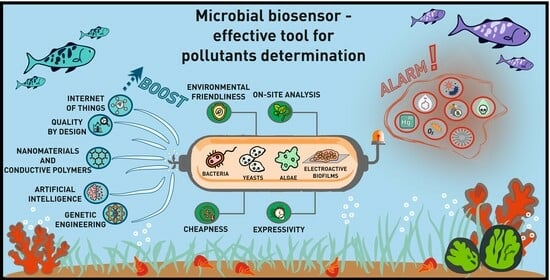
Whole Cells of Microorganisms—A Powerful Bioanalytical Tool for Measuring Integral Parameters of Pollution: A Review
Abstract
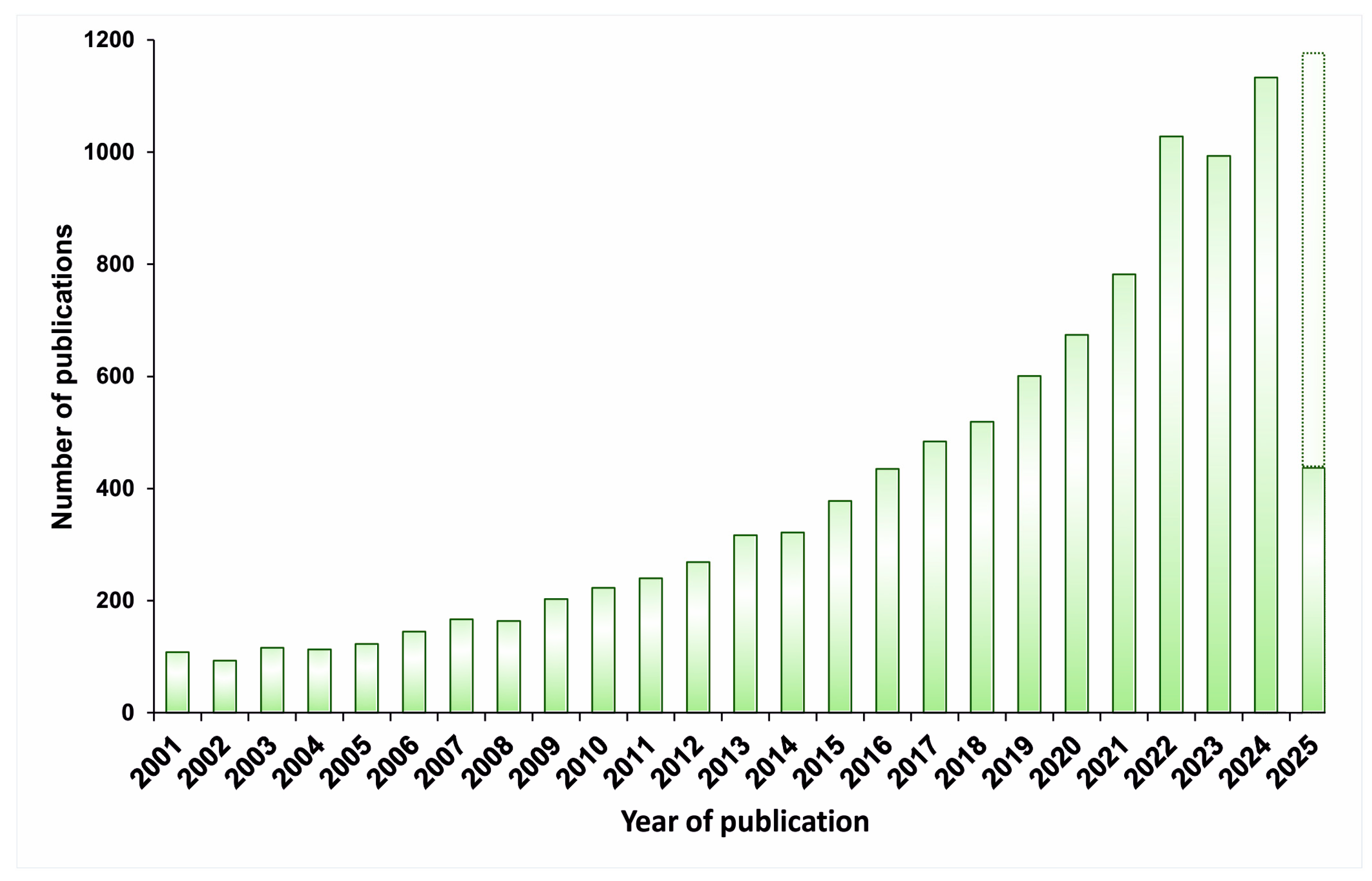
1. Introduction
2. Approaches to Improving Characteristics of Bioanalytical Systems Based on Whole Microbial Cells
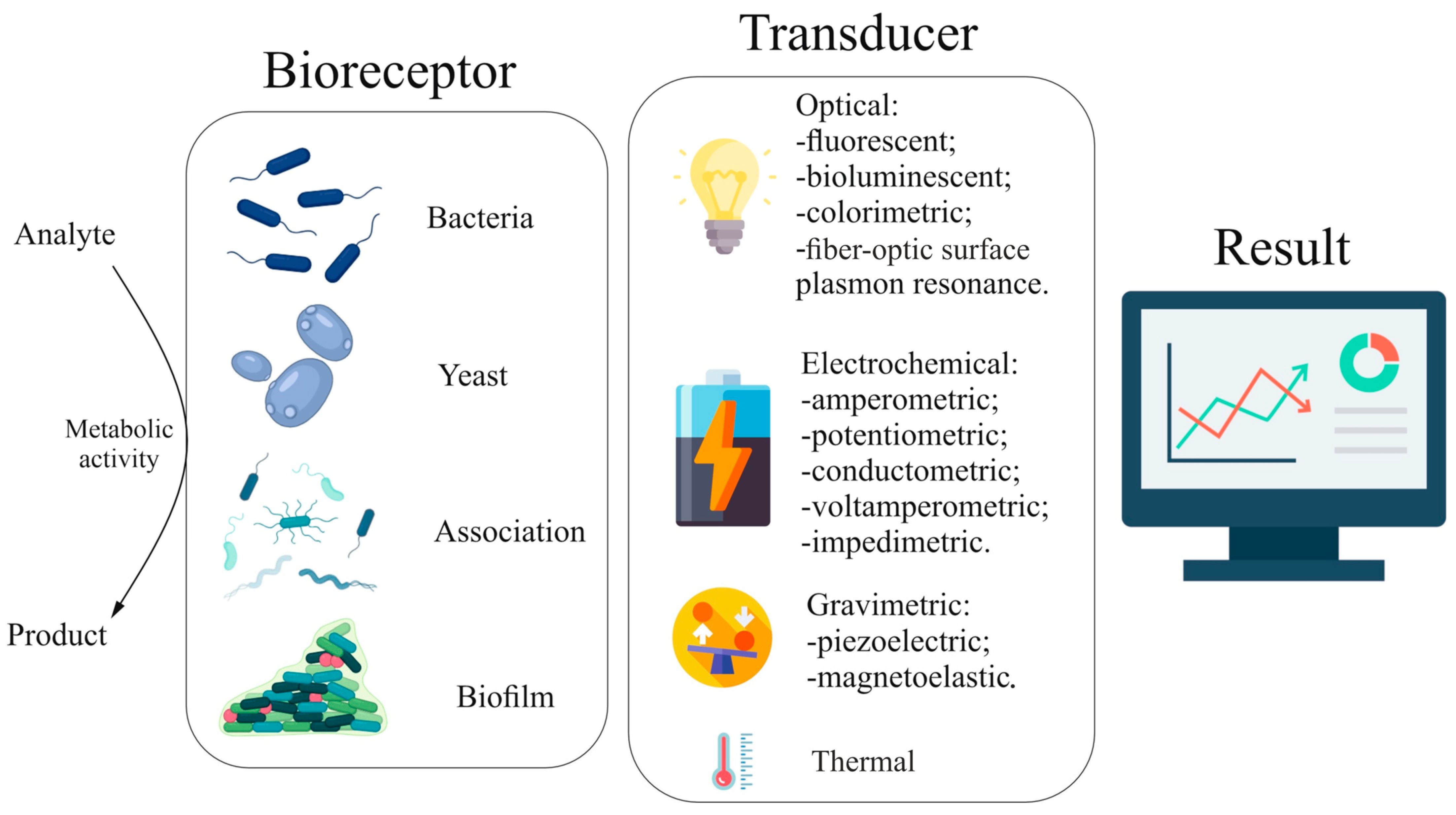
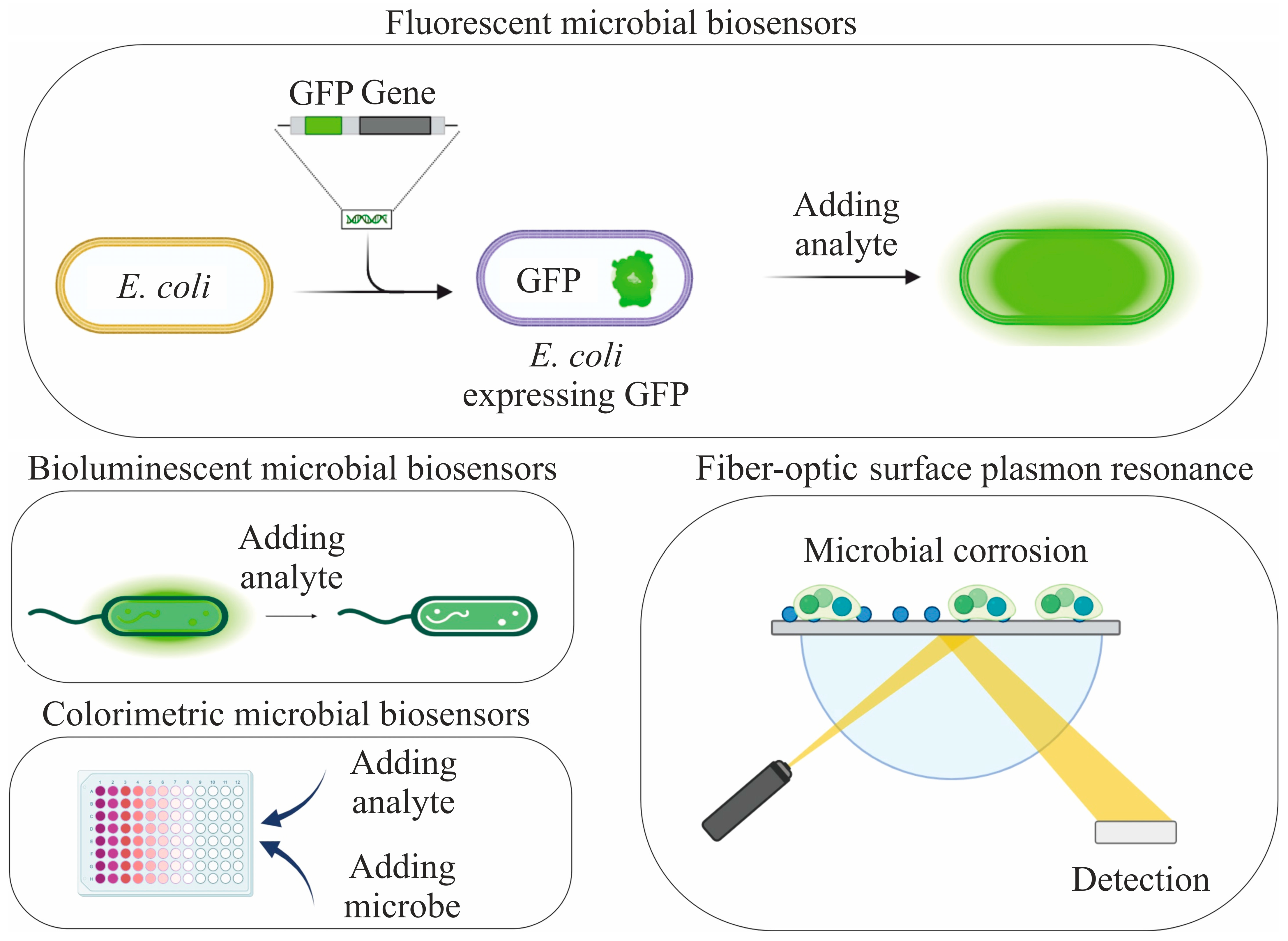
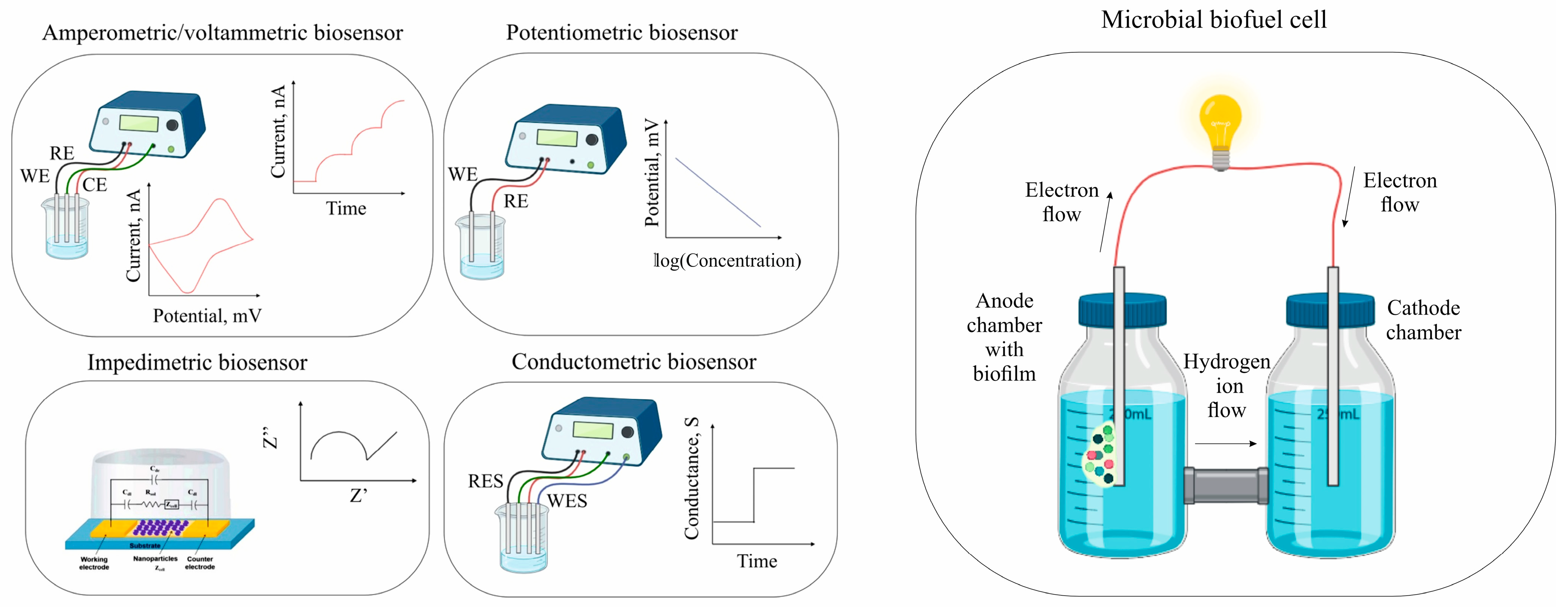
3. Determination of Pollutants
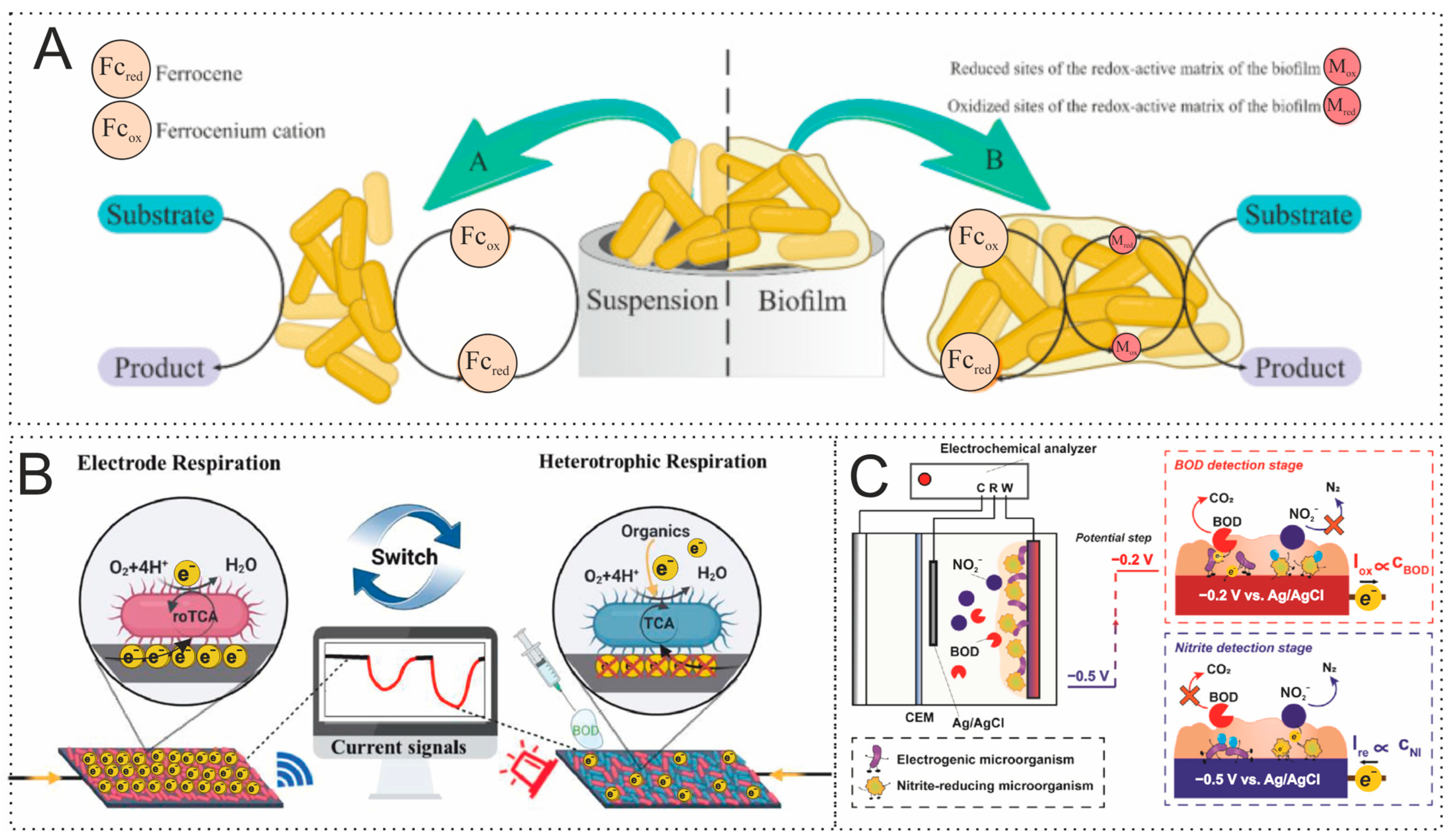
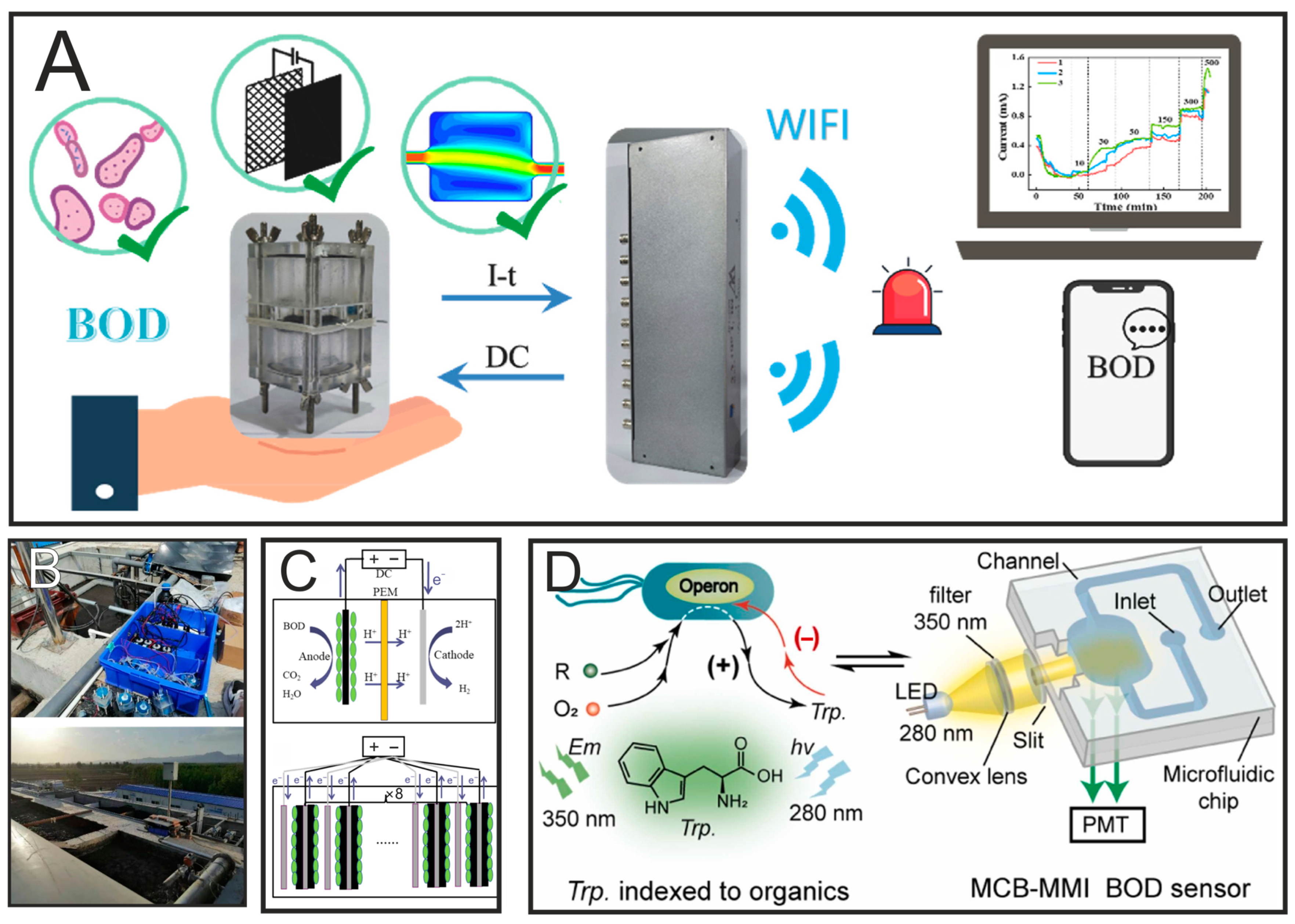
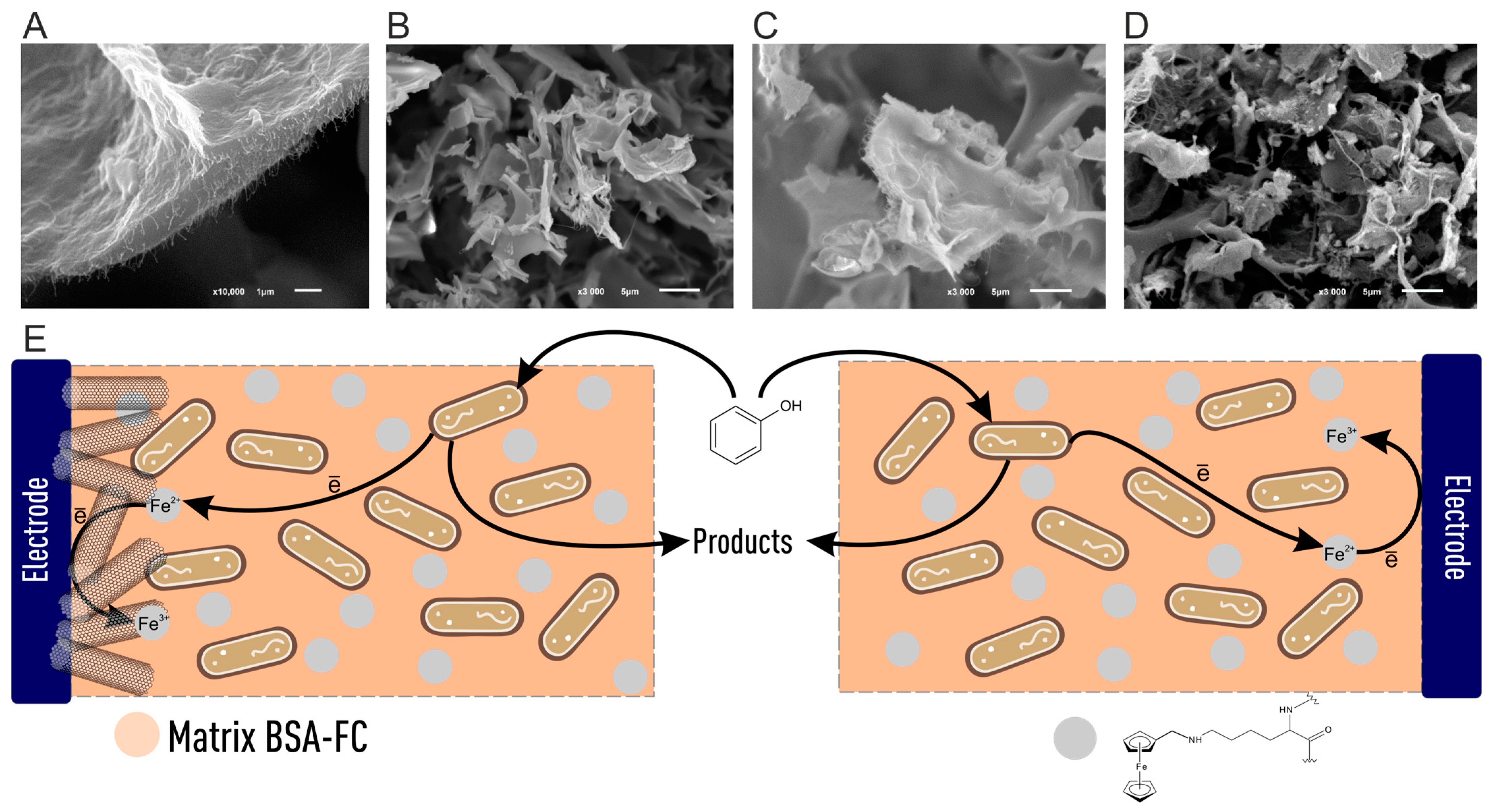
3.1. BOD
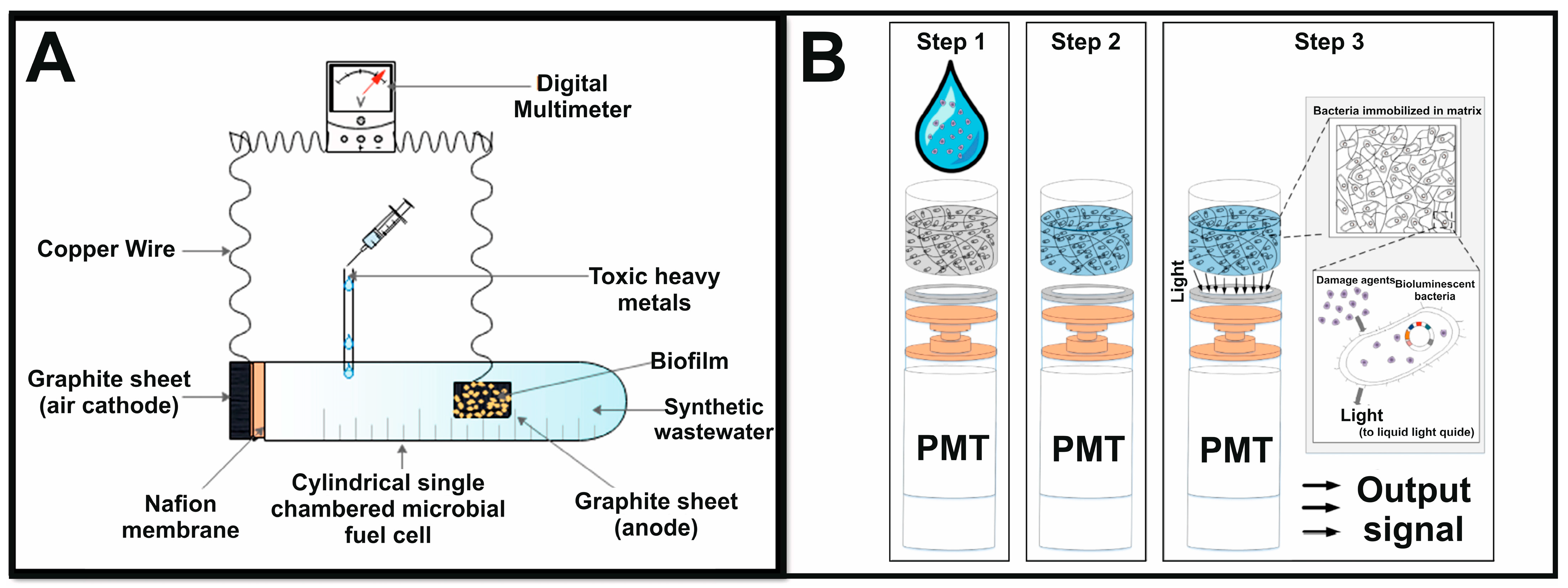
3.2. Toxicity
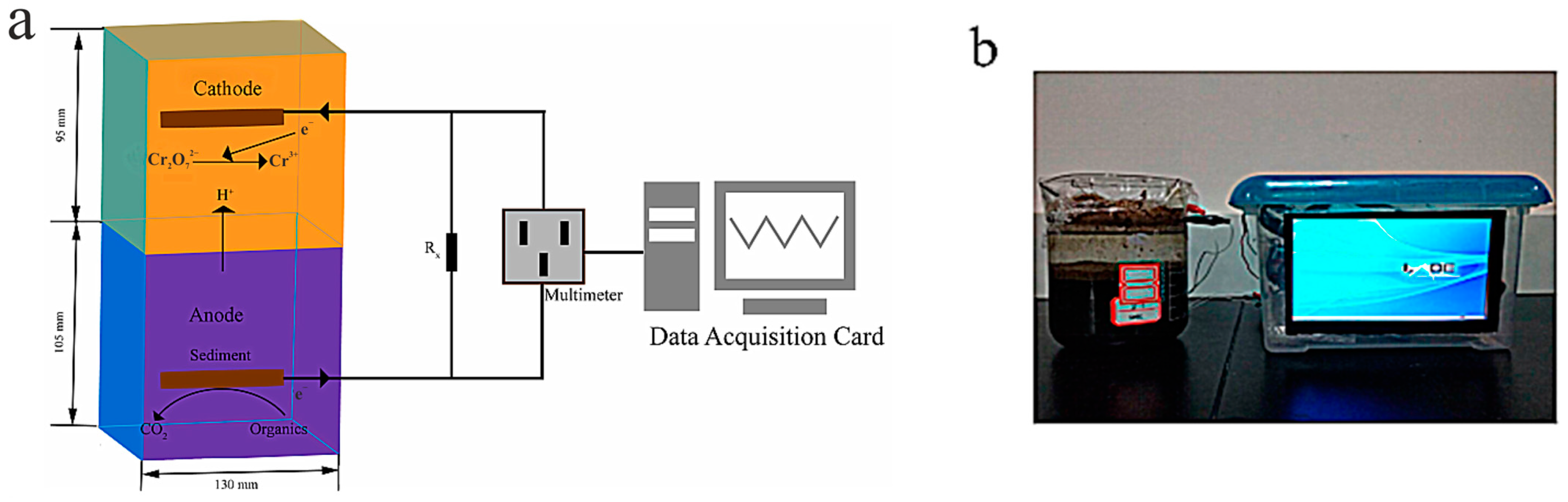
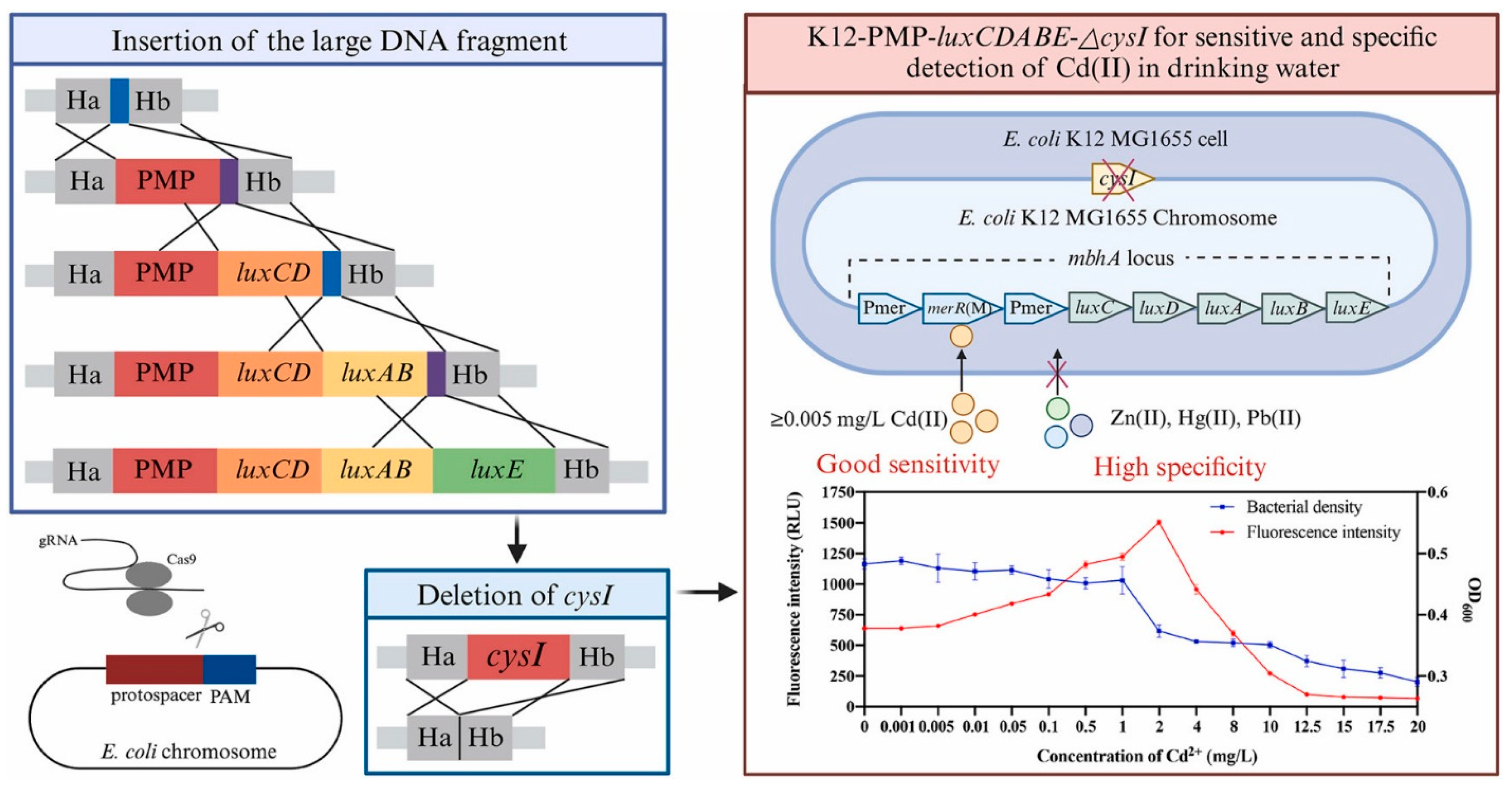
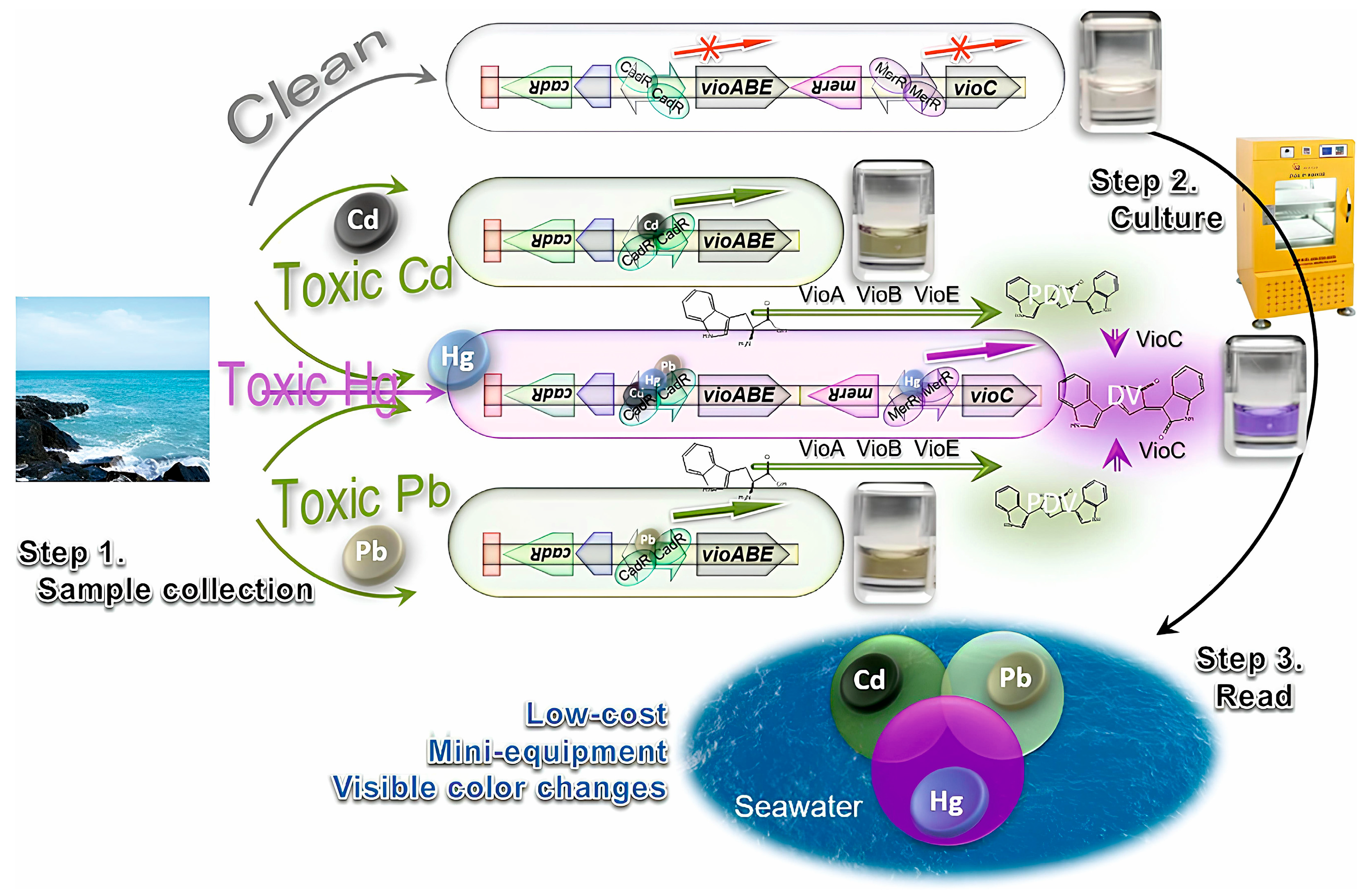
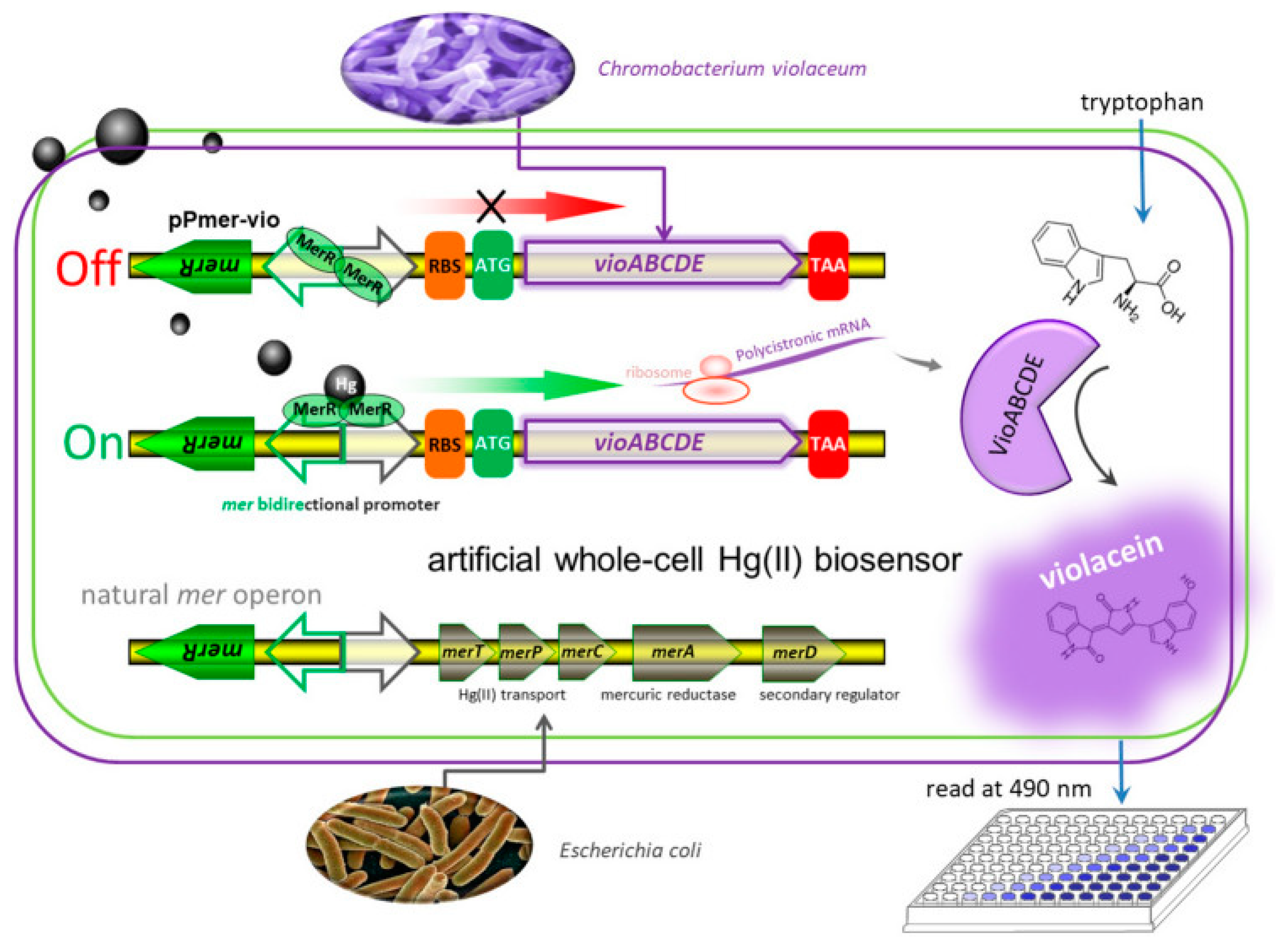
3.3. Heavy Metals
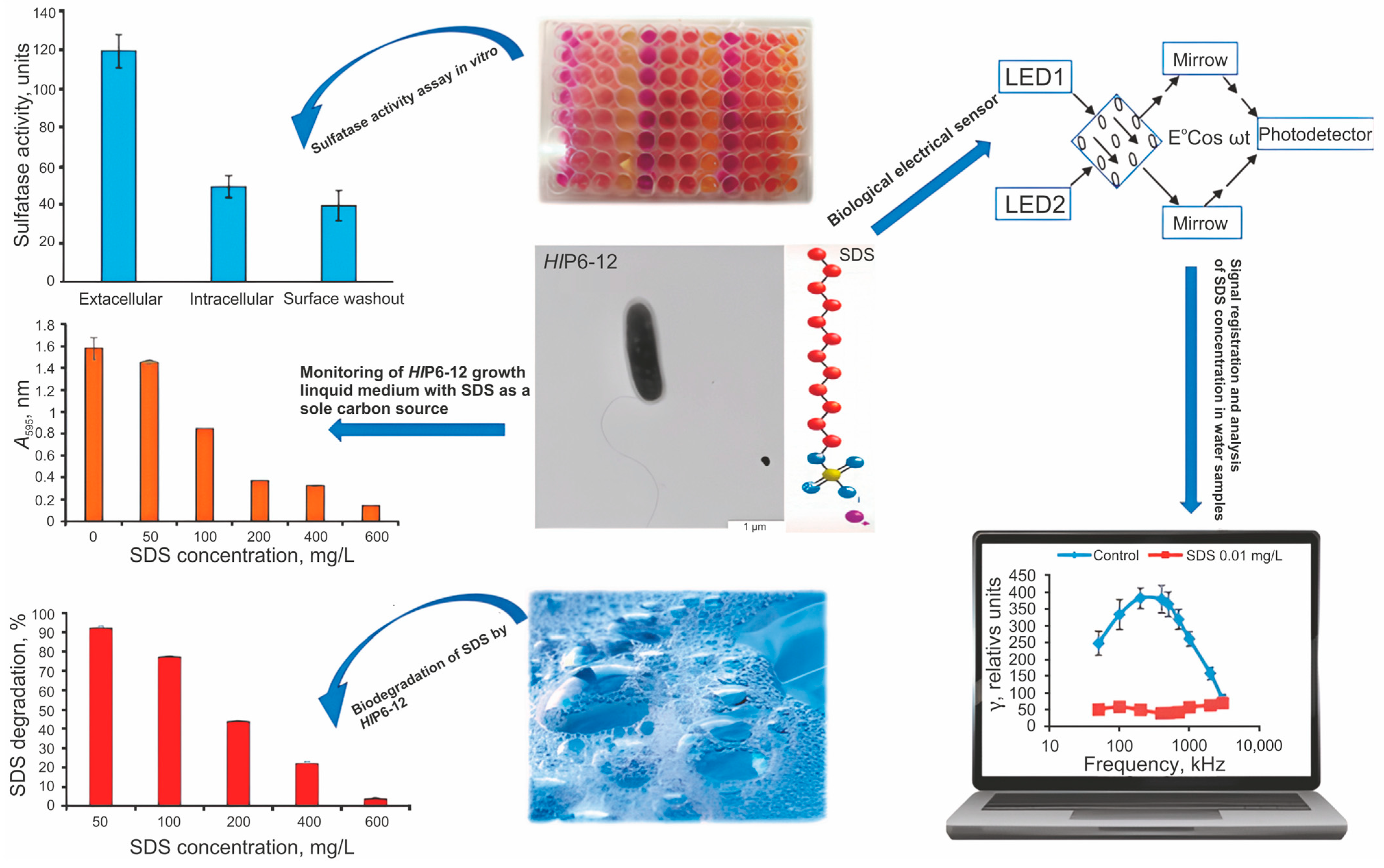
3.4. Surfactants
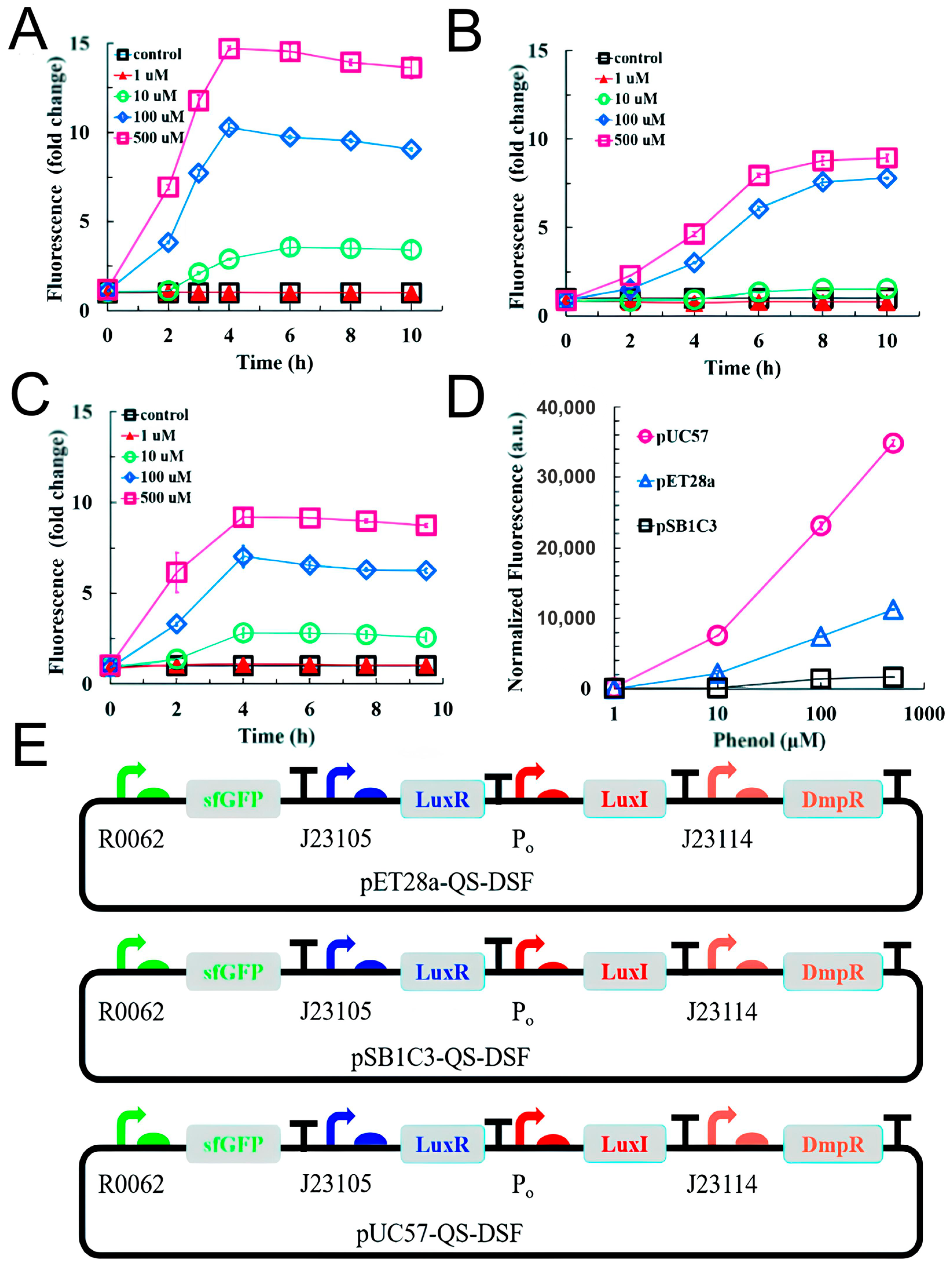
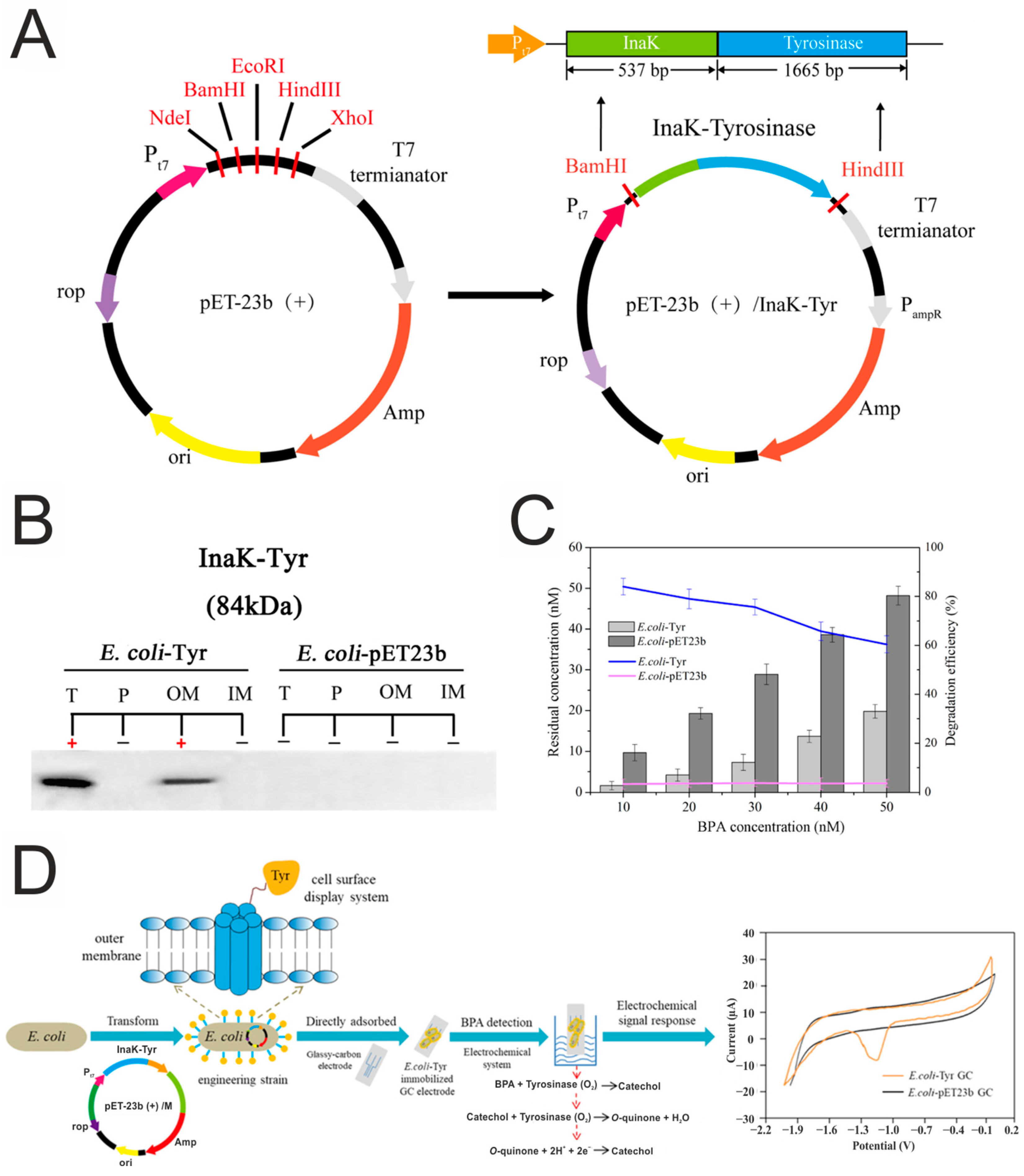
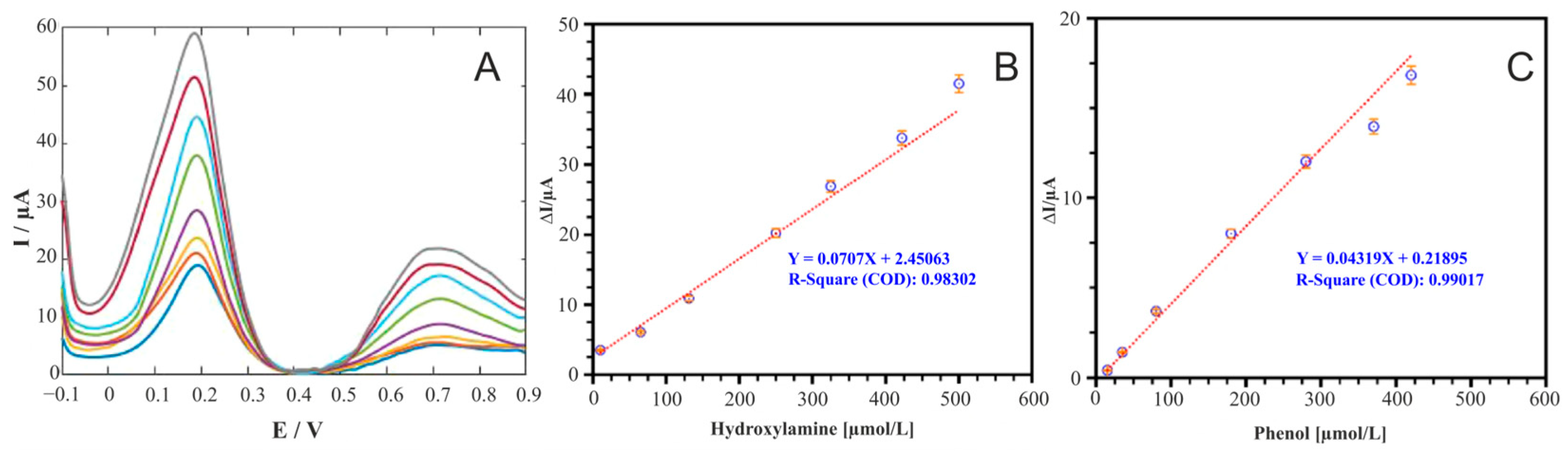
3.5. Phenols
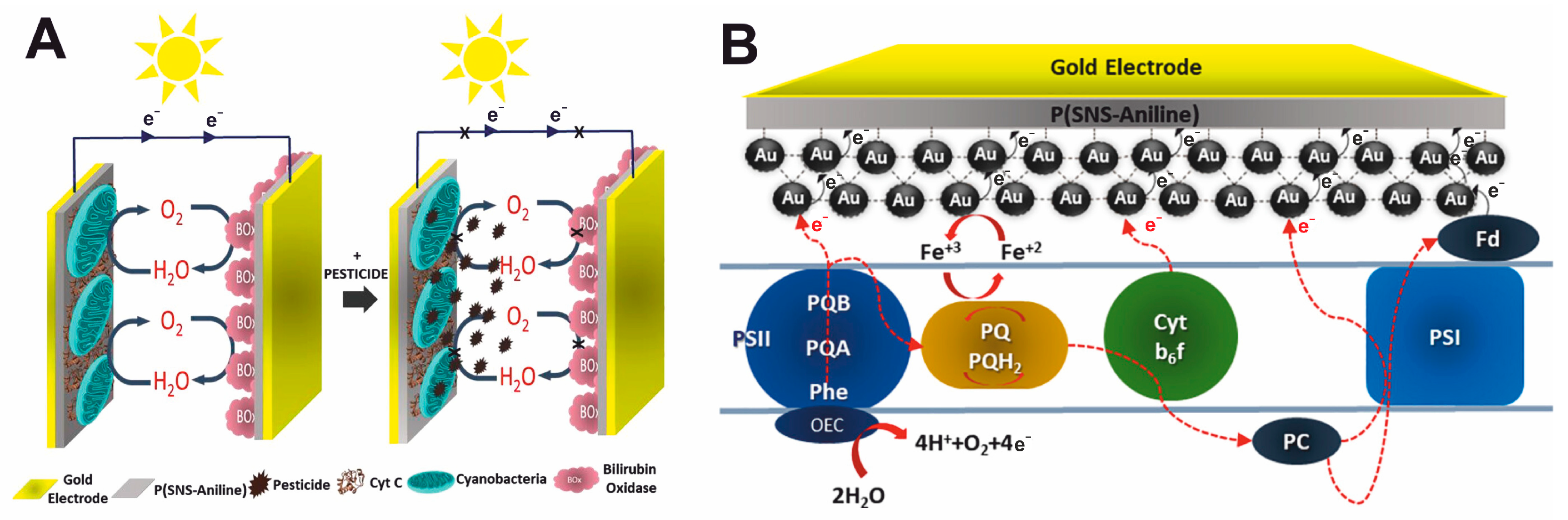
3.6. Pesticides
3.7. Inorganic Pollutants
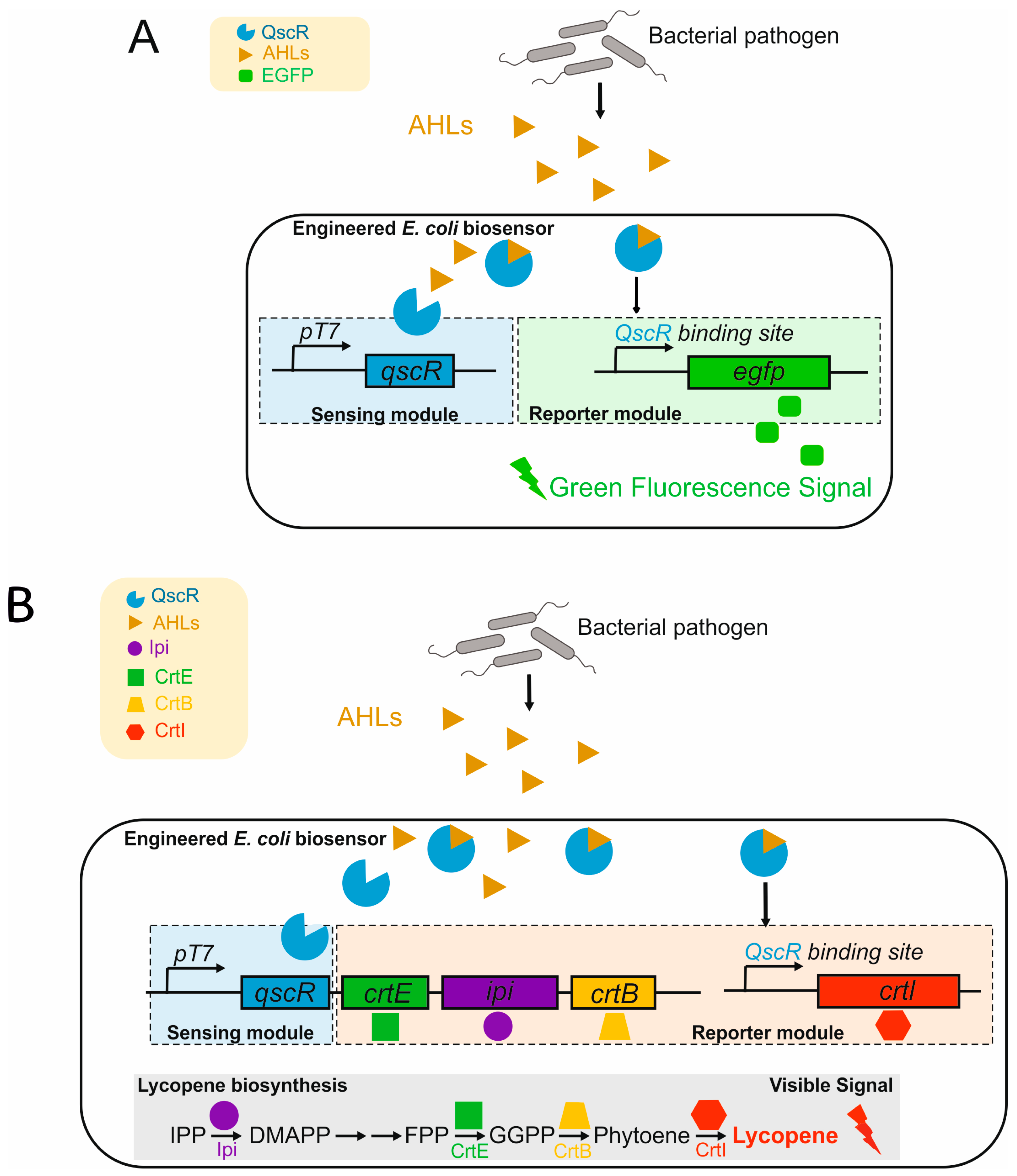
3.8. Viral and Microbiological Contamination
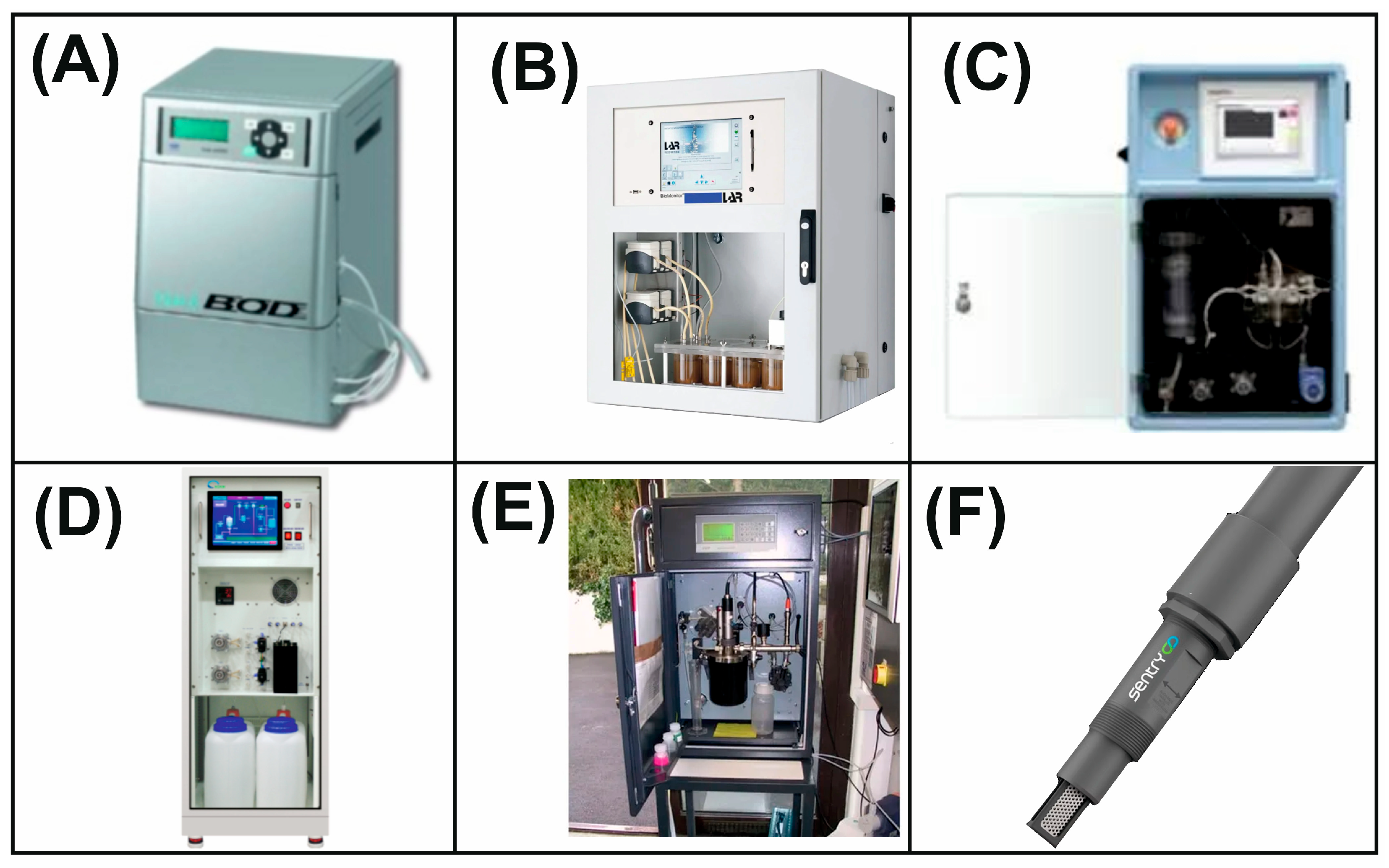
4. Commercial Bioanalytical Systems Based on Whole Cells of Microorganisms
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EIS | Electrochemical impedance spectroscopy |
| MFC | Microbial fuel cell |
| AI | Artificial intelligence |
| IoTs | Internet of Things |
| BESs | Bioelectrochemical systems |
| QbD | Quality by Design |
| BOD | Biological oxygen demand |
| EAMs | Electroactive microorganisms |
| EABs | Electroactive biofilms |
| SWCNTs | Single-walled carbon nanotubes |
| MEC | Microbial electrolytic cell |
| AAS | Atomic absorption spectroscopy |
| OES | Optical emission spectroscopy |
| SDS | Sodium dodecyl sulfate |
| MPC | Maximum permissible concentration |
| HPLC | High-performance liquid chromatography |
| MWCNTs | Multi-walled carbon nanotubes |
| SEM | Scanning electron microscopy |
| BPA | Bisphenol A |
| OPs | Organophosphate pesticides |
| MP | Methylparation |
| p-NP | p-Nitrophenol |
| IPTG | Isopropyl-β-D-thiogalactopyranoside |
| QS | Quorum sensing |
| AHL | N-acylhomoserinlactone |
| AChE | Acetylcholinesterase |
| FGE | Formylglycine generating enzyme |
| OPH | Organophosphate hydrolase |
| BPV | Biological photovoltaic cell |
| Box | Bilirubinoxidase |
| 2,4-D | 2,4-dichlorophenoxyacetic acid |
| TEB | Tebuconazole |
| RSD | Relative standard deviation |
References
- Mathur, S.; Singh, D.; Ranjan, R. Genetic Circuits in Microbial Biosensors for Heavy Metal Detection in Soil and Water. Biochem. Biophys. Res. Commun. 2023, 652, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Meliana, C.; Munawaroh, H.S.H.; Karaman, C.; Karimi-Maleh, H.; Low, S.S.; Show, P.L. Recent Advances in the Analytical Strategies of Microbial Biosensor for Detection of Pollutants. Chemosphere 2022, 306, 135515. [Google Scholar] [CrossRef] [PubMed]
- Shivaram, K.B.; Bhatt, P.; Verma, M.S.; Clase, K.; Simsek, H. Bacteriophage-Based Biosensors for Detection of Pathogenic Microbes in Wastewater. Sci. Total Environ. 2023, 901, 165859. [Google Scholar] [CrossRef]
- Abena, T. Biosensors Technological Advancement and Their Biomedical, Agricultural, Environmental and Food Industrial Applications: A Review. Int. J. Food Agric. Nat. Resour. 2023, 4, 46–57. [Google Scholar] [CrossRef]
- Neelam, A.; Tabassum, S. Optical Sensing Technologies to Elucidate the Interplay between Plant and Microbes. Micromachines 2023, 14, 195. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sengupta, S.; Kumar, L.; Upadhyay, T.; Kabra, A.; Lalhlenmawia, H.; Kumar, D.; Singh, J. Recent Advancements in Photodynamic Therapy and Cancer Biosensor Using Natural Products. Talanta Open 2023, 8, 100261. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, M. Optical Biosensors for Environmental Monitoring: Recent Advances and Future Perspectives in Bacterial Detection. Environ. Res. 2023, 236, 116826. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, J.; Tao, H.; Lv, X.; Deng, Y.; Li, X.E. Coli Biosensor Based on Modular GFP and LuxI/LuxR Cyclic Amplification Circuit for Sensitive Detection of Lysine. Anal. Bioanal. Chem. 2022, 414, 8299–8307. [Google Scholar] [CrossRef]
- Li, Y.; He, X.; Zhu, W.; Li, H.; Wang, W. Bacterial Bioluminescence Assay for Bioanalysis and Bioimaging. Anal. Bioanal. Chem. 2022, 414, 75–83. [Google Scholar] [CrossRef]
- Celik, C.; Kalin, G.; Cetinkaya, Z.; Ildiz, N.; Ocsoy, I. Recent Advances in Colorimetric Tests for the Detection of Infectious Diseases and Antimicrobial Resistance. Diagnostics 2023, 13, 2427. [Google Scholar] [CrossRef]
- He, Y.; Xu, Z.; Kasputis, T.; Zhao, X.; Ibañez, I.; Pavan, F.; Bok, M.; Malito, J.P.; Parreno, V.; Yuan, L.; et al. Development of Nanobody-Displayed Whole-Cell Biosensors for the Colorimetric Detection of SARS-CoV-2. ACS Appl. Mater. Interfaces 2023, 15, 37184–37192. [Google Scholar] [CrossRef]
- Rakhimbekova, A.; Kudaibergenov, B.; Moldabay, D.; Zharylgap, A.; Ajunwa, O.M.; Marsili, E.; Tosi, D. Biofilm Detection by a Fiber-Tip Ball Resonator Optical Fiber Sensor. Biosensors 2022, 12, 481. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Ju, C.; Liu, P.; Li, Z.; Fan, Y.; Zhang, Y.; Zhao, Y.; Gu, T.; Wang, F.; Xu, D. Corrosive Pseudomonas Aeruginosa Detection by Measuring Pyocyanin with a Lab-on-Fiber Optical Surface Plasmon Resonance Biosensor in Aquatic Environments. Biosens. Bioelectron. 2024, 261, 116521. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Mahajan, P.; Alsubaie, A.S.; Khanna, V.; Chahal, S.; Thakur, A.; Yadav, A.; Arya, A.; Singh, A.; Singh, G. Next-Generation Nanomaterials-Based Biosensors: Real-Time Biosensing Devices for Detecting Emerging Environmental Pollutants. Mater. Today Sustain. 2025, 29, 101068. [Google Scholar] [CrossRef]
- Umar, L.; Rosandi, V.A.; Setiadi, R.N.; Agustirandi, B.; Linda, T.M.; Kuswandi, B. Amperometric Microbial Biosensor for Sugars and Sweetener Classification Using Principal Component Analysis in Beverages. J. Food Sci. Technol. 2023, 60, 382–392. [Google Scholar] [CrossRef]
- Lopez-Tellez, J.; Ramirez-Montes, S.; Ferreira, T.A.; Santos, E.M.; Rodriguez, J.A. Application of Voltammetric Sensors for Pathogen Bacteria Detection: A Review. Chemosensors 2022, 10, 424. [Google Scholar] [CrossRef]
- Burge, S.R.; Hristovski, K.D.; Burge, R.G.; Hoffman, D.A.; Saboe, D.; Chao, P.F.; Taylor, E.; Koenigsberg, S.S. Microbial Potentiometric Sensor: A New Approach to Longstanding Challenges. Sci. Total Environ. 2020, 742, 140528. [Google Scholar] [CrossRef]
- Berketa, K.; Saiapina, O.; Fayura, L.; Sibirny, A.; Dzyadevych, S.; Soldatkin, O. Novel Highly Sensitive Conductometric Biosensor Based on Arginine Deiminase from Mycoplasma Hominis for Determination of Arginine. Sens. Actuators B Chem. 2022, 367, 132023. [Google Scholar] [CrossRef]
- Chen, T.; Xu, Y.H.; Li, J.H. Recent Advances in Electrochemical Impedance Spectroscopy-Based Pathogenic Bacteria Sensing. J. Electrochem. 2023, 29, 5. [Google Scholar] [CrossRef]
- Chung, T.H.; Dhar, B.R. Paper-Based Platforms for Microbial Electrochemical Cell-Based Biosensors: A Review. Biosens. Bioelectron. 2021, 192, 113485. [Google Scholar] [CrossRef]
- Irkham, I.; Ibrahim, A.U.; Pwavodi, P.C.; Al-Turjman, F.; Hartati, Y.W. Smart Graphene-Based Electrochemical Nanobiosensor for Clinical Diagnosis. Sensors 2023, 23, 2240. [Google Scholar] [CrossRef] [PubMed]
- Skládal, P. Piezoelectric Biosensors: Shedding Light on Principles and Applications. Microchim. Acta 2024, 191, 184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; He, F. Mycobacterium Tuberculosis Piezoelectric Sensor Based on AuNPs-Mediated Enzyme Assisted Signal Amplification. Talanta 2022, 236, 122902. [Google Scholar] [CrossRef] [PubMed]
- Balcıoğlu, S.; İnan, O.O.; Kolak, S.; Ateş, B.; Atalay, S. Diagnosis, Bacterial Density, Food, and Agricultural Applications of Magnetoelastic Biosensors: Theory, Instrumentation, and Progress. J. Supercond. Nov. Magn. 2024, 37, 1299–1322. [Google Scholar] [CrossRef]
- Polat, E.O.; Cetin, M.M.; Tabak, A.F.; Güven, E.B.; Uysal, B.Ö.; Arsan, T.; Kabbani, A.; Hamed, H.; Gül, S.B. Transducer Technologies for Biosensors and Their Wearable Applications. Biosensors 2022, 12, 385. [Google Scholar] [CrossRef]
- Gertsen, M.M.; Perelomov, L.V.; Arlyapov, V.A.; Atroshchenko, Y.M.; Meshalkin, V.P.; Chistyakova, T.B.; Reverberi, A. Pietro Degradation of Oil and Petroleum Products in Water by Bioorganic Compositions Based on Humic Acids. Energies 2023, 16, 5320. [Google Scholar] [CrossRef]
- Ai, C.; Hou, S.; Yan, Z.; Zheng, X.; Amanze, C.; Chai, L.; Qiu, G.; Zeng, W. Recovery of Metals from Acid Mine Drainage by Bioelectrochemical System Inoculated with a Novel Exoelectrogen, Pseudomonas sp. E8. Microorganisms 2020, 8, 41. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Khatoon, A.; Setapar, S.H.M.; Umar, K.; Parveen, T.; Ibrahim, M.N.M.; Ahmad, A.; Rafatullah, M. Outlook on the Role of Microbial Fuel Cells in Remediation of Environmental Pollutants with Electricity Generation. Catalysts 2020, 10, 819. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Umar, K.; Bhawani, S.A.; Khan, A.; Asiri, A.M.; Khan, M.R.; Azam, M.; Alammari, A.M. Cellulose Derived Graphene/Polyaniline Nanocomposite Anode for Energy Generation and Bioremediation of Toxic Metals via Benthic Microbial Fuel Cells. Polymers 2021, 13, 135. [Google Scholar] [CrossRef]
- Cui, L.; Xin, Y.; Yang, K.; Li, H.; Tan, F.; Zhang, Y.; Li, X.; Zhu, Z.; Yang, J.; Kao, S.J.; et al. Live Tracking Metabolic Networks and Physiological Responses within Microbial Assemblages at Single-Cell Level. PNAS Nexus 2023, 2, pgad006. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, W.E.; Cui, L.; Wagner, M. Single Cell Stable Isotope Probing in Microbiology Using Raman Microspectroscopy. Curr. Opin. Biotechnol. 2016, 41, 34–42. [Google Scholar] [CrossRef]
- Ilchenko, O.; Pilhun, Y.; Kutsyk, A.; Slobodianiuk, D.; Goksel, Y.; Dumont, E.; Vaut, L.; Mazzoni, C.; Morelli, L.; Boisen, S.; et al. Optics Miniaturization Strategy for Demanding Raman Spectroscopy Applications. Nat. Commun. 2024, 15, 3049. [Google Scholar] [CrossRef]
- González-Cabaleiro, R.; Mitchell, A.M.; Smith, W.; Wipat, A.; Ofiteru, I.D. Heterogeneity in Pure Microbial Systems: Experimental Measurements and Modeling. Front. Microbiol. 2017, 8, 1813. [Google Scholar] [CrossRef]
- Rubbens, P.; Props, R.; Kerckhof, F.-M.; Boon, N.; Waegeman, W. PhenoGMM: Gaussian Mixture Modeling of Cytometry Data Quantifies Changes in Microbial Community Structure. mSphere 2021, 6, 10–1128. [Google Scholar] [CrossRef]
- Beg, S.; Chaudhary, V.; Sharma, G.; Garg, B.; Panda, S.S.; Singh, B. QbD-Oriented Development and Validation of a Bioanalytical Method for Nevirapine with Enhanced Liquid-Liquid Extraction and Chromatographic Separation. Biomed. Chromatogr. 2016, 30, 818–828. [Google Scholar] [CrossRef]
- Schröder, H.M.; Niebergall-Roth, E.; Norrick, A.; Esterlechner, J.; Ganss, C.; Frank, M.H.; Kluth, M.A. Drug Regulatory-Compliant Validation of a QPCR Assay for Bioanalysis Studies of a Cell Therapy Product with a Special Focus on Matrix Interferences in a Wide Range of Organ Tissues. Cells 2023, 12, 1788. [Google Scholar] [CrossRef]
- Ananikov, V.P. Top 20 Influential AI-Based Technologies in Chemistry. Artif. Intell. Chem. 2024, 2, 100075. [Google Scholar] [CrossRef]
- Rybin, V.; Butusov, D.; Babkin, I.; Pesterev, D.; Arlyapov, V. Some Properties of a Discrete Lorenz System Obtained by Variable Midpoint Method and Its Application to Chaotic Signal Modulation. Int. J. Bifurc. Chaos 2024, 34, 2450009. [Google Scholar] [CrossRef]
- Pennacchio, A.; Giampaolo, F.; Cafaro, V.; Cicatiello, P.; Della Ventura, B.; Giardina, P.; Rosanova, R.; Savoia, M.; Velotta, R.; Piccialli, F.; et al. A Bacterial Biosensor Based on Gold Nanoparticles Functionalized by a Hydrophobin-Chimera and Combined with Machine Learning for User-Friendly Detection. Sens. Actuators B Chem. 2024, 410, 135645. [Google Scholar] [CrossRef]
- Bhatlawande, A.R.; Ghatge, P.U.; Shinde, G.U.; Anushree, R.K.; Patil, S.D. Unlocking the Future of Smart Food Packaging: Biosensors, IoT, and Nano Materials. Food Sci. Biotechnol. 2024, 33, 1075–1091. [Google Scholar] [CrossRef]
- Verma, D.; Singh, K.R.; Yadav, A.K.; Nayak, V.; Singh, J.; Solanki, P.R.; Singh, R.P. Internet of Things (IoT) in Nano-Integrated Wearable Biosensor Devices for Healthcare Applications. Biosens. Bioelectron. X 2022, 11, 100153. [Google Scholar] [CrossRef]
- Lavrova, T.; Kharkova, A.; Perchikov, R.; Gertsen, M.; Shadrin, A.; Arlyapov, V. A Highly Sensitive Test System for Measuring the Phenolic Index of Wastewater Based on a Biocompatible Composite Material with Carbon Nanotubes. J. Polym. Environ. 2024, 33, 63–77. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.; Bian, C.; Xia, T.; Gao, Y.; Zhang, X.; Wang, H.; Ma, H.; Hu, Y.; Wang, X. Highly Sensitive Microbial Biosensor Based on Recombinant Escherichia coli Overexpressing Catechol 2,3-Dioxygenase for Reliable Detection of Catechol. Biosens. Bioelectron. 2019, 126, 51–58. [Google Scholar] [CrossRef]
- Scognamiglio, V.; Antonacci, A.; Arduini, F.; Moscone, D.; Campos, E.V.R.; Fraceto, L.F.; Palleschi, G. An Eco-Designed Paper-Based Algal Biosensor for Nanoformulated Herbicide Optical Detection. J. Hazard. Mater. 2019, 373, 483–492. [Google Scholar] [CrossRef]
- Ooi, L.; Heng, L.Y.; Ahmad, A. Toxicity Biosensor for Sodium Dodecyl Sulfate Using Immobilized Green Fluorescent Protein Expressing Escherichia coli. J. Sensors 2015, 2015, 809065. [Google Scholar] [CrossRef]
- Kuznetsova, L.S.; Ivanova, K.D.; Lantsova, E.A.; Saverina, E.A.; Kharkova, A.S.; Guetnga, C.; Lipkin, M.S.; Kamanina, O.A.; Kozlova, T.V.; Popova, N.M. Cross-Disciplinary Glucose Biosensors: An ORMOSIL/Enzyme Material for Enhanced Detection. ACS Appl. Polym. Mater. 2024, 6, 12405–12419. [Google Scholar] [CrossRef]
- Ma, T.; Zhao, N.; Ni, Y.; Yi, J.; Wilson, J.P.; He, L.; Du, Y.; Pei, T.; Zhou, C.; Song, C.; et al. China’s Improving Inland Surface Water Quality since 2003. Sci. Adv. 2020, 6, eaau3798. [Google Scholar] [CrossRef]
- Jiao, N.; Liu, J.; Edwards, B.; Lv, Z.; Cai, R.; Liu, Y.; Xiao, X.; Wang, J.; Jiao, F.; Wang, R.; et al. Correcting a Major Error in Assessing Organic Carbon Pollution in Natural Waters. Sci. Adv. 2021, 7, eabc7318. [Google Scholar] [CrossRef]
- Salvian, A.; Farkas, D.; Ramirez-Moreno, M.; Torruella-Salas, D.; Berná, A.; Avignone-Rossa, C.; Varcoe, J.R.; Esteve-Núñez, A.; Gadkari, S. Resilience of Anodic Biofilm in Microbial Fuel Cell Biosensor for BOD Monitoring of Urban Wastewater. Npj Clean Water 2024, 7, 53. [Google Scholar] [CrossRef]
- ISO 5815-1:2019; Water Quality—Determination of Biochemical Oxygen Demand After n Days (BODn)-Part 1: Dilution and Seeding Method with Allylthiourea Addition. ISO: London, UK, 2019.
- Karube, I.; Matsunaga, T.; Mitsuda, S.; Suzuki, S. Microbial Electrode BOD Sensors. Biotechnol. Bioeng. 1977, 19, 1535–1547. [Google Scholar] [CrossRef]
- Arlyapov, V.A.; Plekhanova, Y.V.; Kamanina, O.A.; Nakamura, H.; Reshetilov, A.N. Microbial Biosensors for Rapid Determination of Biochemical Oxygen Demand: Approaches, Tendencies and Development Prospects. Biosensors 2022, 12, 842. [Google Scholar] [CrossRef]
- Yudina, N.Y.; Arlyapov, V.A.; Chepurnova, M.A.; Alferov, S.V.; Reshetilov, A.N. A Yeast Co-Culture-Based Biosensor for Determination of Waste Water Contamination Levels. Enzyme Microb. Technol. 2015, 78, 46–53. [Google Scholar] [CrossRef]
- Arlyapov, V.; Kamanin, S.; Ponamoreva, O.; Reshetilov, A. Biosensor Analyzer for BOD Index Express Control on the Basis of the Yeast Microorganisms Candida Maltosa, Candida Blankii, and Debaryomyces Hansenii. Enzyme Microb. Technol. 2012, 50, 215–220. [Google Scholar] [CrossRef]
- Zaitseva, A.S.; Arlyapov, V.A.; Yudina, N.Y.; Alferov, S.V.; Reshetilov, A.N. Use of One- and Two-Mediator Systems for Developing a BOD Biosensor Based on the Yeast Debaryomyces Hansenii. Enzyme Microb. Technol. 2017, 98, 43–51. [Google Scholar] [CrossRef]
- Arlyapov, V.A.; Yudina, N.Y.; Asulyan, L.D.; Alferov, S.V.; Alferov, V.A.; Reshetilov, A.N. BOD Biosensor Based on the Yeast Debaryomyces Hansenii Immobilized in Poly(Vinyl Alcohol) Modified by N-Vinylpyrrolidone. Enzyme Microb. Technol. 2013, 53, 257–262. [Google Scholar] [CrossRef]
- Melnikov, P.V.; Alexandrovskaya, A.Y.; Naumova, A.O.; Arlyapov, V.A.; Kamanina, O.A.; Popova, N.M.; Zaitsev, N.K.; Yashtulov, N.A. Optical Oxygen Sensing and Clark Electrode: Face-to-Face in a Biosensor Case Study. Sensors 2022, 22, 7626. [Google Scholar] [CrossRef]
- Arlyapov, V.A.; Yudina, N.Y.; Machulin, A.V.; Alferov, V.A.; Ponamoreva, O.N.; Reshetilov, A.N. A Biosensor Based Microorganisms Immobilized in Layer-by-Layer Films for the Determination of Biochemical Oxygen Demand. Appl. Biochem. Microbiol. 2021, 57, 133–141. [Google Scholar] [CrossRef]
- Kharkova, A.S.; Arlyapov, V.A.; Turovskaya, A.D.; Avtukh, A.N.; Starodumova, I.P.; Reshetilov, A.N. Mediator BOD Biosensor Based on Cells of Microorganisms Isolated from Activated Sludge. Appl. Biochem. Microbiol. 2019, 55, 189–197. [Google Scholar] [CrossRef]
- Arlyapov, V.A.; Kharkova, A.S.; Kurbanaliyeva, S.K.; Kuznetsova, L.S.; Machulin, A.V.; Tarasov, S.E.; Melnikov, P.V.; Ponamoreva, O.N.; Alferov, V.A.; Reshetilov, A.N. Use of Biocompatible Redox-Active Polymers Based on Carbon Nanotubes and Modified Organic Matrices for Development of a Highly Sensitive BOD Biosensor. Enzyme Microb. Technol. 2021, 143, 109706. [Google Scholar] [CrossRef]
- Kharkova, A.S.; Medvedeva, A.S.; Kuznetsova, L.S.; Gertsen, M.M.; Kolesov, V.V.; Arlyapov, V.A.; Reshetilov, A.N. A “2-in-1” Bioanalytical System Based on Nanocomposite Conductive Polymers for Early Detection of Surface Water Pollution. Polymers 2024, 16, 1431. [Google Scholar] [CrossRef]
- Kurbanalieva, S.; Arlyapov, V.; Kharkova, A.; Perchikov, R.; Kamanina, O.; Melnikov, P.; Popova, N.; Machulin, A.; Tarasov, S.; Saverina, E.; et al. Electroactive Biofilms of Activated Sludge Microorganisms on a Nanostructured Surface as the Basis for a Highly Sensitive Biochemical Oxygen Demand Biosensor. Sensors 2022, 22, 6049. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Liu, Y.; Liu, H. Hibernations of Electroactive Bacteria Provide Insights into the Flexible and Robust BOD Detection Using Microbial Fuel Cell-Based Biosensors. Sci. Total Environ. 2021, 753, 142244. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liao, C.; Meng, X.; Zhao, Q.; Yan, X.; Tian, L.; Liu, Y.; Li, N.; Wang, X. Switchover of Electrotrophic and Heterotrophic Respirations Enables the Biomonitoring of Low Concentration of BOD in Oxygen-Rich Environment. Water Res. 2023, 235, 119897. [Google Scholar] [CrossRef]
- Yan, X.; Wang, Z.; Liao, C.; Su, H.; Zhao, Q.; Tian, L.; Li, N.; Wang, X. Understanding the Tail Current Behavior of Electroactive Biofilms Realizes the Rapid Measurement of Biochemical Oxygen Demand. Environ. Sci. Technol. 2024, 58, 2881–2890. [Google Scholar] [CrossRef] [PubMed]
- Perchikov, R.; Cheliukanov, M.; Plekhanova, Y.; Tarasov, S.; Kharkova, A.; Butusov, D.; Arlyapov, V.; Nakamura, H.; Reshetilov, A. Microbial Biofilms: Features of Formation and Potential for Use in Bioelectrochemical Devices. Biosensors 2024, 14, 302. [Google Scholar] [CrossRef]
- Kharkova, A.; Perchikov, R.; Kurbanalieva, S.; Osina, K.; Popova, N.; Machulin, A.; Kamanina, O.; Saverina, E.; Saltanov, I.; Melenkov, S.; et al. Targeted Formation of Biofilms on the Surface of Graphite Electrodes as an Effective Approach to the Development of Biosensors for Early Warning Systems. Biosensors 2024, 14, 239. [Google Scholar] [CrossRef]
- Wang, L.; Lv, H.; Yang, Q.; Chen, Y.; Wei, J.; Chen, Y.; Peng, C.; Liu, C.; Xu, X.; Jia, J. A Universal Biofilm Reactor Sensor for the Determination of Biochemical Oxygen Demand of Different Water Areas. Molecules 2022, 27, 5046. [Google Scholar] [CrossRef]
- Yi, Y.; Zhao, T.; Xie, B.; Zang, Y.; Liu, H. Dual Detection of Biochemical Oxygen Demand and Nitrate in Water Based on Bidirectional Shewanella loihica Electron Transfer. Bioresour. Technol. 2020, 309, 123402. [Google Scholar] [CrossRef]
- Cheng, S.; Lin, Z.; Sun, Y.; Li, H.; Ren, X. Fast and Simultaneous Detection of Dissolved BOD and Nitrite in Wastewater by Using Bioelectrode with Bidirectional Extracellular Electron Transport. Water Res. 2022, 213, 118186. [Google Scholar] [CrossRef]
- Kharkova, A.S.; Arlyapov, V.A.; Ilyukhina, A.S.; Ponamoreva, O.N.; Alferov, V.A.; Reshetilov, A.N. A Kinetic Approach to the Formation of Two-Mediator Systems for Developing Microbial Biosensors as Exemplified by a Rapid Biochemical Oxygen Demand Assay. 3 Biotech 2021, 11, 222. [Google Scholar] [CrossRef]
- Kuznetsova, L.S.; Arlyapov, V.A.; Plekhanova, Y.V.; Tarasov, S.E.; Kharkova, A.S.; Saverina, E.A.; Reshetilov, A.N. Conductive Polymers and Their Nanocomposites: Application Features in Biosensors and Biofuel Cells. Polymers 2023, 15, 3783. [Google Scholar] [CrossRef] [PubMed]
- Arlyapov, V.A.; Khar’kova, A.S.; Abramova, T.N.; Kuznetsova, L.S.; Ilyukhina, A.S.; Zaitsev, M.G.; Machulin, A.V.; Reshetilov, A.N. A Hybrid Redox-Active Polymer Based on Bovine Serum Albumin, Ferrocene, Carboxylated Carbon Nanotubes, and Glucose Oxidase. J. Anal. Chem. 2020, 75, 1189–1200. [Google Scholar] [CrossRef]
- Algethami, F.K.; Rabti, A.; Mastouri, M.; Ben Aoun, S.; Alqarni, L.S.; Elamin, M.R.; Raouafi, N. Sub-Femtomolar Capacitance-Based Biosensing of Kanamycin Using Screen-Printed Electrodes Coated with Redox-Active Polymeric Films. Microchim. Acta 2023, 190, 434. [Google Scholar] [CrossRef]
- Navarro-Nateras, L.; Diaz-Gonzalez, J.; Aguas-Chantes, D.; Coria-Oriundo, L.L.; Battaglini, F.; Ventura-Gallegos, J.L.; Zentella-Dehesa, A.; Oza, G.; Arriaga, L.G.; Casanova-Moreno, J.R. Development of a Redox-Polymer-Based Electrochemical Glucose Biosensor Suitable for Integration in Microfluidic 3D Cell Culture Systems. Biosensors 2023, 13, 582. [Google Scholar] [CrossRef]
- Medvedeva, A.S.; Dyakova, E.I.; Kuznetsova, L.S.; Mironov, V.G.; Gurkin, G.K.; Rogova, T.V.; Kharkova, A.S.; Melnikov, P.V.; Naumova, A.O.; Butusov, D.N.; et al. A Two-Mediator System Based on a Nanocomposite of Redox-Active Polymer Poly(Thionine) and SWCNT as an Effective Electron Carrier for Eukaryotic Microorganisms in Biosensor Analyzers. Polymers 2023, 15, 3335. [Google Scholar] [CrossRef] [PubMed]
- Arlyapov, V.A.; Yudina, N.Y.; Asulyan, L.D.; Kamanina, O.A.; Alferov, S.V.; Shumsky, A.N.; Machulin, A.V.; Alferov, V.A.; Reshetilov, A.N. Registration of BOD Using Paracoccus Yeei Bacteria Isolated from Activated Sludge. 3 Biotech 2020, 10, 207. [Google Scholar] [CrossRef]
- Kamanina, O.A.; Lantsova, E.A.; Rybochkin, P.V.; Arlyapov, V.A.; Saverina, E.A.; Kulikovskaya, N.S.; Perepukhov, A.M.; Vereshchagin, A.N.; Ananikov, V.P. “3-in-1” Hybrid Biocatalysts: Association of Yeast Cells Immobilized in a Sol-Gel Matrix for Determining Sewage Pollution. ACS Appl. Mater. Interfaces 2023, 15, 47779–47789. [Google Scholar] [CrossRef]
- Kamanina, O.A.; Lantsova, E.A.; Rybochkin, P.V.; Arlyapov, V.A.; Plekhanova, Y.V.; Reshetilov, A.N. The Use of Diethoxydimethylsilane as the Basis of a Hybrid Organosilicon Material for the Production of Biosensitive Membranes for Sensory Devices. Membranes 2022, 12, 983. [Google Scholar] [CrossRef]
- Kamanina, O.; Arlyapov, V.; Rybochkin, P.; Lavrova, D.; Podsevalova, E.; Ponamoreva, O. Application of Organosilicate Matrix Based on Methyltriethoxysilane, PVA and Bacteria Paracoccus Yeei to Create a Highly Sensitive BOD. 3 Biotech 2021, 11, 331. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Wan, N.; Fu, T.; Wang, L.; Li, C.; Qie, Z.; Zhu, A. A Current Sensing Biosensor for BOD Rapid Measurement. Archaea 2020, 2020, 8894925. [Google Scholar] [CrossRef]
- Gao, Y.; Yin, F.; Ma, W.; Wang, S.; Liu, Y.; Liu, H. Rapid Detection of Biodegradable Organic Matter in Polluted Water with Microbial Fuel Cell Sensor: Method of Partial Coulombic Yield. Bioelectrochemistry 2020, 133, 107488. [Google Scholar] [CrossRef]
- Alferov, S.V.; Arlyapov, V.A.; Alferov, V.A.; Reshetilov, A.N. Biofuel Cell Based on Bacteria of the Genus Gluconobacter as a Sensor for Express Analysis of Biochemical Oxygen Demand. Appl. Biochem. Microbiol. 2018, 54, 689–694. [Google Scholar] [CrossRef]
- Wang, S.; Tian, S.; Zhang, P.; Ye, J.; Tao, X.; Li, F.; Zhou, Z.; Nabi, M. Enhancement of Biological Oxygen Demand Detection with a Microbial Fuel Cell Using Potassium Permanganate as Cathodic Electron Acceptor. J. Environ. Manag. 2019, 252, 109682. [Google Scholar] [CrossRef]
- Ma, Y.; Deng, D.; Zhan, Y.; Cao, L.; Liu, Y. A Systematic Study on Self-Powered Microbial Fuel Cell Based BOD Biosensors Running under Different Temperatures. Biochem. Eng. J. 2022, 180, 108372. [Google Scholar] [CrossRef]
- Xiao, N.; Wu, R.; Huang, J.J.; Selvaganapathy, P.R. Development of a Xurographically Fabricated Miniaturized Low-Cost, High-Performance Microbial Fuel Cell and Its Application for Sensing Biological Oxygen Demand. Sens. Actuators B Chem. 2020, 304, 127432. [Google Scholar] [CrossRef]
- Xiao, N.; Selvaganapathy, P.R.; Wu, R.; Huang, J.J. Influence of Wastewater Microbial Community on the Performance of Miniaturized Microbial Fuel Cell Biosensor. Bioresour. Technol. 2020, 302, 122777. [Google Scholar] [CrossRef]
- Wang, Z.; Liao, C.; Zhong, Z.; Liu, S.; Li, M.; Wang, X. Design, Optimization and Application of a Highly Sensitive Microbial Electrolytic Cell-Based BOD Biosensor. Environ. Res. 2023, 216, 114533. [Google Scholar] [CrossRef]
- Lin, H.; Xu, N.; Xing, G.; Shang, Y.; Wang, X.; Lin, L. Microfluidic Chip-Based Microbial Metabolism-Indexed BOD Sensor for Rapid Determination of Biochemical Oxygen Demand. Sens. Actuators B Chem. 2024, 400, 134868. [Google Scholar] [CrossRef]
- Ching, P.M.L.; Zou, X.; Wu, D.; So, R.H.Y.; Chen, G.H. Development of a Wide-Range Soft Sensor for Predicting Wastewater BOD5 Using an EXtreme Gradient Boosting (XGBoost) Machine. Environ. Res. 2022, 210, 112953. [Google Scholar] [CrossRef]
- Medvedev, I.; Kornaukhova, M.; Galazis, C.; Lóránt, B.; Tardy, G.M.; Losev, A.; Goryanin, I. Using AI and BES/MFC to Decrease the Prediction Time of BOD5 Measurement. Environ. Monit. Assess. 2023, 195, 1018. [Google Scholar] [CrossRef]
- Eltzov, E.; Slobodnik, V.; Ionescu, R.E.; Marks, R.S. On-Line Biosensor for the Detection of Putative Toxicity in Water Contaminants. Talanta 2015, 132, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Azizullah, A.; Häder, D.-P. A Comparison of Commonly Used and Commercially Available Bioassays for Aquatic Ecosystems. Bioassays 2018, 347–368. [Google Scholar] [CrossRef]
- Naik, S.; Jujjavarapu, S.E. Self-Powered and Reusable Microbial Fuel Cell Biosensor for Toxicity Detection in Heavy Metal Polluted Water. J. Environ. Chem. Eng. 2021, 9, 105318. [Google Scholar] [CrossRef]
- Eltzov, E.; Yehuda, A.; Marks, R.S. Creation of a New Portable Biosensor for Water Toxicity Determination. Sens. Actuators B Chem. 2015, 221, 1044–1054. [Google Scholar] [CrossRef]
- Bose, S.; Maity, S.; Sarkar, A. Review of Microbial Biosensor for the Detection of Mercury in Water. Environ. Qual. Manag. 2022, 31, 29–40. [Google Scholar] [CrossRef]
- Adekunle, A.; Bambace, S.; Tanguay-Rioux, F.; Tartakovsky, B. Microbial Fuel Cell Biosensor with Capillary Carbon Source Delivery for Real-Time Toxicity Detection. Sensors 2023, 23, 7065. [Google Scholar] [CrossRef]
- Yudina, N.Y.; Kozlova, T.N.; Bogachikhin, D.A.; Kosarenina, M.M.; Arlyapov, V.A.; Alferov, S.V. Electrochemical Biosensors for Express Analysis of the Integral Toxicity of Polymer Materials. Biosensors 2023, 13, 1011. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Fang, D.; Yu, Y.; Wu, L.; Wang, Y.; Zhi, J. A Double-Mediator Based Whole Cell Electrochemical Biosensor for Acute Biotoxicity Assessment of Wastewater. Talanta 2017, 167, 208–216. [Google Scholar] [CrossRef]
- Kharkova, A.S.; Arlyapov, V.A.; Turovskaya, A.D.; Shvets, V.I.; Reshetilov, A.N. A Mediator Microbial Biosensor for Assaying General Toxicity. Enzyme Microb. Technol. 2020, 132, 109435. [Google Scholar] [CrossRef]
- Kharkova, A.; Arlyapov, V.; Medvedeva, A.; Lepikash, R.; Melnikov, P.; Reshetilov, A. Mediator Microbial Biosensor Analyzers for Rapid Determination of Surface Water Toxicity. Sensors 2022, 22, 8522. [Google Scholar] [CrossRef]
- Liu, X.; Germaine, K.J.; Ryan, D.; Dowling, D.N. Whole-Cell Fluorescent Biosensors for Bioavailability and Biodegradation of Polychlorinated Biphenyls. Sensors 2010, 10, 1377–1398. [Google Scholar] [CrossRef] [PubMed]
- Stenzler, B.R.; Gaudet, J.; Poulain, A.J. An Anaerobic Biosensor Assay for the Detection of Mercury and Cadmium. J. Vis. Exp. 2018, 2018, 58324. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, P.; Niu, Y.; Chen, Z.; Khan, A.; Zhang, P.; Li, X. A Novel Early Warning System Based on a Sediment Microbial Fuel Cell for in Situ and Real Time Hexavalent Chromium Detection in Industrial Wastewater. Sensors 2018, 18, 642. [Google Scholar] [CrossRef]
- Käkinen, A.; Bondarenko, O.; Ivask, A.; Kahru, A. The Effect of Composition of Different Ecotoxicological Test Media on Free and Bioavailable Copper from CuSO4 and CuO Nanoparticles: Comparative Evidence from a Cu-Selective Electrode and a Cu-Biosensor. Sensors 2011, 11, 10502–10521. [Google Scholar] [CrossRef]
- McCourt, K.M.; Cochran, J.; Abdelbasir, S.M.; Carraway, E.R.; Tzeng, T.R.J.; Tsyusko, O.V.; Vanegas, D.C. Potential Environmental and Health Implications from the Scaled-Up Production and Disposal of Nanomaterials Used in Biosensors. Biosensors 2022, 12, 1082. [Google Scholar] [CrossRef]
- Aynalem, B.; Muleta, D. Microbial Biosensors as Pesticide Detector: An Overview. J. Sensors 2021, 2021, 5538857. [Google Scholar] [CrossRef]
- Qin, J.; Niu, A.; Liu, Y.; Lin, C. Arsenic in Leafy Vegetable Plants Grown on Mine Water-Contaminated Soils: Uptake, Human Health Risk and Remedial Effects of Biochar. J. Hazard. Mater. 2021, 402, 123488. [Google Scholar] [CrossRef]
- Anjum, M.; Siddique, N.; Younis, H.; Faiz, Y.; Shafique, M.A.; Mahnoor, A.; Sajid, A.; Altaf, M. Heavy Metals and Radionuclides in Islamabad’s Industrial Area: A Comprehensive Analysis of Soil and Water Pollution, Source Apportionment and Health Effects Using Statistical and Geospatial Tools. J. Trace Elem. Miner. 2024, 8, 100127. [Google Scholar] [CrossRef]
- Saeedi, R.; Sadeghi, S.; Massoudinejad, M.; Oroskhan, M.; Mohagheghian, A.; Mohebbi, M.; Abtahi, M. Assessing Drinking Water Quality Based on Water Quality Indices, Human Health Risk, and Burden of Disease Attributable to Heavy Metals in Rural Communities of Yazd County, Iran, 2015–2021. Heliyon 2024, 10, e33984. [Google Scholar] [CrossRef]
- Perelomov, L.; Sizova, O.; Gertsen, M.; Perelomova, I.; Arlyapov, V.; Atroshchenko, Y. Antibiotic Resistance in Metal-Tolerant Microorganisms from Treatment Facilities. Antibiotics 2023, 12, 1678. [Google Scholar] [CrossRef]
- Fu, Z.; Xi, S. The Effects of Heavy Metals on Human Metabolism. Toxicol. Mech. Methods 2020, 30, 167–176. [Google Scholar] [CrossRef]
- Tang, B.; Xu, H.; Song, F.; Ge, H.; Yue, S. Effects of Heavy Metals on Microorganisms and Enzymes in Soils of Lead–Zinc Tailing Ponds. Environ. Res. 2022, 207, 112174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, Z.; Guo, K.; Huo, Y.; He, G.; Sun, H.; Guan, Y.; Xu, N.; Yang, W.; Sun, G. Toxic Effects of Heavy Metal Cd and Zn on Chlorophyll, Carotenoid Metabolism and Photosynthetic Function in Tobacco Leaves Revealed by Physiological and Proteomics Analysis. Ecotoxicol. Environ. Saf. 2020, 202, 110856. [Google Scholar] [CrossRef]
- Duan, W.; Xu, C.; Liu, Q.; Xu, J.; Weng, Z.; Zhang, X.; Basnet, T.B.; Dahal, M.; Gu, A. Levels of a Mixture of Heavy Metals in Blood and Urine and All-Cause, Cardiovascular Disease and Cancer Mortality: A Population-Based Cohort Study. Environ. Pollut. 2020, 263, 114630. [Google Scholar] [CrossRef]
- PRC Minestry of Health. Standards for Drinking Water Quality GB 5749-2006; National Standard of the People’s Republic of China: Beijing, China, 2006.
- Zhong, J.; Wang, Z.; Chen, Y.; Huan, W.; Shi, M.; Lei, L.; Yu, X.; Chen, L. Determination of Trace Heavy Metal Elements in Litterfall by Inductively Coupled Plasma Optical Emission Spectrometry after Extraction Using Choline Chloride-Based Deep Eutectic Solvents. RSC Adv. 2024, 14, 22497–22503. [Google Scholar] [CrossRef] [PubMed]
- Emily, E.C.; de Castro Dantas, T.N.; da Silva, D.R.; Maranhão, T.A. A New Method for Extraction and Pre-Concentration of the Heavy Metals Using a Microemulsion System in Winsor II Equilibrium and Determination by HR-CS AAS. Chemosphere 2023, 338, 139428. [Google Scholar] [CrossRef]
- Hu, S.; Hui, C.; Wu, C.; Gao, C.; Huang, Z.; Guo, Y. Dual-Colored Bacterial Biosensor Responsive to Cadmium, Mercury, and Lead for Detecting Heavy Metal Pollution in Seawater. Ecol. Indic. 2024, 166, 112244. [Google Scholar] [CrossRef]
- Adekunle, A.; Raghavan, V.; Tartakovsky, B. On-Line Monitoring of Heavy Metals-Related Toxicity with a Microbial Fuel Cell Biosensor. Biosens. Bioelectron. 2019, 132, 382–390. [Google Scholar] [CrossRef]
- Li, J.; Cui, M.; Zhao, J.; Wang, J.; Fang, X. A Self-Amplifying Plasmid Based Ultrasensitive Biosensor for the Detection of As(III) in Water. Biosens. Bioelectron. 2023, 221, 114937. [Google Scholar] [CrossRef]
- Wang, J.; Yang, X.; Cui, M.; Liu, Y.; Li, X.; Zhang, L.; Zhan, G. A High-Sensitive and Durable Electrochemical Sensor Based on Geobacter-Dominated Biofilms for Heavy Metal Toxicity Detection. Biosens. Bioelectron. 2022, 206, 114146. [Google Scholar] [CrossRef]
- Liu, M.; Dong, J.; Suo, Z.; Wang, Q.; Wei, M.; He, B.; Jin, H. A Convenient Fluorescent/Electrochemical Dual-Mode Biosensor for Accurate Detection of Pb2+ Based on DNAzyme Cycle. Bioelectrochemistry 2023, 152, 108452. [Google Scholar] [CrossRef] [PubMed]
- Rathnayake, I.V.N.; Megharaj, M.; Naidu, R. Green Fluorescent Protein Based Whole Cell Bacterial Biosensor for the Detection of Bioavailable Heavy Metals in Soil Environment. Environ. Technol. Innov. 2021, 23, 101785. [Google Scholar] [CrossRef]
- Wei, Y.; Shi, D.; Chen, T.; Zhou, S.; Yang, Z.; Li, H.; Yang, D.; Li, J.; Jin, M. CRISPR/Cas9-Based Engineered Escherichia coli Biosensor for Sensitive and Specific Detection of Cd(II) in Drinking Water. Chemosphere 2024, 362, 142607. [Google Scholar] [CrossRef]
- Iravani, S.; Ahmadzadeh, H.; Sajjadizadeh, H.S.; Rounaghi, G. Scenedesmus Sp. MN738556 as a Whole Cell Biosensor for Sub-Part per Billion Detections of Cd2+ Cations in Freshwater Sources. Microchem. J. 2024, 197, 109836. [Google Scholar] [CrossRef]
- Guo, Y.; Hui, C.; Liu, L.; Chen, M.; Huang, H. Development of a Bioavailable Hg(II) Sensing System Based on MerR-Regulated Visual Pigment Biosynthesis. Sci. Rep. 2021, 11, 13516. [Google Scholar] [CrossRef]
- Köksaldı, İ.Ç.; Avcı, E.; Köse, S.; Özkul, G.; Kehribar, E.Ş.; Şafak Şeker, U.Ö. Genetically Engineered Bacterial Biofilm Materials Enhances Portable Whole Cell Sensing. Biosens. Bioelectron. 2024, 264, 116644. [Google Scholar] [CrossRef] [PubMed]
- Cowan-Ellsberry, C.; Belanger, S.; Dorn, P.; Dyer, S.; Mcavoy, D.; Sanderson, H.; Versteeg, D.; Ferrer, D.; Stanton, K. Environmental Safety of the Use of Major Surfactant Classes in North America. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1893–1993. [Google Scholar] [CrossRef]
- Perelomov, L.; Gertsen, M.; Burachevskaya, M.; Hemalatha, S.; Vijayalakshmi, A.; Perelomova, I.; Atroshchenko, Y. Organoclays Based on Bentonite and Various Types of Surfactants as Heavy Metal Remediants. Sustainability 2024, 16, 4804. [Google Scholar] [CrossRef]
- Wen, T.; Li, N.B.; Luo, H.Q. A Turn-on Fluorescent Sensor for Sensitive and Selective Detection of Sodium Dodecyl Sulfate Based on the Eosin Y/Polyethyleneimine System. Anal. Chem. 2013, 85, 10863–10868. [Google Scholar] [CrossRef]
- Gertsen, M.; Perelomov, L.; Kharkova, A.; Burachevskaya, M.; Hemalatha, S.; Atroshchenko, Y. Removal of Lead Cations by Novel Organoclays Derived from Bentonite and Amphoteric and Nonionic Surfactants. Toxics 2024, 12, 713. [Google Scholar] [CrossRef]
- Liwarska-Bizukojc, E.; Miksch, K.; Malachowska-Jutsz, A.; Kalka, J. Acute Toxicity and Genotoxicity of Five Selected Anionic and Nonionic Surfactants. Chemosphere 2005, 58, 1249–1253. [Google Scholar] [CrossRef] [PubMed]
- Rebello, S.; Asok, A.K.; Mundayoor, S.; Jisha, M.S. Surfactants: Toxicity, Remediation and Green Surfactants. Environ. Chem. Lett. 2014, 12, 275–287. [Google Scholar] [CrossRef]
- Rosety, M.; Ordóñez, F.J.; Rosety-Rodríguez, M.; Rosety, J.M.; Rosety, I.; Carrasco, C.; Ribelles, A. Acute Toxicity of Anionic Surfactants Sodium Dodecyl Sulphate (SDS) and Linear Alkylbenzene Sulphonate (LAS) on the Fertilizing Capability of Gilthead (Sparus aurata L.) Sperm. Histol. Histopathol. 2001, 16, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Baba, S.A.; Bhatt, A.; Dhyani, R.; Navani, N.K. Transcription Factor Based Whole-Cell Biosensor for Specific and Sensitive Detection of Sodium Dodecyl Sulfate. Biosens. Bioelectron. 2020, 170, 112659. [Google Scholar] [CrossRef]
- Badmus, S.O.; Amusa, H.K.; Oyehan, T.A.; Saleh, T.A. Environmental Risks and Toxicity of Surfactants: Overview of Analysis, Assessment, and Remediation Techniques. Environ. Sci. Pollut. Res. 2021, 28, 62085–62104. [Google Scholar] [CrossRef]
- Uhan, H.; Panhwar, S.; Boyaci, I.H.; Tamer, U. Optical Based Transducers for Biosensors. In Biosensors: Fundamentals, Emerging Technologies, and Applications; CRC Press: Boca Raton, FL, USA, 2022; pp. 57–87. [Google Scholar] [CrossRef]
- Velichko, N.S.; Guliy, O.I.; Kanevsky, M.V.; Kupryashina, M.A.; Fedonenko, Y.P. Whole-Cell Electric Sensor for Determination of Sodium Dodecyl Sulfate. World J. Microbiol. Biotechnol. 2022, 38, 118. [Google Scholar] [CrossRef]
- Keane, A.; Lau, P.C.K.; Ghoshal, S. Use of a Whole-Cell Biosensor to Assess the Bioavailability Enhancement of Aromatic Hydrocarbon Compounds by Nonionic Surfactants. Biotechnol. Bioeng. 2008, 99, 86–98. [Google Scholar] [CrossRef]
- Pardhi, D.S.; Panchal, R.R.; Raval, V.H.; Joshi, R.G.; Poczai, P.; Almalki, W.H.; Rajput, K.N. Microbial Surfactants: A Journey from Fundamentals to Recent Advances. Front. Microbiol. 2022, 13, 982603. [Google Scholar] [CrossRef]
- Lim, J.W.; Ha, D.; Lee, J.; Lee, S.K.; Kim, T. Review of Micro/Nanotechnologies for Microbial Biosensors. Front. Bioeng. Biotechnol. 2015, 3, 61. [Google Scholar] [CrossRef]
- Kumari, R.; Singha, L.P.; Shukla, P. Biotechnological Potential of Microbial Bio-Surfactants, Their Significance, and Diverse Applications. FEMS Microbes 2023, 4, xtad015. [Google Scholar] [CrossRef]
- Nomura, Y.; Ikebukuro, K.; Yokoyama, K.; Takeuchi, T.; Karube, I.; Arikawa, Y.; Ohno, S. A Novel Microbial Sensor for Anionic Surfactant Determination. Anal. Lett. 1994, 27, 3095–3108. [Google Scholar] [CrossRef]
- Taranova, L.; Semenchuk, I.; Manolov, T.; Iliasov, P.; Reshetilov, A. Bacteria-Degraders as the Base of an Amperometric Biosensor for Detection of Anionic Surfactants. Biosens. Bioelectron. 2002, 17, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Moutcine, A.; Laghlimi, C.; Ziat, Y.; Isaad, J.; El Bahraoui, S.; Chtaini, A. Electroanalytical Analysis of Phenol Oxidation Using Bacteria Immobilized by a Polycaprolactone Coating on the Copper Electrode Surface. Sci. Rep. 2024, 14, 13136. [Google Scholar] [CrossRef] [PubMed]
- Manan, F.A.A.; Hong, W.W.; Abdullah, J.; Yusof, N.A.; Ahmad, I. Nanocrystalline Cellulose Decorated Quantum Dots Based Tyrosinase Biosensor for Phenol Determination. Mater. Sci. Eng. C 2019, 99, 37–46. [Google Scholar] [CrossRef]
- Duan, W.; Meng, F.; Cui, H.; Lin, Y.; Wang, G.; Wu, J. Ecotoxicity of Phenol and Cresols to Aquatic Organisms: A Review. Ecotoxicol. Environ. Saf. 2018, 157, 441–456. [Google Scholar] [CrossRef]
- Mohamad Said, K.A.; Ismail, A.F.; Abdul Karim, Z.; Abdullah, M.S.; Hafeez, A. A Review of Technologies for the Phenolic Compounds Recovery and Phenol Removal from Wastewater. Process Saf. Environ. Prot. 2021, 151, 257–289. [Google Scholar] [CrossRef]
- Huang, L.; Liang, J.; Zhang, J. Screening of an Extremely Efficient Phenol-Degrading Bacterial Consortia HQ-01 and Its Superior Adaptability to Temperature and Phenol Concentration. J. Water Process Eng. 2024, 59, 104970. [Google Scholar] [CrossRef]
- PND F 14.1:2.105-97; Quantitative Chemical Analysis of Waters. A Method For Measuring The Mass Concentration of Volatile Phenols in Natural and Treated Wastewater by Photometric Method After Steam Distillation. Gosstandart of Russia: Moscow, Russia, 1997; pp. 1–19.
- MUK 4.1.737-99-4.1.754-99; Determination of Concentrations of Chemicals in the Water of Centralized Drinking Water Supply Systems. Federal Center for State Sanitary and Epidemiological Supervision of the Ministry of Health of the Russian Federation: Moscow, Russia, 1999.
- E.R.F. The Method of Measuring the Mass Concentration (Total) of Volatile Phenols in Samples of Natural and Treated Wastewater by Accelerated Extraction-Photometric Method without Distillation. E.R.F. 14.1:2.104-97; E.R.F.: College Park, MD, USA, 2004; ISBN 4215001455. [Google Scholar]
- Bounegru, A.V.; Iticescu, C.; Georgescu, L.P.; Apetrei, C. Development of an Innovative Biosensor Based on Graphene/PEDOT/Tyrosinase for the Detection of Phenolic Compounds in River Waters. Int. J. Mol. Sci. 2024, 25, 4419. [Google Scholar] [CrossRef]
- Perchikov, R.N.; Provotorova, D.V.; Kharkova, A.S.; Arlyapov, V.A.; Medvedeva, A.S.; Machulin, A.V.; Filonov, A.E.; Reshetilov, A.N. Bioanalytical System for Determining the Phenol Index Based on Pseudomonas Putida BS394(PBS216) Bacteria Immobilized in a Redox-Active Biocompatible Composite Polymer “Bovine Serum Albumin–Ferrocene–Carbon Nanotubes”. Polymers 2022, 14, 5366. [Google Scholar] [CrossRef]
- Kolahchi, N.; Ebrahimipour, G.H.; Ranaei Siadat, S.O.; Jaffrezic-Renault, N. Application of Multi Walled Carbon Nanotubes (MWCNTs) in Phenol Biosensor Based on Bacterial Cells. Biol. J. Microorg. 2019, 8, 165–176. [Google Scholar]
- He, J.; Zhang, X.; Qian, Y.; Wang, Q.; Bai, Y. An Engineered Quorum-Sensing-Based Whole-Cell Biosensor for Active Degradation of Organophosphates. Biosens. Bioelectron. 2022, 206, 114085. [Google Scholar] [CrossRef]
- Jin, W.Y.; Guo, J.X.; Tang, R.; Wang, J.; Zhao, H.; Zhang, M.; Teng, L.Z.; Sansonetti, P.J.; Gao, Y.Z. In Vivo Detection of Endogenous Toxic Phenolic Compounds of Intestine. J. Hazard. Mater. 2024, 478, 135526. [Google Scholar] [CrossRef] [PubMed]
- Iswantini, D.; Ghozali, A.A.; Kusmana, C.; Nurhidayat, N. Evaluation of Bacterial Biofilm as Biosensor for Detecting Phenol, Catechol, and 1,2-Dihydroxynaphthalene. HAYATI J. Biosci. 2021, 28, 262–270. [Google Scholar] [CrossRef]
- Naik, S.; Eswari, J.S. Experimental and Validation with Neural Network Time Series Model of Microbial Fuel Cell Bio-Sensor for Phenol Detection. J. Environ. Manag. 2021, 290, 112594. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhou, T.; Khan, A.; Chen, Z.; Liu, P.; Li, X. A Novel Electrochemical Biosensor for Bisphenol A Detection Based on Engineered Escherichia coli Cells with a Surface-Display of Tyrosinase. Sens. Actuators B Chem. 2022, 353, 131063. [Google Scholar] [CrossRef]
- Benvidi, A.; Naserpour, F.; Zarnousheh Farahani, K.; Farasati Far, B.; Karooby, E.; Akbari, A. Sensitive Electrochemical Sensor Modified by Hydroquinone Derivative and Magnesium Oxide Nanoparticles for Simultaneous Determination of Hydroxylamine and Phenol. Arab. J. Sci. Eng. 2024, 49, 9307–9322. [Google Scholar] [CrossRef]
- Li, R.; Wan, Y.; Li, T.; Zhang, X.; Wang, J.; Zhou, L.; Li, N.; Wang, X. Establishment of Low-Carbon Microbial Network to Enhance Phenol Removal in Microbial Electrochemical Systems with Whole Process Carbon Source Limitation. Chem. Eng. J. 2023, 452, 139530. [Google Scholar] [CrossRef]
- Rajmohan, K.S.; Chandrasekaran, R.; Varjani, S. A Review on Occurrence of Pesticides in Environment and Current Technologies for Their Remediation and Management. Indian J. Microbiol. 2020, 60, 125–138. [Google Scholar] [CrossRef]
- Kalyabina, V.P.; Esimbekova, E.N.; Kopylova, K.V.; Kratasyuk, V.A. Pesticides: Formulants, Distribution Pathways and Effects on Human Health—A Review. Toxicol. Reports 2021, 8, 1179–1192. [Google Scholar] [CrossRef]
- Samsidar, A.; Siddiquee, S.; Shaarani, S.M. A Review of Extraction, Analytical and Advanced Methods for Determination of Pesticides in Environment and Foodstuffs. Trends Food Sci. Technol. 2018, 71, 188–201. [Google Scholar] [CrossRef]
- Farré, M.; Gonçalves, C.; Lacorte, S.; Barceló, D.; Alpendurada, M.F. Pesticide Toxicity Assessment Using an Electrochemical Biosensor with Pseudomonas Putida and a Bioluminescence Inhibition Assay with Vibrio Fischeri. Anal. Bioanal. Chem. 2002, 373, 696–703. [Google Scholar] [CrossRef] [PubMed]
- May, K.M.L.; Wang, Y.; Bachas, L.G.; Anderson, K.W. Development of a Whole-Cell-Based Biosensor for Detecting Histamine as a Model Toxin. Anal. Chem. 2004, 76, 4156–4161. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Mulchandani, P.; Wang, J.; Chen, W.; Mulchandani, A. Highly Sensitive and Selective Amperometric Microbial Biosensor for Direct Determination of P-Nitrophenyl-Substituted Organophosphate Nerve Agents. Environ. Sci. Technol. 2005, 39, 8853–8857. [Google Scholar] [CrossRef] [PubMed]
- Shing, W.L.; Heng, L.Y.; Surif, S. Performance of a Cyanobacteria Whole Cell-Based Fluorescence Biosensor for Heavy Metal and Pesticide Detection. Sensors 2013, 13, 6394–6404. [Google Scholar] [CrossRef]
- Yong, D.; Liu, C.; Yu, D.; Dong, S. A Sensitive, Rapid and Inexpensive Way to Assay Pesticide Toxicity Based on Electrochemical Biosensor. Talanta 2011, 84, 7–12. [Google Scholar] [CrossRef]
- Whangsuk, W.; Thiengmag, S.; Dubbs, J.; Mongkolsuk, S.; Loprasert, S. Specific Detection of the Pesticide Chlorpyrifos by a Sensitive Genetic-Based Whole Cell Biosensor. Anal. Biochem. 2016, 493, 11–13. [Google Scholar] [CrossRef]
- Geng, F.; Ding, J.; Jia, C.; Ding, B.; Qin, W. A Potentiometric Biosensing System Based on an Isolated Degrading Bacterium Klebsiella Sp. MP-6 for the Determination of Methyl Parathion. RSC Adv. 2015, 5, 34475–34480. [Google Scholar] [CrossRef]
- Ma, Z.; Li, Y.; Lu, C.; Li, M. On-Site Screening Method for Bioavailability Assessment of the Organophosphorus Pesticide, Methyl Parathion, and Its Primary Metabolite in Soils by Paper Strip Biosensor. J. Hazard. Mater. 2023, 457, 131725. [Google Scholar] [CrossRef]
- Kesik, M.; Ekiz Kanik, F.; Turan, J.; Kolb, M.; Timur, S.; Bahadir, M.; Toppare, L. An Acetylcholinesterase Biosensor Based on a Conducting Polymer Using Multiwalled Carbon Nanotubes for Amperometric Detection of Organophosphorous Pesticides. Sens. Actuators B Chem. 2014, 205, 39–49. [Google Scholar] [CrossRef]
- Dzudzevic Cancar, H.; Soylemez, S.; Akpinar, Y.; Kesik, M.; Göker, S.; Gunbas, G.; Volkan, M.; Toppare, L. A Novel Acetylcholinesterase Biosensor: Core-Shell Magnetic Nanoparticles Incorporating a Conjugated Polymer for the Detection of Organophosphorus Pesticides. ACS Appl. Mater. Interfaces 2016, 8, 8058–8067. [Google Scholar] [CrossRef]
- Wei, M.; Wang, J. A Novel Acetylcholinesterase Biosensor Based on Ionic Liquids-AuNPs-Porous Carbon Composite Matrix for Detection of Organophosphate Pesticides. Sens. Actuators B Chem. 2015, 211, 290–296. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, X.; Ma, L.; Gao, J.; Jiang, Y. Acetylcholinesterase/Chitosan-Transition Metal Carbides Nanocomposites-Based Biosensor for the Organophosphate Pesticides Detection. Biochem. Eng. J. 2017, 128, 243–249. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, M. Development of Acetylcholinesterase Biosensor Based on Platinum-Carbon Aerogels Composite for Determination of Organophosphorus Pesticides. Food Control 2014, 36, 49–54. [Google Scholar] [CrossRef]
- Liang, B.; Wang, G.; Yan, L.; Ren, H.; Feng, R.; Xiong, Z.; Liu, A. Functional Cell Surface Displaying of Acetylcholinesterase for Spectrophotometric Sensing Organophosphate Pesticide. Sens. Actuators B Chem. 2019, 279, 483–489. [Google Scholar] [CrossRef]
- Hoyano, Y.; Tamashiro, I.; Akimoto, T. Fusion Proteins of Organophosphorus Hydrolase and PHluorin for a Whole-Cell Biosensor for Organophosphorus Pesticide Measurement. Anal. Sci. 2023, 39, 1515–1520. [Google Scholar] [CrossRef] [PubMed]
- Chouichit, P.; Whangsuk, W.; Sallabhan, R.; Mongkolsuk, S.; Loprasert, S. A Highly Sensitive Biosensor with a Single-Copy Evolved Sensing Cassette for Chlorpyrifos Pesticide Detection. Microbiology 2020, 166, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Tucci, M.; Grattieri, M.; Schievano, A.; Cristiani, P.; Minteer, S.D. Microbial Amperometric Biosensor for Online Herbicide Detection: Photocurrent Inhibition of Anabaena Variabilis. Electrochim. Acta 2019, 302, 102–108. [Google Scholar] [CrossRef]
- Buyukharman, M.; Mulazimoglu, I.E.; Yildiz, H.B. Construction of a Conductive Polymer/AuNP/Cyanobacteria-Based Biophotovoltaic Cell Harnessing Solar Energy to Generate Electricity via Photosynthesis and Its Usage as a Photoelectrochemical Pesticide Biosensor: Atrazine as a Case Study. ACS Omega 2024, 9, 16249–16261. [Google Scholar] [CrossRef]
- Chouler, J.; Di Lorenzo, M. Pesticide Detection by a Miniature Microbial Fuel Cell under Controlled Operational Disturbances. Water Sci. Technol. 2019, 79, 2231–2241. [Google Scholar] [CrossRef]
- Ritcharoon, B.; Sallabhan, R.; Toewiwat, N.; Mongkolsuk, S.; Loprasert, S. Detection of 2,4-Dichlorophenoxyacetic Acid Herbicide Using a FGE-Sulfatase Based Whole-Cell Agrobacterium Biosensor. J. Microbiol. Methods 2020, 175, 105997. [Google Scholar] [CrossRef]
- Mendes, F.; Miranda, E.; Amaral, L.; Carvalho, C.; Castro, B.B.; Sousa, M.J.; Chaves, S.R. Novel Yeast-Based Biosensor for Environmental Monitoring of Tebuconazole. Appl. Microbiol. Biotechnol. 2024, 108, 10. [Google Scholar] [CrossRef] [PubMed]
- Vanarsdale, E.; Tsao, C.Y.; Liu, Y.; Chen, C.Y.; Payne, G.F.; Bentley, W.E. Redox-Based Synthetic Biology Enables Electrochemical Detection of the Herbicides Dicamba and Roundup via Rewired Escherichia coli. ACS Sensors 2019, 4, 1180–1184. [Google Scholar] [CrossRef]
- Stöckel, S.; Kirchhoff, J.; Neugebauer, U.; Rösch, P.; Popp, J. The Application of Raman Spectroscopy for the Detection and Identification of Microorganisms. J. Raman Spectrosc. 2016, 47, 89–109. [Google Scholar] [CrossRef]
- Bittel, M.; Cordella, C.B.Y.; Assaf, A.; Jouanneau, S.; Durand, M.J.; Thouand, G. Potential of Raman Spectroscopy to Monitor Arsenic Toxicity on Bacteria: Insights toward Multiparametric Bioassays. Environ. Sci. Technol. 2015, 49, 12324–12332. [Google Scholar] [CrossRef]
- Madhu, R.; Veeramani, V.; Chen, S.M.; Veerakumar, P.; Liu, S.B.; Miyamoto, N. Functional Porous Carbon-ZnO Nanocomposites for High-Performance Biosensors and Energy Storage Applications. Phys. Chem. Chem. Phys. 2016, 18, 16466–16475. [Google Scholar] [CrossRef] [PubMed]
- Zu, T.N.K.; Athamneh, A.I.M.; Wallace, R.S.; Collakova, E.; Senger, R.S. Near-Real-Time Analysis of the Phenotypic Responses of Escherichia coli to 1-Butanol Exposure Using Raman Spectroscopy. J. Bacteriol. 2014, 196, 3983–3991. [Google Scholar] [CrossRef]
- Yang, Q.; Li, G.; Jin, N.; Zhang, D. Synergistic/Antagonistic Toxicity Characterization and Source-Apportionment of Heavy Metals and Organophosphorus Pesticides by the Biospectroscopy-Bioreporter-Coupling Approach. Sci. Total Environ. 2023, 905, 167057. [Google Scholar] [CrossRef]
- Bandeliuk, O.; Assaf, A.; Bittel, M.; Durand, M.J.; Thouand, G. Development and Automation of a Bacterial Biosensor to the Targeting of the Pollutants Toxic Effects by Portable Raman Spectrometer. Sensors 2022, 22, 4352. [Google Scholar] [CrossRef]
- Jia, X.; Bu, R.; Zhao, T.; Wu, K. Sensitive and Specific Whole-Cell Biosensor for Arsenic Detection. Appl. Environ. Microbiol. 2019, 85, e00694-19. [Google Scholar] [CrossRef]
- Valenzuela-García, L.I.; Alarcón-Herrera, M.T.; Ayala-García, V.M.; Barraza-Salas, M.; Salas-Pacheco, J.M.; Díaz-Valles, J.F.; Pedraza-Reyes, M. Design of a Whole-Cell Biosensor Based on Bacillus Subtilis Spores and the Green Fluorescent Protein To Monitor Arsenic. Microbiol. Spectr. 2023, 11, e00432-23. [Google Scholar] [CrossRef]
- Amaro, F.; Turkewitz, A.P.; Martín-González, A.; Gutiérrez, J.C. Whole-Cell Biosensors for Detection of Heavy Metal Ions in Environmental Samples Based on Metallothionein Promoters from Tetrahymena Thermophila. Microb. Biotechnol. 2011, 4, 513–522. [Google Scholar] [CrossRef]
- Gui, Q.; Lawson, T.; Shan, S.; Yan, L.; Liu, Y. The Application of Whole Cell-Based Biosensors for Use in Environmental Analysis and in Medical Diagnostics. Sensors 2017, 17, 1623. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, B.; Zhu, Z.; Xin, X.; Wu, H.; Chen, B. Microfluidic Based Whole-Cell Biosensors for Simultaneously On-Site Monitoring of Multiple Environmental Contaminants. Front. Bioeng. Biotechnol. 2021, 9, 622108. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.B.; Mitchell, R.J.; Kim, B.C. Whole-Cell-Based Biosensors for Environmental Biomonitoring and Application. Adv. Biochem. Eng. Biotechnol. 2004, 87, 269–305. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.C.; Amaro, F.; Martín-González, A. Heavy Metal Whole-Cell Biosensors Using Eukaryotic Microorganisms: An Updated Critical Review. Front. Microbiol. 2015, 6, 48. [Google Scholar] [CrossRef]
- Gao, Y.-Z.; Wang, Y.; Ji, M.; Zhou, N.-Y.; Huang, W.E. A Whole-Cell Hydrogen Peroxide Biosensor and Its Application in Visual Food Analysis. Innov. Life 2023, 1, 100011. [Google Scholar] [CrossRef]
- Ben-Yoav, H.; Biran, A.; Sternheim, M.; Belkin, S.; Freeman, A.; Shacham-Diamand, Y. Functional Modeling of Electrochemical Whole-Cell Biosensors. Sens. Actuators, B Chem. 2013, 181, 479–485. [Google Scholar] [CrossRef]
- Chen, S.; Chen, X.; Su, H.; Guo, M.; Liu, H. Advances in Synthetic-Biology-Based Whole-Cell Biosensors: Principles, Genetic Modules, and Applications in Food Safety. Int. J. Mol. Sci. 2023, 24, 7989. [Google Scholar] [CrossRef]
- Mohamed, E.F.; Awad, G. Development of Nano-Sensor and Biosensor as an Air Pollution Detection Technique for the Foreseeable Future. Compr. Anal. Chem. 2022, 99, 163–188. [Google Scholar] [CrossRef]
- Baek, S.H.; Kim, M.W.; Park, C.Y.; Choi, C.S.; Kailasa, S.K.; Park, J.P.; Park, T.J. Development of a Rapid and Sensitive Electrochemical Biosensor for Detection of Human Norovirus via Novel Specific Binding Peptides. Biosens. Bioelectron. 2019, 123, 223–229. [Google Scholar] [CrossRef]
- Cesewski, E.; Johnson, B.N. Electrochemical Biosensors for Pathogen Detection. Biosens. Bioelectron. 2020, 159, 112214. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, C.W.; Wang, D.; Wei, N. A Whole-Cell Biosensor for Point-of-Care Detection of Waterborne Bacterial Pathogens. ACS Synth. Biol. 2021, 10, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Rushworth, J.V.; Hirst, N.A.; Millner, P.A. Biosensors for Whole-Cell Bacterial Detection. Clin. Microbiol. Rev. 2014, 27, 631–646. [Google Scholar] [CrossRef]
- Kaya, H.O.; Cetin, A.E.; Azimzadeh, M.; Topkaya, S.N. Pathogen Detection with Electrochemical Biosensors: Advantages, Challenges and Future Perspectives. J. Electroanal. Chem. 2021, 882, 114989. [Google Scholar] [CrossRef]
- Majer-Baranyi, K.; Adányi, N.; Székács, A. Current Trends in Mycotoxin Detection with Various Types of Biosensors. Toxins 2023, 15, 645. [Google Scholar] [CrossRef]
- Szelenberger, R.; Cichoń, N.; Zajaczkowski, W.; Bijak, M. Application of Biosensors for the Detection of Mycotoxins for the Improvement of Food Safety. Toxins 2024, 16, 249. [Google Scholar] [CrossRef]
- Luka, G.; Samiei, E.; Dehghani, S.; Johnson, T.; Najjaran, H.; Hoorfar, M. Label-Free Capacitive Biosensor for Detection of Cryptosporidium. Sensors 2019, 19, 258. [Google Scholar] [CrossRef] [PubMed]
- Mathelié-Guinlet, M.; Cohen-Bouhacina, T.; Gammoudi, I.; Martin, A.; Béven, L.; Delville, M.H.; Grauby-Heywang, C. Silica Nanoparticles-Assisted Electrochemical Biosensor for the Rapid, Sensitive and Specific Detection of Escherichia coli. Sens. Actuators B Chem. 2019, 292, 314–320. [Google Scholar] [CrossRef]
- Jafari, H.; Amiri, M.; Abdi, E.; Navid, S.L.; Bouckaert, J.; Jijie, R.; Boukherroub, R.; Szunerits, S. Entrapment of Uropathogenic E. Coli Cells into Ultra-Thin Sol-Gel Matrices on Gold Thin Films: A Low Cost Alternative for Impedimetric Bacteria Sensing. Biosens. Bioelectron. 2019, 124–125, 161–166. [Google Scholar] [CrossRef]
- Divagar, M.; Sriramprabha, R.; Sornambikai, S.; Ponpandian, N.; Viswanathan, C. Surface Imprinted Ag Decorated MnO 2 Thin Film Electrodes for the Synergic Electrochemical Detection of Bacterial Pathogens. J. Electrochem. Soc. 2019, 166, G1–G9. [Google Scholar] [CrossRef]
- JIS K3602; Apparatus for the Estimation of Biochemical Oxygen Demand (BODs) with Microbial Sensor. Japan Industrial Standards Committee: Tokyo, Japan, 1990.
- Biosensores. Available online: https://www.biosensores.com/biochemical-oxygen-demand/ (accessed on 26 February 2025).
- Biosensores. Available online: https://www.biosensores.com/toxicity-mb-politox/ (accessed on 26 February 2025).
- Honest Industrial. Available online: https://www.materialsupplies.com.hk/central-kagaku-biosensor-analyzer-bod-1000 (accessed on 26 February 2025).
- Process Insights. Available online: https://www.process-insights.com/products-3/products-industrial/water-quality-analyzers/biomonitor/ (accessed on 26 February 2025).
- Direct Industry. Available online: https://www.directindustry.com/prod/applitek/product-22379-1620211.html (accessed on 26 February 2025).
- KORBI. Available online: http://www.korbi.com/eng/products_type/habs-2000/?k&ckattempt=2 (accessed on 26 February 2025).
- ENVIROPRO. Available online: https://www.enviropro.co.uk/entry/37668/Envitech/CSys-Biox-1010-insitu-BOD-analyser/ (accessed on 26 February 2025).
- SENTRY Water Tech. Available online: https://www.sentrywatertech.com/products (accessed on 26 February 2025).
- AQUALABO. Available online: https://www.aqualabo.fr/en/analyzers/toxicity/ (accessed on 26 February 2025).
- Biosensores. Available online: https://www.biosensores.com/biocarte/ (accessed on 26 February 2025).
- Modern Water. Available online: https://www.modernwater.com/monitoring/toxicity/laboratory-microtox-lx/ (accessed on 26 February 2025).
- Wang, H.; Wang, X.J.; Zhao, J.F.; Chen, L. Toxicity Assessment of Heavy Metals and Organic Compounds Using CellSense Biosensor with E. coli. Chinese Chem. Lett. 2008, 19, 211–214. [Google Scholar] [CrossRef]
- BIOTOXICITY. Available online: https://www.biotoxicity.com/index.php/ebpi-toxicity-tests/sos-genotoxicity-tests/sos-chromotest-kit (accessed on 26 February 2025).
- RE-Place. Available online: https://www.re-place.be/method/vitotox-assay (accessed on 26 February 2025).
- BIOTOXICITY. Available online: https://www.biotoxicity.com/index.php/ebpi-toxicity-tests/sos-genotoxicity-tests/umu-chromotest (accessed on 26 February 2025).
- Huang, Z.; Gustave, W.; Bai, S.; Li, Y.; Li, B.; Elçin, E.; Jiang, B.; Jia, Z.; Zhang, X.; Shaheen, S.M. Challenges and Opportunities in Commercializing Whole-Cell Bioreporters in Environmental Application. Environ. Res. 2024, 262, 119801. [Google Scholar] [CrossRef]
- Medvedeva, A.S.; Gudkova, E.I.; Titova, A.S.; Kharkova, A.S.; Kuznetsova, L.S.; Perchikov, R.N.; Ivanov, V.R.; Ryabkov, Y.D.; Tikhonova, A.A.; Fomina, E.D.; et al. Nanostructured Copper Electrodes—A New Step in the Development of Microbial Bioelectrochemical Systems. Environ. Sci. Nano 2024, 11, 4562–4576. [Google Scholar] [CrossRef]
- Taranova, L.A.; Fesay, A.P.; Ivashchenko, G.V.; Reshetilov, A.N.; Winther-Nielsen, M.; Emneus, J. Comamonas Testosteroni Strain TI as a Potential Base for a Microbial Sensor Detecting Surfactants. Appl. Biochem. Microbiol. 2004, 40, 404–408. [Google Scholar] [CrossRef]
- Reshetilov, A.N.; Semenchuk, I.N.; Iliasov, P.V.; Taranova, L.A. The Amperometric Biosensor for Detection of Sodium Dodecyl Sulfate. Anal. Chim. Acta 1997, 347, 19–26. [Google Scholar] [CrossRef]
- Tecon, R.; Beggah, S.; Czechowska, K.; Sentchilo, V.; Chronopoulou, P.M.; Mcgenity, T.J.; Van Der Meer, J.R. Development of a Multistrain Bacterial Bioreporter Platform for the Monitoring of Hydrocarbon Contaminants in Marine Environments. Environ. Sci. Technol. 2010, 44, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhao, X.; Zhang, D.; Ding, A.; Chen, C.; Huang, W.E.; Zhang, H. New Naphthalene Whole-Cell Bioreporter for Measuring and Assessing Naphthalene in Polycyclic Aromatic Hydrocarbons Contaminated Site. Chemosphere 2017, 186, 510–518. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, J.; Sallach, J.B.; Hu, X.; Gao, Y. Whole-Cell Paper Strip Biosensors to Semi-Quantify Tetracycline Antibiotics in Environmental Matrices. Biosens. Bioelectron. 2020, 168, 112528. [Google Scholar] [CrossRef]



| Metal | MPC, mg/L (China) | MPC, mg/L (Europe) |
|---|---|---|
| Cd | 0.005 | 0.005 |
| Pb | 0.01 | 0.01 |
| Hg | 0.001 | 0.001 |
| As | 0.01 | 0.01 |
| Cr | 0.05 | 0.025 |
| Zn | 1.0 | 5.0 |
| Determination of BOD | ||||||
| Biomaterial | Type of Electrode/Modification of the Electrode | Range of Determined Concentrations, mg O2/L | Sensor Stability, % | Detection Mode | Testing on Real Samples | Reference |
| P. yeei SPB1 | Ferrocene | 1.3–360 | 2.9 | Amperometry | - | [61] |
| O. polymorpha and B. adeninivorans | Ferrocene | 2–140 | - | Amperometry | - | [61] |
| Biofilm | Carbon nanotubes | 0.41 | 5.96 | Amperometry | R2 = 0.9901 Natural and waste water | [62] |
| E. coli biofilm | Ferrocene | 0.87–14 | 7.69 | Amperometry | R2 = 0.9862 Freshwater and wastewater | [67] |
| Shewanella loihica PV-4 | - | 0–435 | - | Amperometry | - | [69] |
| B. adeninivorans | Ferrocene–neutral red | 0.16–2.7 | 1.5 | Amperometry | R2 = 0.9693 Surface waters | [71] |
| B. adeninivorans | Carbon nanotubes and poly(thionine)-NR | 0.4–62 | 3.2 | Amperometry | R2 = 0.9998 Wastewater | [76] |
| Paracoccus yeei VKM B-3302 | Polyvinyl alcohol matrix | 0.05–5 | 7 | Amperometry | R2 = 0.9990 Natural and waste water | [77] |
| Active sludge | - | 25–500 | 10 | MFC | - | [84] |
| Electroactive microorganisms | - | 10–500 | - | MEC | - | [88] |
| E. coli 0157 | - | 0–27 | - | Fluorescence detection | R2 = 0.91 for River A and R2 = 0.93 for River B | [89] |
| Debaryomyces hansenii VKM Y-2482 | Nanostructured electrochemical sensor, ferrocene-methylene blue | 2.0–190 | 3.5 | Amperometry | R2 > 0.98 Surface waters | [234] |
| Determination of Toxicity | ||||||
| Biomaterial | Type of Electrode/Modification of the Electrode | IC50. mg/L | Detection Mode | Testing on Real Samples | Reference | |
| S. cerevisiae S288C | Glassy carbon (GC) electrode covered with chitosan hydrogel polymer film with boron-doped nanocrystalline diamond (BND). two-mediator system (K3[Fe(CN)6]. menadione) | 10.12 (Cu2+) 13.88 (Cd2+) 17.06 (Ni2+) 34.56 (Pb2+) 44.55 (phenol) 34.40 (4-chlorophenol) 16.48 (DCP) | Amperometry | Landfill, electroplanting, and laboratory wastewater | [99] | |
| G. oxydans VKM B-1280 | Oxygen electrode | 16.5 (Fe3+) >200 (Cd2+) 13.9 (Cr3+) 7.2 (Zn2+) 12 (Mn2+) >200 (TCA) >200 (phenol) >200 (salicylic acid) 2.9 (2,4-dinitrophenol) | Amperometry | Aqueous extracts of samples of industrially produced goods | [98] | |
| Graphite-paste electrode/ferrocene | 7.8 (Fe3+) 1.6 (Cd2+) 0.8 (Cr3+) 2.4 (Zn2+) 0.3 (Mn2+) 15.7 (TCA) 17.5 (phenol) 19.0 (salicylic acid) 6.8 (2,4-dinitrophenol) | Amperometry | ||||
| MFC (2.6-DCPIP) | 1.2 (Cd2+) 4.5 (Zn2+) 1.6 (Mn2+) 24.2 (phenol) 0.9 (2,4-dinitrophenol) | MFC | ||||
| P. yeei VKM B-3302 | Ferrocene | 9.9 (Pb2+) 18.2 (Cd2+) 21.1 (Cu2+) 47.5 (Zn2+) 9.9 (phenol) 2.1 (p-nitrophenol) | Amperometry | Perfumery and cosmetics samples | [100] | |
| Association of S. cerevisiae VKM Y-1173/P. yeei VKM B-3302 | BSA-NR-CNT/COOH nanocomposite | 3.2 (Pb2+) 7.6 (Cd2+) 8.9 (Cu2+) 22.1 (Zn2+) 7.5 (phenol) 5 (p-nitrophenol) | Amperometry | Natural waters | [61] | |
| Association of P. yeei VKM B-3302/E. coli K-802 | Ferrocene | 7.3 (Pb2+) 6.6 (Cd2+) 23.8 (Cu2+) 2.3 (Zn2+) 8.1 (phenol) 29.2 (p-nitrophenol) | Amperometry | Wastewater | [101] | |
| Determination of Heavy Metals | ||||||
| Biomaterial | Heavy Metal | Range of Determined Concentrations, mg/L | Limit of Detection, mg/L | Detection Mode | Testing on Real Samples | Reference |
| E. coli K12-PMP-luxCDABE-△cysI | Cd (II) | 0.005–2 | 0.005 | Fluorescence detection | Drinking water | [125] |
| GFP Bacillus megaterium VR1/SiNa/LUDOX | Cd | 0–10 | 1.42 × 10−4 | Fluorescence detection | - | [124] |
| Cu | 0–20 | 3.16 × 10−4 | ||||
| Zn | 0–100 | 2.42 × 10−4 | ||||
| Dual-colored bacterial biosensor (CadR-regulated vioABE and a MerR-regulated VioC expression module) | Cd | 5.5 × 10−4–4.5 | 5.5 × 10−4 | Colorimetric | Seawater | [119] |
| Pb | 5.06 × 10−3–41.4 | 5.06 × 10−3 | ||||
| Hg | 7.42 × 10−4–9.4 × 10−4 | 7.42 × 10−4 | ||||
| Determination of Surfactants | ||||||
| Biomaterial | Range of Determined Concentrations, mg/L | Response Measurement Time, min | Detection Mode | Testing on Real Samples | Reference | |
| Herbaspirillum lusitanum P6–12 | 0.01–0.1 | 1–5 | Electric polarizability detection | - | [139] | |
| Comamonas testosteroni TI | 0.25–0.5 | 12–15 | Amperometry | - | [235] | |
| Pseudomonas rathonis | 0.25–0.75 | 1.7–2.5 | Amperometry | - | [236] | |
| Biosensor based on whole cell transcription factor | 0.48–62.5 | - | Fluorescence detection | Sewage (RSD = 2.4%) and pond (RSD = 2.8%) water | [136] | |
| E. coli | 1.7 | 1 | Fluorescence detection | Tap water, river water, and drinking water | [45] | |
| Determination of Phenols | ||||||
| Biomaterial | Type of Electrode/Modification of the Electrode | Detectable Compound | Range of Determined Concentrations, M | Detection Mode | Testing on Real Samples | Reference |
| Staphylococcus aureus | Copper electrode modified with a poly-caprolactone film | Phenol | 0.01–0.05 | Square-wave voltammetry | - | [146] |
| Pseudomonas putida BS394 (PBS216) (adapted for phenol) | BSA-FC/CNT Redox-Active Biocompatible Composite Polymer “Bovine Serum Albumin–Ferrocene–Carbon Nanotubes”. | Phenol, 2,4-dinitrophenol | 1.063 × 10−8–0.002 (phenol) | Amperometry | River water | [155] |
| Pseudomonas SP. (GSN23) (adapted for phenol) | IDEs-MWCNTs | Phenol ƿ-Nitrophenol Bisphenol-A 4-chlorophenol 2,3,6-trichlorophenol 2,4,6-trichlorophenol | 1 × 10−5–0.003187 (phenol) | Conductometry | - | [156] |
| pUC57-QS-DSF-F42 L/E coli DH5α | - | Phenol, p-nitrophenol | 1 × 10−7–5 × 10−4 (phenol) | Fluorescence detection | - | [157] |
| E. coli-Tyrosinase | Glassy carbon (GCE) electrode | Bisphenol | 1 × 10−11–1 × 10−7 | Amperometry | Tea and juice | [161] |
| E. coli BL21 | Nanoporous gold (NPG)/GCE | Catechol | 1 × 10−6–5 × 10−4 | Differential pulse voltammetry | Synthetic wastewater | [43] |
| Determination of Pesticides | ||||||
| Biomaterial | Detectable Pesticide | Range of Determined Concentrations, µM | Limit of Detection, nM | Detection Mode | Testing on Real Samples | Reference |
| E. coli BL21/pPNP-LacZ | Methylparation | 0.04–38 | 20 | Colorimetric | R2 = 0.997 Soil | [174] |
| S. cerevisae EBY100 | Parathion | 0.017–34.4 | 12.8 | Visible spectrophotometry | Tap water (RSD = 2.7%), seawater (RSD = 5.9%), and sewage (RSD = 3.2%) | [180] |
| Paraoxon | 0.018–36.3 | 0.49 | Tap water (RSD = 4.5%), seawater (RSD = 2.7%), and sewage (RSD = 6.3%) | |||
| E. coli BL21 | Paraoxon | - | 2000 | Fluorescence detection | - | [181] |
| E. coli PC16 | Chlorpyrifos | - | 5 | Fluorescence detection | Seawater, wastewater | [182] |
| A. variabilis SA-1 | Atrazine | 0–1.31 | 70 | Photoelectrochemical (amperometry) | - | [183] |
| Leptolyngbia sp. | Atrazine | 0.1–1.2 | 14 | Photoelectrochemical (amperometry) | Tap water | [184] |
| Chlamydomonas reinhardtii | Nanocapsulated atrazine | 0.010–0.150 | 0.004 | Fluorescence detection | Tap water | [44] |
| Microorganisms of anaerobic sludge | Atrazine | 0.2–1 | 200 | MFC | - | [185] |
| Agrobacterium tumefaciens NTL4 | 2,4-dichlorophenoxyacetic acid | 0–100 | 1560 | Fluorescence detection | Environmental water | [186] |
| S. cerevisiae BY4741 | Tebuconazole | - | 20 | Bioluminescence detection | Environmental water | [187] |
| E. coli | Dicamba | 0–4500 | - | Cyclic voltammetry | Environmental water | [188] |
| E. coli MG1655 | 3,5-dichlorophenol | 20–1500 | - | Raman spectroscopy | - | [194] |
| Determination of Other Organic Pollutants | ||||||
| Biomaterial | Detectable Compound | Range of Determined Concentrations, µg/L | Limit of Detection, µM | Detection Mode | Testing on Real Samples | Reference |
| E. coli DH5α | Benzene. Toluene, and xylene | - | 0.24 | Bioluminescence detection | Seawater | [237] |
| B. sartisoli RP007 | Naphthalene and phenanthrene | - | 0.17 | Seawater | ||
| Acinetobacter ADPWH_Nah | Naphthalene | - | 0.01 | Colorimetric | Groundwater and soil | [238] |
| E. coli/pMTLacZ. | Tetracyclines | 75–10,000 | 0.011 (chlorotetracycline) 0.012 (deoxytetracycline) 0.013 (tetracycline) 0.037 (minocycline) 0.039 (methacycline) | Bioluminescence detection | Environmental water | [239] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheliukanov, M.; Gurkin, G.; Perchikov, R.; Medvedeva, A.; Lavrova, T.; Belousova, T.; Titova, A.; Plekhanova, Y.; Tarasov, S.; Kharkova, A.; et al. Whole Cells of Microorganisms—A Powerful Bioanalytical Tool for Measuring Integral Parameters of Pollution: A Review. Biosensors 2025, 15, 290. https://doi.org/10.3390/bios15050290
Cheliukanov M, Gurkin G, Perchikov R, Medvedeva A, Lavrova T, Belousova T, Titova A, Plekhanova Y, Tarasov S, Kharkova A, et al. Whole Cells of Microorganisms—A Powerful Bioanalytical Tool for Measuring Integral Parameters of Pollution: A Review. Biosensors. 2025; 15(5):290. https://doi.org/10.3390/bios15050290
Chicago/Turabian StyleCheliukanov, Maxim, George Gurkin, Roman Perchikov, Anastasia Medvedeva, Tatyana Lavrova, Tatyana Belousova, Aleksandra Titova, Yulia Plekhanova, Sergei Tarasov, Anna Kharkova, and et al. 2025. "Whole Cells of Microorganisms—A Powerful Bioanalytical Tool for Measuring Integral Parameters of Pollution: A Review" Biosensors 15, no. 5: 290. https://doi.org/10.3390/bios15050290
APA StyleCheliukanov, M., Gurkin, G., Perchikov, R., Medvedeva, A., Lavrova, T., Belousova, T., Titova, A., Plekhanova, Y., Tarasov, S., Kharkova, A., Arlyapov, V., Mandin, P., Nakamura, H., & Reshetilov, A. (2025). Whole Cells of Microorganisms—A Powerful Bioanalytical Tool for Measuring Integral Parameters of Pollution: A Review. Biosensors, 15(5), 290. https://doi.org/10.3390/bios15050290