Development of a Colloidal Gold-Based Immunochromatographic Strip Targeting the Nucleoprotein for Rapid Detection of Canine Distemper Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Viruses, Cells, and Animals
2.2. Chemicals and Reagents
2.3. Plasmid Construction
2.4. Recombinant Expression and Purification of Truncated N Proteins
2.5. Western Blot Identification of Recombinant Truncated N Protein
2.6. Animal Immunization Strategy and Selection of Hybridoma Cells Producing mAbs Against the Truncated N Protein
2.7. Preparation of Mouse Ascites and Purification of mAbs
2.8. Western Blot Identification of mAbs
2.9. Validation of mAbs Using Indirect Immunofluorescence Assay
2.10. Preliminary Identification of the Antigenic Epitope of the Generated mAb
2.11. Synthesis of Colloidal Gold and Optimization of Labeling Conditions
2.12. Preparation of Colloidal Gold–Labeled Antibody
2.13. Preparation and Assembly of the Colloidal Gold ICS
2.14. Specificity and Sensitivity Assessment of the Colloidal Gold Immunochromatographic Strip (ICS)
2.15. Repeatability and Stability of the Colloidal Gold ICS
2.16. Clinical Application of the Colloidal Gold ICS
2.17. Biological Information Analysis
3. Results
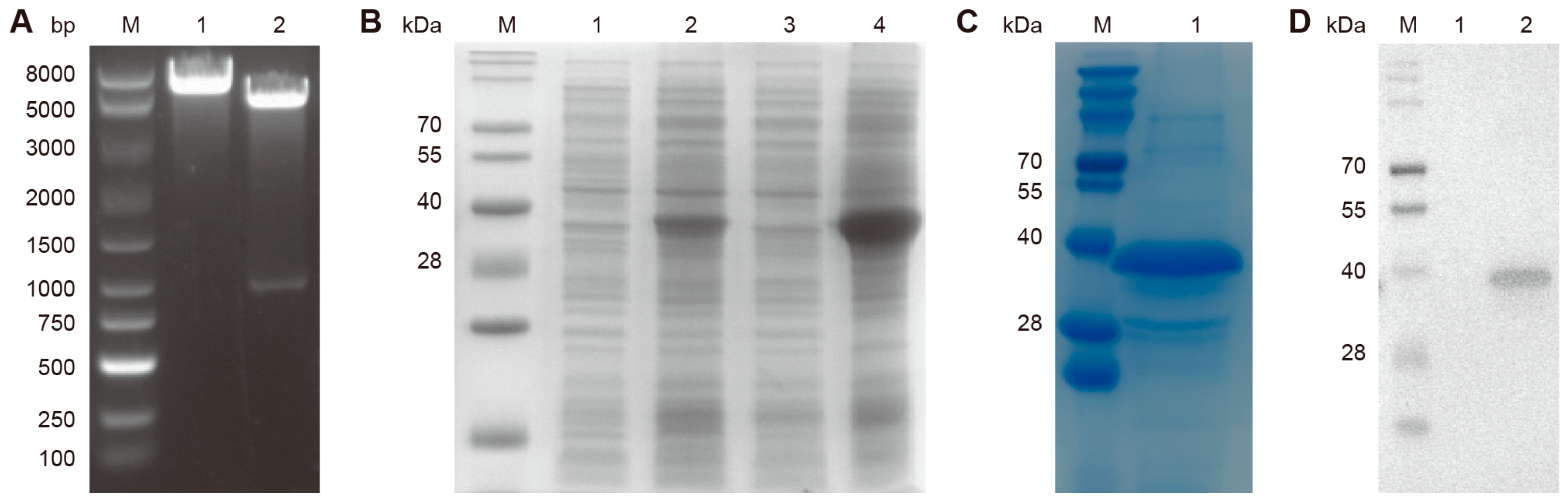
3.1. Construction of the Truncated N Protein Expression Plasmid and Purification of the Recombinant Protein
3.2. Development and Validation of Monoclonal Antibodies Against the CDV Recombinant N Protein
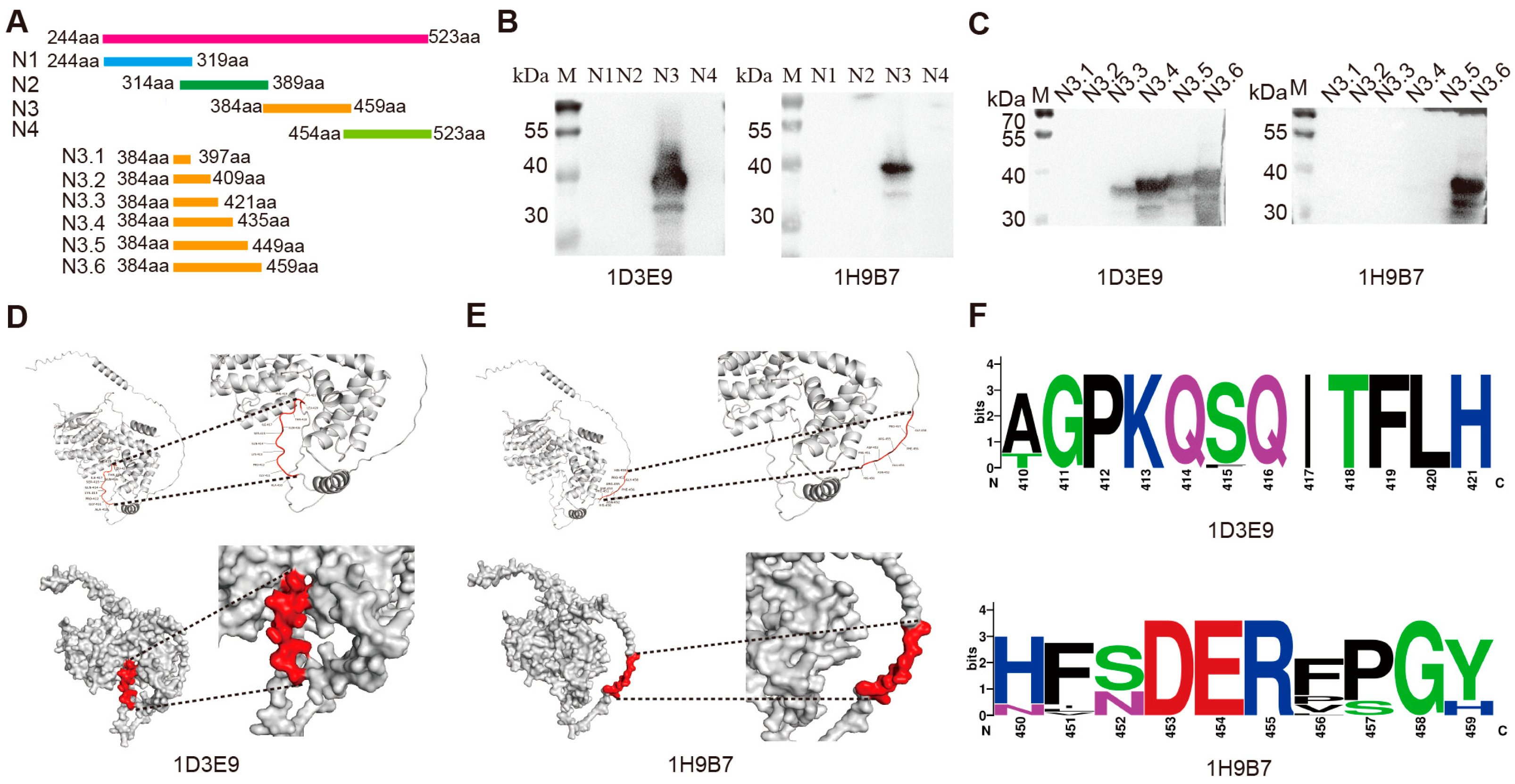
3.3. Epitope Recognition Ability of the mAbs and Spatial Location of the Epitope
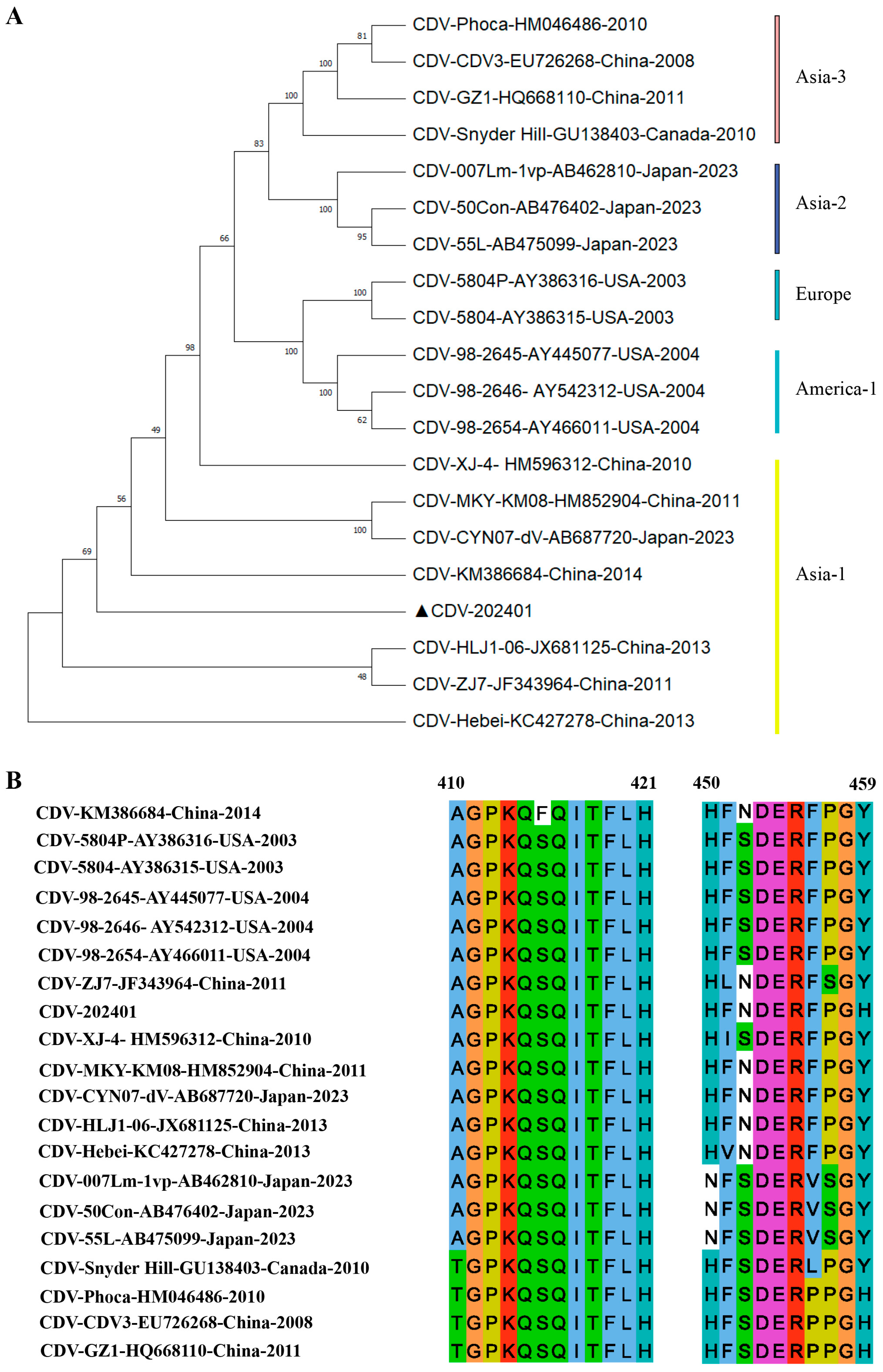
3.4. Characterization of Epitope Conservation Among CDV Strains and Their Recognition by Monoclonal Antibodies
3.5. Colloidal Gold–Antibody Conjugate Preparation
3.6. Evaluation of the Specificity and Sensitivity of the Immunochromatographic Strips
3.7. Repeatability and Stability of the CDV Test Strips
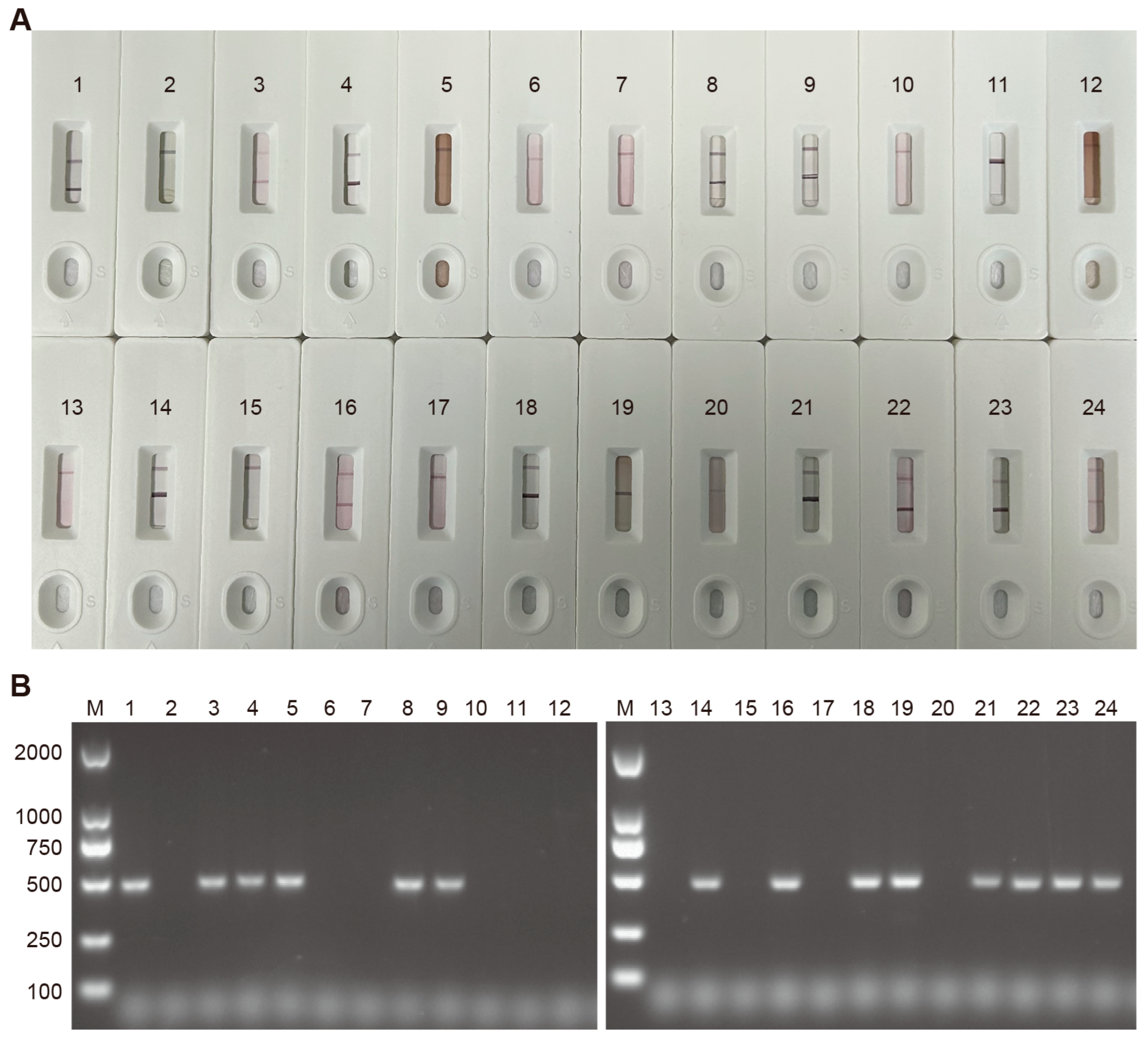
3.8. Evaluation of the Consistency Between Colloidal Gold Test Strips and RT-PCR in Detecting CDV
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviations | Meaning |
| CDV | Canine Distemper Virus |
| CD | Canine Distemper |
| mAb | Monoclonal Antibody |
| N protein | Nucleoprotein |
| ELISA | Indirect Fluorescent Antibody Testing |
| ICS | Immunochromatographic Strip |
| CAV | Canine Adenovirus |
| CCV | Canine Coronavirus |
| CPV | Canine Parvovirus |
| CPIV | Canine Parainfluenza Virus |
| MERS-CoV | Middle East Respiratory Syndrome Coronavirus |
| IFA | Indirect Immunofluorescence Assay |
References
- Martinez-Gutierrez, M.; Ruiz-Saenz, J. Diversity of susceptible hosts in canine distemper virus infection: A systematic review and data synthesis. BMC Vet. Res. 2016, 12, 78. [Google Scholar] [CrossRef]
- Karki, M.; Rajak, K.K.; Singh, R.P. Canine morbillivirus (CDV): A review on current status, emergence and the diagnostics. Virusdisease 2022, 33, 309–321. [Google Scholar] [CrossRef]
- Duque-Valencia, J.; Sarute, N.; Olarte-Castillo, X.A.; Ruiz-Saenz, J. Evolution and Interspecies Transmission of Canine Distemper Virus-An Outlook of the Diverse Evolutionary Landscapes of a Multi-Host Virus. Viruses 2019, 11, 582. [Google Scholar] [CrossRef] [PubMed]
- Bieringer, M.; Han, J.W.; Kendl, S.; Khosravi, M.; Plattet, P.; Schneider-Schaulies, J. Experimental adaptation of wild-type canine distemper virus (CDV) to the human entry receptor CD150. PLoS ONE 2013, 8, e57488. [Google Scholar] [CrossRef]
- Martella, V.; Cirone, F.; Elia, G.; Lorusso, E.; Decaro, N.; Campolo, M.; Desario, C.; Lucente, M.S.; Bellacicco, A.L.; Blixenkrone-Møller, M.; et al. Heterogeneity within the hemagglutinin genes of canine distemper virus (CDV) strains detected in Italy. Vet. Microbiol. 2006, 116, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, O.V.; Botelho, C.V.; Ferreira, C.G.; Scherer, P.O.; Soares-Martins, J.A.; Almeida, M.R.; Silva Júnior, A. Immunopathogenic and neurological mechanisms of canine distemper virus. Adv. Virol. 2012, 2012, 163860. [Google Scholar] [CrossRef] [PubMed]
- Martella, V.; Elia, G.; Buonavoglia, C. Canine distemper virus. Vet. Clin. N. Am. Small Anim. Pract. 2008, 38, 787–797. [Google Scholar] [CrossRef]
- Appel, M.J.; Summers, B.A. Pathogenicity of morbilliviruses for terrestrial carnivores. Vet. Microbiol. 1995, 44, 187–191. [Google Scholar] [CrossRef]
- Blixenkrone-Möller, M.; Svansson, V.; Appel, M.; Krogsrud, J.; Have, P.; Orvell, C. Antigenic relationships between field isolates of morbilliviruses from different carnivores. Arch. Virol. 1992, 123, 279–294. [Google Scholar] [CrossRef]
- Stokholm, I.; Puryear, W.; Sawatzki, K.; Knudsen, S.W.; Terkelsen, T.; Becher, P.; Siebert, U.; Olsen, M.T. Emergence and radiation of distemper viruses in terrestrial and marine mammals. Proc. Biol. Sci. 2021, 288, 20211969. [Google Scholar] [CrossRef]
- Cao, Z.; Yi, L.; Liu, X.; Shang, J.; Cheng, Y.; Feng, E.; Liu, X.; Fan, Y.; Hu, X.; Cai, W.; et al. Rapid lateral flow immunoassay for fluorescence detection of canine distemper virus (CDV). Front. Vet. Sci. 2024, 11, 1413420. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Xu, H.; Zhao, X.; Zhang, K.; Yin, D.; Ma, S.; Li, W.; Li, S.; Ren, J.; Wen, J. Multiplex one-step RT–qPCR assays for simultaneous detection of AMDV, MEV and CDV. BMC Vet. Res. 2025, 21, 18. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Su, J.; Hu, B.; Bai, X. A method for screening CDV microneutralization activity in microvolume samples. J. Virol. Methods 2024, 325, 114883. [Google Scholar] [CrossRef] [PubMed]
- Meazzi, S.; Filipe, J.; Fiore, A.; Di Bella, S.; Mira, F.; Dall’Ara, P. Agreement between In-Clinics and Virus Neutralization Tests in Detecting Antibodies against Canine Distemper Virus (CDV). Viruses 2022, 14, 517. [Google Scholar] [CrossRef]
- Fenwick, C.; Croxatto, A.; Coste, A.T.; Pojer, F.; Andre, C.; Pellaton, C.; Farina, A.; Campos, J.; Hacker, D.; Lau, K.; et al. Changes in SARS-CoV-2 Spike versus Nucleoprotein Antibody Responses Impact the Estimates of Infections in Population-Based Seroprevalence Studies. J. Virol. 2021, 95, e01828-20. [Google Scholar] [CrossRef]
- An, D.J.; Kim, T.Y.; Song, D.S.; Kang, B.K.; Park, B.K. An immunochromatography assay for rapid antemortem diagnosis of dogs suspected to have canine distemper. J. Virol. Methods 2008, 147, 244–249. [Google Scholar] [CrossRef]
- Bi, Z.; Wang, Y.; Pan, Q.; Xia, X.; Xu, L. Development of CDV-specific monoclonal antibodies for differentiation of variable epitopes of nucleocapsid protein. Vet. Microbiol. 2017, 211, 84–91. [Google Scholar] [CrossRef]
- Shahjahan, T.; Javed, B.; Sharma, V.; Tian, F. pH and NaCl Optimisation to Improve the Stability of Gold and Silver Nanoparticles’ Anti-Zearalenone Antibody Conjugates for Immunochromatographic Assay. Methods Protoc. 2023, 6, 93. [Google Scholar] [CrossRef]
- Ruiz, G.; Tripathi, K.; Okyem, S.; Driskell, J.D. pH Impacts the Orientation of Antibody Adsorbed onto Gold Nanoparticles. Bioconjug. Chem. 2019, 30, 1182–1191. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Y.; Li, Y.; Wang, J.; Cheng, L.; Deng, L. Effects of pH on the properties of colloidal gold labeling monoclonal antibody. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2014, 30, 1170–1173. [Google Scholar]
- Zhu, Y.; Li, L.; Wang, Z.; Chen, Y.; Zhao, Z.; Zhu, L.; Wu, X.; Wan, Y.; He, F.; Shen, J. Development of an immunochromatography strip for the rapid detection of 12 fluoroquinolones in chicken muscle and liver. J. Agric. Food Chem. 2008, 56, 5469–5474. [Google Scholar] [CrossRef] [PubMed]
- Preechakasedkit, P.; Pinwattana, K.; Dungchai, W.; Siangproh, W.; Chaicumpa, W.; Tongtawe, P.; Chailapakul, O. Development of a one-step immunochromatographic strip test using gold nanoparticles for the rapid detection of Salmonella typhi in human serum. Biosens. Bioelectron. 2012, 31, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, G.; Liu, Q.; Teng, M.; Yang, J.; Wang, J. Development of a lateral flow colloidal gold immunoassay strip for the rapid detection of enrofloxacin residues. J. Agric. Food Chem. 2008, 56, 12138–12142. [Google Scholar] [CrossRef]
- Gas, F.; Baus, B.; Pinto, L.; Compere, C.; Tanchou, V.; Quéméneur, E. One step immunochromatographic assay for the rapid detection of Alexandrium minutum. Biosens. Bioelectron. 2010, 25, 1235–1239. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gaddi, A.V.; Capello, F.; Aluigi, L.; Antignani, P.L.; Callegaro, A.; Casu, G.; Cipolla, E.; Cipolla, M.; Cosco, L.; Culzoni, F.; et al. The Strategic Alliance between Clinical and Molecular Science in the War against SARS-CoV-2, with the Rapid-Diagnostics Test as an Indispensable Weapon for Front Line Doctors. Int. J. Mol. Sci. 2020, 21, 4446. [Google Scholar] [CrossRef]
- Cherpillod, P.; Tipold, A.; Griot-Wenk, M.; Cardozo, C.; Schmid, I.; Fatzer, R.; Schobesberger, M.; Zurbriggen, R.; Bruckner, L.; Roch, F.; et al. DNA vaccine encoding nucleocapsid and surface proteins of wild type canine distemper virus protects its natural host against distemper. Vaccine 2000, 18, 2927–2936. [Google Scholar] [CrossRef]
- Muriaux, D.; Darlix, J.L. Properties and functions of the nucleocapsid protein in virus assembly. RNA Biol. 2010, 7, 744–753. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, L.; Du, K.; Zhang, Y.; Wang, J.; Chen, L.; Lyu, Y.; Li, J.; Liu, H.; Huo, J.; et al. Foundation and Clinical Evaluation of a New Method for Detecting SARS-CoV-2 Antigen by Fluorescent Microsphere Immunochromatography. Front. Cell Infect. Microbiol. 2020, 10, 553837. [Google Scholar] [CrossRef]
- Wernike, K.; Brocchi, E.; Cordioli, P.; Sénéchal, Y.; Schelp, C.; Wegelt, A.; Aebischer, A.; Roman-Sosa, G.; Reimann, I.; Beer, M. A novel panel of monoclonal antibodies against Schmallenberg virus nucleoprotein and glycoprotein Gc allows specific orthobunyavirus detection and reveals antigenic differences. Vet. Res. 2015, 46, 27. [Google Scholar] [CrossRef]
- Oluka, G.K.; Namubiru, P.; Kato, L.; Ankunda, V.; Gombe, B.; Cotten, M.; Musenero, M.; Kaleebu, P.; Fox, J.; Serwanga, J. Optimisation and Validation of a conventional ELISA and cut-offs for detecting and quantifying anti-SARS-CoV-2 Spike, RBD, and Nucleoprotein IgG, IgM, and IgA antibodies in Uganda. Front. Immunol. 2023, 14, 1113194. [Google Scholar] [CrossRef]
- Lombe, B.P.; Miyamoto, H.; Saito, T.; Yoshida, R.; Manzoor, R.; Kajihara, M.; Shimojima, M.; Fukushi, S.; Morikawa, S.; Yoshikawa, T.; et al. Purification of Crimean-Congo hemorrhagic fever virus nucleoprotein and its utility for serological diagnosis. Sci. Rep. 2021, 11, 2324. [Google Scholar] [CrossRef] [PubMed]
- SowjanyaKumari, S.; Bokade, P.P.; Kumar, K.V.; Bharath, V.; Shome, B.R.; Balamurugan, V. Potential diagnostic application of the baculovirus-expressed recombinant truncated nucleocapsid protein of peste des petits ruminants virus in ELISA. J. Immunol. Methods 2023, 516, 113469. [Google Scholar] [CrossRef] [PubMed]
- Leirs, K.; Tewari Kumar, P.; Decrop, D.; Pérez-Ruiz, E.; Leblebici, P.; Van Kelst, B.; Compernolle, G.; Meeuws, H.; Van Wesenbeeck, L.; Lagatie, O.; et al. Bioassay Development for Ultrasensitive Detection of Influenza A Nucleoprotein Using Digital ELISA. Anal. Chem. 2016, 88, 8450–8458. [Google Scholar] [CrossRef]
- Masuda, M.; Sato, H.; Kamata, H.; Katsuo, T.; Takenaka, A.; Miura, R.; Yoneda, M.; Tsukiyama-Kohara, K.; Mizumoto, K.; Kai, C. Characterization of monoclonal antibodies directed against the canine distemper virus nucleocapsid protein. Comp. Immunol. Microbiol. Infect. Dis. 2006, 29, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Cheng, Y.; Zhang, M.; Cao, Z.; Tong, M.; Wang, J.; Zhao, H.; Lin, P.; Cheng, S. Identification of a novel canine distemper virus B-cell epitope using a monoclonal antibody against nucleocapsid protein. Virus Res. 2016, 213, 1–5. [Google Scholar] [CrossRef]
- Yi, L.; Cheng, S. A monoclonal antibody against truncated N protein (aa 277-471) of canine distemper virus. Monoclon. Antibodies Immunodiagn. Immunother. 2014, 33, 52–56. [Google Scholar] [CrossRef]



| Primers | Sequence (5′-3′) |
|---|---|
| pEGFP-C3-N1-F | TACAAGTACTCAGATCTCGAGTTGGCATCACCAAG |
| pEGFP-C3-N1-R | CGACTGCAGAATTCGAAGCTTAGAATTTTCCAGAATAACCAT |
| pEGFP-C3-N2-F | TACAAGTACTCAGATCTCGAGGTTATTCTGGAAAATTCTGTTCAG |
| pEGFP-C3-N2-R | CGTCGACTGCAGAATTCGAAGCTTTTCCTTGGTGATGCC |
| pEGFP-C3-N3-F | TACAAGTACTCAGATCTCGAGCTTGGCATCACCAAG |
| pEGFP-C3-N3-R | CCGTCGACTGCAGAATTCGAAGCTTGTGCCCTGGAAAGCG |
| pEGFP-C3-N4-F | TACAAGTACTCAGATCTCGAGGAACGGTTTTCCAGG |
| pEGFP-C3-N4-R | CGTCGACTGCAGAATTCGAAGCTTGATGGTTGGGGGTTGTTG |
| pEGFP-C3-N3.1-F | TACAAGTACTCAGATCTCGAGCTTGGCATCACCAAG |
| pEGFP-C3-N3.1-R | CGTCGACTGCAGAATTCGAAGCTTTATTTCTGGCACTAGC |
| pEGFP-C3-N3.2-R | CCGTCGACTGCAGAATTCGAAGCTTAGTGCGAATCGTCCGGT |
| pEGFP-C3-N3.3-R | CCGTCGACTGCAGAATTCGAAGCTTGTGCAGAAAAGTGAT |
| pEGFP-C3-N3.4-R | CGTCGACTGCAGAATTCGAAGCTTGATGGTTGGGGGTTGTTG |
| pEGFP-C3-N3.5-R | CCGTCGACTGCAGAATTCGAAGCTTGATGGGGTATTTGTC |
| pEGFP-C3-N3.6-R | CGTCGACTGCAGAATTCGAAGCTTGATGGTTGGGGGTTGTTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Bi, Z.; Du, Q.; Zhang, M.; Cai, L.; Fan, Y.; Tang, J.; Hu, M.; Zhu, S.; Tang, A.; et al. Development of a Colloidal Gold-Based Immunochromatographic Strip Targeting the Nucleoprotein for Rapid Detection of Canine Distemper Virus. Biosensors 2025, 15, 432. https://doi.org/10.3390/bios15070432
Zhang Z, Bi Z, Du Q, Zhang M, Cai L, Fan Y, Tang J, Hu M, Zhu S, Tang A, et al. Development of a Colloidal Gold-Based Immunochromatographic Strip Targeting the Nucleoprotein for Rapid Detection of Canine Distemper Virus. Biosensors. 2025; 15(7):432. https://doi.org/10.3390/bios15070432
Chicago/Turabian StyleZhang, Zichen, Zhuangli Bi, Qingqing Du, Miao Zhang, Linying Cai, Yiming Fan, Jingjie Tang, Mingxing Hu, Shiqiang Zhu, Aoxing Tang, and et al. 2025. "Development of a Colloidal Gold-Based Immunochromatographic Strip Targeting the Nucleoprotein for Rapid Detection of Canine Distemper Virus" Biosensors 15, no. 7: 432. https://doi.org/10.3390/bios15070432
APA StyleZhang, Z., Bi, Z., Du, Q., Zhang, M., Cai, L., Fan, Y., Tang, J., Hu, M., Zhu, S., Tang, A., Wang, G., Liu, G., & Zhu, Y. (2025). Development of a Colloidal Gold-Based Immunochromatographic Strip Targeting the Nucleoprotein for Rapid Detection of Canine Distemper Virus. Biosensors, 15(7), 432. https://doi.org/10.3390/bios15070432





