Heavy Metal Ion Detection Based on Lateral Flow Assay Technology: Principles and Applications
Abstract
1. Introduction
2. Detection Strategies for Heavy Metal Ions Based on LFA Technology
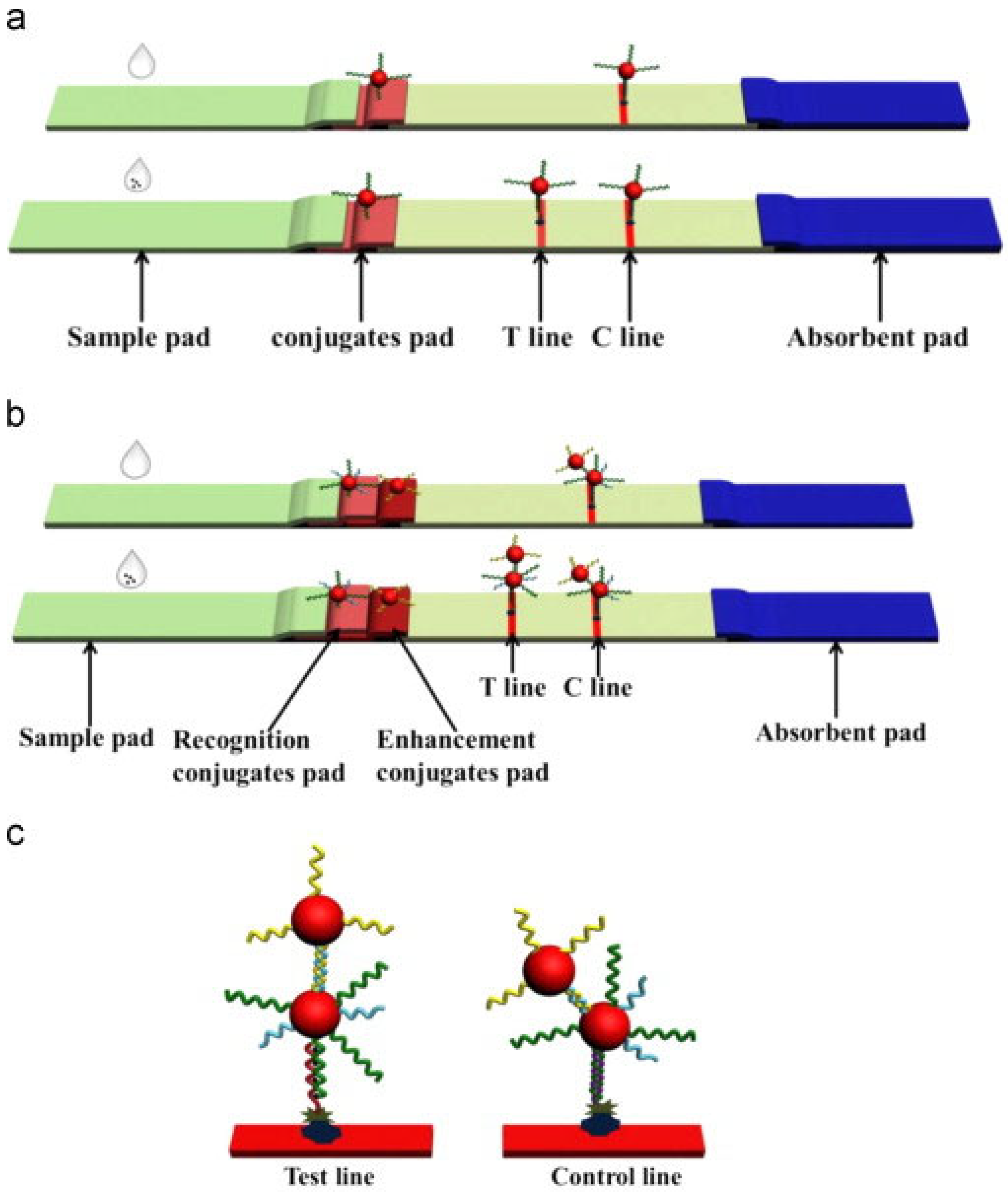
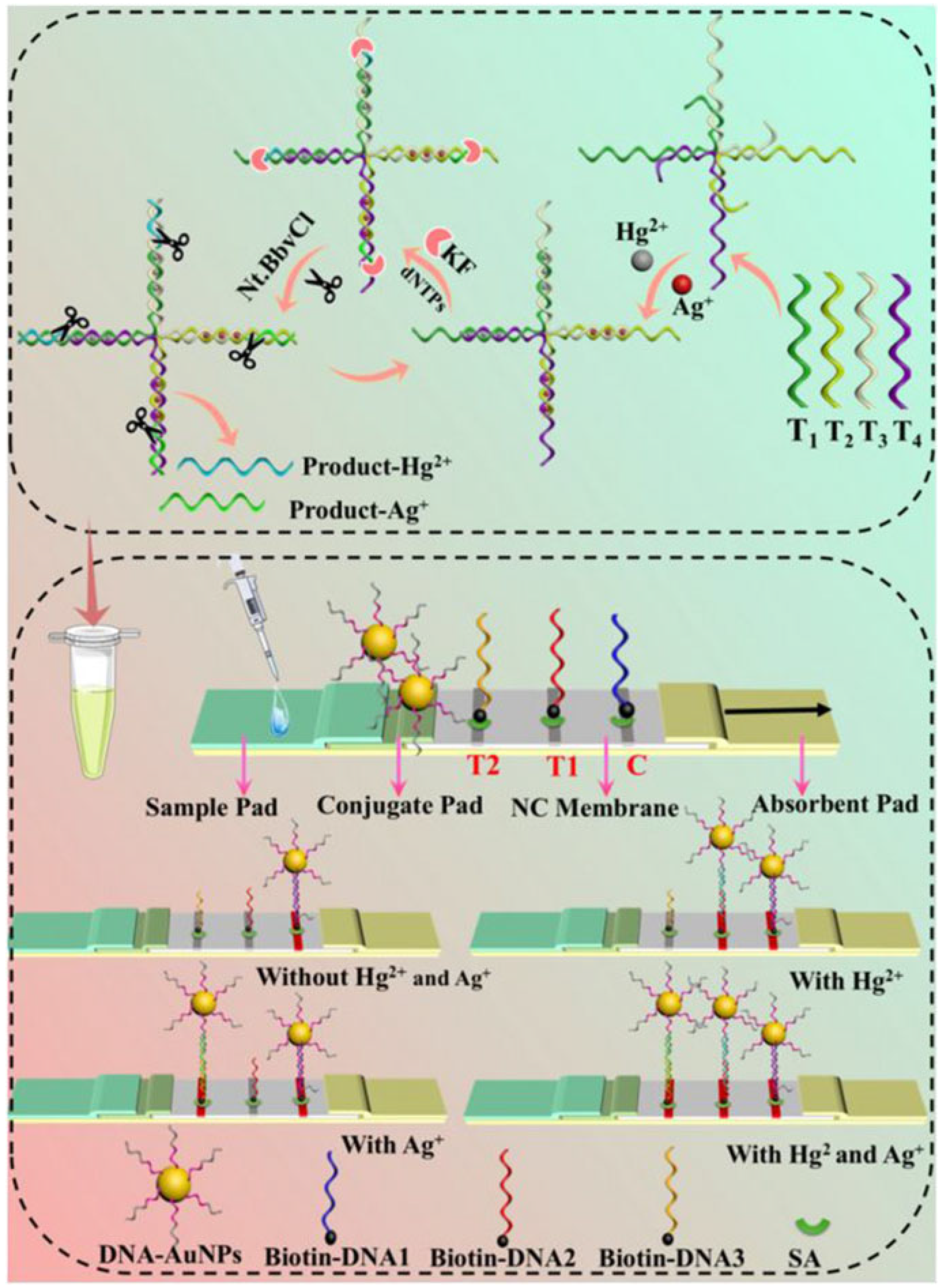
2.1. DNA Probe-Based LFA Technology
- (i)
- Specific binding: DNA probes (e.g., T-rich sequences) form stable complexes or chemical bonds with target ions (e.g., Hg2+ through T-Hg2+-T coordination).
- (ii)
- Signal labeling: Gold nanoparticles (AuNPs) are labeled by a thiol terminated probing DNA sequence, which contains the recognition bases or groups for target metal ions.
- (iii)
- Visual readout: The labeled gold nanoparticle will be captured by the complementary DNA probes at the T line by forming stale double DNA structures, producing distinct red bands whose intensity correlates with target concentration.
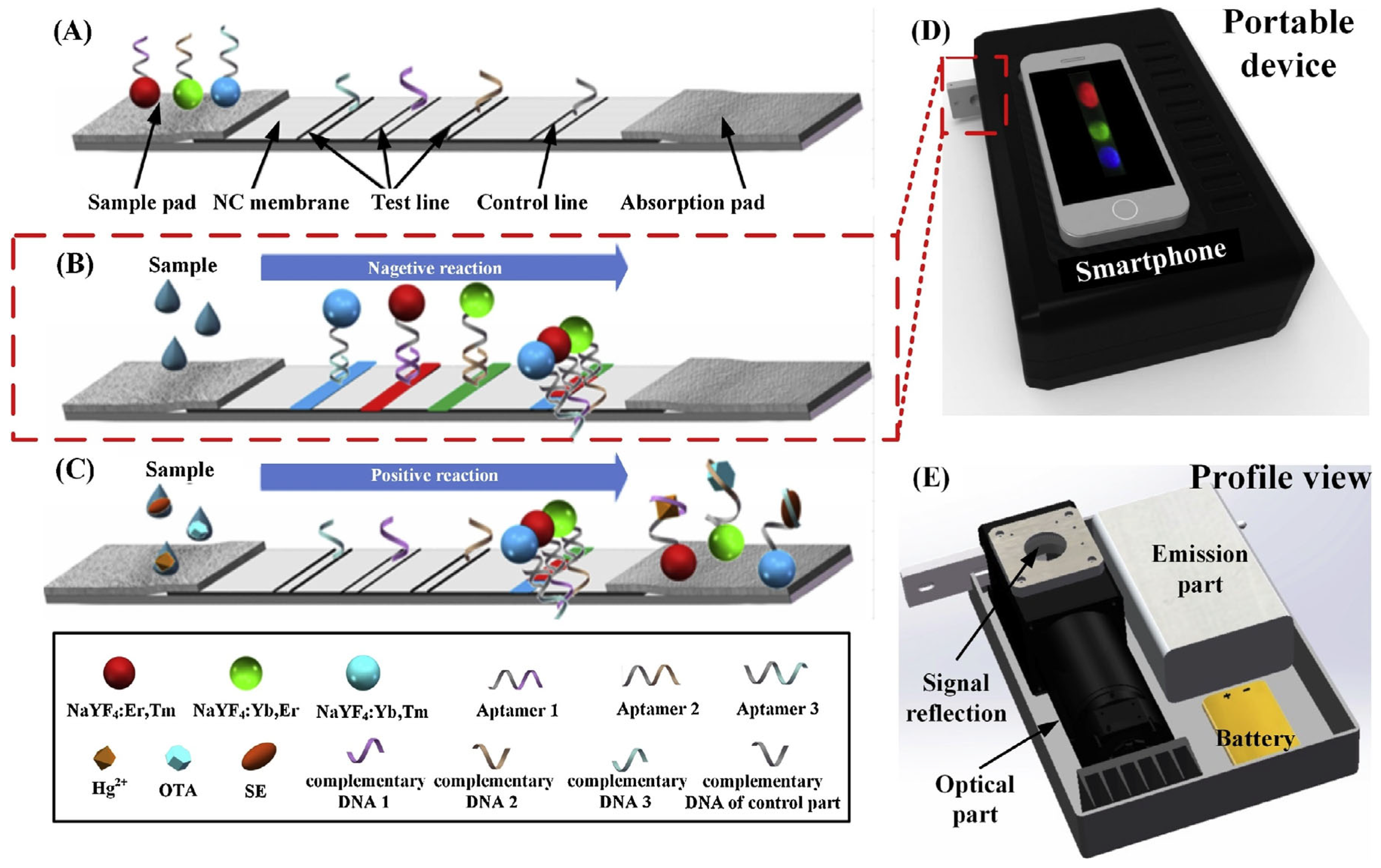
2.2. Aptamer-Based LFA Technology
- (i)
- Specific binding: Aptamers bind to target heavy metal ions (e.g., Hg2+, Cd2+) with high affinity through their unique three-dimensional structures, inducing significant conformational changes.
- (ii)
- Signal labeling: The spatial distribution or aggregation state of reporter molecules (e.g., gold nanoparticles, fluorescent dyes) conjugated to aptamers is altered by these conformational changes.
- (iii)
- Visual readout: Signal molecules accumulate at the test line (T-line), producing colored bands whose intensity correlates with target concentration, while the control line (C-line) validates assay performance.
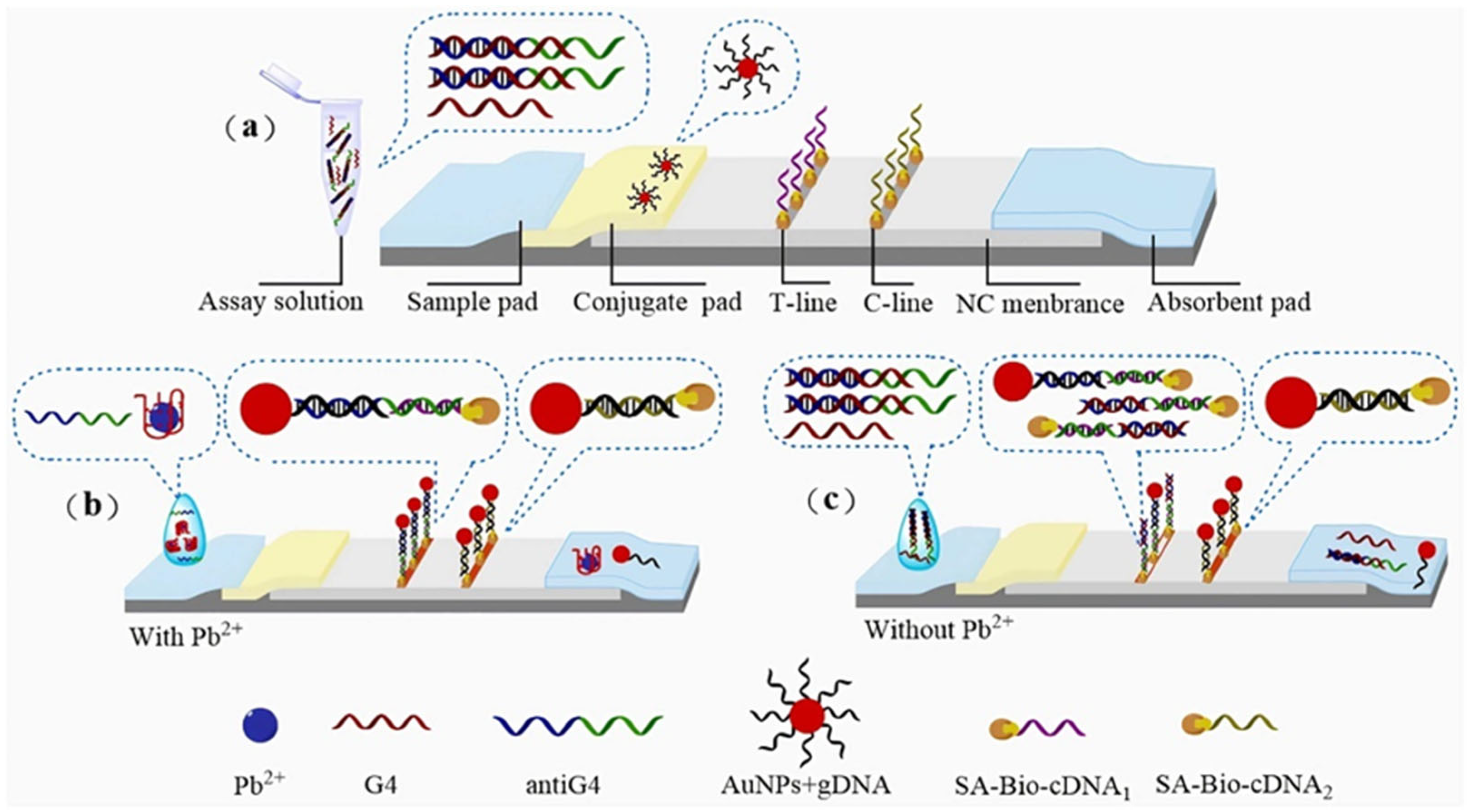
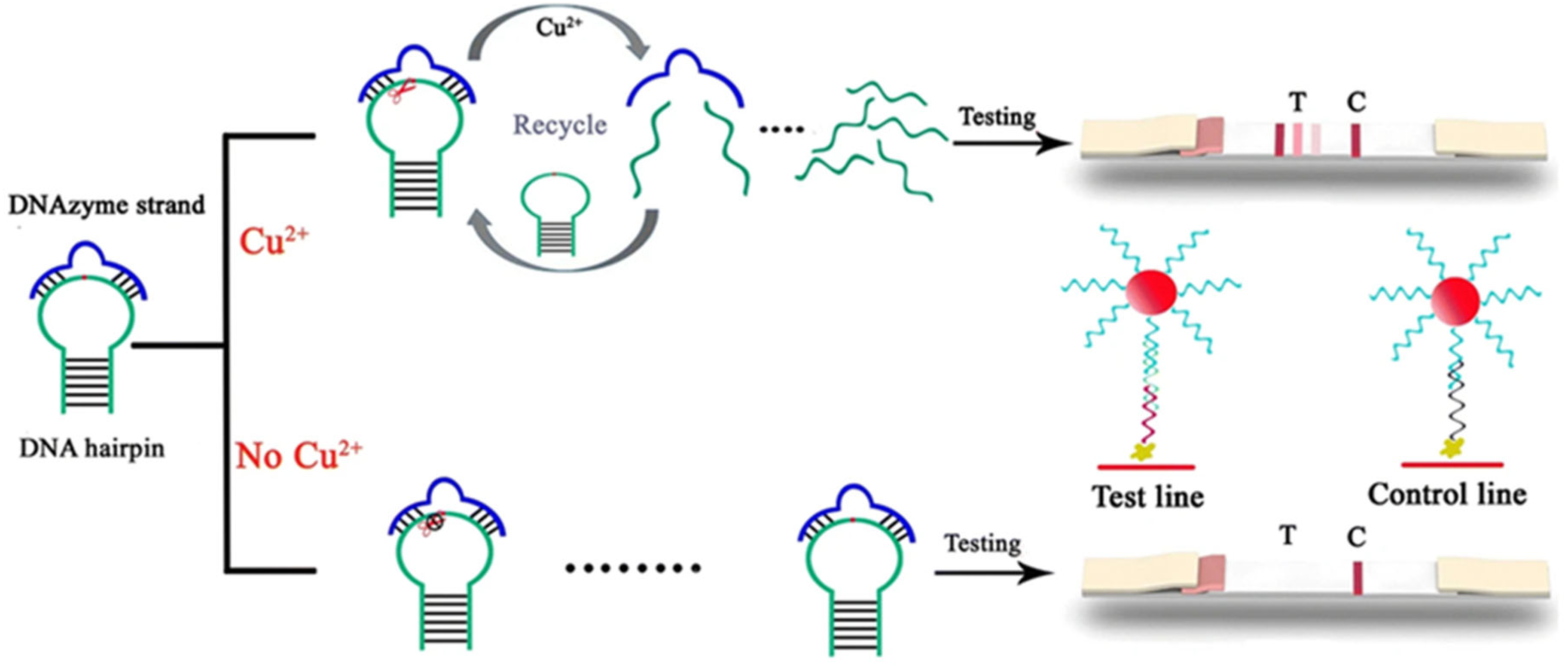
2.3. Nucleic Acid Enzyme-Based LFA Technology
- (i)
- Specific binding: Target heavy metal ions (e.g., Pb2+) specifically activate nucleic acid enzymes (e.g., 8–17 DNAzyme), initiating cleavage of substrate DNA strands.
- (ii)
- Signal labeling: The cleavage reaction releases labeled fragments (e.g., fluorophore-conjugated or gold nanoparticle-tagged DNA segments).
- (iii)
- Visual readout: Released markers are captured at the T-line, generating visible signals (e.g., red bands) with intensity proportional to ion concentration.
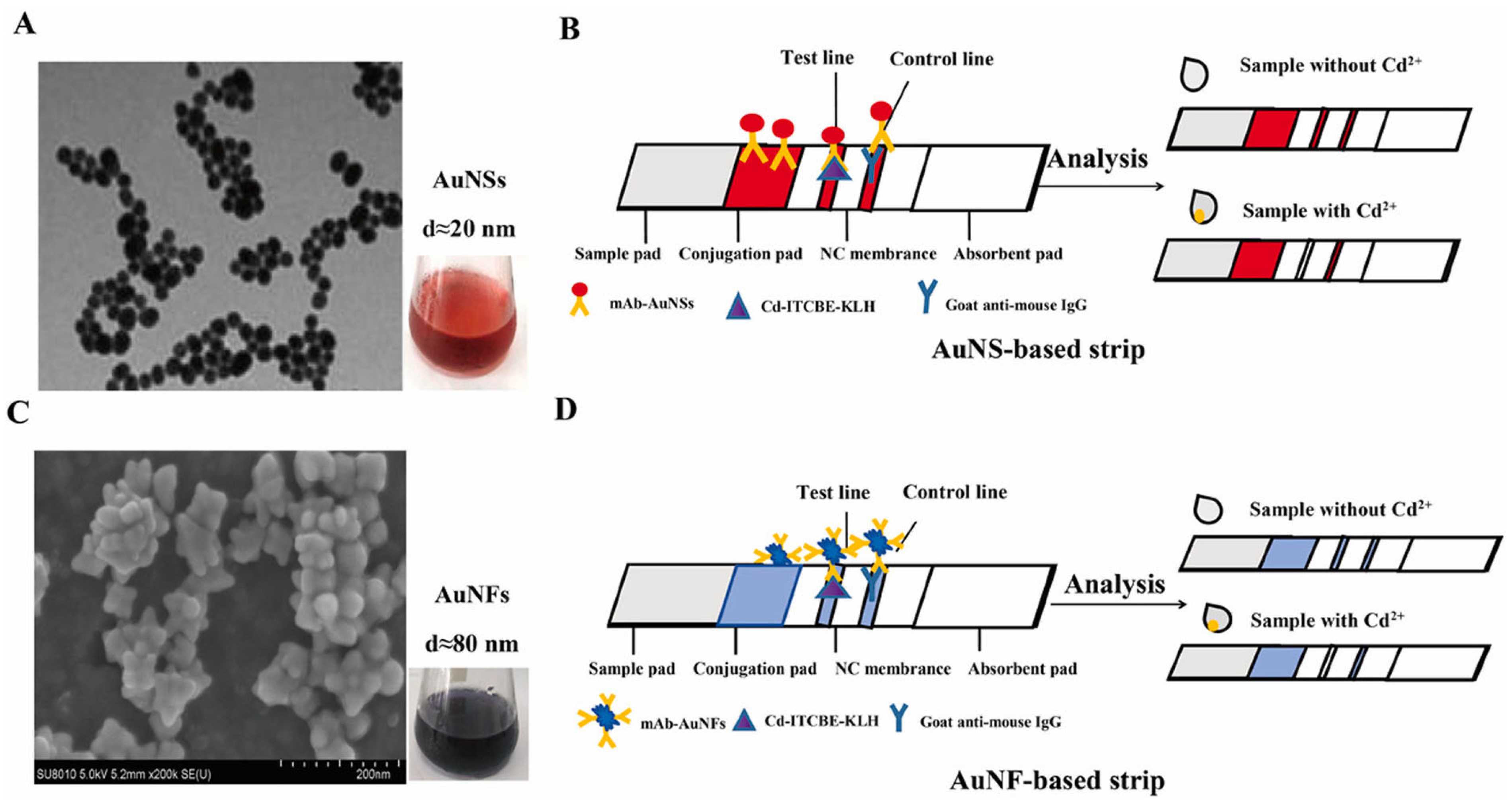
2.4. Antigen–Antibody-Based LFA Technology
- (i)
- Specific binding: Immobilized antibodies (or antigens) on the test strip specifically bind to target analytes (e.g., metal ion–carrier protein complexes) in samples.
- (ii)
- Signal labeling: Gold-conjugated antibodies form immunocomplexes that migrate via capillary action.
- (iii)
- Visual readout: Immunocomplexes accumulate at the T-line, producing colored bands, while the C-line confirms assay validity.
3. Conclusions and Future Perspectives
- (i)
- Multiplex Detection
- (ii)
- Signal Amplification Optimization
- (iii)
- Integration with Portable Devices
- (iv)
- Standardization and Commercialization
- (v)
- SERS-Microdevice Integration
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koller, M.; Saleh, H.M. Introductory chapter: Introducing heavy metals. HMs 2018, 1, 3–11. [Google Scholar]
- Igwe, E.; Onoja, S.; Nwodo, P.; Baharane, V.; Diakite, S.; Saquee, F.; Ugwu, B.; Amechi, O.; Niambe, O.; Shaibu, O. Identification of sources of some priority heavy metallic pollutants caus-ing environmental degradation and it’s health implications. J. Ind. Pollut. Control 2023, 39, 1–13. [Google Scholar]
- Tiwari, S.M. Impact of heavy metal pollution on the biotic and abiotic components of the environment. South Asian J. Res. Microbiol. 2023, 13, 38–54. [Google Scholar]
- He, K.; Sun, Z.; Hu, Y.; Zeng, X.; Yu, Z.; Cheng, H. Comparison of soil heavy metal pollution caused by e-waste recycling activities and traditional industrial operations. Environ. Sci. Pollut. Res. 2017, 24, 9387–9398. [Google Scholar] [CrossRef] [PubMed]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Upadhyay, R. Heavy metals in our ecosystem. In Heavy Metals in Plants; CRC Press: Boca Raton, FL, USA, 2022; pp. 1–15. [Google Scholar]
- Olufemi, A.C.; Mji, A.; Mukhola, M.S. Potential health risks of lead exposure from early life through later life: Implications for public health education. Int. J. Environ. Res. Public Health 2022, 19, 16006. [Google Scholar] [CrossRef]
- Collin, M.S.; Venkatraman, S.K.; Vijayakumar, N.; Kanimozhi, V.; Arbaaz, S.M.; Stacey, R.S.; Anusha, J.; Choudhary, R.; Lvov, V.; Tovar, G.I. Bioaccumulation of lead (Pb) and its effects on human: A review. J. Hazard. Mater. Adv. 2022, 7, 100094. [Google Scholar] [CrossRef]
- Sanders, T.; Liu, Y.; Buchner, V.; Tchounwou, P.B. Neurotoxic effects and biomarkers of lead exposure: A review. Rev. Environ. Health 2009, 24, 15–46. [Google Scholar] [CrossRef]
- Huang, Y.; He, C.; Shen, C.; Guo, J.; Mubeen, S.; Yuan, J.; Yang, Z. Toxicity of cadmium and its health risks from leafy vegetable consumption. Food Funct. 2017, 8, 1373–1401. [Google Scholar] [CrossRef]
- Yang, Y.; Hassan, M.F.; Ali, W.; Zou, H.; Liu, Z.; Ma, Y. Effects of Cadmium Pollution on Human Health: A Narrative Review. Atmosphere 2025, 16, 225. [Google Scholar] [CrossRef]
- Charkiewicz, A.E.; Omeljaniuk, W.J.; Nowak, K.; Garley, M.; Nikliński, J. Cadmium toxicity and health effects—A brief summary. Molecules 2023, 28, 6620. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-S.; Osman, A.I.; Hosny, M.; Elgarahy, A.M.; Eltaweil, A.S.; Rooney, D.W.; Chen, Z.; Rahim, N.S.; Sekar, M.; Gopinath, S.C. The toxicity of mercury and its chemical compounds: Molecular mechanisms and environmental and human health implications: A comprehensive review. ACS Omega 2024, 9, 5100–5126. [Google Scholar] [CrossRef] [PubMed]
- Carocci, A.; Rovito, N.; Sinicropi, M.S.; Genchi, G. Mercury toxicity and neurodegenerative effects. Rev. Environ. Contam. Toxicol. 2014, 229, 1–18. [Google Scholar]
- Alvarez, C.C.; Gómez, M.E.B.; Zavala, A.H. Hexavalent chromium: Regulation and health effects. J. Trace Elem. Med. Biol. 2021, 65, 126729. [Google Scholar] [CrossRef]
- Hossini, H.; Shafie, B.; Niri, A.D.; Nazari, M.; Esfahlan, A.J.; Ahmadpour, M.; Nazmara, Z.; Ahmadimanesh, M.; Makhdoumi, P.; Mirzaei, N. A comprehensive review on human health effects of chromium: Insights on induced toxicity. Environ. Sci. Pollut. Res. 2022, 29, 70686–70705. [Google Scholar] [CrossRef]
- Desai, V.; Kaler, S.G. Role of copper in human neurological disorders. Am. J. Clin. Nutr. 2008, 88, 855S–858S. [Google Scholar] [CrossRef]
- Crisponi, G.; Nurchi, V.M.; Fanni, D.; Gerosa, C.; Nemolato, S.; Faa, G. Copper-related diseases: From chemistry to molecular pathology. Coord. Chem. Rev. 2010, 254, 876–889. [Google Scholar] [CrossRef]
- Faa, A.; Gerosa, C.; Fanni, D.; Floris, G.; Eyken, P.V.; Lachowicz, J.I.; Nurchi, V.M. Depleted uranium and human health. Curr. Med. Chem. 2018, 25, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, M.; Metin, M.; Altay, V.; Bhat, R.A.; Ejaz, M.; Gul, A.; Unal, B.T.; Hasanuzzaman, M.; Nibir, L.; Nahar, K. Arsenic and human health: Genotoxicity, epigenomic effects, and cancer signaling. Biol. Trace Elem. Res. 2022, 200, 988–1001. [Google Scholar] [CrossRef]
- Peter, A.J.; Viraraghavan, T. Thallium: A review of public health and environmental concerns. Environ. Int. 2005, 31, 493–501. [Google Scholar] [CrossRef]
- Huang, X.; Wu, X.; Tang, X.; Zhang, Z.; Ma, J.; Zhang, J.; Liu, H. Distribution characteristics and risk of heavy metals and microbial community composition around the Wanshan mercury mine in Southwest China. Ecotoxicol. Environ. Saf. 2021, 227, 112897. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhang, L.; Xia, T.; Jia, X.; Wang, S. Heavy metal pollution and human health risk assessment at mercury smelting sites in Wanshan district of Guizhou Province, China. Rsc Adv. 2020, 10, 23066–23079. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Hu, W.; Wang, X.; Liu, Y.; Jiang, Y.; Meng, Y.; Xiao, Q.; Guo, X.; Zhou, Y.; Bi, Y. Analysis of heavy metal contamination of agricultural soils and related effect on population health—A case study for East River Basin in China. Int. J. Environ. Res. Public Health 2020, 17, 1996. [Google Scholar] [CrossRef]
- Guo, C.; Lv, L.; Liu, Y.; Ji, M.; Zang, E.; Liu, Q.; Zhang, M.; Li, M. Applied analytical methods for detecting heavy metals in medicinal plants. Crit. Rev. Anal. Chem. 2023, 53, 339–359. [Google Scholar] [CrossRef]
- Malik, L.A.; Bashir, A.; Qureashi, A.; Pandith, A.H. Detection and removal of heavy metal ions: A review. Environ. Chem. Lett. 2019, 17, 1495–1521. [Google Scholar] [CrossRef]
- Chen, W.; Yang, Y.; Fu, K.; Zhang, D.; Wang, Z. Progress in ICP-MS analysis of minerals and heavy metals in traditional medicine. Front. Pharmacol. 2022, 13, 891273. [Google Scholar] [CrossRef]
- Voica, C.; Kovacs, M.H.; Dehelean, A.; Ristoiu, D.; Iordache, A. ICP-MS determinations of heavy metals in surface waters from Transylvania. Rom. J. Phys. 2012, 57, 1184–1193. [Google Scholar]
- Hu, Q.; Yang, G.; Zhao, Y.; Yin, J. Determination of copper, nickel, cobalt, silver, lead, cadmium, and mercury ions in water by solid-phase extraction and the RP-HPLC with UV-Vis detection. Anal. Bioanal. Chem. 2003, 375, 831–835. [Google Scholar] [CrossRef]
- Yang, Y.; Guangyu, Y.; Lin, Q. Determination of heavy metal ions in Chinese herbal medicine by microwave digestion and RP-HPLC with UV-Vis detection. Microchim. Acta 2004, 144, 297–302. [Google Scholar] [CrossRef]
- Lu, Y.; Liang, X.; Niyungeko, C.; Zhou, J.; Xu, J.; Tian, G. A review of the identification and detection of heavy metal ions in the environment by voltammetry. Talanta 2018, 178, 324–338. [Google Scholar] [CrossRef]
- Zheng, J.; Rahim, M.A.; Tang, J.; Allioux, F.M.; Kalantar-Zadeh, K. Post-transition metal electrodes for sensing heavy metal ions by stripping voltammetry. Adv. Mater. Technol. 2022, 7, 2100760. [Google Scholar] [CrossRef]
- Borrill, A.J.; Reily, N.E.; Macpherson, J.V. Addressing the practicalities of anodic stripping voltammetry for heavy metal detection: A tutorial review. Analyst 2019, 144, 6834–6849. [Google Scholar] [CrossRef] [PubMed]
- Guselnikova, O.; Postnikov, P.; Erzina, M.; Kalachyova, Y.; Švorčík, V.; Lyutakov, O. Pretreatment-free selective and reproducible SERS-based detection of heavy metal ions on DTPA functionalized plasmonic platform. Sens. Actuators B Chem. 2017, 253, 830–838. [Google Scholar] [CrossRef]
- Yan, S.; Chu, F.; Zhang, H.; Yuan, Y.; Huang, Y.; Liu, A.; Wang, S.; Li, W.; Li, S.; Wen, W. Rapid, one-step preparation of SERS substrate in microfluidic channel for detection of molecules and heavy metal ions. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2019, 220, 117113. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Feng, S.; Peng, Y.; Li, B.; Zhao, J.; Xu, H.; Meng, X.; Zhai, W.; Pang, H. Emerging insights into the application of metal-organic framework (MOF)-based materials for electrochemical heavy metal ion detection. Food Chem. 2024, 463, 141387. [Google Scholar] [CrossRef] [PubMed]
- Yarur, F.; Macairan, J.-R.; Naccache, R. Ratiometric detection of heavy metal ions using fluorescent carbon dots. Environ. Sci. Nano 2019, 6, 1121–1130. [Google Scholar] [CrossRef]
- Yadav, P.; Yadav, L.; Laddha, H.; Agarwal, M.; Gupta, R. Upsurgence of smartphone as an economical, portable, and consumer-friendly analytical device/interface platform for digital sensing of hazardous environmental ions. Trends Environ. Anal. Chem. 2022, 36, e00177. [Google Scholar] [CrossRef]
- Chiu, R.Y.T.; Jue, E.; Yip, A.T.; Berg, A.R.; Wang, S.J.; Kivnick, A.R.; Nguyen, P.T.; Kamei, D.T. Simultaneous concentration and detection of biomarkers on paper. Lab Chip 2014, 14, 3021–3028. [Google Scholar] [CrossRef]
- Choi, D.H.; Lee, S.K.; Oh, Y.K.; Bae, B.W.; Lee, S.D.; Kim, S.; Shin, Y.B.; Kim, M.G. A dual gold nanoparticle conjugate-based lateral flow assay (LFA) method for the analysis of troponin I. Biosens. Bioelectron. 2010, 25, 1999–2002. [Google Scholar] [CrossRef]
- Posthuma-Trumpie, G.A.; Korf, J.; van Amerongen, A. Lateral flow (immuno) assay: Its strengths, weaknesses, opportunities and threats. A literature survey. Anal. Bioanal. Chem. 2009, 393, 569–582. [Google Scholar] [CrossRef]
- Rohrman, B.A.; Richards-Kortum, R.R. A paper and plastic device for performing recombinase polymerase amplification of HIV DNA. Lab Chip 2012, 12, 3082–3088. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.; Kawde, A.-N.; Daud, M. Designs, formats and applications of lateral flow assay: A literature review. J. Saudi Chem. Soc. 2015, 19, 689–705. [Google Scholar] [CrossRef]
- Guo, J.C.; Chen, S.Q.; Guo, J.H.; Ma, X. Nanomaterial labels in lateral flow immunoassays for point-of-care-testing. J. Mater. Sci. Technol. 2021, 60, 90–104. [Google Scholar] [CrossRef]
- Panferov, V.G.; Zherdev, A.V.; Dzantiev, B.B. Post-Assay chemical enhancement for highly sensitive lateral flow immunoassays: A critical review. Biosensors 2023, 13, 866. [Google Scholar] [CrossRef]
- Najib, M.A.; Selvam, K.; Khalid, M.F.; Ozsoz, M.; Aziah, I. Quantum dot-based lateral flow immunoassay as point-of-care testing for infectious diseases: A narrative review of its principle and performance. Diagnostics 2022, 12, 2158. [Google Scholar] [CrossRef] [PubMed]
- Panferov, V.G.; Ivanov, N.A.; Mazzulli, T.; Brinc, D.; Kulasingam, V.; Krylov, S.N. Electrophoresis-assisted multilayer assembly of nanoparticles for sensitive lateral flow immunoassay. Angew. Chem. Int. Ed. 2023, 62, e202215548. [Google Scholar] [CrossRef]
- Loynachan, C.N.; Thomas, M.R.; Gray, E.R.; Richards, D.A.; Kim, J.; Miller, B.S.; Brookes, J.C.; Agarwal, S.; Chudasama, V.; McKendry, R.A.; et al. Platinum nanocatalyst amplification: Redefining the gold standard for lateral flow immunoassays with ultrabroad dynamic range. ACS Nano 2018, 12, 279–288. [Google Scholar] [CrossRef]
- Cao, Q.Q.; Liu, Y.Q.; Yang, L.; Tan, T.; He, J.; Chen, W.W.; Li, R.H.; Wang, W.G. Popcorn-like bimetallic palladium/platinum exhibiting enhanced peroxidase-like activity for signal enhancement in lateral flow immunoassay. Anal. Chim. Acta 2024, 1309, 342698. [Google Scholar] [CrossRef]
- Ma, D.D.; Wang, Y.T.; Zhang, Q.J.; Wang, C.; Du, Y.X.; Liang, D.B.; Shen, J.C.; Pan, X.; Sheng, E.Z.; Zhu, D. Hierarchical magneto-colorimetric labels for immediate lateral flow immunoassay of chlorothalonil residues. Talanta 2024, 280, 126743. [Google Scholar] [CrossRef]
- Angela, S.; Hsiao, W.W.W.; Fadhilah, G.; Le, T.N.; Chiang, W.H. Detection of avian influenza virus utilizing fluorescent nanodiamonds for lateral flow immunoassay enhanced by magnetic modulation. J. Taiwan Inst. Chem. Eng. 2025, 169, 105945. [Google Scholar] [CrossRef]
- Wen, C.Y.; Zhao, L.J.; Wang, Y.; Wang, K.; Li, H.W.; Li, X.; Zi, M.; Zeng, J.B. Colorimetric and photothermal dual-mode lateral flow immunoassay based on Au-Fe3O4 multifunctional nanoparticles for detection of Salmonella typhimurium. Microchim. Acta 2023, 190, 57. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Ge, C.; Wang, L.; Xing, X.; Zeng, L. A simple lateral flow biosensor for the rapid detection of copper (II) ions based on click chemistry. RSC Adv. 2015, 5, 75722–75727. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Xue, J.; Dong, J.; Cai, J.; Hua, X.; Wang, M.; Zhang, C.; Liu, F. Signal-amplified lateral flow test strip for visual detection of Cu2+. PLoS ONE 2017, 12, e0169345. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, Y.; Deng, Y.; Yao, L.; Adeloju, S.B.; Pan, D.; Xue, F.; Wu, Y.; Zheng, L.; Chen, W. Ultrasensitive detection of mercury with a novel one-step signal amplified lateral flow strip based on gold nanoparticle-labeled ssDNA recognition and enhancement probes. Biosens. Bioelectron. 2014, 61, 14–20. [Google Scholar] [CrossRef]
- Liu, J.; Chen, L.; Chen, J.; Ge, C.; Fang, Z.; Wang, L.; Xing, X.; Zeng, L. An autonomous T-rich DNA machine based lateral flow biosensor for amplified visual detection of mercury ions. Anal. Methods 2014, 6, 2024–2027. [Google Scholar] [CrossRef]
- Guo, Z.; Kang, Y.; Liang, S.; Zhang, J. Detection of Hg (II) in adsorption experiment by a lateral flow biosensor based on streptavidin-biotinylated DNA probes modified gold nanoparticles and smartphone reader. Environ. Pollut. 2020, 266, 115389. [Google Scholar] [CrossRef]
- Cheng, N.; Xu, Y.; Huang, K.; Chen, Y.; Yang, Z.; Luo, Y.; Xu, W. One-step competitive lateral flow biosensor running on an independent quantification system for smart phones based in-situ detection of trace Hg (II) in tap water. Food Chem. 2017, 214, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Chen, Y.; Wang, R.; Yan, C.; Xu, J.; Yao, B.; Cheng, J.; Chen, W. Rapid and sensitive detection of Hg2+ with a SERS-enhanced lateral flow strip. Analyst 2022, 147, 4337–4347. [Google Scholar] [CrossRef]
- Wang, Z.; Cheng, Y.; Tang, T.; Zhang, X.; Yuan, X. Simultaneous or separate detection of heavy metal ions Hg2+ and Ag+ based on lateral flow assays. Microchim. Acta 2025, 192, 97. [Google Scholar] [CrossRef]
- Ren, L.; Sun, J.; Ma, S.; Wang, D.; Qi, R.; Zhang, P.; Zhang, Q.; Qin, Z.; Jiang, L. A novel lateral flow assay for lead ion detection based on G-quadruplex. Biochem. Eng. J. 2025, 213, 109562. [Google Scholar] [CrossRef]
- Wang, D.; Ge, C.; Lv, K.; Zou, Q.; Liu, Q.; Liu, L.; Yang, Q.; Bao, S. A simple lateral flow biosensor for rapid detection of lead(II) ions based on G-quadruplex structure-switching. Chem. Commun. 2018, 54, 13718–13721. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, Y.; Ding, J.; Hayat, K.; Zhan, X.; Zhou, P.; Zhang, D. Label-free colorimetric assay for arsenic (III) determination based on a truncated short ssDNA and gold nanoparticles. Microchim. Acta 2021, 188, 38. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.; Yang, Y.; He, R.; Park, Y.I.; Lee, A.; Bai, D.; Li, F.; Lu, T.J.; Xu, F.; Lin, M. Lateral flow aptamer assay integrated smartphone-based portable device for simultaneous detection of multiple targets using upconversion nanoparticles. Sens. Actuators B Chem. 2018, 276, 48–56. [Google Scholar] [CrossRef]
- Irfan, M.; Murtaza, G.; Fu, S.; Chen, A.; Qu, F.; Su, X. Molecular simulation-guided aptasensor design of robust and sensitive lateral flow strip for cadmium ion detection. Analyst 2023, 148, 1961–1969. [Google Scholar] [CrossRef]
- Wu, Z.; Shen, H.; Hu, J.; Fu, Q.; Yao, C.; Yu, S.; Xiao, W.; Tang, Y. Aptamer-based fluorescence-quenching lateral flow strip for rapid detection of mercury (II) ion in water samples. Anal. Bioanal. Chem. 2017, 409, 5209–5216. [Google Scholar] [CrossRef]
- Srinivasan, S.; Ranganathan, V.; McConnell, E.M.; Murari, B.M.; DeRosa, M.C. Aptamer-based colorimetric and lateral flow assay approaches for the detection of toxic metal ions, thallium (i) and lead (ii). RSC Adv. 2023, 13, 20040–20049. [Google Scholar] [CrossRef]
- Berlina, A.N.; Komova, N.S.; Zherdev, A.V.; Boris, B.D. Combination of phenylboronic acid and oligocytosine for selective and specific detection of lead(ii) by lateral flow test strip. Anal. Chim. Acta 2021, 1155, 338318. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, Y.; Kim, S.; Lee, E.S.; Cha, B.S.; Park, K.S. MNAzyme-Assisted Nucleic Acid Lateral Flow Assay for Cost-Effective, On-Site Mercury Detection. Biosensors 2024, 14, 454. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Zhang, C.; Liu, F. A lateral flow assay for copper (II) utilizing catalytic and stem-loop based signal amplification. Microchim. Acta 2019, 186, 82. [Google Scholar] [CrossRef]
- Mazumdar, D.; Lan, T.; Lu, Y. “Dipstick” colorimetric detection of metal ions based on immobilization of DNAzyme and gold nanoparticles onto a lateral flow device. In Biosensors and Biodetection; Humana Press: New York, NY, USA, 2017; pp. 389–406. [Google Scholar]
- Wang, Z.; Chen, B.; Duan, J.; Hao, T.; Jiang, X.; Guo, Z.; Wang, S. A test strip for lead (II) based on gold nanoparticles multi-functionalized by DNAzyme and barcode DNA. J. Anal. Chem. 2015, 70, 339–345. [Google Scholar] [CrossRef]
- Pei, X. Nanogold-signalized lateral-flow strip for visual detection of lead ion based on cleavage of metal-ion-induced DNAzyme. Chem. Lett. 2014, 43, 1643–1644. [Google Scholar] [CrossRef]
- Fang, Z.; Huang, J.; Lie, P.; Xiao, Z.; Ouyang, C.; Wu, Q.; Wu, Y.; Liu, G.; Zeng, L. Lateral flow nucleic acid biosensor for Cu2+ detection in aqueous solution with high sensitivity and selectivity. Chem. Commun. 2010, 46, 9043–9045. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Liu, F. Antibody developments for metal ions and their applications. Food Agric. Immunol. 2020, 31, 1079–1103. [Google Scholar] [CrossRef]
- Ling, S.; Zhao, Q.; Iqbal, M.N.; Dong, M.; Li, X.; Lin, M.; Wang, R.; Lei, F.; He, C.; Wang, S. Development of immunoassay methods based on monoclonal antibody and its application in the determination of cadmium ion. J. Hazard. Mater. Adv. 2021, 411, 124992. [Google Scholar] [CrossRef] [PubMed]
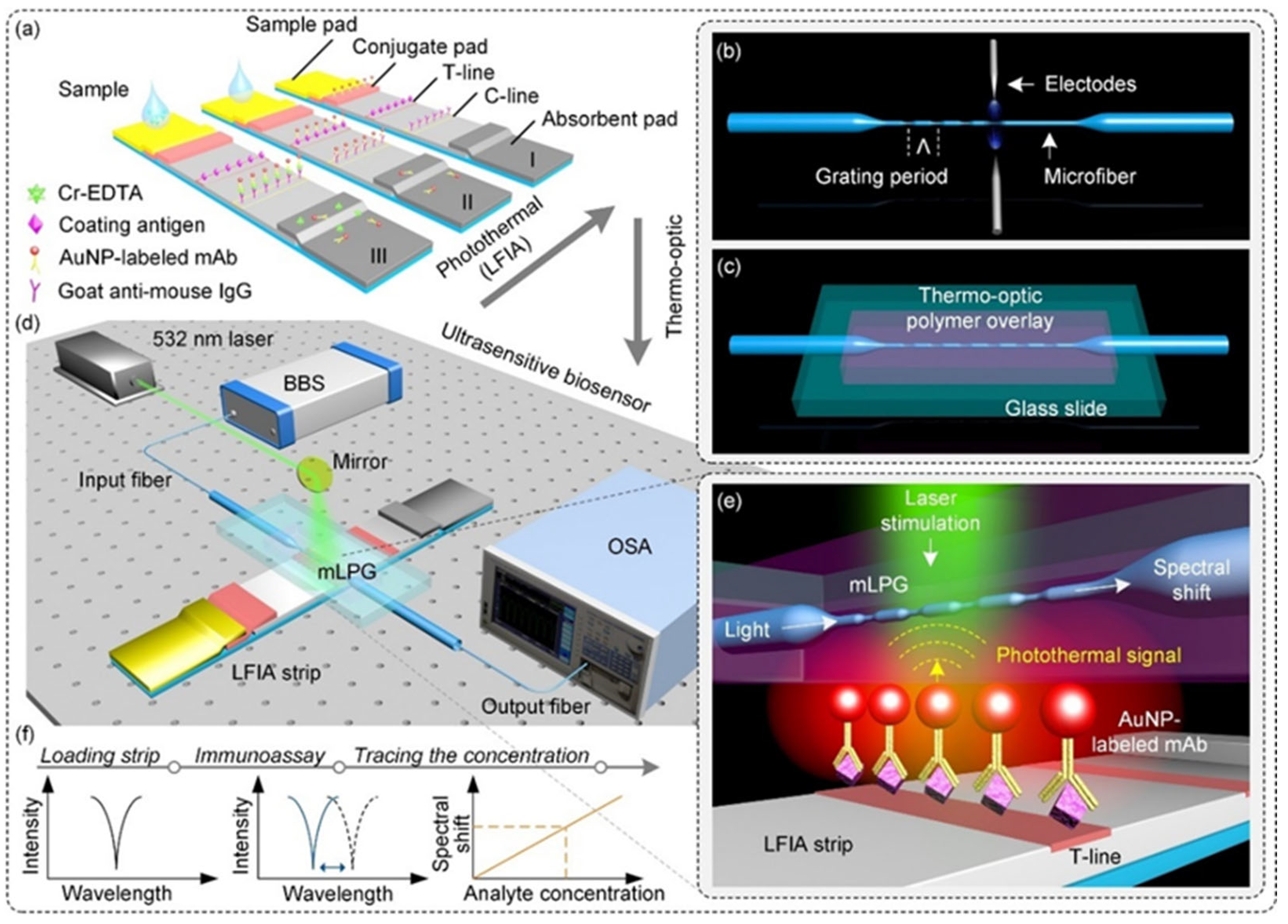
- Huang, T.; Fu, Q.; Sun, L.P.; Liu, P.; Wu, Z.; Li, K.; Xiao, R.; Yang, X.; Huang, Y.; Lin, W.; et al. Photothermal lateral flow immunoassay using microfiber long-period grating. Sens. Actuators B Chem. 2021, 344, 130283. [Google Scholar] [CrossRef]
- Liang, J.; Liu, H.; Lan, C.; Fu, Q.; Huang, C.; Luo, Z.; Jiang, T.; Tang, Y. Silver nanoparticle enhanced Raman scattering-based lateral flow immunoassays for ultra-sensitive detection of the heavy metal chromium. Nanotechnology 2014, 25, 495501. [Google Scholar] [CrossRef]
- Quesada-González, D.; Jairo, G.A.; Blake, R.C.; Blake, D.A.; Merkoçi, A. Uranium (VI) detection in groundwater using a gold nanoparticle/paper-based lateral flow device. Sci. Rep. 2018, 8, 16157. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xiang, J.-J.; Tang, Y.; Zhang, X.-L.; Fu, Q.-Q.; Zou, J.-H.; Lin, Y. Colloidal gold nanoparticle probe-based immunochromatographic assay for the rapid detection of chromium ions in water and serum samples. Anal. Chim. Acta 2012, 745, 99–105. [Google Scholar] [CrossRef]
- Tang, Y.; Zhai, Y.-F.; Xiang, J.-J.; Wang, H.; Liu, B.; Guo, C.-W. Colloidal gold probe-based immunochromatographic assay for the rapid detection of lead ions in water samples. Environ. Pollut. 2010, 158, 2074–2077. [Google Scholar] [CrossRef]
- Song, S.; Zou, S.; Zhu, J.; Liu, L.; Kuang, H. Immunochromatographic paper sensor for ultrasensitive colorimetric detection of cadmium. Food Agric. Immunol. 2018, 29, 3–13. [Google Scholar] [CrossRef]
- Xing, Y.; Wu, X.; Liu, L.; Zhu, J.; Xu, L.; Kuang, H. Development of a fluorescent immunoassay strip for the rapid quantitative detection of cadmium in rice. Food Agric. Immunol. 2020, 31, 501–512. [Google Scholar] [CrossRef]
- Zhu, Z.; Jia, J.; Xu, N.; Zhu, J.; Wang, J.; Wang, Y. Comparative analysis of three immunochromatographic test strips for rapid detection of cadmium ion in Asparagus. Int. J. Food Sci. Nutr. 2023, 58, 6598–6608. [Google Scholar] [CrossRef]
- López_Marzo, A.M.; Pons, J.; Blake, D.A.; Merkoçi, A. High sensitive gold-nanoparticle based lateral flow Immunodevice for Cd2+ detection in drinking waters. Biosens. Bioelectron. 2013, 47, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, Y.-S.; Meng, X.-Y.; Zhang, Y.-Y.; Yang, L.; Zhang, J.-H.; Wang, X.-R.; Lu, S.-Y.; Ren, H.-L.; Liu, Z.-S. Development of an immunochromatographic strip and its application in the simultaneous determination of Hg (II), Cd (II) and Pb (II). Sens. Actuators B Chem. 2013, 183, 303–309. [Google Scholar] [CrossRef]
- Liu, W.; Wang, H.; Zhong, W.; Zhang, Y.; Liu, Y.; Gao, X.; Yan, M.; Zhu, C. The development and application of SERS-based lateral flow immunochromatography in the field of food safety. Microchim. Acta 2025, 192, 246. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Dang, H.; Moon, J.-I.; Kim, K.; Joung, Y.; Park, S.; Yu, Q.; Chen, J.; Lu, M.; Chen, L. SERS-based microdevices for use as in vitro diagnostic biosensors. Chem. Soc. Rev. 2024, 53, 5394–5427. [Google Scholar] [CrossRef]



| Metal Type | Material | Identification Unit | Signal Readout | Detection Limit | Detection Samples | Reference |
|---|---|---|---|---|---|---|
| Cu2+ | AuNPs | Azide-DNA and alkyne/biotin-DNA | Colorimetric/Reading device | 100 nM | Tap water and human serum | [53] |
| Cu2+ | AuNPs | Azide- and alkyne-modified ssDNA | Colorimetric/Reading device | 5 nM | Municipal water and river water | [54] |
| Hg2+ | AuNPs | ssDNA | Colorimetric/Image J | 25 pM | Tap water, tea water, and lake water | [55] |
| Hg2+ | AuNPs | T-Hg2+-T | Colorimetric/Reading device | 5 nM | River water | [56] |
| Hg2+ | AuNPs | T-Hg2+-T | Colorimetric/biosensor | 2.53 nM | Hg(II)-containing aqueous solution | [57] |
| Hg2+ | AuNPs | T-Hg2+-T | Colorimetric/Mobile analysis | 4 nM | Tap water | [58] |
| Hg2+ | Au@AgNPs | T-Hg2+-T | SERS/LabRAM HR Evolution system | 0.36 pM | Tea | [59] |
| Hg2+ Ag+ | AuNPs | T-Hg2+-T and C-Ag+-C | Colorimetric/ImageJ | Hg2+: 2.19 pM Ag+: 5.41 pM | River water and Tap water | [60] |
| Pb2+ | AuNPs | antiG4 single-stranded DNA | Colorimetric/Image J | 20 nM | Drinking water | [61] |
| Pb2+ | AuNPs | Domain 2 (G-rich sequence) in DNA 1-2 probe | Colorimetric/Colorimetric card | 25 nM | Solutions containing different concentrations of lead ions | [62] |
| As3+ | AuNPs | As3+-Apt-21 complex | Colorimetric | 2.4 nM | Tap water and River water | [63] |
| Metal Type | Material | Identification Unit | Signal Readout | Detection Limit | Detection Samples | Reference |
|---|---|---|---|---|---|---|
| Hg2+ | Upconversion nanoparticles (UCNPs) | Aptamer | Fluorescence/ImageJ | 25 nM | Tap water | [64] |
| Cd2+ | 30 nt DNA probe | Cy5-labeled aptamer | Fluorescence | 30 nM | River water | [65] |
| Hg2+ | AuNPs | Specific oligonucleotide probe | Fluorescence/Fluorescence reader | 0.65 nM | River water | [66] |
| Tl+ | AuNPs AgNPs | Aptamer | Colorimetric/ImageJ | AuNPs: 7.4 µM, AgNPs: 6.3 µM | Distilled water, River water, Human serum | [67] |
| Pb2+ | AuNPs | Phenylboronic acid and oligocytosine | Colorimetric/TotalLab TL120 | 4.8 nM | Drinking water | [68] |
| Metal Type | Material | Identification Unit | Signal Readout | Detection Limit | Detection Samples | Reference |
|---|---|---|---|---|---|---|
| Hg2+ | AuNPs | MNAzyme | Colorimetric/ImageJ | 9.34 nM | Tap water | [69] |
| Cu2+ | AuNPs | DNAzyme | Colorimetric | 31.5 nM | Tap water and river water | [70] |
| Pb2+ | AuNPs | 8–17 DNAzyme | Colorimetric | 5 μM | Paint | [71] |
| Pb2+ | AuNPs | 17E DNAzyme | Colorimetric | 20 nM | Tap water, river water, and pool water | [72] |
| Pb2+ | AuNPs | 8–17 DNAzyme | Colorimetric | 0.05 nM | Drinking water | [73] |
| Cu2+ | AuNPs | DNAzyme | Colorimetric/Strip reader | 10 nM | Aqueous solution | [74] |
| Metal Type | Material | Identification Unit | Signal Readout | Detection Limit | Detection Samples | Reference |
|---|---|---|---|---|---|---|
| Cd2+ | AuNS AuNF | Monoclonal antibody (MAb) | Colorimetric/Reading device | AuNS: 3.34 nM, AuNF: 0.27 nM | Drinking water, tap water and laboratory deionized water | [76] |
| Cr3+ | AuNPs | Monoclonal antibody (MAb) | measure the spectral shift in microfiber long-period gratings | 4.21 nM | solutions containing different concentrations of Cr(III) ions | [77] |
| Cr3+ | AgNPs | Anti-Cr3+-EDTA monoclonal antibody | SERS | 0.192 pM | Distilled water, tap water, and environmental water samples | [78] |
| U4+ | AuNPs | Monoclonal antibody (12F6) | Colorimetric/Image J | 6 nM | Groundwater | [79] |
| Cr3+ Cr6+ | AuNPs | Monoclonal antibody (McAb) | Colorimetric/Reading device | 0.962 μM | Water samples from the Pearl River and West Lake | [80] |
| Pb2+ | AuNPs | Anti-Pb-DTPA monoclonal antibody | Colorimetric | 0.241 μM | Water samples from the Pearl River | [81] |
| Cd2+ | AuNPs | Anti-Cd(II)-ITCBE monoclonal antibody (3A9) | Colorimetric/Scanning reading device | 1.78 nM | Tap water | [82] |
| Cd2+ | FMs | Monoclonal antibody (mAb 2F7) | Colorimetric and fluorescence/Reading device | 17.17 nM | Rice | [83] |
| Cd2+ | AuNPs LMs PDA | Anti-cadmium monoclonal antibody (4A9) | Colorimetric/Reading device | 44.48 nM, 0.89 nM, 0.89 nM | Asparagus | [84] |
| Cd2+ | AuNPs | Monoclonal antibody (2A81G5) | Colorimetric/Reading device | 0.89 nM | Tap water | [85] |
| Hg2+ Cd2+ Pb2+ | AuNPs | Monoclonal antibody | Colorimetric/Epson 3200 Photo Scanner | 8 nM, 6 nM, and 6 nM | Mineral water, tap water, and lake water | [86] |
| Detection Strategy | Recognition Mechanism | Core Advantages |
|---|---|---|
| DNA Probe-Based LFA Technology | Specific binding of DNA sequences (e.g., T-rich, G-quadruplex) to metal ions (e.g., T-Hg2+-T coordination) |
|
| Aptamer-Based LFA Technology | High-affinity binding of aptamers to metal ions via conformational changes |
|
| Nucleic Acid Enzyme-Based LFA Technology | Metal ions activate DNAzyme catalytic activity to cleave substrates and release signal molecules |
|
| Antigen–Antibody-Based LFA Technology | Immunological binding of antibodies to metal ion–carrier protein complexes |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, X.; Hu, X.; Cao, X.; Zhou, Q.; Yang, W.; Yu, R.; Liu, S.; Hu, H.; Qi, J.; Zhang, Z. Heavy Metal Ion Detection Based on Lateral Flow Assay Technology: Principles and Applications. Biosensors 2025, 15, 438. https://doi.org/10.3390/bios15070438
Xie X, Hu X, Cao X, Zhou Q, Yang W, Yu R, Liu S, Hu H, Qi J, Zhang Z. Heavy Metal Ion Detection Based on Lateral Flow Assay Technology: Principles and Applications. Biosensors. 2025; 15(7):438. https://doi.org/10.3390/bios15070438
Chicago/Turabian StyleXie, Xiaobo, Xinyue Hu, Xin Cao, Qianhui Zhou, Wei Yang, Ranran Yu, Shuaiqi Liu, Huili Hu, Ji Qi, and Zhiyang Zhang. 2025. "Heavy Metal Ion Detection Based on Lateral Flow Assay Technology: Principles and Applications" Biosensors 15, no. 7: 438. https://doi.org/10.3390/bios15070438
APA StyleXie, X., Hu, X., Cao, X., Zhou, Q., Yang, W., Yu, R., Liu, S., Hu, H., Qi, J., & Zhang, Z. (2025). Heavy Metal Ion Detection Based on Lateral Flow Assay Technology: Principles and Applications. Biosensors, 15(7), 438. https://doi.org/10.3390/bios15070438






