Integrating Near-Infrared Spectroscopy and Proteomics for Semen Quality Biosensing
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Semen Collection and Sample Preparation
2.3. Sperm Motion and Morphology Analyses
2.4. Boar Seminal Plasma Collection
2.5. NIR Spectral Signature Collection
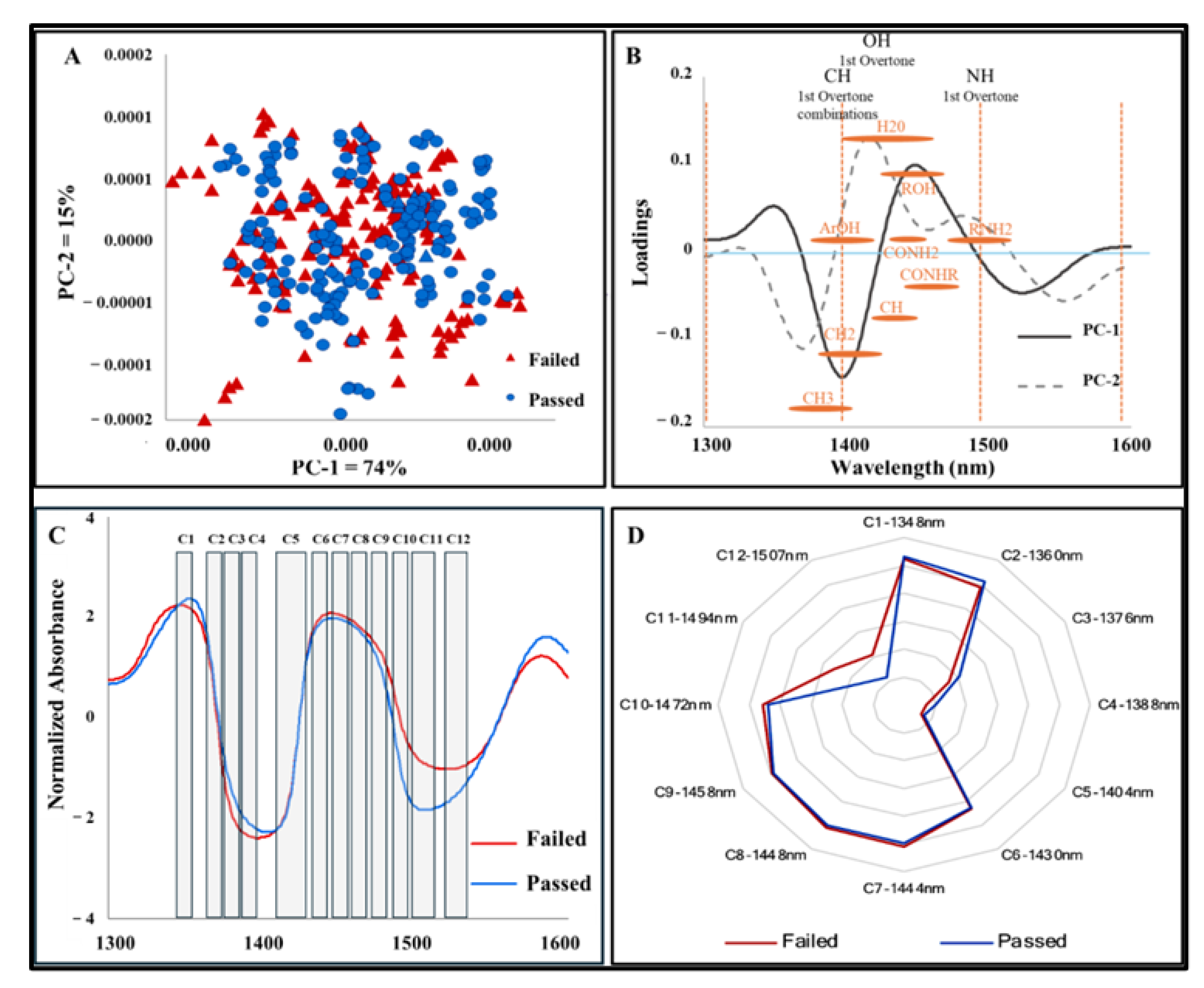
2.5.1. Principal Component Analysis
2.5.2. Aquaphotomics
2.6. Isolation of Seminal Plasma EVs
2.7. Characterization of Isolated SP-EVs
2.7.1. Transmission Electron Microscopy (TEM)
2.7.2. Nanoparticle Tracking Analysis (NTA) of Isolated SP-EVs
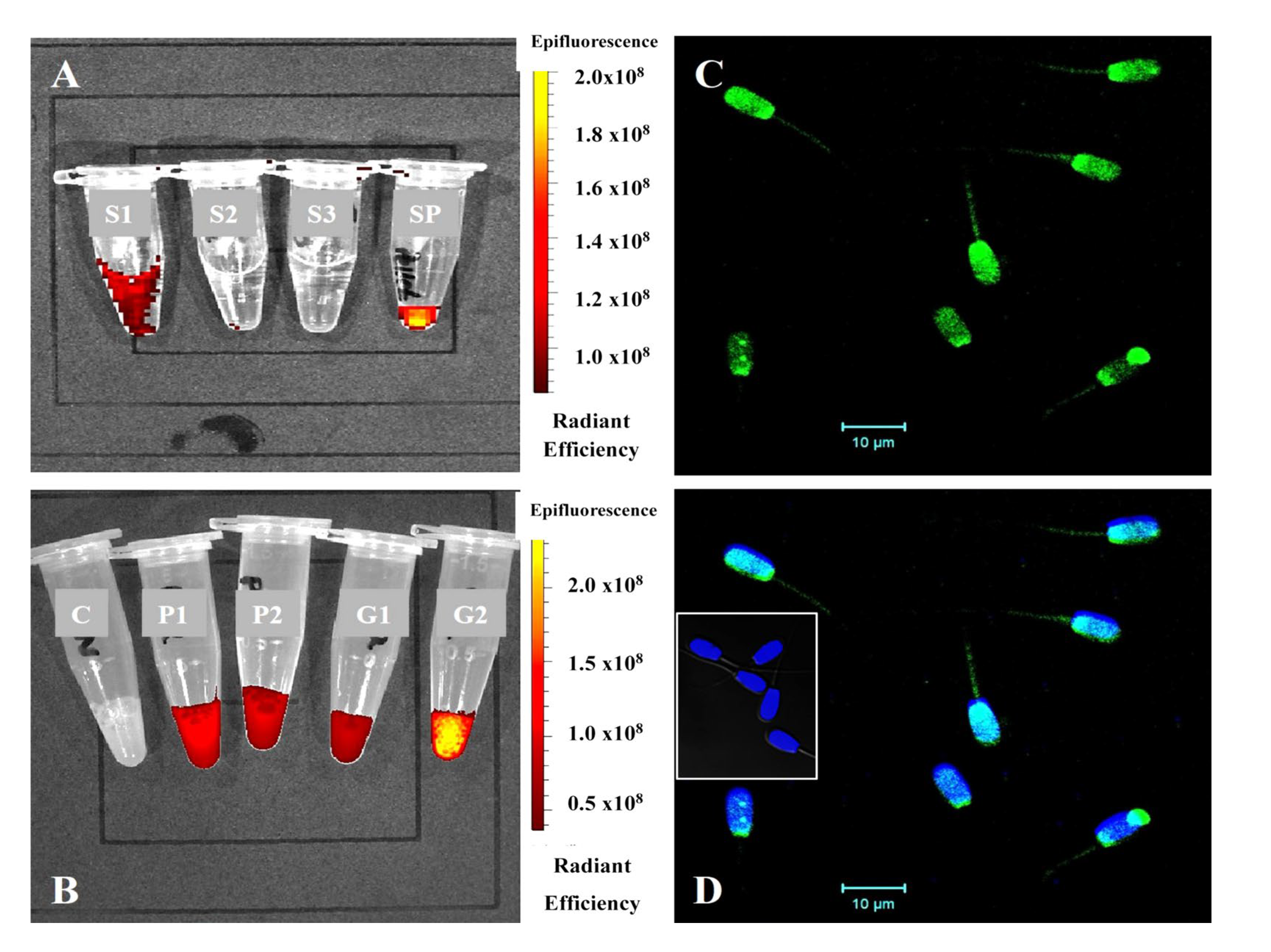
2.8. Seminal Plasma EVs Labeling and Indirect Labeling of Spermatozoa
2.9. Protein Analyses
2.9.1. SP-EV Sample Preparation for NanoLC-MS/MS Analysis
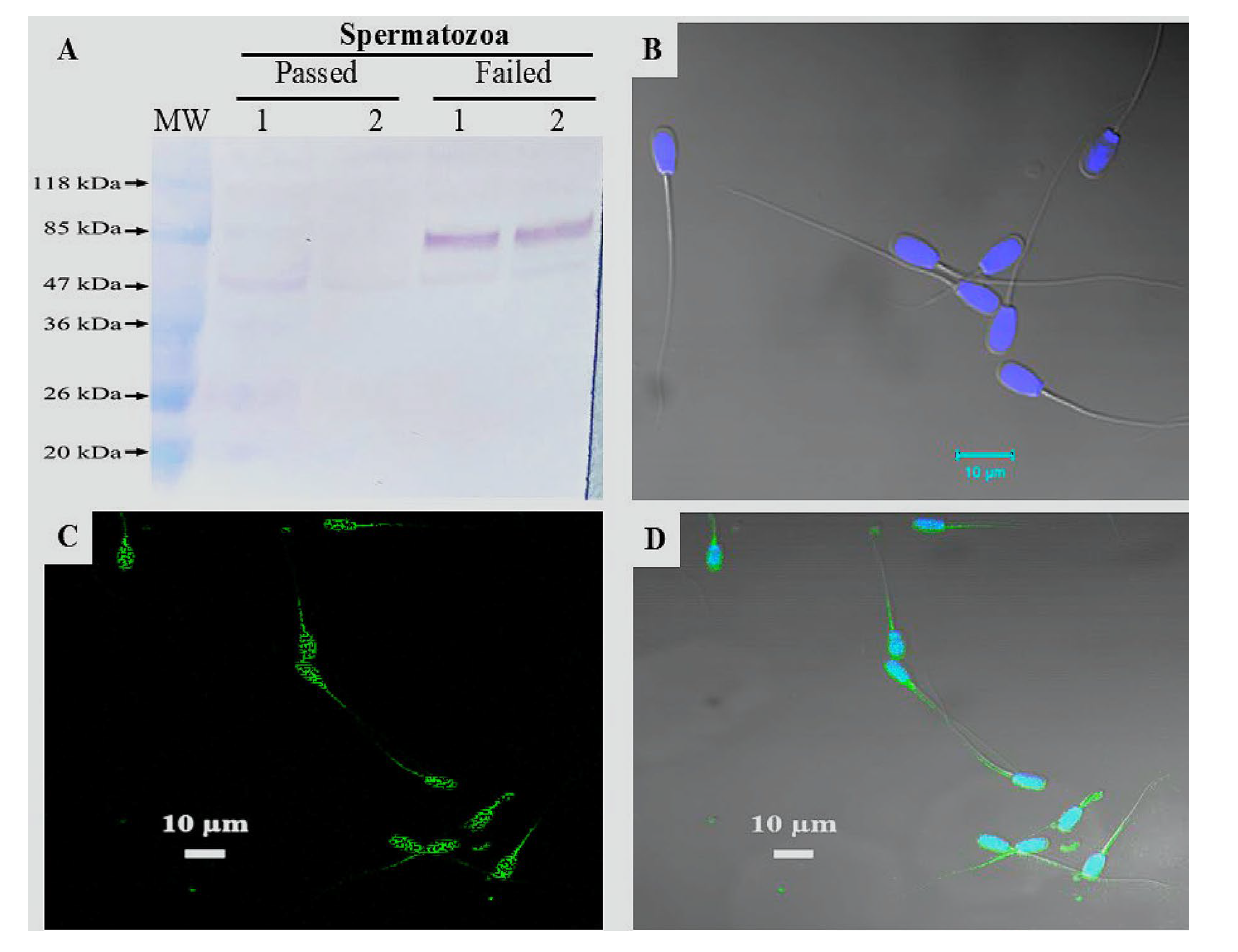
2.9.2. Sperm Immunoprotein Detection
2.9.3. Bioinformatics Analysis
2.10. Statistical Analysis
3. Results
3.1. Sperm Quality Evaluation
3.2. NIRS Findings
3.3. Discriminant Analysis
3.4. Morphological and Molecular Characterization of SP-EVs
3.4.1. TEM and NTA Measurements of SP-EVs
3.4.2. Evaluation of Co-Incubation of SP-EVs with Boar Spermatozoa
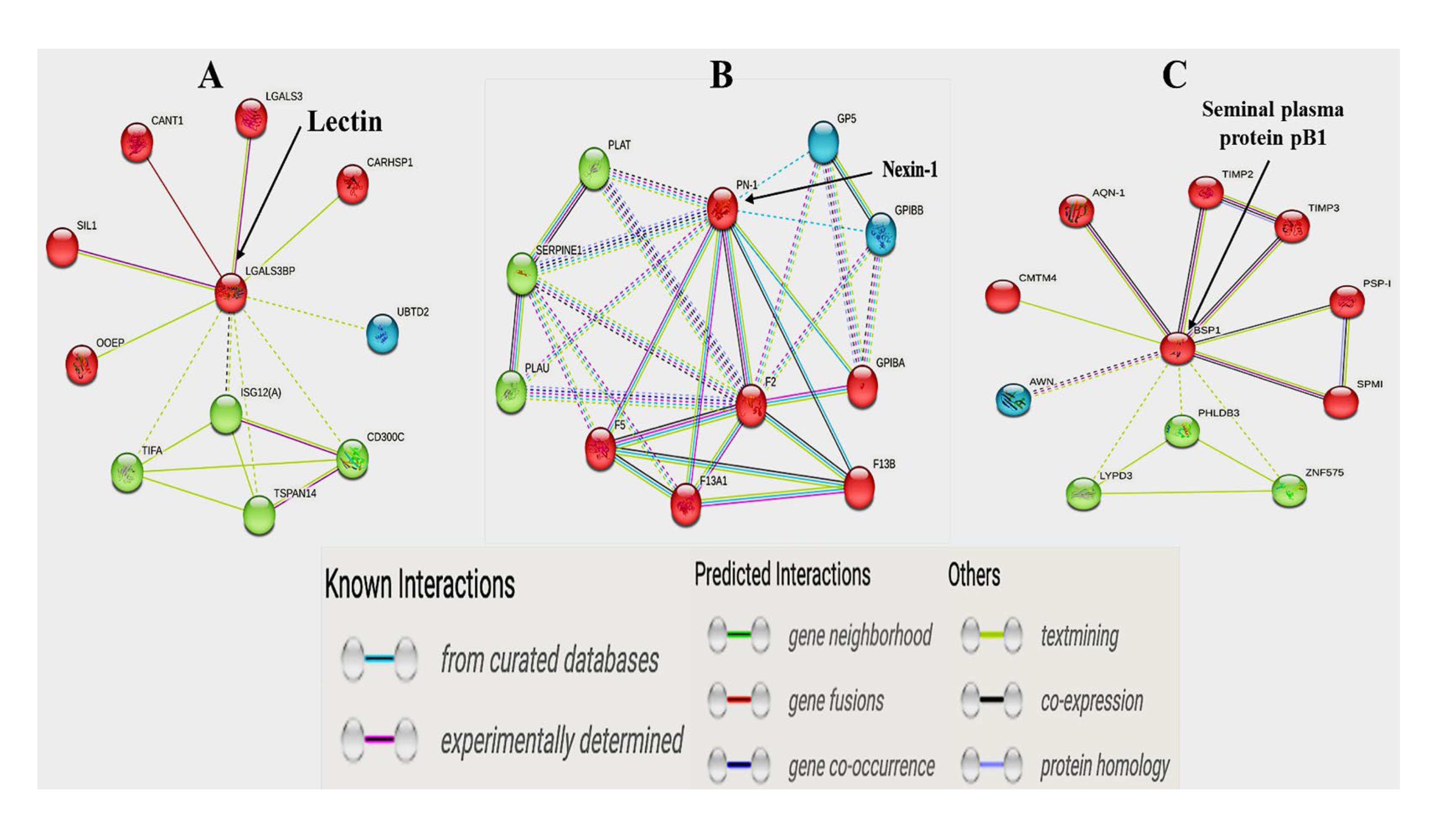
3.5. Proteome of SP-EVs and Differential Expressions
3.6. Functional Enrichment Analysis
3.7. Immunodetection of LGALS3BP Protein
3.8. Protein–to–Protein Interaction (PPI) Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Artificial insemination |
| ALH | Amplitude head |
| BCF | Beat cross frequency |
| CASA | Computer-assisted sperm analysis |
| EVs | Extracellular vesicles |
| GO | Gene ontology |
| IF | Immunofluorescence |
| IVF | In vitro fertilization |
| KEGG | Kyoto encyclopedia of genes and genomes |
| LC-MS/MS | Liquid chromatography-mass spectrometry |
| LDA | Linear discriminant analysis |
| LGALS3BP | Lectin, galactoside-binding, soluble, 3-binding |
| MFG-E8 | Milk fat globule-EGF factor |
| NIRS | Near infrared spectroscopy |
| NOA | Non-obstructive azoospermia |
| NTA | Nanoparticle tracking analysis |
| PBS | Phosphate-buffered saline |
| PCA | Principal component analysis |
| PN-1 | Nexin-1 |
| PPI | Protein–to–protein interaction |
| PVDF | Polyvinylidene difluoride |
| SD | Standard deviation |
| SP | Seminal plasma |
| SVD | Singular value decomposition |
| TEM | Transmission electron microscopy |
| TESE | Testicular sperm extraction |
| VAP | Average path velocity |
| VCL | Curvilinear velocity |
| VSL | Straight line velocity |
| WABS | Water absorbance bands |
| WAMACS | Water Matrix Coordinates |
| WASPS | Water absorbance spectral patterns |
References
- Maes, D.; Nauwynck, H.; Rijsselaere, T.; Mateusen, B.; Vyt, P.; de Kruif, A.; Van Soom, A. Diseases in swine transmitted by artificial insemination: An overview. Theriogenology 2008, 70, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Flowers, W. Selection for boar fertility and semen quality—The way ahead. Soc. Reprod. Fertil. 2009, 66, 67–78. [Google Scholar] [CrossRef]
- Mann, T.; Lutwak-Mann, C. Male reproductive function and the composition of semen: General considerations. In Male Reproductive Function and Semen; Springer: Berlin/Heidelberg, Germany, 1981; pp. 1–37. [Google Scholar]
- Maxwell, W.; De Graaf, S.; Ghaoui, R.-H.; Evans, G. Seminal plasma effects on sperm handling and female fertility. Soc. Reprod. Fertil. Suppl. 2007, 64, 13. [Google Scholar] [CrossRef] [PubMed]
- de Souza, A.P.B.; Schorr-Lenz, Â.M.; Lucca, F.; Bustamante-Filho, I.C. The epididymis and its role on sperm quality and male fertility. Anim. Reprod. (AR) 2018, 14 (Suppl. 1), 1234–1244. [Google Scholar] [CrossRef]
- Xu, Z.; Xie, Y.; Wu, C.; Gu, T.; Zhang, X.; Yang, J.; Yang, H.; Zheng, E.; Huang, S.; Xu, Z.; et al. The effects of boar seminal plasma extracellular vesicles on sperm fertility. Theriogenology 2024, 213, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Dlamini, N.H.; Nguyen, T.; Gad, A.; Tesfaye, D.; Liao, S.F.; Willard, S.T.; Ryan, P.L.; Feugang, J.M. Characterization of Extracellular Vesicle-Coupled miRNA Profiles in Seminal Plasma of Boars with Divergent Semen Quality Status. Int. J. Mol. Sci. 2023, 24, 3194. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, E.R.; Vasconcelos, F.R.; Paula-Marinho, S.d.O.; Daloso, D.d.M.; Guerreiro, D.D.; Martins, J.A.M.; Gomes-Filho, E.; Moura, A.A. Metabolomic profile of seminal plasma from Guzerá bulls (Bos indicus) with contrasting sperm freezability phenotypes. Reprod. Domest. Anim. 2023, 58, 1379–1392. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Martinez, H.; Roca, J. Extracellular vesicles in seminal plasma: A safe and relevant tool to improve fertility in livestock? Anim. Reprod. Sci. 2022, 244, 107051. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Rodríguez, M.; Martinez-Pastor, F. Martinez-Pastor, Molecular determinants of seminal plasma on sperm biology and fertility. Int. J. Mol. Sci. 2021, 22, 3555. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.A.; Mantsch, H.H. Infrared spectroscopy of biological fluids in clinical and diagnostic analysis. In Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation; Wiley Online Library: Hoboken, NJ, USA, 2006. [Google Scholar]
- Pasquini, C. Near infrared spectroscopy: Fundamentals, practical aspects and analytical applications. J. Braz. Chem. Soc. 2003, 14, 198–219. [Google Scholar] [CrossRef]
- Williams, P.; Manley, M.; Antoniszyn, J. Near Infrared Technology: Getting the Best out of Light; African Sun Media: Stellenbosch, South Africa, 2019. [Google Scholar]
- Tsenkova, R. AquaPhotomics: Water absorbance pattern as a biological marker for disease diagnosis and disease understanding. NIR News 2007, 18, 14–16. [Google Scholar] [CrossRef]
- Sutovsky, P.; Lovercamp, K. Molecular markers of sperm quality. In Reproduction in Domestic Ruminants VII: Proceedings of the 8th International Symposium on Reproduction in Domestic Ruminants, Anchorage, Alaska, September 2021; Nottingham University Press: Nottingham, UK, 2011; Volume 247. [Google Scholar]
- Candenas, L.; Chianese, R. Exosome composition and seminal plasma proteome: A promising source of biomarkers of male infertility. Int. J. Mol. Sci. 2020, 21, 7022. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Feugang, J.; Liao, S.; Crenshaw, M.; Clemente, H.; Willard, S.; Ryan, P. Lectin-functionalized magnetic iron oxide nanoparticles for reproductive improvement. JFIV Reprod. Med. Genet. 2015, 3, 17–19. [Google Scholar]
- Gebremedhn, S.; Gad, A.; Aglan, H.S.; Laurincik, J.; Prochazka, R.; Salilew-Wondim, D.; Hoelker, M.; Schellander, K.; Tesfaye, D. Extracellular vesicles shuttle protective messages against heat stress in bovine granulosa cells. Sci. Rep. 2020, 10, 15824. [Google Scholar] [CrossRef] [PubMed]
- Feugang, J.M.; Liao, S.F.; Willard, S.T.; Ryan, P.L. In-depth proteomic analysis of boar spermatozoa through shotgun and gel-based methods. BMC Genom. 2018, 19, 62. [Google Scholar] [CrossRef] [PubMed]
- Feugang, J.M.; Rodriguez-Munoz, J.C.; Willard, S.T.; Bathgate, R.A.; Ryan, P.L. Examination of relaxin and its receptors expression in pig gametes and embryos. Reprod. Biol. Endocrinol. 2011, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Feugang, J.M.; Greene, J.M.; Sanchez-Rodríguez, H.L.; Stokes, J.V.; Crenshaw, M.A.; Willard, S.T.; Ryan, P.L. Profiling of relaxin and its receptor proteins in boar reproductive tissues and spermatozoa. Reprod. Biol. Endocrinol. 2015, 13, 46. [Google Scholar] [CrossRef] [PubMed]
- Althouse, G.; Kuster, C.; Clark, S.; Weisiger, R. Field investigations of bacterial contaminants and their effects on extended porcine semen. Theriogenology 2000, 53, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Moreno, A.; Rigau, T.; Rodríguez-Gil, J.E. Regression analyses and motile sperm subpopulation structure study as improving tools in boar semen quality analysis. Theriogenology 2004, 61, 673–690. [Google Scholar] [CrossRef] [PubMed]
- Carreira, J.T.; Mingoti, G.Z.; Rodrigues, L.H.; Silva, C.; Perri, S.H.; Koivisto, M.B. Impact of proximal cytoplasmic droplets on quality traits and in-vitro embryo production efficiency of cryopreserved bull spermatozoa. Acta Veter. Scand. 2012, 54, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hirano, Y.; Shibahara, H.; Obara, H.; Suzuki, T.; Takamizawa, S.; Yamaguchi, C.; Tsunoda, H.; Sato, I. Andrology: Relationships between sperm motility characteristics assessed by the computer-aided sperm analysis (CASA) and fertilization rates in vitro. J. Assist. Reprod. Genet. 2001, 18, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.L.; Van Soom, A.; Arsenakis, I.; Maes, D. Boar management and semen handling factors affect the quality of boar extended semen. Porc. Health Manag. 2017, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Tsenkova, R.; Munćan, J.; Pollner, B.; Kovacs, Z. Essentials of aquaphotomics and its chemometrics approaches. Front. Chem. 2018, 6, 363. [Google Scholar] [CrossRef] [PubMed]
- Muncan, J.; Tsenkova, R. Aquaphotomics—From Innovative Knowledge to Integrative Platform in Science and Technology. Molecules 2019, 24, 2742. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Olivares, J.A.; Adriaens, I.; Stevens, E.; Saeys, W.; Aernouts, B. Online milk composition analysis with an on-farm near-infrared sensor. Comput. Electron. Agric. 2020, 178, 105734. [Google Scholar] [CrossRef]
- Sullivan, R.; Frenette, G.; Girouard, J. Epididymosomes are involved in the acquisition of new sperm proteins during epididymal transit. Asian J. Androl. 2007, 9, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Vojtech, L.; Woo, S.; Hughes, S.; Levy, C.; Ballweber, L.; Sauteraud, R.P.; Strobl, J.; Westerberg, K.; Gottardo, R.; Tewari, M.; et al. Exosomes in human semen carry a distinctive repertoire of small non-coding RNAs with potential regulatory functions. Nucleic Acids Res. 2014, 42, 7290–7304. [Google Scholar] [CrossRef] [PubMed]
- Barranco, I.; Padilla, L.; Parrilla, I.; Álvarez-Barrientos, A.; Pérez-Patiño, C.; Peña, F.J.; Martínez, E.A.; Rodriguez-Martínez, H.; Roca, J. Extracellular vesicles isolated from porcine seminal plasma exhibit different tetraspanin expression profiles. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Shen, J.; Wang, Y.; Pan, C.; Pang, W.; Diao, H.; Dong, W. Boar seminal plasma exosomes maintain sperm function by infiltrating into the sperm membrane. Oncotarget 2016, 7, 58832. [Google Scholar] [CrossRef] [PubMed]
- Rowlison, T.; Comizzoli, P. Transfer of Galectin-3-Binding Protein via Epididymal Extracellular Vesicles Promotes Sperm Fertilizing Ability and Developmental Potential in the Domestic Cat Model. Int. J. Mol. Sci. 2023, 24, 3077. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Xie, Y.; Zhou, C.; Hu, Q.; Gu, T.; Yang, J.; Zheng, E.; Huang, S.; Xu, Z.; Cai, G.; et al. Expression pattern of seminal plasma extracellular vesicle small RNAs in boar semen. Front. Vet. Sci. 2020, 7, 585276. [Google Scholar] [CrossRef] [PubMed]
- Al-Dossary, A.A.; Strehler, E.E.; Martin-DeLeon, P.A.; Drevet, J.R. Expression and secretion of plasma membrane Ca2+-ATPase 4a (PMCA4a) during murine estrus: Association with oviductal exosomes and uptake in sperm. PLoS ONE 2013, 8, e80181. [Google Scholar] [CrossRef] [PubMed]
- Alcântara-Neto, A.S.; Schmaltz, L.; Caldas, E.; Blache, M.-C.; Mermillod, P.; Almiñana, C. Porcine oviductal extracellular vesicles interact with gametes and regulate sperm motility and survival. Theriogenology 2020, 155, 240–255. [Google Scholar] [CrossRef] [PubMed]
- Roca, J.; Rodriguez-Martinez, H.; Padilla, L.; Lucas, X.; Barranco, I. Extracellular vesicles in seminal fluid and effects on male reproduction. Overv. Farm Anim. Pets. Anim. Reprod. Sci. 2021, 16, 106853. [Google Scholar]
- Baskaran, S.; Selvam, M.K.P.; Agarwal, A. Exosomes of male reproduction. Adv. Clin. Chem. 2020, 95, 149–163. [Google Scholar] [PubMed]
- Wang, D.; Jueraitetibaike, K.; Tang, T.; Wang, Y.; Jing, J.; Xue, T.; Ma, J.; Cao, S.; Lin, Y.; Li, X.; et al. Seminal plasma and seminal plasma exosomes of aged male mice affect early embryo implantation via immunomodulation. Front. Immunol. 2021, 12, 723409. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, E.L.; Fuentes, F.B.; Felmer, R.N.; Yeste, M.; Arias, M.E. Extracellular vesicles in mammalian reproduction: A review. Zygote 2022, 30, 440–463. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, L.; Marcianò, V.; Carpino, A. Prostasome-like vesicles stimulate acrosome reaction of pig spermatozoa. Reprod. Biol. Endocrinol. 2008, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dostàlovà, Z.; Calvete, J.J.; Töpfer-Petersen, E. Töpfer-Petersen, Interaction of non-aggregated boar AWN-1 and AQN-3 with phospholipid matrices. A model for coating of spermadhesins to the sperm surface. Biol. Chem. Hoppe-Seyler 1995, 376, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Gatti, J.-L.; MétAyer, S.; Belghazi, M.; Dacheux, F.; Dacheux, J.-L. Identification, proteomic profiling, and origin of ram epididymal fluid exosome-like vesicles. Biol. Reprod. 2005, 72, 1452–1465. [Google Scholar] [CrossRef] [PubMed]
- Kaddour, H.; Lyu, Y.; Welch, J.L.; Paromov, V.; Mandape, S.N.; Sakhare, S.S.; Pandhare, J.; Stapleton, J.T.; Pratap, S.; Dash, C.; et al. Proteomics profiling of autologous blood and semen exosomes from HIV-infected and uninfected individuals reveals compositional and functional variabilities. Mol. Cell. Proteom. 2020, 19, 78–100. [Google Scholar] [CrossRef] [PubMed]
- Llavanera, M.; Delgado-Bermúdez, A.; Olives, S.; Mateo-Otero, Y.; Recuero, S.; Bonet, S.; Fernández-Fuertes, B.; Yeste, M.; Barranco, I. Glutathione S-Transferases Play a Crucial Role in Mitochondrial Function, Plasma Membrane Stability and Oxidative Regulation of Mammalian Sperm. Antioxidants 2020, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Stampolidis, P.; Ullrich, A.; Iacobelli, S. LGALS3BP, lectin galactoside-binding soluble 3 binding protein, promotes oncogenic cellular events impeded by antibody intervention. Oncogene 2015, 34, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Block, A.S.; Saraswati, S.; Lichti, C.F.; Mahadevan, M.; Diekman, A.B. Co-purification of Mac-2 binding protein with galectin-3 and association with prostasomes in human semen. Prostate 2011, 71, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Freour, T.; Com, E.; Barriere, P.; Bouchot, O.; Jean, M.; Masson, D.; Pineau, C. Comparative proteomic analysis coupled with conventional protein assay as a strategy to identify predictors of successful testicular sperm extraction in patients with non-obstructive azoospermia. Andrology 2013, 1, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, F.; Dong, L.; Chang, D.; Yu, X. Seminal plasma biomarkers for predicting successful sperm retrieval in patients with nonobstructive azoospermia: A narrative review of human studies. Basic Clin. Androl. 2023, 33, 9. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, P.; Ho-Tin-Noé, B.; Vranckx, R.; Bouton, M.-C.; Meilhac, O.; Lijnen, H.R.; Guillin, M.-C.; Michel, J.-B.; Anglés-Cano, E. Protease Nexin-1 Inhibits Plasminogen Activation-induced Apoptosis of Adherent Cells. J. Biol. Chem. 2004, 279, 10346–10356. [Google Scholar] [CrossRef] [PubMed]
- Murer, V.; Spetz, J.F.; Hengst, U.; Altrogge, L.M.; de Agostini, A.; Monard, D. Male fertility defects in mice lacking the serine protease inhibitor protease nexin-1. Proc. Natl. Acad. Sci. USA 2001, 98, 3029–3033. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-H.; Lee, R.K.-K.; Hwu, Y.-M.; Chu, S.-L.; Chen, Y.-J.; Chang, W.-C.; Lin, S.-P.; Li, S.-H. SERPINE2, a serine protease inhibitor extensively expressed in adult male mouse reproductive tissues, may serve as a murine sperm decapacitation factor. Biol. Reprod. 2011, 84, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Perez-Patiño, C.; Barranco, I.; Parrilla, I.; Valero, M.L.; Martinez, E.A.; Rodriguez-Martinez, H.; Roca, J. Characterization of the porcine seminal plasma proteome comparing ejaculate portions. J. Proteom. 2016, 142, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Durfey, C.L.; Swistek, S.E.; Liao, S.F.; Crenshaw, M.A.; Clemente, H.J.; Thirumalai, R.V.K.G.; Steadman, C.S.; Ryan, P.L.; Willard, S.T.; Feugang, J.M. Nanotechnology-based approach for safer enrichment of semen with best spermatozoa. J. Anim. Sci. Biotechnol. 2019, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Feugang, J.M.; Youngblood, R.C.; Greene, J.M.; Fahad, A.S.; Monroe, W.A.; Willard, S.T.; Ryan, P.L. Application of quantum dot nanoparticles for potential non-invasive bio-imaging of mammalian spermatozoa. J. Nanobiotechnology 2012, 10, 45. [Google Scholar] [CrossRef] [PubMed]


| Sperm Characteristics | Passed Semen | Failed Semen | p-Values |
|---|---|---|---|
| Total motility (%) | 87.5 ± 0.9 a | 56.3 ± 4.3 b | <0.001 |
| Progressive motility (%) | 51.9 ± 3.4 a | 36.0 ± 4.4 b | 0.002 |
| Normal morphology (%) | 84.0 ± 1.1 a | 53.2 ± 2.4 b | <0.001 |
| Average path velocity (VAP; μm/s) | 74.1 ± 3.3 a | 63.3 ± 4.3 b | 0.045 |
| Straight-line velocity (VSL; μm/s) | 51.0 ± 2.4 | 44.0 ± 3.4 | 0.077 |
| Curvilinear velocity (VCL; μm/s) | 146.8 ± 6.7 a | 124.8 ± 8.3 b | 0.026 |
| Bent tail (%) | 0.7 ± 0.2 | 1.8 ± 0.4 | 0.071 |
| Coiled tail (%) | 3.4 ± 0.5 | 5.3 ± 0.8 | 0.057 |
| Distal droplet (%) | 6.5 ± 0.7 | 8.1 ± 0.8 | 0.156 |
| Proximal droplet (%) | 16.5 ± 1.7 a | 24.6 ± 2.5 b | 0.019 |
| Linearity (%) | 38.3 ± 1.1 | 38.8 ± 1.9 | 0.705 |
| Straightness (%) | 71.5 ± 1.2 | 71.4 ± 1.7 | 0.414 |
| Amplitude head (ALH; μm) | 6.5 ± 0.2 | 6.1 ± 0.3 | 0.169 |
| Beat cross frequency (BCF; Hz) | 34.8 ± 0.6 | 33.6 ± 1.0 | 0.167 |
| Time (h) | Total Motility (%) | Progressive Motility (%) | Norm. Morphology (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | P-EVs | F-EVs | Control | P-EVs | F-EVs | Control | P-EVs | F-EVs | |
| 0 | 65.0 ± 3.8 a | 64.5 ± 3.3 a | 64.5 ± 3.3 a | 26.2 ± 2.5 a | 22.4 ± 6.3 a | 22.4 ± 6.3 a | 86.3 ± 2.0 a | 84 ± 4.3 a | 84 ± 4.3 a |
| 1 | 49.1 ± 3.4 b | 40.9 ± 7.8 b | 36.2 ± 1.7 b | 27.5 ± 0.9 a | 20.2 ± 6.2 a | 19.8 ± 2.8 a | 82.8 ± 0.3 a | 79 ± 7.1 a | 80 ± 0.3 a |
| 2 | 53.7 ± 0.6 ab | 42.6 ± 4.6 ab | 41.1 ± 2.4 ab | 28.7 ± 1.1 a | 30.7 ± 4.4 a | 23.5 ± 0.1 a | 82.1 ± 1.9 a | 85 ± 1.8 a | 86.6 ± 1.2 a |
| Gene Accession No. | Protein Name | Gene ID | p Value |
|---|---|---|---|
| SP-EV downregulated proteins (In Passed vs. Failed) | |||
| gi|456752927 | Lectin, galactoside-binding, soluble, 3 binding protein | LGALS3BP | <0.001 |
| SP-EV upregulated proteins (In Passed vs. Failed) | |||
| gi|28435507 | Nexin-1 | PN-1 | 0.01 |
| gi|3599989 | Seminal plasma protein pB1 precursor | BSP1 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dlamini, N.H.; Santos-Rivera, M.; Vance-Kouba, C.K.; Pechanova, O.; Pechan, T.; Feugang, J.M. Integrating Near-Infrared Spectroscopy and Proteomics for Semen Quality Biosensing. Biosensors 2025, 15, 456. https://doi.org/10.3390/bios15070456
Dlamini NH, Santos-Rivera M, Vance-Kouba CK, Pechanova O, Pechan T, Feugang JM. Integrating Near-Infrared Spectroscopy and Proteomics for Semen Quality Biosensing. Biosensors. 2025; 15(7):456. https://doi.org/10.3390/bios15070456
Chicago/Turabian StyleDlamini, Notsile H., Mariana Santos-Rivera, Carrie K. Vance-Kouba, Olga Pechanova, Tibor Pechan, and Jean M. Feugang. 2025. "Integrating Near-Infrared Spectroscopy and Proteomics for Semen Quality Biosensing" Biosensors 15, no. 7: 456. https://doi.org/10.3390/bios15070456
APA StyleDlamini, N. H., Santos-Rivera, M., Vance-Kouba, C. K., Pechanova, O., Pechan, T., & Feugang, J. M. (2025). Integrating Near-Infrared Spectroscopy and Proteomics for Semen Quality Biosensing. Biosensors, 15(7), 456. https://doi.org/10.3390/bios15070456








