1. Introduction
Mammalian cell culture is one of the pillars of the life sciences. It supports fundamental and applied research, particularly the production of advanced therapy medicinal products. In all cases, cells are cultivated in specific media to increase their quantity and functionality. However, contamination could occur during this process and lead to the termination of cell cultures. Therefore, early, rapid, and sensitive quality control and pathogen detection strategies are crucial throughout the production process. The goal is to monitor production in real time and stop cell cultivation as soon as bacteria are detected. This could reduce production costs and make these promising medicines more widely available. More generally, this type of detection is one of the most challenging aspects in the fields of human health and the agri-food industry [
1]. Quality controls are required during industrial drug and biosimilar production processes. Numerous physics-, biology-, and chemistry-based techniques have been developed to detect, identify, and enumerate bacteria [
2,
3].
The polymerase chain reaction (PCR), which is based on DNA amplification, is currently the gold standard for detecting bacteria. Quantitative PCR (QPCR) can quantify targeted pathogens [
4]. However, PCR is highly specific to the target DNA of a given microorganism. More recent techniques based on molecular biotechnology are also employed for rapid, real-time, sensitive, and specific detection. These techniques include nucleic acid amplification tests, real-time polymerase chain reaction (RT-PCR), and loop-mediated isothermal amplification (LAMP) [
5]. Methods such as the plate culture approach are still used because they are effective and cost-effective; however, they are time-consuming and have limited sensitivity. Other techniques are based on growth to make detection possible, but these are reserved for cultivable bacteria. These techniques measure different changes in gas to detect bacteria in blood for sepsis diagnosis [
6], such as charged ionic metabolites measured by impedance and adenosine triphosphate (ATP). However, filtration is required to distinguish bacteria from other sources. Other techniques measure temperature by microcalorimetry and optical density at specific wavelengths [
7]. The sensitivity of each method depends on the microorganism. Although a variety of chromogenic and fluorogenic culture media have been developed for the selective isolation and differentiation of Gram-positive (G+) and Gram-negative (G−) bacteria, they have longer detection times and limited sensitivity [
8,
9]. Fluorescence enables more sensitive and accurate techniques, as well as high-speed and rapid techniques that allow for simultaneous detection without the need for bacterial growth [
10]. However, it requires the labeling of bacteria with fluorophores. Recently, new techniques have emerged, such as metal–organic frameworks (MOFs) and molecularly imprinted polymers (MIPs). This combination of materials results in a composite that significantly improves the sensitivity, selectivity, antibacterial efficiency, and environmental friendliness of microbial detection [
11].
Other detection techniques include immunological assays, such as ELISA. These tests aim to detect a specific molecule using a labeled capturing agent specific to one target. While these methods have a short response time, they are less sensitive than PCR, and they are difficult to apply to unknown samples due to their specificity. Traditional methods are often complex, time-consuming, and labor-intensive. Alternative, innovative techniques based on the recognition of ligands and bacteria have been developed. These techniques enable the detection of bacteria without the need for cultures, allowing for faster detection and improved sensitivity and specificity. They also allow for automation and the creation of cost-effective devices. Ligands can be classical antibodies, aptamers [
12], bacteriophages [
13], antimicrobial peptides (AMPs) derived from ATP or ADP, or fluorogenic RNA-cleaving DNAzymes. This enables discrimination between bacteria and detection at very low concentrations. These techniques can be used with optical methods, such as Raman spectroscopy [
14], or acoustic techniques, such as QCM (quartz crystal microbalance) [
15], in bulk systems or on microfluidic chips. These methods enable label-free detection of bacteria and allow for online, real-time measurements as well as integration into biosensors [
16]. Advancements in nanomaterials and biosensors enhance their sensitivity and specificity [
17]. Techniques based on microfluidics (e.g., acoustophoresis and microdroplets), mass spectrometry, and microscopy are also used [
18,
19]. However, these techniques can be expensive and require expertise. In recent years, various commercial systems for detecting bacteria have emerged. These systems use either oxygen-coupled fluorescence compounds (BACTEC, Becton Dickinson [
20]) or pH changes due to CO
2 emitted by bacteria (BactAlert
®, BioMérieux [
21]).
All of the above techniques require culturing steps prior to analysis in order to increase bacteria concentration. These techniques also require sampling, which breaks the sterility of closed cultivation systems. They also require adding compounds and/or fluorophores to increase sensitivity. These requirements highlight the need for rapid, effective, reproducible, label-free, cost-effective, sampling-less, real-time, automatable techniques for detecting bacteria without prior identification. Currently, no technique fulfills all these criteria. In recent years, white light spectroscopy has proven to be a powerful technique for monitoring and counting mammalian cells and bacteria, including those of the ESKAPEE group [
22,
23,
24,
25]. This group includes highly virulent, antibiotic-resistant bacteria that cause hospital-acquired diseases. These bacteria include both Gram-positive and Gram-negative types [
26]. Both B- and T-type lymphocytes, as well as primary T-cells isolated from healthy donors, can be monitored [
27]. Furthermore, this technique can be easily integrated into a contactless system for real-time, noninvasive measurements. These recent publications prove the concept of using white light spectroscopy in CAR-T cell production. They also describe how this method can easily be integrated into a real-time, sampling-free device.
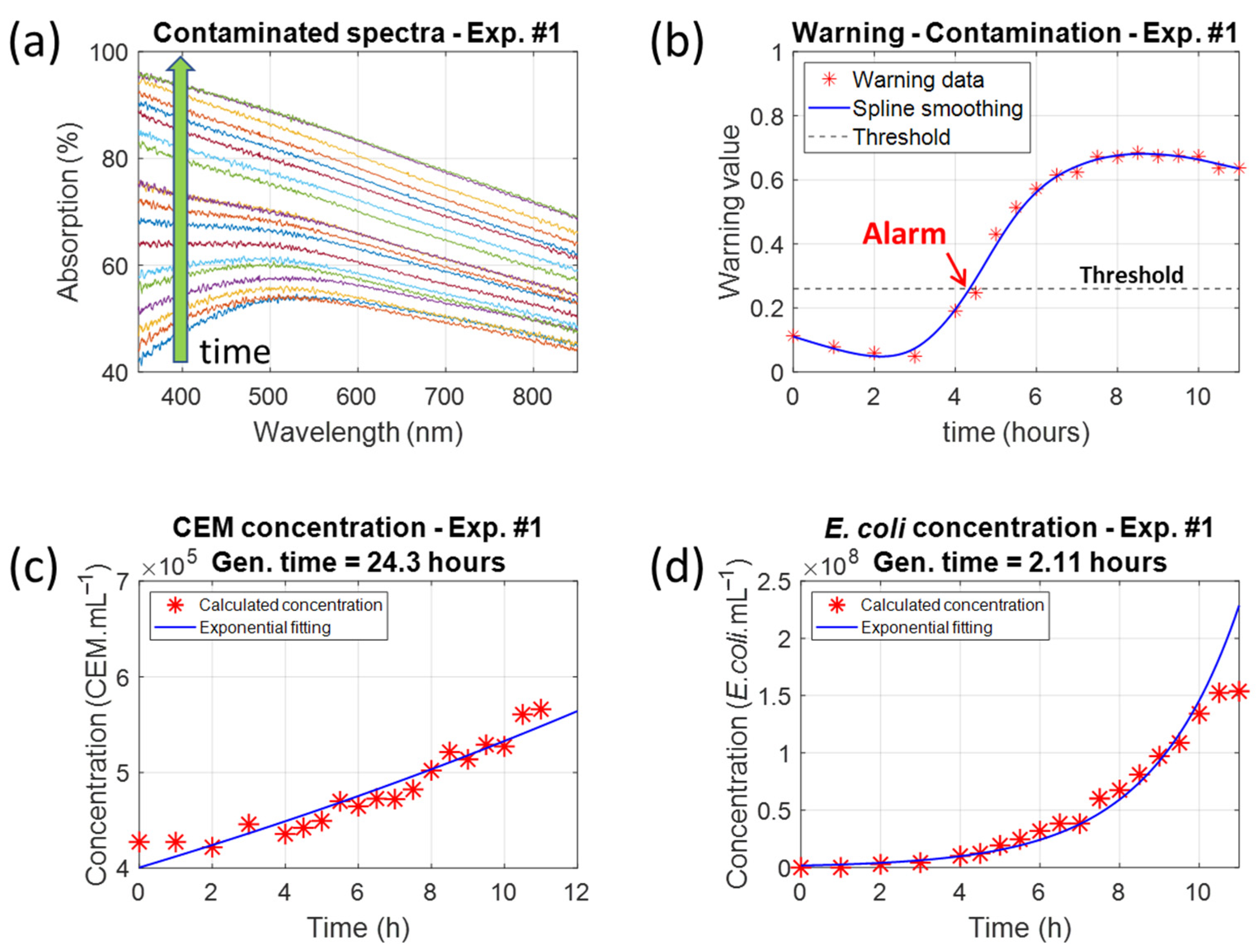
This paper describes using white light spectroscopy to detect bacterial contamination during mammalian cell cultivation monitoring. Contamination was induced using
Escherichia coli, a well-known bacterium, but this method could be applied to other ESKAPEE group bacteria and possibly other contaminants. Mathematical descriptions of the absorption spectra of both species enable cell and bacterial concentrations to be measured simultaneously.
Section 2 describes the materials and methods employed in this study.
Section 3, “Experimental Results,” is divided into two parts. First, it proposes a spectroscopic marker for detecting contamination in mammalian cell cultures. This marker is based on analyzing the shapes of absorption spectra and, more precisely, how they change when contamination develops. A warning value is defined that triggers an alert when contamination is detected. Using synthetic spectra obtained from experimental spectra of CEM-C1 T-cell lines and bacteria, the minimum detectable concentration of bacteria can be calculated for various cell concentrations and for bacteria from the ESKAPEE group. Second, the marker detects contamination in a cell culture infected with
E. coli. The evolution of the warning value during the development of contamination was clearly observed.
Section 4 discusses the experimental results, the position of this work in relation to others, and the real-time, sampling-less possibilities offered by white light spectroscopy.
Section 5 draws conclusions.
2. Materials and Methods
The objective of the present work was to perform bacterial contamination of mammalian cell cultures using the well-known bacterium Escherichia coli and follow these contaminations by spectral measurements using white light spectroscopy.
2.1. Mammalian Cell Culture
T lymphoblasts (CEM-C1 cells, ATCC
® CRL-2265
TM) were provided by the French Blood Agency (EFS BFC, Besançon, France). The cells were grown in a phenol red-free RPMI-1640 medium supplemented with 25 mM HEPES, 10% heat-inactivated fetal bovine serum (FBS), and 1% penicillin (10 kU.mL
−1)/streptomycin (10 mg.mL
−1). The cells were maintained at 37 °C in a humidified atmosphere containing 5% CO
2.
Table 1 summarizes reagents, suppliers, and references for cell culture.
2.2. Bacteria Culture
The E. coli bacteria (strain 18265017, Fischer Scientific™, Illkirch, France) were stored long-term at −80 °C in LB medium/30% glycerol (v/v). They were thawed on a TSA gelose and incubated overnight. Precultures were made with 4 different clones in 10 mL in the same RPMI medium used for cells but without antibiotics (RPMI-atb) and incubated for at least 20 h at 37 °C, 200 rpm. Then, they were centrifuged at 7180 g for 10 min at RT, and DO was measured at a wavelength of 600 nm using a spectrophotometer (Biowave DNA, Biochrom Ltd., Cambridge, UK). Afterwards, the pellets were resuspended in an appropriate volume of RPMI-atb to adjust bacteria to a concentration of 1 × 107 bact.mL−1.
Suspensions of bacteria were diluted in 0.85% NaCl at dilutions allowing bacteria measurements. A volume of 54.3 µL was plated on a Petri dish containing TSB medium with an automatic seeder (Spiral Platter Eddy Jet, I&L Biosystems, Konigswinter, Germany). The plates were incubated overnight before performing a manual enumeration following the manufacturer’s instructions.
Table 2 summarizes reagents, suppliers, and references for bacteria culture.
2.3. Bacterial Contamination of Mammalian Cell Experiments
In total, 6 different experiments have been performed during 3 experimental sessions. For each session one control cuvette with 5 × 105 cell.mL−1 was used as a control. For each experiment, two mixtures of the CEM cells and bacteria were prepared in duplicates with the following species concentrations in RPMI media without antibiotics:
Prior to the preparation of the mixtures, the species’ concentrations were measured optically using the Luna counter for mammalian cells and the optical density at 600 nm for bacteria. Then, mixtures were incubated within an incubator at 37 °C during 11 h.
2.4. Spectral Measurements
We performed spectral measurements of CEM suspensions with and without bacteria using the experimental setup described in [
24,
26]. Our spectroscopic system consisted of a light source connected to a cuvette holder via optical fibers. After propagating through the spectroscopic cuvette, the light was transmitted to the spectrophotometer for spectral acquisition.
Before each measurement, the reference spectrum was acquired using only RPMI-atb. Cell suspensions with or without bacteria were homogenized with several gentle inversions before each spectroscopic measurement. Measurements were performed every 30 min. The spectra were recorded in transmission in the wavelength range of 177–892 nm with a step size of 0.22 nm using OceanView software (see
Table 3). Each experiment lasted 11 h. Spectra were acquired hourly, except during session 1, when they were recorded twice an hour between 4 and 11 h.
Table 3 summarizes the equipment, suppliers, and references for the spectral measurements.
2.5. Spectral Data Processing
A total of 129 spectra were acquired during the six experiments, as detailed in
Table 4. The transmission data were converted to absorption, and all calculations were performed using MATLAB™ software (version number R2020b; MATLAB™, USA; supplier: Meudon, France). Only wavelengths between 350 and 850 nm, for which the signal-to-noise ratio was high enough, were considered. As previously mentioned, artifacts due to the energetic emission peaks of the deuterium lamp were numerically removed [
24]. The additional signal at 410 nm due to the varying FSB concentration was also removed numerically, as explained in [
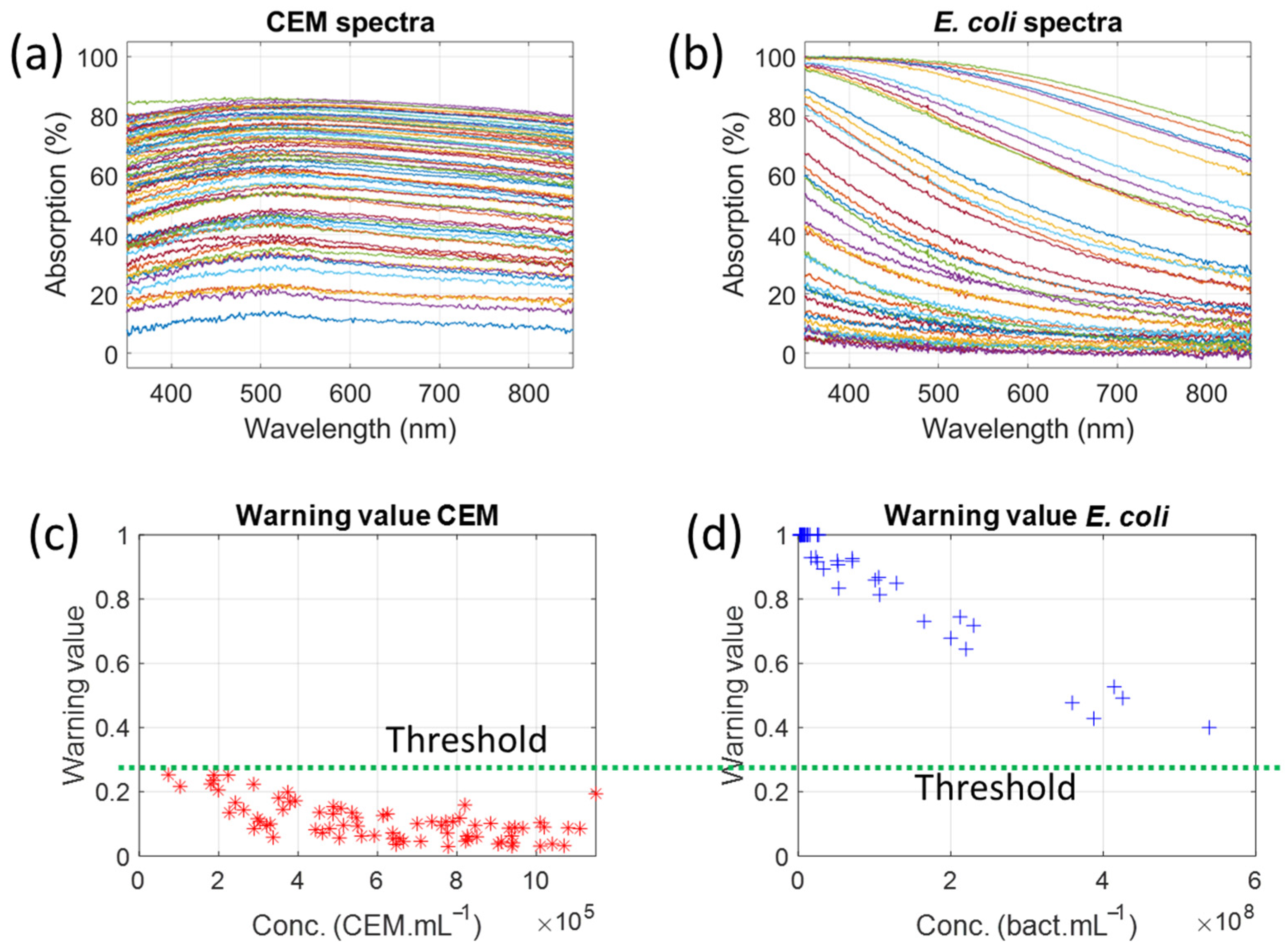
26]. Absorption spectra of neutral densities (Thorlabs, USA; Maisons Laffitte, France; part numbers NE05B and NE10B) were regularly recorded and compared to the supplier’s data to ensure correct absorption spectrum measurements. Only spectra containing useful information within the considered spectral range were kept; these spectra had an absorption range of 3% to 94% at a wavelength of 600 nm.
2.6. Synthetic Spectra Mimicking CEM-C1 Contaminated with ESKAPEE Bacteria
ESKAPEE is an acronym that refers to a model group of model bacteria involved in nosocomial diseases: Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, Enterococcus faecium, and Enterobacter cloacae.
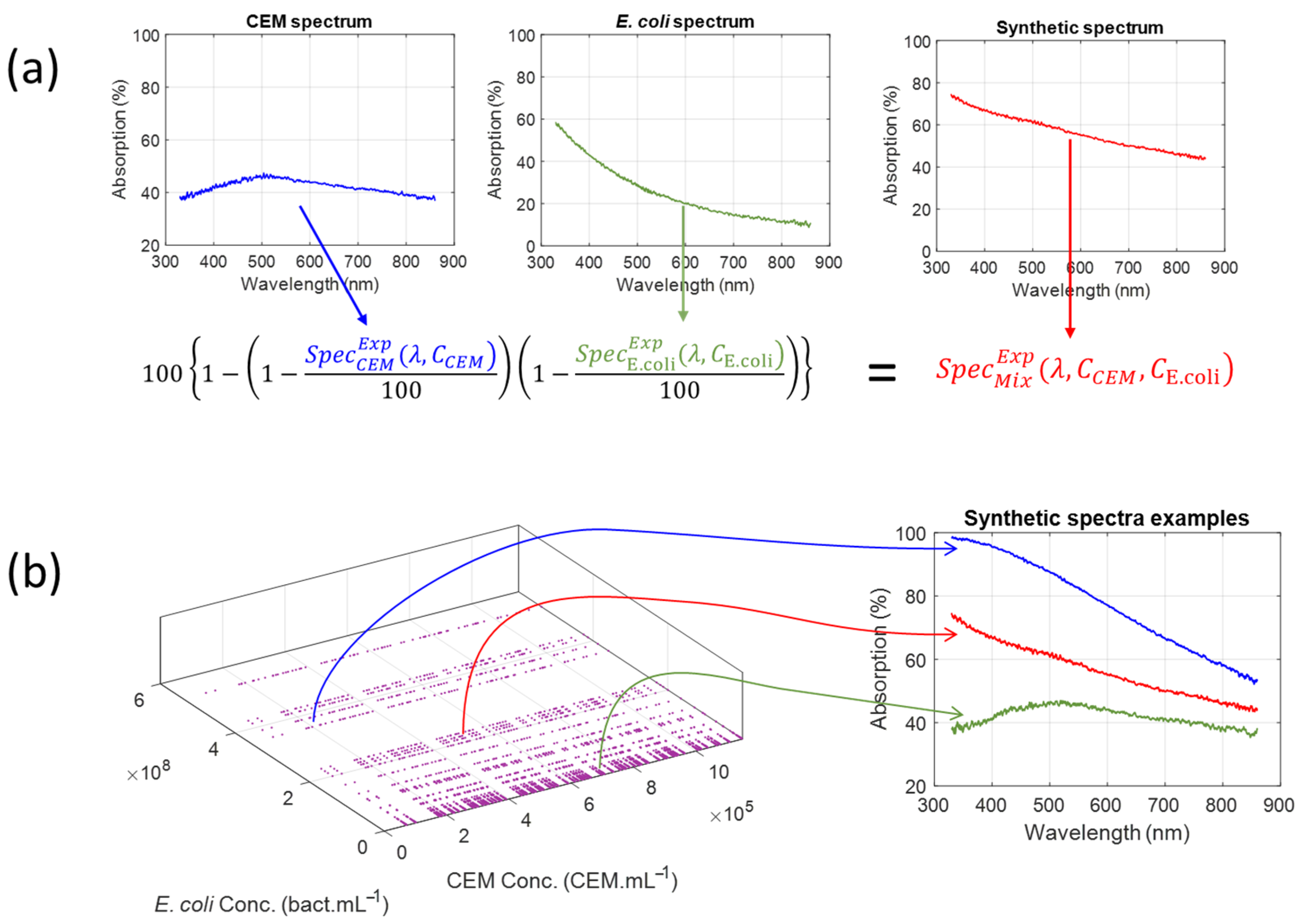
Synthetic spectra are artificial yet realistic data sets generated by algorithms or simulations rather than collected from real-world events. This type of data is used more and more in machine learning, AI development, testing, and privacy-sensitive environments. The goal is to produce data that preserves the key characteristics of the original data. In this study, synthetic spectra were computed using a spectral composition equation derived from the law of optical densities additivity (Equation (2) in [
24]). This equation is obtained as follows: Equation (1) shows the law of additivity of optical densities for a mixture of “n” elements.
Here,
is the optical density of the mixture and
the optical density of element “i”. Using the relationship between optical densities and transmittance yields the following:
Here,
is the transmittance of the mixture and
the transmittance of element “i”. Transmittance is related to the absorption, expressed as a percentage, by the following equation:
Here,
is the absorption of element “i”. The absorption spectrum of a mixture is then given by Equation (4), which includes wavelength and concentration dependencies.
Synthetic spectra were obtained by considering the 75 experimental spectra of the CEM dilution ranges [
24] and the experimental dilution range spectra of the ESKAPEE bacteria [
26].
Table 5 summarizes the number of synthetic spectra generated for each type of bacterial contamination. Overall, 19,125 synthetic spectra were generated.
4. Discussion
4.1. Accuracy of Species Concentrations Measurements
Figure 7c shows the calculated CEM concentration obtained by fitting the experimental spectra to Equation (8). This function enables the simultaneous calculation of bacteria and CEM concentrations, useful for monitoring the evolution of both populations in a co-culture. The dispersion was 1.6 × 10
4 CEM.mL
−1, representing about 2.8% accuracy. Since only CEM is present in the control cuvettes, one might think that fitting CEM spectra with an equation that calculates two different concentrations would reduce the accuracy of both. However, fitting the spectra shown in
Figure 7a with Equation (9) (the spectra shape of pure CEM) results in a dispersion of 1.5 × 10
4 CEM.mL
−1, or 2.7%. Therefore, using the “mixture” function (Equation (8)) did not reduce the measurement accuracy.
Previously published studies reported measurement accuracies of 9% for T-cell lines and 10% for primary T-cells [
24,
27]. These values corresponded to the accuracies obtained for dilution ranges where spectroscopy cuvettes were changed at each measurement. These basic plastic cuvettes show dispersion of about 5%. However, when conducting monitoring experiments in which the cuvette is used throughout, accuracies of about 3% were obtained [
24,
26]. The same high accuracy was achieved in the present study.
Indeed, the “mixture” function was only used to monitor both species concentrations during the contamination experiments. Under normal circumstances and in an automated device (see below), the CEM function would be used to calculate the warning value and stop production early. After production is stopped, identification of bacterial contamination or other causes of poor-quality culture would be performed. At this stage, conventional identification and enumeration methods would be used.
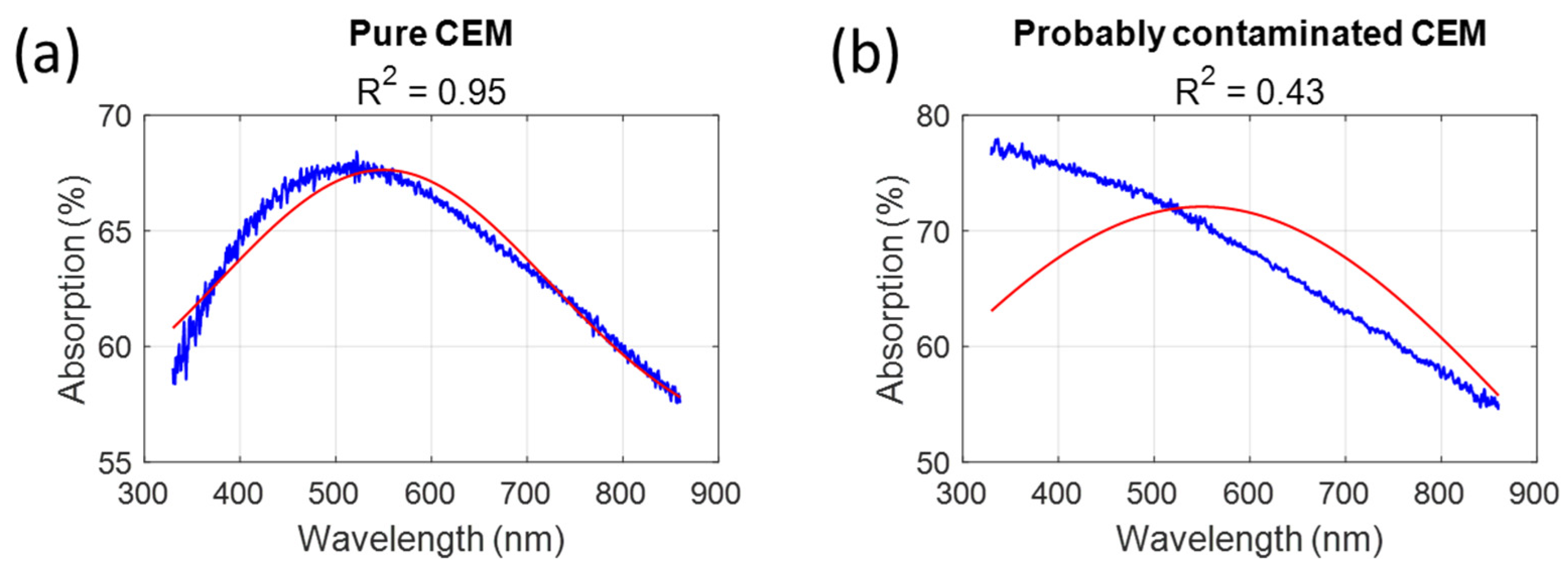
4.2. Modified CEM Function and Non-Unicity of Functions Describing Absorption Spectra Shapes
Here, we consider the equation describing shapes of the CEM absorption spectra. The same remark holds for the shape functions of bacteria.
The function describing the CEM spectrum shape and its evolution with concentration was originally determined in two steps. First, a set of sequential fittings was used to find the general form of the CEM shape function. Then, a minimization algorithm was used to calculate the final function parameters [
24]. The function consists of a concentration-dependent Gaussian plus a fixed Gaussian acting as a baseline, which is centered in the near-infrared region (936.1 nm) with a relatively large amplitude (12.21%).
Using this function in
Section 3.1.3 was not ideal for low CEM concentrations because the large amplitude of the fixed Gaussian resulted in a high warning value (low R
2) even with very low bacterial concentrations. We then calculated a new set of parameters using a global fitting algorithm (3D fitting in MATLAB™), limiting the possible amplitude of the fixed Gaussian. This was not possible with other methods because the algorithms do not allow it. This method produced the parameters listed in
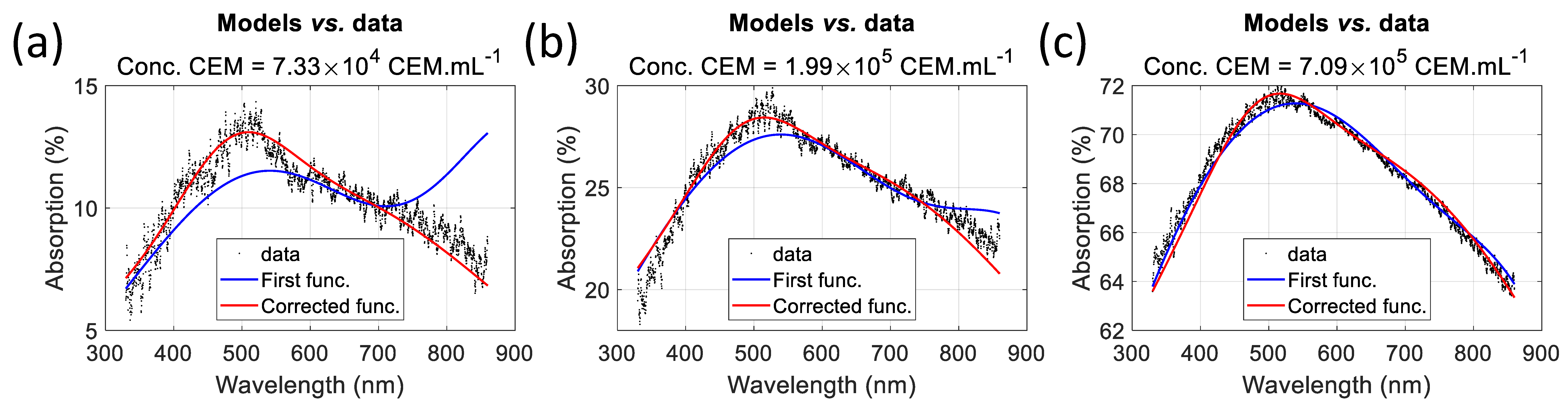
Table 7, which allowed us to prove the concept of a warning value for low CEM concentrations, i.e., at the beginning of CAR-T cell production. We compared the fittings obtained using the initial and modified functions for very low, average, and high CEM concentrations (
Figure 14). The influence of the large amplitude of the fixed Gaussian in the initial function is clearly visible at low concentrations (
Figure 14b) and gradually diminishes as the CEM concentration increases.
Therefore, there is no unique set of parameters that can accurately determine the CEM concentration.
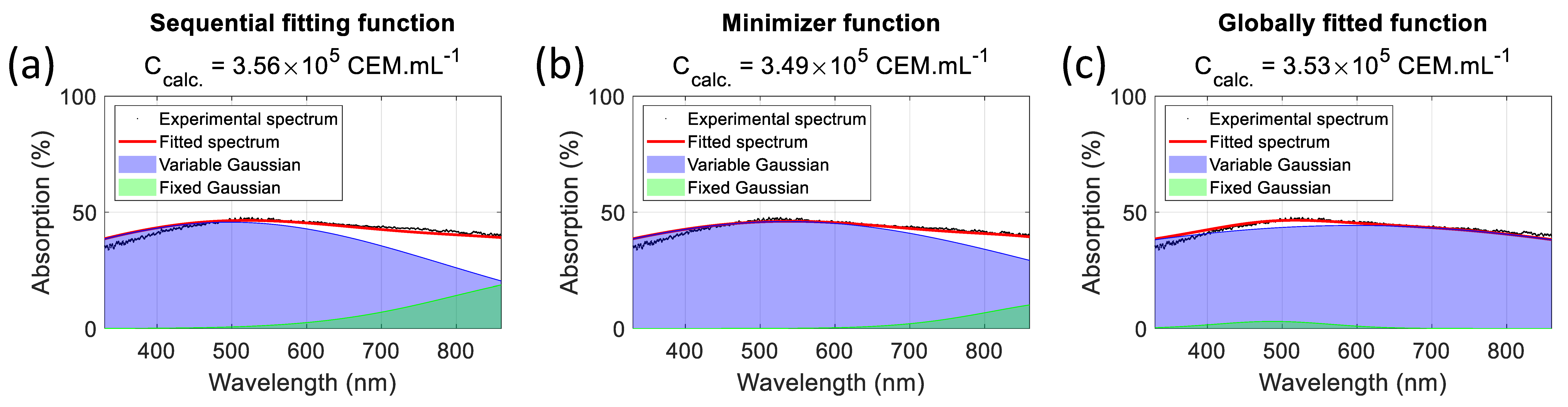
Figure 15 shows examples of spectra fitting using various sets of parameters.
Table 10 summarizes the method used to calculate the parameters, their values in each case, and the dispersion obtained over the 75 spectra of the dilution ranges used in [
24]. The “sequential fitting” and “minimization algorithm” methods were published earlier and produce the “approximated” and “final” parameters [
24]. The “globally fitted function” method is used in the present paper. Regardless of the method, the spectra are perfectly fitted. However, the characteristics of the fixed Gaussian differ. Using sequential fitting (
Figure 15a), the fixed Gaussian is centered in the infrared region at 976.9 nm and is 253.9 nm wide. The same is true for the minimization algorithm with a fixed Gaussian centered at 936.1 nm and 177.2 nm wide (
Figure 15b). As the concentration decreases, the fixed Gaussian becomes predominant, and the spectra cannot be efficiently fitted because of the infrared contribution (
Figure 14a). Using the globally fitted function yields a fixed Gaussian centered at 487.2 nm and 107.5 nm wide (
Figure 15c). Fitting spectra with this function is now efficient, even for absorptions below 15%, because the infrared component no longer exists (
Figure 14a).
The CEM concentration of the experimental spectrum was measured using an automatic cell counter. 3.51 × 10
5 CEM.mL
−1. The titles of the subfigures in
Figure 15 indicate the concentrations measured using the corresponding methods. The respective errors were +1.4%, −0.5%, and +0.5%, showing that the functions are equivalent in terms of concentration measurement accuracy.
In summary, the functions describing shapes of absorption spectra are not unique and can be adapted to each specific study.
4.3. Warning Threshold and Comparison with Statistical Species Classification
The warning threshold was set at 0.26, which is just above the maximum warning value for pure CEM (0.25). A Receiver Operating Characteristic (ROC) curve analysis confirmed the mathematical warning threshold of 0.25 (
Figure 16).
Figure 16a shows the evolution of the false and true positive rates (FPR and TPR), and
Figure 16b shows the ROC curve and the optimal warning threshold. The perfect shape of the ROC curve is due to the fact that the two populations (CEM and bacteria) are perfectly separated. The threshold of 0.26 was used to ensure that pure CEM is not considered contaminated.
Figure 16 was obtained using CEM and
E. coli data. The threshold value is the same for other ESKAPEE bacteria because the warning threshold is mainly governed by the warning values of CEM, and all ESKAPEE bacteria behave similarly spectrally [
26].
A convenient and descriptive way to describe the shape of an absorption spectrum is to use principal component analysis (PCA, [
26]). Furthermore, linear or quadratic discriminant analysis (LDA or QDA) can be applied to separate spectra corresponding to different species.
This was applied to the CEM and
E. coli dilution ranges (
Figure 17). Only the first two principal components were considered, as they represent 99.8% of the information contained in the spectra. Black stars and blue circles represent the
E. coli and CEM spectra, respectively. Increasing species concentrations are shown from left to right in the figure. Green disks represent extreme spectral shapes: flat spectra at 0% absorption for cuvettes filled with culture medium only (disk on the left) and flat spectra at 100% absorption for cuvettes filled with extremely high concentrations of either species. The red line in
Figure 17 represents synthetic spectra corresponding to the contour line in
Figure 5a, which represents the warning threshold. The green line represents the CEM-
E. coli separation frontier obtained by QDA. Only QDA is represented here because it produces a diagonal confusion chart, while LDA does not.
Our method is equivalent to or better than QDA for medium and high concentrations even if QDA is slightly better for very low concentrations. Therefore, our method is quite competitive with the conventional method of species separation using QDA. Indeed, QDA determines the optimal separation between two classes. Our method determines the outer frontier of the CEM region.
4.4. Concerning the Time to Trigger the Alarm and Bacteria Generation Times
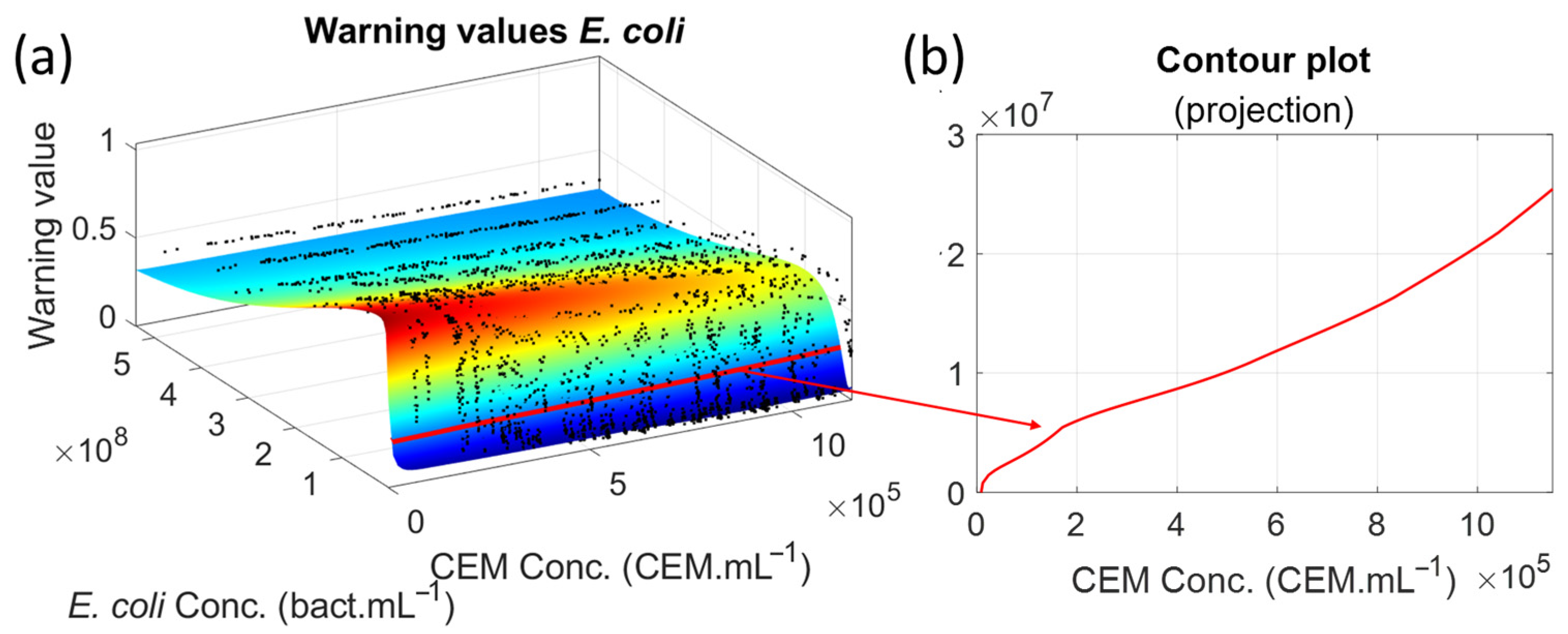
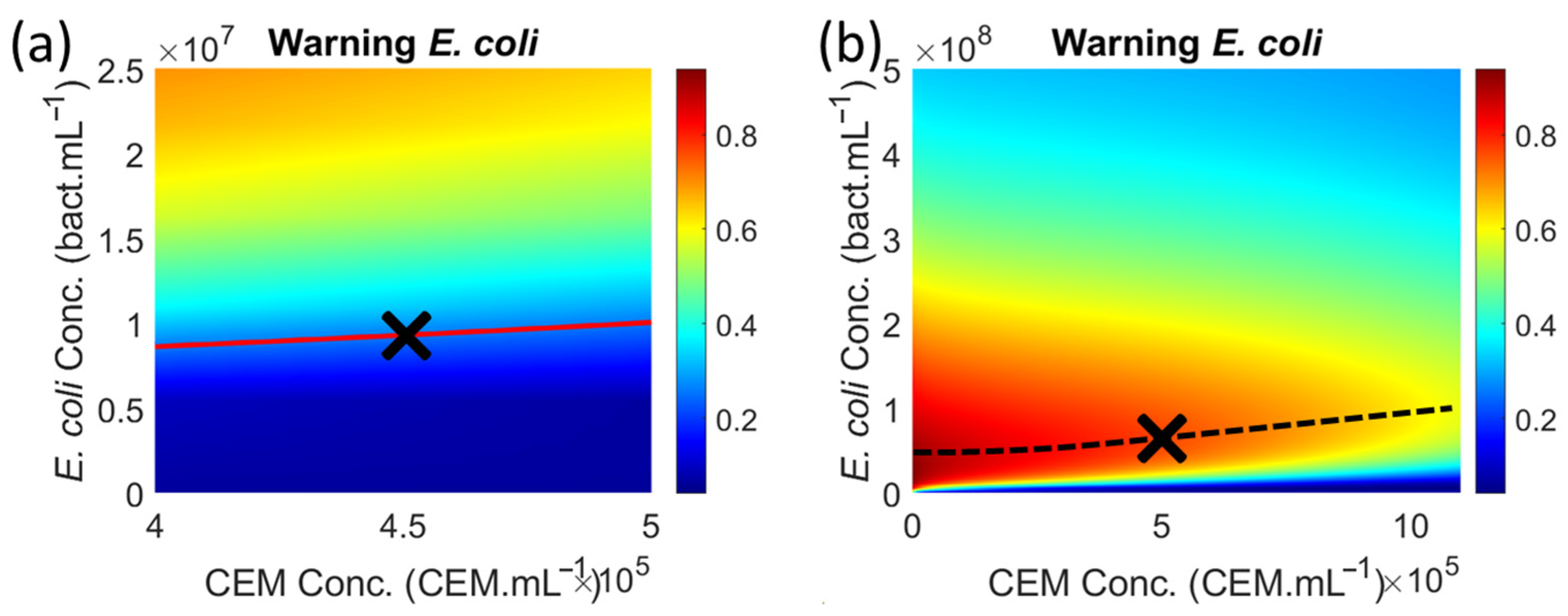
Determining a minimum bacterial concentration makes it possible to trigger an alarm (
Figure 6). We acknowledge that the pertinent question is “How long does it take to trigger the alarm after possible contamination?”.
To answer this question, ESKAPEE bacteria would need to be cultivated in a mammalian cell culture medium to determine their generation times under these conditions. While this could be performed, the added value would be minimal. A better approach would be to perform these experiments in co-culture with mammalian cells at various initial concentrations. This would allow considering competition between species during co-culture experiments. However, this method would not allow determining the lag time of bacteria in non-optimum culture media.
The six experiments presented here showed generation times of several hours instead of the usual tens of minutes for ESKAPEE bacteria. In these conditions, the time required to reach the warning thresholds was about 5 h. This time should be compared to the 7–10-day duration of the CAR-T cell production expansion phase. We recall that the goal of our work is to detect contamination during production as early as possible without sampling, thus avoiding the added risk of contamination due to quality control itself (see below for more information on the integration and real-time potential of the method). Five hours appears to be nearly real-time compared to current methods, which consist of sampling and cultivating the sample prior to measurement and potential detection. Our method consists of real-time measurements and direct monitoring of cultures and contaminations that could occur in cuvettes, rather than the detection of priming contamination.
As described in the introduction, most traditional techniques (e.g., PCR, ELISA, and culture) require sampling and cultivation prior to measurement. This process takes at least 18–20 h, or one night, to obtain sufficient bacteria for detection [
3,
4]. In addition to this incubation time, the time required for measurement and analysis should also be considered. Furthermore, the BacT/ALERT 3D system requires up to five or six days to confirm the absence of microbial growth [
20]. However, this equipment is used to certify the absence of contamination. Our method simply triggers an alarm as soon as contamination is detectable during the expansion phase.
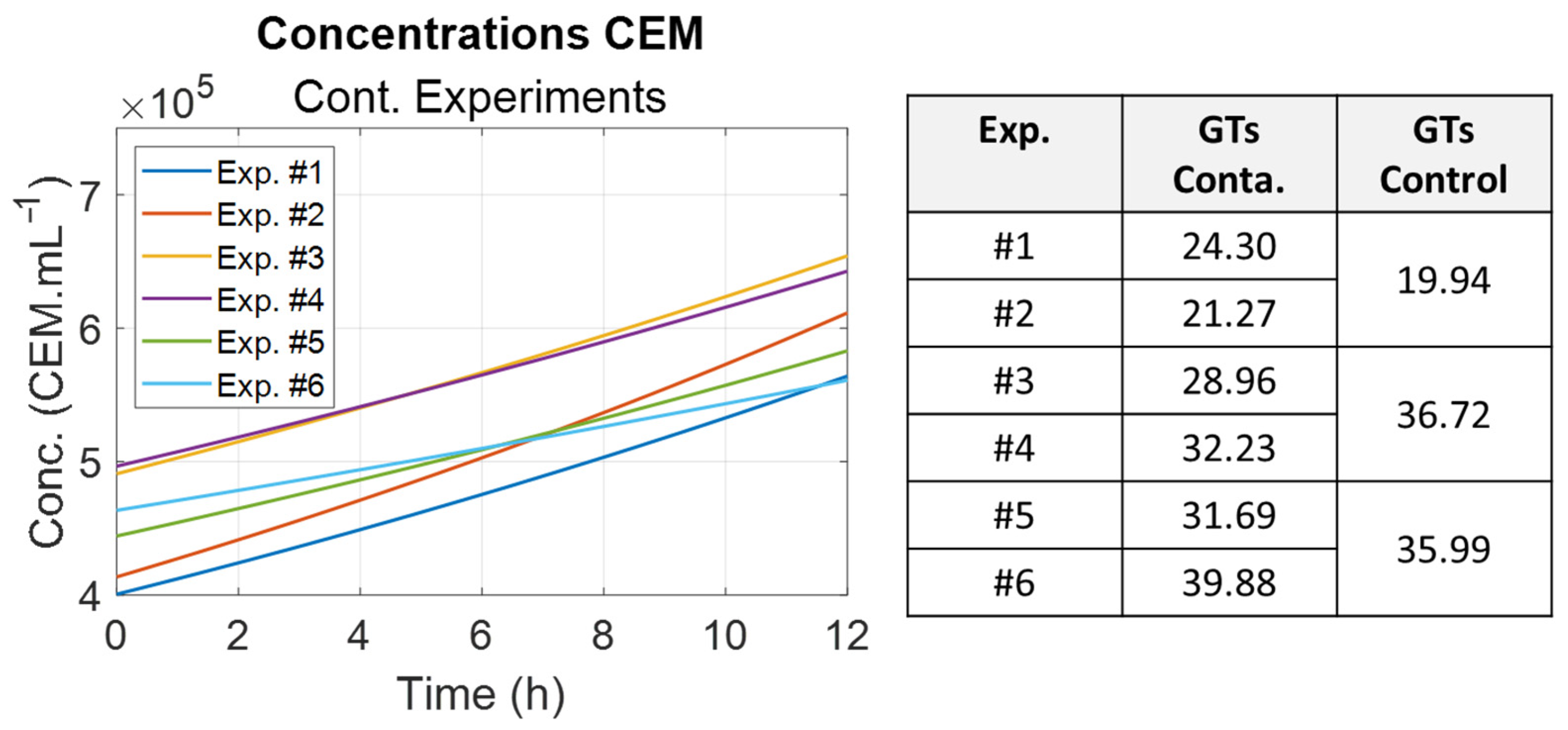
It was noted that the
E. coli generation times were different and increased between experimental sessions. They were inversely proportional to the optical density measured for the bacteria preculture used for contamination (
Figure 11). Indeed, it is commonly observed that a bacteria’s ability to grow is directly related to the preculture optical density [
29].
However, the warning threshold was reached even with very long generation times. This efficiency is due to the chosen threshold value of 0.26, which is just above the warning value for pure CEM, and to the contamination concentration of 106 E. coli mL−1. When the contamination was at 102 E. coli mL−1, the warning threshold was never reached, and no realistic E. coli concentration could be measured using Equation (8). It was due to an E. coli concentration below the inoculum threshold in this non-optimum culture medium. Therefore, the method described here cannot detect contamination at low concentrations of contaminants. Nevertheless, the method remains valid in the context of CAR-T cell production mentioned above because it only addresses the expansion phase of the fabrication process for which automated, sampling-less controls are desired. The main points are listed below.
Contamination may occur during the expansion phase.
The development of contamination depends on the culture medium used for cell culture, which may be more or less favorable to the contaminating microorganism.
Our goal is to detect this contamination without sampling to provide a rapid response and to stop production as early as possible.
We can only detect contamination if it has developed enough to be detected by our method. In our experiments, inoculation at 102 bact.mL−1 was insufficient for contamination to develop in the CEM-C1 culture medium. An inoculation of 106 bact.mL−1 was sufficient for the contamination to develop and for us to prove the concept of our method.
Inoculation at concentrations too low for contamination to develop cannot be detected by the closed system and real-time technique we propose, but contamination does exist. In any case, detecting contamination is mandatory and must be performed at the end of ATMP production using any available sensitive method. At this stage of production, the need for rapid detection without sampling is no longer an issue. This ultimate control is not the subject of our study.
Another aspect concerns the generation of small particles, such as apoptotic and necrotic bodies and vesicles, when cells grow normally (see
Section 3.3). The CEM model was originally established using cells after centrifugation and resuspension in PBS [
24]. Consequently, there were no small particles in the spectra shown in
Figure 3. The contamination experiments presented in this paper were performed directly in the culture medium without centrifugation. Consequently, small particles were not removed before recording the spectra. Although small particles are produced in limited quantities, they slightly modify the shape of the cells’ absorption spectra, though not as much as bacterial contamination. This will slightly increase the warning values and, consequently, the warning threshold. Contamination will still be detectable, but it will take longer to reach the threshold and trigger an alarm, and the minimum detectable bacteria concentration will increase. For practical applications, the warning values and threshold must be determined.
4.5. Origin of the Contaminated Spectra Shape Evolution and General Use of the Warning Function
From an optical perspective, the shape of an absorption spectrum depends on the intrinsic absorption and the size of the particles interacting with light. For large particles, such as CEM, light is primarily absorbed by the intracellular constituents and propagates in a straightforward manner. For CEM, this results in a Gaussian-like absorption spectrum, which is described by Equation (9) (
Figure 1a). For small particles (bacteria, for example), light does not propagate in a straight line but rather undergoes diffusion. For particles of bacteria size, diffusion evolves according to 1/λ, producing characteristic absorption spectra described by Equation (10) [
26]. During contamination, the absorption spectra evolve from a Gaussian-like shape to a shape deformed by the 1/λ diffusion efficiency (
Figure 8a). We calculated these equations describing the shapes of both species using dilution ranges of pure particles, i.e., CEM resuspended in PBS and bacteria in their culture medium.
The mixture function (Equations (7) and (8)) enables the determination of the concentrations of different suspended species. Optically, this means that the mixture function enables the simultaneous calculation of the concentrations of large and small particles. As CEM cells grow, they produce new cells and small particles (e.g., vesicles, debris), and some cells die, producing other types of small particles (e.g., necrotic or apoptotic bodies). Small particles are produced more abundantly when CEM multiplication is suboptimal, such as in the case of contamination. Since the mixture function only accounts for large and small particles, the measured CEM concentration is correct, but the measured bacterial concentration is overestimated due to the presence of small particles produced by the CEM. This is why the
E. coli concentration was overestimated in
Section 3.3. Since the initial CEM and
E. coli concentrations were similar for all experiments, the overestimation remained consistent across all experiments, as summarized in
Table 9.
When used to fit absorption spectra, the warning function (Equation (5)) produces an R
2 value, which is a measure of the Gaussian-like aspect of the absorption spectra. It is a general Gaussian-like marker, regardless of the reasons spectra deviate from a Gaussian shape. This warning function was used to correlate the Gaussian aspect of the absorption spectra of dying cells to cell viability, although in a slightly different manner. In fact, cells produce small particles when they die [
30]. Indeed, this Gaussian-like marker is a more general indicator of abnormal cell culture, whether due to contamination or abnormal cell mortality.
Regarding CAR-T cell production, the warning function and the corresponding R2 value serve as a “quality” marker that triggers an alarm to stop production as early as possible when the expansion phase is abnormal.
4.6. Extension of the Method to Other Bacteria or Contaminants
This method can be applied to other types of contaminants. The only restriction is that the absorption spectra of the contaminants must differ significantly from that of CEM in order to compute a high warning value, which is often related to the contaminants’ size.
ESKAPEE bacteria range in size from approximately 0.5 to 2 µm. Diffusion is predominant for them, and the method can easily be applied, as shown in
Figure 6. The position of ESKAPEE bacteria in the PCA representation has been reported previously [
26]. They are all situated in the same area as
E. coli, i.e., far from the threshold limit in the PCA representation.
Yeasts are similar in size to CEM. Their length ranges from 2–3 µm to 20–50 µm, and their diameter ranges from 1 to 10 µm [
31]. Only two yeasts were tested. Several absorption spectra of
Candida albicans (5–6 µm) and
Saccharomyces cerevisiae (5.5–7.5 µm) were recorded and included in the PCA representation (
Figure 18).
Although they are different, their spectra are similar to those of CEM (
Figure 18a). However, the shape of their spectra above 600 nm suggests that these yeasts diffuse light slightly. These similarities with CEM spectra are clearly visible in the PCA representation (
Figure 18b). Nevertheless, at certain concentrations, they exceed the threshold frontier (1.1 × 10
6 cells mL
−1 for
C. albicans and 0.4 × 10
6 cells mL
−1 for
S. cerevisiae). This demonstrates that this type of contamination can be detected, albeit not as easily as bacterial contamination. However, the wide disparity in yeast sizes makes it difficult to generalize the ability to detect contamination in all yeasts.
Fungal contamination is probably much more difficult to detect. Most microscopic fungi are 2 to 10 µm in diameter and millimeters in length, though some, like
Fungal hyphae, are only 5 to 50 µm in length [
32]. Without performing absorption spectra measurements, it is difficult to estimate the detectability of contamination by such microorganisms.
In conclusion, specific experiments must be conducted for each type of non-bacterial contaminant. Contamination can only be detected if the absorption spectra of the contaminants differ sufficiently from those of mammalian cells to exceed the alert threshold.
4.7. Integration Possibilities and First Real-Time Experiment
The integration possibilities of this method and other proofs of concept on different subjects have been discussed extensively [
22,
23,
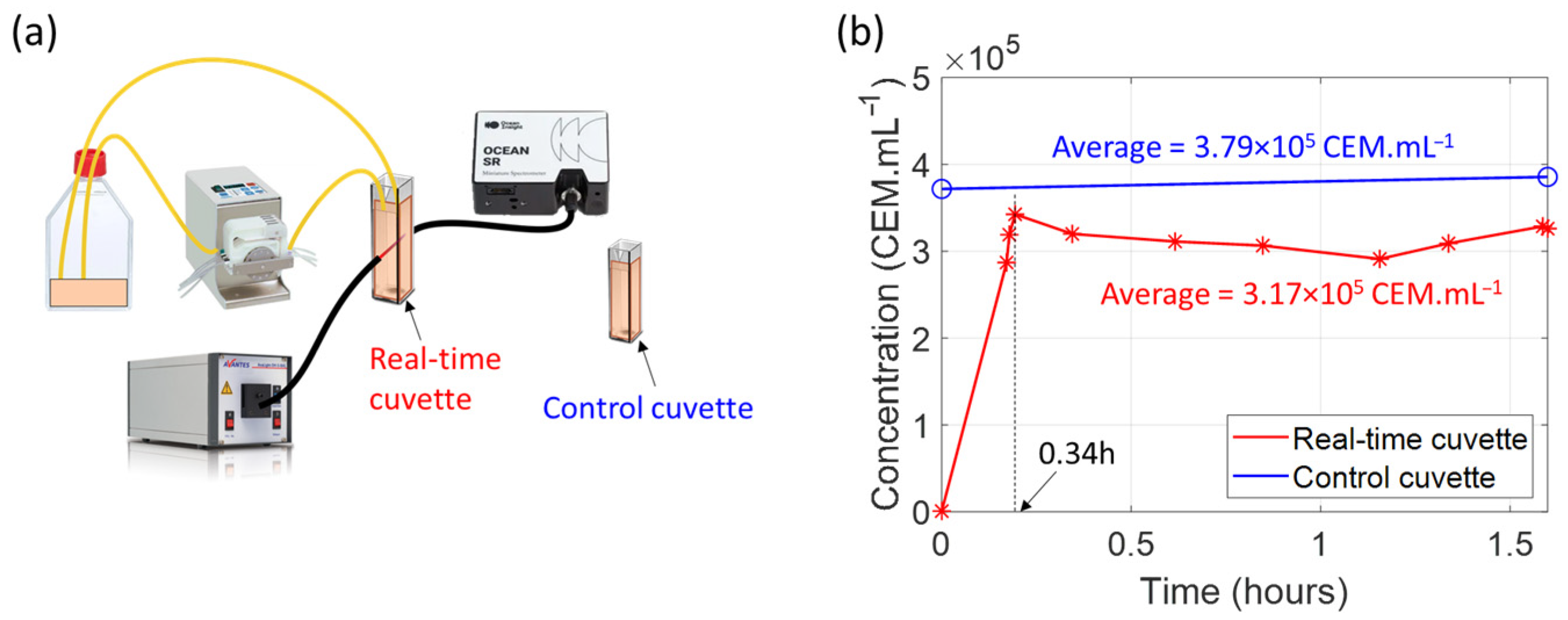
27]. For this study, we began integrating a real-time, closed-loop system based on a previously published schematic setup (
Figure 19a, [
23]). In a recent preliminary experiment, we replaced the bioreactor with a culture flask containing a CEM suspension at a concentration of 3.8 × 10
5 CEM.mL
−1 and an additional control cuvette containing the same concentration of CEM. Fluid was driven using a peristaltic pump. Spectroscopically measured concentrations were recorded regularly during the 1.5 h experiment at room temperature. At the beginning of the experiment, the spectroscopy cuvette was filled with culture medium only for the reference measurement (
Figure 19b).
As mentioned above, at t = 0, the spectroscopy cuvette is filled only with culture medium, and the measured concentration is 0.
After turning on the peristaltic pump, the medium in the spectroscopy cuvette is progressively replaced by the cell suspension. The measured concentration stabilizes after 0.34 h. The concentration in the spectroscopy cuvette remained relatively constant throughout the experiment, demonstrating the safety of using a peristaltic pump for cells.
The concentration in the spectroscopy cuvette is lower than the concentration in the control cuvette. This is due to the slight dilution of the culture flask’s contents when the spectroscopy cuvette’s culture medium is mixed in. The culture flask originally contained 20 mL of cell suspension, and the spectroscopy cuvette was filled with 3 mL of culture medium. The initial concentration of 3.8 × 105 CEM.mL−1 decreases by a factor of 1.19 during the experiment, while the total volume increases from 20 mL to 23 mL, representing an increase of 1.15, which is consistent with the change in concentration.
Although contamination has not yet been tested, these preliminary results demonstrate the feasibility of a real-time, sampling-less monitoring device based on white light spectroscopy. In the future, the automated experiment shown above will enable us to conduct a large number of experiments under various culture conditions and with different contaminants. This will allow us to estimate the reliability of our white light spectroscopy method.
4.8. Position of Our Studies Compared to Others
The method we developed in recent years, based on white light spectroscopy, has allowed us to measure cell and bacterial concentrations, monitor growth, and determine concentrations of both cells and bacteria in mixtures [
24,
26]. The present study highlights another application: detecting bacterial contamination in mammalian cell cultures in real time based on defining a warning value and corresponding warning threshold. Below, we present the advantages and disadvantages of this technique compared to those described in the literature.
Measurements are performed in a large volume, which leads to a high level of accuracy in determining cell and bacterial concentrations, even during growth. This is superior to other techniques that require sampling and/or working with a small volume, which could be poorly representative of the bioreactor’s contents. This large measurement volume is acceptable in a closed system because no suspension volume is sacrificed. This is not the case in conventional laboratory practice. The large volume can be obtained using a derivation directly in the bioreactor with a reflection probe.
Classical detection techniques based on DNA [
4] or ligand immobilization [
12,
13] often require additional time for experiments and/or bacterial cultivation. Our system enables the detection of cell culture contamination in nearly real time. Indeed, the experiments presented here were performed in spectroscopic cuvettes, but preliminary experiments demonstrate the feasibility of real-time detection in cell flasks.
Most techniques used for bacterial detection only reveal the presence of bacteria and do not provide information about the state of mammalian cells. Very few studies have employed simultaneous detection methods that combine Raman spectroscopy and advanced signal processing [
33]. Our system is powerful because it can measure the concentration of both species in a solution without sampling and trigger an alarm to stop cell cultivation as quickly as possible.
In recent years, bacterial detection has improved with the help of fluorescence, such as with flow cytometry [
34], or other techniques [
10]. One advantage is that our system allows for direct, label-free detection of bacteria, as opposed to indirect measurements of metabolites, ATP, and gases secreted or emitted by bacteria [
35,
36]. Moreover, this simple method, based on the old technique of white light spectroscopy, can be easily transferred and does not require complex technologies, such as microfluidics or mass spectrometry.
The methods used for bacterial detection can identify the bacteria responsible for contamination. However, this does not correspond to the goal of the present study, which is to detect contamination in mammalian cell cultures, since most of the time, the only necessary application is to stop the culture. Other methods involving immobilization on ligands (e.g., ELISA, SPR, and QCM) require prior knowledge of the bacteria [
14,
15,
16]. In contrast, our system can rapidly detect bacteria regardless of their origin. All of the bacteria tested displayed similar spectra profiles, which were completely different from those of mammalian cells [
26], and generated different spectra while growing within cell media.
Developments in the health and agri-food industries require the detection of bacteria at very low concentrations. Our experiments were performed with a relatively high bacterial concentration for inoculation, i.e., 106 bact.mL−1. Depending on the bacteria and/or cell media, this concentration can be reached within a day if there are enough bacteria present to develop. Specific experiments can determine the minimum bacterial inoculation that leads to detectable contamination. In all cases, 106 bact.mL−1 is acceptable for monitoring CAR-T cell production during the expansion phase. To our knowledge, this is the first study to allow real-time determination of both cell and bacterial concentrations. More importantly, it is the first proposal of a real-time warning method to stop CAR-T cell production as soon as contamination occurs.
More generally, CAR-T cell therapy is a groundbreaking approach to treating currently incurable diseases. While this technology shows great promise, it is difficult to predict which conditions will respond best or how many people will benefit. Currently, several CAR-T cell therapies are available, but they are costly due to the complex manufacturing and administration processes. While these therapies have demonstrated effectiveness in treating various hematologic malignancies, they also incur substantial additional expenses, particularly those associated with hospital stays and managing adverse effects, such as cytokine release syndrome (CRS). Consequently, the total cost per patient can approach or even exceed
$1 million [
37,
38].
Introducing automation into CAR-T cell production could play a key role in reducing expenses. Automation decreases reliance on specialized personnel and minimizes human error. It has the potential to streamline manufacturing, improve reproducibility, and increase access. Automation could also accelerate production timelines, enabling patients to begin therapy sooner and potentially reducing the need for interim treatments. Ultimately, these improvements could lessen the strain on healthcare systems and improve the affordability and accessibility of CAR-T cell therapies.
5. Conclusions
We presented here a white light spectroscopy method for detecting bacterial contamination in T cell cultures. It is based on the difference in absorption spectra shape between pure T cells and bacteria due to the difference in light interaction with small and large particles. We proposed a warning function to analyze the absorption spectra of potentially contaminated cultures. This enabled defining a warning value based on the degree of resemblance between the shape of the suspension absorption spectra and the Gaussian distribution. Contamination is detected when the warning value reaches a threshold.
We presented numerical proof of concept using synthetic spectra generated from actual experimental spectra of T cells and ESKAPEE group bacteria, employing the law of optical density additivity. Fitting the spectra with the warning function yielded an R2 value inversely proportional to the proposed warning value. A warning threshold of 0.26 was defined. The minimum bacterial concentrations required to trigger a contamination alarm were calculated for bacteria of the ESKAPEE group.
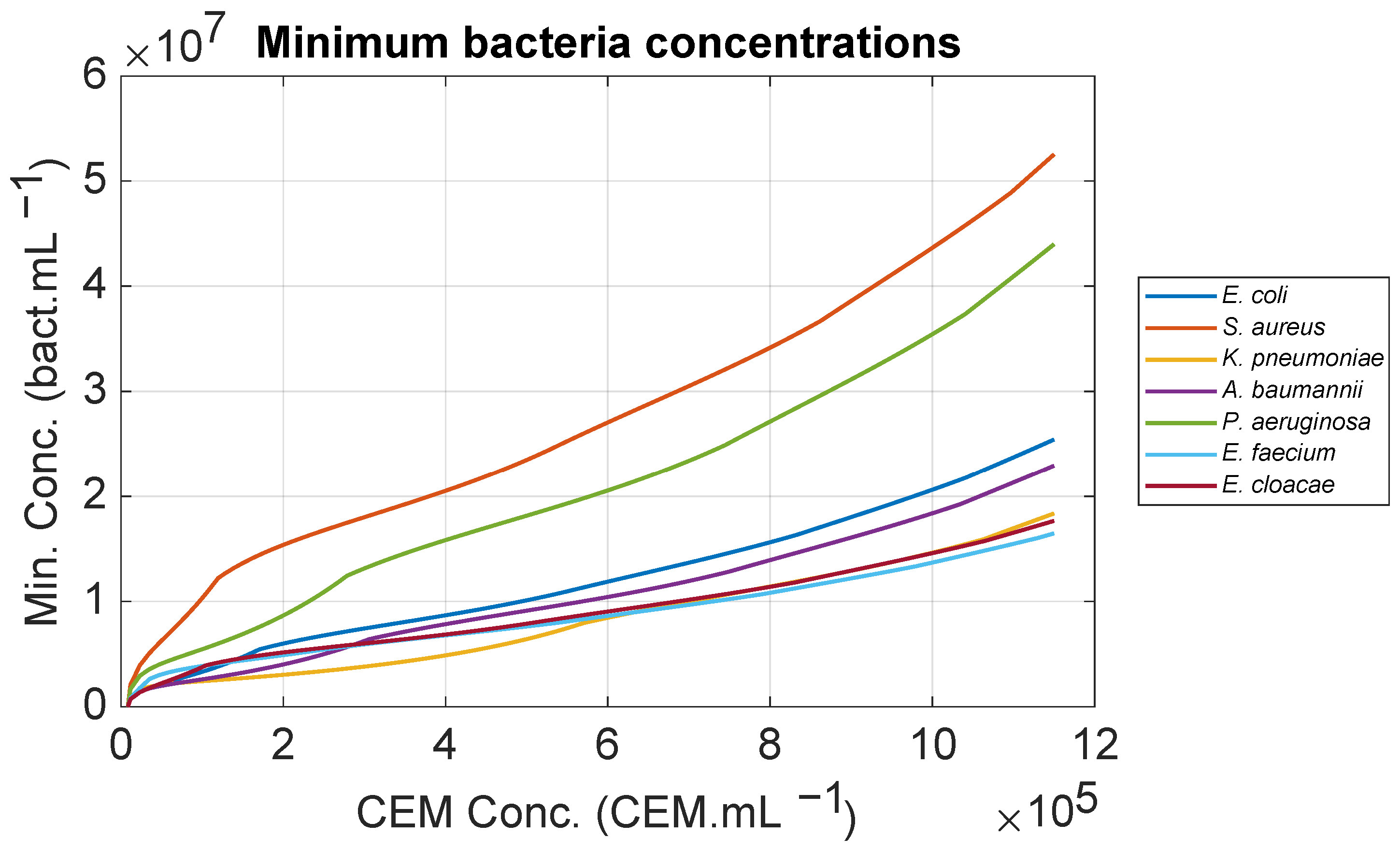
Experiments conducted with CEM-C1 cells (a T cell line) and E. coli bacteria demonstrated the efficiency of contamination detection. The warning threshold was reached between 3.55 and 5.8 h after inoculation at 106 bact.mL−1, corresponding to E. coli concentrations between 1.24 × 107 and 1.48 × 107 bact.mL−1. These values were slightly higher than the calculated theoretical values (0.94 × 107 and 1.08 × 107 bact.mL−1), due to the generation of apoptotic and necrotic bodies during T cell growth, which was not taken into account in synthetic computations. When the threshold was reached, CEM concentrations were between 4.34 × 105 and 5.08 × 105 CEM.mL−1. For CEM concentration at 5 × 105 CEM.mL−1, the theoretical minimum detectable bacteria concentration was approximately 0.9 × 107 bact.mL−1 for all ESKAPEE bacteria except for P. aeruginosa (2 × 107 bact.mL−1) and S. aureus (2.7 × 107 bact.mL−1). PCA analysis confirmed that the method is more effective than QDA-based techniques. A discussion of the presented results was proposed and included a demonstration of the first attempt at real-time, sampling-less measurements.
Using white light spectroscopy makes it possible to reduce unnecessary sampling and the time required to stop ATMP production when contamination is detected. This should lower the cost of these promising medicines and make them more accessible.