Assessment of Microvascular Disturbances in Children with Type 1 Diabetes—A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
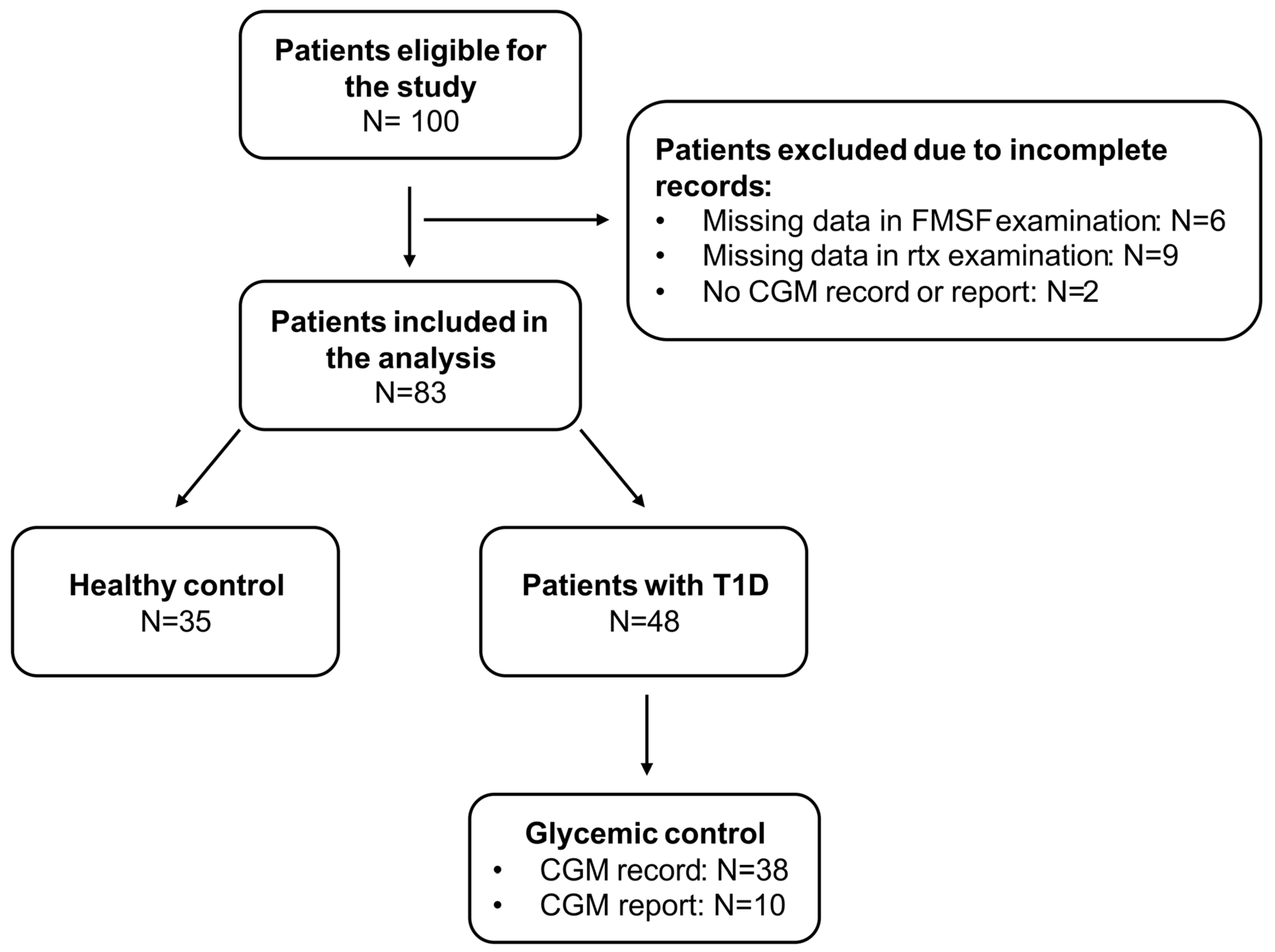
2.1. Study Group
2.2. Laboratory Tests
2.3. Flow-Mediated Skin Fluorescence (FMSF)
2.3.1. Technical Principles of FMSF Method
2.3.2. Implementation of the FMSF Technique in the Present Study
2.4. Adaptive Optics Retinal Camera (Rtx)
2.5. Carotid Intima-Media Thickness (cIMT)
2.6. Continuous Glucose Monitoring (CGM)
2.7. Statistical Analysis
3. Results
3.1. Clinical Characteristics of Patients and CGM Metrics
3.2. Comparisons of cIMT, FMSF, and Rtx Examination
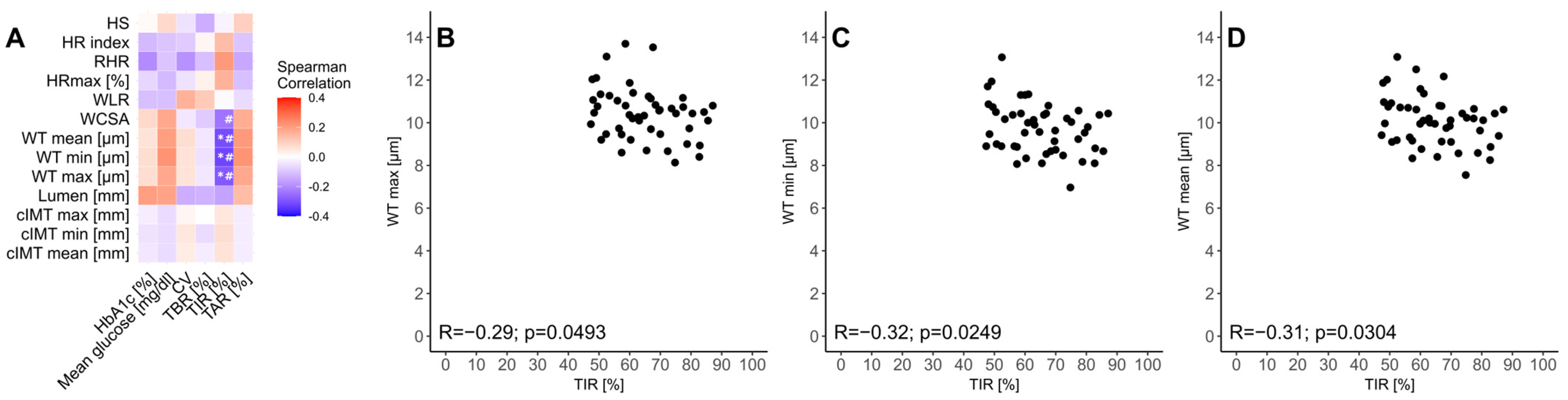
3.3. Relationship Between Glycemic Control and cIMT, FMSF, and Rtx Examinations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bjornstad, P.; Dart, A.; Donaghue, K.C.; Dost, A.; Feldman, E.L.; Tan, G.S.; Wadwa, R.P.; Zabeen, B.; Marcovecchio, M.L. ISPAD Clinical Practice Consensus Guidelines 2022: Microvascular and macrovascular complications in children and adolescents with diabetes. Pediatr. Diabetes 2022, 23, 1432–1450. [Google Scholar] [CrossRef] [PubMed]
- Bertoluci, M.C.; Cé, G.V.; da Silva, A.M.; Wainstein, M.V.; Boff, W.; Puñales, M. Endothelial dysfunction as a predictor of cardiovascular disease in type 1 diabetes. World J. Diabetes 2015, 6, 679. [Google Scholar] [CrossRef]
- Lespagnol, E.; Dauchet, L.; Pawlak-Chaouch, M.; Balestra, C.; Berthoin, S.; Feelisch, M.; Roustit, M.; Boissière, J.; Fontaine, P.; Heyman, E. Early Endothelial Dysfunction in Type 1 Diabetes Is Accompanied by an Impairment of Vascular Smooth Muscle Function: A Meta-Analysis. Front. Endocrinol. 2020, 11, 203. [Google Scholar] [CrossRef]
- Katarzynska, J.; Cholewinski, T.; Sieron, L.; Marcinek, A.; Gebicki, J. Flowmotion Monitored by Flow Mediated Skin Fluorescence (FMSF): A Tool for Characterization of Microcirculatory Status. Front. Physiol. 2020, 11, 538470. [Google Scholar] [CrossRef]
- Lombardo, M.; Serrao, S.; Devaney, N.; Parravano, M.; Lombardo, G. Adaptive Optics Technology for High-Resolution Retinal Imaging. Sensors 2012, 13, 334–366. [Google Scholar] [CrossRef]
- Baltǎ, F.; Cristescu, I.E.; Mirescu, A.E.; Baltǎ, G.; Zemba, M.; Tofolean, I.T. Investigation of Retinal Microcirculation in Diabetic Patients Using Adaptive Optics Ophthalmoscopy and Optical Coherence Angiography. J. Diabetes Res. 2022, 2022, 1516668. [Google Scholar] [CrossRef]
- Katarzynska, J.; Borkowska, A.; Czajkowski, P.; Los, A.; Szczerbinski, L.; Milewska-Kranc, A.; Marcinek, A.; Kretowski, A.; Cypryk, K.; Gebicki, J. Flow Mediated Skin Fluorescence technique reveals remarkable effect of age on microcirculation and metabolic regulation in type 1 diabetes. Microvasc. Res. 2019, 124, 19–24. [Google Scholar] [CrossRef]
- Katarzynska, J.; Borkowska, A.; Los, A.; Marcinek, A.; Cypryk, K.; Gebicki, J. Flow-Mediated Skin Fluorescence (FMSF) Technique for Studying Vascular Complications in Type 2 Diabetes. J. Diabetes Sci. Technol. 2020, 14, 693–694. [Google Scholar] [CrossRef]
- Hellmann, M.; Tarnawska, M.; Dudziak, M.; Dorniak, K.; Roustit, M.; Cracowski, J.L. Reproducibility of flow mediated skin fluorescence to assess microvascular function. Microvasc. Res. 2017, 113, 60–64. [Google Scholar] [CrossRef]
- Tarnawska, M.; Dorniak, K.; Kaszubowski, M.; Dudziak, M.; Hellmann, M. A pilot study with flow mediated skin fluorescence: A novel device to assess microvascular endothelial function in coronary artery disease. Cardiol. J. 2018, 25, 120–127. [Google Scholar] [CrossRef]
- Romanowska-Kocejko, M.; Dudziak, M.; Hellmann, M. Nicotinamide adenine dinucleotide fluorescence to assess microvascular disturbances in post-COVID-19 patients. Cardiol. J. 2022, 29, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Romanowska-Kocejko, M.; Braczko, A.; Jędrzejewska, A.; Żarczyńska-Buchowiecka, M.; Kocejko, T.; Kutryb-Zając, B.; Hellmann, M. Follow-up assessment of the microvascular function in patients with long COVID. Microvasc. Res. 2025, 157, 104748. [Google Scholar] [CrossRef] [PubMed]
- Rechciński, T.; Cieślik-Guerra, U.; Siedlecki, P.; Uznańska-Loch, B.; Trzos, E.; Wierzbowska-Drabik, K.; Szymczyk, E.; Wejner-Mik, P.; Kurpesa, M.; Lipiec, P.; et al. Flow-mediated skin fluorescence: A novel method for the estimation of sleep apnea risk in healthy persons and cardiac patients. Cardiol. J. 2022, 29, 948–953. [Google Scholar] [CrossRef]
- Gebicki, J.; Filipiak, T.; Marcinek, A.; Wozniacka, A. Assessment of NADH/NAD+ Redox Imbalance in Psoriatic Lesions Using the FMSF Technique: Therapeutic Aspects. Sensors 2023, 23, 8718. [Google Scholar] [CrossRef]
- Zaleska-Żmijewska, A.; Wawrzyniak, Z.M.; Dąbrowska, A.; Szaflik, J.P. Adaptive Optics (rtx1) High-Resolution Imaging of Photoreceptors and Retinal Arteries in Patients with Diabetic Retinopathy. J. Diabetes Res. 2019, 2019, 9548324. [Google Scholar] [CrossRef]
- Mariotti, L.; Devaney, N.; Lombardo, G.; Lombardo, M. Analysis of Cone Mosaic Reflectance Properties in Healthy Eyes and in Eyes With Nonproliferative Diabetic Retinopathy Over Time. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4057–4067. [Google Scholar] [CrossRef][Green Version]
- Cristescu, I.-E.; Ochinciuc, R.; Balta, F.; Zagrean, L. High-resolution imaging of diabetic retinopathy lesions using an adaptive optics retinal camera. Rom. J. Ophthalmol. 2019, 63, 29–34. [Google Scholar] [CrossRef]
- Libman, I.; Haynes, A.; Lyons, S.; Pradeep, P.; Rwagasor, E.; Tung, J.Y.; Jefferies, C.A.; Oram, R.A.; Dabelea, D.; Craig, M.E. ISPAD Clinical Practice Consensus Guidelines 2022: Definition, epidemiology, and classification of diabetes in children and adolescents. Pediatr. Diabetes 2022, 23, 1160–1174. [Google Scholar] [CrossRef]
- Różdżyńska-Świątkowska, A.; Kułaga, Z.; Grajda, A.; Gurzkowska, B.; Góźdź, M.; Wojtyło, M.; Świąder, A.; Litwin, M.; OLA OLAF group. Height, weight and body mass index references for growth and nutritional status assessment in children and adolescents 3–18 year of age. Stand. Med. Pediatr 2013, 1, 11–21. [Google Scholar]
- Godara, P.; Dubis, A.M.; Roorda, A.; Duncan, J.L.; Carroll, J. Adaptive optics retinal imaging: Emerging clinical applications. Optom. Vis. Sci. 2010, 87, 930–941. [Google Scholar] [CrossRef]
- Nardin, M.; Coschignano, M.A.; Rossini, C.; Ciuceis, C.D.; Caletti, S.; Rizzoni, M.; Docchio, F.; Porteri, E.; Rizzoni, D. Methods of evaluation of microvascular structure: State of the art. Eur. J. Transl. Clin. Med. 2018, 1, 9–19. [Google Scholar]
- Rosenbaum, D.; Mattina, A.; Koch, E.; Rossant, F.; Gallo, A.; Kachenoura, N.; Paques, M.; Redheuil, A.; Girerd, X. Effects of age, blood pressure and antihypertensive treatments on retinal arterioles remodeling assessed by adaptive optics. J. Hypertens. 2016, 34, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Koch, E.; Rosenbaum, D.; Brolly, A.; Sahel, J.A.; Chaumet-Riffaud, P.; Girerd, X.; Rossant, F.; Paques, M. Morphometric analysis of small arteries in the human retina using adaptive optics imaging: Relationship with blood pressure and focal vascular changes. J. Hypertens. 2014, 32, 890–898. [Google Scholar] [CrossRef]
- Matuszewski, W.; Szklarz, M.; Wołos-Kłosowicz, K.; Harazny, J.M.; Bandurska-Stankiewicz, E. High-Resolution Imaging of Cones and Retinal Arteries in Patients with Diabetes Mellitus Type 1 Using Adaptive Optics (rtx1). Biomedicines 2024, 12, 863. [Google Scholar] [CrossRef]
- de Bock, M.; Agwu, J.C.; Deabreu, M.; Dovc, K.; Maahs, D.M.; Marcovecchio, M.L.; Mahmud, F.H.; Nóvoa-Medina, Y.; Priyambada, L.; Smart, C.E. ISPAD Clinical Practice Consensus Guidelines 2024: Glycemic Targets. Horm. Res. Paediatr. 2024, 97, 546–554. [Google Scholar] [CrossRef]
- Cherubini, V.; Bonfanti, R.; Casertano, A.; De Nitto, E.; Iannilli, A.; Lombardo, F.; Maltoni, G.; Marigliano, M.; Bassi, M.; Minuto, N.; et al. Time In Range in Children with Type 1 Diabetes Using Treatment Strategies Based on Nonautomated Insulin Delivery Systems in the Real World. Diabetes Technol. Ther. 2020, 22, 509–515. [Google Scholar] [CrossRef]
- Dovc, K.; Lanzinger, S.; Cardona-Hernandez, R.; Tauschmann, M.; Marigliano, M.; Cherubini, V.; Preikša, R.; Schierloh, U.; Clapin, H.; AlJaser, F. Association of Achieving Time in Range Clinical Targets With Treatment Modality Among Youths With Type 1 Diabetes. JAMA Netw. Open 2023, 6, e230077. [Google Scholar] [CrossRef]
- Araszkiewicz, A.; Bandurska-Stankiewicz, E.; Borys, S.; Broncel, M. Standards of Care in Diabetes. The position of Diabetes Poland–2024. Curr. Top. Diabetes 2024, 3, 1–348. [Google Scholar] [CrossRef]
- Beck, R.W.; Bergenstal, R.M.; Riddlesworth, T.D.; Kollman, C.; Li, Z.; Brown, A.S.; Close, K.L. Validation of Time in Range as an Outcome Measure for Diabetes Clinical Trials. Diabetes Care 2019, 42, 400–405. [Google Scholar] [CrossRef]
- Kupis, M.; Wawrzyniak, Z.M.; Szaflik, J.P.; Zaleska-Żmijewska, A. Retinal Photoreceptors and Microvascular Changes in the Assessment of Diabetic Retinopathy Progression: A Two-Year Follow-Up Study. Diagnostics 2023, 13, 2513. [Google Scholar] [CrossRef]
- Ueno, Y.; Iwase, T.; Goto, K.; Tomita, R.; Ra, E.; Yamamoto, K.; Terasaki, H. Association of changes of retinal vessels diameter with ocular blood flow in eyes with diabetic retinopathy. Sci. Rep. 2021, 11, 4653. [Google Scholar] [CrossRef] [PubMed]
- Stefański, A.; Wolf, J.; Harazny, J.M.; Miszkowska-Nagórna, E.; Wolnik, B.; Murawska, J.; Narkiewicz, K.; Schmieder, R.E. Impact of type 1 diabetes and its duration on wall-to-lumen ratio and blood flow in retinal arterioles. Microvasc. Res. 2023, 147, 104499. [Google Scholar] [CrossRef] [PubMed]
- Sampani, K.; Mujat, M.; Patel, A.H.; Kang, C.; Iftimia, N.; Chatziralli, I.; Sun, J.K. Characterizing Vascular Wall and Lumen Caliber in Eyes with Diabetic Retinopathy Based on Adaptive Optics Scanning Laser Ophthalmoscopy. Diagnostics 2024, 14, 2020. [Google Scholar] [CrossRef]
- Simonett, J.M.; Scarinci, F.; Picconi, F.; Giorno, P.; De Geronimo, D.; Di Renzo, A.; Varano, M.; Frontoni, S.; Parravano, M. Early microvascular retinal changes in optical coherence tomography angiography in patients with type 1 diabetes mellitus. Acta Ophthalmol. 2017, 95, e751–e755. [Google Scholar] [CrossRef]
- Szewczuk, A.; Zaleska-Żmijewska, A.; Dziedziak, J.; Szaflik, J.P. Clinical Application of Adaptive Optics Imaging in Diagnosis, Management, and Monitoring of Ophthalmological Diseases: A Narrative Review. Med. Sci. Monit. 2023, 29, e941926. [Google Scholar] [CrossRef]
- Rizzoni, D.; Rosei, E.A. Small artery remodeling in hypertension and diabetes. Curr. Hypertens. Rep. 2006, 8, 90–95. [Google Scholar] [CrossRef]
- Rizzoni, D.; Rosei, E.A. Small artery remodeling in diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 587–592. [Google Scholar] [CrossRef]
- Rosei, E.A.; Rizzoni, D. Small artery remodelling in diabetes. J. Cell Mol. Med. 2010, 14, 1030–1036. [Google Scholar] [CrossRef]
- Shah, V.N.; Kanapka, L.G.; Akturk, H.K.; Polsky, S.; Forlenza, G.P.; Kollman, C.; Beck, R.W.; Snell-Bergeon, J.K. Time in Range Is Associated with Incident Diabetic Retinopathy in Adults with Type 1 Diabetes: A Longitudinal Study. Diabetes Technol. Ther. 2024, 26, 246–251. [Google Scholar] [CrossRef]
- Zhu, D.D.; Wu, X.; Cheng, X.X.; Ding, N. Time in range as a useful marker for evaluating retinal functional changes in diabetic retinopathy patients. Int. J. Ophthalmol. 2023, 16, 915. [Google Scholar] [CrossRef]
- Bezerra, M.F.; Neves, C.; Neves, J.S.; Carvalho, D. Time in range and complications of diabetes: A cross-sectional analysis of patients with Type 1 diabetes. Diabetol. Metab. Syndr. 2023, 15, 244. [Google Scholar] [CrossRef] [PubMed]
- Gubitosi-Klug, R.; Gao, X.; Pop-Busui, R.; de Boer, I.H.; White, N.; Aiello, L.P.; Miller, R.; Palmer, J.; Tamborlane, W.; Wallia, A.; et al. Associations of Microvascular Complications With the Risk of Cardiovascular Disease in Type 1 Diabetes. Diabetes Care 2021, 44, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Panfilova, V.N.; Panfilov, A.Y.; Doroshchenko, S.N.; Taranushenko, T.E. Ultrasonographic study of common carotid arteries in adolescents with type 1 diabetes mellitus. Diabetes Mellit. 2009, 12, 36–38. [Google Scholar] [CrossRef][Green Version]
- Doyon, A.; Kracht, D.; Bayazit, A.K.; Deveci, M.; Duzova, A.; Krmar, R.T.; Litwin, M.; Niemirska, A.; Oguz, B.; Schmidt, B.M.; et al. Carotid Artery Intima-Media Thickness and Distensibility in Children and Adolescents. Hypertension 2013, 62, 550–556. [Google Scholar] [CrossRef]
- Järvisalo, M.J.; Putto-Laurila, A.; Jartti, L.; Lehtimäki, T.; Solakivi, T.; Rönnemaa, T.; Raitakari, O.T. Carotid Artery Intima-Media Thickness in Children With Type 1 Diabetes. Diabetes 2002, 51, 493–498. [Google Scholar] [CrossRef]
- Novak, L.; Walker, S.; Fonda, S.; Schmidt, V.; Vigersky, R. Clinical Diabetes/Therapeutics. Diabetes 2016, 65, A221–A360. [Google Scholar] [CrossRef][Green Version]
- Wołoszyn-Durkiewicz, A.; Iwaszkiewicz-Grześ, D.; Świętoń, D.; Kujawa, M.J.; Jankowska, A.; Durawa, A.; Glasner, P.; Trzonkowski, P.; Glasner, L.; Szurowska, E.; et al. The Complex Network of Cytokines and Chemokines in Pediatric Patients with Long-Standing Type 1 Diabetes. Int. J. Mol. Sci. 2024, 25, 1565. [Google Scholar] [CrossRef]


| Variable | T1D N = 48 | Control N = 35 | p-Value | |
|---|---|---|---|---|
| Age [years] (N = 48/N = 35) | 13 (11.79–14.96) | 13 (11.35–14.97) | 0.8141 | |
| Body mass [kg] (N = 48/N = 35) | 50.65 (38.05–58.50) | 48.00 (39.00–56.00) | 0.6714 | |
| Body mass [z-score] (N = 48/N = 35) | 0.15 (−0.42–0.77) | 0.16 (−0.43–0.75) | 0.9926 | |
| Body mass [percentile] (N = 48/N = 35) | 55.87 (33.80–77.96) | 56.19 (33.51–77.20) | - | |
| Height [cm] (N = 48/N = 35) | 162 (152.65–170.55) | 160 (150.00–173.00) | 0.7433 | |
| Height [z-score] (N = 48/N = 35) | 0.55 (−0.43–1.07) | 0.72 (−0.56–1.51) | 0.5928 | |
| Height [percentile] (N = 48/N = 35) | 70.78 (33.52–85.72) | 76.41 (28.83–93.49) | - | |
| BMI [kg/m2] (N = 48/N = 35) | 18.78 (16.68–20.43) | 18.75 (17.48–20.08) | 0.8356 | |
| BMI [z-score] (N = 48/N = 35) | −0.10 (−0.79–0.50) | −0.17 (−0.82–0.53) | 0.9596 | |
| BMI [percentile] (N = 48/N = 35) | 45.92 (21.35–69.21) | 43.43 (20.73–70.14) | - | |
| UACR (N = 48/N = 35) | 6.88 (4.17–14.63) | 5.60 (0.00–8.47) | 0.1457 | |
| HbA1c [%] (N = 48/N = 35) | 7.20 (6.30–7.60) | 5.30 (5.20–5.50) | <0.0001 | |
| TSH [uU/mL] (N = 47/N = 35) | 1.65 (1.22–2.14) | 1.58 (1.27–2.08) | >0.9999 | |
| FT4 [pmol/L] (N = 47/N = 34) | 11.60 (11.07–12.65) | 11.34 (10.69–12.74) | 0.4298 | |
| TC [mg/dL] (N = 46/N = 35) | 157.00 (139.00–170.00) | 157.00 (144.00–178.00) | 0.6817 | |
| LDL-C [mg/dL] (N = 46/N = 35) | 88.50 (73.00–102.00) | 90.00 (78.00–99.00) | 0.9392 | |
| HDL-C [mg/dL] (N = 46/N = 35) | 57.50 (53.00–63.00) | 55.00 (49.00–63.00) | 0.2521 | |
| TG [mg/dL] (N = 46/N = 35) | 52.00 (44.00–60.00) | 58.00 (40.00–73.00) | 0.2855 | |
| Sex | Female | 25 (52.08%) | 18 (51.43%) | 0.9529 |
| Male | 23 (47.92%) | 17 (48.57%) | ||
| Celiac disease | Yes | 3 (6.25%) | 1 (2.86%) | 0.6348 |
| No | 45 (93.75%) | 34 (97.14%) | ||
| Autoimmune thyroiditis | Yes | 5 (10.42%) | 0 (0.00%) | 0.0765 |
| No | 43 (89.58%) | 35 (100%) | ||
| Albuminuria (UACR > 30 mg/g) | Yes | 12 (25.00%) | 4 (11.43%) | 0.1624 |
| No | 36 (75.00%) | 31 (88.57%) | ||
| Variable | T1D N = 48 | Control N = 35 | p-Value |
|---|---|---|---|
| cIMTmin [mm] (N = 47/N = 32) | 0.40 (0.36–0.42) | 0.37 (0.34–0.40) | 0.0278 |
| cIMTmax [mm] (N = 47/N = 32) | 0.42 (0.38–0.45) | 0.39 (0.37–0.42) | 0.0856 |
| cIMTmean [mm] (N = 47/N = 32) | 0.41 (0.38–0.44) | 0.39 (0.37–0.41) | 0.0472 |
| HRmax [%](N = 48/N = 35) | 17.80 (15.20–21.10) | 19.75 (17.48–21.48) | 0.1388 |
| RHR (N = 48/N = 35) | 28.25 (17.55–37.65) | 27.31 (20.15–37.79) | 0.7574 |
| HRindex [%](N = 48/N = 35) | 11.15 (8.30–12.80) | 11.09 (8.76–12.01) | 0.9963 |
| HS (N = 48/N = 35) | 102.45 (54.10–164.40) | 80.44 (42.13–156.81) | 0.5520 |
| Lumen [μm] (N = 48/N = 35) | 96.00 (90.25–102.08) | 95.67 (83.60–101.47) | 0.5428 |
| WTmin [μm] (N = 48/N = 35) | 9.72 (8.83–10.43) | 9.00 (7.87–10.03) | 0.0187 |
| WTmax [μm] (N = 48/N = 35) | 10.45 (9.58–11.10) | 9.57 (8.07–10.87) | 0.0149 |
| WTmean [μm] (N = 48/N = 35) | 10.11 (9.17–10.73) | 9.30 (7.97–10.20) | 0.0189 |
| WCSA (N = 48/N = 35) | 3346.76 (2958.05–3756.59) | 3116.53 (2601.94–3490.76) | 0.0774 |
| WLR (N = 48/N = 35) | 0.21 (0.20–0.23) | 0.19 (0.18–0.22) | 0.0326 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wołoszyn-Durkiewicz, A.; Dąbrowska, E.; Hellmann, M.; Jankowska, A.; Kujawa, M.J.; Świętoń, D.; Durawa, A.; Kuhn, J.; Szypułowska-Grzyś, J.; Brandt-Varma, A.; et al. Assessment of Microvascular Disturbances in Children with Type 1 Diabetes—A Pilot Study. Biosensors 2025, 15, 439. https://doi.org/10.3390/bios15070439
Wołoszyn-Durkiewicz A, Dąbrowska E, Hellmann M, Jankowska A, Kujawa MJ, Świętoń D, Durawa A, Kuhn J, Szypułowska-Grzyś J, Brandt-Varma A, et al. Assessment of Microvascular Disturbances in Children with Type 1 Diabetes—A Pilot Study. Biosensors. 2025; 15(7):439. https://doi.org/10.3390/bios15070439
Chicago/Turabian StyleWołoszyn-Durkiewicz, Anna, Edyta Dąbrowska, Marcin Hellmann, Anna Jankowska, Mariusz J. Kujawa, Dominik Świętoń, Agata Durawa, Joanna Kuhn, Joanna Szypułowska-Grzyś, Agnieszka Brandt-Varma, and et al. 2025. "Assessment of Microvascular Disturbances in Children with Type 1 Diabetes—A Pilot Study" Biosensors 15, no. 7: 439. https://doi.org/10.3390/bios15070439
APA StyleWołoszyn-Durkiewicz, A., Dąbrowska, E., Hellmann, M., Jankowska, A., Kujawa, M. J., Świętoń, D., Durawa, A., Kuhn, J., Szypułowska-Grzyś, J., Brandt-Varma, A., Burzyński, J., Chrzanowski, J., Michalak, A., Michnowska, A., Trzonek, D., Wolf, J., Narkiewicz, K., Szurowska, E., & Myśliwiec, M. (2025). Assessment of Microvascular Disturbances in Children with Type 1 Diabetes—A Pilot Study. Biosensors, 15(7), 439. https://doi.org/10.3390/bios15070439







