An Ultra-Robust, Highly Compressible Silk/Silver Nanowire Sponge-Based Wearable Pressure Sensor for Health Monitoring
Abstract
1. Introduction
2. Experimental Sections
2.1. Materials
2.2. Silk Sponge Fabrication
2.3. Preparation of AgNW
2.4. SWPS Fabrication and Assembly
2.5. Cytocompatibility Evaluation
2.6. On-Body Physiological Performance Evaluation
2.7. Vehicular Tire Compression Endurance Test
3. Results and Discussion
3.1. Device Design of the SWPS
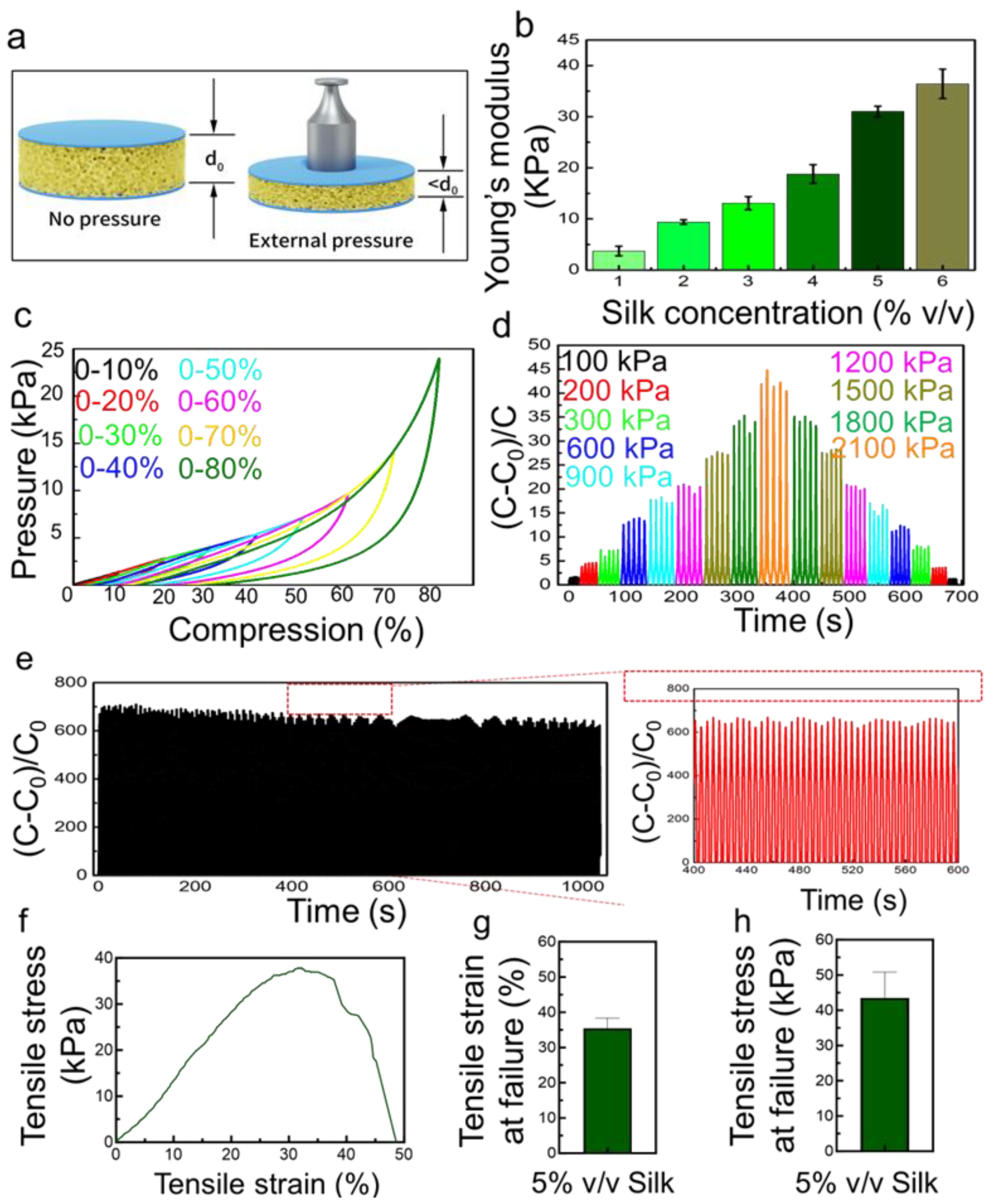
3.2. Benchtop Evaluation of the SWPS
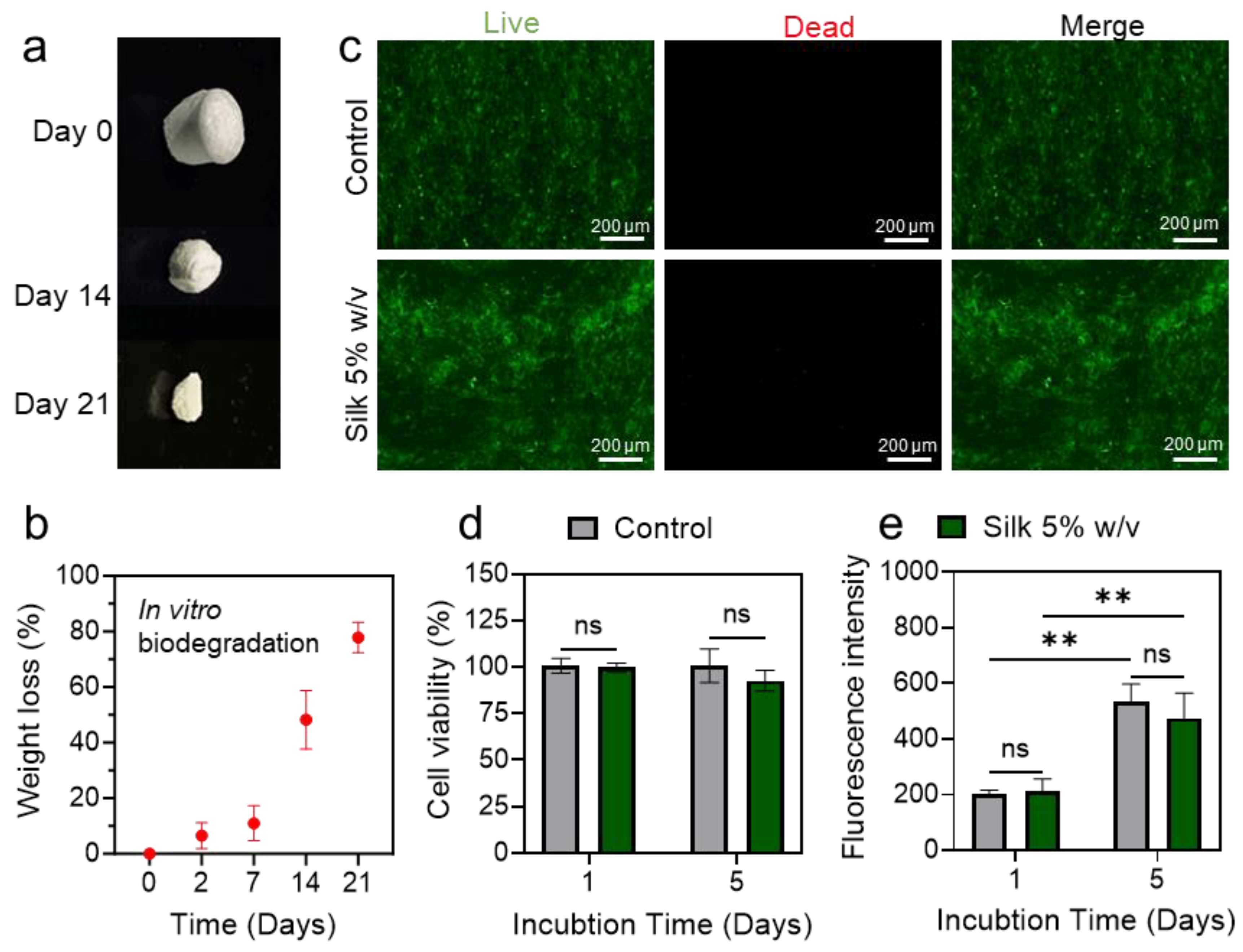
3.3. Assessment of SWPS Biodegradability and Cytocompatibility
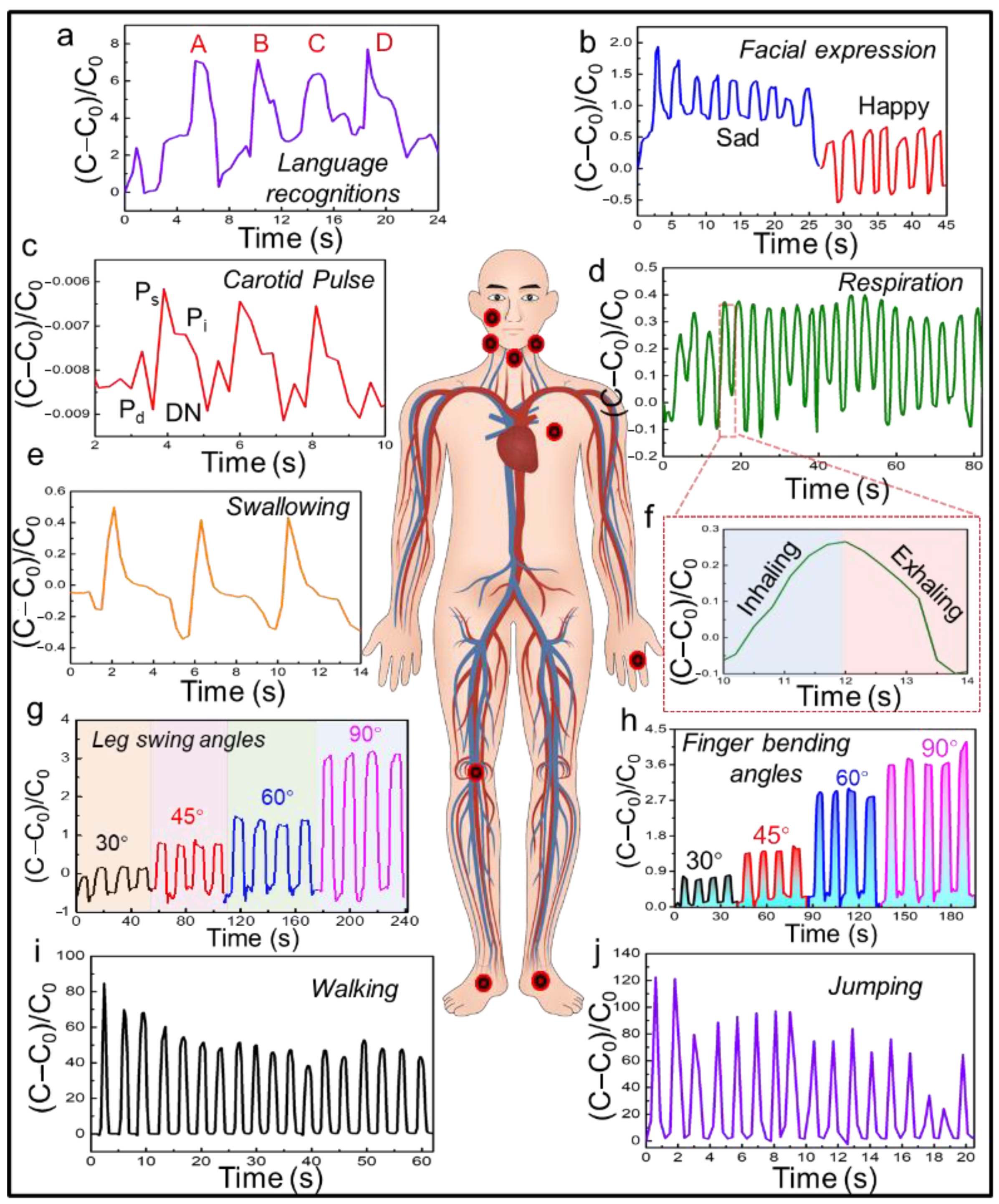
3.4. On-Body Validation of the SWPS
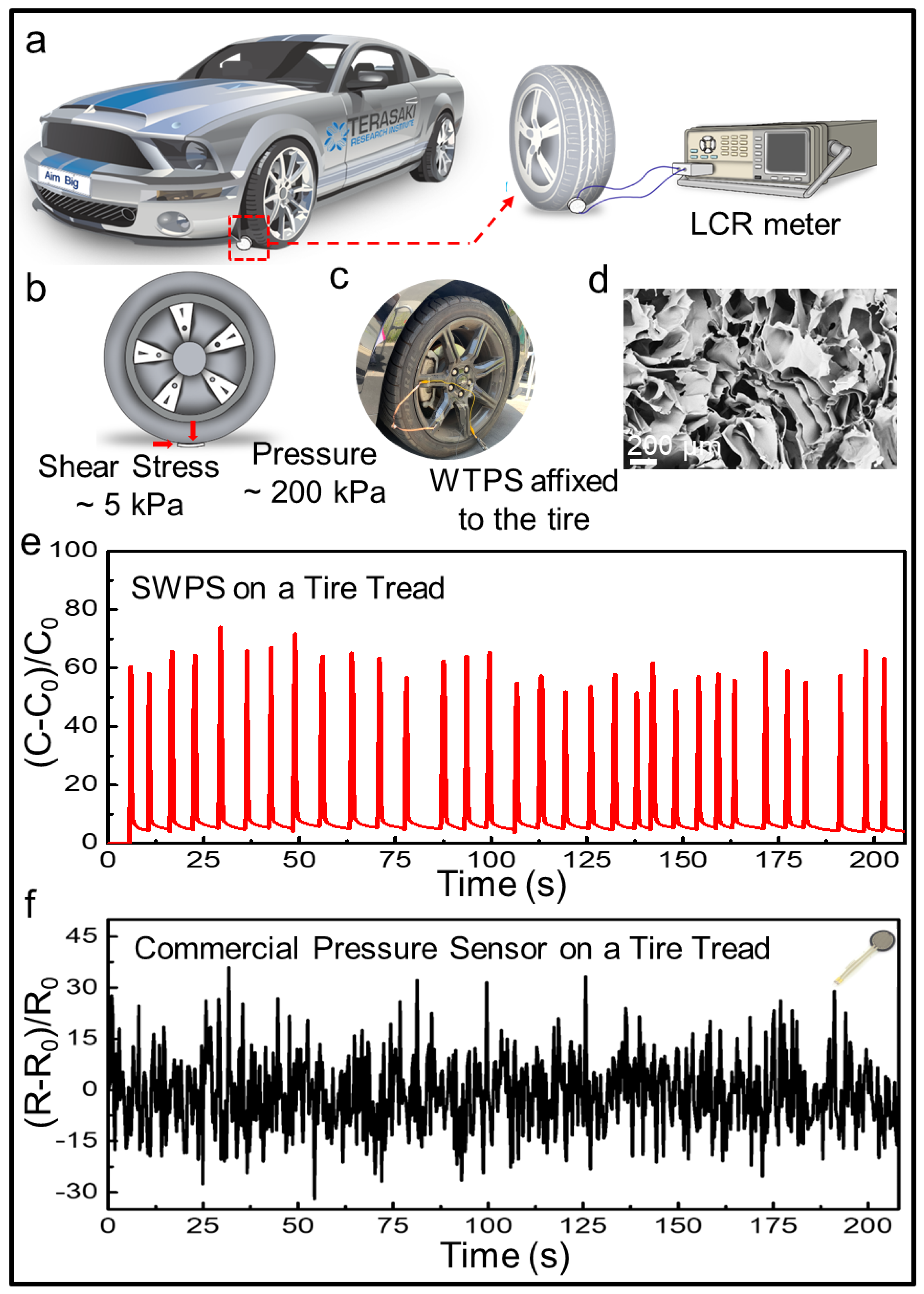
3.5. Extreme Load Endurance Testing of the SWPS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, Y.; Abidian, M.R.; Ahn, J.-H.; Akinwande, D.; Andrews, A.M.; Antonietti, M.; Bao, Z.; Berggren, M.; Berkey, C.A.; Bettinger, C.J.; et al. Technology Roadmap for Flexible Sensors. ACS Nano 2023, 17, 5211–5295. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, J.; Kim, J.; Li, S.; Zhao, Y.; Bahari, J.; Eliahoo, P.; Li, G.; Kawakita, S.; Haghniaz, R.; et al. Skin-interfaced electronics: A promising and intelligent paradigm for personalized healthcare. Biomaterials 2023, 296, 122075. [Google Scholar] [CrossRef]
- Lee, Y.; Tian, X.; Park, J.; Nam, D.H.; Wu, Z.; Choi, H.; Kim, J.; Park, D.-W.; Zhou, K.; Lee, S.W. Rapidly self-healing electronic skin for machine learning–assisted physiological and movement evaluation. Sci. Adv. 2025, 11, eads1301. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Hou, X.; Li, G.; Wang, L.; Bai, N.; Cai, M.; Zhao, L.; Wang, Y.; Zhang, J.; et al. Highly stable flexible pressure sensors with a quasi-homogeneous composition and interlinked interfaces. Nat. Commun. 2022, 13, 1317. [Google Scholar] [CrossRef]
- Han, S.; Kim, J.; Won, S.M.; Ma, Y.; Kang, D.; Xie, Z.; Lee, K.-T.; Chung, H.U.; Banks, A.; Min, S.; et al. Battery-free, wireless sensors for full-body pressure and temperature mapping. Sci. Transl. Med. 2018, 10, eaan4950. [Google Scholar] [CrossRef]
- Park, J.; Seo, B.; Jeong, Y.; Park, I. A Review of Recent Advancements in Sensor-Integrated Medical Tools. Adv. Sci. 2024, 11, 2307427. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, P.; Wu, X.; Zhao, Y.; Zhou, F.; Luo, Y.; Jia, X.; Zhong, W.; Xing, M.; Lyu, G. Negative Pressure Smart Patch to Sense and Heal the Wound. Adv. Sci. 2025, 12, e2408077. [Google Scholar] [CrossRef]
- Li, L.; Zheng, J.; Chen, J.; Luo, Z.; Su, Y.; Tang, W.; Gao, X.; Li, Y.; Cao, C.; Liu, Q.; et al. Flexible Pressure Sensors for Biomedical Applications: From Ex Vivo to In Vivo. Adv. Mater. Interfaces 2020, 7, 2000743. [Google Scholar] [CrossRef]
- Zhu, Y.; Hartel, M.C.; Yu, N.; Garrido, P.R.; Kim, S.; Lee, J.; Bandaru, P.; Guan, S.; Lin, H.; Emaminejad, S.; et al. Epidermis-Inspired Wearable Piezoresistive Pressure Sensors Using Reduced Graphene Oxide Self-Wrapped Copper Nanowire Networks. Small Methods 2022, 6, 2100900. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, B.; Ling, Y.; Fei, Q.; Chen, Z.; Li, X.; Guo, P.; Jeon, N.; Goswami, S.; Liao, Y.; et al. Multiscale porous elastomer substrates for multifunctional on-skin electronics with passive-cooling capabilities. Proc. Natl. Acad. Sci. USA 2020, 117, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, J.; Zhou, J.; Chow, L.; Zhao, G.; Huang, Y.; Ma, Z.; Zhang, Q.; Yang, Y.; Yiu, C.K.; et al. A three-dimensional liquid diode for soft, integrated permeable electronics. Nature 2024, 628, 84–92. [Google Scholar] [CrossRef]
- Vepari, C.; Kaplan, D.L. Silk as a biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef]
- Sahoo, J.K.; Hasturk, O.; Falcucci, T.; Kaplan, D.L. Silk chemistry and biomedical material designs. Nat. Rev. Chem. 2023, 7, 302–318. [Google Scholar] [CrossRef]
- Li, C.; Guo, C.; Fitzpatrick, V.; Ibrahim, A.; Zwierstra, M.J.; Hanna, P.; Lechtig, A.; Nazarian, A.; Lin, S.J.; Kaplan, D.L. Design of biodegradable, implantable devices towards clinical translation. Nat. Rev. Mater. 2020, 5, 61–81. [Google Scholar] [CrossRef]
- Mirbakht, S.S.; Golparvar, A.; Umar, M.; Kuzubasoglu, B.A.; Irani, F.S.; Yapici, M.K. Highly Self-Adhesive and Biodegradable Silk Bioelectronics for All-In-One Imperceptible Long-Term Electrophysiological Biosignals Monitoring. Adv. Sci. 2025, 12, 2405988. [Google Scholar] [CrossRef]
- Haghniaz, R.; Gangrade, A.; Montazerian, H.; Zarei, F.; Ermis, M.; Li, Z.; Du, Y.; Khosravi, S.; de Barros, N.R.; Mandal, K.; et al. An All-In-One Transient Theranostic Platform for Intelligent Management of Hemorrhage. Adv. Sci. 2023, 10, 2301406. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xia, K.; Zhang, Y.; Kaplan, D.L. Silk-Based Advanced Materials for Soft Electronics. Acc. Chem. Res. 2019, 52, 2916–2927. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Heo, D.; Kim, T.; Jung, S.; Choi, M.; Heo, J.; Kwon, J.-S.; Kim, B.-S.; Lee, W.; Koh, W.-G.; et al. Stress Dissipation Encoded Silk Fibroin Electrode for the Athlete-Beneficial Silk Bioelectronics. Adv. Sci. 2022, 9, 2105420. [Google Scholar] [CrossRef]
- Song, Y.; Hu, C.; Wang, Z.; Wang, L. Silk-based wearable devices for health monitoring and medical treatment. iScience 2024, 27, 109604. [Google Scholar] [CrossRef] [PubMed]
- John, D.A.; Parameswaran, C.; Sandhu, S.; Dahiya, R. Silk Nanofibers-Based Soft and Degradable Capacitive Pressure Sensor Arrays. IEEE Sens. Lett. 2023, 7, 2501104. [Google Scholar] [CrossRef]
- Ding, S.; Jin, X.; Guo, J.; Kou, B.; Chai, M.; Dou, S.; Jin, G.; Zhang, H.; Zhao, X.; Ma, J. A biomimetic asymmetric structured intelligent wound dressing with dual-modality humidity-pressure sensing for non-invasive and real-time wound healing monitoring. Adv. Fiber Mater. 2025, 7, 156–171. [Google Scholar] [CrossRef]
- Xing, T.; He, A.; Huang, Z.; Luo, Y.; Zhang, Y.; Wang, M.; Shi, Z.; Ke, G.; Bai, J.; Zhao, S. Silk-based flexible electronics and smart wearable Textiles: Progress and beyond. Chem. Eng. J. 2023, 474, 145534. [Google Scholar] [CrossRef]
- Xing, C.; Luo, M.; Sheng, Q.; Zhu, Z.; Yu, D.; Huang, J.; He, D.; Zhang, M.; Fan, W.; Chen, D. Silk Fabric Functionalized by Nanosilver Enabling the Wearable Sensing for Biomechanics and Biomolecules. ACS Appl. Mater. Interfaces 2024, 16, 51669–51678. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Li, Q.; Cao, P.; Wei, C.; Li, Y.; Wang, Z.; Wang, L. Designing Silk Biomaterials toward Better Future Healthcare: The Development and Application of Silk-Based Implantable Electronic Devices in Clinical Diagnosis and Therapy. Adv. Mater. 2025, 37, 2411946. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.; Li, R.; Jeong, H.; Li, S.; Wu, C.; Tzavelis, A.; Yoo, S.; Kwak, S.S.; Huang, Y.; Rogers, J.A. Bitter Flavored, Soft Composites for Wearables Designed to Reduce Risks of Choking in Infants. Adv. Mater. 2021, 33, 2103857. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yoo, S.; Liu, C.; Kwak, S.S.; Walter, J.R.; Xu, S.; Rogers, J.A. Skin-interfaced wireless biosensors for perinatal and paediatric health. Nat. Rev. Bioeng. 2023, 1, 631–647. [Google Scholar] [CrossRef]
- Liu, C.; Kim, J.-T.; Yang, D.S.; Cho, D.; Yoo, S.; Madhvapathy, S.R.; Jeong, H.; Yang, T.; Luan, H.; Avila, R.; et al. Multifunctional Materials Strategies for Enhanced Safety of Wireless, Skin-Interfaced Bioelectronic Devices. Adv. Funct. Mater. 2023, 33, 2302256. [Google Scholar] [CrossRef]
- Wray, L.S.; Hu, X.; Gallego, J.; Georgakoudi, I.; Omenetto, F.G.; Schmidt, D.; Kaplan, D.L. Effect of processing on silk-based biomaterials: Reproducibility and biocompatibility. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 99, 89–101. [Google Scholar] [CrossRef]
- Wen, D.-L.; Sun, D.-H.; Huang, P.; Huang, W.; Su, M.; Wang, Y.; Han, M.-D.; Kim, B.; Brugger, J.; Zhang, H.-X.; et al. Recent progress in silk fibroin-based flexible electronics. Microsyst. Nanoeng. 2021, 7, 35. [Google Scholar] [CrossRef]
- Zhu, Y.; Kim, S.; Ma, X.; Byrley, P.; Yu, N.; Liu, Q.; Sun, X.; Xu, D.; Peng, S.; Hartel, M.C.; et al. Ultrathin-shell epitaxial Ag@Au core-shell nanowires for high-performance and chemically-stable electronic, optical, and mechanical devices. Nano Res. 2021, 14, 4294–4303. [Google Scholar] [CrossRef]
- Jiang, J.; Song, X.; Qi, Y.; Tao, X.; Zheng, Z.; Huang, Q. Skin-inspired, permeable, structure-gradient fiber mats for pressure sensing in rehabilitation assistance. Adv. Fiber Mater. 2025, 7, 894–907. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, C.; Huang, Y.; Liang, H.; Guan, B.-O. Silk fibroin diaphragm-based fiber-tip Fabry-Perot pressure sensor. Opt. Express 2016, 24, 19600–19606. [Google Scholar] [CrossRef]
- Ge, D.; Mi, Q.; Gong, R.; Li, S.; Qin, C.; Dong, Y.; Yu, H.-Y.; Tam, K.C. Mass-Producible 3D Hair Structure-Editable Silk-Based Electronic Skin for Multiscenario Signal Monitoring and Emergency Alarming System. Adv. Funct. Mater. 2023, 33, 2305328. [Google Scholar] [CrossRef]
- Wang, S.; Zong, Q.; Yang, H.; Tan, C.; Huang, Q.; Liu, X.; Zhang, G.; French, P.; Ye, H. Rapid Fabrication of High-Performance Flexible Pressure Sensors Using Laser Pyrolysis Direct Writing. ACS Appl. Mater. Interfaces 2023, 15, 41055–41066. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Niu, M.; Xie, F.; Zhang, D.; Dai, L.; Cai, X. A Single-Ply and Knit-Only Textile Sensing Matrix for Mapping Body Surface Pressure. IEEE Sens. J. 2024, 24, 26334–26341. [Google Scholar] [CrossRef]


| Ref. | Material Composition | Sensitivity | Detection Range | Biocompatibility | Extreme Condition Stability |
|---|---|---|---|---|---|
| [31] | SEBS/CNT gradient fiber mat | 0.068 kPa−1 (0–53 kPa); 0.013 kPa−1 (53–660 kPa) | 0–660 kPa | N/A | Stable after 30,000 cycles, 20 washes |
| [32] | Silk fibroin diaphragm | 12.3 nm/kPa | N/A | Biocompatible | N/A |
| [33] | P-silk/RG with tunable micropillars | N/A | 0.5–200 g | N/A | Waterproof, 8000 cycles |
| [34] | AgNW electrodes on cotton | N/A | N/A | N/A | N/A |
| [35] | Single-ply knitted yarn | 0.72 kPa−1 | 0.255–35.65 kPa | N/A | Withstands folding/stretching |
| Ours | SWPS (Silk-AgNW) | 18.68 kPa−1 | 0–2.4 kPa (linear) | >90% cell viability; >80% mass loss in 21 days | Tolerates 200 kPa + 5 kPa shear |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Yu, N.; Hartel, M.C.; Haghniaz, R.; Emaminejad, S.; Zhu, Y. An Ultra-Robust, Highly Compressible Silk/Silver Nanowire Sponge-Based Wearable Pressure Sensor for Health Monitoring. Biosensors 2025, 15, 498. https://doi.org/10.3390/bios15080498
Li Z, Yu N, Hartel MC, Haghniaz R, Emaminejad S, Zhu Y. An Ultra-Robust, Highly Compressible Silk/Silver Nanowire Sponge-Based Wearable Pressure Sensor for Health Monitoring. Biosensors. 2025; 15(8):498. https://doi.org/10.3390/bios15080498
Chicago/Turabian StyleLi, Zijie, Ning Yu, Martin C. Hartel, Reihaneh Haghniaz, Sam Emaminejad, and Yangzhi Zhu. 2025. "An Ultra-Robust, Highly Compressible Silk/Silver Nanowire Sponge-Based Wearable Pressure Sensor for Health Monitoring" Biosensors 15, no. 8: 498. https://doi.org/10.3390/bios15080498
APA StyleLi, Z., Yu, N., Hartel, M. C., Haghniaz, R., Emaminejad, S., & Zhu, Y. (2025). An Ultra-Robust, Highly Compressible Silk/Silver Nanowire Sponge-Based Wearable Pressure Sensor for Health Monitoring. Biosensors, 15(8), 498. https://doi.org/10.3390/bios15080498





