A Signal On-Off Ratiometric Molecularly Imprinted Electrochemical Sensor Based on MXene/PEI-MWCNTs Signal Amplification for the Detection of Diuron
Abstract
1. Introduction
2. Experimental Section
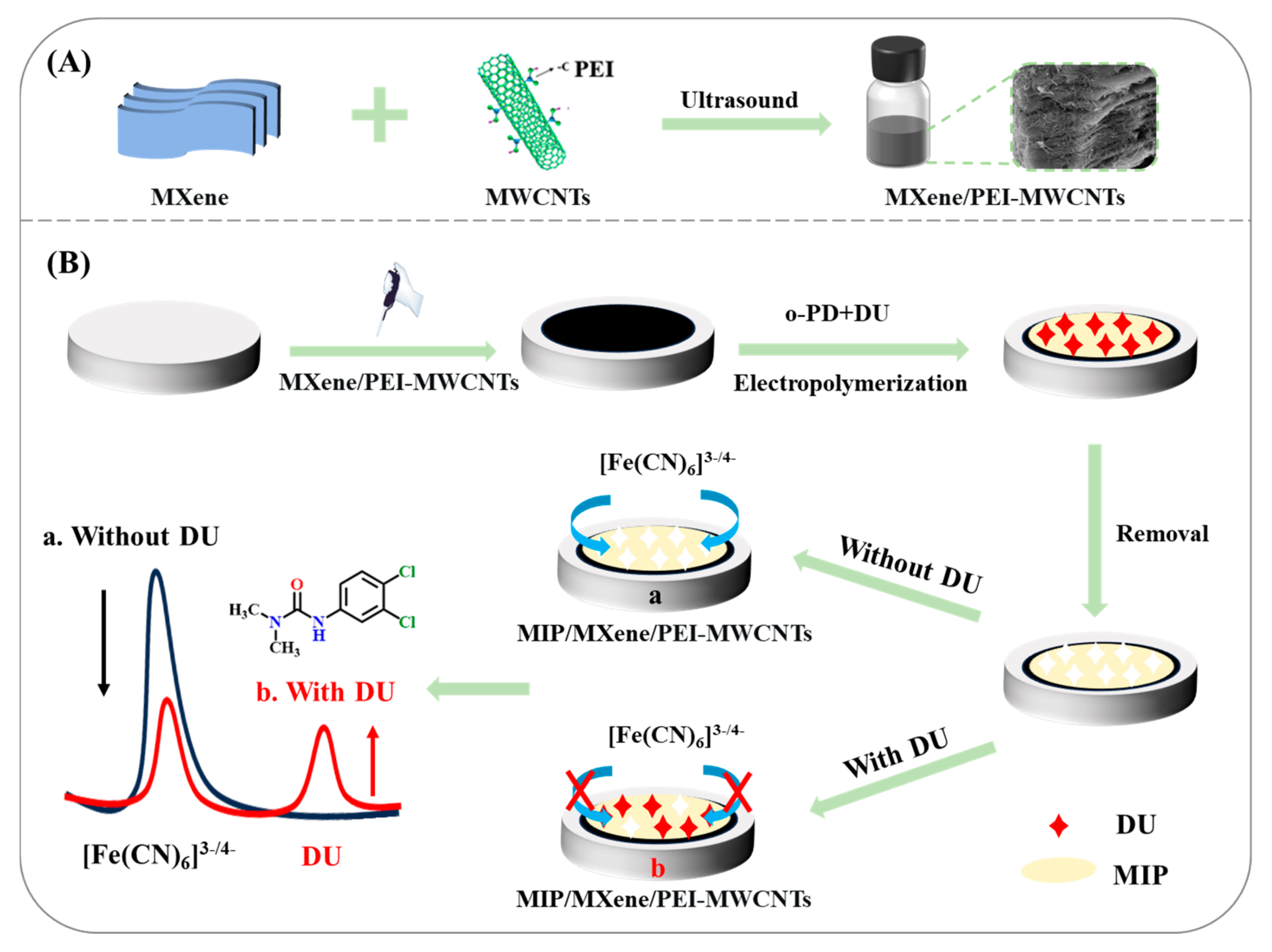
2.1. Synthesis of MXene/PEI-MWCNTs
2.2. Construction of MIP/MXene/PEI-MWCNTs/GCE
2.3. Electrochemical Measurements
2.4. Determination of Real Samples
3. Results and Discussion
3.1. Characterization of the MXene/PEI-MWCNTs Nanocomposites
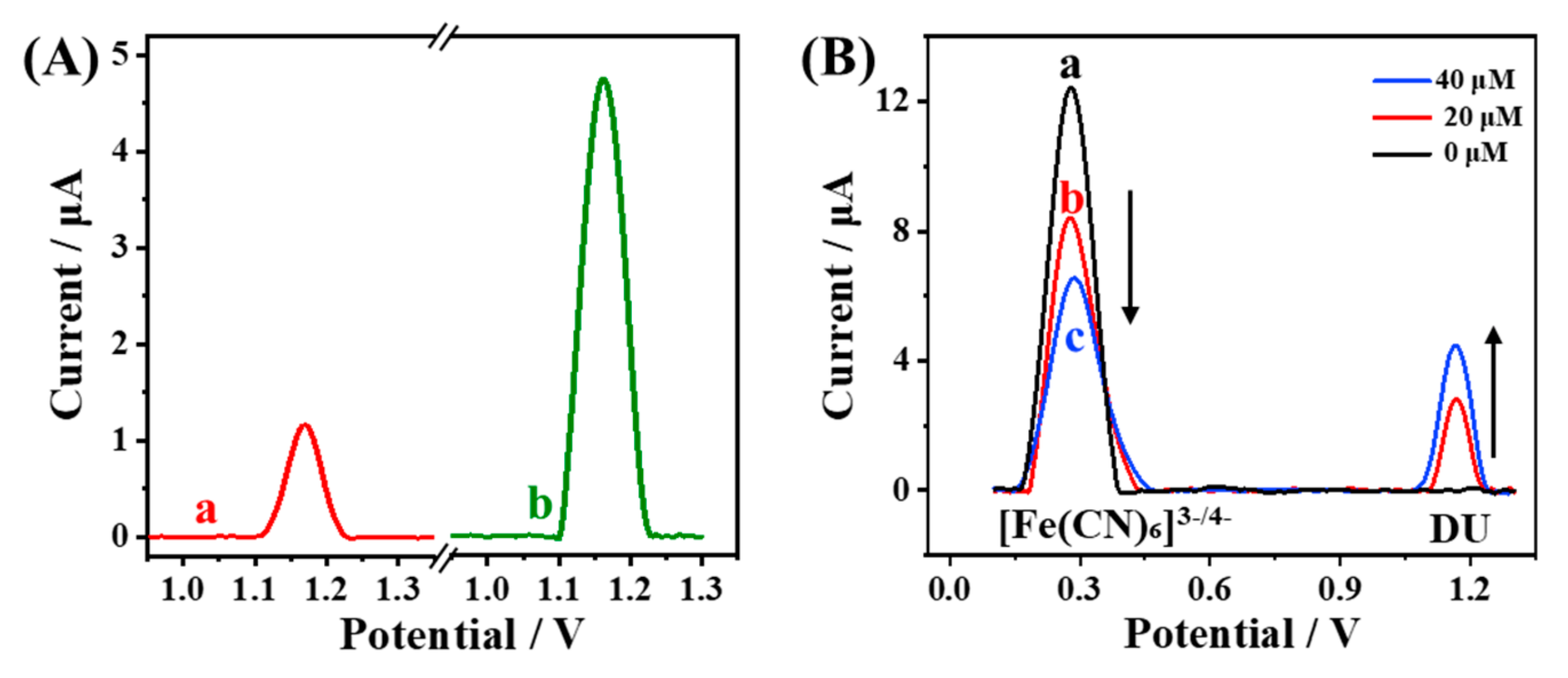
3.2. Electrochemical Response of MIP/MXene/PEI-MWCNTs/GCE Sensor
3.3. Fabrication and Feasibility of the Ratiometric MIP-EC Sensor
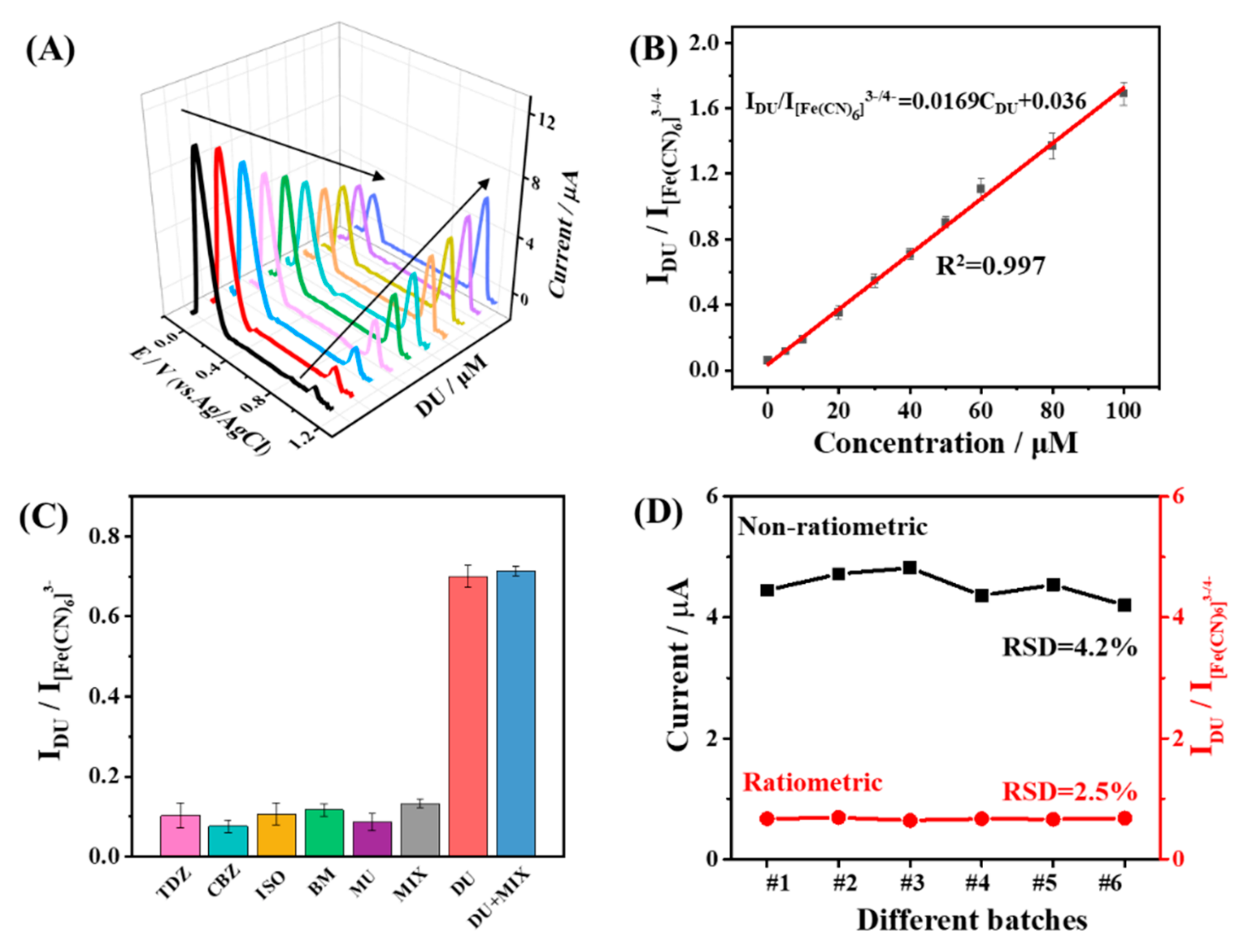
3.4. Analytical Performance of the Ratiometric MIP-EC Sensor
3.5. Real Sample Analysis by the Ratiometric MIP-EC Sensor in Soil
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reyes-Cervantes, A.; Robles-Morales, D.L.; Téllez-Jurado, A.; Huerta-Ochoa, S.; Jiménez-González, A.; Medina-Moreno, S.A. Evaluation in the performance of the biodegradation of herbicide diuron to high concentrations by Lysinibacillus fusiformis acclimatized by sequential batch culture. J. Environ. Manag. 2021, 291, 112688. [Google Scholar] [CrossRef]
- Lee, H.; Depuydt, S.; Shin, K.; Choi, S.; Kim, G.; Lee, Y.H.; Park, J.T.; Han, T.; Park, J. Assessment of various toxicity endpoints in duckweed (Lemna minor) at the physiological, biochemical, and molecular levels as a measure of diuron stress. Biology 2021, 10, 684. [Google Scholar] [CrossRef]
- Li, J.Y.; Zhang, W.P.; Lin, Z.Q.; Huang, Y.; Bhatt, P.; Chen, S. Emerging strategies for the bioremediation of the phenylurea herbicide diuron. Front. Microbiol. 2021, 12, 686509. [Google Scholar] [CrossRef]
- Behrens, D.; Rouxel, J.; Burgeot, T.; Akcha, F. Comparative embryotoxicity and genotoxicity of the herbicide diuron and its metabolites in early life stages of Crassostrea gigas: Implication of reactive oxygen species production. Aquat. Toxicol. 2016, 175, 249–259. [Google Scholar] [CrossRef]
- Tian, F.; Xu, B.; Zhang, T.; Gao, N. Degradation of phenylurea herbicides by chlorine dioxide and formation of disinfection by-products during subsequent chlor(am)ination. Chem. Eng. J. 2014, 258, 210–217. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, Y.; Zhou, R.; He, Y.; Wu, Y.; Yi, Y.; Zhu, G. Highly sensitive electrochemical detection of paraoxon ethyl in water and fruit samples based on defect-engineered graphene nanoribbons modified electrode. J. Food Meas. Charact. 2022, 16, 2596–2603. [Google Scholar] [CrossRef]
- Karuppusamy, N.; Sakthivel, R.; Chen, S.; Prasanna, S.B.; Chung, R. Template-assisted synthesis of Co1Zn0.5–P/CoO/ZnO core-shell heterostructure for the electrochemical detection of carbofuran and diuron. Chem. Eng. J. 2023, 473, 145305. [Google Scholar] [CrossRef]
- Sousa, K.A.P.; Morawski, F.d.M.; de Campos, C.E.M.; Parreira, R.L.T.; Piotrowski, M.J.; Nagurniak, G.R.; Jost, C.L. Electrochemical, theoretical, and analytical investigation of the phenylurea herbicide fluometuron at a glassy carbon electrode. Electrochim. Acta 2022, 408, 139945. [Google Scholar] [CrossRef]
- Qiu, H.; Gao, L.; Wang, J.; Pan, J.; Yan, Y.; Zhang, X. A precise and efficient detection of Beta-Cyfluthrin via fluorescent molecularly imprinted polymers with ally fluorescein as functional monomer in agricultural products. Food Chem. 2017, 217, 620–627. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, X.; Yu, F.; Quan, Y. Preparation of dummy molecularly imprinted polymers based on dextran-modified magnetic nanoparticles Fe3O4 for the selective detection of acrylamide in potato chips. Food Chem. 2020, 317, 126431. [Google Scholar] [CrossRef]
- Su, X.; Zheng, K.; Tian, X.; Zhou, X.; Zou, X.; Xu, X.; Sun, Z.; Zhang, W. An advanced ratiometric molecularly imprinted sensor based on metal ion reoxidation for indirect and ultrasensitive glyphosate detection in fruit. Food Chem. 2023, 429, 136927. [Google Scholar] [CrossRef]
- Liu, Y.; Xia, Y.; Tang, Y.; Chen, Y.; Cao, J.; Zhao, F.; Zeng, B. A ratiometric electrochemical sensor based on Cu-coordinated molecularly imprinted polymer and porous carbon supported Ag nanoparticles for highly sensitive and selective detection of perphenazine. Anal. Chim. Acta 2022, 1227, 340301. [Google Scholar] [CrossRef]
- Li, Y.; Feng, Y.; Chen, S.; Li, R.; Yang, Y.; Guan, J.j.; Ye, B. Signal on–off ratiometric electrochemical sensor coupled with a molecularly imprinted polymer for the detection of carbendazim. Microchim. Acta 2022, 189, 250. [Google Scholar] [CrossRef]
- Lu, Z.; Wei, K.; Ma, H.; Xiong, Q.; Li, Y.; Sun, M.; Wang, X.; Wang, Y.; Wu, C.; Su, G.; et al. Nanoarchitectonics of on–off ratiometric signal amplified electrochemical sensor for chlorpromazine with molecularly imprinted polymer based on Ni-MOF/Fe-MOF-5 hybrid Au nanoparticles. Sep. Purif. Technol. 2023, 327, 124858. [Google Scholar] [CrossRef]
- Haotian, R.; Zhu, Z.; Zhang, H.; Lv, T.; Tang, S.; Zhang, J.; Luo, A.; Liang, A. Engineering conductive covalent-organic frameworks enable highly sensitive and anti-interference molecularly imprinted electrochemical biosensor. Biosens. Bioelectron. 2025, 273, 117195. [Google Scholar] [CrossRef]
- Gao, H.; You, J.; Wu, H.; Tian, M. A dual action electrochemical molecular imprinting sensor based on FeCu-MOF and RGO/PDA@MXene hybrid synergies for trace detection of ribavirin. Food Chem. 2025, 473, 143092. [Google Scholar] [CrossRef]
- Zhu, J.; He, Y.; Luo, L.; Li, L.; You, T. Molecularly imprinted electrochemical sensor based on SnS2 nanoflower and polyethyleneimine modified multiwalled carbon nanotubes for highly sensitive and specific detection of diuron. IEEE Sens. J. 2025, 25, 4167–4174. [Google Scholar] [CrossRef]
- Manoj; Ghrera, A.S. MXene/PEDOT: PSS composite-modified electrode for electrochemical sensing of bilirubin by molecularly imprinted ortho-phenylenediamine. Microchim. Acta 2025, 192, 50. [Google Scholar] [CrossRef]
- Liu, Y.; Meng, X.; Ma, Z.; Gu, H.; Luo, X.; Yin, X.; Yi, H.; Chen, Y. Hybrid recognition-enabled ratiometric electrochemical sensing of Staphylococcus aureus via in-situ growth of MOF/Ti3C2Tx-MXene and a self-reporting bacterial imprinted polymer. Food Chem. 2025, 463, 141496. [Google Scholar] [CrossRef]
- Qi, Y.; Chen, Y.; Li, Q.; Dang, X.; Chen, H. A novel ratiometric electrochemical sensing platform combined with molecularly imprinted polymer and Fe-MOF-NH2/CNTs-NH2/MXene composite for efficient detection of ofloxacin. Anal. Chim. Acta 2024, 1316, 342876. [Google Scholar] [CrossRef]
- He, Y.; Luo, L.J.; Li, L.B.; You, T.; Chen, X. Synergistic signal–amplification effect of silver nanowires and bifunctional monomers on molecularly imprinted electrochemical sensor for diuron analysis. Biosens. Bioelectron. 2024, 262, 116570. [Google Scholar] [CrossRef]
- Gao, F.; Hong, W.; Yang, T.; Qiao, C.; Li, J.; Xiao, X.; Zhao, Z.; Zhang, C.; Tang, J. Expanded interlayer spacing of SnO2 QDs-Decorated MXene for highly selective luteolin detection with Ultra-Low limit of detection. J. Colloid. Interf. Sci. 2024, 653, 561–569. [Google Scholar] [CrossRef]
- Chen, X.; Li, W.; Lu, C.; Chu, J.; Lin, R.; Wang, P.; Xie, G.; Gu, Q.; Wu, D.; Chu, B. Highly sensitive electrochemical detection of carbendazim residues in water by synergistic enhancement of nitrogen-doped carbon nanohorns and polyethyleneimine modified carbon nanotubes. Sci. Total Environ. 2022, 851, 158324. [Google Scholar] [CrossRef]
- Zhong, W.; Gao, F.; Zou, J.; Liu, S.; Li, M.; Gao, Y.; Yu, Y.; Wang, X.; Lu, L. MXene@Ag-based ratiometric electrochemical sensing strategy for effective detection of carbendazim in vegetable samples. Food Chem. 2021, 360, 130006. [Google Scholar] [CrossRef]
- Panda, A.K.; Murugan, K.; Sakthivel, R.; Lin, L.; Duann, Y.; Dhawan, U.; Liu, X.; He, J.; Chung, R. A non-enzymatic, biocompatible electrochemical sensor based on N-doped graphene quantum dot-incorporated SnS2 nanosheets for in situ monitoring of hydrogen peroxide in breast cancer cells. Colloid Surfaces B. 2023, 222, 113033. [Google Scholar] [CrossRef]
- Nehru, R.; Chen, C.; Dong, C. In-situ growth of MoS2 nanosheets on g-C3N4 nanotube: A novel electrochemical sensing platform for vanillin determination in food samples. Carbon 2023, 208, 410–420. [Google Scholar] [CrossRef]
- Shang, T.; Lin, Z.; Qi, C.; Liu, X.; Li, P.; Tao, Y.; Wu, Z.; Li, D.; Simon, P.; Yang, Q.H. 3D macroscopic architectures from self-assembled MXene hydrogels. Adv. Funct. Mater. 2019, 29, 1903960. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, J.; Li, Y. MXene with great adsorption ability toward organic dye: An excellent material for constructing a ratiometric electrochemical sensing platform. ACS Sens. 2019, 4, 2058–2064. [Google Scholar] [CrossRef]
- He, Y.; Bi, X.Y.; Liu, X.H.; Zhu, J.; Li, L.; You, T.; Chen, X. Molecularly imprinted electrochemical sensor based on the self-assembly of cage-like gold nanoparticles and amino-functionalized rGO for the detection of diuron in cotton defoliant. ACS Agric. Sci. Technol. 2024, 4, 223–233. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, Z.; Zhang, C.; Zhang, J.; Zhou, H.; Ning, F.; Wang, D.; Du, X. Ginsenoside Rg3 determination using an electro-synthesized molecularly imprinted polymer on MWCNT-Ti3C2Tx nanocomposite modified electrode. Talanta 2022, 243, 123391. [Google Scholar] [CrossRef]
- Lv, M.; Cao, X.; Tian, M.; Jiang, R.; Gao, C.; Xia, J.; Wang, Z. A novel electrochemical biosensor based on MIL-101-NH2 (Cr) combining target-responsive releasing and self-catalysis strategy for p53 detection. Biosens. Bioelectron. 2022, 214, 114518. [Google Scholar] [CrossRef]
- Deng, X.; Yi, Z.; Xiong, Y.; Gao, X.; Huang, R.; Chen, X.; Deng, D.; Xiong, C.; Zhang, J.; Huang, G. Molecularly imprinted ratiometric electrochemical sensor based on 3D-1D MoS2@CNTs hetero-nanoflower for selective detection of trimethoprim. Microchem. J. 2024, 201, 110522. [Google Scholar] [CrossRef]
- Han, E.; Pan, Y.; Li, L.; Cai, J. Bisphenol A detection based on nano gold-doped molecular imprinting electrochemical sensor with enhanced sensitivity. Food Chem. 2023, 426, 136608. [Google Scholar] [CrossRef]
- Lesueur, C.; Gartner, M.; Mentler, A.; Fuerhacker, M. Comparison of four extraction methods for the analysis of 24 pesticides in soil samples with gas chromatography-mass spectrometry and liquid chromatography-ion trap-mass spectrometry. Talanta 2008, 75, 284–293. [Google Scholar] [CrossRef]
- Chen, B.; Wang, X.M. Combined approach for determining diuron in sugarcane and soil: Ultrasound-assisted extraction, carbon nanotube-mediated purification, and gas chromatography-electron capture detection. J. Food Sci. 2019, 84, 2402–2411. [Google Scholar] [CrossRef]
- Wang, Y.C.; Xiao, L.; Cheng, M.R. Determination of phenylureas herbicides in food stuffs based on matrix solid-phase dispersion extraction and capillary electrophoresis with electrochemiluminescence detection. J. Chromatogr. A 2011, 1218, 9115–9119. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, J.; Song, S.S.; Liu, L.; Xu, C.; Kuang, H.; Ma, W. Development of monoclonal antibodies for the detection of diuron in water and sugarcane and their application in immunochromatographic strips. Anal. Methods 2022, 14, 4202–4208. [Google Scholar] [CrossRef]
- Alves, G.F.; de Faria, L.V.; Lisboa, T.P.; Matos, M.A.C.; Muñoz, R.A.A.; Matos, R.C. Simple and fast batch injection analysis method for monitoring diuron herbicide residues in juice and tap water samples using reduced graphene oxide sensor. J. Food Compos. Anal. 2022, 106, 104284. [Google Scholar] [CrossRef]
- Velmurugan, S.; Anupriya, J.; Chen, S.M.; Hahn, Y. Efficient lock-in CuO/WON heterostructures tailored for highly sensitive electrochemical detection of hazardous herbicide diuron in fruit juices and aqua region. Sensor. Actuat. B Chem. 2023, 375, 132920. [Google Scholar] [CrossRef]
- Kamel, A.H.; Amr, A.E.G.E.; Al Omar, M.A.; Almehizia, A.A. Solid-State membrane sensors based on man-tailored biomimetic receptors for selective recognition of isoproturon and diuron herbicides. Membranes 2020, 10, 279. [Google Scholar] [CrossRef]
- Sharifuzzaman, M.; Barman, S.C.; Rahman, M.T.; Zahed, M.A.; Xuan, X.; Park, J.Y. Green synthesis and layer-by-layer assembly of amino-functionalized graphene oxide/carboxylic surface modified trimetallic nanoparticles nanocomposite for label-free electrochemical biosensing. J. Electrochem. Soc. 2019, 166, B983–B993. [Google Scholar] [CrossRef]




| Detection Methods | Linear Range | LOD | Refs. |
|---|---|---|---|
| HPLC-MS | 0.0858–17.16 µM | 50.6 nM | [34] |
| GC-EC | 0.0429–21.45 µM | 30.0 nM | [35] |
| MSPD-CE-ECL | 0.00858–2.15 µM | 0.858 nM | [36] |
| ICA | 0.858–42.9 nM | 0.3 nM | [37] |
| EC | 5–50 µM | 360 nM | [38] |
| EC | 0.01–764.4 µM | 5.50 nM | [39] |
| EC | 2–149 µM | 2.52 nM | [7] |
| MIP-EC | 0.1–60 µM | 20.0 nM | [17] |
| MIP-EC | 3.2–1000 µM | 1.40 µM | [40] |
| MIP-EC | 10–500 µM | 43.4 µM | [41] |
| Ratiometric MIP-EC | 0.1–100 µM | 30.0 nM | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Zhu, J.; Li, L.; You, T.; Chen, X. A Signal On-Off Ratiometric Molecularly Imprinted Electrochemical Sensor Based on MXene/PEI-MWCNTs Signal Amplification for the Detection of Diuron. Biosensors 2025, 15, 433. https://doi.org/10.3390/bios15070433
He Y, Zhu J, Li L, You T, Chen X. A Signal On-Off Ratiometric Molecularly Imprinted Electrochemical Sensor Based on MXene/PEI-MWCNTs Signal Amplification for the Detection of Diuron. Biosensors. 2025; 15(7):433. https://doi.org/10.3390/bios15070433
Chicago/Turabian StyleHe, Yi, Jin Zhu, Libo Li, Tianyan You, and Xuegeng Chen. 2025. "A Signal On-Off Ratiometric Molecularly Imprinted Electrochemical Sensor Based on MXene/PEI-MWCNTs Signal Amplification for the Detection of Diuron" Biosensors 15, no. 7: 433. https://doi.org/10.3390/bios15070433
APA StyleHe, Y., Zhu, J., Li, L., You, T., & Chen, X. (2025). A Signal On-Off Ratiometric Molecularly Imprinted Electrochemical Sensor Based on MXene/PEI-MWCNTs Signal Amplification for the Detection of Diuron. Biosensors, 15(7), 433. https://doi.org/10.3390/bios15070433





