Photothermal and Photodynamic Strategies for Diagnosis and Therapy of Alzheimer’s Disease by Modulating Amyloid-β Aggregation
Abstract
1. Introduction
2. PTT Methods
2.1. Polymeric and Self-Assembled Nanoparticles
2.2. Carbon-Based Materials
2.3. Metal-Based Materials
2.3.1. Metal Nanomaterials
2.3.2. Metallic Oxides and Sulfides
2.4. Others
3. PDT Methods
3.1. Small Organic Molecules
| Photosensitizers | Ex. (nm) | Application | Ref. |
|---|---|---|---|
| ThT | 442 | Degrading Aβ40 fibrils under light | [95] |
| ThT derivative | 500 | Targeting Aβ42 aggregates to reduce PC12 cytotoxicity | [96] |
| ThT derivative | LED light | Photooxidation of Tyr10, His13, His14, and Met35 in Aβ42 | [97] |
| ThT derivative | 450 | Degrading Aβ42 aggregates | [99] |
| Fullerene | 365 | Inhibiting Aβ42-mediated PC12 cytotoxicity | [100] |
| Riboflavin T | White | Aβ42-mediated PC12 cytotoxicity by oxidation of Tyr10, His13, His14, and Met35 | [101] |
| Methylene blue | 630 | Decomposition of Aβ42 aggregates to reduce drosophila cytotoxicity | [102] |
| Rose bengal | 525 | Inhibiting Aβ42-mediated PC12 cytotoxicity | [103] |
| 1,2,4-Oxadiazole | 260 | Inhibiting Aβ40-mediated LAN-5 cytotoxicity | [105] |
| Porphyrin derivative | 365 | Inhibiting Aβ42-mediated PC12 cytotoxicity | [106] |
| Porphyrin derivative | 450 | Inhibiting neurodegenerative manifestations in AD Drosophila | [108] |
| Chlorin e6 | Visible light | Inhibiting Aβ40-mediated PC12 cytotoxicity | [107] |
| Quinoline derivatives | 614~830 | Degrading Aβ plaques in AD mouse brain | [109] |
| Donor-π-Acceptor | 561 | Photooxygenation of Aβ40 aggregates in PC12 cells | [110] |
| Donor-π-Acceptor | 635 | Photooxygenation of Aβ40 aggregates in PC12 cells | [111] |
| Donor-π-Acceptor | 520 | Photooxygenation of Aβ42 aggregates in SH-SY5Y cells | [112] |
| CRANAD | 780 | Degrading Aβ aggregation in an AD mouse model | [113] |
| Azobenzene-boron | 595 | Degrading brain Aβ42 in AD mice | [115] |
| Leuco ethyl violet | 595 | Oxygenating Aβ in vivo | [116] |
| Anthraquinone series | 467 | Controlling the pathological factors of AD | [117] |
3.2. Metal Complexes
3.3. Nanomaterials
3.3.1. Nanodots
3.3.2. Two-Dimensional Nanomaterials
3.4. UCNPs
4. AIE-Based Strategies for Imaging and Phototherapy of AD
| AIE Probe | Ex. (nm) | Application | Ref. |
|---|---|---|---|
| Quinoline derivative | 570 | Imaging Aβ plaques in AD mouse brain | [152] |
| PD-BZ-OH/PD-NA-OH | 486 | 3D mapping Aβ plaques in Tg mouse brain | [154] |
| PD-NA-TEG | 365 | Imaging Aβ plaques in mouse brain | [155] |
| Cou-AIE-TPP+ | 604 | Imaging Aβ-induced neuronal cell mitochondria | [156] |
| AIE-CNPy-AD | 455 | Tracing Aβ deposits in AD model mice | [157] |
| TPE peptide | 370 | Monitoring of Aβ fibrillation | [162] |
| AIE glyconanoparticle | 374 | Detecting Aβ fibrils | [163] |
| G7-TBA | 450 | Monitoring Aβ aggregation and inhibiting its cytotoxicity | [164] |
| QM-FN-SO3 | 500 | Mapping Aβ plaques in AD mouse brain | [165] |
| QM-FN-SO3 | 500 | Mapping Aβ plaques in AD brain tissues and living mice | [166] |
| ROF2 | 442 | Detecting Aβ aggregates of different sequences | [167] |
| DNTPH | 488 | Imaging/reducing Aβ plaques in AD mouse brain | [168] |
| Ang-AIE NCs | 808 | Imaging Aβ plaques in AD mouse brain and inhibiting its cytotoxicity | [170] |
| T-LD NPs | 600 | Imaging and scavenging Aβ plaques | [171] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- 2024 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2024, 20, 3708–3821. [CrossRef]
- Thawabteh, A.M.; Ghanem, A.W.; AbuMadi, S.; Thaher, D.; Jaghama, W.; Karaman, D.; Karaman, R. Recent advances in therapeutics for the treatment of Alzheimer’s disease. Molecules 2024, 29, 5131. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Mucke, L. Alzheimer mechanisms and therapeutic strategies. Cell Adhes. Commun. 2012, 148, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.A.; Poirier, M.A. Protein aggregation and neurodegenerative disease. Nat. Med. 2004, 10, S10–S17. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Xu, H.; Liu, L.; Ohulchanskyy, T.Y.; Qu, J. Optical imaging of beta-amyloid plaques in Alzheimer’s disease. Biosensors 2021, 11, 255. [Google Scholar] [CrossRef]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef]
- Barage, S.H.; Sonawane, K.D. Amyloid cascade hypothesis: Pathogenesis and therapeutic strategies in Alzheimer’s disease. Neuropeptides 2015, 52, 1–18. [Google Scholar] [CrossRef]
- Selkoe, D.J. Normal and abnormal biology of the β-amyloid precursor protein. Annu. Rev. Neurosci. 1994, 17, 489–517. [Google Scholar] [CrossRef]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Dzyuba, S.V. BODIPY dyesas probes and sensors to study amyloid-related processes. Biosensors 2020, 10, 192. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; Li, Z.-J.; Tang, Y.-H.; Xu, L.; Zhang, D.-T.; Qin, T.-Y.; Wang, Y.-L. Recent research progress in fluorescent probes for detection of amyloid-β in Vivo. Biosensors 2023, 13, 990. [Google Scholar] [CrossRef]
- Paranjape, G.S.; Terrill, S.E.; Gouwens, L.K.; Ruck, B.M.; Nichols, M.R. Amyloid-β(1–42) protofibrils formed in modified artificial cerebrospinal fluid bind and activate microglia. J. Neuroimmune Pharmacol. 2013, 8, 312–322. [Google Scholar] [CrossRef]
- Du, Z.; Li, M.; Ren, J.; Qu, X. Current strategies for modulating Aβ aggregation with multifunctional agents. Acc. Chem. Res. 2021, 54, 2172–2184. [Google Scholar] [CrossRef]
- Preethy, H.A.; Rajendran, K.; Sukumar, A.J.; Krishnan, U.M. Emerging paradigms in Alzheimer’s therapy. Eur. J. Pharmacol. 2024, 981, 176872. [Google Scholar] [CrossRef]
- Self, W.K.; Holtzman, D.M. Emerging diagnostics and therapeutics for Alzheimer disease. Nat. Med. 2023, 29, 2187–2199. [Google Scholar] [CrossRef]
- Yuan, X.; Zhou, J.-L.; Yuan, L.; Fan, J.; Yoon, J.; Zhang, X.-B.; Peng, X.; Tan, W. Phototherapy: Progress, challenges, and opportunities. Sci. China Chem. 2025, 68, 826–865. [Google Scholar] [CrossRef]
- Zhang, Y.; Qian, C.; Chen, Y.; He, W.; Guo, Z. Phototherapy via modulation of β-amyloidin combating Alzheimer’s disease. Aggregate 2025, 6, e70020. [Google Scholar] [CrossRef]
- Li, C.L.; Wang, J.; Liu, L. Alzheimer’s therapeutic strategy: Photoactive platforms for suppressing the aggregation of amyloid β protein. Front. Chem. 2020, 8, 509. [Google Scholar] [CrossRef] [PubMed]
- Su, C.-T.; Wu, J.-H. Photobiomodulation in biological tissues: Light penetration, dosimetry, and potential applications. Opt. Lasers Eng. 2025, 186, 108852. [Google Scholar] [CrossRef]
- Lv, G.; Shen, Y.; Zheng, W.; Yang, J.; Li, C.; Lin, J. Fluorescence detection and dissociation of amyloid-β species for the treatment of Alzheimer’s disease. Adv. Ther. 2019, 2, 1900054. [Google Scholar] [CrossRef]
- Chau, J.H.C.; Lee, M.M.S.; Yu, E.Y.; Kwok, R.T.K.; Lam, J.W.Y.; Sun, J.; Tang, B.Z. Advances in biomimetic AIE nanoparticles for diagnosis and phototherapy. Nanoscale 2024, 16, 14707–14715. [Google Scholar] [CrossRef]
- Mäger, I.; Meyer, A.H.; Li, J.; Lenter, M.; Hildebrandt, T.; Leparc, G.; Wood, M.J.A. Targeting blood-brain-barrier transcytosis–perspectives for drug delivery. Neuropharmacology 2017, 120, 4–7. [Google Scholar] [CrossRef]
- Wang, J.; Gu, Y.; Liu, X.; Fan, Y.; Zhang, Y.; Yi, C.; Cheng, C.; Yang, M. Near-infrared photothermally enhanced photo-oxygenation for inhibition of amyloid-aggregation based on RVG-conjugated porphyrinic metal–organic framework and indocyanine green nanoplatform. Int. J. Mol. Sci. 2022, 23, 10885. [Google Scholar] [CrossRef]
- Yin, T.; Xie, W.; Sun, J.; Yang, L.; Liu, J. Penetratin peptide-functionalized gold nanostars: Enhanced BBB permeability and NIR photothermal treatment of Alzheimer’s disease using ultralow irradiance. ACS Appl. Mater. Interfaces 2016, 8, 19291–19302. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, Y.; Liu, Q. Fluorescent sensing platforms for detecting and imaging the biomarkers of Alzheimer’s disease. Biosensors 2023, 13, 515. [Google Scholar] [CrossRef] [PubMed]
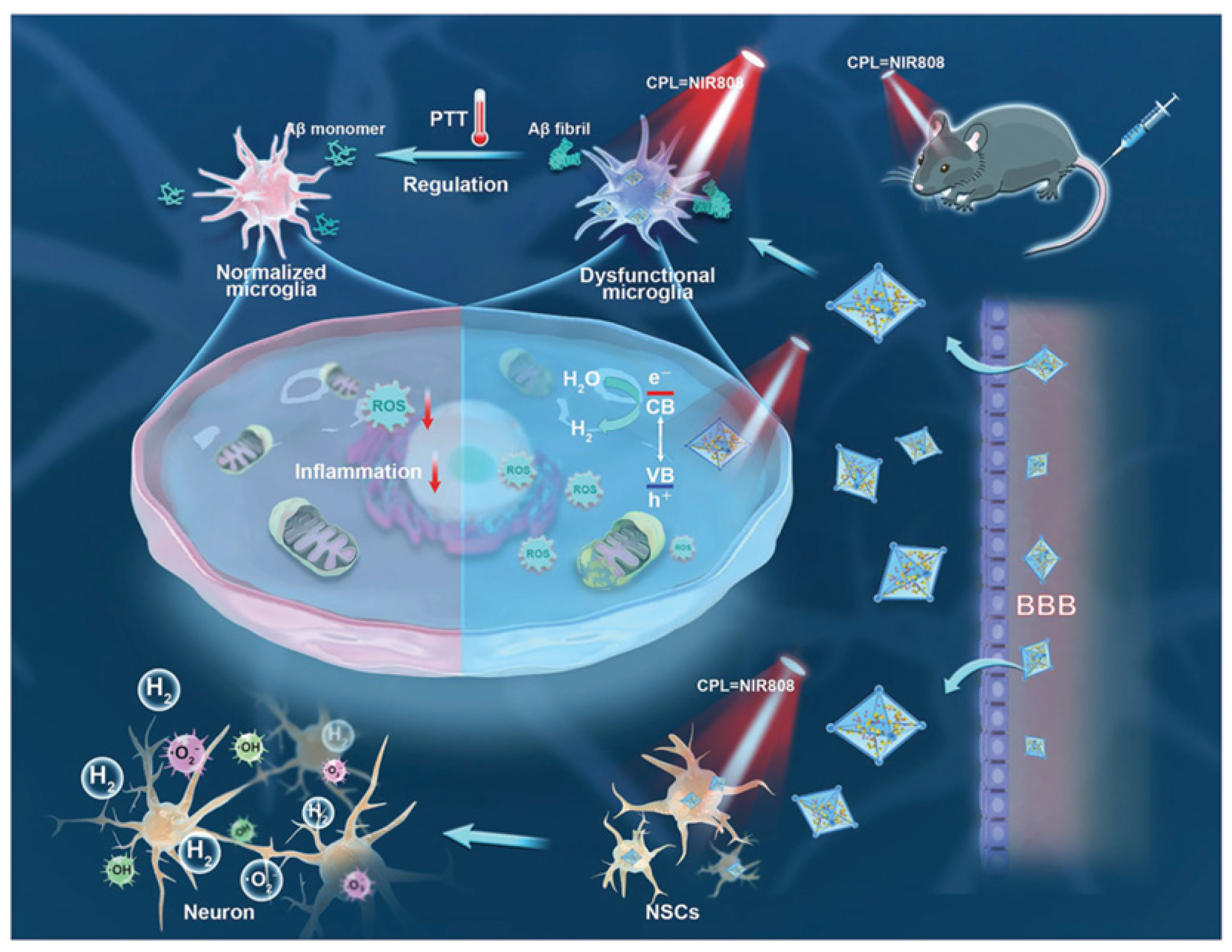
- Chen, W.; Li, J.; Guo, J.; Li, L.; Wu, H. Diagnosis and therapy of Alzheimer’s disease: Light-driven heterogeneous redox processes. Adv. Colloid Interface Sci. 2024, 332, 103253. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.; Gao, D.; Wang, Z.; Liu, X.; Cao, Z.; Xing, C. Strategies for inhibition and disaggregation of amyloid-β fibrillation. Chin. J. Chem. 2021, 40, 524–538. [Google Scholar] [CrossRef]
- Lee, B.I.; Chung, Y.J.; Park, C.B. Photosensitizing materials and platforms for light-triggered modulation of Alzheimer’s β-amyloid self-assembly. Biomaterials 2019, 190, 121–132. [Google Scholar] [CrossRef]
- Liu, W.; Dong, X.; Liu, Y.; Sun, Y. Photoresponsive materials for intensified modulation of Alzheimer’s amyloid-β protein aggregation: A review. Acta Biomater. 2021, 123, 93–109. [Google Scholar] [CrossRef]
- Xu, Y.; Xiong, H.; Zhang, B.; Lee, I.; Xie, J.; Li, M.; Zhang, H.; Seung Kim, J. Photodynamic Alzheimer’s disease therapy: From molecular catalysis to photo-nanomedicine. Coord. Chem. Rev. 2022, 470, 214726. [Google Scholar] [CrossRef]
- Zeng, F.; Peng, K.; Han, L.; Yang, J. Photothermal and photodynamic therapies via NIR-activated nanoagents in combating Alzheimer’s disease. ACS Biomater. Sci. Eng. 2021, 7, 3573–3585. [Google Scholar] [CrossRef]
- Zhou, C.; Zeng, F.; Yang, H.; Liang, Z.; Xu, G.; Li, X.; Liu, X.; Yang, J. Near-infrared II theranostic agents for the diagnosis and treatment of Alzheimer’s disease. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 2953–2969. [Google Scholar] [CrossRef]
- Gao, G.; Sun, X.; Liang, G. Nanoagent-promoted mild-temperature photothermal therapy for cancer treatment. Adv. Funct. Mater. 2021, 31, 2100738. [Google Scholar] [CrossRef]
- Pradhan, N.; Jana, N.R. Nanomodulators that target Alzheimer’s disease: A review. ACS Appl. Nano Mater. 2024, 7, 3515–3545. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, X.; Wang, X.; Guan, X.; Zhang, J.; Ma, J. Recent advances in selective photothermal therapy of tumor. J. Nanobiotechnol. 2021, 19, 335. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Min, S.; Kim, G.; Lee, S. Recent advances in the design of organic photothermal agents for cancer treatment: A review. Coord. Chem. Rev. 2024, 506, 215719. [Google Scholar] [CrossRef]
- Harmon, B.V.; Takano, Y.S.; Winterford, C.M.; Gobé, G.C. The role of apoptosis in the response of cells and tumours to mild hyperthermia. Int. J. Radiat. Biol. 1991, 59, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Kirui, D.K.; Koay, E.J.; Guo, X.; Cristini, V.; Shen, H.; Ferrar, M. Tumor vascular permeabilization using localized mild hyperthermia to improve macromolecule transport. Nanomedicine 2014, 10, 1487–1496. [Google Scholar] [CrossRef] [PubMed]
- Jr Stine, W.B.; Dahlgren, K.N.; Krafft, G.A.; LaDu, M.J. In vitro characterization of conditions for amyloid-β peptide oligomerization and fibrillogenesis. J. Biol. Chem. 2003, 278, 11612–11622. [Google Scholar] [CrossRef]
- Wang, H.; Mu, X.; Yang, J.; Liang, Y.; Zhang, X.-D.; Ming, D. Brain imaging with near-infrared fluorophores. Coord. Chem. Rev. 2019, 380, 550–571. [Google Scholar] [CrossRef]
- Manek, E.; Darvas, F.; Petroianu, G.A. Use of biodegradable, chitosan-based nanoparticles in the treatment of Alzheimer’s disease. Molecules 2020, 25, 4866. [Google Scholar] [CrossRef]
- Tripathi, P.; Shukla, P.; Bieberich, E. Theranostic applications of nanomaterials in Alzheimer’s disease: A multifunctional approach. Curr. Pharm. Des. 2022, 28, 116–132. [Google Scholar] [CrossRef]
- Thangudu, S.; Cheng, F.Y.; Su, C.H. Advancements in the blood-brain barrier penetrating nanoplatforms for brain related disease diagnostics and therapeutic applications. Polymers 2020, 12, 3055. [Google Scholar] [CrossRef]
- Ling, C.; Wang, X.; Shen, Y. Advances in hollow inorganic nanomedicines for photothermal-based therapies. Int. J. Nanomed. 2021, 16, 493–513. [Google Scholar] [CrossRef] [PubMed]
- Fei, J.; Li, Q.; Zhao, J.; Li, J. Optical properties and potential applications of diphenylalanine dipeptide-based assemblies. Prog. Chem. 2019, 31, 30–37. [Google Scholar]
- Song, B.-L.; Zhang, X.-H.; Qiao, Z.-Y.; Wang, H. Peptide-based AIEgens: From molecular design, stimuli responsiveness to biomedical application. CCS Chem. 2022, 4, 437–455. [Google Scholar] [CrossRef]
- Geng, H.; Pan, Y.C.; Zhang, R.; Gao, D.; Wang, Z.; Li, B.; Li, N.; Guo, D.S.; Xing, C. Binding to amyloid-β protein by photothermal blood-brain barrier-penetrating nanoparticles for inhibition and disaggregation of fibrillation. Adv. Funct. Mater. 2021, 31, 2102953. [Google Scholar] [CrossRef]
- Zhang, Z.; Lv, M.; Liu, Y.; Qin, J.; Fan, Z.; Du, J. A peptide-polyphenol coated polypyrrole nanoparticle for synergetic attenuation of aggregation and cytotoxicity of amyloid-β fibrils. Adv. Funct. Mater. 2024, 34, 2401208. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, L.; Gu, Z.; Dai, L. Carbon nanomaterials for phototherapy. Nanophotonics 2022, 11, 4955–4976. [Google Scholar] [CrossRef]
- Bai, M.; Shao, X.; Wang, C.; Wang, J.; Wang, X.; Guan, P.; Hu, X. Application of carbon-based nanomaterials in Alzheimer’s disease. Mater. Horiz. 2025, 12, 673–693. [Google Scholar] [CrossRef]
- Feng, L.; Wu, L.; Qu, X. New horizons for diagnostics and therapeutic applications of graphene and graphene oxide. Adv. Mater. 2013, 25, 168–186. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, X.; Ren, J.; Qu, K.; Qu, X. Using graphene oxide high near-infrared absorbance for photothermal treatment of Alzheimer’s disease. Adv. Mater. 2012, 24, 1722–1728. [Google Scholar] [CrossRef] [PubMed]
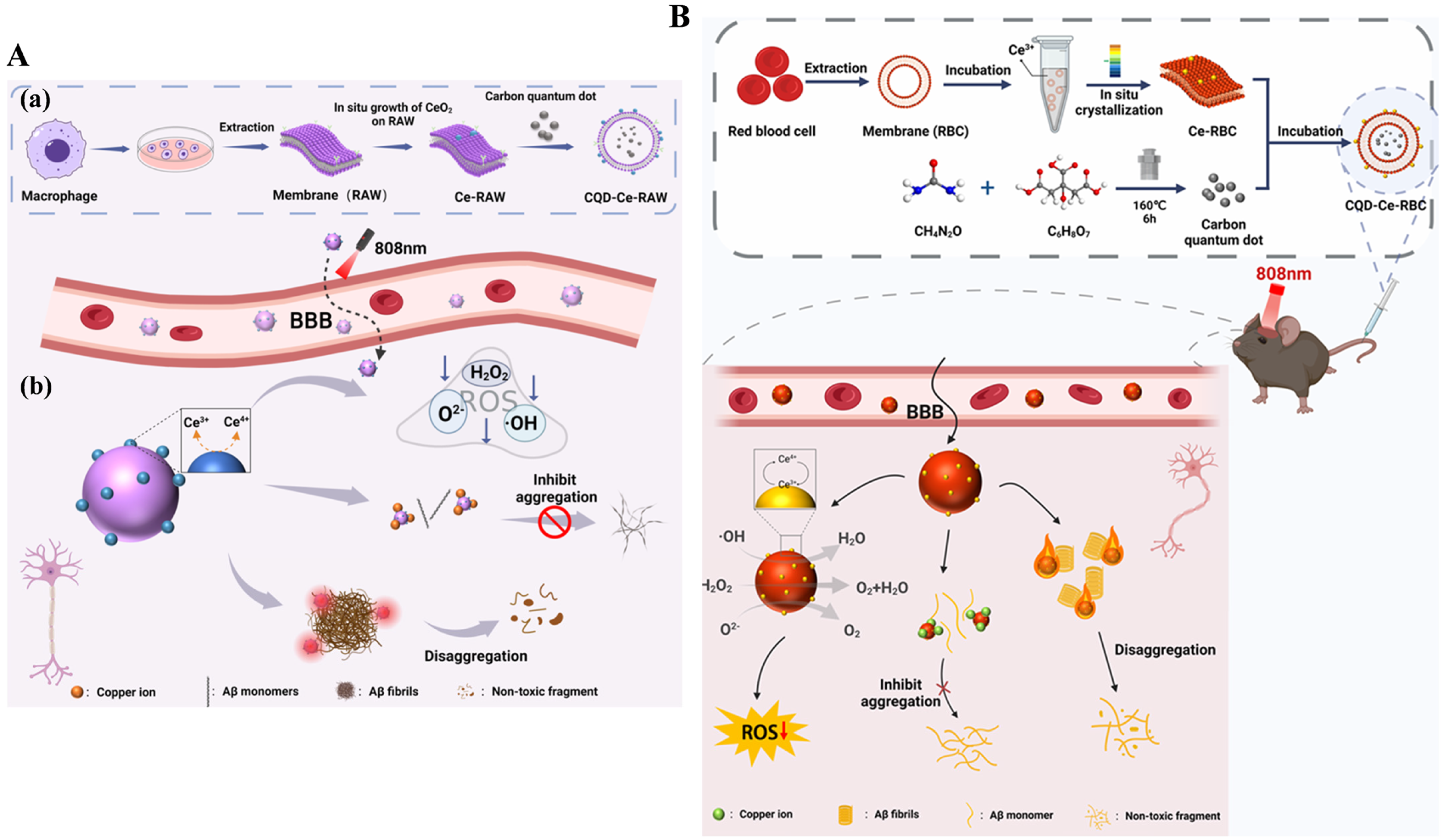
- Ye, P.; Li, L.; Qi, X.; Chi, M.; Liu, J.; Xie, M. Macrophage membrane-encapsulated nitrogen-doped carbon quantum dot nanosystem for targeted treatment of Alzheimer’s disease: Regulating metal ion homeostasis and photothermal removal of β-amyloid. J. Colloid Interface Sci. 2023, 650, 1749–1761. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chi, M.; Li, L.; Zhang, Y.; Xie, M. Erythrocyte membrane coated with nitrogen-doped quantum dots and polydopamine composite nano-system combined with photothermal treatment of Alzheimer’s disease. J. Colloid Interface Sci. 2024, 663, 856–868. [Google Scholar] [CrossRef]
- Chi, M.; Liu, J.; Li, L.; Zhang, Y.; Xie, M. In-situ growth of CeO2 on biofilms: Innovative nanoparticles for photothermal therapy & multi-pronged attack on Alzheimer’s disease. Colloids Surf. B 2024, 238, 113887. [Google Scholar]
- Chi, M.; Liu, J.; Li, L.; Zhang, Y.; Xie, M. CeO2 in situ growth on red blood cell membranes: CQD coating and multipathway Alzheimer’s disease therapy under NIR. ACS Appl. Mater. Interfaces 2024, 16, 35898–35911. [Google Scholar] [CrossRef]
- Feng, S.; Lu, J.; Wang, K.; Di, D.; Shi, Z.; Zhao, Q.; Wang, S. Advances in smart mesoporous carbon nanoplatforms for photothermal–enhanced synergistic cancer therapy. Chem. Eng. J. 2022, 435, 134886. [Google Scholar] [CrossRef]
- Xu, M.; Zhou, H.; Liu, Y.; Sun, J.; Xie, W.; Zhao, P.; Liu, J. Ultrasound-excited protoporphyrin IX-modified multifunctional nanoparticles as a strong inhibitor of Tau phosphorylation and β-amyloid aggregation. ACS Appl. Mater. Interfaces 2018, 10, 32965–32980. [Google Scholar] [CrossRef]
- Ma, M.; Gao, N.; Li, X.; Liu, Z.; Pi, Z.; Du, X.; Ren, J.; Qu, X. A biocompatible second near-infrared nanozyme for spatiotemporal and non-invasive attenuation of amyloid deposition through scalp and skull. ACS Nano 2020, 14, 9894–9903. [Google Scholar] [CrossRef]
- Li, M.; Guan, Y.; Zhao, A.; Ren, J.; Qu, X. Using multifunctional peptide conjugated Au nanorods for monitoring β-amyloid aggregation and chemo-photothermal treatment of Alzheimer’s disease. Theranostics 2017, 7, 2996–3006. [Google Scholar] [CrossRef]
- Prades, R.; Guerrero, S.; Araya, E.; Molina, C.; Salas, E.; Zurita, E.; Selva, J.; Egea, G.; Lopez-Iglesias, C.; Teixido, M.; et al. Delivery of gold nanoparticles to the brain by conjugation with a peptide that recognizes the transferrin receptor. Biomaterials 2012, 33, 7194–7205. [Google Scholar] [CrossRef]
- Ruff, J.; Hassan, N.; Morales-Zavala, F.; Steitz, J.; Araya, E.; Kogan, M.J.; Simon, U. CLPFFD-PEG functionalized NIR-absorbing hollow gold nanospheres and gold nanorods inhibit β-amyloid aggregation. J. Mater. Chem. B 2018, 6, 2432–2443. [Google Scholar] [CrossRef] [PubMed]
- Ruff, J.; Huwel, S.; Kogan, M.J.; Simon, U.; Galla, H.J. The effects of gold nanoparticles functionalized with ss-amyloid specific peptides on an in vitro model of blood-brain barrier. Nanomedicine 2017, 13, 1645–1652. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Xu, N.; Yang, X.; Ling, G.; Zhang, P. The roles of gold nanoparticles in the detection of amyloid-β peptide for Alzheimer’s disease. Colloid Interface Sci. Commun. 2022, 46, 100579. [Google Scholar] [CrossRef]
- Lin, D.; Qian, Z.; Bagnani, M.; Hernández-Rodríguez, M.A.; Corredoira-Vázquez, J.; Wei, G.; Carlos, L.D.; Mezzenga, R. Probing the protein folding energy landscape: Dissociation of amyloid-β fibrils by laser-induced plasmonic heating. ACS Nano 2023, 17, 9429–9441. [Google Scholar] [CrossRef]
- Streich, C.; Akkari, L.; Decker, C.; Bormann, J.; Rehbock, C.; Müller-Schiffmann, A.; Niemeyer, F.C.; Nagel-Steger, L.; Willbold, D.; Sacca, B.; et al. Characterizing the effect of multivalent conjugates composed of Aβ-specific ligands and metal nanoparticles on neurotoxic fibrillar aggregation. ACS Nano 2016, 10, 7582–7597. [Google Scholar] [CrossRef]
- Chuang, Y.-C.; Lee, H.-L.; Chiou, J.-F.; Lo, L.-W. Recent advances in gold nanomaterials for photothermal therapy. J. Nanotheranostics 2022, 3, 117–131. [Google Scholar] [CrossRef]
- Martins, P.A.; Alsaiari, S.; Julfakyan, K.; Nie, Z.; Khashab, N.M. Self-assembled lipoprotein based gold nanoparticles for detection and photothermal disaggregation of β-amyloid aggregates. Chem. Commun. 2017, 53, 2102–2105. [Google Scholar] [CrossRef]
- Zeng, X.; Tang, L.; Zhang, W.; Hong, X.; Xiao, Y. Shape and size effects of gold nanoparticles for tumor photoacoustic imaging and photothermal therapy within the NIR-I and NIR-II biowindows. Small 2025, 21, 2412296. [Google Scholar] [CrossRef]
- Yang, W.; Liang, H.; Ma, S.; Wang, D.; Huang, J. Gold nanoparticle based photothermal therapy: Development and application for effective cancer treatment. Sustain. Mater. Technol. 2019, 22, e00109. [Google Scholar] [CrossRef]
- Liu, D.; Li, W.; Jiang, X.; Bai, S.; Liu, J.; Liu, X.; Shi, Y.; Kuai, Z.; Kong, W.; Gao, R.; et al. Using near-infrared enhanced thermozyme and scFv dual-conjugated Au nanorods for detection and targeted photothermal treatment of Alzheimer’s disease. Theranostics 2019, 9, 2268–2281. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, W.; Dong, X.; Sun, Y. Near-infrared light-powered Janus nanomotor significantly facilitates inhibition of Amyloid-β fibrillogenesis. ACS Appl. Mater. Interfaces 2020, 12, 12618–12628. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Huang, Y.; Chen, X.; Weng, J.; Zheng, N. Optimization of surface coating on small Pd nanosheets for in vivo near-infrared photothermal therapy of tumor. ACS Appl. Mater. Interfaces 2015, 7, 14369–14375. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Luo, W.; Wu, T.; Cai, S.; Pan, Z.; Li, H.; Tu, B.; Fang, Q.; Yan, X.; Yang, R. Atomic-thick porous Pd nanosheets with antioxidant enzyme-like activities and photothermal properties for potential Alzheimer’s disease treatment. Nano Today 2024, 54, 102121. [Google Scholar] [CrossRef]
- Zhang, H.; Hao, C.; Qu, A.; Sun, M.; Xu, L.; Xu, C.; Kuang, H. Light-induced chiral iron copper selenide nanoparticles prevent β-amyloidopathy in vivo. Angew. Chem. Int. Ed. 2020, 59, 7131–7138. [Google Scholar] [CrossRef]
- Yuan, X.; Jia, Z.; Li, J.; Liu, Y.; Huang, Y.; Gong, Y.; Guo, X.; Chen, X.; Cen, J.; Liu, J. A diselenide bond-containing ROS-responsive ruthenium nanoplatform delivers nerve growth factor for Alzheimer’s disease management by repairing and promoting neuron regeneration. J. Mater. Chem. B 2021, 9, 7835–7847. [Google Scholar] [CrossRef]
- Pachaiappan, R.; Manavalan, K. Role of metals, metal oxides, and metal sulfides in the diagnosis and treatment of cancer. In Metal, Metal Oxides and Metal Sulphides for Biomedical Applications; Springer International Publishing: Cham, Switzerland, 2021; pp. 165–207. [Google Scholar]
- Tan, H.; Huang, Y.; Dong, S.; Bai, Z.; Chen, C.; Wu, X.; Chao, M.; Yan, H.; Wang, S.; Geng, D.; et al. A chiral nanocomplex for multitarget therapy to alleviate neuropathology and rescue Alzheimer’s cognitive deficits. Small 2023, 19, e2303530. [Google Scholar] [CrossRef]
- Li, M.; Zhao, A.; Dong, K.; Li, W.; Ren, J.; Qu, X. Chemically exfoliated WS2 nanosheets efficiently inhibit amyloid β-peptide aggregation and can be used for photothermal treatment of Alzheimer’s disease. Nano Res. 2015, 8, 3216–3227. [Google Scholar] [CrossRef]
- Chai, H.; Wang, X.; Liu, Z.; Zhao, Y. Study on the removal of amyloid plaque by nano-gold in the treatment of neurodegenerative disease-alzheimer’s disease. Mater. Express 2021, 11, 1038–1044. [Google Scholar] [CrossRef]
- Ma, M.; Gao, N.; Sun, Y.; Du, X.; Ren, J.; Qu, X. Redox-activated near-infrared-responsive polyoxometalates used for photothermal treatment of Alzheimer’s disease. Adv. Healthc. Mater. 2018, 7, e1800320. [Google Scholar] [CrossRef]
- Gao, N.; Liu, Z.; Qu, X. Site-directed chemical modification of amyloid by polyoxometalates for inhibition of protein misfolding and aggregation. Angew. Chem. Int. Ed. 2022, 61, 202115336. [Google Scholar] [CrossRef]
- Lu, X.-L.; Li, J.-L.; Liu, S.-J. Two-dimensional nanomaterials against β-amyloid in treatment of Alzheimer’s disease. Chin. J. Biochem. Mol. Biol. 2021, 37, 595–602. [Google Scholar]
- Yan, X.; Pan, Y.; Ji, L.; Gu, J.; Hu, Y.; Xia, Y.; Li, C.; Zhou, X.; Yang, D.; Yu, Y. Multifunctional metal-organic framework as a versatile nanoplatform for Aβ oligomer imaging and chemo-photothermal treatment in living cells. Anal. Chem. 2021, 93, 13823–13834. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Hu, Y.; Feng, X.; Wang, Z.; Song, Q.; Dai, C.; Yang, B.; Fu, X.; Sun, D.; Fan, C. Enhanced blood-brain barrier penetrability of BACE1 SiRNA-loaded prussian blue nanocomplexes for Alzheimer’s disease synergy therapy. Exploration 2025, 5, e20230178. [Google Scholar] [CrossRef]
- Li, L.; Xiong, Y.; Zhang, Y.; Yan, Y.; Zhao, R.; Yang, F.; Xie, M. Biofilm-camouflaged Prussian blue synergistic mitochondrial mass enhancement for Alzheimer’s disease based on Cu(2+) chelation and photothermal therapy. J. Control. Release 2024, 375, 269–284. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, X.; Cai, S.; Zhang, W.; Yang, R. Prussian blue nanocages as efficient radical scavengers and photothermal agents for reducing amyloid-beta induced neurotoxicity. Colloids Surf. B 2025, 246, 114369. [Google Scholar] [CrossRef]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Oluwajembola, A.M.; Cleanclay, W.D.; Onyia, A.F.; Chikere, B.N.; Zakari, S.; Ndifreke, E.; De Campos, O.C. Photosensitizers in photodynamic therapy: An advancement in cancer treatment. Results Chem. 2024, 10, 101715. [Google Scholar] [CrossRef]
- Jiang, Y.; Zeng, Z.; Yao, J.; Guan, Y.; Jia, P.; Zhao, X.; Xu, L. Treatment of Alzheimer’s disease with small-molecule photosensitizers. Chin. Chem. Lett. 2023, 34, 107966. [Google Scholar] [CrossRef]
- Nestoros, E.; Sharma, A.; Kim, E.; Kim, J.S.; Vendrell, M. Smart molecular designs and applications of activatable organic photosensitizers. Nat. Rev. Chem. 2025, 9, 46–60. [Google Scholar] [CrossRef]
- Sarabia-Vallejo, Á.; López-Alvarado, P.; Menéndez, J.C. Small-molecule theranostics in Alzheimer’s disease. Eur. J. Med. Chem. 2023, 255, 115382. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Guo, Z. Functionalization of platinum complexes for biomedical applications. Acc. Chem. Res. 2015, 48, 2622–2631. [Google Scholar] [CrossRef]
- Zamora, A.; Vigueras, G.; Rodríguez, V.; Santana, M.D.; Ruiz, J. Cyclometalated iridium(III) luminescent complexes in therapy and phototherapy. Coord. Chem. Rev. 2018, 360, 34–76. [Google Scholar] [CrossRef]
- Yagi, H.; Ozawa, D.; Sakurai, K.; Kawakami, T.; Kuyama, H.; Nishimura, O.; Shimanouchi, T.; Kuboi, R.; Naiki, H.; Goto, Y. Laser-induced propagation and destruction of amyloid β fibrils. J. Biol. Chem. 2010, 285, 19660–19667. [Google Scholar] [CrossRef] [PubMed]
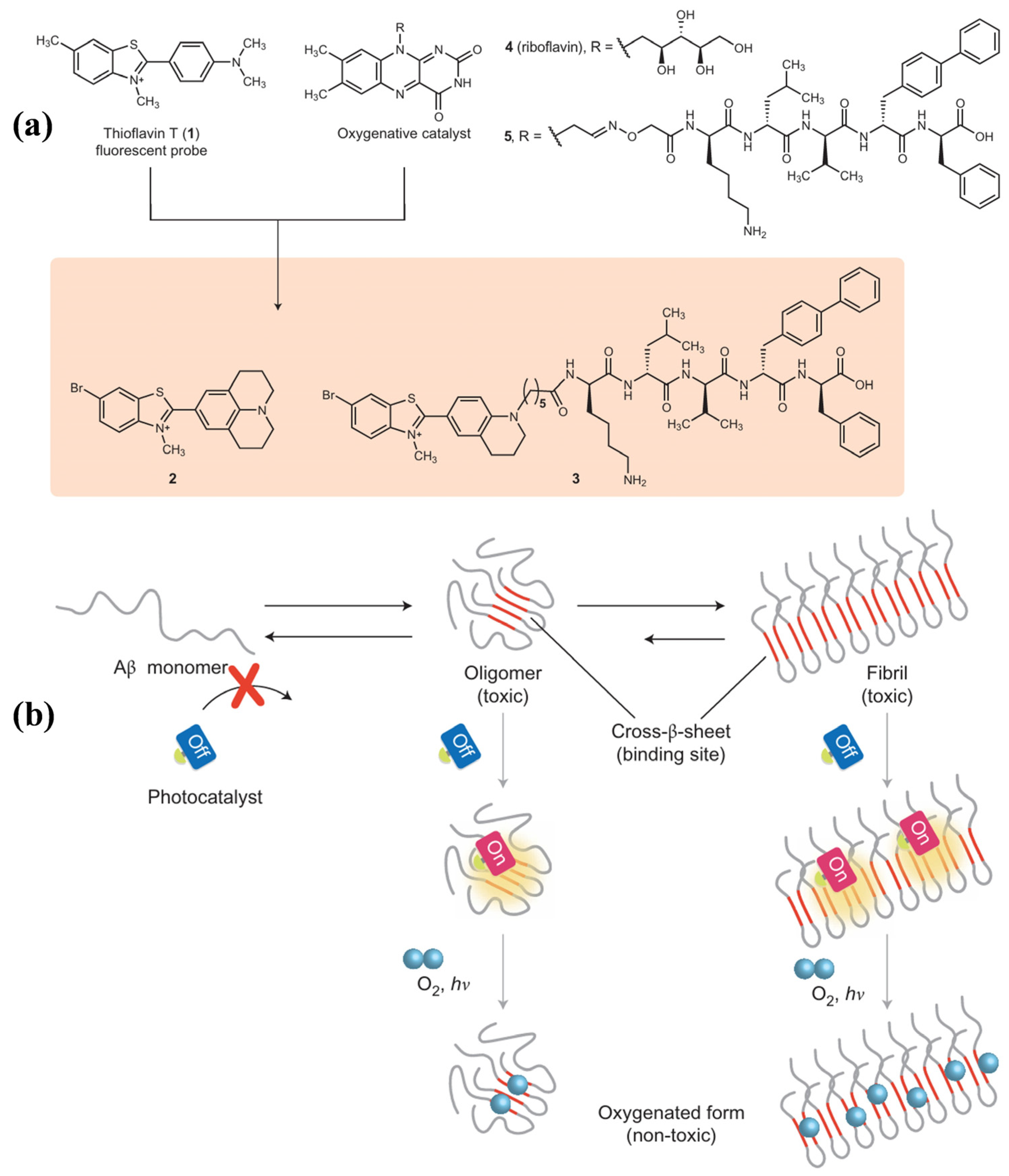
- Taniguchi, A.; Shimizu, Y.; Oisaki, K.; Sohma, Y.; Kanai, M. Switchable photooxygenation catalysts that sense higher-order amyloid structures. Nat. Chem. 2016, 8, 974–982. [Google Scholar] [CrossRef]
- Ahn, M.; Lee, B.I.; Chia, S.; Habchi, J.; Kumita, J.R.; Vendruscolo, M.; Dobson, C.M.; Park, C.B. Chemical and mechanistic analysis of photodynamic inhibition of Alzheimer’s beta-amyloid aggregation. Chem. Commun. 2019, 55, 1152–1155. [Google Scholar] [CrossRef]
- Bondia, P.; Torra, J.; Tone, C.M.; Sawazaki, T.; del Valle, A.; Sot, B.; None, S.; Kanai, M.; Sohma, Y.; Flors, C. Nanoscale view of amyloid photodynamic damage. J. Am. Chem. Soc. 2020, 142, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, Z.; Shao, Y.; Li, G.; Pan, Y.; Wang, L.; Akkaya, E.U. Degradation of amyloid peptide aggregates by targeted singlet oxygen delivery from a benzothiazole functionalized naphthalene endoperoxide. Chem. Commun. 2022, 58, 3747–3750. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Fujii, T.; Oka, K.; Takahashi, D.; Toshima, K. Inhibition of amyloid β aggregation and cytotoxicity by photodegradation using a designed fullerene derivative. Chem. Asian J. 2011, 6, 2312–2315. [Google Scholar] [CrossRef]
- Taniguchi, A.; Sasaki, D.; Shiohara, A.; Iwatsubo, T.; Tomita, T.; Sohma, Y.; Kanai, M. Attenuation of the aggregation and neurotoxicity of amyloid-β peptides by catalytic photooxygenation. Angew. Chem. Int. Ed. 2014, 53, 1382–1385. [Google Scholar] [CrossRef]
- Lee, B.I.; Suh, Y.S.; Chung, Y.J.; Yu, K.; Park, C.B. Shedding light on Alzheimer’s β-amyloidosis: Photosensitized methylene blue inhibits self-assembly of β-amyloid peptides and disintegrates their aggregates. Sci. Rep. 2017, 7, 7523. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Lee, B.I.; Park, C.B. Photo-induced inhibition of Alzheimer’s β-amyloid aggregation in vitro by rose bengal. Biomaterials 2015, 38, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Wang, J.; Jiang, H.; Chen, Q.; Xiao, Y.; Yang, H.; Lin, L. Transcranial deep-tissue phototherapy for Alzheimer’s disease using low-dose X-ray-activated long-afterglow scintillators. Acta Biomater. 2023, 155, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Mangione, M.R.; Piccionello, A.P.; Marino, C.; Ortore, M.G.; Picone, P.; Vilasi, S.; Carlo, M.D.; Buscemi, S.; Bulone, D.; San Biagio, P.L. Photo-inhibition of Aβ fibrillation mediated by a newly designed fluorinated oxadiazole. RSC Adv. 2015, 5, 16540–16548. [Google Scholar] [CrossRef]
- Hirabayashi, A.; Shindo, Y.; Oka, K.; Takahashia, D.; Toshima, K. Photodegradation of amyloid β and reduction of its cytotoxicity to PC12 cells using porphyrin derivatives. Chem. Commun. 2014, 50, 9543–9546. [Google Scholar] [CrossRef]
- Leshem, G.; Richman, M.; Lisniansky, E.; Antman-Passig, M.; Habashi, M.; Graslund, A.; Warmlander, S.; Rahimipour, S. Photoactive chlorin e6 is a multifunctional modulator of amyloid-β aggregation and toxicity via specific interactions with its histidine residues. Chem. Sci. 2019, 10, 208–217. [Google Scholar] [CrossRef]
- Lee, B.I.; Lee, S.; Suh, Y.S.; Lee, J.S.; Kim, A.-K.; Kwon, O.-Y.; Yu, K.; Park, C.B. Photoexcited porphyrins as a strong suppressor of β-amyloid aggregation and synaptic toxicity. Angew. Chem. Int. Ed. 2015, 54, 11472–11476. [Google Scholar] [CrossRef]
- Fu, H.; Cui, M.; Liu, Z.; Tu, P.; Zhou, K.; Dai, J.; Liu, B. Highly sensitive near-infrared fluorophores for in vivo detection of amyloid-β plaques in Alzheimer’s disease. J. Med. Chem. 2015, 58, 6972–6983. [Google Scholar] [CrossRef]
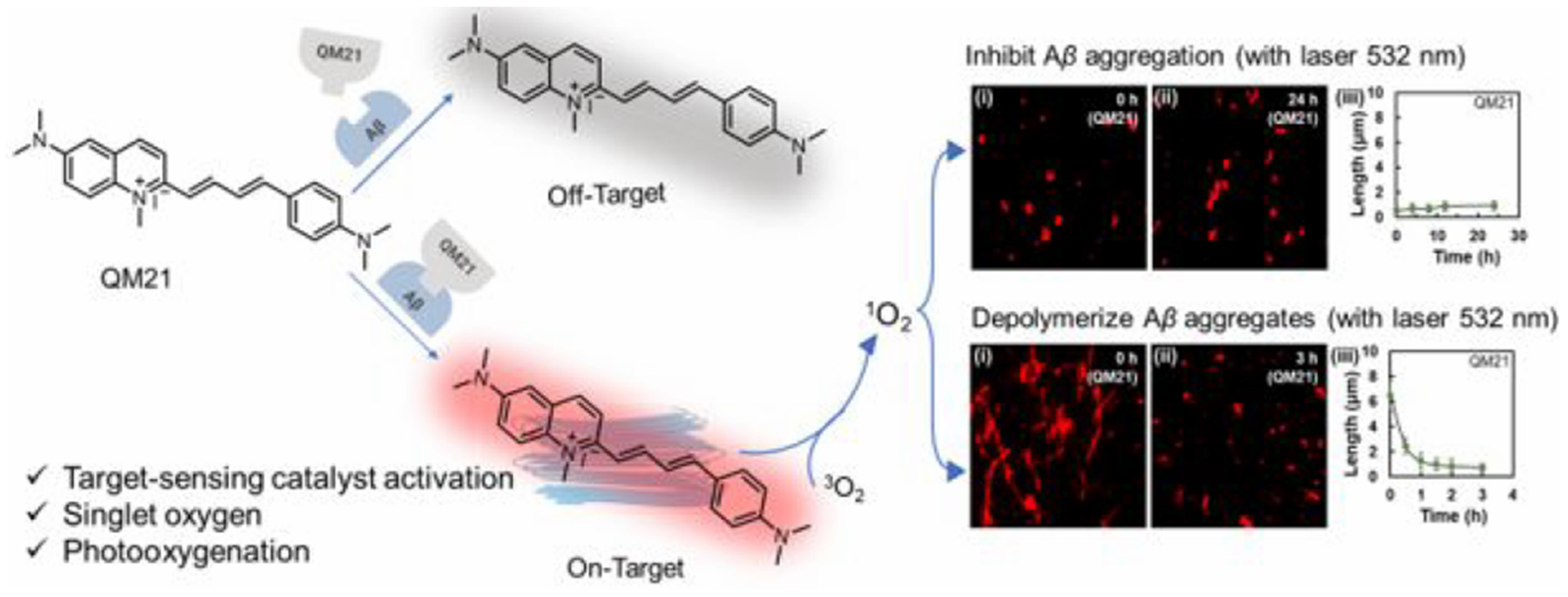
- Yang, J.; Wang, X.; Liu, J.; Chi, W.; Zhang, L.; Xiao, L.; Yan, J.-W. Near-infrared photooxygenation theranostics used for the specific mapping and modulating of amyloid-β aggregation. Anal. Chem. 2022, 94, 15902–15907. [Google Scholar] [CrossRef]
- Liu, H.; Li, Y.; Wei, L.; Ye, Z.; Yuan, J.; Xiao, L. Amyloid β aggregates-restricted rotational motion amplifies photo-catalytic activity from quinolinium derivatives. Nano Today 2024, 58, 102434. [Google Scholar] [CrossRef]
- Xie, Z.; Hu, C.; Li, Y.; Zhang, L.; Yan, J. Optimization of quinolinium-carbazole theranostics for the improved photooxidation of β-amyloid aggregates with the variation of conjugation site. J. Photochem. Photobiol. A Chem. 2024, 456, 115823. [Google Scholar] [CrossRef]
- Ni, J.; Taniguchi, A.; Ozawa, S.; Hori, Y.; Kuninobu, Y.; Saito, T.; Saido, T.C.; Tomita, T.; Sohma, Y.; Kanai, M. Near-infrared photoactivatable oxygenation catalysts of amyloid peptide. Chem 2018, 4, 807–820. [Google Scholar] [CrossRef]
- Ozawa, S.; Hori, Y.; Shimizu, Y.; Taniguchi, A.; Suzuki, T.; Wang, W.; Chiu, Y.W.; Koike, R.; Yokoshima, S.; Fukuyama, T.; et al. Photo-oxygenation by a biocompatible catalyst reduces amyloid-β levels in Alzheimer’s disease mice. Brain 2021, 144, 1884–1897. [Google Scholar] [CrossRef]
- Nagashima, N.; Ozawa, S.; Furuta, M.; Oi, M.; Hori, Y.; Tomita, T.; Sohma, Y.; Kanai, M. Catalytic photooxygenation degrades brain Aβ in vivo. Sci. Adv. 2021, 7, eabc9750. [Google Scholar] [CrossRef] [PubMed]
- Furuta, M.; Arii, S.; Umeda, H.; Matsukawa, R.; Shizu, K.; Kaji, H.; Kawashima, S.A.; Hori, Y.; Tomita, T.; Sohma, Y.; et al. Leuco ethyl violet as self-activating prodrug photocatalystfor in vivo amyloid-selective oxygenation. Adv. Sci. 2024, 11, 2401346. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Kim, M.; Lim, M.H. Excited-state intramolecular hydrogen transfer of compact molecules controls amyloid aggregation profiles. JACS Au 2022, 2, 2001–2012. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yang, J.; Yan, J. Chemiexcitation-triggered photosensitizer activation for photooxidation of Aβ1–42 aggregates. ACS Appl. Mater. Interfaces 2024, 16, 41843–41854. [Google Scholar] [CrossRef]
- Fanni, A.M.; Okoye, D.; Monge, F.A.; Hammond, J.; Maghsoodi, F.; Martin, T.D.; Brinkley, G.; Phipps, M.L.; Evans, D.G.; Martinez, J.S.; et al. Controlled and selective photo-oxidation of amyloid-β fibrils by oligomeric p-phenylene ethynylenes. ACS Appl. Mater. Interfaces 2022, 14, 14871–14886. [Google Scholar] [CrossRef]
- Ma, Y.; Ye, Z.; Zhang, C.; Zhao, Y.; Luo, H.-B.; Xiao, L. Curved carbon photo-oxygenation catalysts for the suppression and nanoscopic imaging of β-amyloid peptides fibrillation. Nano Res. 2022, 15, 3387–3397. [Google Scholar] [CrossRef]
- Son, G.; Lee, B.I.; Chung, Y.J.; Park, C.B. Light-triggered dissociation of self-assembled β-amyloid aggregates into small, nontoxic fragments by ruthenium (II) complex. Acta Biomater. 2018, 67, 147–155. [Google Scholar] [CrossRef]
- Suh, J.-M.; Kim, G.; Kang, J.; Lim, M.H. Strategies employing transition metal complexes to modulate amyloid-β aggregation. Inorg. Chem. 2019, 58, 8–17. [Google Scholar] [CrossRef]
- Xu, W.; Gao, C.; Sun, X.; Tai, W.C.-S.; Lung, H.L.; Law, G.-L. Design, synthesis and comparison of water-soluble phthalocyanine/porphyrin analogues and their inhibition effects on Aβ42 fibrillization. Inorg. Chem. Front. 2021, 8, 3501–3513. [Google Scholar] [CrossRef]
- Babu, E.; Bhuvaneswari, J.; Rajakumar, K.; Sathish, V.; Thanasekaran, P. Non-conventional photoactive transition metal complexes that mediated sensing and inhibition of amyloidogenic aggregates. Coord. Chem. Rev. 2021, 428, 213612. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, M.; Yu, D.; Ren, J.; Qu, X. Target-driven supramolecular self-assembly for selective amyloid-β photooxygenation against Alzheimer’s disease. Chem. Sci. 2020, 11, 11003–11008. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, B.-D.; Peng, X.-H.; Li, S.-Z.; Ying, J.-W.; Zhao, Y.; Huang, J.-D.; Yoon, J. Phthalocyanines as medicinal photosensitizers: Developments in the last five years. Coord. Chem. Rev. 2019, 379, 147–160. [Google Scholar] [CrossRef]
- Zhan, Q.; Shi, X.; Wang, T.; Hu, J.; Zhou, J.; Zhou, L.; Wei, S. Design and synthesis of thymine modified phthalocyanine for Aβ protofibrils photodegradation and Aβ peptide aggregation inhibition. Talanta 2019, 191, 27–38. [Google Scholar] [CrossRef]
- Chen, J.; Fan, T.; Xie, Z.; Zeng, Q.; Xue, P.; Zheng, T.; Chen, Y.; Luo, X.; Zhang, H. Advances in nanomaterials for photodynamic therapy applications: Status and challenges. Biomaterials 2020, 237, 119827. [Google Scholar] [CrossRef]
- Hou, T.; Yang, Q.; Ding, M.; Wang, X.; Mei, K.; Guan, P.; Wang, C.; Hu, X. Blood-brain barrier permeable carbon nano-assemblies for amyloid-β clearance and neurotoxic attenuation. Colloids Surf. B 2024, 244, 114182. [Google Scholar] [CrossRef]
- Lin, X.; Dong, X.; Sun, Y. Dual-carbon dots composite: A multifunctional photo-propelled nanomotor against Alzheimer’s β-amyloid. Small 2024, 20, e2407154. [Google Scholar] [CrossRef]
- Ma, Z.; Song, C.; Yang, K.; Zhu, Z.; Wang, J. Application of PEG-modified copper-cysteamine in photodynamic therapy for Alzheimer’s disease. Mater. Lett. 2022, 328, 133018. [Google Scholar] [CrossRef]
- Shao, X.; Li, M.; Yan, C.; Wang, C.; Wang, X.; Guan, P.; Hu, X.; Fan, L. Photocatalytic, photothermal, and blood-brain barrier-permeable carbon nanodots: A potent multifunctional scavenger for β-amyloid plaque. Colloids Surf. B 2025, 246, 114380. [Google Scholar] [CrossRef]
- Xu, Y.; Xiao, L. Efficient suppression of amyloid-β peptide aggregation and cytotoxicity with photosensitive polymer nanodots. J. Mater. Chem. B 2020, 8, 5776–5782. [Google Scholar] [CrossRef]
- Zhu, J.; Xiao, P.; Li, H.; Carabineiro, S.A.C. Graphitic carbon nitride: Synthesis, properties, and applications in catalysis. ACS Appl. Mater. Interfaces 2014, 6, 16449–16465. [Google Scholar] [CrossRef]
- Chung, Y.J.; Lee, B.I.; Ko, J.W.; Park, C.B. Photoactive g-C3N4 nanosheets for light-induced suppression of Alzheimer’s β-amyloid aggregation and toxicity. Adv. Healthc. Mater. 2016, 5, 1560–1565. [Google Scholar] [CrossRef]
- Wang, J.; Fan, Y.; Tan, Y.; Zhao, X.; Zhang, Y.; Cheng, C.; Yang, M. Porphyrinic metal-organic framework PCN-224 nanoparticles for near-infrared-induced attenuation of aggregation and neurotoxicity of Alzheimer’s amyloid-β peptide. ACS Appl. Mater. Interfaces 2018, 10, 36615–36621. [Google Scholar] [CrossRef]
- Yu, D.; Guan, Y.; Bai, F.; Du, Z.; Gao, N.; Ren, J.; Qu, X. Metal-organic frameworks harness Cu chelating and photooxidation against amyloid β aggregation in vivo. Chem. Eur. J. 2019, 25, 3489–3495. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, A.; Yi, W.; Chen, G.; Huang, S.; Ouyang, G. Nanozyme engineering in structurally explicit framework: Design mechanisms and biosensing applications. Coord. Chem. Rev. 2024, 500, 215517. [Google Scholar] [CrossRef]
- Fu, N.; Liu, Y.; Kang, K.; Tang, X.; Zhang, S.; Yang, Z.; Wang, Y.; Jin, P.; Niu, Y.; Yang, B. Fully sp2 carbon-conjugated covalent organic frameworks with multiple active sites for advanced lithium-ion battery cathodes. Angew. Chem. Int. Ed. 2024, 63, e202412334. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yu, D.; Liu, S.; Liu, C.; Liu, Z.; Ren, J.; Qu, X. NIR-II hydrogen-bonded organic frameworks (HOFs) used for target-specific amyloid-β photooxygenation in an Alzheimer’s disease model. Angew. Chem. Int. Ed. 2022, 61, e202109068. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Gao, N.; Wang, X.; Ren, J.; Qu, X. Near-infrared switchable fullerene-based synergy therapy for Alzheimer’s disease. Small 2018, 14, 1801852. [Google Scholar] [CrossRef]
- Huang, J.; Li, Z.; Liu, Z. Functionalized upconversion nanoparticles for disassembly of β-amyloid aggregation with near-infrared excitation. Acta Chim. Sin. 2021, 79, 1049–1057. [Google Scholar] [CrossRef]
- Qiao, L.; Shen, Y.; Li, G.; Lv, G.; Li, C. Hypochlorous acid-activated UCNPs-LMB/VQIVYK multifunctional nanosystem for Alzheimer’s disease treatment. J. Funct. Biomater. 2023, 14, 207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, J.; Song, Y.; Wang, Z.; Dong, M.; Liu, L. Disassembly of Alzheimer’s amyloid fibrils by functional upconversion nanoparticles under near-infrared light irradiation. Colloids Surf. B 2019, 181, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Kuk, S.; Lee, B.I.; Lee, J.S.; Park, C.B. Rattle-structured upconversion nanoparticles for near-IR-induced suppression of Alzheimer’s β-amyloid aggregation. Small 2017, 13, 1603139. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, W.; Hou, L.; Geng, W.; Wang, J.; Kong, Y.; Liu, C.; Zeng, X.; Kong, D. A biomimetic upconversion nanobait-based near infrared light guided photodynamic therapy alleviates Alzheimer’s disease by inhibiting β-Amyloid aggregation. Adv. Healthc. Mater. 2023, 13, e2303278. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, Q.; Xiang, C.; Liu, G.; Li, J. A novel aggregation-induced emission fluorescent probe for detection of-amyloid based on pyridinyltriphenylamine and quinoline–malononitrile. Biosensors 2023, 13, 610. [Google Scholar] [CrossRef]
- Zhou, Y.; Hua, J.; Ding, D.; Tang, Y. Interrogating amyloid aggregation with aggregation-induced emission fluorescence probes. Biomaterials 2022, 286, 121605. [Google Scholar] [CrossRef]
- Bajad, N.G.; Kumar, A.; Singh, S.K. Recent advances in the development of near-infrared fluorescent probes for the in vivo brain imaging of amyloid-β species in Alzheimer’s disease. ACS Chem. Neurosci. 2023, 14, 2955–2967. [Google Scholar] [CrossRef]
- Wei, W.; Qiu, Z. Diagnostics and theranostics of central nervous system diseases based on aggregation-induced emission luminogens. Biosens. Bioelectron. 2022, 217, 114670. [Google Scholar] [CrossRef]
- Li, K.; Ren, T.B.; Huan, S.; Yuan, L.; Zhang, X.B. Progress and perspective of solid-state organic fluorophores for biomedical applications. J. Am. Chem. Soc. 2021, 143, 21143–21160. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, Y.; Sun, A.; Xiong, Y.; Tan, H.; Shi, Y.; Yu, P.; Roy, G.; Zhang, L.; Yan, J. Dual-functional AIE fluorescent probes for imaging β-amyloid plaques and lipid droplets. Anal. Chim. Acta 2020, 1133, 109–118. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Li, Z.J.; Tang, Y.H.; Hou, T.T.; Xu, L.; Wang, Z.H.; Qin, T.Y.; Wang, Y.L.; Zhu, M.Q. Tailoring near-infrared amyloid-β probes with high-affinity and low background based on CN and amphipathic regulatory strategies and in vivo imaging of AD mice. Talanta 2025, 281, 126858. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Luo, T.; Zhang, J.; Fan, C.; Li, X.; Li, C.; Gong, H.; Luo, Q.; Zhu, M.-Q. AIE-based fluorescent micro-optical sectioning tomography for automatic 3D mapping of β-amyloid plaques in Tg mouse whole brain. Chem. Eng. J. 2022, 446, 136840. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Fan, C.; Xin, B.; Zhang, J.-P.; Luo, T.; Chen, Z.-Q.; Zhou, Q.-Y.; Yu, Q.; Li, X.-N.; Huang, Z.-L.; et al. AIE-based super-resolution imaging probes for β-amyloid plaques in mouse brains. Mater. Chem. Front. 2018, 2, 1554–1562. [Google Scholar] [CrossRef]
- Bera, T.; Mondal, A.; Kar, S.; Mukherjee, A.; Banerjee, S.; Guha, S. A mitochondria targeting, de novo designed, aggregation-induced emission probe for selective detection of neurotoxic amyloid-β aggregates. J. Mater. Chem. B 2024, 12, 11368–11380. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mei, D.; Zhang, X.; Qu, D.-H.; Mei, J. Visualizing Aβ deposits in live young AD model mice with a simple red/near-infrared-fluorescent AIEgen. Sci. China Chem. 2021, 65, 339–352. [Google Scholar] [CrossRef]
- Marzano, N.R.; Wray, K.M.; Johnston, C.L.; Paudel, B.P.; Hong, Y.; van Oijen, A.; Ecroyd, H. An α-cyanostilbene derivative for the enhanced detection and imaging of amyloid fibril aggregates. ACS Chem. Neurosci. 2020, 11, 4191–4202. [Google Scholar] [CrossRef]
- Kumar, M.; Hong, Y.; Thorn, D.C.; Ecroyd, H.; Carver, J.A. Monitoring early-stage protein aggregation by an aggregation-induced emission fluorogen. Anal. Chem. 2017, 89, 9322–9329. [Google Scholar] [CrossRef]
- Salveson, P.J.; Haerianardakani, S.; Thuy-Boun, A.; Yoo, S.; Kreutzer, A.G.; Demeler, B.; Nowick, J.S. Repurposing triphenylmethane dyes to bind to trimers derived from Aβ. J. Am. Chem. Soc. 2018, 140, 11745–11754. [Google Scholar] [CrossRef]
- Arumugam, D.; Jamuna, N.A.; Kamalakshan, A.; Mandal, S. Modulation of AIE and Intramolecular Charge Transfer of a Pyrene-Based Probe for Discriminatory Detection and Imaging of Oligomers and Amyloid Fibrils. ACS Appl. Bio Mater. 2024, 7, 6343–6356. [Google Scholar] [CrossRef]
- Pradhan, N.; Jana, D.; Ghorai, B.K.; Jana, N.R. Detection and monitoring of amyloid fibrillation using a fluorescence switch-on probe. ACS Appl. Mater. Interfaces 2015, 7, 25813–25820. [Google Scholar] [CrossRef]
- Zhang, J.D.; Mei, J.; Hu, X.L.; He, X.P.; Tian, H. Ratiometric detection of β-amyloid and discrimination from lectins by a supramolecular AIE glyconanoparticle. Small 2016, 12, 6562–6567. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhang, D.; Gong, X.; Zheng, J. Dual-functional, multi-targeting GNNQQNY-AIE conjugates as amyloid probes and amyloid modulators via amyloid cross-seeding principle. Adv. Funct. Mater. 2022, 32, 2208022. [Google Scholar] [CrossRef]
- Fu, W.; Yan, C.; Guo, Z.; Zhang, J.; Zhang, H.; Tian, H.; Zhu, W.-H. Rational design of near-infrared aggregation-induced-emission-active probes: In situ mapping of amyloid-β plaques with ultrasensitivity and high-fidelity. J. Am. Chem. Soc. 2019, 141, 3171–3177. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Dai, J.; Yao, Y.; Fu, W.; Tian, H.; Zhu, W.H.; Guo, Z. Preparation of near-infrared AIEgen-active fluorescent probes for mapping amyloid-β plaques in brain tissues and living mice. Nat. Protoc. 2023, 18, 1316–1336. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, D.; Zheng, J. ROF-2 as an aggregation-induced emission (AIE) probe for multi-target amyloid detection and screening of amyloid inhibitors. Small 2024, 20, e2400879. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, X.; Yuan, C.; Pang, X.; Shangguan, P.; Liu, Y.; Han, L.; Sun, J.; Lam, J.W.Y.; Liu, Y.; et al. Near-infrared aggregation-induced emission luminogens for in vivo theranostics of Alzheimer’s disease. Angew. Chem. Int. Ed. 2023, 62, e202211550. [Google Scholar] [CrossRef]
- Jia, M.; Li, Y.; Wang, C.; Gao, X.; Guan, Y.; Ai, H. Fluorescence detection and inhibition mechanisms of DNTPH on Aβ42 oligomers characterized as products in the four stages of aggregation. ACS Chem. Neurosci. 2024, 15, 4220–4228. [Google Scholar] [CrossRef]
- Wang, J.; Shangguan, P.; Chen, X.; Zhong, Y.; Lin, M.; He, M.; Liu, Y.; Zhou, Y.; Pang, X.; Han, L.; et al. A one-two punch targeting reactive oxygen species and fibril for rescuing Alzheimer’s disease. Nat. Commun. 2024, 15, 705. [Google Scholar] [CrossRef]
- Liu, L.; Liu, W.; Sun, Y.; Dong, X. Design of aggregation-induced emission-active fluorogen-based nanoparticles for imaging and scavenging Alzheimer’s β-amyloid by photo-oxygenation. J. Mater. Chem. B 2023, 11, 8994–9004. [Google Scholar] [CrossRef]

























Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, F.; Hou, Y.; Wang, Y.; Liu, L.; Yi, X.; Xia, N. Photothermal and Photodynamic Strategies for Diagnosis and Therapy of Alzheimer’s Disease by Modulating Amyloid-β Aggregation. Biosensors 2025, 15, 480. https://doi.org/10.3390/bios15080480
Gao F, Hou Y, Wang Y, Liu L, Yi X, Xia N. Photothermal and Photodynamic Strategies for Diagnosis and Therapy of Alzheimer’s Disease by Modulating Amyloid-β Aggregation. Biosensors. 2025; 15(8):480. https://doi.org/10.3390/bios15080480
Chicago/Turabian StyleGao, Fengli, Yupeng Hou, Yaru Wang, Linyuan Liu, Xinyao Yi, and Ning Xia. 2025. "Photothermal and Photodynamic Strategies for Diagnosis and Therapy of Alzheimer’s Disease by Modulating Amyloid-β Aggregation" Biosensors 15, no. 8: 480. https://doi.org/10.3390/bios15080480
APA StyleGao, F., Hou, Y., Wang, Y., Liu, L., Yi, X., & Xia, N. (2025). Photothermal and Photodynamic Strategies for Diagnosis and Therapy of Alzheimer’s Disease by Modulating Amyloid-β Aggregation. Biosensors, 15(8), 480. https://doi.org/10.3390/bios15080480






