Cell Observation and Analysis with a Three-Dimensional Optical Wave Field Microscope
Abstract
1. Introduction
2. Materials and Methods
2.1. System of 3D-OWFM
2.2. Convert of OPD Images to OPD Edge and OPD Differential Images
2.3. Analysis Software
2.4. Cell Culture and Observation Condition
2.5. Time-Lapse Imaging and Cell Survival Assay
2.6. Statistical Analysis
2.7. Use of Generative Artificial Intelligence Tools
3. Results
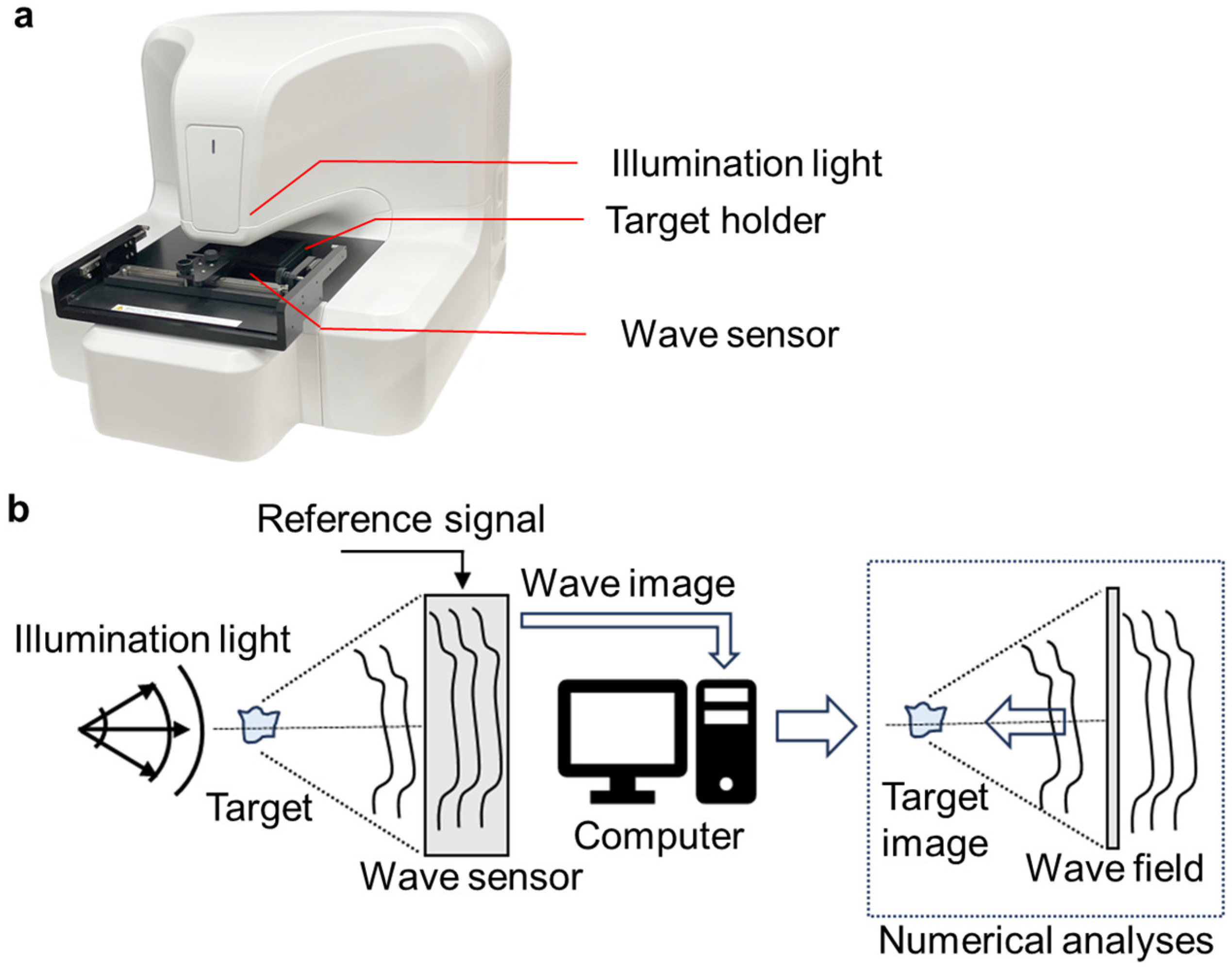
3.1. Appearance of the 3D-OWFM System and the Visualization Principles
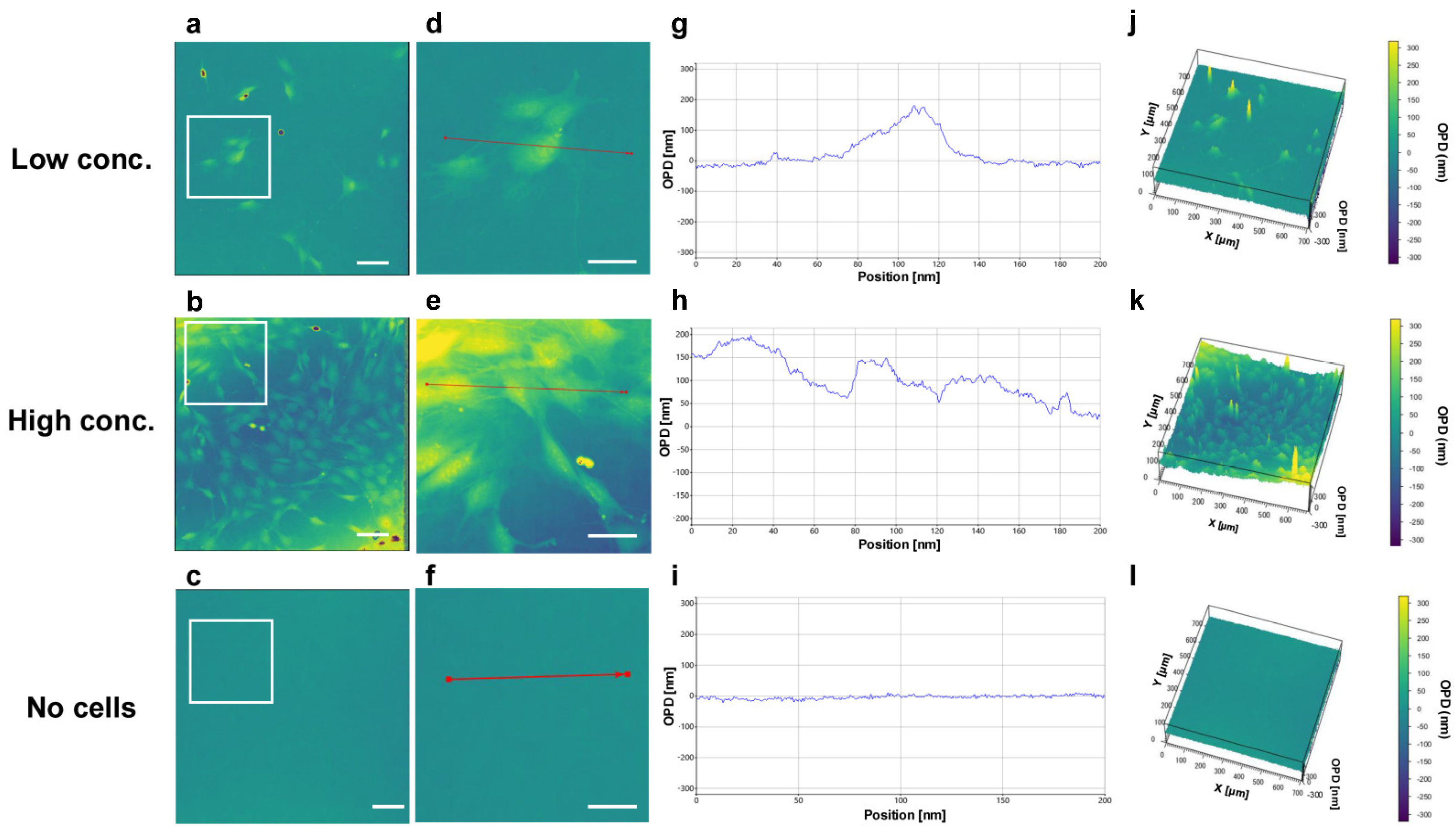
3.2. Observation and Analysis of Cells with 3D-OWFM
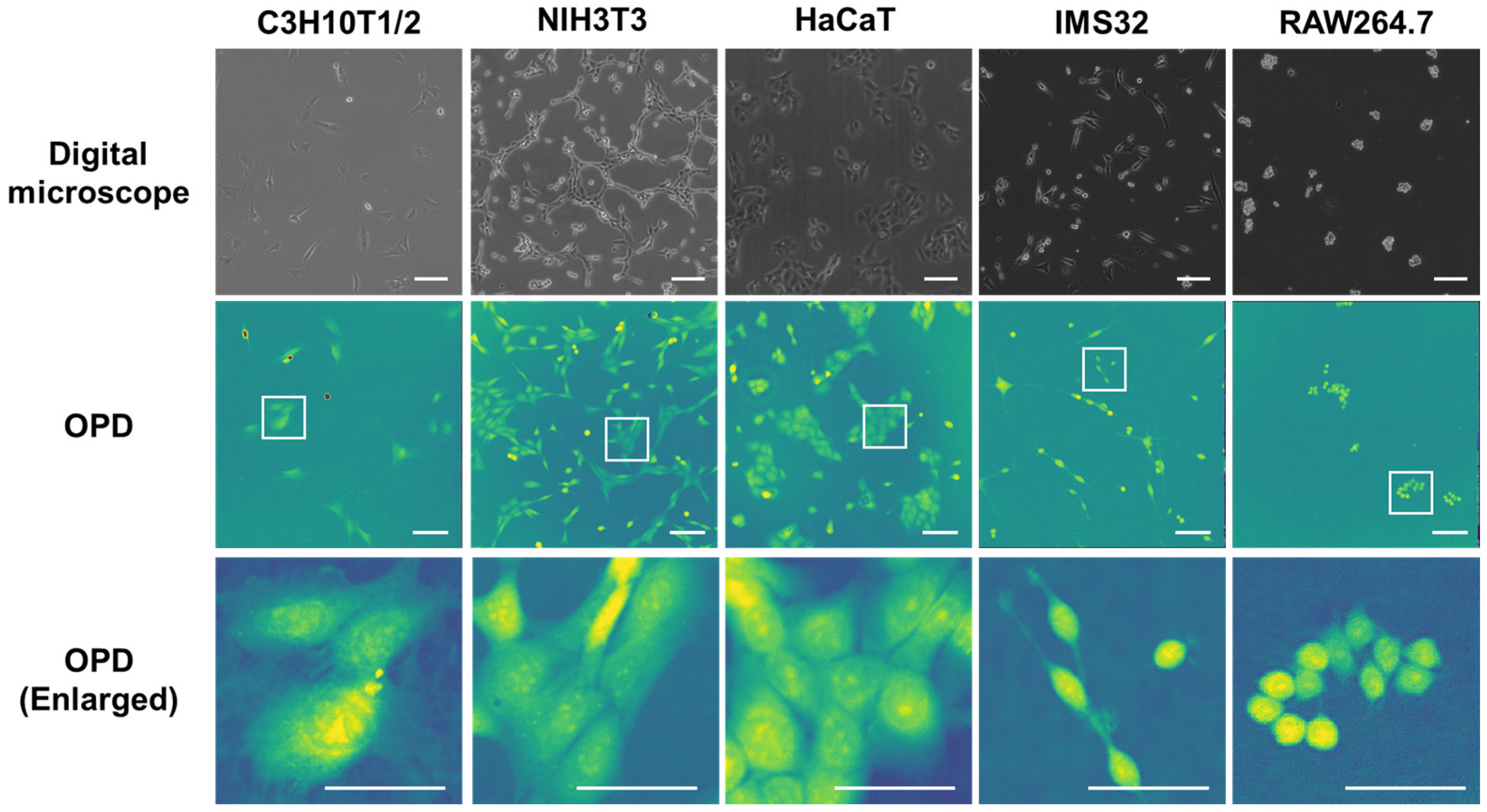
3.3. Observation of Various Cells with 3D-OWFM and Comparison to Digital Microscope
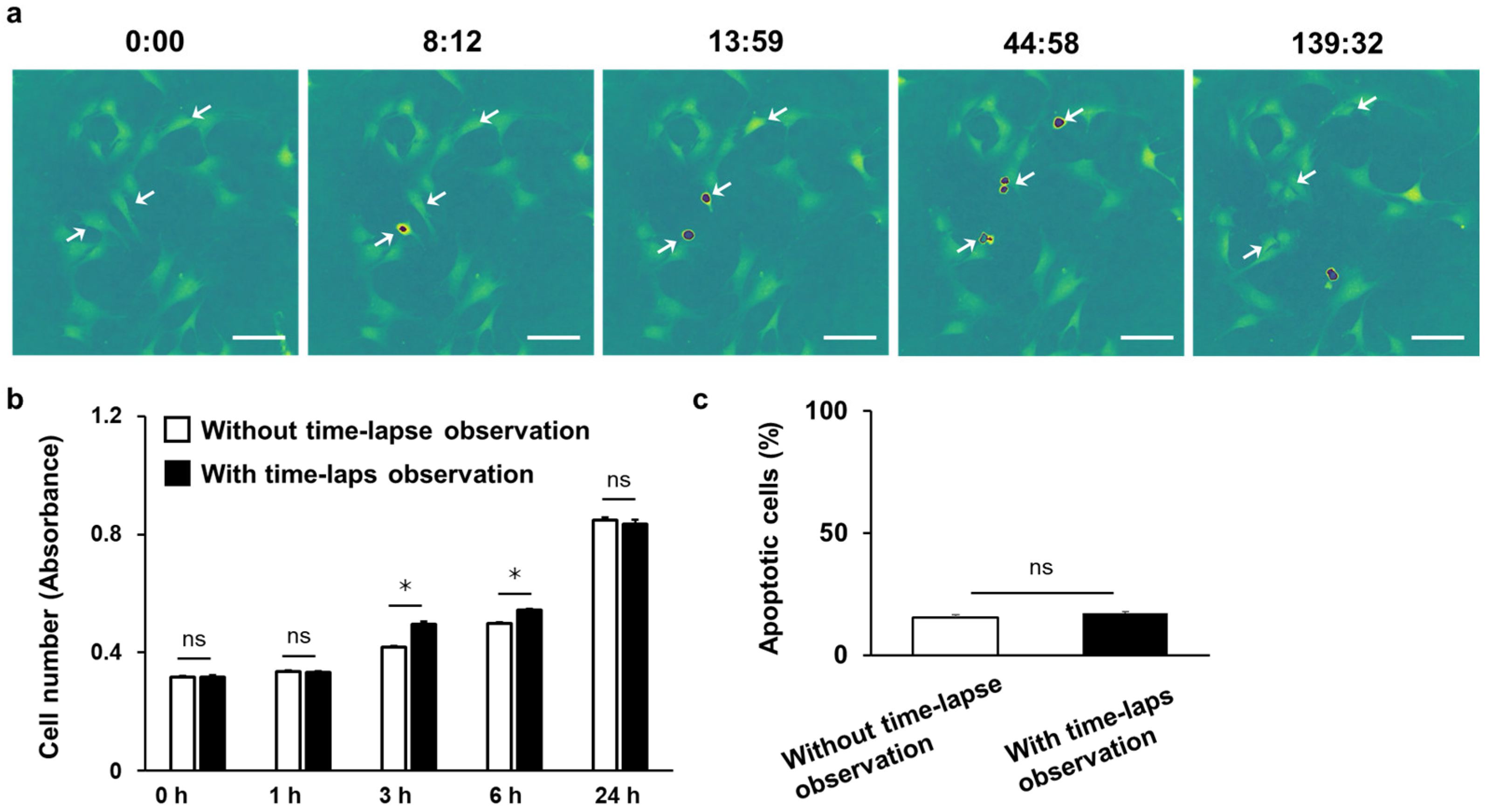
3.4. Time-Lapse Observation of C3H10T1/2 Cells Using 3D-OWFM for the Analysis of Cell Division
4. Discussion
4.1. Technical Advantages of 3D-OWFM
4.2. Interpretation of Key Findings
4.3. Future Applications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alberts, B.; Heald, R.; Johnson, A.; Morgan, D.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 7th ed.; W. W. Norton & Company: New York, NY, USA, 2024. [Google Scholar]
- Wolpert, L.; Tickle, C.; Martinez Arias, A. Principles of Development, 6th ed.; Oxford University Press: Oxford, UK, 2019. [Google Scholar]
- Yewdall, N.A.; Mason, A.F.; van Hest, J.C.M. The hallmarks of living systems: Towards creating artificial cells. Interface Focus 2018, 8, 20180023. [Google Scholar] [CrossRef]
- Zhang, X.; Marjani, S.L.; Hu, Z.; Weissman, S.M.; Pan, X.; Wu, S. Single-cell sequencing for precise cancer research: Progress and prospects. Cancer Res. 2016, 76, 1305–1312. [Google Scholar] [CrossRef]
- Cha, J.; Lee, I. Single-cell network biology for resolving cellular heterogeneity in human diseases. Exp. Mol. Med. 2020, 52, 1798–1808. [Google Scholar] [CrossRef] [PubMed]
- Betzig, E.; Patterson, G.H.; Sougrat, R.; Lindwasser, O.W.; Olenych, S.; Bonifacino, J.S.; Davidson, M.W.; Lippincott-Schwartz, J.; Hess, H.F. Imaging intracellular fluorescent proteins at nanometer resolution. Science 2006, 313, 1642–1645. [Google Scholar] [CrossRef]
- Altschuler, S.J.; Wu, L.F. Cellular heterogeneity: Do differences make a difference? Cell 2010, 141, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.; Zhu, X.; Zhang, S.; Wu, J.; Luo, Y. Phase contrast microscopy of living cells within the whole lens: Spatial correlations and morphological dynamics. Mol. Vis. 2012, 18, 2165–2173. [Google Scholar] [PubMed]
- Elliott, A.D. Confocal microscopy: Principles and modern practices. Curr. Protoc. Cytom. 2020, 92, e68. [Google Scholar] [CrossRef]
- Cole, R. Live-cell imaging: The cell’s perspective. Cell Adhes. Migr. 2014, 8, 452–459. [Google Scholar] [CrossRef]
- Murphy, G.E.; Narayan, K.; Lowekamp, B.C.; Hartnell, L.M.; Heymann, J.A.W.; Fu, J.; Subramaniam, S. Correlative 3D imaging of whole mammalian cells with light and electron microscopy. J. Struct. Biol. 2011, 176, 268–278. [Google Scholar] [CrossRef][Green Version]
- Franken, L.E.; Grünewald, K.; Boekema, E.J.; Stuart, M.C.A. A technical introduction to transmission electron microscopy for soft-matter: Imaging, possibilities, choices, and technical developments. Small 2020, 16, 1906198. [Google Scholar] [CrossRef]
- Popescu, G. Quantitative phase imaging of nanoscale cell structure and dynamics. Methods Cell Biol. 2008, 90, 87–115. [Google Scholar]
- Marquet, P.; Rappaz, B.; Magistretti, P.J.; Cuche, E.; Emery, Y.; Colomb, T.; Depeursinge, C. Digital holographic microscopy: A non-invasive contrast imaging technique allowing quantitative visualization of living cells with subwavelength axial accuracy. Opt. Lett. 2005, 30, 468–470. [Google Scholar] [CrossRef]
- Cacace, T.; Bianco, V.; Ferraro, P. Quantitative phase imaging trends in biomedical applications. Opt. Lasers Eng. 2020, 135, 106188. [Google Scholar] [CrossRef]
- Choi, W.; Fang-Yen, C.; Badizadegan, K.; Oh, S.; Lue, N.; Dasari, R.R.; Feld, M.S. Tomographic phase microscopy. Nat. Methods 2007, 4, 717–719. [Google Scholar] [CrossRef]
- Kuś, A. Real-time, multiplexed holographic tomography. Opt. Lasers Eng. 2022, 149, 106783. [Google Scholar] [CrossRef]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Stavrakis, S.; Holzner, G.; Choo, J.; deMello, A. High-throughput microfluidic imaging flow cytometry. Curr. Opin. Biotechnol. 2019, 55, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Surmacki, J.M.; Woodhams, B.J.; Haslehurst, A.; Ponder, B.A.J.; Bohndiek, S.E. Raman micro-spectroscopy for accurate identification of primary human bronchial epithelial cells. Sci. Rep. 2018, 8, 12604. [Google Scholar] [CrossRef] [PubMed]
- Rappaz, B.; Marquet, P.; Cuche, E.; Emery, Y.; Depeursinge, C.; Magistretti, P.J. Measurement of the integral refractive index and dynamic cell morphometry of living cells with digital holographic microscopy. Opt. Express 2005, 13, 9361–9373. [Google Scholar] [CrossRef] [PubMed]
- Greenbaum, A.; Luo, W.; Su, T.-W.; Göröcs, Z.; Xue, L.; Isikman, S.O.; Coskun, A.F.; Mudanyali, O.; Ozcan, A. Imaging without lenses: Achievements and remaining challenges of wide-field on-chip microscopy. Nat. Methods 2012, 9, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Sobieranski, A.C.; Inci, F.; Tekin, H.C.; Yuksekkaya, M.; Comunello, E.; Cobra, D.; Wangenheim, A.V.; Demirci, U. Portable lensless wide-field microscopy imaging platform based on digital inline holography and multi-frame pixel super-resolution. Light Sci. Appl. 2015, 4, e346. [Google Scholar] [CrossRef] [PubMed]
- Greenbaum, A.; Zhang, Y.; Feizi, A.; Chung, P.L.; Luo, W.; Kandukuri, S.R.; Ozcan, A. Wide-field computational imaging of pathology slides using lens-free on-chip microscopy. Sci. Transl. Med. 2014, 6, 267ra175. [Google Scholar] [CrossRef] [PubMed]
- Maniscalco, B.; Kaminski, P.M.; Walls, J.M. Thin film thickness measurements using scanning white light interferometry. Thin Solid Films 2014, 550, 10–16. [Google Scholar] [CrossRef]
- Schellhaus, A.K.; De Magistris, P.; Antonin, W. Nuclear reformation at the end of mitosis. J. Mol. Biol. 2016, 428, 1962–1985. [Google Scholar] [CrossRef]
- Balasubramani, V.; Kuś, A.; Tu, H.-Y.; Cheng, C.-J.; Baczewska, M.; Krauze, W.; Kujawińska, M. Holographic tomography: Techniques and biomedical applications. Appl. Opt. 2021, 60, B65–B80. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsumoto, S.; Itakura, S.; Minato, J.; Hashimoto, M.; Obana, S.; Kanai, M.; Kobayashi, M.; Nishikawa, M.; Kusamori, K. Cell Observation and Analysis with a Three-Dimensional Optical Wave Field Microscope. Biosensors 2025, 15, 515. https://doi.org/10.3390/bios15080515
Matsumoto S, Itakura S, Minato J, Hashimoto M, Obana S, Kanai M, Kobayashi M, Nishikawa M, Kusamori K. Cell Observation and Analysis with a Three-Dimensional Optical Wave Field Microscope. Biosensors. 2025; 15(8):515. https://doi.org/10.3390/bios15080515
Chicago/Turabian StyleMatsumoto, Shimon, Shoko Itakura, Junta Minato, Masahiro Hashimoto, Shu Obana, Mai Kanai, Masaki Kobayashi, Makiya Nishikawa, and Kosuke Kusamori. 2025. "Cell Observation and Analysis with a Three-Dimensional Optical Wave Field Microscope" Biosensors 15, no. 8: 515. https://doi.org/10.3390/bios15080515
APA StyleMatsumoto, S., Itakura, S., Minato, J., Hashimoto, M., Obana, S., Kanai, M., Kobayashi, M., Nishikawa, M., & Kusamori, K. (2025). Cell Observation and Analysis with a Three-Dimensional Optical Wave Field Microscope. Biosensors, 15(8), 515. https://doi.org/10.3390/bios15080515




