Recent Advances in Metal–Organic Framework-Based Nanozymes for Intelligent Microbial Biosensing: A Comprehensive Review of Biomedical and Environmental Applications
Abstract
1. Introduction
2. Fundamentals of MOF Nanozymes
2.1. MOFs: Structure and Properties
2.2. Nanozymes: Definition and Classification
2.3. MOFs as Nanozyme Platforms
3. Design Strategies for MOF Nanozymes in Microbial Biosensing
3.1. Metal Center Engineering
3.2. Ligand Functionalization
3.3. Morphology and Size Control
3.4. Composite and Hybrid Structures
4. Detection Mechanisms and Signal Transduction
4.1. Colorimetric Detection
4.2. Electrochemical Detection
4.3. Fluorescence and Luminescence
5. Biomedical Applications
5.1. Pathogen Detection in Clinical Samples
5.2. Real-Time and POC Diagnostics
5.3. Wound Monitoring and Infection Control
6. Environmental Applications
6.1. Waterborne Pathogen Detection
6.2. Soil and Air Microbial Sensing
7. Challenges and Future Perspectives
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) |
| AgNP | Silver nanoparticle |
| AuNP | Gold nanoparticle |
| bPEI-g-PEG | Poly(ethylene imine)-graft-poly(ethylene glycol) |
| CAT | Catalase |
| CF | Carbon microfiber |
| CL | Chemiluminescence |
| CNT | Carbon nanotube |
| DAP | Diaminophenazine |
| DHBDC2− | 2,5-dihydroxyterephthalic acid |
| ECL | Electrochemiluminescence |
| FQ | Fluoroquinolone antibiotics |
| GDY-CNT | Graphdiyne/carbon nanotube |
| Gox | Glucose oxidase |
| IFE | Inner filter effect |
| LOD | Limit of detection |
| Lox | Lactate oxidase |
| MN | Microneedle |
| MOF | Metal–organic framework |
| NIR | Near-infrared |
| NP | Nanoparticle |
| OD | Oxidase |
| OMV | Outer membrane vesicle |
| OPD | o-phenylenediamine |
| PAA | Poly(acrylic acid) |
| PPase | Pyrophosphatase |
| PPi | Pyrophosphate |
| POD | Peroxidase |
| PtNP | Platinum nanoparticle |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| TMB | 3,3′,5,5′-tetramethylbenzidine |
| UA | Uric acid |
| ZIF | Zeolitic imidazolate framework |
References
- Oluwaseun, O.; Singleton, I.; Sant, A.S.; January, S. Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus COVID-19. The COVID-19 resource centre is hosted on Elsevier Connect, the company’s public news and information. Food Microbiol. 2020, 73, 177–208. [Google Scholar]
- Taylor, J.; Lai, K.M.; Davies, M.; Clifton, D.; Ridley, I.; Biddulph, P. Flood management: Prediction of microbial contamination in large-scale floods in urban environments. Environ. Int. 2011, 37, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Novikova, N.D. Review of the knowledge of microbial contamination of the russian manned spacecraft. Microb. Ecol. 2004, 47, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Edmiston, C.E.; Seabrook, G.R.; Cambria, R.A.; Brown, K.R.; Lewis, B.D.; Sommers, J.R.; Krepel, C.J.; Wilson, P.J.; Sinski, S.; Towne, J.B. Molecular epidemiology of microbial contamination in the operating room environment: Is there a risk for infection? Surgery 2005, 138, 573–582. [Google Scholar] [CrossRef]
- Dacarro, C.; Picco, A.M.; Grisoli, P.; Rodolfi, M. Determination of aerial microbiological contamination in scholastic sports environments. J. Appl. Microbiol. 2003, 95, 904–912. [Google Scholar] [CrossRef]
- Tropea, A. Microbial Contamination and Public Health: An Overview. Int. J. Environ. Res. Public Health 2022, 19, 7441. [Google Scholar] [CrossRef]
- Fredricks, D.N.; Relman, D.A.; Diseases, I. Application of Polymerase Chain Reaction to the Diagnosis of Infectious Diseases. In Application of Polymerase Chain; David, N.F., David, A., Eds.; Oxford University Press: Oxford, UK, 2018; Volume 29, pp. 475–486. Available online: http://www.jstor.org/stable/4460932 (accessed on 24 May 2025).
- Mendoza, L.G.; McQuary, P.; Mongan, A.; Gangadharan, R.; Brignac, S.; Eggers, M. High-Throughput Microarray-Based Enzyme-Linked Immunosorbent Assay (ELISA). Biotechniques 1999, 27, 778–788. [Google Scholar] [CrossRef]
- Austin, P.; Elia, M. A Systematic Review and Meta-Analysis of the Risk of Microbial Contamination of Aseptically Prepared Doses in Different Environments. J. Pharm. Pharm. Sci. 2009, 12, 233–242. [Google Scholar] [CrossRef]
- Koskinen, M.T.; Wellenberg, G.J.; Sampimon, O.C.; Holopainen, J.; Rothkamp, A.; Salmikivi, L.; van Haeringen, W.A.; Lam, T.J.G.M.; Pyörälä, S. Field comparison of real-time polymerase chain reaction and bacterial culture for identification of bovine mastitis bacteria. J. Dairy Sci. 2010, 93, 5707–5715. [Google Scholar] [CrossRef]
- White, P.L.; Parr, C.; Thornton, C.; Barnes, R.A. Evaluation of Real-Time PCR, Galactomannan Enzyme-Linked Immunosorbent Assay (ELISA), and a Novel Lateral-Flow Device for Diagnosis of Invasive Aspergillosis. J. Clin. Microbiol. 2013, 51, 1510–1516. [Google Scholar] [CrossRef]
- Sutarlie, L.; Ow, S.Y.; Su, X. Nanomaterials-based biosensors for detection of microorganisms and microbial toxins. Biotechnol. J. 2017, 12, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Raza, N.; Bhardwaj, S.K.; Vikrant, K.; Kim, K.H.; Bhardwaj, N. Advances in nanomaterial-based electrochemical biosensors for the detection of microbial toxins, pathogenic bacteria in food matrices. J. Hazard. Mater. 2021, 401, 123379. [Google Scholar] [CrossRef] [PubMed]
- Šefčovičová, J.; Tkac, J. Application of nanomaterials in microbial-cell biosensor constructions. Chem. Pap. 2015, 69, 42–53. [Google Scholar] [CrossRef]
- Khan, M.Q.; Khan, J.; Alvi, M.A.H.; Nawaz, H.; Fahad, M.; Umar, M. Nanomaterial-based sensors for microbe detection: A review. Discov. Nano 2024, 19, 1–24. [Google Scholar] [CrossRef]
- Bhalla, N.; Pan, Y.; Yang, Z.; Payam, A.F. Opportunities and Challenges for Biosensors and Nanoscale Analytical Tools for Pandemics: COVID-19. ACS Nano 2020, 14, 7783–7807. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Jian, M.; Geleta, G.S.; Wang, Z. Two-Dimensional Layered Nanomaterial-Based Electrochemical Biosensors for Detecting Microbial Toxins. Toxins 2019, 12, 20. [Google Scholar] [CrossRef]
- Bobrinetskiy, I.; Radovic, M.; Rizzotto, F.; Vizzini, P.; Jaric, S.; Pavlovic, Z.; Radonic, V.; Nikolic, M.V.; Vidic, J. Advances in Nanomaterials-Based Electrochemical Biosensors for Foodborne Pathogen Detection. Nanomaterials 2021, 11, 2700. [Google Scholar] [CrossRef]
- Mehta, D.; Gupta, D.; Kafle, A.; Kaur, S.; Nagaiah, T.C. Advances and Challenges in Nanomaterial-Based Electrochemical Immunosensors for Small Cell Lung Cancer Biomarker Neuron-Specific Enolase. ACS Omega 2024, 9, 33–51. [Google Scholar] [CrossRef]
- Khor, S.M.; Choi, J.; Won, P.; Ko, S.H. Challenges and Strategies in Developing an Enzymatic Wearable Sweat Glucose Biosensor as a Practical Point-Of-Care Monitoring Tool for Type II Diabetes. Nanomaterials 2022, 12, 221. [Google Scholar] [CrossRef]
- Bedendi, G.; De Torquato, L.D.M.; Webb, S.; Cadoux, C.; Kulkarni, A.; Sahin, S.; Maroni, P.; Milton, R.D.; Grattieri, M. Enzymatic and Microbial Electrochemistry: Approaches and Methods. ACS Meas. Sci. Au 2022, 2, 517–541. [Google Scholar] [CrossRef]
- Lim, J.W.; Ha, D.; Lee, J.; Lee, S.K.; Kim, T. Review of micro/nanotechnologies for microbial biosensors. Front. Bioeng. Biotechnol. 2015, 3, 61. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Du, Z.; Zhang, Y.; Bai, R.; Zhu, L.; Xu, W. Exploring Nucleic Acid Nanozymes: A New Frontier in Biosensor Development. Biosensors 2025, 15, 142. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Fang, X.; Cheng, J.; Yang, S.; Chen, Z.; Tong, Y. Research progress of metal–organic framework nanozymes in bacterial sensing, detection, and treatment. RSC Med. Chem. 2023, 15, 380–398. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Li, X.; Lyu, Z.; Pan, J.; Ding, S.; Ruan, X.; Zhu, W.; Du, D.; Lin, Y. Metal–organic framework based nanozymes: Promising materials for biochemical analysis. Chem. Commun. 2020, 56, 11338–11353. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Amira, M.F.; Seleim, S.M.; Mohamed, A.K. Amino-decorated magnetic metal-organic framework as a potential novel platform for selective removal of chromium (Vl), cadmium (II) and lead (II). J. Hazard. Mater. 2020, 381, 120979. [Google Scholar] [CrossRef]
- Li, M.; Zhang, G.; Boakye, A.; Chai, H.; Qu, L.; Zhang, X. Recent Advances in Metal-Organic Framework-Based Electrochemical Biosensing Applications. Front. Bioeng. Biotechnol. 2021, 9, 797067. [Google Scholar] [CrossRef]
- Wang, H.S.; Wang, Y.H.; Ding, Y. Development of biological metal–organic frameworks designed for biomedical applications: From bio-sensing/bio-imaging to disease treatment. Nanoscale Adv. 2020, 2, 3788–3797. [Google Scholar] [CrossRef]
- Miller, S.E.; Teplensky, M.H.; Moghadam, P.Z.; Fairen-Jimenez, D. Metal-organic frameworks as biosensors for luminescence-based detection and imaging. Interface Focus 2016, 6, 20160027. [Google Scholar] [CrossRef]
- Wang, X.; Lan, P.C.; Ma, S. Metal-Organic Frameworks for Enzyme Immobilization: Beyond Host Matrix Materials. ACS Central Sci. 2020, 6, 1497–1506. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.; Hou, M.; Li, X.; Wu, X.; Ge, J. Immobilization on Metal-Organic Framework Engenders High Sensitivity for Enzymatic Electrochemical Detection. ACS Appl. Mater. Interfaces 2017, 9, 13831–13836. [Google Scholar] [CrossRef]
- Aggarwal, V.; Solanki, S.; Malhotra, B.D. Applications of metal-organic framework-based bioelectrodes. Chem. Sci. 2022, 13, 8727–8743. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Xing, G.; Lin, H.; Chen, S.; Xie, T.; Lin, J.M. Portable Biosensor with Bimetallic Metal-Organic Frameworks for Visual Detection and Elimination of Bacteria. Anal. Chem. 2023, 95, 13368–13375. [Google Scholar] [CrossRef] [PubMed]
- Elgazar, A.; Sabouni, R.; Ghommem, M.; Majdalawieh, A.F. Novel metal-organic framework biosensing platform for detection of COVID-19 RNA. Sci. Rep. 2024, 14, 25437. [Google Scholar] [CrossRef]
- Leelasree, T.; Selamneni, V.; Akshaya, T.; Sahatiya, P.; Aggarwal, H. MOF based flexible, low-cost chemiresistive device as a respiration sensor for sleep apnea diagnosis. J. Mater. Chem. B 2020, 8, 10182–10189. [Google Scholar] [CrossRef]
- Zhang, T.; Tang, M.; Yang, S.Y.; Fa, H.; Wang, Y.; Huo, D.; Hou, C.; Yang, M. Development of a novel ternary MOF nanozyme-based smartphone-integrated colorimetric and microfluidic paper-based analytical device for trace glyphosate detection. Food Chem. 2024, 464, 141780. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Yuan, Z.; Wu, L.; Ma, J.; Tan, W.; Sun, Y.; Zhang, G.; Chai, H. MOF nanozymes: Active sites and sensing applications. Inorg. Chem. Front. 2024, 12, 400–429. [Google Scholar] [CrossRef]
- Wang, D.; Jana, D.; Zhao, Y. Metal-Organic Framework Derived Nanozymes in Biomedicine. Acc. Chem. Res. 2020, 53, 1389–1400. [Google Scholar] [CrossRef]
- Liang, J.; Liang, Z.; Zou, R.; Zhao, Y. Heterogeneous Catalysis in Zeolites, Mesoporous Silica, and Metal-Organic Frameworks. Adv. Mater. 2017, 29, 1701139. [Google Scholar] [CrossRef]
- Zorlu, T.; Hetey, D.; Reithofer, M.R.; Chin, J.M. Physicochemical Methods for the Structuring and Assembly of MOF Crystals. Acc. Chem. Res. 2024, 57, 2105–2116. [Google Scholar] [CrossRef]
- Redfern, L.R.; Farha, O.K. Mechanical properties of metal–organic frameworks. Chem. Sci. 2019, 10, 10666–10679. [Google Scholar] [CrossRef]
- Stavila, V.; Talin, A.A.; Allendorf, M.D. MOF-based electronic and opto-electronic devices. Chem. Soc. Rev. 2014, 43, 5994–6010. [Google Scholar] [CrossRef] [PubMed]
- Burtch, N.C.; Heinen, J.; Bennett, T.D.; Dubbeldam, D.; Allendorf, M.D. Mechanical Properties in Metal-Organic Frameworks: Emerging Opportunities and Challenges for Device Functionality and Technological Applications. Adv. Mater. 2018, 30, 1704124. [Google Scholar] [CrossRef] [PubMed]
- Kidanemariam, A.; Cho, S. Recent Advances in the Application of Metal-Organic Frameworks and Coordination Polymers in Electrochemical Biosensors. Chemosensors 2024, 12, 135. [Google Scholar] [CrossRef]
- Gharib, G.; Bütün, I.; Muganlı, Z.; Kozalak, G.; Namlı, İ.; Sarraf, S.S.; Ahmadi, V.E.; Toyran, E.; van Wijnen, A.J.; Koşar, A. Biomedical Applications of Microfluidic Devices: A Review. Biosensors 2022, 12, 1023. [Google Scholar] [CrossRef]
- Liu, X.; Fang, Y.; Chen, X.; Shi, W.; Wang, X.; He, Z.; Wang, F.; Li, C. Cascaded nanozyme-based high-throughput microfluidic device integrating with glucometer and smartphone for point-of-care pheochromocytoma diagnosis. Biosens. Bioelectron. 2024, 251, 116105. [Google Scholar] [CrossRef]
- Zhu, N.; Liu, C.; Liu, R.; Niu, X.; Xiong, D.; Wang, K.; Yin, D.; Zhang, Z. Biomimic Nanozymes with Tunable Peroxidase-like Activity Based on the Confinement Effect of Metal-Organic Frameworks (MOFs) for Biosensing. Anal. Chem. 2022, 94, 4821–4830. [Google Scholar] [CrossRef]
- Huang, Y.; Ren, J.; Qu, X. Nanozymes: Classification, Catalytic Mechanisms, Activity Regulation, and Applications. Chem. Rev. 2019, 119, 4357–4412. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef]
- Abdullah, K.A.; Tahir, T.F.; Qader, A.F.; Omer, R.A.; Othman, K.A. Nanozymes: Classification and Analytical Applications—A Review. J. Fluoresc. 2024. [Google Scholar] [CrossRef]
- Cui, C.; Wang, Q.; Liu, Q.; Deng, X.; Liu, T.; Li, D.; Zhang, X. Porphyrin-based porous organic framework: An efficient and stable peroxidase-mimicking nanozyme for detection of H2O2 and evaluation of antioxidant. Sens. Actuators B Chem. 2018, 277, 86–94. [Google Scholar] [CrossRef]
- Smutok, O.; Kavetskyy, T.; Prokopiv, T.; Serkiz, R.; Šauša, O.; Novák, I.; Švajdlenková, H.; Maťko, I.; Gonchar, M.; Katz, E. Biosensor Based on Peroxidase-Mimetic Nanozyme and Lactate Oxidase for Accurate L-Lactate Analysis in Beverages. Biosensors 2022, 12, 1042. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Wang, H.; Xu, B.; Liang, L.; Shen, L.; Lin, Q. Regenerative cerium oxide nanozymes alleviate oxidative stress for efficient dry eye disease treatment. Regen. Biomater. 2022, 9, rbac070. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Sikdar, S.; Basak, S.; Mondal, M.; Mallick, K.; Haydar, S.M.; Ghosh, S.; Roy, M.N. Assemble multi-enzyme mimic tandem Mn3O4@ g-C3N4 for augment ROS elimination and label free detection. Chem. Eng. J. 2023, 463, 142355. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rathod, V.K. Magnetic-metal organic framework (magnetic-MOF): A novel platform for enzyme immobilization and nanozyme applications. Int. J. Biol. Macromol. 2018, 120, 2293–2302. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Wang, Q.; Lin, A.; Wei, H. Nanozyme-Enabled Analytical Chemistry. Anal. Chem. 2022, 94, 312–323. [Google Scholar] [CrossRef]
- Qiu, Y.; Tan, G.; Fang, Y.; Liu, S.; Zhou, Y.; Kumar, A.; Trivedi, M.; Liu, D.; Liu, J. Biomedical applications of metal-organic framework (MOF)-based nano-enzymes. New J. Chem. 2021, 45, 20987–21000. [Google Scholar] [CrossRef]
- Zhang, X.; Li, G.; Wu, D.; Li, X.; Hu, N.; Chen, J.; Chen, G.; Wu, Y. Recent progress in the design fabrication of metal-organic frameworks-based nanozymes and their applications to sensing and cancer therapy. Biosens. Bioelectron. 2019, 137, 178–198. [Google Scholar] [CrossRef]
- Li, S.; Hou, Y.; Chen, Q.; Zhang, X.; Cao, H.; Huang, Y. Promoting Active Sites in MOF-Derived Homobimetallic Hollow Nanocages as a High-Performance Multifunctional Nanozyme Catalyst for Biosensing and Organic Pollutant Degradation. ACS Appl. Mater. Interfaces 2020, 12, 2581–2590. [Google Scholar] [CrossRef]
- Ren, G.; Dong, F.; Zhao, Z.; Li, K.; Lin, Y. Structure Defect Tuning of Metal–Organic Frameworks as a Nanozyme Regulatory Strategy for Selective Online Electrochemical Analysis of Uric Acid. ACS Appl. Mater. Interfaces 2021, 13, 52987–52997. [Google Scholar] [CrossRef]
- Baranwal, A.; Polash, S.A.; Aralappanavar, V.K.; Behera, B.K.; Bansal, V.; Shukla, R. Recent Progress and Prospect of Metal-Organic Framework-Based Nanozymes in Biomedical Application. Nanomaterials 2024, 14, 244. [Google Scholar] [CrossRef]
- Khan, S.; Cho, W.C.; Sepahvand, A.; Hosseinali, S.H.; Hussain, A.; Babadaei, M.M.N.; Sharifi, M.; Falahati, M.; Jaragh-Alhadad, L.A.; Hagen, T.L.M.T.; et al. Electrochemical aptasensor based on the engineered core-shell MOF nanostructures for the detection of tumor antigens. J. Nanobiotechnology 2023, 21, 136. [Google Scholar] [CrossRef] [PubMed]
- Ling, P.; Cheng, S.; Chen, N.; Qian, C.; Gao, F. Nanozyme-Modified Metal-Organic Frameworks with Multienzymes Activity as Biomimetic Catalysts and Electrocatalytic Interfaces. ACS Appl. Mater. Interfaces 2020, 12, 17185–17192. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Luo, S.; Fan, Y.; Ma, Y.; Wang, L.; Fu, Z. Preparation of Co dual atomic site catalysts loaded on defect-engineered MOFs material with superb chemiluminescent enhancement effect for sensitive detection of bacteria. Anal. Chim. Acta 2023, 1282, 341909. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Zhang, C.; Xu, Y.; Wang, W.; Hu, H.; Chen, K.; Yang, J.; He, S.; Sun, H.; Ye, Y. Electrochemical detection of S. typhimurium based on peroxidase-like activity of gold nanoparticle-doped CuZr-MOF nanozyme. Microchim. Acta 2025, 192, 296. [Google Scholar] [CrossRef]
- Li, Z.; Dai, G.; Luo, F.; Lu, Y.; Zhang, J.; Chu, Z.; He, P.; Zhang, F.; Wang, Q. An electrochemical sensor for bacterial lipopolysaccharide detection based on dual functional Cu2+-modified metal–organic framework nanoparticles. Microchim. Acta 2020, 187, 415. [Google Scholar] [CrossRef]
- Kabak, B.; Soyuçok, A. Fe-MOF based turn-on fluorescence sensor for the rapid detection of foodborne pathogens in multiple matrices. Food Biosci. 2025, 69, 106910. [Google Scholar] [CrossRef]
- Shen, H.; Tang, Y.; Ma, H. Accelerated peroxidase-like activity of ultrathin amine-tagged bimetallic MOF-74 nanozyme for construction of versatile bioassays from small molecules to enzymes and bacteria. Microchem. J. 2024, 201, 110706. [Google Scholar] [CrossRef]
- Li, T.; Wang, X.; Wang, Y.; Zhang, Y.; Li, S.; Liu, W.; Liu, S.; Liu, Y.; Xing, H.; Otake, K.I.; et al. Microenvironmental modulation breaks intrinsic pH limitations of nanozymes to boost their activities. Nat. Commun. 2024, 15, 10861. [Google Scholar] [CrossRef]
- Zheng, S.; Sun, Y.; Xue, H.; Braunstein, P.; Huang, W.; Pang, H. Dual-ligand and hard-soft-acid-base strategies to optimize metal-organic framework nanocrystals for stable electrochemical cycling performance. Natl. Sci. Rev. 2022, 9, nwab197. [Google Scholar] [CrossRef]
- Jahani, P.M.; Tajik, S.; Dourandish, Z. Electrochemical Sensor Based on Ce-MOF Modified Screen Printed Electrode for Metronidazole Determination. Chem. Methodol. 2024, 8, 123–132. [Google Scholar] [CrossRef]
- Ameen, S.S.M.; Bedair, A.; Hamed, M.; Mansour, F.R.; Omer, K.M. Recent Advances in Metal-Organic Frameworks as Oxidase Mimics: A Comprehensive Review on Rational Design and Modification for Enhanced Sensing Applications. ACS Appl. Mater. Interfaces 2024, 17, 110–129. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, J.; Wu, W.; Fang, Y.; Dong, S. Oxidase-like MOF-818 Nanozyme with High Specificity for Catalysis of Catechol Oxidation. J. Am. Chem. Soc. 2020, 142, 15569–15574. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wu, S.; Peng, Y.; Wang, M.; Jalalah, M.; Al-Assiri, M.; Harraz, F.A.; Yang, J.; Li, G. Sensor array for rapid pathogens identification fabricated with peptide-conjugated 2D metal-organic framework nanosheets. Chem. Eng. J. 2021, 405, 126707. [Google Scholar] [CrossRef]
- Zuo, W.; Liang, L.; Ye, F.; Zhao, S. An integrated platform for label-free fluorescence detection and inactivation of bacteria based on boric acid functionalized Zr-MOF. Sens. Actuators B Chem. 2021, 345, 130345. [Google Scholar] [CrossRef]
- Sethi, S.; Rathod, V. Isolation and chemical immobilization of E. coli-specific bacteriophage with NH2-MIL-101 (Fe) MOF, a high photoluminescence rod-shaped microcrystals for low-level bacteria detection. Appl. Organomet. Chem. 2024, 38, e7624. [Google Scholar] [CrossRef]
- Bhatt, D.; Singh, S.; Singhal, N.; Bhardwaj, N.; Deep, A. Glyco-conjugated metal-organic framework biosensor for fluorescent detection of bacteria. Anal. Bioanal. Chem. 2023, 415, 659–667. [Google Scholar] [CrossRef]
- Tolentino, M.Q.; Hartmann, A.K.; Loe, D.T.; Rouge, J.L. Controlled release of small molecules and proteins from DNA-surfactant stabilized metal organic frameworks. J. Mater. Chem. B 2020, 8, 5627–5635. [Google Scholar] [CrossRef]
- Shahrokhian, S.; Ranjbar, S. Aptamer immobilization on amino-functionalized metal-organic frameworks: An ultrasensitive platform for the electrochemical diagnostic of Escherichia coli O157:H7. Analyst 2018, 143, 3191–3201. [Google Scholar] [CrossRef]
- Li, C.; Hang, T.; Jin, Y. Atomically Fe-anchored MOF-on-MOF nanozyme with differential signal amplification for ultrasensitive cathodic electrochemiluminescence immunoassay. Exploration 2023, 3, 20220151. [Google Scholar] [CrossRef]
- Rohra, N.; Gaikwad, G.; Dandekar, P.; Jain, R. Microfluidic Synthesis of a Bioactive Metal-Organic Framework for Glucose-Responsive Insulin Delivery. ACS Appl. Mater. Interfaces 2022, 14, 8251–8265. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y. Metal-Organic Frameworks as Sensors of Biomolecules. In ACS Symposium Series; ACS Publications: Washington, DC, USA, 2021; Volume 1394, pp. 1–31. [Google Scholar] [CrossRef]
- Usman, K.A.S.; Maina, J.W.; Seyedin, S.; Conato, M.T.; Payawan, L.M.; Dumée, L.F.; Razal, J.M. Downsizing metal-organic frameworks by bottom-up and top-down methods. NPG Asia Mater. 2020, 12, 58. [Google Scholar] [CrossRef]
- Singh, R.; Musameh, M.; Gao, Y.; Ozcelik, B.; Mulet, X.; Doherty, C.M. Stable MOF@enzyme composites for electrochemical biosensing devices. J. Mater. Chem. C 2021, 9, 7677–7688. [Google Scholar] [CrossRef]
- Nataraj, N.; Chen, T.W.; Gan, Z.W.; Chen, S.; Hatshan, M.; Ali, M. Bifunctional 3D-MOF-based nanoprobes for electrochemical sensing and nanozyme enhanced with peroxidase mimicking for colorimetric detection of acetaminophen. Mater. Today Chem. 2022, 23, 100725. [Google Scholar] [CrossRef]
- Wang, K.; Li, N.; Zhang, J.; Zhang, Z.; Dang, F. Size-selective QD@MOF core-shell nanocomposites for the highly sensitive monitoring of oxidase activities. Biosens. Bioelectron. 2017, 87, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, Z.; Hu, H.; Zhou, X.; You, F.; Yao, C.; Liu, F.J.; Yu, P.; Wu, D.; Yao, J.; et al. Advanced Metal–Organic Frameworks-Based Catalysts in Electrochemical Sensors. Front. Chem. 2022, 10, 881172. [Google Scholar] [CrossRef]
- Jayaramulu, K.; Mukherjee, S.; Morales, D.M.; Dubal, D.P.; Nanjundan, A.K.; Schneemann, A.; Masa, J.; Kment, S.; Schuhmann, W.; Otyepka, M.; et al. Graphene-Based Metal-Organic Framework Hybrids for Applications in Catalysis, Environmental, and Energy Technologies. Chem. Rev. 2022, 122, 17241–17338. [Google Scholar] [CrossRef]
- Tian, J.Y.; Liu, X.; Zhang, S.; Chen, K.; Zhu, L.; Song, Y.; Wang, M.; Zhang, Z.; Du, M. Novel aptasensing strategy for efficiently quantitative analyzing Staphylococcus aureus based on defective copper-based metal-organic framework. Food Chem. 2023, 402, 134357. [Google Scholar] [CrossRef]
- Yang, S.; Guo, Y.; Fan, J.; Yang, Y.; Zuo, C.; Bai, S.; Sheng, S.; Li, J.; Xie, G. A fluorometric assay for rapid enrichment and determination of bacteria by using zirconium-metal organic frameworks as both capture surface and signal amplification tag. Microchim. Acta 2020, 187, 188. [Google Scholar] [CrossRef]
- Li, R.; Yan, J.; Feng, B.; Sun, M.; Ding, C.; Shen, H.; Zhu, J.; Yu, S. Ultrasensitive Detection of Multidrug-Resistant Bacteria Based on Boric Acid-Functionalized Fluorescent MOF@COF. ACS Appl. Mater. Interfaces 2023, 15, 18663–18671. [Google Scholar] [CrossRef]
- Chai, H.; Yu, K.; Zhao, Y.; Zhang, Z.; Wang, S.; Huang, C.; Zhang, X.; Zhang, G. MOF-On-MOF Dual Enzyme-Mimic Nanozyme with Enhanced Cascade Catalysis for Colorimetric/Chemiluminescent Dual-Mode Aptasensing. Anal. Chem. 2023, 95, 10785–10794. [Google Scholar] [CrossRef]
- Ma, S.; Xiao, S.; Hong, Y.; Bao, Y.; Xu, Z.; Chen, D.; Huang, X. Coupling metal organic frameworks nanozyme with carbon nanotubes on the gradient porous hollow fiber membrane for nonenzymatic electrochemical H2O2 detection. Anal. Chim. Acta 2024, 1293, 342285. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, W.; Mao, Y.; Ling, G.; Zhang, P. Enhanced enzyme-like DNAzyme@MOFs for non-enzymatic uric acid detection in interstitial fluid. Chem. Eng. J. 2024, 500, 156837. [Google Scholar] [CrossRef]
- Guan, H.; Zhang, Y.; Liu, S. A novel enhanced electrochemical sensor based on the peroxidase-like activity of Fe3O4@Au/MOF for the detection of p-aminophenol. J. Appl. Electrochem. 2022, 52, 989–1002. [Google Scholar] [CrossRef]
- Tajwar, M.A.; Qi, L. Dual Stimulus-Responsive Enzyme@Metal–Organic Framework-Polymer Composites toward Enhanced Catalytic Performance for Visual Detection of Glucose. ACS Appl. Bio. Mater. 2024, 7, 325–331. [Google Scholar] [CrossRef]
- Chandio, I.; Ai, Y.; Wu, L.; Liang, Q. Recent progress in MOFs-based nanozymes for biosensing. Nano Res. 2023, 17, 39–64. [Google Scholar] [CrossRef]
- Wang, Z.; Li, M.; Bu, H.; Zia, D.S.; Dai, P.; Liu, J. Nanomaterials for molecular recognition: Specific adsorption and regulation of nanozyme activities. Mater. Chem. Front. 2023, 7, 3625–3640. [Google Scholar] [CrossRef]
- Ma, T.; Huang, K.; Cheng, N. Recent Advances in Nanozyme-Mediated Strategies for Pathogen Detection and Control. Int. J. Mol. Sci. 2023, 24, 13342. [Google Scholar] [CrossRef]
- Hou, H.; Wang, L.; Gao, Y.; Ping, J.; Zhao, F. Recent advances in metal-organic framework-based nanozymes and their enabled optical biosensors for food safety analysis. TrAC Trends Anal. Chem. 2024, 173, 117602. [Google Scholar] [CrossRef]
- Teymouri, Z.; Shekarchizadeh, H.; Karami, K. Innovative real-time monitoring of fish freshness via agar-based film embedded with Cu-MOF colorimetric indicators. Chem. Eng. J. 2025, 510, 161751. [Google Scholar] [CrossRef]
- Wang, S.; Deng, W.; Yang, L.; Tan, Y.; Xie, Q.; Yao, S. Copper-Based Metal-Organic Framework Nanoparticles with Peroxidase-Like Activity for Sensitive Colorimetric Detection of Staphylococcus aureus. ACS Appl. Mater. Interfaces 2017, 9, 24440–24445. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, W.; Gao, Y.; Li, X.; Yuan, L.; Zhu, G.; Gu, X.; Yang, Z. Nanozyme colourimetry based on temperate bacteriophage for rapid and sensitive detection of Staphylococcus aureus in food matrices. Int. J. Food Microbiol. 2024, 416, 110657. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xiao, S.; Zeng, M.; Xie, H.; Gan, N. Dual-mode colorimetric-electrochemical biosensor for Vibrio parahaemolyticus detection based on CuO2 nanodot-encapsulated metal-organic framework nanozymes. Sens. Actuators B Chem. 2023, 387, 133835. [Google Scholar] [CrossRef]
- Li, R.; Qian, G.; Shen, H.; Yu, S. Self-cascade MOF@MOF nanozyme for ultrasensitive and low-background detection of multidrug-resistant bacteria. Microchem. J. 2025, 209, 112695. [Google Scholar] [CrossRef]
- Hao, M.; Huang, W.; Feng, B.; Teng, M.; Ding, C.; Shen, H.; Yu, S.; Wang, L. Layered Double Hydroxide-Derived Two-Dimensional Bimetallic Metal–Organic Framework Nanozymes for Microorganism Identification. ACS Appl. Nano Mater. 2023, 6, 4610–4618. [Google Scholar] [CrossRef]
- Ren, M.; Zhang, Y.; Yu, L.; Qu, L.; Li, Z.; Zhang, L. A Co-based MOF as nanozyme with enhanced oxidase-like activity for highly sensitive and selective colorimetric differentiation of aminophenol isomers. Talanta 2023, 255, 124219. [Google Scholar] [CrossRef]
- Abedeen, Z.; Yadav, L.; Gupta, R. Sensitive electrochemical determination of hydrogen peroxide in milk and water utilizing AgNPs@Sn MOF nanozyme. Microchim. Acta 2025, 192, 152. [Google Scholar] [CrossRef]
- Mansouri, S. Detection of pathogenic bacteria with nanozymes-based colorimetric biosensors: Advances, challenges and future prospects. Microchem. J. 2024, 205, 111392. [Google Scholar] [CrossRef]
- Mo, F.; Zhong, S.; You, T.; Lu, J.; Sun, D. Aptamer and DNAzyme-Functionalized Cu-MOF Hybrid Nanozymes for the Monitoring and Management of Bacteria-Infected Wounds. ACS Appl. Mater. Interfaces 2023, 15, 52114–52127. [Google Scholar] [CrossRef]
- Gao, H.; Yu, H.; Yang, S.; Chai, F.; Wu, H.; Tian, M. Ultrasensitive detection of H2O2 via electrochemical sensor by graphene synergized with MOF-on-MOF nanozymes. Microchim. Acta 2024, 191, 482. [Google Scholar] [CrossRef]
- Chen, S.; Xie, Y.; Guo, X.; Sun, D. Self-supporting electrochemical sensors for monitoring of cell-released H2O2 based on metal nanoparticle/MOF nanozymes. Microchem. J. 2022, 181, 107715. [Google Scholar] [CrossRef]
- Yasin, G.; Ibrahim, S.; Ajmal, S.; Ibraheem, S.; Ali, S.; Nadda, A.K.; Zhang, G.; Kaur, J.; Maiyalagan, T.; Gupta, R.K.; et al. Tailoring of electrocatalyst interactions at interfacial level to benchmark the oxygen reduction reaction. Coord. Chem. Rev. 2022, 469, 214669. [Google Scholar] [CrossRef]
- Wei, J.J.; Li, H.B.; Wang, G.Q.; Zheng, J.Y.; Wang, A.J.; Mei, L.P.; Zhao, T.; Feng, J.J. Novel Ultrasensitive Photoelectrochemical Cytosensor Based on Hollow CdIn2S4/In2S3 Heterostructured Microspheres for HepG2 Cells Detection and Inhibitor Screening. Anal. Chem. 2022, 94, 12240–12247. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, H.; Liu, P.; Wang, M.; Pan, J.; Qiu, F.; Ni, L.; Niu, X. Realizing selective detection with nanozymes: Strategies and trends. TrAC-Trends Anal. Chem. 2021, 143, 116379. [Google Scholar] [CrossRef]
- Sradha, S.A.; George, L.; Keerthana, P.; Varghese, A. Recent advances in electrochemical and optical sensing of the organophosphate chlorpyrifos: A review. Crit. Rev. Toxicol. 2022, 52, 431–448. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, F.; Ren, J.; Qu, X. A series of MOF/Ce-based nanozymes with dual enzyme-like activity disrupting biofilms and hindering recolonization of bacteria. Biomaterials 2019, 208, 21–31. [Google Scholar] [CrossRef]
- Ye, T.; Bai, L.; Yu, J.; Shi, W.; Yuan, M.; Wang, J.; Cao, H.; Hao, L.; Wu, X.; Yin, F.; et al. Fluorescent nanozyme for the dual-mode detection of tetracycline: Aggregation-induced luminescence and boosting peroxidase-like activity. Food Control 2025, 171, 111109. [Google Scholar] [CrossRef]
- Du, J.; Shi, F.; Wang, K.; Han, Q.; Shi, Y.; Zhang, W.; Gao, Y.; Dong, B.; Wang, L.; Xu, L. Metal-organic framework-based biosensing platforms for diagnosis of bacteria-induced infectious diseases. TrAC-Trends Anal. Chem. 2024, 175, 117707. [Google Scholar] [CrossRef]
- Quijia, C.R.; Alves, R.C.; Hanck-Silva, G.; Frem, R.C.G.; Arroyos, G.; Chorilli, M. Metal-organic frameworks for diagnosis and therapy of infectious diseases. Crit. Rev. Microbiol. 2022, 48, 161–196. [Google Scholar] [CrossRef]
- Cesewski, E.; Johnson, B.N. Electrochemical biosensors for pathogen detection. Biosens. Bioelectron. 2020, 159, 112214. [Google Scholar] [CrossRef]
- Hu, W.C.; Zhao, X.P.; Wang, J.; Wang, C.; Xia, X.H. Recent progress in metal-organic frameworks-based biosensors for pathogen detection. TrAC-Trends Anal. Chem. 2024, 178, 117857. [Google Scholar] [CrossRef]
- Gu, W.; Miller, S.; Chiu, C.Y. Clinical Metagenomic Next-Generation Sequencing for Pathogen Detection. Annu. Rev. Pathol. Mech. Dis. 2019, 14, 319–338. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, R.; Sheng, S.; Yang, H.; Tang, X.; Wang, J.; Wang, F.; Zhang, Q.; Bai, L.; Chen, X.; et al. MOF nanozyme mediated bacterial metabolic regulation to intervene MRSA antibiotic tolerance for enhanced antimicrobial efficacy. Nano Today 2025, 63, 102753. [Google Scholar] [CrossRef]
- Li, X.; Li, G.; Pan, Q.; Xue, F.; Wang, Z.; Peng, C. Rapid and ultra-sensitive lateral flow assay for pathogens based on multivalent aptamer and magnetic nanozyme. Biosens. Bioelectron. 2024, 250, 116044. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yu, H.; Zhang, B.; Liu, S.; Liu, C.G.; Li, F.; Song, H. Engineering whole-cell microbial biosensors: Design principles and applications in monitoring and treatment of heavy metals and organic pollutants. Biotechnol. Adv. 2022, 60, 108019. [Google Scholar] [CrossRef]
- Gao, L.; Wen, J.; Huang, Z.; Sheng, S.; Xu, F.; Ma, G.; Tan, H. pH Meter-Assisted Biosensor Based on Glucose Oxidase-Conjugated Magnetic Metal–Organic Framework for On-Site Evaluation of Bacterial Contamination. ACS Appl. Mater. Interfaces 2023, 15, 31224–31232. [Google Scholar] [CrossRef]
- Ren, Z.; Wang, X.; Jia, B.; Liu, X.; Qu, Y.; Li, W.; Zhao, M.; Li, Y.Q. Nanozyme-Energized SERS Sensor for Ultrasensitive Dual-Mode Bacterial Detection. ACS Appl. Nano Mater. 2025, 8, 8385–8396. [Google Scholar] [CrossRef]
- Fu, H.; Li, H.; He, Q.; Du, G.; Li, J.; Zhang, Y.; Liang, Y.; Lin, B. Flexible fiber composite functional materials based on Fe-MOFs nanozyme catalytic activity for efficient point-of-use water disinfection. Chem. Eng. J. 2024, 490, 151879. [Google Scholar] [CrossRef]
- Feng, Q.; Wang, C.; Miao, X.; Wu, M. A novel paper-based electrochemiluminescence biosensor for non-destructive detection of pathogenic bacteria in real samples. Talanta 2024, 267, 125224. [Google Scholar] [CrossRef]
- Liu, Y.F.; Ni, P.W.; Huang, Y.; Xie, T. Therapeutic strategies for chronic wound infection. Chin. J. Traumatol. 2022, 25, 11–16. [Google Scholar] [CrossRef]
- Chao, D.; Dong, Q.; Yu, Z.; Qi, D.; Li, M.; Xu, L.; Liu, L.; Fang, Y.; Dong, S. Specific Nanodrug for Diabetic Chronic Wounds Based on Antioxidase-Mimicking MOF-818 Nanozymes. J. Am. Chem. Soc. 2022, 144, 23438–23447. [Google Scholar] [CrossRef]
- Le, G.; Li, Y.; Cai, L.; Zhang, L.; Pei, W.; Zhu, X.; Xu, S.; Zhang, J.; Chen, J. Lysozyme-based nanozyme encapsulated in double-network hydrogel for monitoring and repair of MRSA infected wounds. Chem. Eng. J. 2023, 477, 146421. [Google Scholar] [CrossRef]
- Magar, H.S.; Hemdan, B.A.; Rashdan, H.R.M.; Hassan, R.Y.A. Rapid and Selective Detection of Foodborne Pathogens Using a Disposable Bio-sensing System Designed by Stepwise Antibody Immobilization on AuNPs@Cu-MOF Nanocomposite. J. Anal. Test. 2024, 8, 478–492. [Google Scholar] [CrossRef]
- Fan, M.; Kiefer, P.; Charki, P.; Hedberg, C.; Seibel, J.; Vorholt, J.A.; Hilbi, H. The Legionella autoinducer LAI-1 is delivered by outer membrane vesicles to promote interbacterial and interkingdom signaling. J. Biol. Chem. 2023, 299, 105376. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Lu, S. Effects of microbial organic fertilizer (MOF) application on cadmium uptake of rice in acidic paddy soil: Regulation of the iron oxides driven by the soil microorganisms. Environ. Pollut. 2022, 307, 119447. [Google Scholar] [CrossRef]
- Guselnikova, O.; Postnikov, P.; Elashnikov, R.; Miliutina, E.; Svorcik, V.; Lyutakov, O. Metal-organic framework (MOF-5) coated SERS active gold gratings: A platform for the selective detection of organic contaminants in soil. Anal. Chim. Acta 2019, 1068, 70–79. [Google Scholar] [CrossRef]
- Souza, J.E.d.S.; de Oliveira, G.P.; Alexandre, J.Y.N.H.; Neto, J.G.L.; Sales, M.B.; Junior, P.G.d.S.; de Oliveira, A.L.B.; de Souza, M.C.M.; dos Santos, J.C.S. A Comprehensive Review on the Use of Metal–Organic Frameworks (MOFs) Coupled with Enzymes as Biosensors. Electrochem 2022, 3, 89–113. [Google Scholar] [CrossRef]
- Sun, L.; Chen, Y.; Duan, Y.; Ma, F. Electrogenerated Chemiluminescence Biosensor Based on Functionalized Two-Dimensional Metal–Organic Frameworks for Bacterial Detection and Antimicrobial Susceptibility Assays. ACS Appl. Mater. Interfaces 2021, 13, 38923–38930. [Google Scholar] [CrossRef]
- Falcone, M.; De Angelis, B.; Pea, F.; Scalise, A.; Stefani, S.; Tasinato, R.; Zanetti, O.; Paola, L.D. Challenges in the management of chronic wound infections. J. Glob. Antimicrob. Resist. 2021, 26, 140–147. [Google Scholar] [CrossRef]
- Zhang, L.; Ouyang, M.; Zhang, Y.; Zhang, L.; Huang, Z.; He, L.; Lei, Y.; Zou, Z.; Feng, F.; Yang, R. The fluorescence imaging and precise suppression of bacterial infections in chronic wounds by porphyrin-based metal-organic framework nanorods. J. Mater. Chem. B 2021, 9, 8048–8055. [Google Scholar] [CrossRef]
- Zhao, X.; Chang, L.; Hu, Y.; Xu, S.; Liang, Z.; Ren, X.; Mei, X.; Chen, Z. Preparation of Photocatalytic and Antibacterial MOF Nanozyme Used for Infected Diabetic Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 18194–18208. [Google Scholar] [CrossRef]
- Ren, X.; Chang, L.; Hu, Y.; Zhao, X.; Xu, S.; Liang, Z.; Mei, X.; Chen, Z. Au@MOFs used as peroxidase-like catalytic nanozyme for bacterial infected wound healing through bacterial membranes disruption and protein leakage promotion. Mater. Des. 2023, 229, 111890. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, X.; Wang, Z.; Zhao, Y.; Li, R.; Chen, X.; Chen, H.; Wan, M.; Wang, X. Metal-organic framework-modulated Fe3O4 composite au nanoparticles for antibacterial wound healing via synergistic peroxidase-like nanozymatic catalysis. J. Nanobiotechnology 2023, 21, 427. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Yan, Y.; Xie, M.; Liu, Y.; Deng, L.; Wang, H. A critical review on metal organic frameworks (MOFs)-based sensors for foodborne pathogenic bacteria detection. Talanta 2025, 281, 126918. [Google Scholar] [CrossRef] [PubMed]
- Oon, Y.L.; Ayaz, M.; Deng, M.; Li, L.; Song, K. Waterborne pathogens detection technologies: Advances, challenges, and future perspectives. Front. Microbiol. 2023, 14, 1286923. [Google Scholar] [CrossRef]
- Zhou, Q.; Natarajan, B.; Kannan, P. Nanostructured biosensing platforms for the detection of food- and water-borne pathogenic Escherichia coli. Anal. Bioanal. Chem. 2023, 415, 3111–3129. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Wang, J.; Hao, H.G.; Yang, H.; Yang, Q.S.; Zhao, W.Y. A water-stable europium-MOF sensor for the selective, sensitive ratiometric fluorescence detection of anthrax biomarker. Microchem. J. 2021, 166, 106253. [Google Scholar] [CrossRef]
- Su, H.; Zhang, Y.; Lu, Z.; Wang, Q. A mechanism of microbial sensitivity regulation on interventional remediation by nanozyme manganese oxide in soil heavy metal pollution. J. Clean. Prod. 2022, 373, 133825. [Google Scholar] [CrossRef]
- Kumar, M.; Kaur, N.; Singh, N. Colorimetric Nanozyme Sensor Array Based on Metal Nanoparticle-Decorated CNTs for Quantification of Pesticides in Real Water and Soil Samples. ACS Sustain. Chem. Eng. 2024, 12, 728–736. [Google Scholar] [CrossRef]
- Ju, P.; Zhang, G.; Lu, W.; Wang, S.; Li, A.; Zhang, Q.; Li, B.; Fei, S.; Jiang, L.; Zhang, E. A new hydroxyl group functionalized Zn-MOF as an efficient fluorescent probe for sulfasalazine residue detection in water, milk and soil. J. Mol. Struct. 2024, 1311, 138437. [Google Scholar] [CrossRef]
- Li, Q.Q.; Wen, M.J.; Zhang, Y.S.; Guo, Z.S.; Bai, X.; Song, J.X.; Liu, P.; Wang, Y.Y.; Li, J.L. Multiple fluorescence response behaviors towards antibiotics and bacteria based on a highly stable Cd-MOF. J. Hazard. Mater. 2022, 423, 127132. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, Y.; Bao, L.; Chen, J.; Duan, S.; Chen, S.C.; Xu, P.; Wang, W.N. Self-decontaminating nanofibrous filters for efficient particulate matter removal and airborne bacteria inactivation. Environ. Sci. Nano 2021, 8, 1081–1095. [Google Scholar] [CrossRef]
- Pan, M.M.; Feng, D.; Ouyang, Y.; Yang, D.; Yu, X.; Xu, L.; Willner, I. MOF-templated synthesis of cobalt-doped zinc oxide superparticles for detection of the 3-hydroxy-2-butanone microbial biomarker. Sens. Actuators B Chem. 2022, 358, 131482. [Google Scholar] [CrossRef]
- Ali, A.; Ovais, M.; Zhou, H.; Rui, Y.; Chen, C. Tailoring metal-organic frameworks-based nanozymes for bacterial theranostics. Biomaterials 2021, 275, 120951. [Google Scholar] [CrossRef]
- Qin, J.; Guo, N.; Yang, J.; Wei, J. Recent advances in metal oxide nanozyme-based optical biosensors for food safety assays. Food Chem. 2024, 447, 139019. [Google Scholar] [CrossRef]
- Zhao, Y.; Zeng, H.; Zhu, X.W.; Lu, W.; Li, D. Metal-organic frameworks as photoluminescent biosensing platforms: Mechanisms and applications. Chem. Soc. Rev. 2021, 50, 4484–4513. [Google Scholar] [CrossRef]
- Leoi, M.W.N.; Zheng, X.T.; Yu, Y.; Gao, J.; Ong, D.H.S.; Koh, C.Z.H.; Chen, P.; Yang, L. Redefining Metal Organic Frameworks in Biosensors: Where Are We Now? ACS Appl. Mater. Interfaces 2025, 17, 13246–13278. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, C.; Qian, W.; Lei, F.; Chen, Z.; Wu, X.; Lin, Y.; Wang, F. Recent advances in MOF-based nanozymes: Synthesis, activities, and bioapplications. Biosens. Bioelectron. 2024, 263, 116593. [Google Scholar] [CrossRef]
- Xu, Z.; Long, L.L.; Chen, Y.Q.; Chen, M.L.; Cheng, Y.H. A nanozyme-linked immunosorbent assay based on metal–organic frameworks (MOFs) for sensitive detection of aflatoxin B1. Food Chem. 2021, 338, 128039. [Google Scholar] [CrossRef]
- Ahmadi, S.; Rahimizadeh, K.; Shafiee, A.; Rabiee, N.; Iravani, S. Nanozymes and their emerging applications in biomedicine. Process Biochem. 2023, 131, 154–174. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, M.; Fan, Z. Current trends in colorimetric biosensors using nanozymes for detecting biotoxins (bacterial food toxins, mycotoxins, and marine toxins). Anal. Methods 2024, 16, 6771–6792. [Google Scholar] [CrossRef]
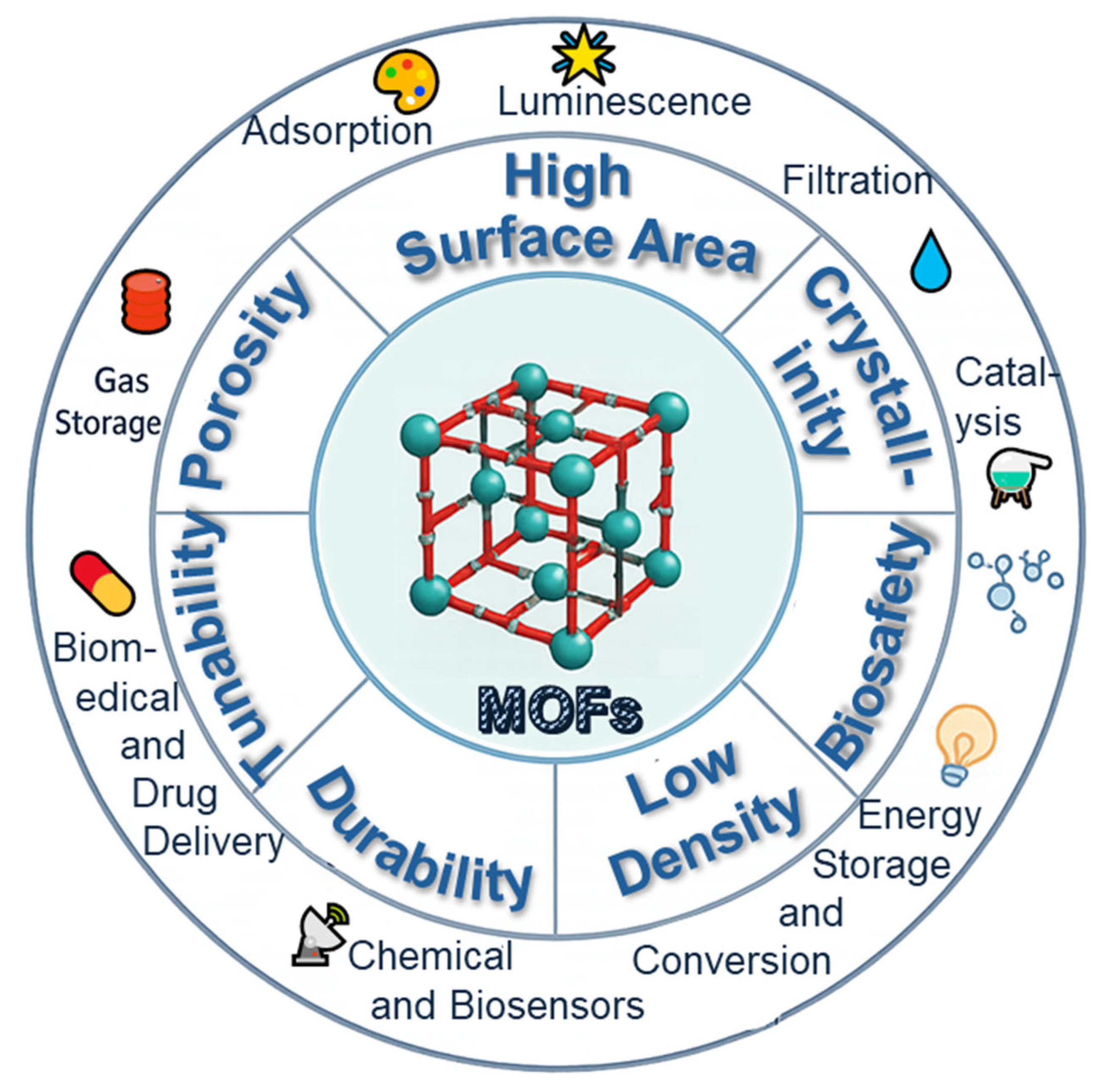
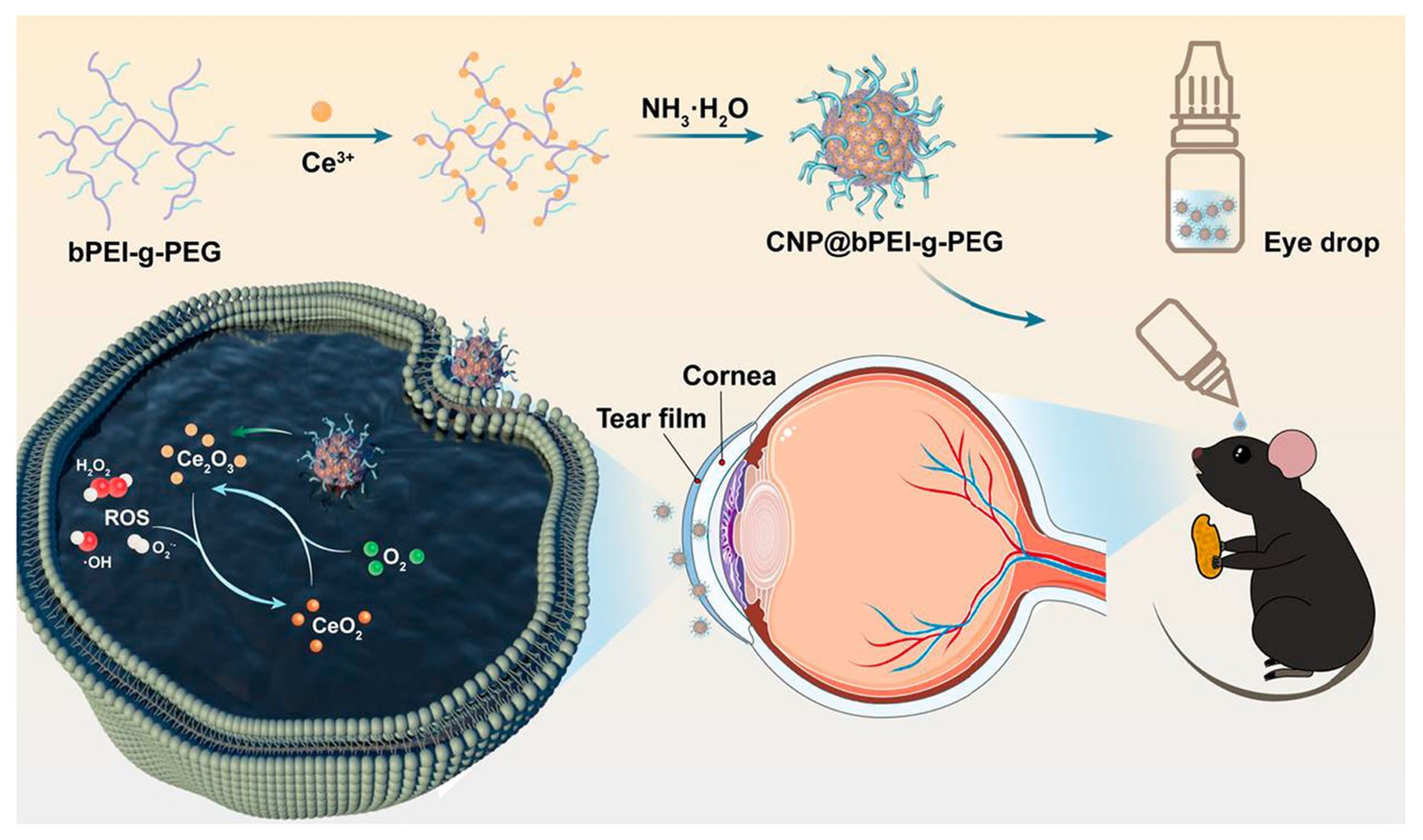
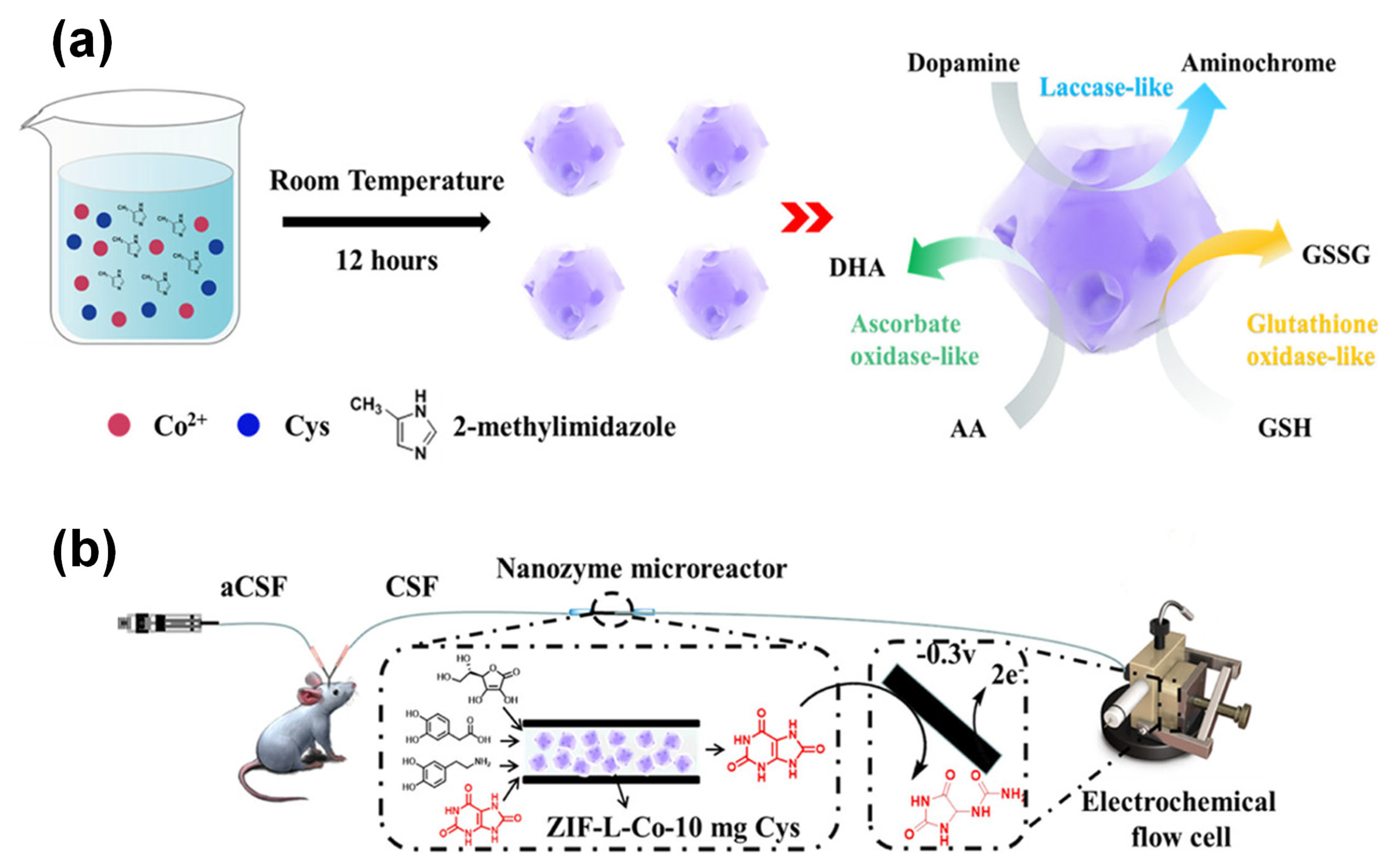
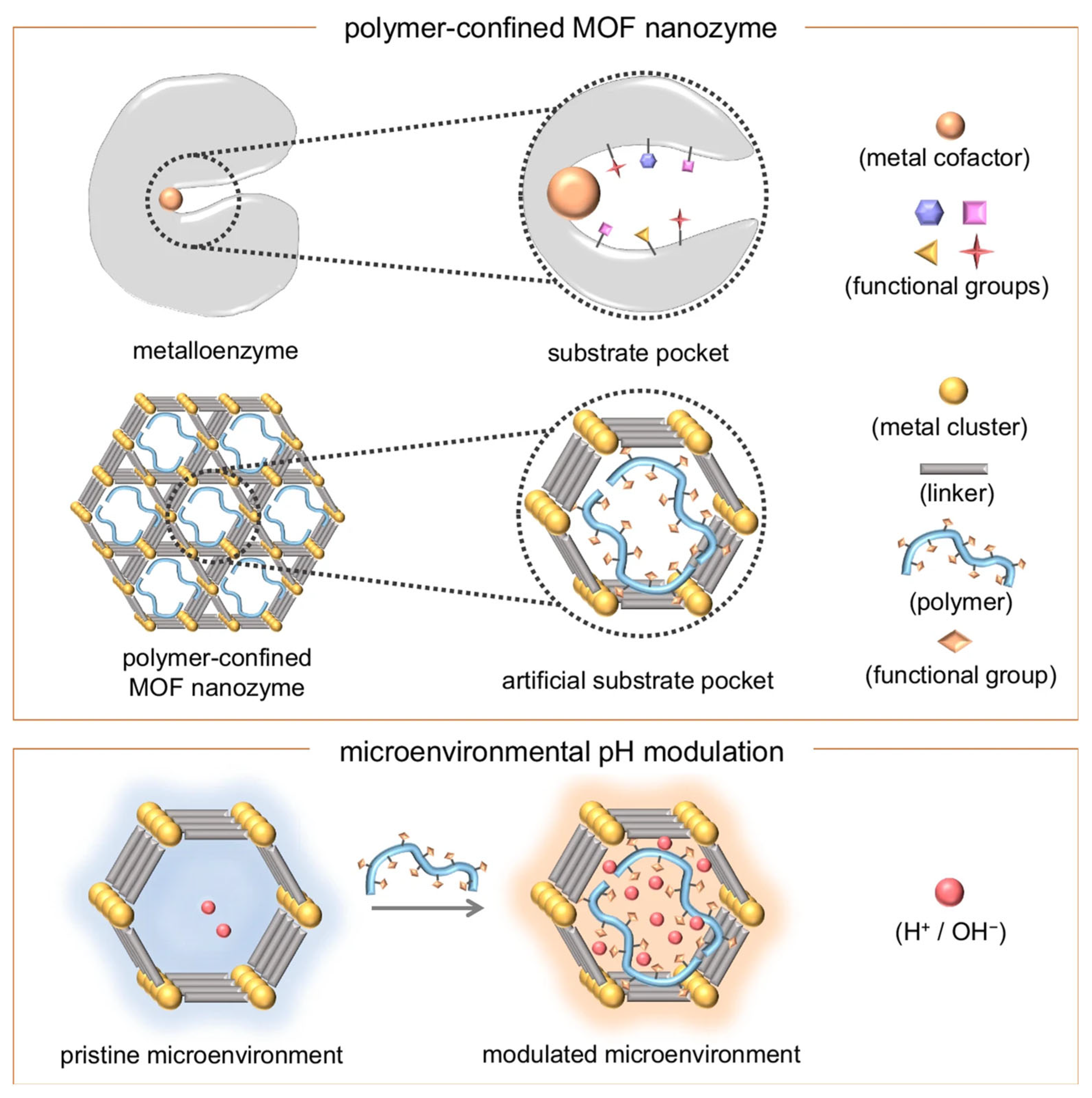
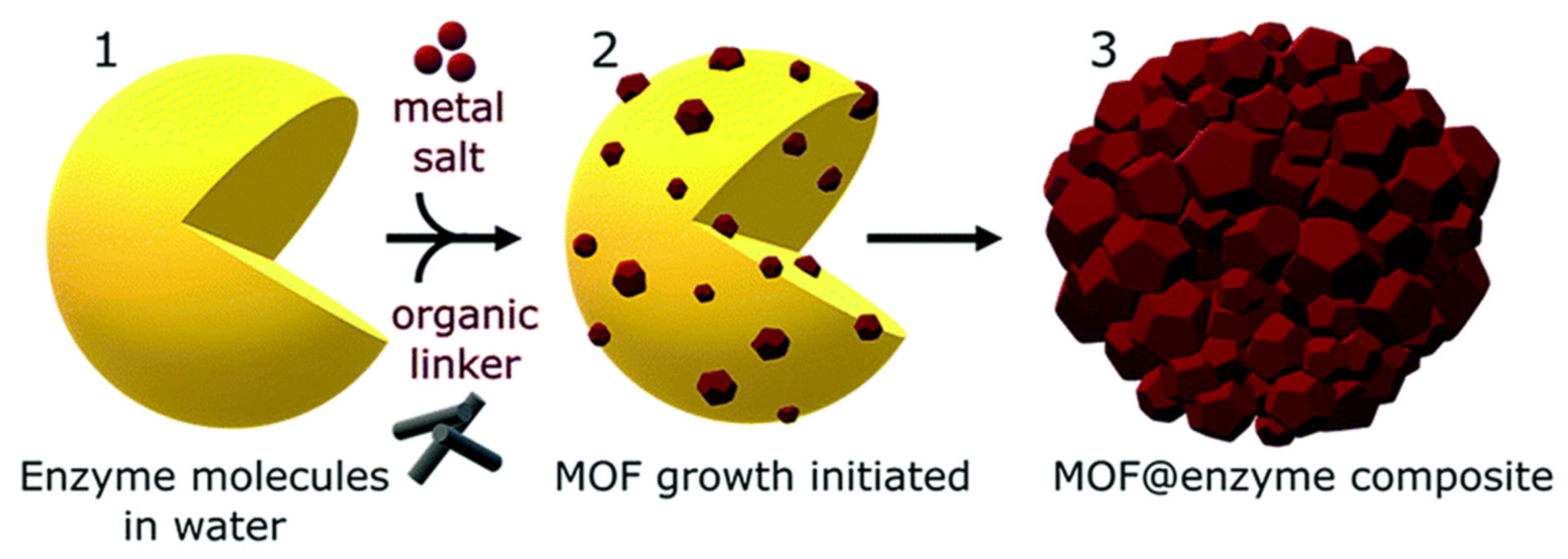
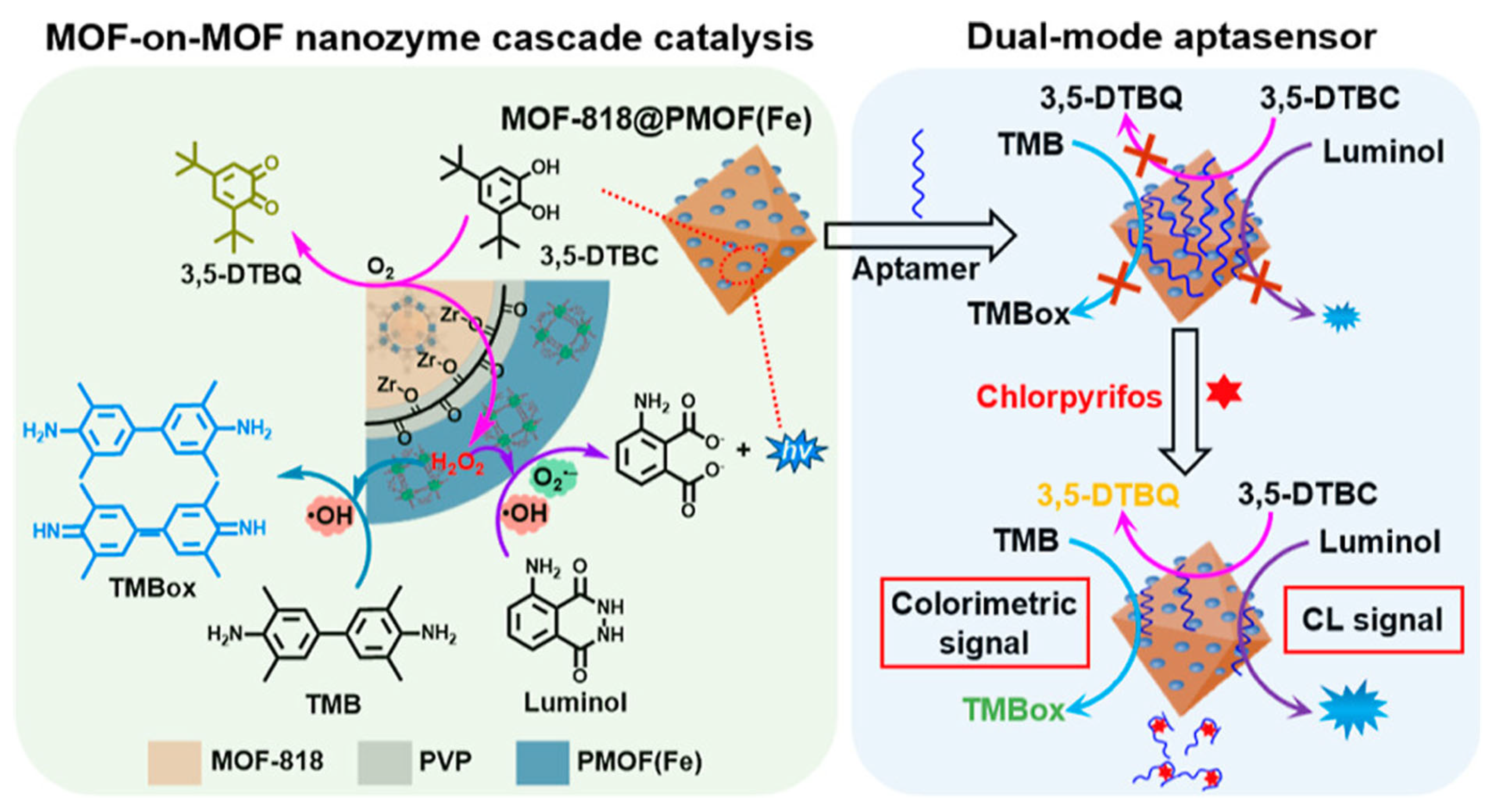

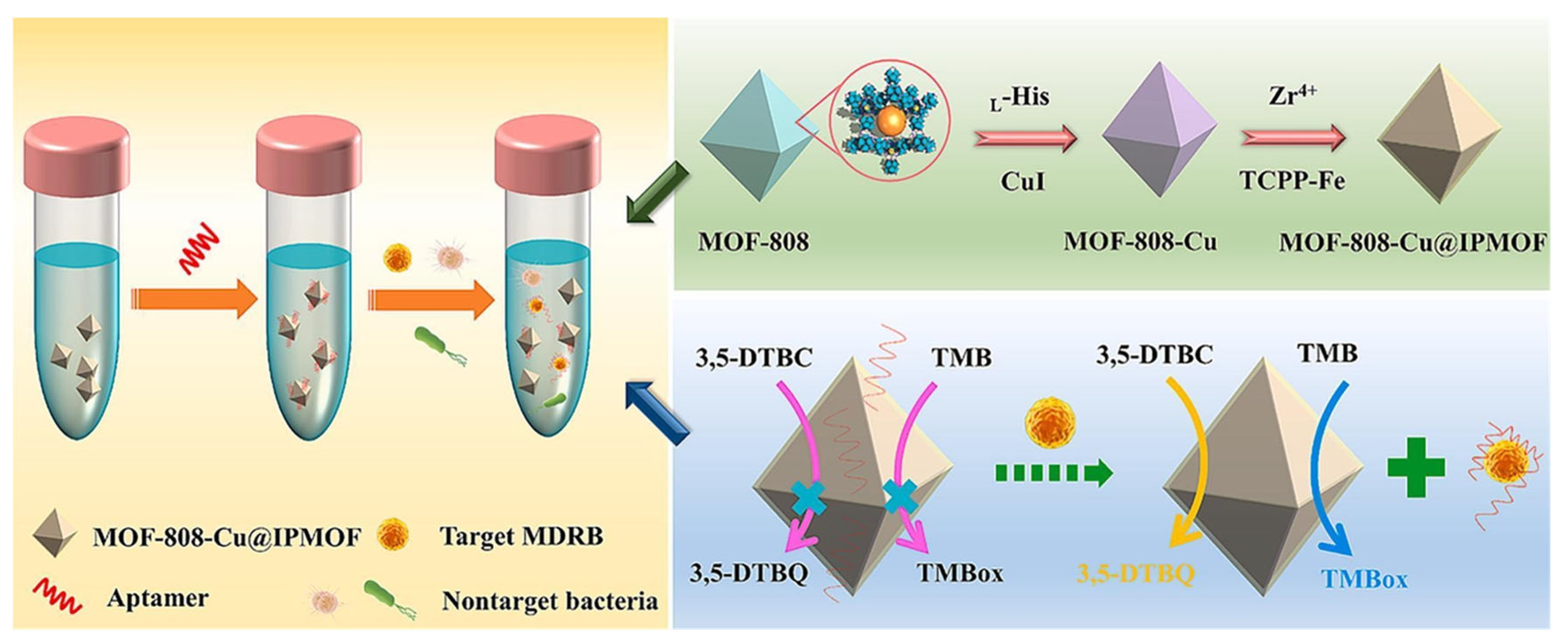
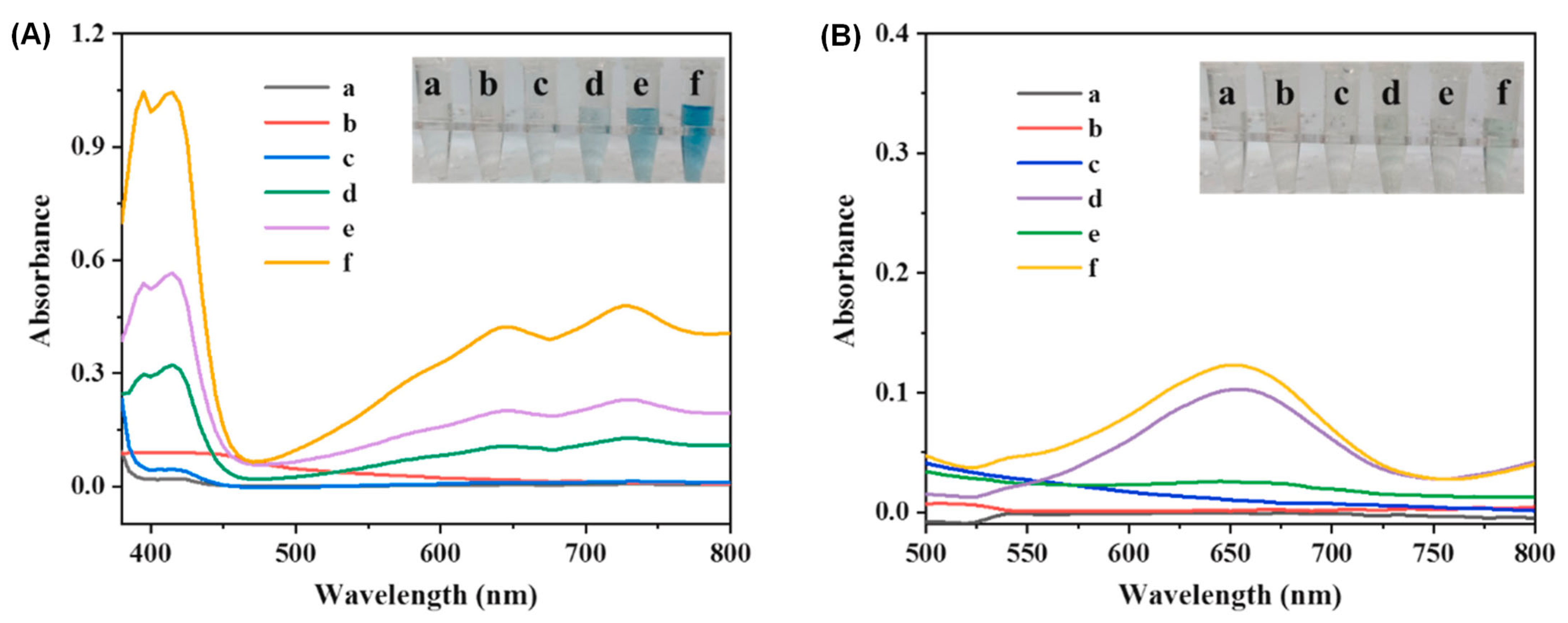

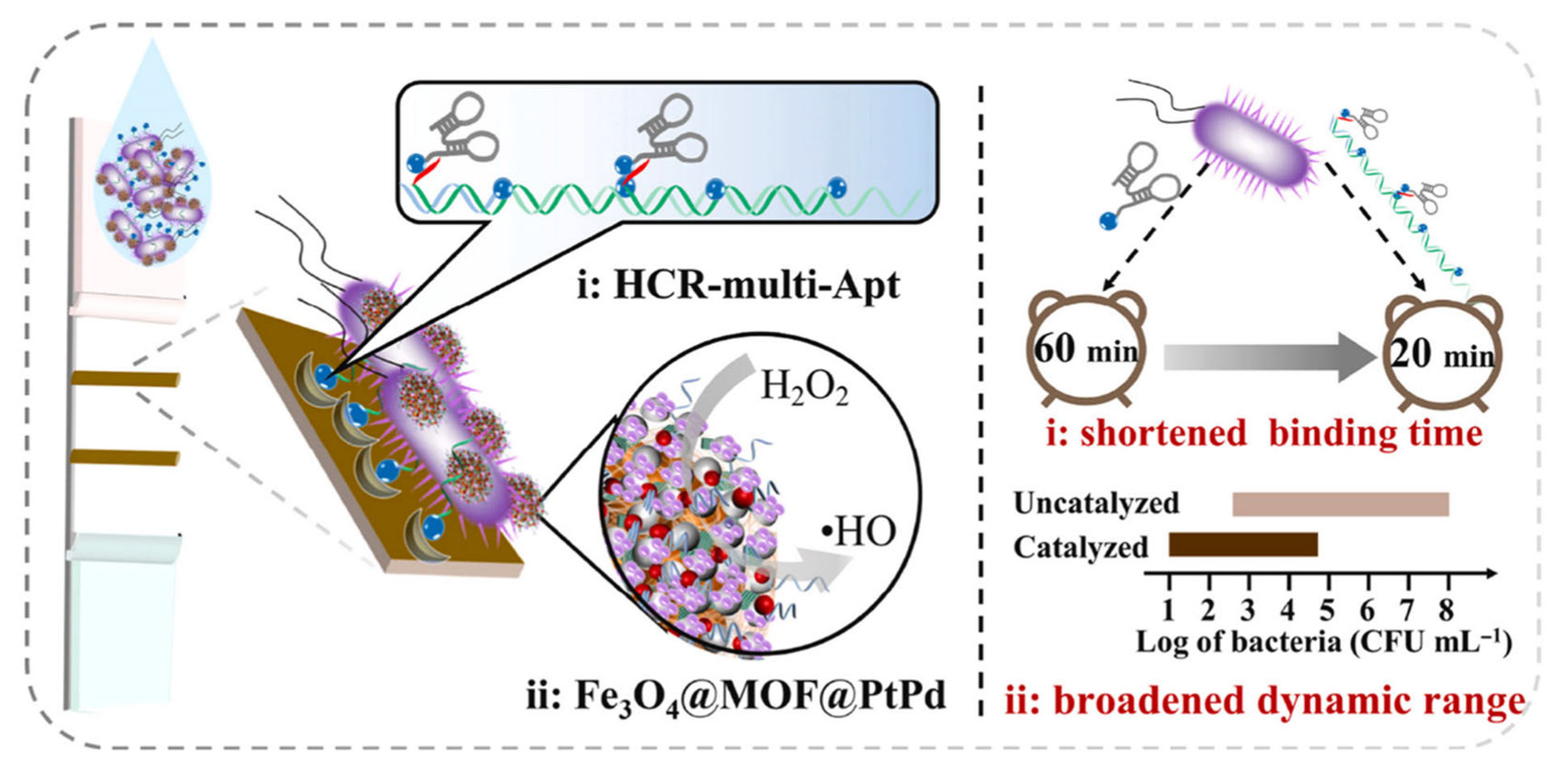
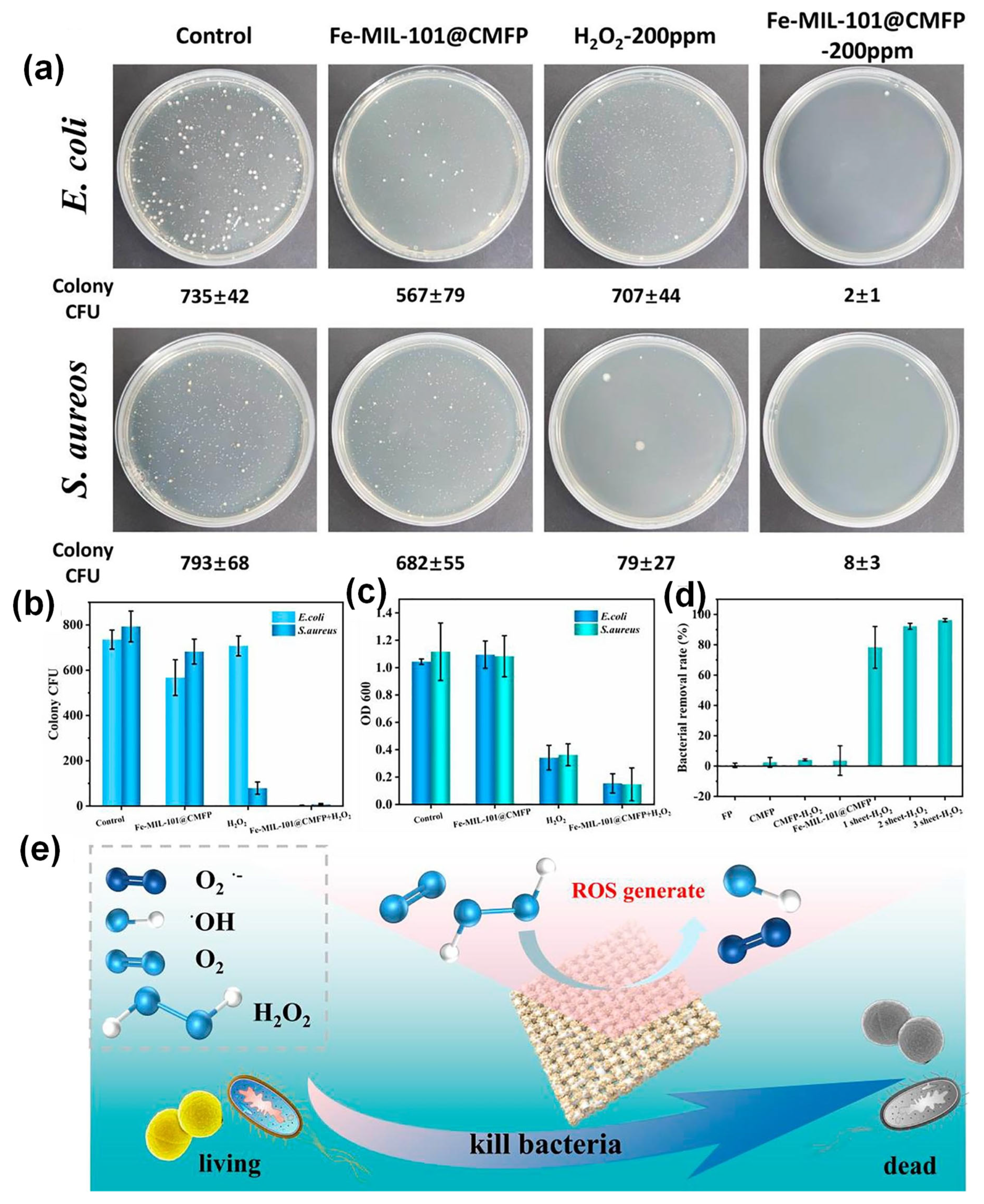
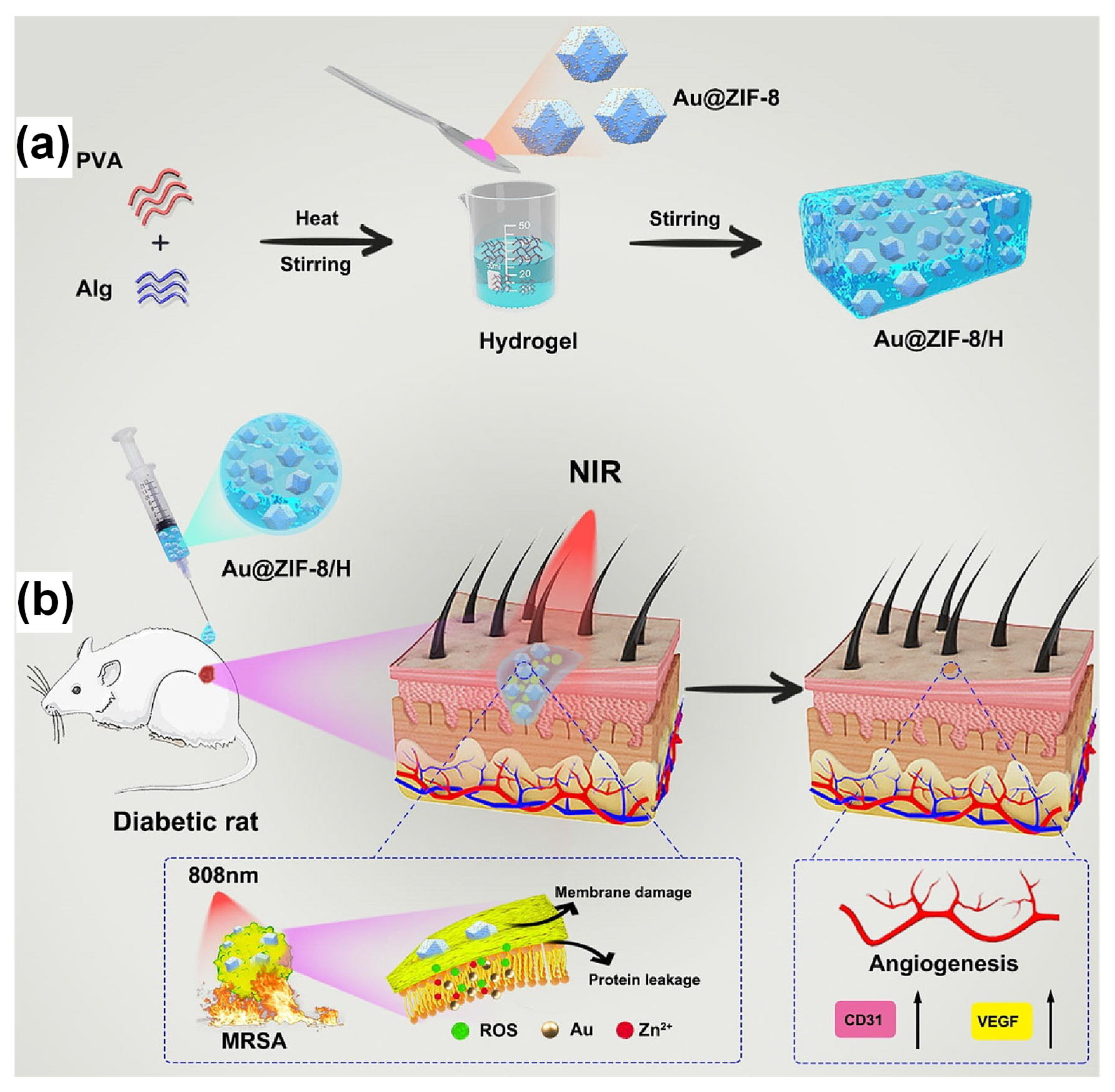
| Key Concept | Description | Relevance to Biosensing | Representative Examples | Refs. |
|---|---|---|---|---|
| Structural Features of MOFs | MOFs are crystalline porous materials formed by metal ions/clusters and organic ligands, offering ultrahigh surface area, ordered networks, and tunable pore sizes. | Enable selective adsorption, diffusion of analytes, and high loading of active sites, forming the structural basis for biosensing and nanozyme integration. | Use of porous MOFs to concentrate microbial analytes or catalytic substrates. | [40,41,42,43,44] |
| Chemical Tunability of MOFs | MOFs can be customized at both the metal center and linker level, allowing the modulation of surface chemistry and catalytic activity. | Facilitates precise design of nanozyme activity, target specificity, and sensing selectivity. | Functionalized MOFs with redox-active metals or tailored linkers enhancing specificity for microbial detection. | [41,42,43,44] |
| Classification of Nanozymes | Nanozymes are classified into metal-based, carbon-based, and composite types based on material and catalytic mechanism. | Understanding classification aids in selecting the most suitable catalytic system for microbial biosensing applications. | FePPOP-1 for peroxidase-like sensing; Smutok et al.’s composite biosensor for L-lactate; cerium oxide nanozymes for ROS-related diagnostics; Mn3O4@g-C3N4 for pollutant sensing. | [45,46,47,48,49,50,51,52,53,54] |
| Advantages of MOFs as Nanozyme Hosts | MOFs offer high porosity, active site confinement, and chemical compatibility for hosting nanozymes. | Improves catalytic efficiency, stability, and substrate accessibility, enhancing detection sensitivity and robustness. | Use of MOFs to stabilize catalytic centers and provide porous diffusion pathways for microbial analytes. | [55,56,57] |
| Integration of MOFs and Nanozymes | Rational design of MOF–nanozyme hybrids leverages both structural and catalytic advantages, enabling multifunctional biosensing platforms. | Enhances sensitivity, selectivity, and potential for intelligent (e.g., point-of-care or responsive) microbial biosensing platforms. | Cerium oxide nanozymes within polymer-modified MOFs for ROS scavenging; multifunctional Mn3O4@g-C3N4 composites for pollutant sensing with enzyme-mimetic properties. | [53,54,58] |
| Design Strategy | Target Microorganism | Type of Enzyme-mimetic Activity | Advantages | Representative Examples | Detection Method | Ref. |
|---|---|---|---|---|---|---|
| Active Center Engineering | Methicillin-resistant Staphylococcus aureus (MRSA) | Peroxidase-like | High catalytic activity; ultrasensitive detection; antibiotic susceptibility testing | Co2–O10 dual atomic sites in MOF-808; amplified chemiluminescence (~5800-fold) | Chemiluminescence | [64] |
| Salmonella Typhimurium | Peroxidase-mimetic electrocatalysis | High sensitivity; synergistic catalysis via Cu, Zr, and AuNPs | AuNP-doped CuZr-MOF functionalized with DNA probes | Electrochemical | [65] | |
| Lipopolysaccharide (LPS) (endotoxin) | Dopamine oxidation catalysis | Ultra-low LOD; electrostatic recognition; high specificity | Cu2+-modified nanoscale MOFs | Electrochemical | [66] | |
| Escherichia coli, Staphylococcus aureus | Fluorescence modulation via surface interaction | Rapid detection; multiplex pathogen detection; applicable in food samples | Turn-on Fe-MOF fluorescence biosensor | Fluorescence | [67] | |
| Staphylococcus aureus | Peroxidase-like | Synergistic metal doping; enhanced catalytic activity | Amine-functionalized bimetallic Fe–Ni MOF-74 | Colorimetric/Electrochemical | [68] | |
| — | Oxidase-like (ascorbate, glutathione, laccase) | Defect engineering enhances multi-enzyme mimicry; improved oxygen adsorption | Cysteine-deficient Co-based MOF (ZIF-L-Co) | Electrochemical (uric acid sensing) | [60] | |
| — | Peroxidase-like | Local pH regulation improves activity at physiological pH | PAA embedded in PCN-222-Fe | Colorimetric | [69] | |
| Pore Environment Modulation | — | Peroxidase-like | Microenvironment tuning for physiological stability | PAA-modulated PCN-222-Fe | Colorimetric | [69] |
| Surface Functionalization | Multiple microbial species | Fluorescence quenching/recovery | Rapid microbial fingerprinting; high classification accuracy in complex matrices | 2D-MOFs with fluorescent dye-labeled peptides | Fluorescence | [74] |
| Bacteria (general) | ROS generation (photocatalytic antibacterial) | Dual detection and disinfection capability | Boronic acid-modified UiO-66 (Zr-UiO-66-B(OH)2) | Fluorescence | [75] | |
| Escherichia coli | Peroxidase-like | Rapid, specific detection; enhanced phage stability and signal amplification | NH2-MIL-101(Fe) conjugated with lytic bacteriophages | Colorimetric/Fluorescence | [76] | |
| Pseudomonas aeruginosa, Escherichia coli | Luminescent detection | Label-free, specific carbohydrate binding; stable in environment | Glycosylated NH2-MIL-53(Fe) with galactose/mannose ligands | Luminescence | [77] | |
| — | Dual-gated enzymatic activity | Programmable, tunable biosensing behavior | ZIF-8 functionalized with DNA surfactant micelles | Fluorescence/Enzymatic | [78] | |
| Escherichia coli O157:H7 | Peroxidase-like | Wide detection range; improved electron transfer via polyaniline | Amino-functionalized MOF aptasensor | Electrochemical | [79] | |
| — | Fenton-like catalytic activity | Enhanced ECL signal; improved electron transfer | CoNi-MOF@PCN-224/Fe dual MOF-on-MOF system | Electrochemiluminescence | [80] | |
| Hybridization with Other Materials | Staphylococcus aureus | Peroxidase-like | Improved conductivity and aptamer immobilization; ultralow detection limits | ML-Cu2O@Cu-MOF composite nanozyme | Electrochemical (EIS, DPV) | [89] |
| Acinetobacter baumannii | Fluorescence amplification | Magnetic enrichment; rapid detection; high recovery efficiency | Zr-mMOF magnetic + fluorescein-loaded MOF aptasensing platform | Fluorescence | [90] | |
| A. baumannii, Pseudomonas aeruginosa | Peroxidase-like | Dual-mode detection; good performance in complex fluids | MOF–COF composite with boric acid and DNA aptamer scaffold | Fluorescence/Colorimetric | [91] | |
| Chlorpyrifos (pesticide, microbial sensor) | Cascade catalysis (ROS generation) | Signal amplification via in situ H2O2 generation | MOF-818@PMOF(Fe) nanozyme | Chemiluminescence/Colorimetric | [92] | |
| Live microbial cells (H2O2 secretion) | Peroxidase-like | Real-time metabolic monitoring; high stability | CNT/MOF composite on 3D gradient porous fiber membrane | Electrochemical | [93] | |
| — | Peroxidase-like | Wearable sensing; minimally invasive; colorimetric detection | DNAzyme@MOF composite in hydrogel microneedle tip | Colorimetric | [94] | |
| — | Peroxidase-like | Enhanced recyclability and electron transfer | Fe3O4@Au/MOF dual nanoparticle composite | Electrochemical | [95] | |
| — | Peroxidase-like | Responsive microenvironment; enhanced catalytic efficiency | PDM grafted UiO-66-NH2 polymer hybrid | Colorimetric | [96] |
| Detection Mode | MOF Nanozyme Used | Target Analyte | Limit of Detection (LOD) | Advantage | Limitations | Ref. |
|---|---|---|---|---|---|---|
| Colorimetric | Cu-MOF@AF film | H2S, NH3 (spoilage gases) | - | Visible signal, pH/TVB-N/TVC correlation, field-deployable | Semi-quantitative, limited to volatile markers | [101] |
| Colorimetric | Aptamer-functionalized Cu-MOF | Staphylococcus aureus | - | High selectivity, magnetic separation | May require aptamer regeneration | [102] |
| Colorimetric | SapYZUs8@Cu-MOF | Viable Staphylococcus aureus | 1.09 × 102 CFU/mL | High specificity, effective in pork and milk | Target-specific, may not detect non-viable cells | [103] |
| Colorimetric + Electrochemical | CP@MOF (CuO2 nanodots in MOF) | V. parahaemolyticus | - | Dual mode improves accuracy (88.7%) | Complex setup | [104] |
| Colorimetric | MOF@MOF (Cu-MOF-808 + Fe-porphyrin MOFs) | MRSA, Pseudomonas aeruginosa | 5 CFU/mL (MRSA), 2 CFU/mL (Pseudomonas aeruginosa ) | Self-cascade amplifies signal, aptamer specificity | Complex synthesis | [105] |
| Colorimetric (Multichannel) | 2D Ni–Co bimetallic MOF | Multiple microbes | - | Label-free, multichannel readout, 30 min detection | Qualitative/semi-quantitative | [106] |
| Colorimetric | MVCM@β-CD (2D Co-MOF) | m-Aminophenol (proxy for microbial metabolites) | 0.16 μM | High catalytic efficiency, tunable surface | Not yet applied directly to microbes | [107] |
| Electrochemical + Colorimetric | GATC (Cu-ZIF + Au-TA + G-quadruplex/hemin aptamer) | MRSA | - | Therapeutic + diagnostic, dual-mode | Potential toxicity, multi-step synthesis | [110] |
| Electrochemical + Colorimetric | Gr/FeCu-NZs (Graphene + FeCu-MOF-on-MOF) | H2O2 (microbial oxidative marker) | 0.06 μM | Synergistic conductivity, broad range (0.1–3800 μM) | Requires precise material tuning | [111] |
| Electrochemical | Ag/2D Zn-MOF | H2O2 (cellular secretion) | 1.67 μM | Real time, broad range (5 μM–70 mM) | Selectivity could be improved | [112] |
| Fluorescence-Based | Ce-MOF | Biofilm matrix (eDNA) + H2O2 | - | Therapeutic + detection, biofilm disruption | No quantification of microbial load | [117] |
| Fluorescence + Colorimetric (Ratiometric) | Hemin@MOF (blue-fluorescent MOF + hemin) | Tetracycline (residue) | 27.2 nM (colorimetric), 4.1 nM (fluorescence) | Dual-readout, high selectivity | May be limited to specific antibiotic residues | [118] |
| Nanozyme Type | Biomedical Application | Environmental Application | Ref. |
|---|---|---|---|
| MOF-based | Zr@ICG-NH2@HPW/OVA MOF nanozyme for Pseudomonas aeruginosa detection through GSH-depletion and photodynamic/photothermal therapy | Ce-FMA MOF nanozyme for colorimetric detection of Escherichia coli in water via peroxidase-like activity | [86,93] |
| Carbon-based | PrGO/Fe-N-C nanozyme integrated with aptamer for colorimetric detection of Salmonella typhimurium | 3D-rGO@Au–Pt hybrid used in colorimetric paper sensor for Salmonella enteritidis detection in contaminated milk | [120,122] |
| Metal oxide-based | MnFe2O4@SiO2 NPs with peroxidase-like activity for sensitive detection of Staphylococcus aureus in infected wounds | Fe3O4 NPs in lateral flow immunoassay for Escherichia coli O157:H7 detection in water and food samples | [106,110] |
| Noble metal-based | PtCo@Au NPs integrated with aptasensor for electrochemical detection of Salmonella typhimurium | Au@Pt NPs for electrochemical impedance spectroscopy detection of Listeria monocytogenes in food | [128,131] |
| Transition metal-based | CoFe-LDH nanozyme modified electrode for electrochemical detection of Listeria monocytogenes | CuFe2O4 nanozyme-based immunosensor for Salmonella enteritidis detection in milk | [132,133] |
| Metal sulfide-based | CuS@BSA nanocluster with peroxidase-like activity for colorimetric detection of Staphylococcus aureus | CdS QDs@MOF hybrid for visible-light-induced photocatalytic detection of Escherichia coli in water | [134,135] |
| Hybrid nanozymes | Ag/Cu-TCPP MOF nanozyme for fluorescence and colorimetric dual-mode detection of Helicobacter pylori | Au-Pt@CeO2-rGO hybrid nanozyme for ultrasensitive Escherichia coli O157:H7 detection in wastewater | [95,126] |
| Polymer-based | Chitosan–Cu nanozyme for selective detection of Escherichia coli and Salmonella via smartphone-integrated colorimetry | Molecularly imprinted polymer-coated Fe3O4 nanozymes for target-specific bacterial detection in water | [136,137] |
| 2D material-based | MoS2–CeO2 heterostructure for fluorescence biosensing of Pseudomonas aeruginosa via GSH-level detection | Graphene oxide/Au nanozyme composite for colorimetric detection of Escherichia coli in environmental water | [138,139] |
| Challenges | Implications | Proposed Solutions | Refs. |
|---|---|---|---|
| Stability and Reusability | Structural degradation and loss of catalytic activity after repeated use or harsh conditions. | Enhance structural robustness and catalytic longevity via ligand engineering, post-synthetic modifications, composites with graphene or polymers. | [139,155] |
| Selectivity and Specificity | Limited ability to precisely identify microbial strains in complex biological matrices. | Integrate selective biorecognition elements (aptamers, antibodies, molecularly imprinted polymers); exploit MOFs’ tunable structures for PET, RET, structural transformations. | [156,157] |
| Scalability and Cost | High production costs, batch inconsistencies, and difficulty in large-scale manufacturing. | Develop green, low-cost, scalable synthesis methods ensuring reproducibility and sustainability. | [158] |
| Smart System Integration | Technical challenges in interfacing MOFs with electronics, data processing, and communication units. | Innovate interdisciplinary solutions to integrate MOFs with AI, IoT, and smart responsive materials for adaptive biosensors. | [159] |
| Biosafety and Regulatory Approval | Potential cytotoxicity and environmental impact; lack of standardized testing and clear guidelines. | Conduct comprehensive toxicity and biocompatibility assessments; establish standardized protocols and regulatory frameworks. | [160,161,162] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kidanemariam, A.; Cho, S. Recent Advances in Metal–Organic Framework-Based Nanozymes for Intelligent Microbial Biosensing: A Comprehensive Review of Biomedical and Environmental Applications. Biosensors 2025, 15, 437. https://doi.org/10.3390/bios15070437
Kidanemariam A, Cho S. Recent Advances in Metal–Organic Framework-Based Nanozymes for Intelligent Microbial Biosensing: A Comprehensive Review of Biomedical and Environmental Applications. Biosensors. 2025; 15(7):437. https://doi.org/10.3390/bios15070437
Chicago/Turabian StyleKidanemariam, Alemayehu, and Sungbo Cho. 2025. "Recent Advances in Metal–Organic Framework-Based Nanozymes for Intelligent Microbial Biosensing: A Comprehensive Review of Biomedical and Environmental Applications" Biosensors 15, no. 7: 437. https://doi.org/10.3390/bios15070437
APA StyleKidanemariam, A., & Cho, S. (2025). Recent Advances in Metal–Organic Framework-Based Nanozymes for Intelligent Microbial Biosensing: A Comprehensive Review of Biomedical and Environmental Applications. Biosensors, 15(7), 437. https://doi.org/10.3390/bios15070437







