Polymeric Composite-Based Electrochemical Sensing Devices Applied in the Analysis of Monoamine Neurotransmitters
Abstract
1. Introduction to Electrochemical Sensors
2. Conducting Polymers in Sensors: Overview
3. Sensors Using Conducting Polymers/Metallic Nanoparticles as Sensing Materials
3.1. Sensors Based on Electrodes of Conventional Size
3.2. Microelectrode-Based Sensors and In Vivo Applications
4. Sensors Using Carbonaceous Materials
4.1. Carbon Nanotube-Based Sensors
4.2. Sensors Based on Either Graphene or Graphene Oxide Quantum Dots
5. Conclusions and Further Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baranwal, J.; Barse, B.; Gatto, G.; Broncova, G.; Kumar, A. Electrochemical sensors and their applications: A review. Chemosensors 2022, 10, 363. [Google Scholar] [CrossRef]
- Brett, C.M.A. Electrochemical impedance spectroscopy in the characterisation and application of modified electrodes for electrochemical sensors and biosensors. Molecules 2022, 27, 1497. [Google Scholar] [CrossRef] [PubMed]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Pan, C.; Wei, H.; Han, Z.; Wu, F.; Mao, L. Enzymatic electrochemical biosensors for in situ neurochemical measurement. Curr. Opin. Electrochem. 2020, 19, 162–167. [Google Scholar] [CrossRef]
- Terán-Alcocer, Á.; Bravo-Plascencia, F.; Cevallos-Morillo, C.; Palma-Cando, A. Electrochemical sensors based on conducting polymers for the aqueous detection of biologically relevant molecules. Nanomaterials 2021, 11, 252. [Google Scholar] [CrossRef]
- Yang, M.; Wang, L.; Lu, H.; Dong, Q. Advances in MXene-based electrochemical (bio)sensors for neurotransmitter detection. Micromachines 2023, 14, 1088. [Google Scholar] [CrossRef]
- Fredj, Z.; Singh, B.; Bahri, M.; Qin, P.; Sawan, M. Enzymatic electrochemical biosensors for neurotransmitters detection: Recent achievements and trends. Chemosensors 2023, 11, 388. [Google Scholar] [CrossRef]
- Leau, S.A.; Lete, C.; Lupu, S. Nanocomposite materials based on metal nanoparticles for the electrochemical sensing of neurotransmitters. Chemosensors 2023, 11, 179. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, J.; Guo, X.; Li, Y.; Li, L.; Pan, L. Recent advances in conductive polymers-based electrochemical sensors for biomedical and environmental applications. Polymers 2024, 16, 1597. [Google Scholar] [CrossRef]
- Kaushik, P.; Bharti, R.; Sharma, R.; Verma, M.; Olsson, R.T.; Pandey, A. Progress in synthesis and applications of polyaniline-coated nanocomposites: A comprehensive review. Eur. Polym. J. 2024, 221, 113574. [Google Scholar] [CrossRef]
- Pilo, M.I.; Sanna, G.; Spano, N. Conducting polymers in amperometric sensors: A state of the art over the last 15 years with a focus on polypyrrole-, polythiophene-, and poly(3,4-ethylenedioxythiophene)-based materials. Chemosensors 2024, 12, 81. [Google Scholar] [CrossRef]
- Karasu, T.; Armutcu, C.; Elkhoury, K.; Özgür, E.; Maziz, A.; Uzun, L. Conducting polymers as a functional recognition interface to design sensors for pathogen and cancer diagnosis. TrAC Trends Anal. Chem. 2024, 175, 117705. [Google Scholar] [CrossRef]
- Karabozhikova, V.; Tsakova, V. Electrochemically obtained poly(3,4-ethylenedioxythiophene) layers for electroanalytical determination of lipoic acid. Coatings 2023, 13, 2014. [Google Scholar] [CrossRef]
- Gonçalves, R.; Paiva, R.S.; Pereira, E.C. Calculation of real growth current using variable electroactive area obtained during polypyrrole synthesis. J. Solid State Electrochem. 2021, 25, 1567–1577. [Google Scholar] [CrossRef]
- Janardhanan, J.A.; Yu, H.H. Recent advances in PEDOT/PProDOT-derived nano biosensors: Engineering nano assemblies for fostering advanced detection platforms for biomolecule detection. Nanoscale 2024, 16, 17202–17229. [Google Scholar] [CrossRef]
- Yadav, P.; Patra, A. Recent advances in poly(3,4-ethylenedioxyselenophene) and related polymers. Polym. Chem. 2020, 11, 7275–7292. [Google Scholar] [CrossRef]
- Gueye, M.N.; Carella, A.; Faure-Vincent, J.; Demadrille, R.; Simonato, J.P. Progress in understanding structure and transport properties of PEDOT-based materials: A critical review. Progr. Mater. Sci. 2020, 108, 100616. [Google Scholar] [CrossRef]
- Mahalakshmi, S.; Sridevi, V. In situ electrodeposited gold nanoparticles on polyaniline-modified electrode surface for the detection of dopamine in presence of ascorbic acid and uric acid. Electrocatalysis 2021, 12, 415–435. [Google Scholar] [CrossRef]
- Holze, R. Overoxidation of intrinsically conducting polymers. Polymers 2022, 14, 1584. [Google Scholar] [CrossRef]
- Song, M.J.; Lee, S.K.; Kim, J.H.; Lim, D.S. Dopamine sensor based on a boron-doped diamond electrode modified with a polyaniline/Au nanocomposites in the presence of ascorbic acid. Anal. Sci. 2012, 28, 583–587. [Google Scholar] [CrossRef][Green Version]
- Lin, L.; Li, M.; Li, P.; Ye, C.; Zhuang, H.; Weng, S.; Chen, F. Simultaneous determination of dopamine and uric acid based on electrocatalytic oxidation of Cu2O-Au and polyaniline nanocomposites. Microchem. J. 2024, 196, 109602. [Google Scholar] [CrossRef]
- Bhattacharyya, A.S. Conducting polymers in biosensing: A review. Chem. Phys. Impact 2024, 8, 100642. [Google Scholar] [CrossRef]
- Le, C.V.; Yoon, H. Advances in the use of conducting polymers for healthcare monitoring. Int. J. Mol. Sci. 2024, 25, 1564. [Google Scholar] [CrossRef]
- Amiri, M.; Golmohammadi, F.; Safari, M.; Sadeq, T.W. Electrochemical synthesis of polypyrrole composite with modified gold nanoparticles for electrochemical detection of ascorbic acid, dopamine and acetaminophen in different pharmaceutical samples. Polym. Bull. 2024, 81, 9775–9793. [Google Scholar] [CrossRef]
- Seiti, M.; Giuri, A.; Corcione, C.E.; Ferraris, E. Advancements in tailoring PEDOT: PSS properties for bioelectronic applications: A comprehensive review. Biomater. Adv. 2023, 154, 213655. [Google Scholar] [CrossRef]
- Du, X.; Yang, L.; Liu, N. Recent progress on poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) bioelectrodes. Small Sci. 2023, 3, 2300008. [Google Scholar] [CrossRef]
- Zhao, Z.; Hou, Y.; Zhang, H.; Guo, J.; Wang, J. A PEDOT: PSS/GO fiber microelectrode fabricated by microfluidic spinning for dopamine detection in human serum and PC12 cells. Microchim. Acta 2024, 191, 362. [Google Scholar] [CrossRef]
- Nuh, S.; Numnuam, A.; Thavarungkul, P.; Phairatana, T. A novel microfluidic-based OMC-PEDOT-PSS composite electrochemical sensor for continuous dopamine monitoring. Biosensors 2023, 13, 68. [Google Scholar] [CrossRef]
- Karabozhikova, V.; Tsakova, V. Electroanalytical determination of caffeic acid—Factors controlling the oxidation reaction in the case of PEDOT-modified electrodes. Electrochim. Acta 2019, 293, 439–446. [Google Scholar] [CrossRef]
- Nandhini, C.; Huang, C.-H.; Arul, P.; Huang, S.-T.; Lin, C.-M. Fabrication of ternary hybrid composites of Co-MOFs/MoS2/PEDOTs for sensitive detection of dopamine and norepinephrine in biological samples. Sens. Actuator B Chem. 2024, 417, 136147. [Google Scholar] [CrossRef]
- Dunham, K.E.; Venton, B.J. Electrochemical and biosensor techniques to monitor neurotransmitter changes with depression. Anal. Bioanal. Chem. 2024, 416, 2301–2318. [Google Scholar] [CrossRef]
- Puthongkham, P.; Venton, B.J. Recent advances in fast-scan cyclic voltammetry. Analyst 2020, 145, 1087–1102. [Google Scholar] [CrossRef] [PubMed]
- Majdi, S.; Larsson, A.; Hoang Philipsen, M.; Ewing, A.G. Electrochemistry in and of the fly brain. Electroanalysis 2018, 30, 99–1010. [Google Scholar] [CrossRef]
- Rodeberg, N.T.; Sandberg, S.G.; Johnson, J.A.; Phillips, P.E.M.; Wightman, R.M. Hitchhiker’s guide to voltammetry: Acute and chronic electrodes for in vivo fast-scan cyclic voltammetry. ACS Chem. Neurosci. 2017, 8, 221–234. [Google Scholar] [CrossRef]
- Schenk, J.O.; Miller, E.; Rice, M.E.; Adams, R.N. Chronoamperometry in brain slices: Quantitative evaluations of in vivo electrochemistry. Brain Res. 1983, 277, 1–8. [Google Scholar] [CrossRef]
- Baur, J.E.; Kristensen, E.W.; May, L.J.; Wiedemann, D.J.; Wightman, R.M. Fast-scan voltammetry of biogenic amines. Anal. Chem. 1988, 60, 1268–1272. [Google Scholar] [CrossRef]
- Amatore, C.; Maisonhaute, E.; Simonneau, G. Ultrafast cyclic voltammetry: Performing in the few megavolts per second range without ohmic drop. Electrochem. Commun. 2000, 2, 81–84. [Google Scholar] [CrossRef]
- Rafi, H.; Zestos, A.G. Review–recent advances in FSCV detection of neurochemicals via waveform and carbon microelectrode modification. J. Electrochem. Soc. 2021, 168, 057520. [Google Scholar] [CrossRef]
- Oleinick, A.; Álvarez-Martos, I.; Svir, I.; Ferapontova, E.E.; Amatore, C. Surface heterogeneities matter in fast scan cyclic voltammetry investigations of catecholamines in brain with carbon microelectrodes of high-aspect ratio: Dopamine oxidation at conical carbon microelectrodes. J. Electrochem. Soc. 2018, 165, G3057. [Google Scholar] [CrossRef]
- Denno, M.E.; Privman, E.; Venton, B.J. Analysis of neurotransmitter tissue content of Drosophila melanogaster in different life stages. ACS Chem. Neurosci. 2015, 6, 117–123. [Google Scholar] [CrossRef]
- Shao, Z.; Zhao, H.; Dunham, K.E.; Cao, Q.; Lavrik, N.V.; Venton, B.J. 3D-printed carbon nanoneedle electrodes for dopamine detection in Drosophila. Angew. Chem. 2024, 63, e202405634. [Google Scholar] [CrossRef]
- Desagani, D.; Ben-Yoav, H. Chemometrics meets electrochemical sensors for intelligent in vivo bioanalysis. TrAC Trends Anal. Chem. 2023, 164, 117089. [Google Scholar] [CrossRef]
- Shrestha, K.; Venton, B.J. Transient adenosine modulates serotonin release indirectly in the dorsal raphe nuclei. ACS Chem. Neurosci. 2024, 15, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Vreeland, R.F.; Atcherley, C.W.; Russell, W.S.; Xie, J.Y.; Lu, D.; Laude, N.D.; Porreca, F.; Heien, M.L. Biocompatible PEDOT:Nafion composite electrode coatings for selective detection of neurotransmitters in vivo. Anal. Chem. 2015, 87, 2600–2607. [Google Scholar] [CrossRef]
- Ostertag, B.J.; Porshinsky, E.J.; Nawarathne, C.P.; Ross, A.E. Surface-roughened graphene oxide microfibers enhance electrochemical reversibility. Langmuir 2024, 40, 12124–12136. [Google Scholar] [CrossRef]
- Forderhase, A.G.; Ligons, L.A.; Norwood, E.; McCarty, G.S.; Sombers, L.A. Optimized fabrication of carbon-fiber nicrobiosensors for codetection of glucose and dopamine in brain tissue. ACS Sens. 2024, 9, 2662–2672. [Google Scholar] [CrossRef]
- Darroudi, M.; White, K.A.; Crocker, M.A.; Kim, B.N. Dopamine measurement using engineered CNT–CQD–Polymer coatings on Pt microelectrodes. Sensors 2024, 24, 1893. [Google Scholar] [CrossRef]
- Zhang, P.; Zhu, B.; Du, P.; Travas-Sejdic, J. Electrochemical and electrical biosensors for wearable and implantable electronics based on conducting polymers and carbon-based materials. Chem. Rev. 2024, 124, 722–767. [Google Scholar] [CrossRef]
- Li, J.; Cheng, Y.; Gu, M.; Yang, Z.; Zhan, L.; Du, Z. Sensing and stimulation applications of carbon nanomaterials in implantable brain-computer interface. Int. J. Mol. Sci. 2023, 24, 5182. [Google Scholar] [CrossRef]
- Xu, X.; Zuo, Y.; Chen, S.; Hatami, A.; Gu, H. Advancements in brain research: The in vivo/in vitro electrochemical detection of neurochemicals. Biosensors 2024, 14, 125. [Google Scholar] [CrossRef]
- Jeong, Y.H.; Kwon, M.; Shin, S.; Lee, J.; Kim, K.S. Biomedical applications of CNT-based fibers. Biosensors 2024, 14, 137. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Reddy, S.; Xiao, Q.; Liu, H.; Li, C.; Chen, S.; Wang, C.; Chiu, K.; Chen, N.; Tu, Y.; Ramakrishna, S.; et al. Bionanotube/poly(3,4-ethylenedioxythiophene) nanohybrid as an electrode for the neural interface and dopamine sensor. ACS Appl. Mater. Interfaces 2019, 11, 18254–18267. [Google Scholar] [CrossRef] [PubMed]
- Rajarathinam, T.; Jayaraman, S.; Seol, J.; Lee, J.; Chang, S.-C. Utilizing a disposable sensor with polyaniline-doped multi-walled carbon nanotubes to enable dopamine detection in ex vivo mouse brain tissue homogenates. Biosensors 2024, 14, 262. [Google Scholar] [CrossRef]
- Sun, D.; Li, H.; Li, M.; Li, C.; Dai, H.; Sun, D.; Yang, B. Electrodeposition synthesis of a NiO/CNT/PEDOT composite for simultaneous detection of dopamine, serotonin, and tryptophan. Sens. Actuator. B Chem. 2018, 259, 433–442. [Google Scholar] [CrossRef]
- Hsine, Z.; Mlika, R.; Jaffrezic-Renault, N.; Korri-Youssoufi, H. Review-recent progress in graphene based modified electrodes for electrochemical detection of dopamine. Chemosensors 2022, 10, 249. [Google Scholar] [CrossRef]
- Liu, R.; Feng, Z.Y.; Li, D.; Jin, B.; Lan, Y.; Meng, L.Y. Recent trends in carbon-based microelectrodes as electrochemical sensors for neurotransmitter detection: A review. TrAC Trends Anal. Chem. 2022, 148, 116541. [Google Scholar] [CrossRef]
- Zarepour, A.; Karasu, Ç.; Mir, Y.; Nematollahi, M.H.; Iravani, S.; Zarrabi, A. Graphene- and MXene-based materials for neuroscience: Diagnostic and therapeutic applications. Biomater. Sci. 2023, 11, 6687–6710. [Google Scholar] [CrossRef]
- Alimohammadzadeh, E.; Hedley, J. Advances in graphene field effect transistors (FETs) for amine neurotransmitter sensing. Appl. Sci. 2024, 14, 10109. [Google Scholar] [CrossRef]
- Karim, A.; Yasser, M.; Ahmad, A.; Natsir, H.; Wahab, A.W.; Fauziah, S.; Taba, P.; Pratama, I.; Rajab, A.; Abubakar, A.N.F.; et al. A review: Progress and trend advantage of dopamine electrochemical sensor. J. Electroanal. Chem. 2024, 959, 118157. [Google Scholar] [CrossRef]
- Ko, S.H.; Kim, S.W.; Lee, S.H.; Lee, Y.J. Electrodeposited reduced graphene oxide-PEDOT:PSS/Nafion hybrid interface for the simultaneous determination of dopamine and serotonin. Sci. Rep. 2023, 13, 20274. [Google Scholar] [CrossRef]
- Chen, J.; Li, D.; Ding, X.; Zhang, D. Sensitive and selective electrochemical aptasensing method for the voltammetric determination of dopamine based on AuNPs/PEDOT-ERGO nanocomposites. Bioelectrochemistry 2024, 157, 108653. [Google Scholar] [CrossRef]
- Luhana, C.; Mashazi, P. Simultaneous detection of dopamine and paracetamol on electroreduced graphene oxide–cobalt phthalocyanine polymer nanocomposite electrode. Electrocatalysis 2023, 14, 406–417. [Google Scholar] [CrossRef]
- Tian, P.; Tang, L.; Teng, K.-S.; Lau, S.-P. Graphene quantum dots: Preparations, properties, functionalizations and applications. Mater. Futures 2024, 3, 022301. [Google Scholar] [CrossRef]
- Baruah, A.; Newar, R.; Das, S.; Kalita, N.; Nath, M.; Ghosh, P.; Chinnam, S.; Sarma, H.; Narayan, M. Biomedical applications of graphene-based nanomaterials: Recent progress, challenges, and prospects in highly sensitive biosensors. Discov. Nano 2024, 19, 103. [Google Scholar] [CrossRef]
- Şimşek, N.; Gözde Aydoğdu, T.I.Ğ. Graphene quantum dot-poly(l-lysine)-gold nanoparticles nanocomposite for electrochemical determination of dopamine and serotonin. Electroanalysis 2022, 34, 61–73. [Google Scholar] [CrossRef]
- Balkourani, G.; Brouzgou, A.; Tsiakaras, P. A review on recent advancements in electrochemical detection of dopamine using carbonaceous nanomaterials. Carbon 2023, 213, 118281. [Google Scholar] [CrossRef]
- He, C.; Tao, M.; Zhang, C.; He, Y.; Xu, W.; Liu, Y.; Zhu, W. Microelectrode-based electrochemical sensing technology for in vivo detection of dopamine: Recent developments and future prospects. Crit. Rev. Anal. Chem. 2022, 52, 544–554. [Google Scholar] [CrossRef]
- Burns, G.; Ali, M.Y.; Howlader, M.M.R. Advanced functional materials for electrochemical dopamine sensors. TrAC Trends Anal. Chem. 2023, 169, 117367. [Google Scholar] [CrossRef]
- Song, H.; Liu, Y.; Fang, Y.; Zhang, D. Carbon-based electrochemical sensors for in vivo and in vitro neurotransmitter detection. Crit. Rev. Anal. Chem. 2023, 53, 955–974. [Google Scholar] [CrossRef]
- Leau, S.A.; Lete, C.; Matei, C.; Lupu, S. Electrochemical sensing platform based on metal nanoparticles for epinephrine and serotonin. Biosensors 2023, 13, 781. [Google Scholar] [CrossRef]
- Leau, S.A.; Diaconu, I.; Lete, C.; Matei, C.; Lupu, S. Electroanalysis of serotonin at platinum nanoparticles modified electrode. Univ. Politeh. Buchar. Bull. Ser. B-Chem. Mater. Sci. 2024, 86, 3–14. [Google Scholar]
- Leau, S.A.; Marin, M.; Toader, A.M.; Anastasescu, M.; Matei, C.; Lete, C.; Lupu, S. MeNPs-PEDOT composite-based detection platforms for epinephrine and quercetin. Biosensors 2024, 14, 320. [Google Scholar] [CrossRef]
- Han, J.; Ho, T.-W.; Stine, J.M.; Overtone, S.N.; Herberholz, J.; Ghodssi, R. Simultaneous dopamine and serotonin monitoring in freely moving crayfish using a wireless electrochemical sensing system. ACS Sens. 2024, 9, 2346–2355. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wei, H.; Ma, W.; Wu, W.; Ji, W.; Mao, J.; Yu, P.; Mao, L. Inflammation-free electrochemical in vivo sensing of dopamine with atomic-level engineered antioxidative single-atom catalyst. Nat. Commun. 2024, 15, 7915. [Google Scholar] [CrossRef]
- Castagnola, E.; Robbins, E.M.; Krahe, D.D.; Wu, B.; Pwint, M.Y.; Cao, Q.; Cui, X.T. Stable in-vivo electrochemical sensing of tonic serotonin levels using PEDOT/CNT-coated glassy carbon flexible microelectrode arrays. Biosens. Bioelectron. 2023, 230, 115242. [Google Scholar] [CrossRef]
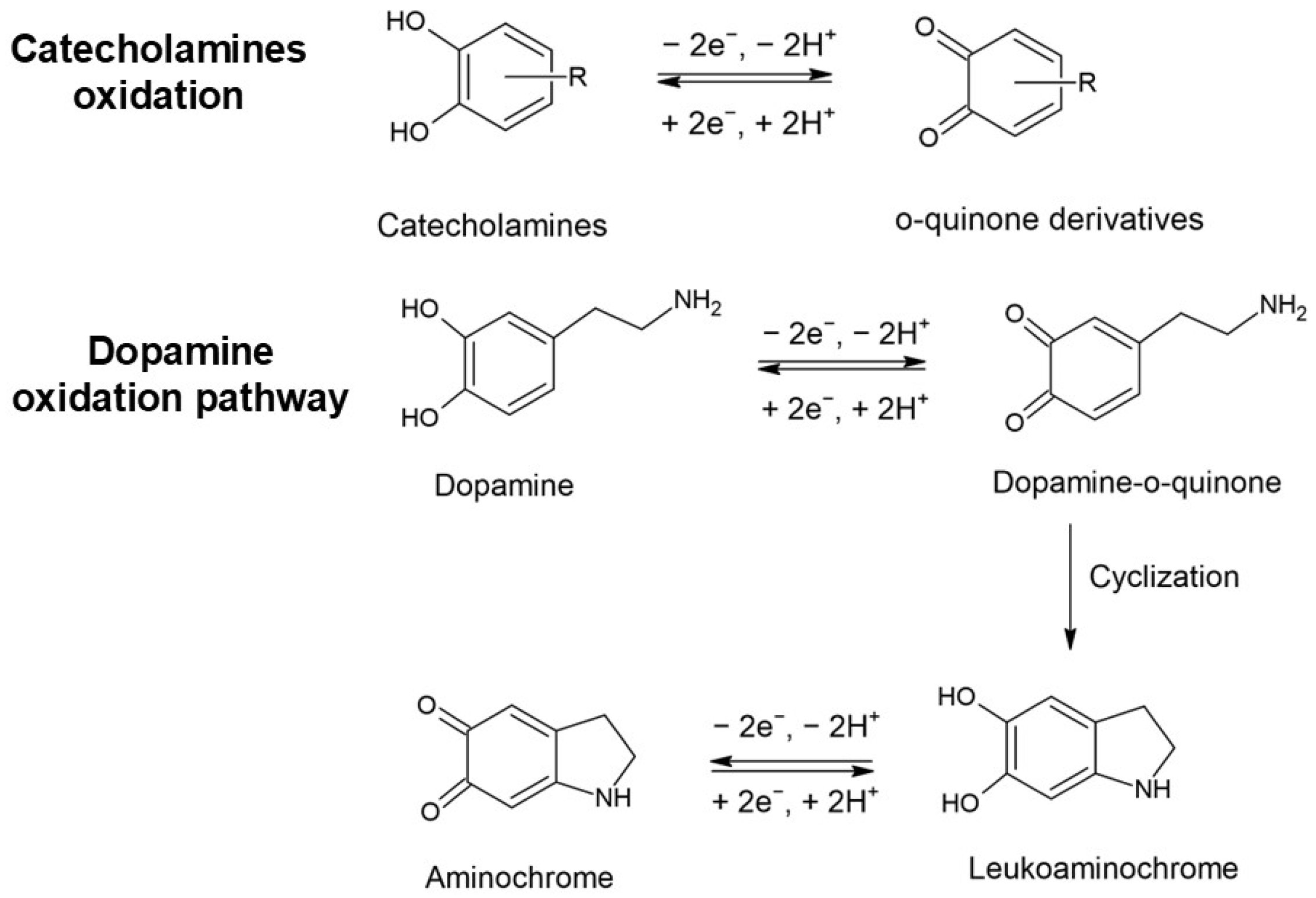

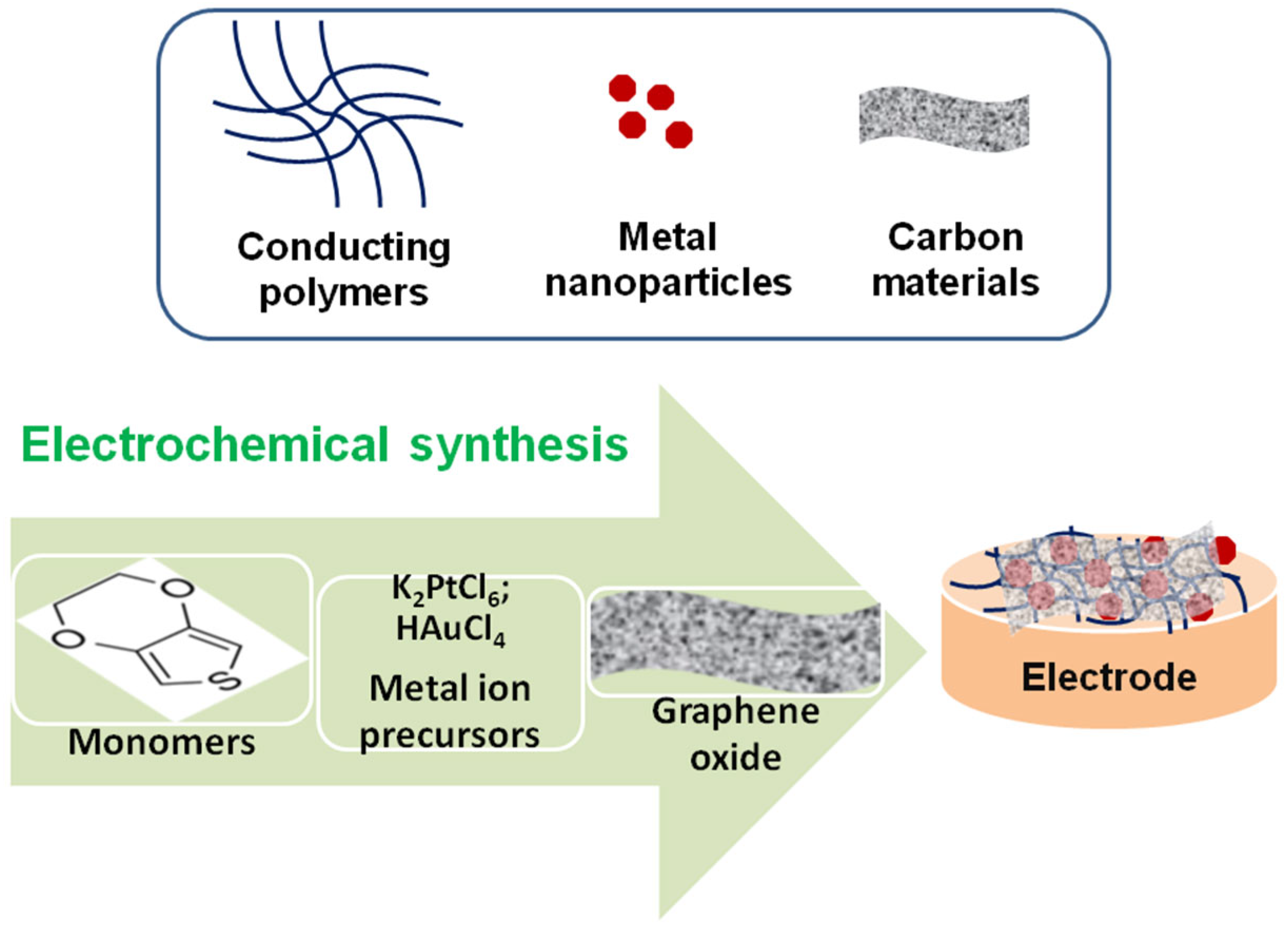
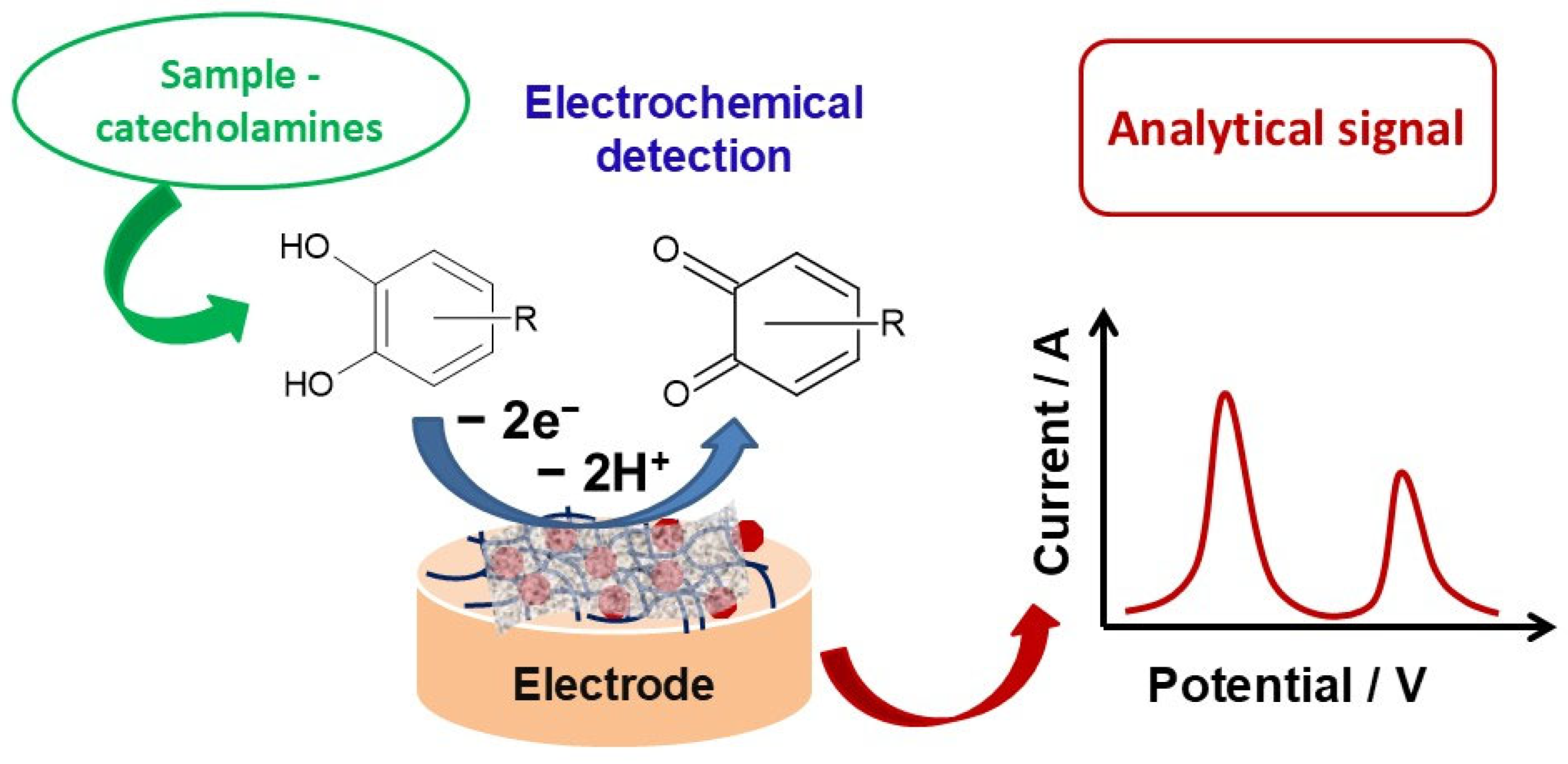
| Composite Material-Based Sensor | Analyte | Det. Technique | Working/Peak Potential | Sensitivity (μA/μM) | Linear Response (μM) | LOD (μM) | Sample | Ref. |
|---|---|---|---|---|---|---|---|---|
| PANI-AuNPs | dopamine | DPV | 0.168 V vs. Ag/AgCl | 0.0279 | 20–100 | 16 | PBS (pH 7) | [18] |
| PANIox/AuNPs/BDD | dopamine | SWV | 0.27 V vs. Ag/AgCl | 0.131 | 0.15–500 | 0.03 | PBS (pH 7) | [20] |
| PANI/Cu2O-Au | dopamine | DPV | 0.260 V vs. Ag/AgCl | 0.05457 | 0.01–1; 1–200 | 0.0076 | urine | [21] |
| PPy-AuNPs | dopamine | DPV | 0.30 V vs. Ag/AgCl | 0.027 | 0.1–8 | 0.078 | human serum | [24] |
| PEDOT:PSS/GO | dopamine | DPV | ~0.10 V vs. SCE | 0.1147 | 0.01–8.0 | 0.00456 | human serum and PC12 cells | [27] |
| PEDOT-PSS | dopamine | DPV | 0.17 V vs. Ag | 20.2 ± 0.6 | 2–100 | 0.0216 | PBS (pH 7.4) | [28] |
| Co-MOF/MoS2/PEDOT | dopamine norepinephrine | CA | 0.20 V vs. Ag/AgCl 0.20 V vs. Ag/AgCl | 0.1043 0.0922 | 0.002–350 0.02–1000 | 0.00023 0.00218 | PC12 cells and blood | [30] |
| PDA-CNT/PEDOT | dopamine | DPV | 0.159 V vs. SCE | 7.35 | 1–22 | 0.62 | human serum | [53] |
| PANI-MWCNTs | dopamine | CV, CA | 0.10 V vs. Ag/AgCl | 0.0284 | 1–200 | 0.05 | ex vivo mouse brain tissue homogenate | [54] |
| NiO/CNT/PEDOT | dopamine serotonin tryptophan | DPV | 0.15 V vs. SCE 0.3 V vs. SCE 0.6 V vs. SCE | 0.52 0.215 0.065 | 0.03–20 0.3–35 1–41 | 0.026 0.063 0.210 | human serum | [55] |
| rGO-PP/NF | dopamine serotonin | DPV | 0.09 V vs. Ag/AgCl 0.25 V vs. Ag/AgCl | 99.3 µA/µM cm2 86 µA/µM cm2 | 0.5–75 0.05–50 | 0.17 0.16 | PBS (pH 7.4) | [61] |
| AuNPs/PEDOT-ERGO/GCE | dopamine | DPV | 0.160 V vs. Ag/AgCl | 0.073 | 5–200 | 1.0 | fetal bovine serum | [62] |
| rGO/polyCoTAPC | dopamine paracetamol | DPV | 0.186 V vs. Ag/AgCl 0.373 V vs. Ag/AgCl | 1.01; 0.158 | 2–100 7–90 | 0.095 0.104 | urine | [63] |
| PGE/AuNPs/poly(L-lysine)GQDs | dopamine serotonin | DPV | 0.13 V vs. Ag/AgCl 0.32 V vs. Ag/AgCl | 0.360 1.558 | 0.1–80 0.05–200 | 0.03 0.017 | fetal bovine serum/dopamine injection | [66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lupu, S. Polymeric Composite-Based Electrochemical Sensing Devices Applied in the Analysis of Monoamine Neurotransmitters. Biosensors 2025, 15, 440. https://doi.org/10.3390/bios15070440
Lupu S. Polymeric Composite-Based Electrochemical Sensing Devices Applied in the Analysis of Monoamine Neurotransmitters. Biosensors. 2025; 15(7):440. https://doi.org/10.3390/bios15070440
Chicago/Turabian StyleLupu, Stelian. 2025. "Polymeric Composite-Based Electrochemical Sensing Devices Applied in the Analysis of Monoamine Neurotransmitters" Biosensors 15, no. 7: 440. https://doi.org/10.3390/bios15070440
APA StyleLupu, S. (2025). Polymeric Composite-Based Electrochemical Sensing Devices Applied in the Analysis of Monoamine Neurotransmitters. Biosensors, 15(7), 440. https://doi.org/10.3390/bios15070440




