukocyte Adhesion Molecules in Diabetic Retinopathy. J. Ophthalmol. 2012, 2012, 279037. [Google Scholar] [CrossRef]
- Davis, J.L.; Haft, P.; Hartley, K. Retinal Arteriolar Occlusions Due to Cytomegalovirus Retinitis in Elderly Patients without HIV. J. Ophthalmic Inflamm. Infect. 2013, 3, 17. [Google Scholar] [CrossRef]
- Fonseca, G.H.H.; Souza, R.; Salemi, V.M.C.; Jardim, C.V.P.; Gualandro, S.F.M. Pulmonary Hypertension Diagnosed by Right Heart Catheterisation in Sickle Cell Disease. Eur. Respir. J. 2011, 39, 112–118. [Google Scholar] [CrossRef]
- Gladwin, M.T.; Sachdev, V.; Jison, M.L.; Shizukuda, Y.; Plehn, J.F.; Minter, K.; Brown, B.; Coles, W.A.; Nichols, J.S.; Ernst, I.; et al. Pulmonary Hypertension as a Risk Factor for Death in Patients with Sickle Cell Disease. N. Engl. J. Med. 2004, 350, 886–895. [Google Scholar] [CrossRef]
- Machado, R.F.; Anthi, A.; Steinberg, M.H.; Bonds, D.; Sachdev, V.; Kato, G.J.; Taveira-DaSilva, A.M.; Ballas, S.K.; Blackwelder, W.; Xu, X.; et al. N-Terminal Pro-Brain Natriuretic Peptide Levels and Risk of Death in Sickle Cell Disease. JAMA 2006, 296, 310–318. [Google Scholar] [CrossRef]
- Mehari, A.; Alam, S.; Tian, X.; Cuttica, M.J.; Barnett, C.F.; Miles, G.; Xu, D.; Seamon, C.; Adams-Graves, P.; Castro, O.L.; et al. Hemodynamic Predictors of Mortality in Adults with Sickle Cell Disease. Am. J. Respir. Crit. Care Med. 2013, 187, 840–847. [Google Scholar] [CrossRef]
- Sachdev, V.; Kato, G.J.; Gibbs, J.S.R.; Barst, R.J.; Machado, R.F.; Nouraie, M.; Hassell, K.L.; Little, J.A.; Schraufnagel, D.E.; Krishnamurti, L.; et al. Echocardiographic Markers of Elevated Pulmonary Pressure and Left Ventricular Diastolic Dysfunction Are Associated With Exercise Intolerance in Adults and Adolescents With Homozygous Sickle Cell Anemia in the United States and United Kingdom. Circulation 2011, 124, 1452–1460. [Google Scholar] [CrossRef]
- Evans, E.A.; Waugh, R.; Melnik, L. Elastic Area Compressibility Modulus of Red Cell Membrane. Biophys. J. 1976, 16, 585–595. [Google Scholar] [CrossRef]
- Hochmuth, R.M.; Waugh, R.E. Erythrocyte Membrane Elasticity and Viscosity. Annu. Rev. Physiol. 1987, 49, 209–219. [Google Scholar] [CrossRef]
- Evans, E.A. Bending Elastic Modulus of Red Blood Cell Membrane Derived from Buckling Instability in Micropipet Aspiration Tests. Biophys. J. 1983, 43, 27–30. [Google Scholar] [CrossRef]
- Yoon, Y.-Z.; Hong, H.; Brown, A.; Kim, D.C.; Kang, D.J.; Lew, V.L.; Cicuta, P. Flickering Analysis of Erythrocyte Mechanical Properties: Dependence on Oxygenation Level, Cell Shape, and Hydration Level. Biophys. J. 2009, 97, 1606–1615. [Google Scholar] [CrossRef]
- Tomaiuolo, G.; Guido, S. Start-up Shape Dynamics of Red Blood Cells in Microcapillary Flow. Microvasc. Res. 2011, 82, 35–41. [Google Scholar] [CrossRef]
- Baskurt, O.K.; Hardeman, M.R.; Uyuklu, M.; Ulker, P.; Cengiz, M.; Nemeth, N.; Shin, S.; Alexy, T.; Meiselman, H.J. Parameterization of Red Blood Cell Elongation Index—Shear Stress Curves Obtained by Ektacytometry. Scand. J. Clin. Lab. Investig. 2009, 69, 777–788. [Google Scholar] [CrossRef]
- Tomaiuolo, G. Biomechanical Properties of Red Blood Cells in Health and Disease towards Microfluidics. Biomicrofluidics 2014, 8, 051501. [Google Scholar] [CrossRef]
- Waugh, R.E.; Narla, M.; Jackson, C.W.; Mueller, T.J.; Suzuki, T.; Dale, G.L. Rheologic Properties of Senescent Erythrocytes: Loss of Surface Area and Volume with Red Blood Cell Age. Blood 1992, 79, 1351–1358. [Google Scholar] [CrossRef]
- Linderkamp, O.; Wu, P.Y.K.; Meiselman, H.J. Geometry of Neonatal and Adult Red Blood Cells. Pediatr. Res. 1983, 17, 250–253. [Google Scholar] [CrossRef]
- Linderkamp, O.; Friederichs, E.; Meiselman, H.J. Mechanical and Geometrical Properties of Density-Separated Neonatal and Adult Erythrocytes. Pediatr. Res. 1993, 34, 688–693. [Google Scholar] [CrossRef][Green Version]
- Linderkamp, O.; Meiselman, H.J. Geometric, Osmotic, and Membrane Mechanical Properties of Density-Separated Human Red Cells. Blood 1982, 59, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Tomaiuolo, G.; Rossi, D.; Caserta, S.; Cesarelli, M.; Guido, S. Comparison of Two Flow-based Imaging Methods to Measure Individual Red Blood Cell Area and Volume. Cytom. Part A 2012, 81A, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Nash, G.B.; Meiselman, H.J. Red Cell and Ghost Viscoelasticity. Effects of Hemoglobin Concentration and in Vivo Aging. Biophys. J. 1983, 43, 63–73. [Google Scholar] [CrossRef]
- Sirs, J.A. Erythrocyte Flexibility and Whole-Blood Viscosity. In Clinical Aspects of Blood Viscosity and Cell Deformability; Springer: London, UK, 1981; pp. 9–18. [Google Scholar] [CrossRef]
- Secomb, T.W.; Hsu, R. Analysis of Red Blood Cell Motion through Cylindrical Micropores: Effects of Cell Properties. Biophys. J. 1996, 71, 1095–1101. [Google Scholar] [CrossRef][Green Version]
- Evans, E.A.; Hochmuth, R.M. Membrane Viscoelasticity. Biophys. J. 1976, 16, 1–11. [Google Scholar] [CrossRef]
- Meiselman, H.J.; Evans, E.A.; Hochmuth, R.M. Membrane Mechanical Properties of ATP-Depleted Human Erythrocytes. Blood 1978, 52, 499–504. [Google Scholar] [CrossRef][Green Version]
- Hochmuth, R.M.; Buxbaum, K.L.; Evans, E.A. Temperature Dependence of the Viscoelastic Recovery of Red Cell Membrane. Biophys. J. 1980, 29, 177–182. [Google Scholar] [CrossRef][Green Version]
- Hochmuth, R.M.; Worthy, P.R.; Evans, E.A. Red Cell Extensional Recovery and the Determination of Membrane Viscosity. Biophys. J. 1979, 26, 101–114. [Google Scholar] [CrossRef]
- Tomaiuolo, G.; Barra, M.; Preziosi, V.; Cassinese, A.; Rotoli, B.; Guido, S. Microfluidics Analysis of Red Blood Cell Membrane Viscoelasticity. Lab. Chip 2010, 11, 449–454. [Google Scholar] [CrossRef]
- Bazzoni, G.; Rasia, M. Effects of an Amphipathic Drug on the Rheological Properties of the Cell Membrane. Blood Cells Mol. Dis. 1998, 24, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.A. New Membrane Concept Applied to the Analysis of Fluid Shear- and Micropipette-Deformed Red Blood Cells. Biophys. J. 1973, 13, 941–954. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.-M.; Discher, D.E. Deformation-Enhanced Fluctuations in the Red Cell Skeleton with Theoretical Relations to Elasticity, Connectivity, and Spectrin Unfolding. Biophys. J. 2001, 81, 3178–3192. [Google Scholar] [CrossRef] [PubMed]
- Pinho, D.; Campo-Deaño, L.; Lima, R.; Pinho, F.T. In Vitro Particulate Analogue Fluids for Experimental Studies of Rheological and Hemorheological Behavior of Glucose-Rich RBC Suspensions. Biomicrofluidics 2017, 11, 054105. [Google Scholar] [CrossRef]
- Lenormand, G.; Hénon, S.; Richert, A.; Siméon, J.; Gallet, F. Direct Measurement of the Area Expansion and Shear Moduli of the Human Red Blood Cell Membrane Skeleton. Biophys. J. 2001, 81, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Hénon, S.; Lenormand, G.; Richert, A.; Gallet, F. A New Determination of the Shear Modulus of the Human Erythrocyte Membrane Using Optical Tweezers. Biophys. J. 1999, 76, 1145–1151. [Google Scholar] [CrossRef]
- Hwang, W.C.; Waugh, R.E. Energy of Dissociation of Lipid Bilayer from the Membrane Skeleton of Red Blood Cells. Biophys. J. 1997, 72, 2669–2678. [Google Scholar] [CrossRef]
- Scheffer, L.; Bitler, A.; Ben-Jacob, E.; Korenstein, R. Atomic Force Pulling: Probing the Local Elasticity of the Cell Membrane. Eur. Biophys. J. 2001, 30, 83–90. [Google Scholar] [CrossRef]
- Betz, T.; Lenz, M.; Joanny, J.-F.; Sykes, C. ATP-Dependent Mechanics of Red Blood Cells. Proc. Natl. Acad. Sci. USA 2009, 106, 15320–15325. [Google Scholar] [CrossRef]
- Williamson, J.R.; Gardner, R.A.; Boylan, C.W.; Carroll, G.L.; Chang, K.; Marvel, J.S.; Gonen, B.; Kilo, C.; Tran-Son-Tay, R.; Sutera, S.P. Microrheologic Investigation of Erythrocyte Deformability in Diabetes Mellitus. Blood 1985, 65, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Bremmell, K.E.; Evans, A.; Prestidge, C.A. Deformation and Nano-Rheology of Red Blood Cells: An AFM Investigation. Colloids Surf. B Biointerfaces 2006, 50, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Waugh, R.; Evans, E.A. Thermoelasticity of Red Blood Cell Membrane. Biophys. J. 1979, 26, 115–131. [Google Scholar] [CrossRef] [PubMed]
- Dulińska, I.; Targosz, M.; Strojny, W.; Lekka, M.; Czuba, P.; Balwierz, W.; Szymoński, M. Stiffness of Normal and Pathological Erythrocytes Studied by Means of Atomic Force Microscopy. J. Biochem. Biophys. Methods 2006, 66, 1–11. [Google Scholar] [CrossRef]
- Rab, M.A.E.; van Oirschot, B.A.; Bos, J.; Kanne, C.K.; Sheehan, V.A.; van Beers, E.J.; van Wijk, R. Characterization of Sickling During Controlled Automated Deoxygenation with Oxygen Gradient Ektacytometry. J. Vis. Exp. 2019, e60213. [Google Scholar] [CrossRef]
- Costa, L.D.; Suner, L.; Galimand, J.; Bonnel, A.; Pascreau, T.; Couque, N.; Fenneteau, O.; Mohandas, N.; On behalf of the Group of Société d’Hématologie et d’Immunologie Pédiatrique (SHIP); The Société Française d’Hématologie (SFH). Diagnostic Tool for Red Blood Cell Membrane Disorders: Assessment of a New Generation Ektacytometer. Blood Cells Mol. Dis. 2016, 56, 9–22. [Google Scholar] [CrossRef]
- Saadat, A.; Huyke, D.A.; Oyarzun, D.I.; Escobar, P.V.; Øvreeide, I.H.; Shaqfeh, E.S.G.; Santiago, J.G. A System for the High-Throughput Measurement of the Shear Modulus Distribution of Human Red Blood Cells. Lab. Chip 2020, 20, 2927–2936. [Google Scholar] [CrossRef]
- Wu, P.-H.; Aroush, D.R.-B.; Asnacios, A.; Chen, W.-C.; Dokukin, M.E.; Doss, B.L.; Durand-Smet, P.; Ekpenyong, A.; Guck, J.; Guz, N.V.; et al. A Comparison of Methods to Assess Cell Mechanical Properties. Nat. Methods 2018, 15, 491–498. [Google Scholar] [CrossRef]
- Evans, E.; Celle, P.L. Intrinsic Material Properties of the Erythrocyte Membrane Indicated by Mechanical Analysis of Deformation. Blood 1975, 45, 29–43. [Google Scholar] [CrossRef]
- Hochmuth, R.M. Micropipette Aspiration of Living Cells. J. Biomech. 2000, 33, 15–22. [Google Scholar] [CrossRef]
- Chien, S.; Sung, K.L.; Skalak, R.; Usami, S.; Tözeren, A. Theoretical and Experimental Studies on Viscoelastic Properties of Erythrocyte Membrane. Biophys. J. 1978, 24, 463–487. [Google Scholar] [CrossRef] [PubMed]
- Dao, M.; Lim, C.T.; Suresh, S. Mechanics of the Human Red Blood Cell Deformed by Optical Tweezers. J. Mech. Phys. Solids 2003, 51, 2259–2280. [Google Scholar] [CrossRef]
- Massiera, G.; Citters, K.M.V.; Biancaniello, P.L.; Crocker, J.C. Mechanics of Single Cells: Rheology, Time Dependence, and Fluctuations. Biophys. J. 2007, 93, 3703–3713. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Diez-Silva, M.; Popescu, G.; Lykotrafitis, G.; Choi, W.; Feld, M.S.; Suresh, S. Refractive Index Maps and Membrane Dynamics of Human Red Blood Cells Parasitized by Plasmodium Falciparum. Proc. Natl. Acad. Sci. USA 2008, 105, 13730–13735. [Google Scholar] [CrossRef]
- Guck, J.; Ananthakrishnan, R.; Mahmood, H.; Moon, T.J.; Cunningham, C.C.; Käs, J. The Optical Stretcher: A Novel Laser Tool to Micromanipulate Cells. Biophys. J. 2001, 81, 767–784. [Google Scholar] [CrossRef]
- Guck, J.; Schinkinger, S.; Lincoln, B.; Wottawah, F.; Ebert, S.; Romeyke, M.; Lenz, D.; Erickson, H.M.; Ananthakrishnan, R.; Mitchell, D.; et al. Optical Deformability as an Inherent Cell Marker for Testing Malignant Transformation and Metastatic Competence. Biophys. J. 2005, 88, 3689–3698. [Google Scholar] [CrossRef]
- Stigter, D.; Mingins, J.; Dill, K.A. Phospholipid Interactions in Model Membrane Systems. II. Theory. Biophys. J. 1992, 61, 1616–1629. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.; Wang, S.; Wang, C.; Xie, J.; Chen, X.; Xu, Y.; Mao, P. Detection of Human Erythrocytes Influenced by Iron Deficiency Anemia and Thalassemia Using Atomic Force Microscopy. Micron 2012, 43, 1287–1292. [Google Scholar] [CrossRef]
- Wang, K.; Li, Z.; Egini, O.; Wadgaonkar, R.; Jiang, X.-C.; Chen, Y. Atomic Force Microscopy Reveals Involvement of the Cell Envelope in Biomechanical Properties of Sickle Erythrocytes. BMC Biol. 2023, 21, 31. [Google Scholar] [CrossRef]
- Buys, A.V.; Rooy, M.-J.V.; Soma, P.; Papendorp, D.V.; Lipinski, B.; Pretorius, E. Changes in Red Blood Cell Membrane Structure in Type 2 Diabetes: A Scanning Electron and Atomic Force Microscopy Study. Cardiovasc. Diabetol. 2013, 12, 25. [Google Scholar] [CrossRef]
- LI, Y.; Lu, L.; LI, J. Topological Structures and Membrane Nanostructures of Erythrocytes after Splenectomy in Hereditary Spherocytosis Patients via Atomic Force Microscopy. Cell Biochem. Biophys. 2016, 74, 365–371. [Google Scholar] [CrossRef]
- Butt, H.-J.; Cappella, B.; Kappl, M. Force Measurements with the Atomic Force Microscope: Technique, Interpretation and Applications. Surf. Sci. Rep. 2005, 59, 1–152. [Google Scholar] [CrossRef]
- Wang, N.; Butler, J.P.; Ingber, D.E. Mechanotransduction Across the Cell Surface and Through the Cytoskeleton. Science 1993, 260, 1124–1127. [Google Scholar] [CrossRef] [PubMed]
- Puig-De-Morales, M.; Grabulosa, M.; Alcaraz, J.; Mullol, J.; Maksym, G.N.; Fredberg, J.J.; Navajas, D. Measurement of Cell Microrheology by Magnetic Twisting Cytometry with Frequency Domain Demodulation. J. Appl. Physiol. 2001, 91, 1152–1159. [Google Scholar] [CrossRef] [PubMed]
- Debnath, S.K.; Park, Y. Real-Time Quantitative Phase Imaging with a Spatial Phase-Shifting Algorithm. Opt. Lett. 2011, 36, 4677–4679. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zavadil, J.; Martin, L.; Parisi, F.; Friedman, E.; Levy, D.; Harding, H.; Ron, D.; Gardner, L.B. Inhibition of Nonsense-Mediated RNA Decay by the Tumor Microenvironment Promotes Tumorigenesis. Mol. Cell Biol. 2011, 31, 3670–3680. [Google Scholar] [CrossRef]
- Popescu, G.; Park, Y.; Lue, N.; Best-Popescu, C.; Deflores, L.; Dasari, R.R.; Feld, M.S.; Badizadegan, K. Optical Imaging of Cell Mass and Growth Dynamics. Am. J. Physiol. Physiol. 2008, 295, C538–C544. [Google Scholar] [CrossRef]
- Park, Y.; Depeursinge, C.; Popescu, G. Quantitative Phase Imaging in Biomedicine. Nat. Photonics 2018, 12, 578–589. [Google Scholar] [CrossRef]
- BARER, R. Determination of Dry Mass, Thickness, Solid and Water Concentration in Living Cells. Nature 1953, 172, 1097–1098. [Google Scholar] [CrossRef]
- Jung, J.; Matemba, L.E.; Lee, K.; Kazyoba, P.E.; Yoon, J.; Massaga, J.J.; Kim, K.; Kim, D.-J.; Park, Y. Optical Characterization of Red Blood Cells from Individuals with Sickle Cell Trait and Disease in Tanzania Using Quantitative Phase Imaging. Sci. Rep. 2016, 6, 31698. [Google Scholar] [CrossRef]
- Scheven, C. Ektacytometry: A Method for Characterizing Erythrocyte Deformability. Folia Haematol. (Leipz. Ger. 1928) 1989, 116, 653–669. [Google Scholar]
- Niss, O.; Chonat, S.; Dagaonkar, N.; Almansoori, M.O.; Kerr, K.; Rogers, Z.R.; McGann, P.T.; Quarmyne, M.-O.; Risinger, M.; Zhang, K.; et al. Genotype-Phenotype Correlations in Hereditary Elliptocytosis and Hereditary Pyropoikilocytosis. Blood Cells Mol. Dis. 2016, 61, 4–9. [Google Scholar] [CrossRef]
- Llaudet-Planas, E.; Vives-Corrons, J.L.; Rizzuto, V.; Gómez-Ramírez, P.; Navarro, J.S.; Sibina, M.T.C.; García-Bernal, M.; Llobet, A.R.; Badell, I.; Velasco-Puyó, P.; et al. Osmotic Gradient Ektacytometry: A Valuable Screening Test for Hereditary Spherocytosis and Other Red Blood Cell Membrane Disorders. Int. J. Lab. Hematol. 2018, 40, 94–102. [Google Scholar] [CrossRef]
- Allard, C.; Mohandas, N.; Bessis, M. Red Cell Deformability Changes in Hemolytic Anemias Estimated by Diffractometric Methods (Ektacytometry) Preliminary Results. In Red Cell Rheology; Springer: Berlin/Heidelberg, Germany, 1978; pp. 209–221. [Google Scholar] [CrossRef]
- Guo, Q.; Park, S.; Ma, H. Microfluidic Micropipette Aspiration for Measuring the Deformability of Single Cells. Lab. Chip 2012, 12, 2687–2695. [Google Scholar] [CrossRef] [PubMed]
- Darras, A.; Peikert, K.; Rabe, A.; Yaya, F.; Simionato, G.; John, T.; Dasanna, A.K.; Buvalyy, S.; Geisel, J.; Hermann, A.; et al. Acanthocyte Sedimentation Rate as a Diagnostic Biomarker for Neuroacanthocytosis Syndromes: Experimental Evidence and Physical Justification. Cells 2021, 10, 788. [Google Scholar] [CrossRef] [PubMed]
- Barber, E.M.; Muenger, J.R.; Villforth, F.J. High Rate of Shear Rotational Viscometer. Anal. Chem. 1955, 27, 425–429. [Google Scholar] [CrossRef]
- Parrow, N.L.; Violet, P.-C.; Tu, H.; Nichols, J.; Pittman, C.A.; Fitzhugh, C.; Fleming, R.E.; Mohandas, N.; Tisdale, J.F.; Levine, M. Measuring Deformability and Red Cell Heterogeneity in Blood by Ektacytometry. J. Vis. Exp. 2018, e56910. [Google Scholar] [CrossRef]
- Koutsouris, D.; Guillet, R.; Lelièvre, J.C.; Guillemin, M.T.; Bertholom, P.; Beuzard, Y.; Boynard, M. Determination of Erythrocyte Transit Times through Micropores. I-Basic Operational Principles. Biorheology 1988, 25, 763–772. [Google Scholar] [CrossRef]
- Picot, J.; Ndour, P.A.; Lefevre, S.D.; Nemer, W.E.; Tawfik, H.; Galimand, J.; Costa, L.D.; Ribeil, J.; de Montalembert, M.; Brousse, V.; et al. A Biomimetic Microfluidic Chip to Study the Circulation and Mechanical Retention of Red Blood Cells in the Spleen. Am. J. Hematol. 2015, 90, 339–345. [Google Scholar] [CrossRef]
- Kenny, M.W.; Meakin, M.; Worthington, D.J.; Stuart, J. Erythrocyte Deformability in Sickle-Cell Crisis. Br. J. Haematol. 1981, 49, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Tillmann, W.; Schröter, W. Rheological Properties of Erythrocytes in Heterozygous and Homozygous β Thalassaemia. Br. J. Haematol. 1979, 43, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Al-Marri, M.R.; Kirkpatrick, M.B. Erythrocyte Sedimentation Rate in Childhood Tuberculosis: Is It Still Worthwhile? Int. J. Tuberc. Lung Dis. Off. J. Int. Union Against Tuberc. Lung Dis. 2000, 4, 237–239. [Google Scholar]
- Grzybowski, A.; Sak, J. Edmund Biernacki (1866–1911): Discoverer of the Erythrocyte Sedimentation Rate. On the 100th Anniversary of His Death. Clin. Dermatol. 2011, 29, 697–703. [Google Scholar] [CrossRef]
- Rabe, A.; Kihm, A.; Darras, A.; Peikert, K.; Simionato, G.; Dasanna, A.K.; Glaß, H.; Geisel, J.; Quint, S.; Danek, A.; et al. The Erythrocyte Sedimentation Rate and Its Relation to Cell Shape and Rigidity of Red Blood Cells from Chorea-Acanthocytosis Patients in an Off-Label Treatment with Dasatinib. Biomolecules 2021, 11, 727. [Google Scholar] [CrossRef]
- Gossett, D.R.; Tse, H.T.K.; Lee, S.A.; Ying, Y.; Lindgren, A.G.; Yang, O.O.; Rao, J.; Clark, A.T.; Carlo, D.D. Hydrodynamic Stretching of Single Cells for Large Population Mechanical Phenotyping. Proc. Natl. Acad. Sci. USA 2012, 109, 7630–7635. [Google Scholar] [CrossRef]
- Liang, M.; Yang, D.; Zhou, Y.; Li, P.; Zhong, J.; Ai, Y. Single-Cell Stretching in Viscoelastic Fluids with Electronically Triggered Imaging for Cellular Mechanical Phenotyping. Anal. Chem. 2021, 93, 4567–4575. [Google Scholar] [CrossRef]
- Henon, Y.; Sheard, G.J.; Fouras, A. Erythrocyte Deformation in a Microfluidic Cross-Slot Channel. RSC Adv. 2014, 4, 36079–36088. [Google Scholar] [CrossRef]
- Otto, O.; Rosendahl, P.; Mietke, A.; Golfier, S.; Herold, C.; Klaue, D.; Girardo, S.; Pagliara, S.; Ekpenyong, A.; Jacobi, A.; et al. Real-Time Deformability Cytometry: On-the-Fly Cell Mechanical Phenotyping. Nat. Methods 2015, 12, 199–202. [Google Scholar] [CrossRef]
- Myrand-Lapierre, M.-E.; Deng, X.; Ang, R.R.; Matthews, K.; Santoso, A.T.; Ma, H. Multiplexed Fluidic Plunger Mechanism for the Measurement of Red Blood Cell Deformability. Lab. Chip 2014, 15, 159–167. [Google Scholar] [CrossRef]
- Xu, T.; Lizarralde-Iragorri, M.A.; Roman, J.; Ghasemi, R.; Lefèvre, J.-P.; Martincic, E.; Brousse, V.; Français, O.; Nemer, W.E.; Pioufle, B.L. Characterization of Red Blood Cell Microcirculatory Parameters Using a Bioimpedance Microfluidic Device. Sci. Rep. 2020, 10, 9869. [Google Scholar] [CrossRef] [PubMed]
- Zeng, N.F.; Ristenpart, W.D. Mechanical Response of Red Blood Cells Entering a Constriction. Biomicrofluidics 2014, 8, 064123. [Google Scholar] [CrossRef] [PubMed]
- Reichel, F.; Mauer, J.; Nawaz, A.A.; Gompper, G.; Guck, J.; Fedosov, D.A. High-Throughput Microfluidic Characterization of Erythrocyte Shapes and Mechanical Variability. Biophys. J. 2019, 117, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Doan, M.; Sebastian, J.A.; Caicedo, J.C.; Siegert, S.; Roch, A.; Turner, T.R.; Mykhailova, O.; Pinto, R.N.; McQuin, C.; Goodman, A.; et al. Objective Assessment of Stored Blood Quality by Deep Learning. Proc. Natl. Acad. Sci. USA 2020, 117, 21381–21390. [Google Scholar] [CrossRef]
- Bow, H.; Pivkin, I.V.; Diez-Silva, M.; Goldfless, S.J.; Dao, M.; Niles, J.C.; Suresh, S.; Han, J. A Microfabricated Deformability-Based Flow Cytometer with Application to Malaria. Lab. Chip 2011, 11, 1065–1073. [Google Scholar] [CrossRef]
- Tsai, C.-H.D.; Sakuma, S.; Arai, F.; Taniguchi, T.; Ohtani, T.; Sakata, Y.; Kaneko, M. Geometrical Alignment for Improving Cell Evaluation in a Microchannel with Application on Multiple Myeloma Red Blood Cells. RSC Adv. 2014, 4, 45050–45058. [Google Scholar] [CrossRef]
- Abkarian, M.; Faivre, M.; Stone, H.A. High-Speed Microfluidic Differential Manometer for Cellular-Scale Hydrodynamics. Proc. Natl. Acad. Sci. USA 2006, 103, 538–542. [Google Scholar] [CrossRef]
- Rosenbluth, M.J.; Lam, W.A.; Fletcher, D.A. Analyzing Cell Mechanics in Hematologic Diseases with Microfluidic Biophysical Flow Cytometry. Lab. Chip 2008, 8, 1062–1070. [Google Scholar] [CrossRef]
- Man, Y.; Maji, D.; An, R.; Ahuja, S.P.; Little, J.A.; Suster, M.A.; Mohseni, P.; Gurkan, U.A. Microfluidic Electrical Impedance Assessment of Red Blood Cell-Mediated Microvascular Occlusion. Lab. Chip 2021, 21, 1036–1048. [Google Scholar] [CrossRef]
- Byun, S.; Son, S.; Amodei, D.; Cermak, N.; Shaw, J.; Kang, J.H.; Hecht, V.C.; Winslow, M.M.; Jacks, T.; Mallick, P.; et al. Characterizing Deformability and Surface Friction of Cancer Cells. Proc. Natl. Acad. Sci. USA 2013, 110, 7580–7585. [Google Scholar] [CrossRef]
- Liang, M.; Zhong, J.; Ai, Y. A Systematic Study of Size Correlation and Young’s Modulus Sensitivity for Cellular Mechanical Phenotyping by Microfluidic Approaches. Adv. Healthc. Mater. 2022, 11, 2200628. [Google Scholar] [CrossRef]
- Lange, J.R.; Steinwachs, J.; Kolb, T.; Lautscham, L.A.; Harder, I.; Whyte, G.; Fabry, B. Microconstriction Arrays for High-Throughput Quantitative Measurements of Cell Mechanical Properties. Biophys. J. 2015, 109, 26–34. [Google Scholar] [CrossRef]
- Tahiri, N.; Biben, T.; Ez-Zahraouy, H.; Benyoussef, A.; Misbah, C. On the Problem of Slipper Shapes of Red Blood Cells in the Microvasculature. Microvasc. Res. 2013, 85, 40–45. [Google Scholar] [CrossRef]
- McWhirter, J.L.; Noguchi, H.; Gompper, G. Flow-Induced Clustering and Alignment of Vesicles and Red Blood Cells in Microcapillaries. Proc. Natl. Acad. Sci. USA 2009, 106, 6039–6043. [Google Scholar] [CrossRef] [PubMed]
- Tomaiuolo, G.; Simeone, M.; Martinelli, V.; Rotoli, B.; Guido, S. Red Blood Cell Deformation in Microconfined Flow. Soft Matter 2009, 5, 3736–3740. [Google Scholar] [CrossRef]
- Xiong, W.; Zhang, J. Shear Stress Variation Induced by Red Blood Cell Motion in Microvessel. Ann. Biomed. Eng. 2010, 38, 2649–2659. [Google Scholar] [CrossRef] [PubMed]
- Recktenwald, S.M.; Graessel, K.; Maurer, F.M.; John, T.; Gekle, S.; Wagner, C. Red Blood Cell Shape Transitions and Dynamics in Time-Dependent Capillary Flows. Biophys. J. 2022, 121, 23–36. [Google Scholar] [CrossRef]
- Agarwal, D.; Biros, G. Stable Shapes of Three-Dimensional Vesicles in Unconfined and Confined Poiseuille Flow. Phys. Rev. Fluids 2020, 5, 013603. [Google Scholar] [CrossRef]
- Trejo-Soto, C.; Lázaro, G.R.; Pagonabarraga, I.; Hernández-Machado, A. Microfluidics Approach to the Mechanical Properties of Red Blood Cell Membrane and Their Effect on Blood Rheology. Membranes 2022, 12, 217. [Google Scholar] [CrossRef]
- Noguchi, H.; Gompper, G. Shape Transitions of Fluid Vesicles and Red Blood Cells in Capillary Flows. Proc. Natl. Acad. Sci. USA 2005, 102, 14159–14164. [Google Scholar] [CrossRef]
- Lanotte, L.; Mauer, J.; Mendez, S.; Fedosov, D.A.; Fromental, J.-M.; Claveria, V.; Nicoud, F.; Gompper, G.; Abkarian, M. Red Cells’ Dynamic Morphologies Govern Blood Shear Thinning under Microcirculatory Flow Conditions. Proc. Natl. Acad. Sci. USA 2016, 113, 13289–13294. [Google Scholar] [CrossRef]
- Fedosov, D.A.; Peltomäki, M.; Gompper, G. Deformation and Dynamics of Red Blood Cells in Flow through Cylindrical Microchannels. Soft Matter 2014, 10, 4258–4267. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, A.M.; Wan, J.; Owrutsky, P.D.; Abkarian, M.; Stone, H.A. Multiscale Approach to Link Red Blood Cell Dynamics, Shear Viscosity, and ATP Release. Proc. Natl. Acad. Sci. USA 2011, 108, 10986–10991. [Google Scholar] [CrossRef] [PubMed]
- Kaoui, B.; Tahiri, N.; Biben, T.; Ez-Zahraouy, H.; Benyoussef, A.; Biros, G.; Misbah, C. Complexity of Vesicle Microcirculation. Phys. Rev. E 2011, 84, 041906. [Google Scholar] [CrossRef]
- Guckenberger, A.; Kihm, A.; John, T.; Wagner, C.; Gekle, S. Numerical–Experimental Observation of Shape Bistability of Red Blood Cells Flowing in a Microchannel. Soft Matter 2018, 14, 2032–2043. [Google Scholar] [CrossRef]
- Dupire, J.; Socol, M.; Viallat, A. Full Dynamics of a Red Blood Cell in Shear Flow. Proc. Natl. Acad. Sci. USA 2012, 109, 20808–20813. [Google Scholar] [CrossRef]
- Kaoui, B.; Biros, G.; Misbah, C. Why Do Red Blood Cells Have Asymmetric Shapes Even in a Symmetric Flow? Phys. Rev. Lett. 2009, 103, 188101. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, J.; Cui, T.; Shehata, N.; Wang, C.; Sun, Y. Characterization of Red Blood Cell Deformability Change during Blood Storage. Lab. Chip 2013, 14, 577–583. [Google Scholar] [CrossRef]
- Pallotta, V.; Gevi, F.; D’alessandro, A.; Zolla, L. Storing Red Blood Cells with Vitamin C and N-Acetylcysteine Prevents Oxidative Stress-Related Lesions: A Metabolomics Overview. Blood Transfus. Trasfus. Del. Sangue 2013, 12, 376–387. [Google Scholar] [CrossRef]
- Chaudhary, R.; Katharia, R. Oxidative Injury as Contributory Factor for Red Cells Storage Lesion during Twenty Eight Days of Storage. Blood Transfus. Trasfus. Del. Sangue 2010, 10, 59–62. [Google Scholar] [CrossRef]
- Yang, Y.; He, H.; Wang, J.; Chen, L.; Xu, Y.; Ge, C.; Li, S. Blood Quality Evaluation via On-Chip Classification of Cell Morphology Using a Deep Learning Algorithm. Lab. Chip 2023, 23, 2113–2121. [Google Scholar] [CrossRef]
- Bochkovskiy, A.; Wang, C.-Y.; Liao, H.-Y.M. YOLOv4: Optimal Speed and Accuracy of Object Detection. arXiv 2020, arXiv:10.48550/arXiv.2004.10934. [Google Scholar]
- Redmon, J.; Divvala, S.; Girshick, R.; Farhadi, A. You Only Look Once: Unified, Real-Time Object Detection. arXiv 2015, arXiv:10.48550/arxiv.1506.02640. [Google Scholar]


| Disorder | Pathophysiology | Deformity in Cell | Test to Detect the Disorder |
|---|---|---|---|
| Storage lesions | ATP depletion and calcium accumulation | Viscosity increase | Microfluidic-based method [12] |
| Sickle cell anemia | Autosomal recessive disease with a mutation in β globin chain. Normal HbA is replaced by HbS. On oxidation stress the abnormal HbS polymerizes to cause sickle cell deformity and leading to sickle cell crisis [13]. | Increase in the plasma viscosity. Increase in the aggregation of RBC causing occlusion of the vessels [14]. | Electrophoresis [15] |
| Malaria | Caused by either of the five varieties of single celled parasites named Plasmodium through the bite of female anopheles mosquito. | Loss of discoid shape of the cell. Increase in the rigidity of the membrane. Increased adhesiveness to endothelial surfaces leading to aggregation and obstruction in microcirculatory flow [16]. | Blood smears, Rapid antigen detection test [17]. |
| Diabetes mellitus | Type 1 is caused by autoimmune destruction of pancreatic β cells. Type 2 is caused by peripheral resistance to insulin. GDM: hyperglycemia or hyperinsulinemia in utero | Impaired glucose metabolism thereby deforming the RBC membrane. An increase in blood viscosity increases RBC aggregation [18]. | Random blood glucose levels. Fasting blood glucose levels. Postprandial blood glucose levels HbA1c levels. GDM: membrane flickering [19] |
| Sepsis | Infectious insult results in a local inflammatory response which spills over and causes systemic symptoms secondary to various cytokines. It is called systemic inflammatory response syndrome. These cytokines also activate the extrinsic coagulation pathway thereby causing coagulopathy [20]. | Changes in microcirculation [21]. Increased RBC aggregation [21,22]. Decrease in RBC and WBC deformability [23]. Loss of capillary density [23]. Change in microvascular reactivity. WBC-endothelial cell adhesion and leaking of the vasculature coagulation disturbances [24]. | Blood culture. Coagulation profile. Total blood count. Peripheral smear. |
| Occlusive disorders | Caused by ischemia secondary to atherosclerosis and post ischemic reperfusion injuries | Increase in RBC aggregation and blood viscosity [25]. Decrease in RBC deformity [26]. | Computed tomography. Magnetic resonance angiography. |
| Micropipette | Viscometer | Microfluidics | Ektacytometry | AFM | Optical Tweezer | |
|---|---|---|---|---|---|---|
| Volume | ☑ [62,63,64,65] | ☑ [66,67] | ||||
| Surfaces area | ☑ [62,63,64,65] | ☑ [66,67] | ||||
| Cytoplasmatic viscosity | ☑ [68,69] | |||||
| Surface viscosity | ☑ [55,70,71,72,73] | ☑ [74] | ☑ [75] | |||
| Shear elastic modulus | ☑ [70,71,72,76,77] | ☑ [78] | ☑ [75] | ☑ [79,80] | ||
| Bending modulus | ☑ [57,81] | ☑ [82] | ☑ [83] | |||
| Relaxation constant | ☑ [70,72,73] | ☑ [84] | ☑ [74] | ☑ [85] | ☑ [80] | |
| Area compressibility | ☑ [55,71,86] | |||||
| Yong’s modulus | ☑ [87] | |||||
| Deformation index | ☑ [88,89] | ☑ [74,90] |
| Category | Technique | Principle | Key Pros | Key Cons |
|---|---|---|---|---|
| Section 4.1 Single cell Measurement | Section 4.1.1 Micropipette Aspiration | Deform cell into a constriction | Straightforward and economical | Time-consuming |
| Section 4.1.2 Optical Tweezers | Momentum and force of photon | Non-invasive, versatile, and highly accurate | Requirement of optical alignment is pretty high | |
| Section 4.1.3 Atomic Force Microscopy | Poking the cell membrane with a probe | Can detect forces at the piconewton level and investigate nanoscale cellular structures | Limited in its ability to probe multiple points in a cell | |
| Section 4.1.4 Magnetic Twisting Cytometry | Apply oscillating magnetic fields | Flexibility in bead attachment | Non-uniform stress | |
| Section 4.1.5 Quantitative Phase Imaging | Laser interference | Dynamic membrane fluctuations can be observed | Thick specimen, shade-off and halo effect | |
| Section 4.2 Bulk Measurement Techniques | Section 4.2.1 Blood Viscometry and Ektacytometry | Detecting the torque required to spin an object | User-friendly | Require substantial amounts of reagents |
| Section 4.2.2 Filtration Tests | Encourage whole blood to pass through a membrane filter | Simplicity of instrumentation | Lower sensitivity to increments in cytoplasmic viscosity | |
| Section 4.2.3 Erythrocyte Sedimentation Rate | RBC tend to stack into rouleaux and sediment quick | Low cost | Cannot be used as a sole diagnostic tool | |
| Section 4.3 Microfluidic-Based Measurement | Section 4.3.1 Hydrodynamic Stretching | Hydrodynamic forces deform cell | Non-contact working principle | Only suitable for sphere cell |
| Section 4.3.2 Passing Through Constrictions | Squeeze through a small constriction | Integrating multiple techniques | Suffer from the clogging issue | |
| Section 4.3.3 Motion and Shapes | Due to the deformable disk shape interacting with the fluids | Convenient and gentle method | Difficulties in tracking the motion or categorized shapes | |
| Section 4.3.4 Machine Learning | RBC morphology indicates the biochemical changes | Reduces human labor and minimizes subjective bias | Training of the model requires a large quantity of labeled data |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, M.; Ming, D.; Zhong, J.; Shannon, C.S.; Rojas-Carabali, W.; Agrawal, K.; Ai, Y.; Agrawal, R. Pathophysiological Associations and Measurement Techniques of Red Blood Cell Deformability. Biosensors 2025, 15, 566. https://doi.org/10.3390/bios15090566
Liang M, Ming D, Zhong J, Shannon CS, Rojas-Carabali W, Agrawal K, Ai Y, Agrawal R. Pathophysiological Associations and Measurement Techniques of Red Blood Cell Deformability. Biosensors. 2025; 15(9):566. https://doi.org/10.3390/bios15090566
Chicago/Turabian StyleLiang, Minhui, Dawei Ming, Jianwei Zhong, Choo Sheriel Shannon, William Rojas-Carabali, Kajal Agrawal, Ye Ai, and Rupesh Agrawal. 2025. "Pathophysiological Associations and Measurement Techniques of Red Blood Cell Deformability" Biosensors 15, no. 9: 566. https://doi.org/10.3390/bios15090566
APA StyleLiang, M., Ming, D., Zhong, J., Shannon, C. S., Rojas-Carabali, W., Agrawal, K., Ai, Y., & Agrawal, R. (2025). Pathophysiological Associations and Measurement Techniques of Red Blood Cell Deformability. Biosensors, 15(9), 566. https://doi.org/10.3390/bios15090566







