Carboxymethyl Dextran-Based Biosensor for Simultaneous Determination of IDO-1 and IFN-Gamma in Biological Material
Abstract
1. Introduction
2. Experimental Process
2.1. Materials and Reagents
2.2. SPRi Apparatus
2.3. Biological Material
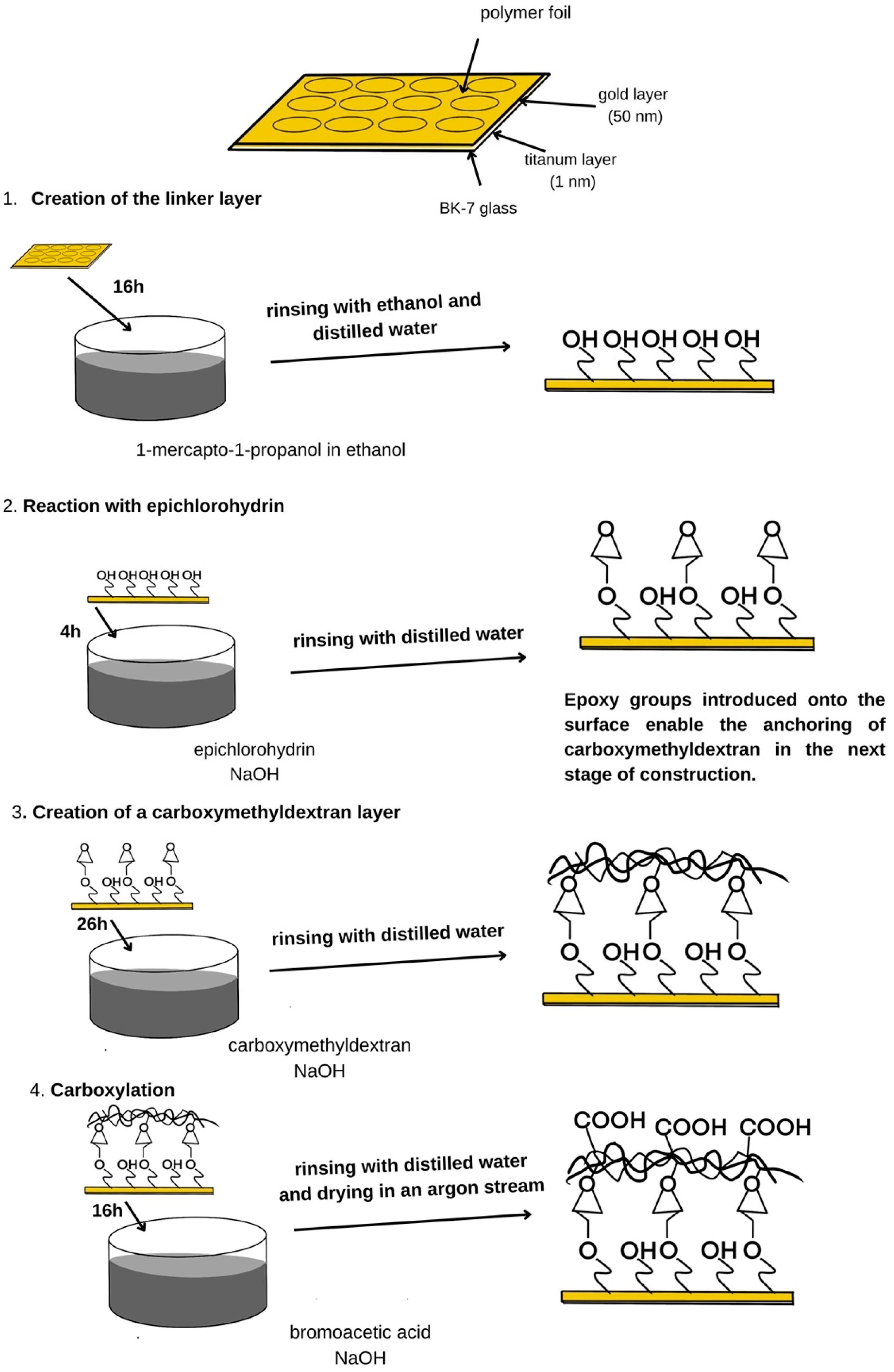
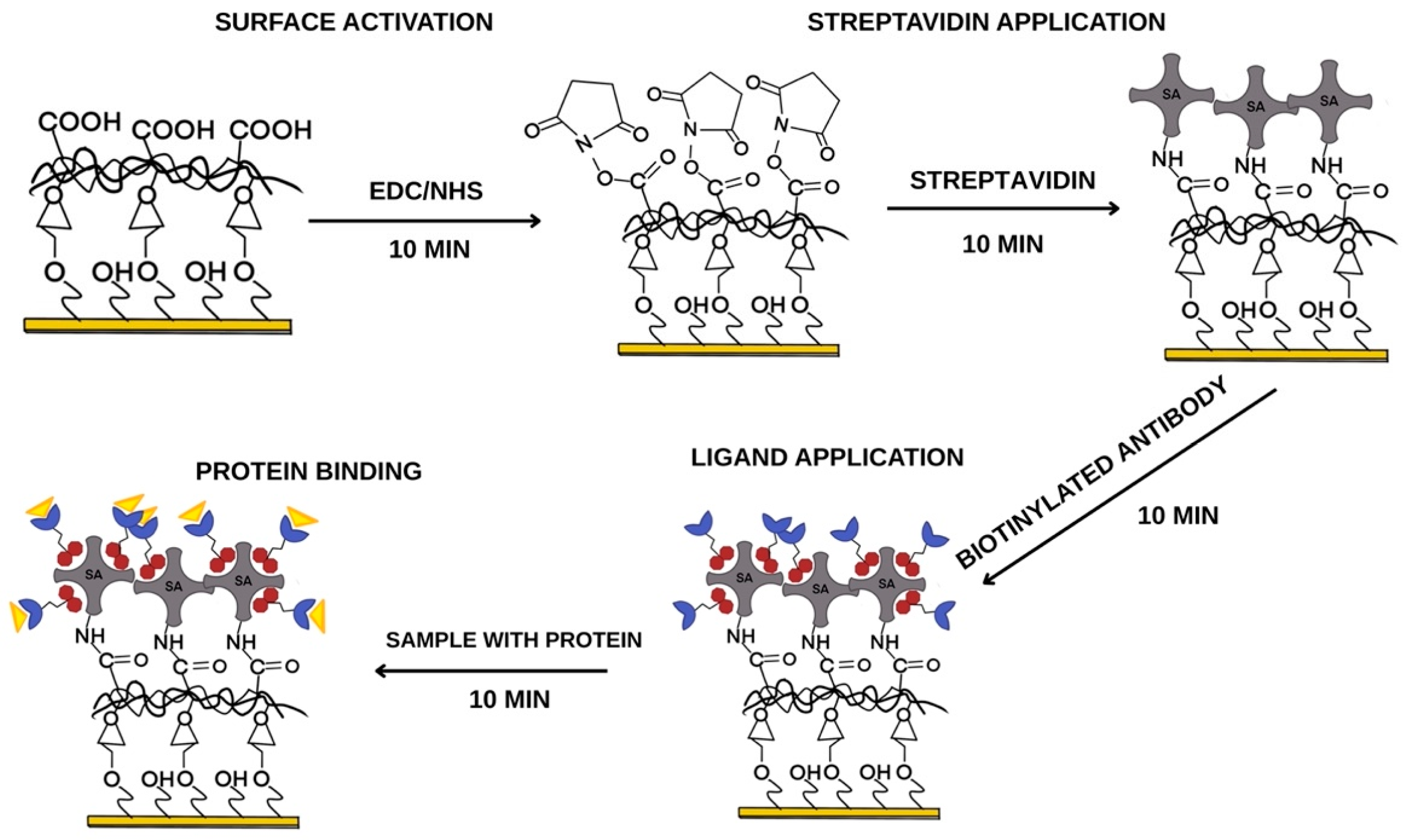
2.4. Construction of the Biosensor
2.4.1. Receptor Layer Preparation
2.4.2. SPRi Measurement
3. Optimization of Method Conditions
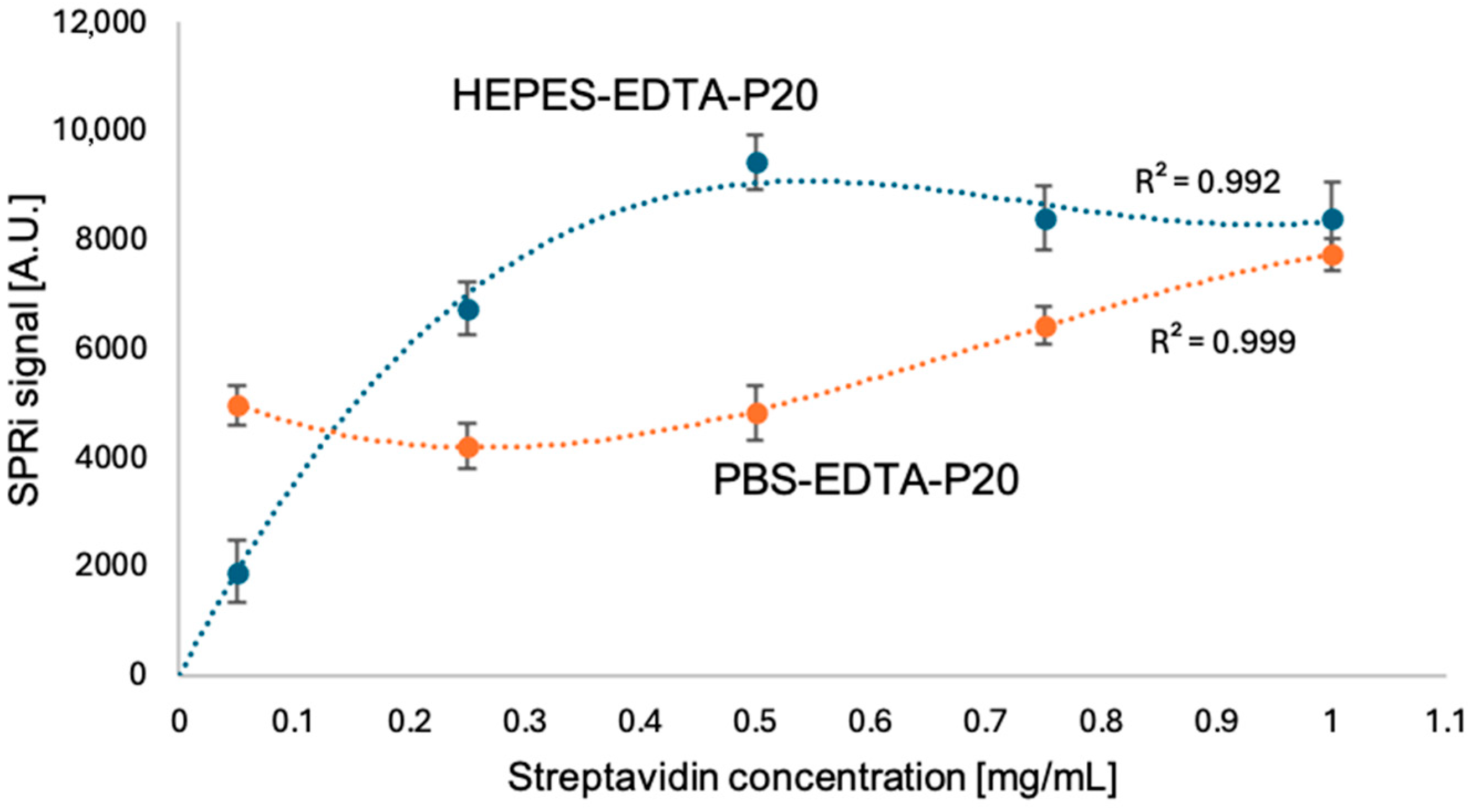
3.1. Selecting the Appropriate Streptavidin Concentration
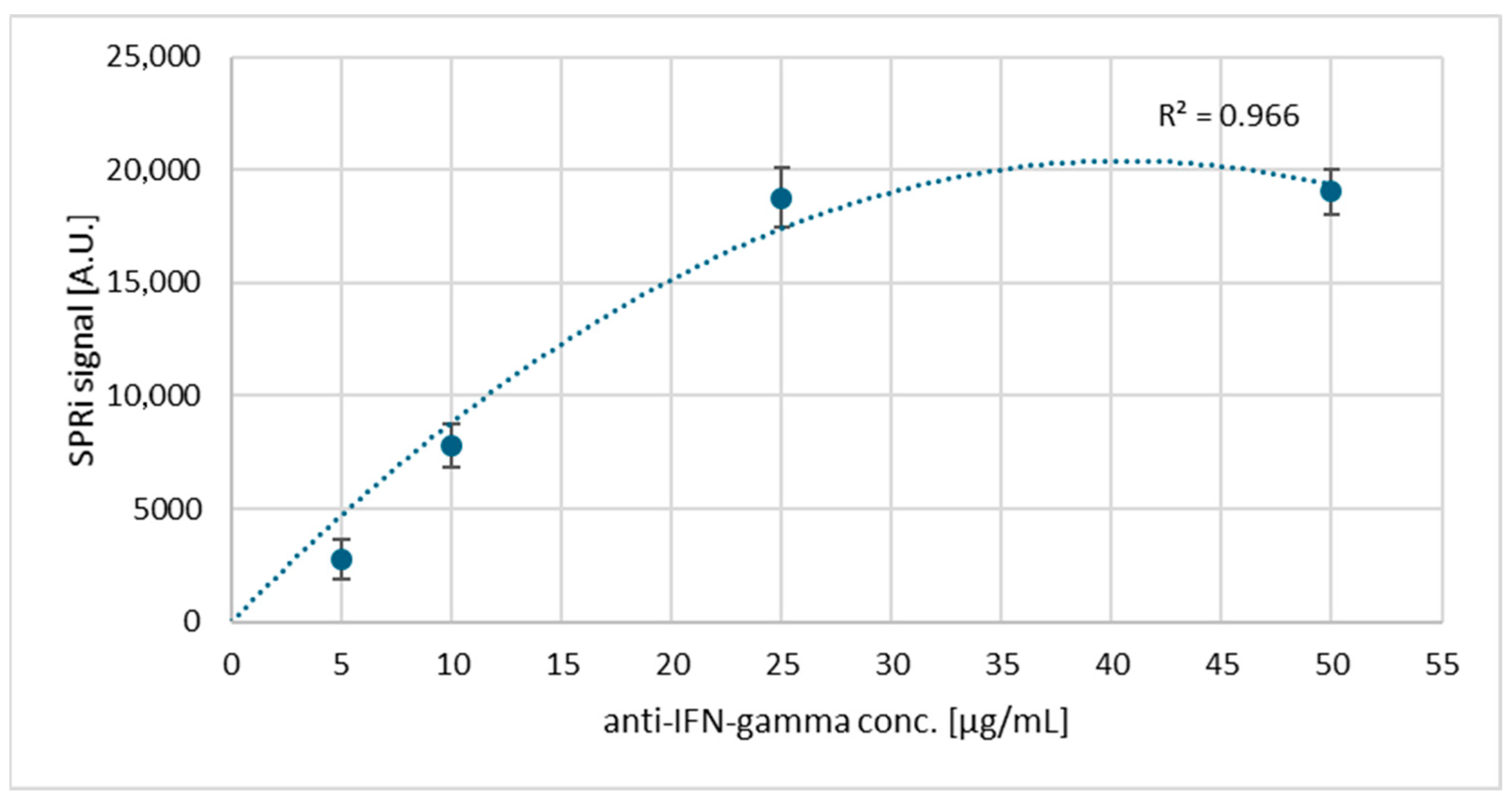
3.2. Saturation of the Biosensor Surface with Antibodies: Selection of the Appropriate Ligand Concentration
3.3. Selection of Rinse Frequency
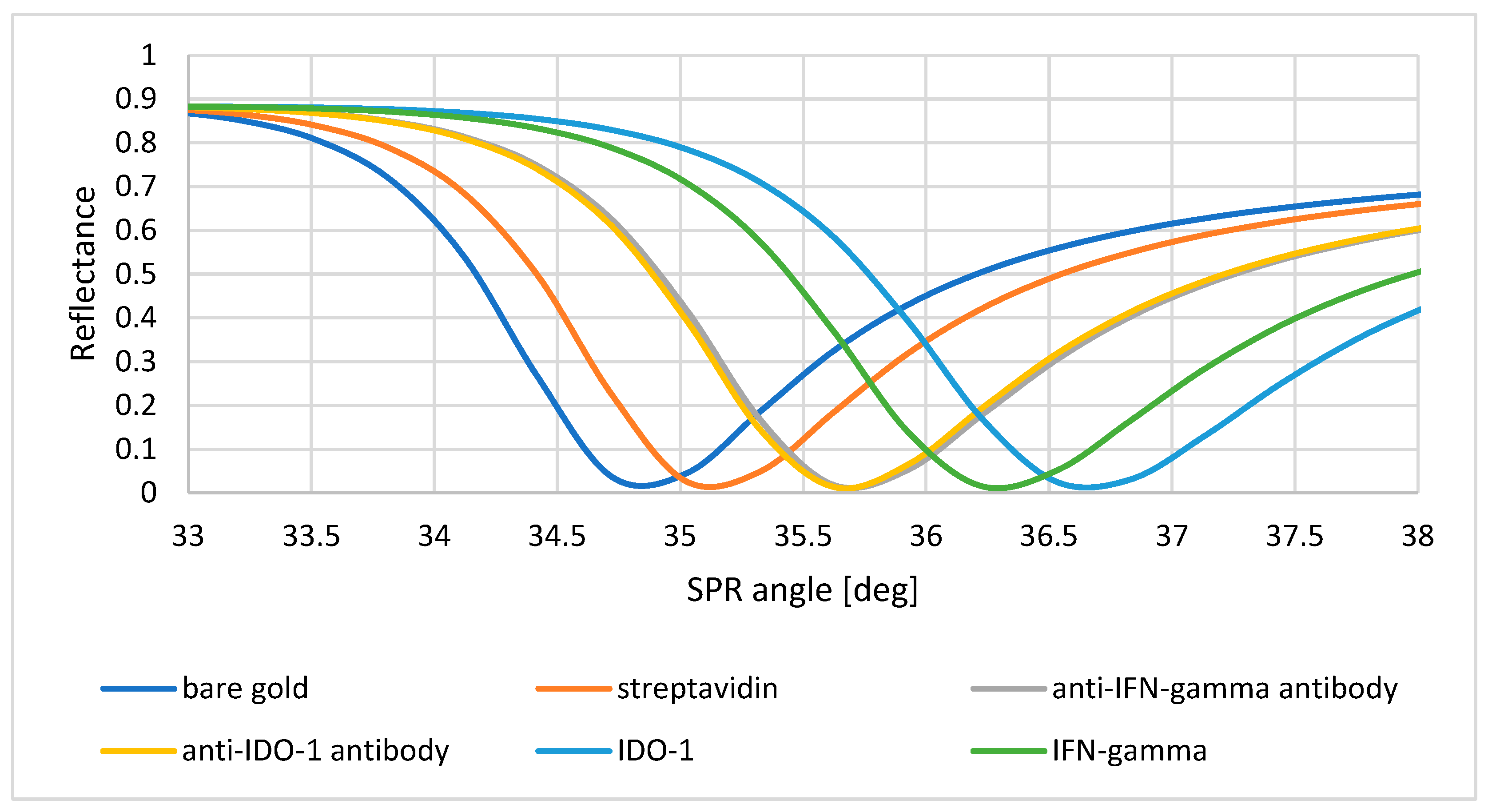
3.4. Proof of Layer Formation on the Sensor Surface Using SPR Curves
3.5. Association Time and Regeneration Cycles
4. Validation of the Analytical Method
4.1. Analytical Response of the Biosensor, Limit of Detection and Quantification
4.2. Precision and Accuracy
4.3. Repeatability and Selectivity of the Method
4.4. Measurement in Biological Material
- (A)
- plasma samples were diluted 5-fold for IFN-γ determinations and 2-fold for IDO-1;
- (B)
- urine samples were diluted 5-fold for IFN-γ determinations and 1-fold for IDO-1.
Analysis of the Obtained Results Using the SPRi and ELISA Method
5. Results and Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1
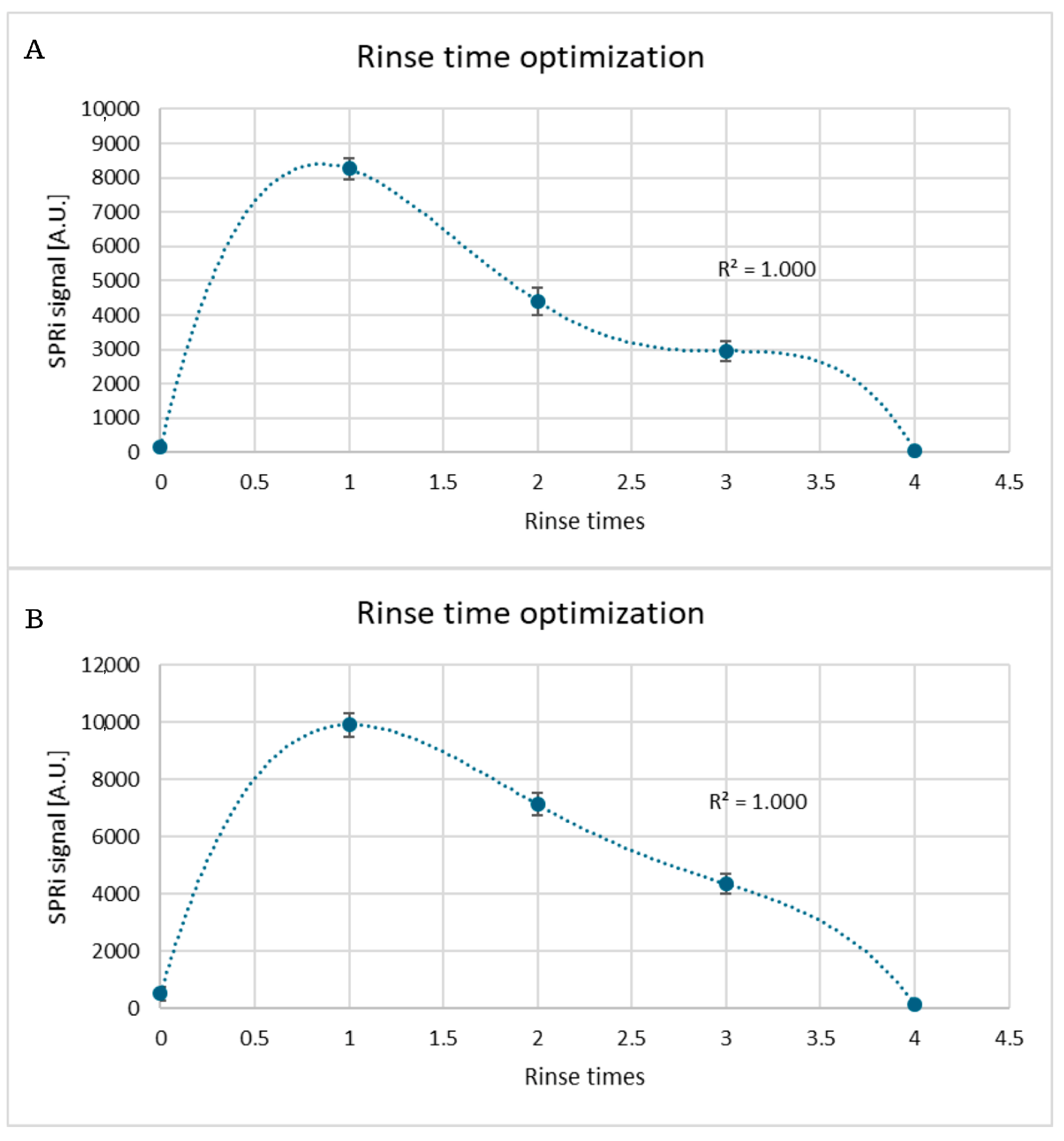
Appendix A.2
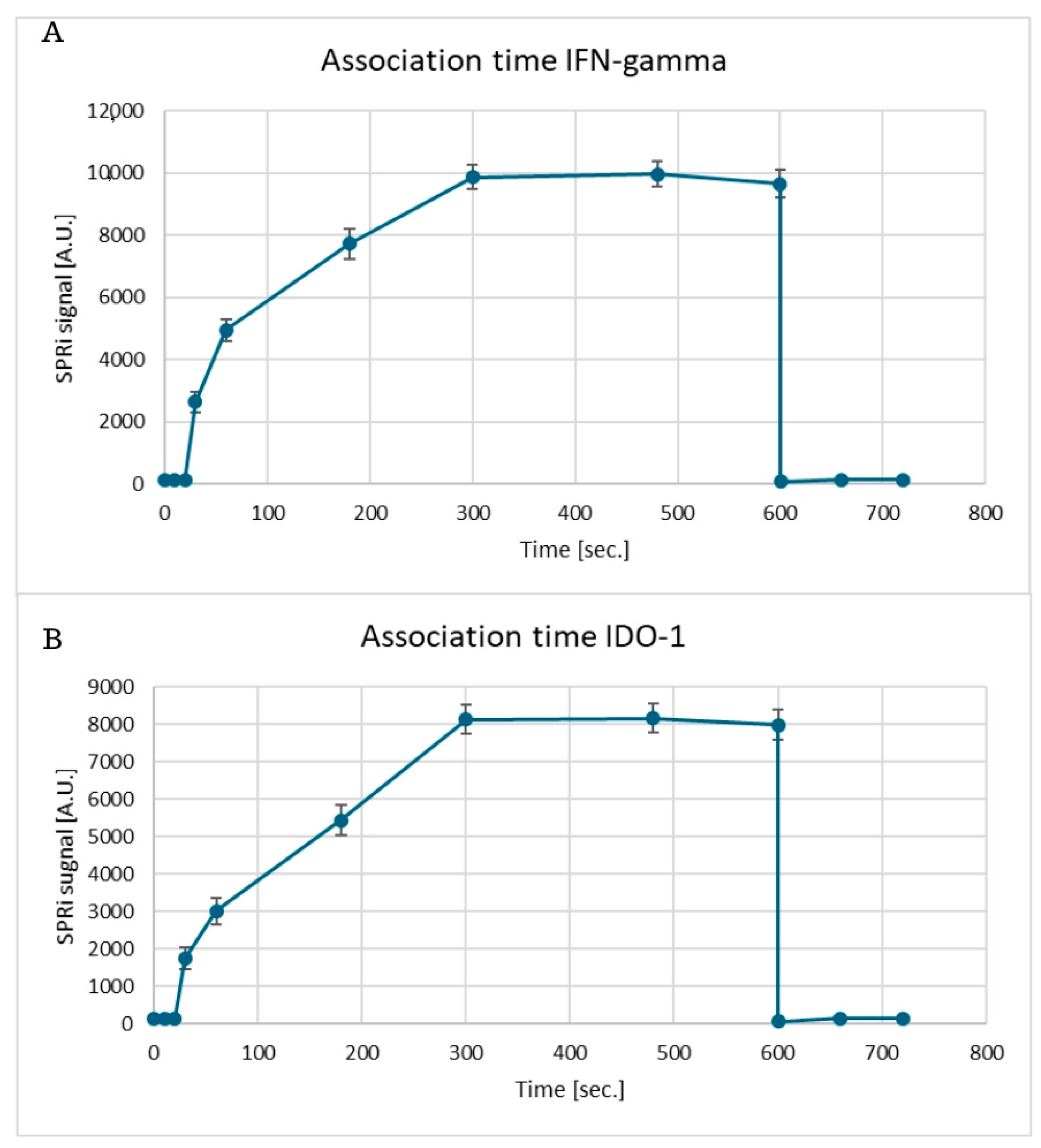
Appendix A.3
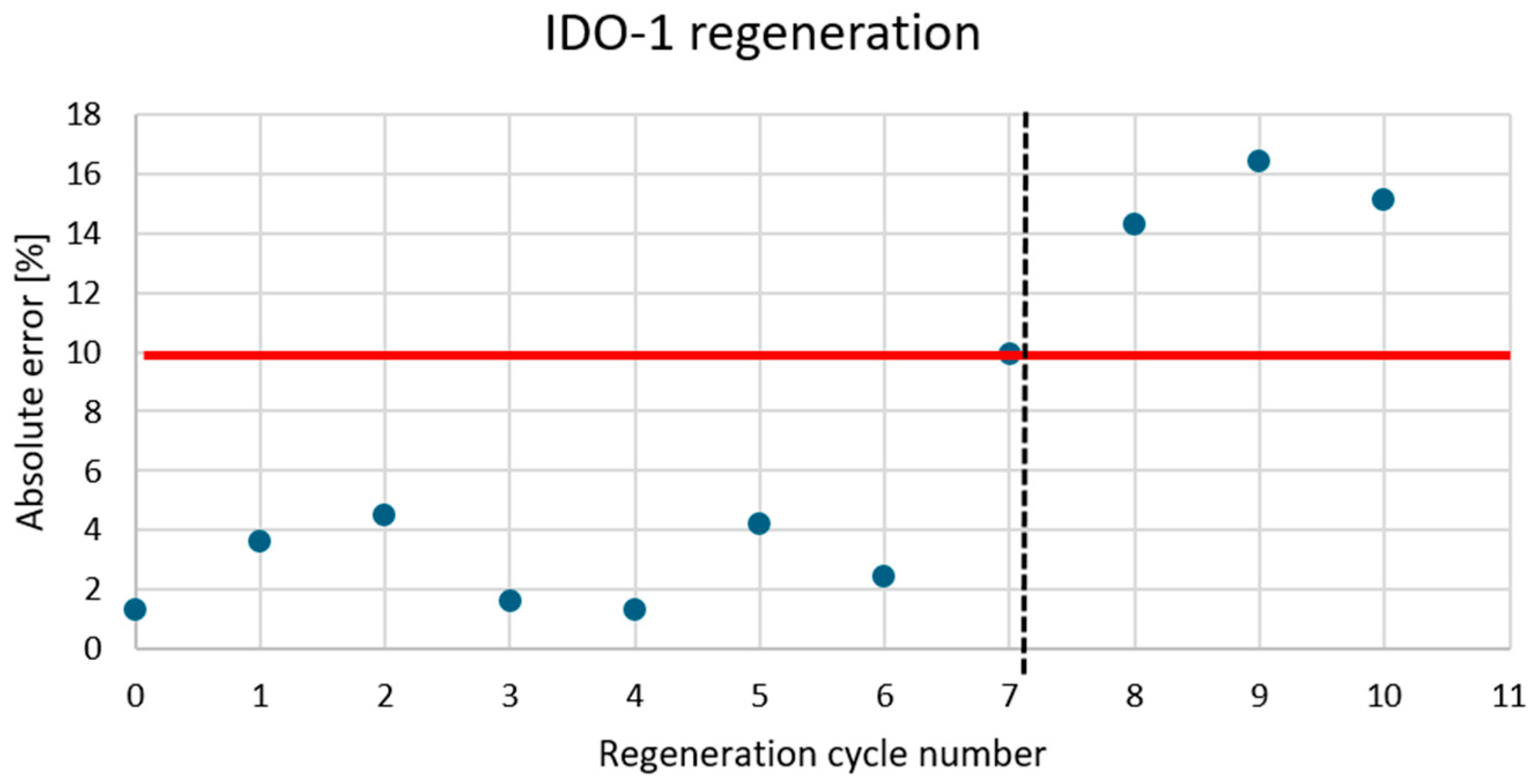
Appendix A.4
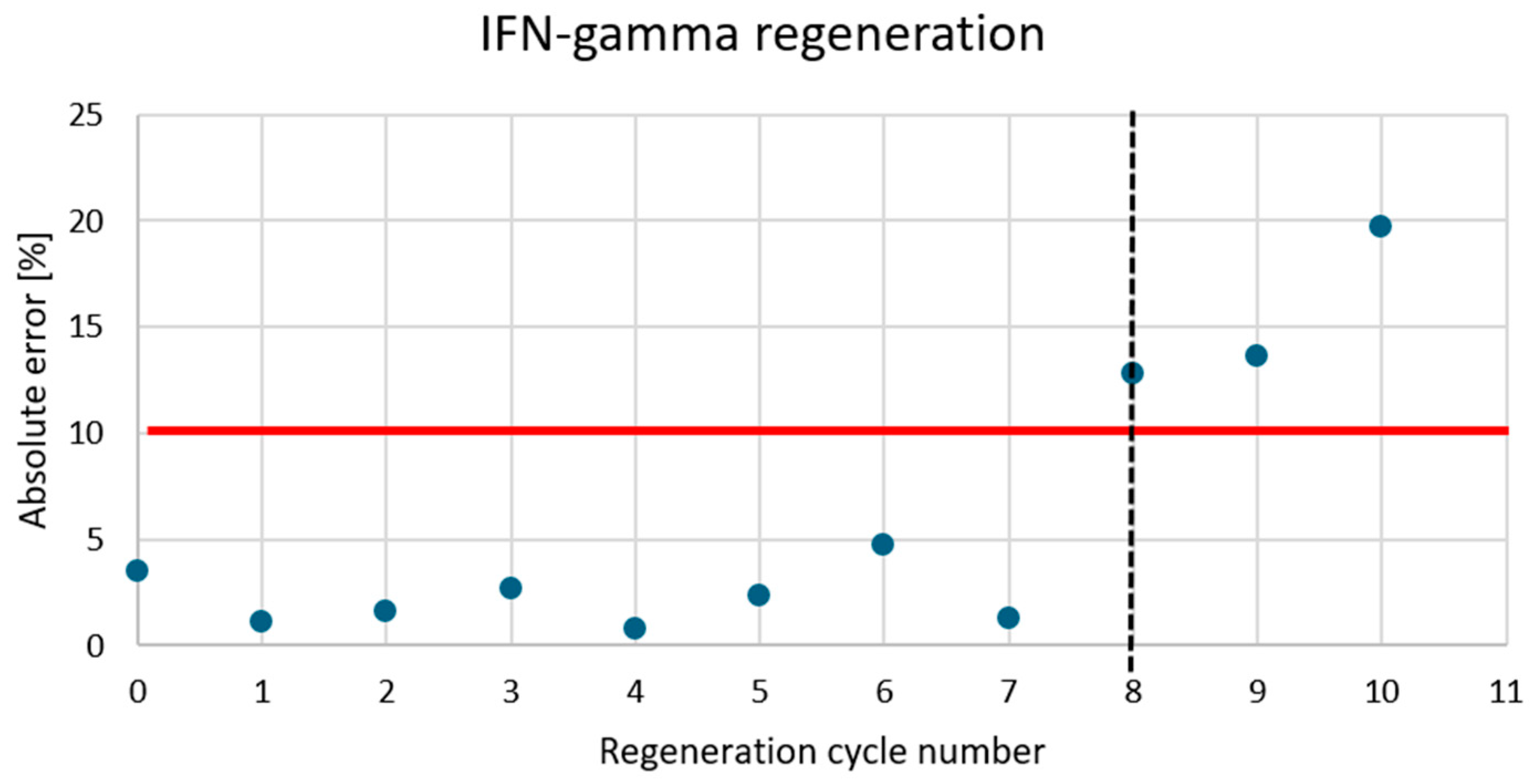
Appendix A.5
| Selectivity of the Method | |||||||
|---|---|---|---|---|---|---|---|
| IDO-1 (antibody) | SPRi signal | Mean | Concentration [ng/mL] | <LOD | |||
| I | II | III | |||||
| IFN-γ | 5 ng/mL | 1539.71 | 1531.35 | 1544.79 | 1538.62 | 0.01 | |
| VEGF-A | 1541.59 | 1536.93 | 1556.67 | 1545.06 | 0.02 | ||
| VEGF-R2 | 1537.2 | 1545.28 | 1540.48 | 1540.99 | 0.02 | ||
| FGF-2 | 1539.88 | 1538.97 | 1537.41 | 1538.75 | 0.01 | ||
| NRP-1 | 1531.05 | 1544.03 | 1547.76 | 1540.95 | 0.02 | ||
| KAT B | 1544.18 | 1531.47 | 1534.76 | 1536.80 | 0.01 | ||
| KAT S | 1546.58 | 1545.14 | 1535.12 | 1542.28 | 0.02 | ||
| IFN-γ (antibody) | SPRi signal | Mean | Concentration [pg/mL] | <LOD | |||
| I | II | III | |||||
| IDO-1 | 5 ng/mL | 3240.09 | 3240.19 | 3238.08 | 3239.45 | 1.54 | |
| VEGF-A | 3236.85 | 3240.61 | 3239.24 | 323,890 | 0.73 | ||
| VEGF-R2 | 3240.66 | 3238.8 | 3239.12 | 3239.53 | 1.64 | ||
| FGF-2 | 3239.65 | 3240.11 | 3237.06 | 3238.94 | 0.79 | ||
| NRP-1 | 3240.68 | 3237.89 | 3237.38 | 3238.65 | 0.36 | ||
| KAT B | 3238.01 | 3241.37 | 3236.94 | 3238.77 | 0.54 | ||
| KAT S | 3235.86 | 3240.96 | 3240.63 | 3239.15 | 1.09 | ||
Appendix A.6
| Plasma Sample Number | Concentration of IDO-1 [pg/mL] | Concentration of IDO-1 ELISA Test [pg/mL] | Concentration of IFN-γ [pg/mL] | Concentration of IFN-γ ELISA Test [pg/mL] |
|---|---|---|---|---|
| 1 | 1509.72 | 1520 | 19.85 | 113.7 |
| 2 | 456.70 | 200 | 2.20 | 1.63 |
| 3 | 1628.80 | 1830 | 1.00 | 0.54 |
| 4 | 689.50 | 560 | 15.72 | 165 |
| 5 | 679.99 | 730 | 3.59 | 4.88 |
| 6 | 662.44 | 710 | 5.12 | 4.34 |
| 7 | 2070.24 | 1663 | 9.64 | 10.21 |
| 8 | 1891.92 | 1800 | 2.50 | 2.17 |
| 9 | 732.97 | 490 | 7.46 | 7.05 |
| 10 | 663.85 | 320 | 3.04 | 3.28 |
| 11 | 502.35 | 440 | 3.12 | 3.79 |
| 12 | 3013.72 | 2980 | 14.55 | 215.2 |
| 13 | 41,110.98 | 45,870 | 6.66 | 4.93 |
| 14 | 5616.21 | 5890 | 3.49 | 4.31 |
| 15 | 8132.62 | 8330 | 17.65 | 236.8 |
| 16 | 556.04 | 380 | 2.15 | 2.17 |
| 17 | 882.31 | 830 | 9.76 | 9.76 |
| 18 | 870.71 | 570 | 6.52 | 7.59 |
| 19 | 538.88 | 590 | 5.64 | 3.31 |
| 20 | 539.17 | 580 | 0.99 | 1.17 |
| 21 | 681.42 | 600 | 3.51 | 2.17 |
| 22 | 857.27 | 470 | 10.78 | 11.11 |
| 23 | 877.36 | 950 | 2.41 | 3.22 |
| 24 | 665.94 | 460 | 3.24 | 2.58 |
| 25 | 720.20 | 670 | 1.85 | 2.17 |
| 26 | 950.90 | 320 | 1.51 | 1.31 |
| 27 | 457.22 | 640 | 0.91 | 0.59 |
| 1C | 1419.58 | 320 | 0.94 | 1.08 |
| 2C | 1070.46 | 330 | 13.61 | 55.34 |
| 3C | 2591.03 | 3220 | 3.81 | 2.71 |
Appendix A.7
| Urine Sample Number | Concentration of IDO-1 [pg/mL] | Concentration of IDO-1 ELISA Test [pg/mL] | Concentration of IFN-γ [pg/mL] | Concentration of IFN-γ ELISA Test [pg/mL] |
|---|---|---|---|---|
| 1 | 19,510.56 | 23,750 | 1.99 | 1.63 |
| 2 | 25,945.57 | 30,060 | 1.72 | 1.14 |
| 3 | 7502.61 | 8290 | 3.27 | 3.21 |
| 4 | 919.71 | 860 | 1.79 | 1.17 |
| 5 | 814.21 | 640 | 2.96 | 3.25 |
| 6 | 1344.73 | 1750 | 0.78 | 1.08 |
| 7 | 2048.75 | 1640 | 0.51 | 0.54 |
| 8 | 2586.19 | 2370 | 1.84 | 1.08 |
| 9 | 642.32 | 950 | 0.94 | 1.17 |
| 10 | 1005.24 | 630 | 0.83 | 0.93 |
| 11 | 560.36 | 580 | 2.90 | 2.12 |
| 12 | 647.91 | 780 | 3.48 | 3.18 |
| 13 | 452.52 | 310 | 3.21 | 3.13 |
| 14 | 615.57 | 440 | 3.26 | 2.91 |
| 15 | 1122.50 | 1530 | 3.35 | 3.27 |
| 16 | 493.11 | 600 | 8.35 | 103.2 |
| 17 | 1954.25 | 1840 | 8.85 | 12.17 |
| 18 | 689.10 | 780 | 6.99 | 14.27 |
| 19 | 728.06 | 800 | 5.72 | 4.19 |
| 20 | 621.15 | 670 | 5.55 | 5.14 |
| 21 | 831.24 | 67,350 | 4.26 | 3.29 |
| 22 | 1853.03 | 1690 | 2.89 | 2.17 |
| 23 | 1308.13 | 1480 | 5.71 | 5.11 |
| 24 | 999.90 | 650 | 3.33 | 3.22 |
| 25 | 7620.39 | 7810 | 4.30 | 4.36 |
| 26 | 772.79 | 730 | 2.37 | 2.17 |
| 1C | 686.73 | 660 | 5.62 | 616.8 |
| 2C | 500.58 | 260 | 1.45 | 1.08 |
| 3C | 613.92 | 840 | 0.72 | 0.54 |
| 4C | 645.75 | 750 | 4.22 | 6.23 |
References
- Yeung, A.W.S.; Terentis, A.C.; King, N.J.C.; Thomas, S.R. Role of Indoleamine 2,3-Dioxygenase in Health and Disease. Clin. Sci. 2015, 129, 601–672. [Google Scholar] [CrossRef] [PubMed]
- Rios-Avila, L.; Nijhout, H.F.; Reed, M.C.; Sitren, H.S.; Gregory, J.F. A Mathematical Model of Tryptophan Metabolism via the Kynurenine Pathway Provides Insights into the Effects of Vitamin B-6 Deficiency, Tryptophanloading, and Induction of Tryptophan 2,3-Dioxygenase on Tryptophan Metabolites. J. Nutr. 2013, 143, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Gong, J.; Liu, Y. Indoleamine 2, 3-Dioxygenase Regulation of Immune Response (Review). Mol. Med. Rep. 2018, 17, 4867–4873. [Google Scholar] [CrossRef]
- Lancellotti, S.; Novarese, L.; De Cristofaro, R. Biochemical Properties of Indoleamine 2,3-Dioxygenase: From Structure to Optimized Design of Inhibitors. Curr. Med. Chem. 2011, 18, 2205–2214. [Google Scholar] [CrossRef] [PubMed]
- King, N.J.C.; Thomas, S.R. Molecules in Focus: Indoleamine 2,3-Dioxygenase. Int. J. Biochem. Cell Biol. 2007, 39, 2167–2172. [Google Scholar] [CrossRef]
- Liu, M.; Wang, X.; Wang, L.; Ma, X.; Gong, Z.; Zhang, S.; Li, Y. Targeting the IDO1 Pathway in Cancer: From Bench to Bedside. J. Hematol. Oncol. 2018, 11, 100. [Google Scholar] [CrossRef]
- Ding, S.; Yang, R.; Meng, J.; Guan, X.; Hong, Y.; Xu, J.; Qu, L.; Ji, J.; Yi, W.; Zou, Q.; et al. Prognostic and Immune Correlation of IDO1 Promoter Methylation in Breast Cancer. Sci. Rep. 2024, 14, 27836. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, L.; Yang, J.; Wu, Q.; Sun, X.; Zhang, J.; Yu, Y.; Zhang, L.; Gao, J.; Zhou, Q.; et al. Integrated Analyses Reveal IDO1 as a Prognostic Biomarker Coexpressed with PD-1 on Tumor-Associated Macrophages in Esophageal Squamous Cell Carcinoma. Front. Pharmacol. 2024, 15, 1466779. [Google Scholar] [CrossRef]
- Guangzhao, L.; Xin, W.; Miaoqing, W.; Wenjuan, M.; Ranyi, L.; Zhizhong, P.; Rongxin, Z.; Gong, C. IDO1 Inhibitor Enhances the Effectiveness of PD-1 Blockade in Microsatellite Stable Colorectal Cancer by Promoting Macrophage pro-Inflammatory Phenotype Polarization. Cancer Immunol. Immunother. CII 2025, 74, 71. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, F.; Wang, X.; Liu, K. The Role of Indoleamine 2, 3-Dioxygenase 1 in Regulating Tumor Microenvironment. Cancers 2022, 14, 2756. [Google Scholar] [CrossRef]
- Zaidi, M.R.; Merlino, G. The Two Faces of Interferon-γ in Cancer. Clin. Cancer Res. 2011, 17, 6118–6124. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Hertzog, P.J.; Ravasi, T.; Hume, D.A. Interferon-γ: An Overview of Signals, Mechanisms and Functions. J. Leukoc. Biol. 2004, 75, 163–189. [Google Scholar] [CrossRef] [PubMed]
- Werner, E.R.; Bitterlich, G.; Fuchs, D.; Hausen, A.; Reibnegger, G.; Szabo, G.; Dierich, M.P.; Wachter, H. Human macrophages degrade tryptophan upon induction by interferon-gamma. Life Sci. 1987, 41, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Moretti, S.; Menicali, E.; Nucci, N.; Voce, P.; Colella, R.; Melillo, R.M.; Liotti, F.; Morelli, S.; Fallarino, F.; Macchiarulo, A.; et al. Signal Transducer and Activator of Transcription 1 Plays a Pivotal Role in RET/PTC3 Oncogene-Induced Expression of Indoleamine 2,3-Dioxygenase 1. J. Biol. Chem. 2017, 292, 1785–1797. [Google Scholar] [CrossRef]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-γ in Tumor Progression and Regression: A Review. Biomark. Res. 2020, 8, 49. [Google Scholar] [CrossRef]
- Yang, S.L.; Tan, H.X.; Niu, T.T.; Liu, Y.K.; Gu, C.J.; Li, D.J.; Li, M.Q.; Wang, H.Y. The IFN-γ-IDO1-kynureine pathway-induced autophagy in cervical cancer cell promotes phagocytosis of macrophage. Int. J. Biol. Sci. 2022, 17, 339–352. [Google Scholar] [CrossRef]
- Mohammadi, S.; Sedighi, S.; Memarian, A.; Yazdani, Y. Overexpression of interferon-γ and indoleamine 2, 3-dioxygenase in systemic lupus erythematosus: Relationship with the disease activity. LaboratoriumsMedizin 2017, 41, 41–47. [Google Scholar] [CrossRef]
- Hornyák, L.; Dobos, N.; Koncz, G.; Karányi, Z.; Páll, D.; Szabó, Z.; Halmos, G.; Székvölgyi, L. The Role of Indoleamine-2,3-Dioxygenase in Cancer Development, Diagnostics, and Therapy. Front. Immunol. 2018, 9, 151. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, B.; Liu, J.; Sun, P.; Liu, H. An Overview on the Methods of Determining the Activity of Indoleamine 2, 3-Dioxygenase 1. J. Drug Target. 2019, 27, 724–731. [Google Scholar] [CrossRef]
- Aktivite, E.I.-; Belirlenmesi, D. Determination of Indoleamine 2,3 Dioxygenase Enzyme (IDO-1) Activity Levels in COVID-19 Patients According to Disease Severity, and Relationship with Inflammatory Markers. FLORA 2024, 29, 502–508. [Google Scholar] [CrossRef]
- Bao, Y.S.; Ji, Y.; Zhao, S.L.; Ma, L.L.; Xie, R.J.; Na, S.P. Serum Levels and Activity of Indoleamine2,3-Dioxygenase and Tryptophanyl-TRNA Synthetase and Their Association with Disease Severity in Patients with Chronic Kidney Disease. Biomarkers 2013, 18, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Huang, X.; Zeng, C.; Sun, D.; Liu, F.; Zhang, J.; Liao, Q.; Luo, S.; Xu, W.; Xiao, Y.; et al. The role of indoleamine 2,3-dioxygenase 1 in early-onset post-stroke depression. Front. Immunol. 2023, 14, 1125634. [Google Scholar] [CrossRef] [PubMed]
- Carvajal-Hausdorf, D.E.; Mani, N.; Velcheti, V.; Schalper, K.A.; Rimm, D.L. Objective Measurement and Clinical Significance of IDO1 Protein in Hormone Receptor-Positive Breast Cancer. J. Immunother. Cancer 2017, 5, 81. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; She, Q.; You, R.; Huang, Y.; Chen, J.; Su, H.; Lu, Y. “On-off” SERS sensor triggered by IDO for non-interference and ultrasensitive quantitative detection of IDO. Sens. Actuators B Chem. 2021, 344, 130166. [Google Scholar] [CrossRef]
- Yerrapragada, R.M.; Mampallil, D. Interferon-γ Detection in Point of Care Diagnostics: Short Review. Talanta 2022, 245, 123428. [Google Scholar] [CrossRef]
- Sánchez-Tirado, E.; González-Cortés, A.; Yáñez-Sedeño, P.; Pingarrón, J.M. Electrochemical Immunosensor for the Determination of the Cytokine Interferon Gamma (IFN-γ) in Saliva. Talanta 2020, 211, 120761. [Google Scholar] [CrossRef]
- Pohanka, M. Immunoassay of Interferon Gamma by Quartz Crystal Microbalance Biosensor. Talanta 2020, 218, 121167. [Google Scholar] [CrossRef]
- Kim, H.J.; Jang, C.H. Liquid Crystal-Based Aptasensor for the Detection of Interferon-Γ and Its Application in the Diagnosis of Tuberculosis Using Human Blood. Sens. Actuators B Chem. 2019, 282, 574–579. [Google Scholar] [CrossRef]
- Saputra, H.A.; Chung, J.H.; Kwon, R.J.; Jannath, K.A.; Park, D.S.; Shim, Y.B. Ultrasensitive Interferon-Gamma Sensor with a Bifunctionalized Conducting Polymer Nanobioconjugate for Therapy-Progress Monitoring of Cancer Patients. Sens. Actuators B Chem. 2024, 398, 134739. [Google Scholar] [CrossRef]
- Kwon, M.; Ko, S.K.; Jang, M.; Kim, G.H.; Ryoo, I.J.; Son, S.; Ryu, H.W.; Oh, S.R.; Lee, W.K.; Kim, B.Y.; et al. Inhibitory Effects of Flavonoids Isolated from Sophora Flavescens on Indoleamine 2,3-Dioxygenase 1 Activity. J. Enzym. Inhib. Med. Chem. 2019, 34, 1481–1488. [Google Scholar] [CrossRef]
- Šípová, H.; Ševců, V.; Kuchař, M.; Ahmad, J.N.; Mikulecký, P.; Šebo, P.; Malý, P.; Homola, J. Sensitive Detection of Interferon-Gamma with Engineered Proteins and Surface Plasmon Resonance Biosensor. Procedia Eng. 2011, 25, 940–943. [Google Scholar] [CrossRef][Green Version]
- Stigter, E.C.A.; de Jong, G.J.; van Bennekom, W.P. An Improved Coating for the Isolation and Quantitation of Interferon-Gamma in Spiked Plasma Using Surface Plasmon Resonance (SPR). Biosens. Bioelectron. 2005, 21, 474–482. [Google Scholar] [CrossRef]
- Chuang, T.L.; Chang, C.C.; Chu-Su, Y.; Wei, S.C.; Zhao, X.H.; Hsueh, P.R.; Lin, C.W. Disposable Surface Plasmon Resonance Aptasensor with Membrane-Based Sample Handling Design for Quantitative Interferon-Gamma Detection. Lab Chip 2014, 14, 2968–2977. [Google Scholar] [CrossRef] [PubMed]
- Takemura, K. Surface Plasmon Resonance (SPR)- and Localized SPR (LSPR)-Based Virus Sensing Systems: Optical Vibration of Nano- and Micro-Metallic Materials for the Development of Next-Generation Virus Detection Technology. Biosensors 2021, 11, 250. [Google Scholar] [CrossRef]
- Oldak, L.; Sankiewicz, A.; Żelazowska-Rutkowska, B.; Cylwik, B.; Lukaszewski, Z.; Skoczylas, M.; Gorodkiewicz, E. Two SPRi Biosensors for the Determination of Cathepsin S in Blood Plasma. Talanta 2021, 225, 121900. [Google Scholar] [CrossRef]
- Matuszczak, E.; Tylicka, M.; Dębek, W.; Tokarzewicz, A.; Gorodkiewicz, E.; Hermanowicz, A. Concentration of UHCL1 in the Serum of Children with Acute Appendicitis, Before and After Surgery, and Its Correlation with CRP and Prealbumin. J. Investig. Surg. 2018, 31, 136–141. [Google Scholar] [CrossRef]
- Gorodkiewicz, E.; Regulska, E.; Wojtulewski, K. Development of an SPR imaging biosensor for determination of cathepsin G in saliva and white blood cells Microchim. Acta 2011, 173, 407–413. [Google Scholar] [CrossRef][Green Version]
- Zhang, Q.; Li, Y.; Hu, Q.; Xie, R.; Zhou, W.; Liu, X.; Wang, Y. Smartphone Surface Plasmon Resonance Imaging for the Simultaneous and Sensitive Detection of Acute Kidney Injury Biomarkers with Noninvasive Urinalysis. Lab Chip 2022, 22, 4941–4949. [Google Scholar] [CrossRef]
- Nakano, S.; Nagao, M.; Yamasaki, T.; Morimura, H.; Hama, N.; Iijima, Y.; Shinomiya, H.; Tanaka, M.; Yamamoto, M.; Matsumura, Y.; et al. Evaluation of a Surface Plasmon Resonance Imaging-Based Multiplex O-Antigen Serogrouping for Escherichia coli Using Eleven Major Serotypes of Shiga-Toxin-Producing E. Coli. J. Infect. Chemother. 2018, 24, 443–448. [Google Scholar] [CrossRef]
- Hassan, N.; Mohamed, A. Determination of Interferon Gamma Protein in serum of Breast Cancer Patients Using the ELISA. J. Appl. Sci. Nanotechnol. 2021, 2, 37–48. [Google Scholar] [CrossRef]
- Koper-Lenkiewicz, O.M.; Gińdzieńska-Sieśkiewicz, E.; Kamińska, J.; Milewska, A.J.; Kowal-Bielecka, O.; Matowicka-Karna, J. Could IL-1β, IL-6, IFN-γ, and sP-selectin serum levels be considered as objective and quantifiable markers of rheumatoid arthritis severity and activity? Reumatologia 2022, 60, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Arinola, G.O.; Abdullahi, I.; Rahamon, S.K.; Fasasi, Z.B.; Adedeji, O.O.; Kehinde, A.; Bakare, A.A. Activities of plasma indoleamine-2, 3-dioxygenase (IDO) enzyme in Nigerian patients with lung diseases: Basis for tryptophan supplementation or IDO inhibitor use. Egypt. J. Bronchol. 2023, 17, 2. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Kato, S.; Nesline, M.K.; Conroy, J.M.; DePietro, P.; Pabla, S.; Kurzrock, R. Indoleamine 2,3-dioxygenase (IDO) inhibitors and cancer immunotherapy. Cancer Treat. Rev. 2022, 110, 102461. [Google Scholar] [CrossRef] [PubMed]


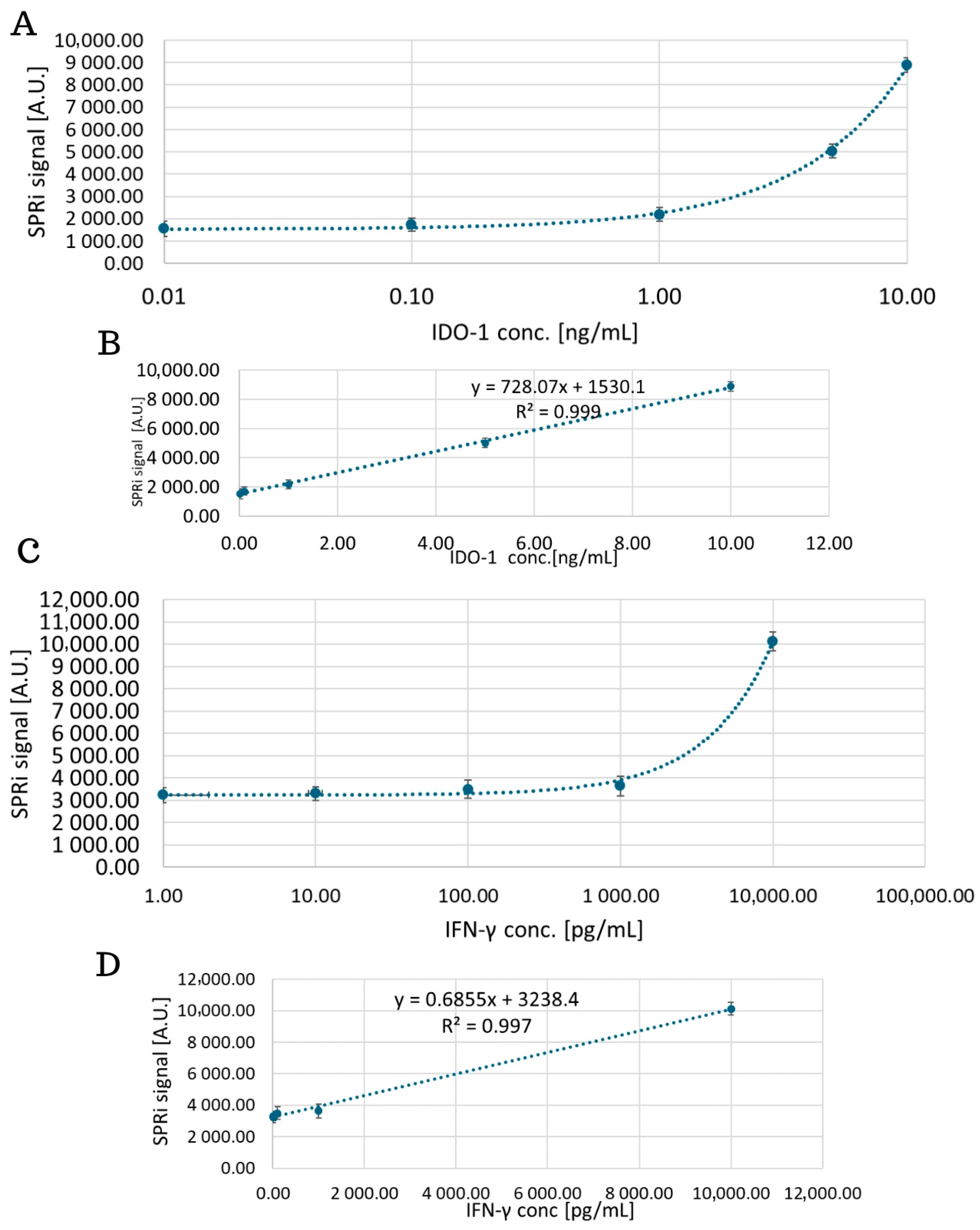

| Analyte | LOD | LOQ |
|---|---|---|
| IDO-1 | 0.27 ng/mL (0.1 ng/mL [22] 0.278 ng/mL [25]) | 0.81 ng/mL |
| IFN-γ | 1.76 pg/mL (5.7 pg/mL [27] 17 pg/mL [28]) | 5.29 pg/mL |
| Precision and Accuracy | |||||
|---|---|---|---|---|---|
| IDO-1 | Concentration [ng/mL] | ||||
| 0.81 | 1.00 | 5.00 | 10.00 | ||
| I measurement | 0.87 | 1.01 | 5.03 | 9.89 | |
| II measurement | 0.94 | 0.89 | 4.93 | 9.94 | |
| III measurement | 0.83 | 0.98 | 5.01 | 9.96 | |
| Mean concentration [ng/mL] | 0.88 | 0.98 | 4.99 | 9.93 | |
| SD [ng/mL] | 0.06 | 0.06 | 0.05 | 0.04 | Mean |
| CV [%] | 6.33 | 6.51 | 1.06 | 0.36 | 3.56 |
| %error | 8.64 | 4.00 | 0.20 | 0.70 | 3.39 |
| IFN-γ | Concentration [pg/mL] | ||||
| 5.29 | 10.00 | 1000.00 | 10,000.00 | ||
| I measurement | 5.78 | 9.92 | 996.76 | 9998.64 | |
| II measurement | 5.69 | 10.46 | 1002.45 | 9985.35 | |
| III measurement | 5.34 | 9.79 | 993.32 | 9997.75 | |
| Mean concentration [pg/mL] | 5.60 | 10.06 | 997.51 | 9993.91 | |
| SD [pg/mL] | 0.23 | 0.36 | 4.61 | 7.43 | Mean |
| CV [%] | 4.15 | 3.53 | 0.46 | 0.07 | 2.05 |
| %error | 5.92 | 0.57 | 0.25 | 0.06 | 1.70 |
| Blood Serum | |||
|---|---|---|---|
| IDO-1 | Concentration [ng/mL] | IFN-γ | Concentration [pg/mL] |
| I | 38.28 | I | 190.1 |
| II | 35.97 | II | 188.76 |
| III | 37.64 | III | 195.03 |
| IV | 39.76 | IV | 183.54 |
| V | 37.55 | V | 197.37 |
| Mean concentration [ng/mL] | 37.84 | Mean concentration [pg/mL] | 190.96 |
| SD [ng/mL] | 1.37 | SD [pg/mL] | 5.44 |
| CV [%] | 3.62 | CV [%] | 2.85 |
| Tissue homogenate | |||
| IDO-1 | Concentration [ng/mL] | IFN-γ | Concentration [pg/mL] |
| I | 22.66 | I | 2.4 |
| II | 21.75 | II | 2.23 |
| III | 23.02 | III | 2.51 |
| IV | 22.89 | IV | 2.42 |
| V | 20.93 | V | 2.32 |
| Mean concentration [ng/mL] | 22.25 | Mean concentration [pg/mL] | 2.38 |
| SD [ng/mL] | 0.89 | SD [pg/mL] | 0.11 |
| CV [%] | 4.00 | CV [%] | 4.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zielinska, Z.; Sankiewicz, A.; Kalinowska, N.; Zelazowska-Rutkowska, B.; Guszcz, T.; Ambroziak, L.; Kondratiuk, M.; Gorodkiewicz, E. Carboxymethyl Dextran-Based Biosensor for Simultaneous Determination of IDO-1 and IFN-Gamma in Biological Material. Biosensors 2025, 15, 444. https://doi.org/10.3390/bios15070444
Zielinska Z, Sankiewicz A, Kalinowska N, Zelazowska-Rutkowska B, Guszcz T, Ambroziak L, Kondratiuk M, Gorodkiewicz E. Carboxymethyl Dextran-Based Biosensor for Simultaneous Determination of IDO-1 and IFN-Gamma in Biological Material. Biosensors. 2025; 15(7):444. https://doi.org/10.3390/bios15070444
Chicago/Turabian StyleZielinska, Zuzanna, Anna Sankiewicz, Natalia Kalinowska, Beata Zelazowska-Rutkowska, Tomasz Guszcz, Leszek Ambroziak, Miroslaw Kondratiuk, and Ewa Gorodkiewicz. 2025. "Carboxymethyl Dextran-Based Biosensor for Simultaneous Determination of IDO-1 and IFN-Gamma in Biological Material" Biosensors 15, no. 7: 444. https://doi.org/10.3390/bios15070444
APA StyleZielinska, Z., Sankiewicz, A., Kalinowska, N., Zelazowska-Rutkowska, B., Guszcz, T., Ambroziak, L., Kondratiuk, M., & Gorodkiewicz, E. (2025). Carboxymethyl Dextran-Based Biosensor for Simultaneous Determination of IDO-1 and IFN-Gamma in Biological Material. Biosensors, 15(7), 444. https://doi.org/10.3390/bios15070444






