Bioluminescence in Clinical and Point-of-Care Testing
Abstract
1. Introduction
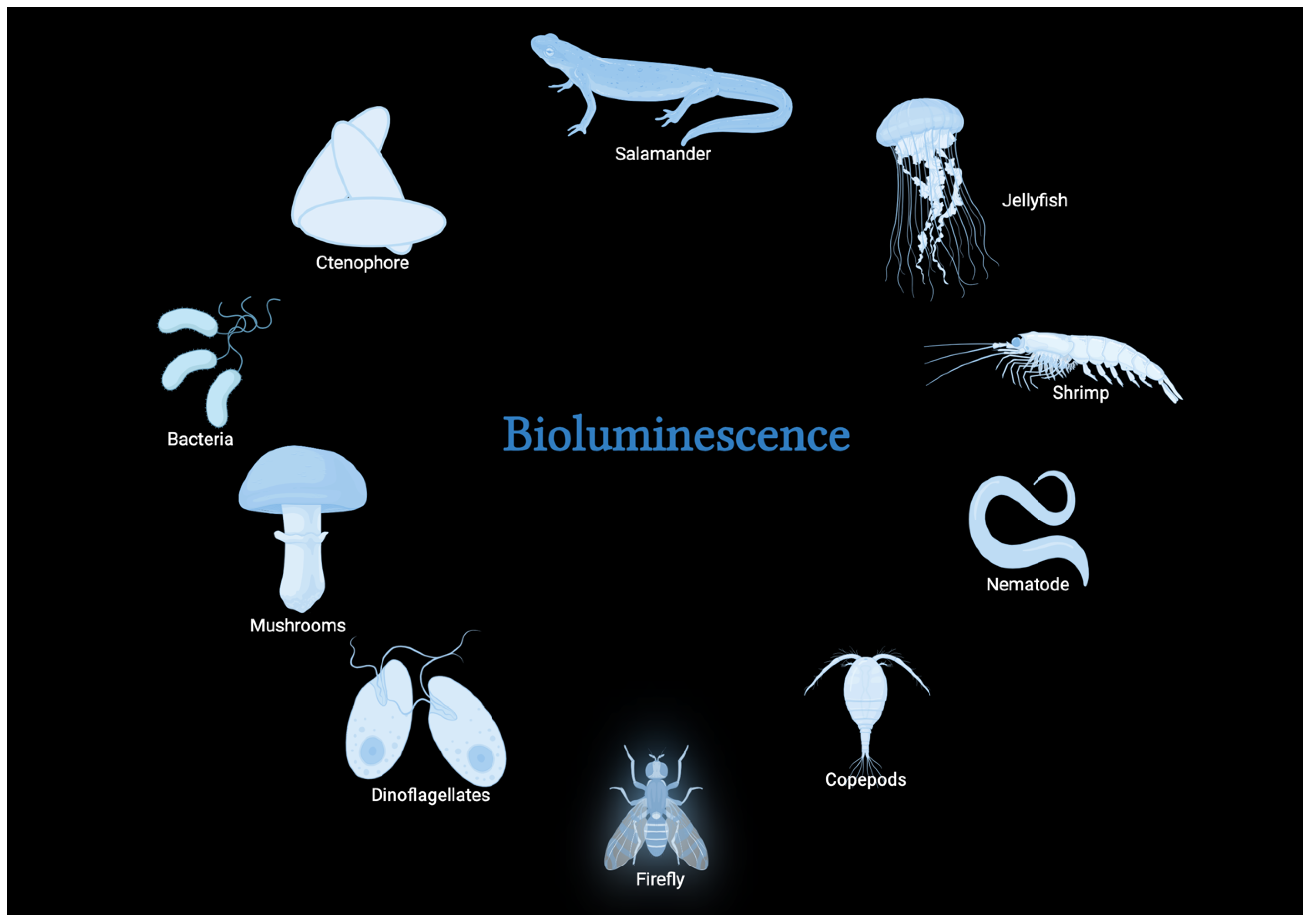
2. Bioluminescence and Its Natural Occurrence
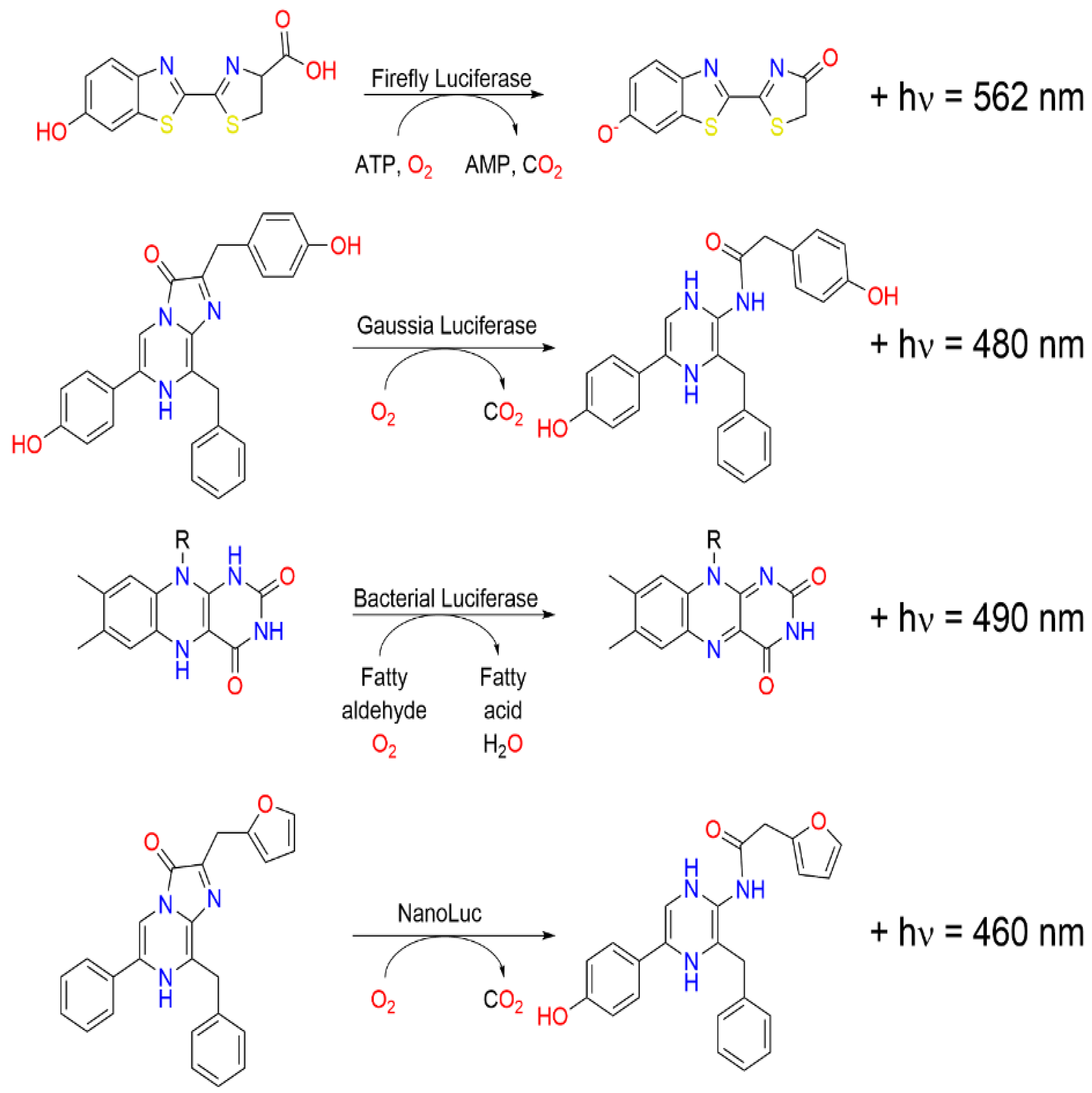
2.1. Bioluminescent Proteins
| Bioluminescent Protein | Protein Data Bank | Crystal Structure | Source | Molecular Weight | Substrate | Co-Factor | Emission Wavelength | Reference |
|---|---|---|---|---|---|---|---|---|
| Bacterial Luciferase | 1LUC |  | Vibrio harveyi | 62 kDa | Reduced flavin | FMNH2 | 490 nm | [42] |
| Firefly Luciferase | 1LCI | 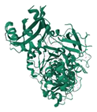 | Photinus pyralis | 60.82 kDa | D-Luciferin | ATP, Mg2+ | 560 nm | [43] |
| Dinoflagellate Luciferase | 1VPR | 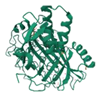 | Lingulodinium polyedra | 42.03 kDa | Luciferin | Oxygen | 475 nm | [44] |
| Renilla Luciferase | 2PSH | 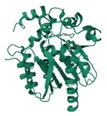 | Renilla reniformis | 36 kDa | Coelenterazine | Oxygen | 480 nm | [45] |
| Gaussia Luciferase | 7D20 | 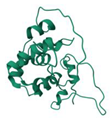 | Gaussia princeps | 20 kDa | Coelenterazine | 480 nm | [46] | |
| Oplophorus (NanoLuc) | 7VXS |  | Oplophorus gracilirostris | 19 kDa | Coelenterazine | 455 nm | [47] | |
| Aequorin | 1SL8 |  | Aequorea victoria | 21 kDa | Coelenterazine | Ca2+ | 465 nm | [48] |
| Berovin | 4MN0 |  | Beroe abyssicola | 25.47 kDa | Coelenterazine | Ca2+ | 491 nm | [49] |
2.2. Application of Bioluminescence in Biological and Biochemical Research
2.3. Exploring Bioluminescence in the Field of Medicine
2.4. Advantages and Limitations of Luciferase-Based POCT Platforms
3. Point-of-Care in Infectious Disease Diagnosis
3.1. Importance of Accurate and Timely Diagnosis
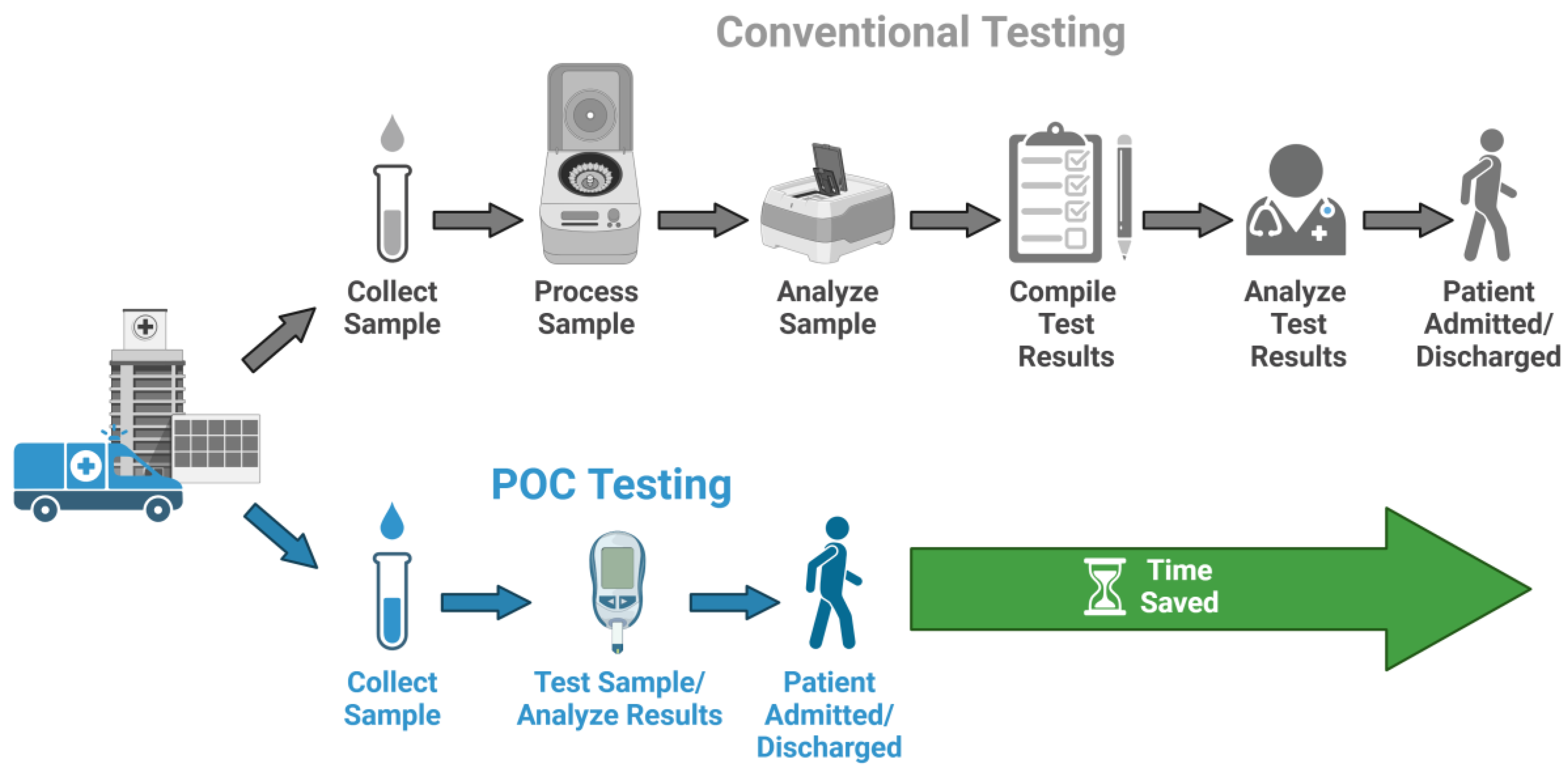
3.2. Requirements of Point-of-Care Tests
3.3. Technical Strategies to Optimize Light Collection in Bioluminescent POC Devices
3.4. Bioluminescence in Point-of-Care Testing
3.5. Advanced Bioluminescent Platforms
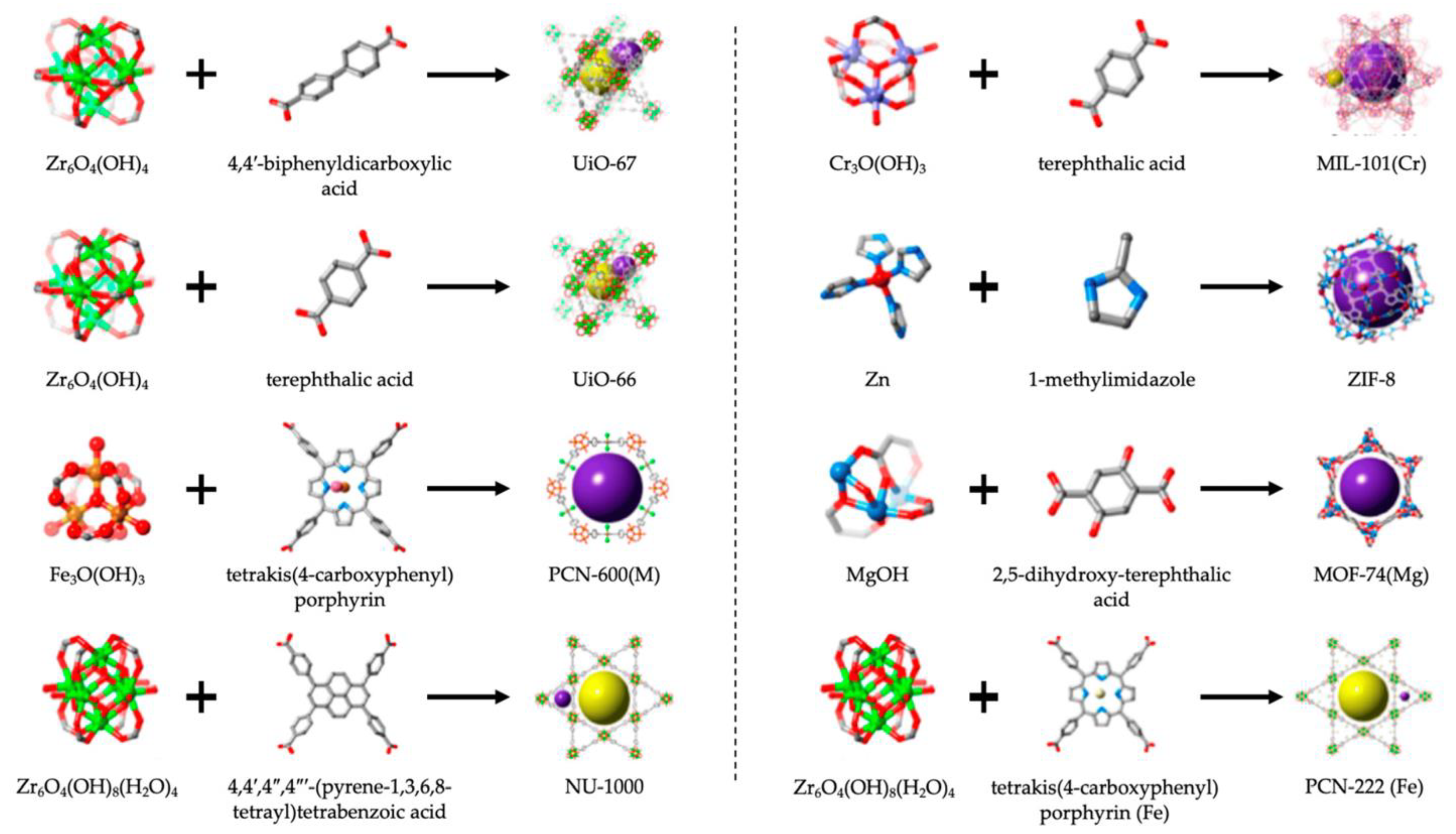
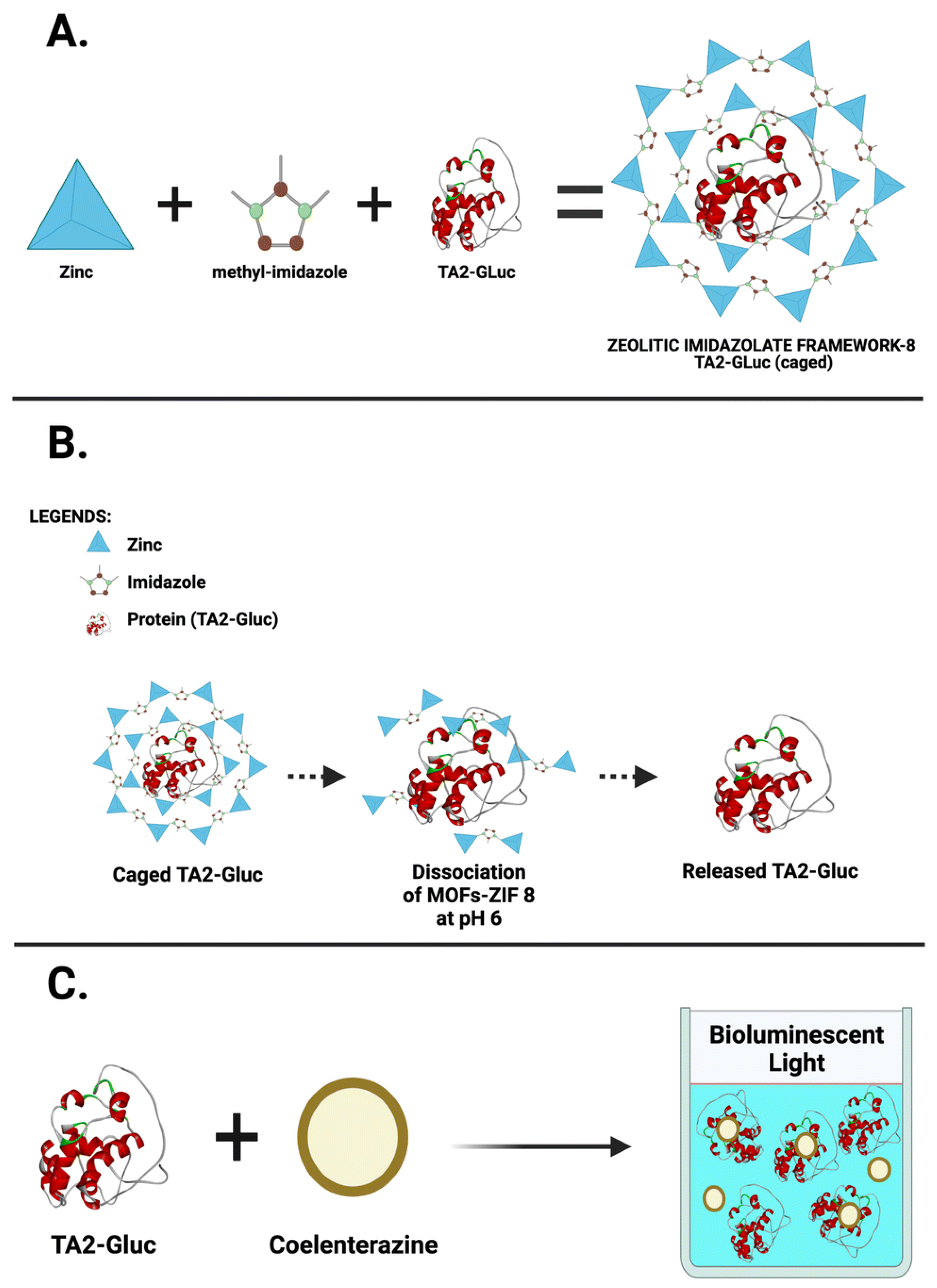
3.6. Metal-Organic Frameworks
4. Challenges and Future Directions
Funding
Conflicts of Interest
References
- Shaw, J.L. Practical challenges related to point of care testing. Pract. Lab. Med. 2016, 4, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Herrador, E.; Cruz-Vera, M.; Valcárcel, M. Analytical connotations of point-of-care testing. Analyst 2010, 135, 2220–2232. [Google Scholar] [CrossRef] [PubMed]
- Hansen, G.T. Point-of-care testing in microbiology: A mechanism for improving patient outcomes. Clin. Chem. 2020, 66, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Luppa, P.B.; Müller, C.; Schlichtiger, A.; Schlebusch, H. Point-of-care testing (POCT): Current techniques and future perspectives. TrAC Trends Anal. Chem. 2011, 30, 887–898. [Google Scholar] [CrossRef]
- Heidt, B.; Siqueira, W.F.; Eersels, K.; Diliën, H.; van Grinsven, B.; Fujiwara, R.T.; Cleij, T.J. Point of care diagnostics in resource-limited settings: A review of the present and future of PoC in its most needed environment. Biosensors 2020, 10, 133. [Google Scholar] [CrossRef]
- Dima, K. Point of Care Testing (POCT) Present and Future. 2021. Available online: https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Dima%2C+K.%2C+Point+of+care+testing+%28POCT%29+present+and+future.+2021&btnG= (accessed on 14 May 2025).
- St John, A.; Price, C.P. Existing and emerging technologies for point-of-care testing. Clin. Biochem. Rev. 2014, 35, 155. [Google Scholar]
- Drain, P.K.; Hyle, E.P.; Noubary, F.; Freedberg, K.A.; Wilson, D.; Bishai, W.R.; Rodriguez, W.; Bassett, I.V. Diagnostic point-of-care tests in resource-limited settings. Lancet Infect. Dis. 2014, 14, 239–249. [Google Scholar] [CrossRef]
- Soh, J.H.; Chan, H.-M.; Ying, J.Y. Strategies for developing sensitive and specific nanoparticle-based lateral flow assays as point-of-care diagnostic device. Nano Today 2020, 30, 100831. [Google Scholar] [CrossRef]
- Hesse, E.A.; Patton, S.A.; Huppert, J.S.; Gaydos, C.A. Using a rapid communication approach to improve a POC Chlamydia test. IEEE Trans. Biomed. Eng. 2010, 58, 837–840. [Google Scholar] [CrossRef]
- Manmana, Y.; Kubo, T.; Otsuka, K. Recent developments of point-of-care (POC) testing platform for biomolecules. TrAC Trends Anal. Chem. 2021, 135, 116160. [Google Scholar] [CrossRef]
- Bartholomeusz, D.A.; Andrade, J.; Frazier, A.B. Bioluminescent based chemchip for point-of-care diagnostics. In Proceedings of the 1st Annual International IEEE-EMBS Special Topic Conference on Microtechnologies in Medicine and Biology, Proceedings (Cat. No. 00EX451), Lyon, France, 12–14 October 2000. [Google Scholar]
- Zarei, M. Portable biosensing devices for point-of-care diagnostics: Recent developments and applications. TrAC Trends Anal. Chem. 2017, 91, 26–41. [Google Scholar] [CrossRef]
- Tomimuro, K.; Tenda, K.; Ni, Y.; Hiruta, Y.; Merkx, M.; Citterio, D. Thread-based bioluminescent sensor for detecting multiple antibodies in a single drop of whole blood. ACS Sens. 2020, 5, 1786–1794. [Google Scholar] [CrossRef] [PubMed]
- Pfleger, K.D.; Seeber, R.M.; Eidne, K.A. Bioluminescence resonance energy transfer (BRET) for the real-time detection of protein-protein interactions. Nat. Protoc. 2006, 1, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Mezzanotte, L.; van‘t Root, M.; Karatas, H.; Goun, E.A.; Löwik, C.W. In vivo molecular bioluminescence imaging: New tools and applications. Trends Biotechnol. 2017, 35, 640–652. [Google Scholar] [CrossRef]
- Griss, R.; Schena, A.; Reymond, L.; Patiny, L.; Werner, D.; Tinberg, C.E.; Baker, D.; Johnsson, K. Bioluminescent sensor proteins for point-of-care therapeutic drug monitoring. Nat. Chem. Biol. 2014, 10, 598–603. [Google Scholar] [CrossRef]
- Calabretta, M.M.; Michelini, E. Current advances in the use of bioluminescence assays for drug discovery: An update of the last ten years. Expert Opin. Drug Discov. 2024, 19, 85–95. [Google Scholar] [CrossRef]
- Kelly, T.; Yang, X. Application of Fluorescence- and Bioluminescence-Based Biosensors in Cancer Drug Discovery. Biosensors 2024, 14, 570. [Google Scholar] [CrossRef]
- Sangeetha, B.; Leroy, K.I.; Udaya Kumar, B. Harnessing Bioluminescence: A Comprehensive Review of In Vivo Imaging for Disease Monitoring and Therapeutic Intervention. Cell Biochem. Funct. 2024, 42, e70020. [Google Scholar] [CrossRef]
- Dunuweera, A.N.; Dunuweera, S.P.; Ranganathan, K. A Comprehensive Exploration of Bioluminescence Systems, Mechanisms, and Advanced Assays for Versatile Applications. Biochem. Res. Int. 2024, 2024, 8273237. [Google Scholar] [CrossRef] [PubMed]
- Roda, B.; Deo, S.K.; O’Connor, G.; Moraskie, M.; Giordani, S.; Marassi, V.; Roda, A.; Daunert, S. Shining light on biosensors: Chemiluminescence and bioluminescence in enabling technologies. Trends Anal. Chem. 2024, 180, 117975. [Google Scholar] [CrossRef]
- Haddock, S.H.; Moline, M.A.; Case, J.F. Bioluminescence in the sea. Annu. Rev. Mar. Sci. 2010, 2, 443–493. [Google Scholar] [CrossRef] [PubMed]
- Desjardin, D.E.; Oliveira, A.G.; Stevani, C.V. Fungi bioluminescence revisited. Photochem. Photobiol. Sci. 2008, 7, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Hastings, J.W. Progress and perspectives on bioluminescence: From luminous organisms to molecular mechanisms. In Chemiluminescence and Bioluminescence; The Royal Society of Chemistry: London, UK, 2010; pp. 91–112. [Google Scholar]
- Sharifian, S.; Homaei, A.; Hemmati, R.; Luwor, R.B.; Khajeh, K. The emerging use of bioluminescence in medical research. Biomed. Pharmacother. 2018, 101, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Syed, A.J.; Anderson, J.C. Applications of bioluminescence in biotechnology and beyond. Chem. Soc. Rev. 2021, 50, 5668–5705. [Google Scholar] [CrossRef]
- Lee, J. Bioluminescence, the Nature of the Light; The University of Gerogia: Athens, GA, USA, 2020. [Google Scholar]
- Lee, J. Perspectives on bioluminescence mechanisms. Photochem. Photobiol. 2017, 93, 389–404. [Google Scholar] [CrossRef]
- Roda, A. A history of bioluminescence and chemiluminescence from ancient times to the present. In Chemiluminescence and Bioluminescence; The Royal Society of Chemistry: London, UK, 2010; pp. 1–50. [Google Scholar]
- Welsh, D.K.; Kay, S.A. Bioluminescence imaging in living organisms. Curr. Opin. Biotechnol. 2005, 16, 73–78. [Google Scholar] [CrossRef]
- Johnson, F.H.; Haneda, Y. Bioluminescence in Progress; Princeton University Press: Princeton, NJ, USA, 2015. [Google Scholar]
- Kotlobay, A.; Kaskova, Z.; Yampolsky, I. Palette of luciferases: Natural biotools for new applications in biomedicine. Acta Naturae (англoязычная версия) 2020, 12, 15–27. [Google Scholar] [CrossRef]
- Watkins, O.C.; Sharpe, M.L.; Perry, N.B.; Krause, K.L. New Zealand glowworm (Arachnocampa luminosa) bioluminescence is produced by a firefly-like luciferase but an entirely new luciferin. Sci. Rep. 2018, 8, 3278. [Google Scholar] [CrossRef]
- Lyons, S.K.; Patrick, P.S.; Brindle, K.M. Imaging mouse cancer models in vivo using reporter transgenes. Cold Spring Harb. Protoc. 2013, 2013, 685–699. [Google Scholar] [CrossRef]
- McCutcheon, D.C.; Porterfield, W.B.; Prescher, J.A. Rapid and scalable assembly of firefly luciferase substrates. Org. Biomol. Chem. 2015, 13, 2117–2121. [Google Scholar] [CrossRef]
- Kato, M.; Tsuchihashi, K.; Kanie, S.; Oba, Y.; Nishikawa, T. A practical, biomimetic, one-pot synthesis of firefly luciferin. Sci. Rep. 2024, 14, 30461. [Google Scholar] [CrossRef] [PubMed]
- Ando, Y.; Niwa, K.; Yamada, N.; Enomoto, T.; Irie, T.; Kubota, H.; Ohmiya, Y.; Akiyama, H. Firefly bioluminescence quantum yield and colour change by pH-sensitive green emission. Nat. Photonics 2008, 2, 44–47. [Google Scholar] [CrossRef]
- Ono, R.; Osawa, K.; Takahashi, Y.; Noguchi, Y.; Kitada, N.; Saito-Moriya, R.; Hirano, T.; Maki, S.A.; Shibata, K.; Akiyama, H. Quantum yield of near-infrared bioluminescence with firefly luciferin analog: AkaLumine. J. Photochem. Photobiol. A Chem. 2023, 434, 114270. [Google Scholar] [CrossRef]
- De, A.; Arora, R.; Jasani, A. Engineering aspects of bioluminescence resonance energy transfer systems. In Engineering in Translational Medicine; Springer: Berlin/Heidelberg, Germany, 2013; pp. 257–300. [Google Scholar]
- Fujii, H.; Noda, K.; Asami, Y.; Kuroda, A.; Sakata, M.; Tokida, A. Increase in bioluminescence intensity of firefly luciferase using genetic modification. Anal. Biochem. 2007, 366, 131–136. [Google Scholar] [CrossRef]
- Fisher, A.J.; Thompson, T.B.; Thoden, J.B.; Baldwin, T.O.; Rayment, I. The 1.5-Å resolution crystal structure of bacterial luciferase in low salt conditions. J. Biol. Chem. 1996, 271, 21956–21968. [Google Scholar] [CrossRef]
- Conti, E.; Franks, N.P.; Brick, P. Crystal structure of firefly luciferase throws light on a superfamily of adenylate-forming enzymes. Structure 1996, 4, 287–298. [Google Scholar] [CrossRef]
- Schultz, L.W.; Liu, L.; Cegielski, M.; Hastings, J.W. Crystal structure of a pH-regulated luciferase catalyzing the bioluminescent oxidation of an open tetrapyrrole. Proc. Natl. Acad. Sci. USA 2005, 102, 1378–1383. [Google Scholar] [CrossRef]
- Loening, A.M.; Fenn, T.D.; Gambhir, S.S. Crystal structures of the luciferase and green fluorescent protein from Renilla reniformis. J. Mol. Biol. 2007, 374, 1017–1028. [Google Scholar] [CrossRef]
- Ho, C.-H.; Takizawa, Y.; Kobayashi, W.; Arimura, Y.; Kimura, H.; Kurumizaka, H. Structural basis of nucleosomal histone H4 lysine 20 methylation by SET8 methyltransferase. Life Sci. Alliance 2021, 4, e202000919. [Google Scholar] [CrossRef]
- Inouye, S.; Sato, J.-i.; Sahara-Miura, Y.; Tomabechi, Y.; Sumida, Y.; Sekine, S.-i.; Shirouzu, M.; Hosoya, T. Reverse mutants of the catalytic 19 kDa mutant protein (nanoKAZ/nanoLuc) from Oplophorus luciferase with coelenterazine as preferred substrate. PLoS ONE 2022, 17, e0272992. [Google Scholar] [CrossRef]
- Deng, L.; Vysotski, E.S.; Markova, S.V.; Liu, Z.J.; Lee, J.; Rose, J.; Wang, B.C. All three Ca2+-binding loops of photoproteins bind calcium ions: The crystal structures of calcium-loaded apo-aequorin and apo-obelin. Protein Sci. 2005, 14, 663–675. [Google Scholar] [CrossRef] [PubMed]
- Stepanyuk, G.A.; Liu, Z.-J.; Burakova, L.P.; Lee, J.; Rose, J.; Vysotski, E.S.; Wang, B.-C. Spatial structure of the novel light-sensitive photoprotein berovin from the ctenophore Beroe abyssicola in the Ca2+-loaded apoprotein conformation state. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2013, 1834, 2139–2146. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Du, L.; Li, M. Lighting up bioluminescence with coelenterazine: Strategies and applications. Photochem. Photobiol. Sci. 2016, 15, 466–480. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Pang, H. Glucuronidation of d-Luciferin In Vitro: Isoform Selectivity and Kinetics Characterization. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 549–556. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, L.; Yang, L.; Ni, W.; Li, Y.; Wu, Y. Tandem reassembly of split luciferase-DNA chimeras for bioluminescent detection of attomolar circulating microRNAs using a smartphone. Biosens. Bioelectron. 2021, 173, 112824. [Google Scholar] [CrossRef]
- Rowe, L.; Dikici, E.; Daunert, S. Engineering bioluminescent proteins: Expanding their analytical potential. Anal. Chem. 2009, 81, 8662–8668. [Google Scholar] [CrossRef]
- Tafreshi, N.K.; Sadeghizadeh, M.; Emamzadeh, R.; Ranjbar, B.; Naderi-Manesh, H.; Hosseinkhani, S. Site-directed mutagenesis of firefly luciferase: Implication of conserved residue (s) in bioluminescence emission spectra among firefly luciferases. Biochem. J. 2008, 412, 27–33. [Google Scholar] [CrossRef]
- Auld, D.S.; Zhang, Y.-Q.; Southall, N.T.; Rai, G.; Landsman, M.; MacLure, J.; Langevin, D.; Thomas, C.J.; Austin, C.P.; Inglese, J. A basis for reduced chemical library inhibition of firefly luciferase obtained from directed evolution. J. Med. Chem. 2009, 52, 1450–1458. [Google Scholar] [CrossRef]
- Frank, L.A.; Krasitskaya, V.V. Application of enzyme bioluminescence for medical diagnostics. In Bioluminescence: Fundamentals and Applications in Biotechnology—Volume 1; Springer: Berlin/Heidelberg, Germany, 2014; pp. 175–197. [Google Scholar]
- Alsawaftah, N.; Farooq, A.; Dhou, S.; Majdalawieh, A.F. Bioluminescence imaging applications in cancer: A comprehensive review. IEEE Rev. Biomed. Eng. 2020, 14, 307–326. [Google Scholar] [CrossRef]
- Luke Mankin, S.; Allen, G.C.; Thompson, W.F. Introduction of a plant intron into the luciferase gene of Photinus pyralis. Plant Mol. Biol. Rep. 1997, 15, 186–196. [Google Scholar] [CrossRef]
- Day, J.; Bailey, M. Structure and evolution of the luciferin-regenerating enzyme (LRE) gene from the firefly Photinus pyralis. Insect Mol. Biol. 2003, 12, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Branchini, B.R.; Southworth, T.L.; Fontaine, D.M.; Davis, A.L.; Behney, C.E.; Murtiashaw, M.H. A Photinus pyralis and Luciola italica chimeric firefly luciferase produces enhanced bioluminescence. Biochemistry 2014, 53, 6287–6289. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.P.; Unch, J.; Binkowski, B.F.; Valley, M.P.; Butler, B.L.; Wood, M.G.; Otto, P.; Zimmerman, K.; Vidugiris, G.; Machleidt, T. Engineered luciferase reporter from a deep sea shrimp utilizing a novel imidazopyrazinone substrate. ACS Chem. Biol. 2012, 7, 1848–1857. [Google Scholar] [CrossRef]
- Reyes-Alcaraz, A.; Lucero Garcia-Rojas, E.Y.; Merlinsky, E.A.; Seong, J.Y.; Bond, R.A.; McConnell, B.K. A NanoBiT assay to monitor membrane proteins trafficking for drug discovery and drug development. Commun. Biol. 2022, 5, 212. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, G.; Zhang, Y.; Lin, X.; Zhao, X.; Cui, Q.; Rong, L.; Du, R. Development of an HiBiT-tagged reporter H3N2 influenza A virus and its utility as an antiviral screening platform. J. Med. Virol. 2023, 95, e28345. [Google Scholar] [CrossRef]
- Nagashima, S.; Primadharsini, P.P.; Nishiyama, T.; Takahashi, M.; Murata, K.; Okamoto, H. Development of a HiBiT-tagged reporter hepatitis E virus and its utility as an antiviral drug screening platform. J. Virol. 2023, 97, e00508-23. [Google Scholar] [CrossRef]
- Calabretta, M.M.; Alvarez-Diduk, R.; Michelini, E.; Merkoçi, A. ATP sensing paper with smartphone bioluminescence-based detection. In Bioluminescence: Methods and Protocols, Volume 2; Springer: Berlin/Heidelberg, Germany, 2022; pp. 297–307. [Google Scholar]
- Reyes, S.; Rizzo, E.; Ting, A.; Dikici, E.; Daunert, S.; Deo, S.K. Metal organic framework encapsulated tamavidin-Gluc reporter: Application in COVID-19 spike antigen bioluminescent immunoassay. Sens. Diagn. 2022, 1, 1198–1208. [Google Scholar] [CrossRef]
- Vysotski, E.S. Bioluminescent and Fluorescent Proteins: Molecular Mechanisms and Modern Applications. Int. J. Mol. Sci. 2023, 24, 281. [Google Scholar] [CrossRef]
- Shimomura, O.; Johnson, F.H.; Saiga, Y. Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. J. Cell. Comp. Physiol. 1962, 59, 223–239. [Google Scholar] [CrossRef]
- Zimmer, M. GFP: From jellyfish to the Nobel prize and beyond. Chem. Soc. Rev. 2009, 38, 2823–2832. [Google Scholar] [CrossRef]
- Choy, G.; O’Connor, S.; Diehn, F.E.; Costouros, N.; Alexander, H.R.; Choyke, P.; Libutti, S.K. Comparison of noninvasive fluorescent and bioluminescent small animal optical imaging. Biotechniques 2003, 35, 1022–1030. [Google Scholar] [CrossRef]
- Celinskis, D.; Friedman, N.; Koksharov, M.; Murphy, J.; Gomez-Ramirez, M.; Borton, D.; Shaner, N.; Hochgeschwender, U.; Lipscombe, D.; Moore, C. Miniaturized devices for bioluminescence imaging in freely behaving animals. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020. [Google Scholar]
- Sadikot, R.T.; Blackwell, T.S. Bioluminescence imaging. Proc. Am. Thorac. Soc. 2005, 2, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Thomulka, K.W.; McGee, D.J.; Lange, J.H. Detection of biohazardous materials in water by measuring bioluminescence reduction with the marine organism Vibrio harveyi. J. Environ. Sci. Health Part A 1993, 28, 2153–2166. [Google Scholar]
- Olsen, O. Rapid food microbiology: Application of bioluminescence in the dairy and food industry—A review. In Physical Methods for Microorganisms Detection; CRC Press: Boca Raton, FL, USA, 2018; pp. 63–80. [Google Scholar]
- De Almeida, P.E.; van Rappard, J.R.; Wu, J.C. In vivo bioluminescence for tracking cell fate and function. Am. J. Physiol.-Heart Circ. Physiol. 2011, 301, H663–H671. [Google Scholar] [CrossRef] [PubMed]
- Zambito, G.; Chawda, C.; Mezzanotte, L. Emerging tools for bioluminescence imaging. Curr. Opin. Chem. Biol. 2021, 63, 86–94. [Google Scholar] [CrossRef] [PubMed]
- De Maesschalck, V.; Gutiérrez, D.; Paeshuyse, J.; Briers, Y.; Vande Velde, G.; Lavigne, R. A Bioluminescence-Based Ex Vivo Burn Wound Model for Real-Time Assessment of Novel Phage-Inspired Enzybiotics. Pharmaceutics 2022, 14, 2553. [Google Scholar] [CrossRef]
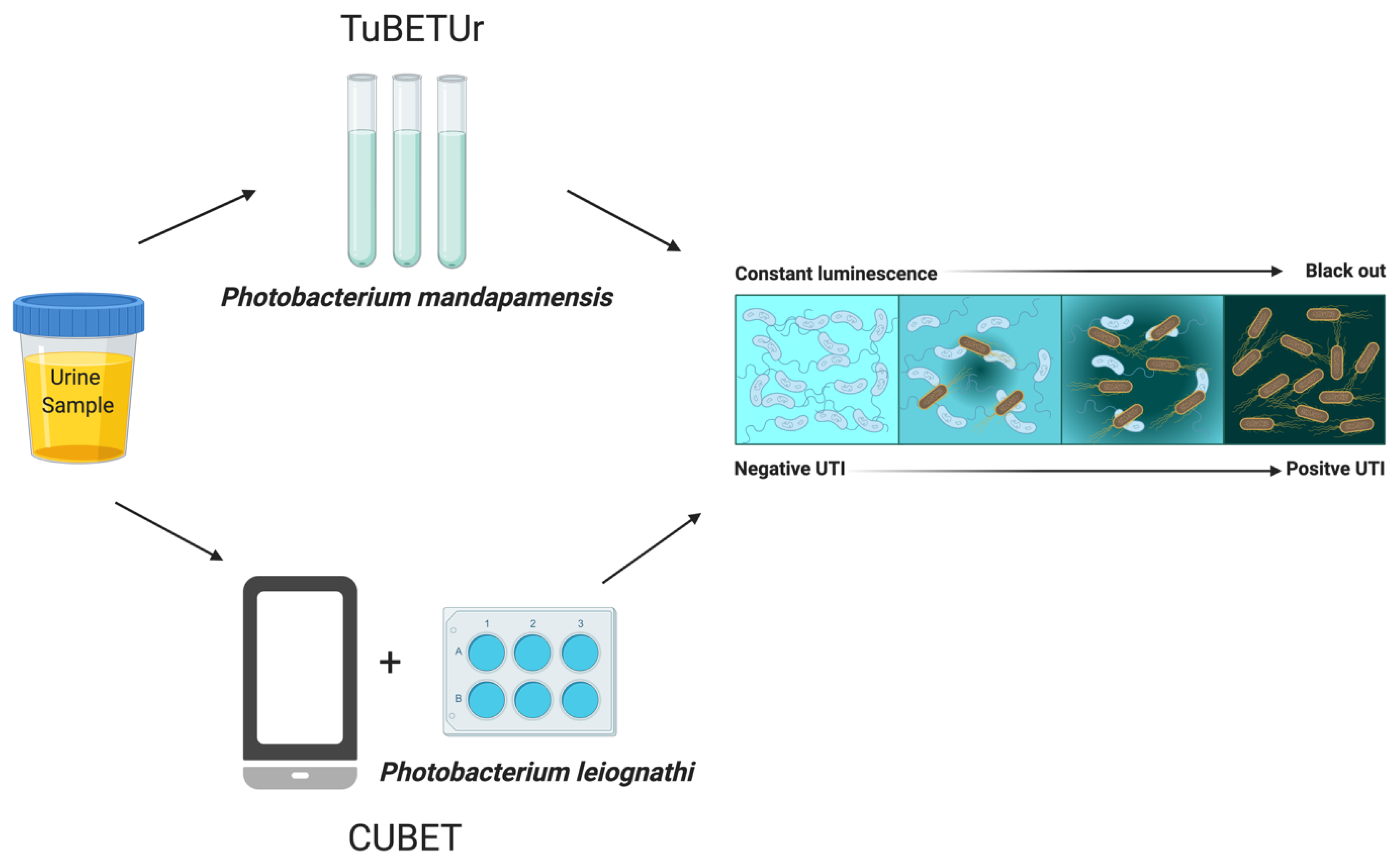
- Reyes, S.; Le, N.; Fuentes, M.D.; Upegui, J.; Dikici, E.; Broyles, D.; Quinto, E.; Daunert, S.; Deo, S.K. An Intact Cell Bioluminescence-Based Assay for the Simple and Rapid Diagnosis of Urinary Tract Infection. Int. J. Mol. Sci. 2020, 21, 5015. [Google Scholar] [CrossRef]
- Welsh, D.K.; Yoo, S.-H.; Liu, A.C.; Takahashi, J.S.; Kay, S.A. Bioluminescence imaging of individual fibroblasts reveals persistent, independently phased circadian rhythms of clock gene expression. Curr. Biol. 2004, 14, 2289–2295. [Google Scholar] [CrossRef]
- Fang, M.; Kang, H.-G.; Park, Y.; Estrella, B.; Zarbl, H. In vitro bioluminescence assay to characterize circadian rhythm in mammary epithelial cells. JoVE (J. Vis. Exp.) 2017, e55832. [Google Scholar] [CrossRef]
- Suff, N.; Waddington, S.N. The power of bioluminescence imaging in understanding host-pathogen interactions. Methods 2017, 127, 69–78. [Google Scholar] [CrossRef]
- Kono, M.; Conlon, E.G.; Lux, S.Y.; Yanagida, K.; Hla, T.; Proia, R.L. Bioluminescence imaging of G protein-coupled receptor activation in living mice. Nat. Commun. 2017, 8, 1163. [Google Scholar] [CrossRef] [PubMed]
- Prescher, J.A.; Contag, C.H. Guided by the light: Visualizing biomolecular processes in living animals with bioluminescence. Curr. Opin. Chem. Biol. 2010, 14, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Dinca, E.B.; Voicu, R.V.; Ciurea, A.V. Bioluminescence imaging of invasive intracranial xenografts: Implications for translational research and targeted therapeutics of brain tumors. Neurosurg. Rev. 2010, 33, 385–394. [Google Scholar] [CrossRef]
- Cabral, F.V.; Sabino, C.P.; Dimmer, J.A.; Sauter, I.P.; Cortez, M.J.; Ribeiro, M.S. Preclinical Investigation of Methylene Blue-mediated Antimicrobial Photodynamic Therapy on Leishmania Parasites Using Real-Time Bioluminescence. Photochem. Photobiol. 2020, 96, 604–610. [Google Scholar] [CrossRef]
- Sato, A.; Klaunberg, B.; Tolwani, R. In vivo bioluminescence imaging. Comp. Med. 2004, 54, 631–634. [Google Scholar]
- Iwano, S.; Sugiyama, M.; Hama, H.; Watakabe, A.; Hasegawa, N.; Kuchimaru, T.; Tanaka, K.Z.; Takahashi, M.; Ishida, Y.; Hata, J. Single-cell bioluminescence imaging of deep tissue in freely moving animals. Science 2018, 359, 935–939. [Google Scholar] [CrossRef]
- Coleman, S.M.; McGregor, A. A bright future for bioluminescent imaging in viral research. Future Virol. 2015, 10, 169–183. [Google Scholar] [CrossRef]
- Vantaggiato, C.; Dell’Omo, G.; Ramachandran, B.; Manni, I.; Radaelli, E.; Scanziani, E.; Piaggio, G.; Maggi, A.; Ciana, P. Bioluminescence imaging of estrogen receptor activity during breast cancer progression. Am. J. Nucl. Med. Mol. Imaging 2016, 6, 32. [Google Scholar]
- Lemmen, J.; Arends, R.; Van Boxtel, A.; Van Der Saag, P.; Van Der Burg, B. Tissue-and time-dependent estrogen receptor activation in estrogen reporter mice. J. Mol. Endocrinol. 2004, 32, 689–701. [Google Scholar] [CrossRef]
- Price, C.P. Point of care testing. BMJ 2001, 322, 1285–1288. [Google Scholar] [CrossRef]
- Song, Q.; Sun, X.; Dai, Z.; Gao, Y.; Gong, X.; Zhou, B.; Wu, J.; Wen, W. Point-of-care testing detection methods for COVID-19. Lab Chip 2021, 21, 1634–1660. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, D.; Roy, A.K.; Khanna, R.C.; Jalali, S.; Panigrahy, B.; Parija, D.C.; Rath, S. Point-of-care rapid antigen testing for COVID-19 at a tertiary eye care facility: Role in commencement of elective surgeries, contact tracing and implementation of back-to-work policy. Indian J. Ophthalmol. 2021, 69, 964–970. [Google Scholar] [CrossRef] [PubMed]
- May, L.; Tran, N.; Ledeboer, N.A. Point-of-care COVID-19 testing in the emergency department: Current status and future prospects. Expert Rev. Mol. Diagn. 2021, 21, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Naseri, M.; Ziora, Z.M.; Simon, G.P.; Batchelor, W. ASSURED-compliant point-of-care diagnostics for the detection of human viral infections. Rev. Med. Virol. 2022, 32, e2263. [Google Scholar] [CrossRef]
- Yamada, K.; Shibata, H.; Suzuki, K.; Citterio, D. Toward practical application of paper-based microfluidics for medical diagnostics: State-of-the-art and challenges. Lab Chip 2017, 17, 1206–1249. [Google Scholar] [CrossRef]
- Cai, R.; Kolabas, Z.I.; Pan, C.; Mai, H.; Zhao, S.; Kaltenecker, D.; Voigt, F.F.; Molbay, M.; Ohn, T.-l.; Vincke, C. Whole-mouse clearing and imaging at the cellular level with vDISCO. Nat. Protoc. 2023, 18, 1197–1242. [Google Scholar] [CrossRef]
- Kim, H.; Jung, Y.; Doh, I.-J.; Lozano-Mahecha, R.A.; Applegate, B.; Bae, E. Smartphone-based low light detection for bioluminescence application. Sci. Rep. 2017, 7, 40203. [Google Scholar] [CrossRef]
- Calabretta, M.M.; Lopreside, A.; Montali, L.; Zangheri, M.; Evangelisti, L.; D’Elia, M.; Michelini, E. Portable light detectors for bioluminescence biosensing applications: A comprehensive review from the analytical chemist’s perspective. Anal. Chim. Acta 2022, 1200, 339583. [Google Scholar] [CrossRef]
- Holland, C.A.; Kiechle, F.L. Point-of-care molecular diagnostic systems—Past, present and future. Curr. Opin. Microbiol. 2005, 8, 504–509. [Google Scholar] [CrossRef]
- Nayak, S.; Blumenfeld, N.R.; Laksanasopin, T.; Sia, S.K. Point-of-care diagnostics: Recent developments in a connected age. Anal. Chem. 2017, 89, 102–123. [Google Scholar] [CrossRef]
- Kinnamon, D.S.; Heggestad, J.T.; Liu, J.; Chilkoti, A. Technologies for frugal and sensitive point-of-care immunoassays. Annu. Rev. Anal. Chem. 2022, 15, 123–149. [Google Scholar] [CrossRef] [PubMed]
- Branchini, B.R.; Southworth, T.L.; Khattak, N.F.; Michelini, E.; Roda, A. Red-and green-emitting firefly luciferase mutants for bioluminescent reporter applications. Anal. Biochem. 2005, 345, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Roda, A.; Michelini, E.; Zangheri, M.; Di Fusco, M.; Calabria, D.; Simoni, P. Smartphone-based biosensors: A critical review and perspectives. TrAC Trends Anal. Chem. 2016, 79, 317–325. [Google Scholar] [CrossRef]
- Sande, M.G.; Rodrigues, J.L.; Ferreira, D.; Silva, C.J.; Rodrigues, L.R. Novel biorecognition elements against pathogens in the design of state-of-the-art diagnostics. Biosensors 2021, 11, 418. [Google Scholar] [CrossRef] [PubMed]
- Anik, Ü. Electrochemical medical biosensors for POC applications. In Medical Biosensors for Point of Care (POC) Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 275–292. [Google Scholar]
- Posthuma-Trumpie, G.A.; Korf, J.; van Amerongen, A. Lateral flow (immuno) assay: Its strengths, weaknesses, opportunities and threats. A literature survey. Anal. Bioanal. Chem. 2009, 393, 569–582. [Google Scholar] [CrossRef]
- Abdelhamid, H.N. Zeolitic imidazolate frameworks (ZIF-8) for biomedical applications: A review. Curr. Med. Chem. 2021, 28, 7023–7075. [Google Scholar] [CrossRef]
- Xue, L.; Yu, Q.; Griss, R.; Schena, A.; Johnsson, K. Bioluminescent Antibodies for Point-of-Care Diagnostics. Angew. Chem. 2017, 129, 7218–7222. [Google Scholar] [CrossRef]
- Tenda, K.; van Gerven, B.; Arts, R.; Hiruta, Y.; Merkx, M.; Citterio, D. Paper-based antibody detection devices using bioluminescent BRET-switching sensor proteins. Angew. Chem. Int. Ed. 2018, 57, 15369–15373. [Google Scholar] [CrossRef]
- Arts, R.; Den Hartog, I.; Zijlema, S.E.; Thijssen, V.; Van Der Beelen, S.H.; Merkx, M. Detection of antibodies in blood plasma using bioluminescent sensor proteins and a smartphone. Anal. Chem. 2016, 88, 4525–4532. [Google Scholar] [CrossRef]
- Chen, Y.; Xianyu, Y.; Wu, J.; Dong, M.; Zheng, W.; Sun, J.; Jiang, X. Double-enzymes-mediated bioluminescent sensor for quantitative and ultrasensitive point-of-care testing. Anal. Chem. 2017, 89, 5422–5427. [Google Scholar] [CrossRef]
- Gandelman, O.A.; Church, V.L.; Moore, C.A.; Kiddle, G.; Carne, C.A.; Parmar, S.; Jalal, H.; Tisi, L.C.; Murray, J.A. Novel bioluminescent quantitative detection of nucleic acid amplification in real-time. PLoS ONE 2010, 5, e14155. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Pandian, V.; Mauk, M.G.; Bau, H.H.; Cherry, S.; Tisi, L.C.; Liu, C. Smartphone-Based Mobile Detection Platform for Molecular Diagnostics and Spatiotemporal Disease Mapping. Anal Chem 2018, 90, 4823–4831. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Mendoza, D.A.; Yasar, A.; Caygara, D.; Kassem, A.; Densmore, D.; Yazicigil, R.T. Integrated Real-Time CMOS Luminescence Sensing and Impedance Spectroscopy in Droplet Microfluidics. IEEE Trans. Biomed. Circuits Syst. 2024, 18, 1233–1252. [Google Scholar] [CrossRef]
- Lomazzi, S.; Caccia, M.; Distasi, C.; Dionisi, M.; Lim, D.; Martemiyanov, A.; Nardo, L.; Ruffinatti, F.; Santoro, R. Assessment of the potential of SiPM-based systems for bioluminescence detection. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2020, 979, 164493. [Google Scholar] [CrossRef]
- Ohashi, H.; Matsumoto, T.; Jeong, H.-J.; Dong, J.; Abe, R.; Ueda, H. Insight into the working mechanism of quenchbody: Transition of the dye around antibody variable region that fluoresces upon antigen binding. Bioconjugate Chem. 2016, 27, 2248–2253. [Google Scholar] [CrossRef]
- Yang, Y.; Inoue, A.; Yasuda, T.; Ueda, H.; Zhu, B.; Kitaguchi, T. BRET Nano Q-Body: A Nanobody-Based Ratiometric Bioluminescent Immunosensor for Point-of-Care Testing. ACS Sens. 2024, 9, 5955–5965. [Google Scholar] [CrossRef]
- Di Carlo, E.; Sorrentino, C. State of the art CRISPR-based strategies for cancer diagnostics and treatment. Biomark. Res. 2024, 12, 156. [Google Scholar] [CrossRef]
- Rahman, M.R.; Hossain, M.A.; Mozibullah, M.; Al Mujib, F.; Afrose, A.; Shahed-Al-Mahmud, M.; Apu, M.A.I. CRISPR is a useful biological tool for detecting nucleic acid of SARS-CoV-2 in human clinical samples. Biomed. Pharmacother. 2021, 140, 111772. [Google Scholar] [CrossRef]
- van der Veer, H.J.; van Aalen, E.A.; Michielsen, C.M.; Hanckmann, E.T.; Deckers, J.; van Borren, M.M.; Flipse, J.; Loonen, A.J.; Schoeber, J.P.; Merkx, M. Glow-in-the-dark infectious disease diagnostics using Crispr-Cas9-based Split luciferase complementation. ACS Cent. Sci. 2023, 9, 657–667. [Google Scholar] [CrossRef]
- Calabretta, M.M.; Zangheri, M.; Calabria, D.; Lopreside, A.; Montali, L.; Marchegiani, E.; Trozzi, I.; Guardigli, M.; Mirasoli, M.; Michelini, E. based immunosensors with bio-chemiluminescence detection. Sensors 2021, 21, 4309. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Kim, H.R.; Jung, J.H.; Lee, K.-B.; Kim, B.C. The development of paper discs immobilized with luciferase/D-luciferin for the detection of ATP from airborne bacteria. Sens. Actuators B Chem. 2018, 260, 274–281. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, F.; Duan, W.; Mu, X.; Fang, S.; Lu, N.; Zhou, X.; Kong, W. Engineering a “PEG-g-PEI/DNA nanoparticle-in-PLGA microsphere” hybrid controlled release system to enhance immunogenicity of DNA vaccine. Mater. Sci. Eng. C 2020, 106, 110294. [Google Scholar] [CrossRef] [PubMed]
- Ugarova, N.; Koksharov, M. Thermostabilization of firefly luciferases using genetic engineering. Genet. Eng.–Basics New Appl. Responsib. 2011, 1, 61–92. [Google Scholar]
- Riahi-Madvar, A.; Hosseinkhani, S. Design and characterization of novel trypsin-resistant firefly luciferases by site-directed mutagenesis. Protein Eng. Des. Sel. 2009, 22, 655–663. [Google Scholar] [CrossRef]
- Gutiérrez-Serpa, A.; Pacheco-Fernández, I.; Pasán, J.; Pino, V. Metal–Organic Frameworks as Key Materials for Solid-Phase Microextraction Devices—A Review. Separations 2019, 6, 47. [Google Scholar] [CrossRef]
- Kitagawa, S. Metal–organic frameworks (MOFs). Chem. Soc. Rev. 2014, 43, 5415–5418. [Google Scholar]
- Safaei, M.; Foroughi, M.M.; Ebrahimpoor, N.; Jahani, S.; Omidi, A.; Khatami, M. A review on metal-organic frameworks: Synthesis and applications. TrAC Trends Anal. Chem. 2019, 118, 401–425. [Google Scholar] [CrossRef]
- Moosavi, S.M.; Nandy, A.; Jablonka, K.M.; Ongari, D.; Janet, J.P.; Boyd, P.G.; Lee, Y.; Smit, B.; Kulik, H.J. Understanding the diversity of the metal-organic framework ecosystem. Nat. Commun. 2020, 11, 4068. [Google Scholar] [CrossRef]
- Jones, C.W. Metal–organic frameworks and covalent organic frameworks: Emerging advances and applications. Jacs Au 2022, 2, 1504–1505. [Google Scholar] [CrossRef]
- Paz, F.A.A.; Klinowski, J.; Vilela, S.M.; Tome, J.P.; Cavaleiro, J.A.; Rocha, J. Ligand design for functional metal–organic frameworks. Chem. Soc. Rev. 2012, 41, 1088–1110. [Google Scholar]
- Tong, P.; Liang, J.; Jiang, X.; Li, J. Research progress on metal-organic framework composites in chemical sensors. Crit. Rev. Anal. Chem. 2020, 50, 376–392. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, V.F.; Malek, N.I.; Kailasa, S.K. Review on Metal–Organic Framework Classification, Synthetic Approaches, and Influencing Factors: Applications in Energy, Drug Delivery, and Wastewater Treatment. ACS Omega 2022, 7, 44507–44531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lv, Y.; Tan, T.; van der Spoel, D. Atomistic simulation of protein encapsulation in metal–organic frameworks. J. Phys. Chem. B 2016, 120, 477–484. [Google Scholar] [CrossRef]
- Wang, A.; Walden, M.; Ettlinger, R.; Kiessling, F.; Gassensmith, J.J.; Lammers, T.; Wuttke, S.; Peña, Q. Biomedical metal–organic framework materials: Perspectives and challenges. Adv. Funct. Mater. 2023, 34, 2308589. [Google Scholar] [CrossRef]
- Felix Sahayaraj, A.; Joy Prabu, H.; Maniraj, J.; Kannan, M.; Bharathi, M.; Diwahar, P.; Salamon, J. Metal–Organic Frameworks (MOFs): The Next Generation of Materials for Catalysis, Gas Storage, and Separation. J. Inorg. Organomet. Polym. Mater. 2023, 33, 1757–1781. [Google Scholar] [CrossRef]
- Kalashgrani, M.Y.; Babapoor, A.; Mousavi, S.M.; Feizpoor, S.; Hashemi, S.A.; Binazadeh, M.; Chiang, W.-H.; Lai, C.W. Synthesis of Isoreticular Metal Organic Framework-3 (IRMOF-3) Porous Nanostructure and Its Effect on Naphthalene Adsorption: Optimized by Response Surface Methodology. Separations 2023, 10, 261. [Google Scholar] [CrossRef]
- Pan, Y.; Zhan, S.; Xia, F. Zeolitic imidazolate framework-based biosensor for detection of HIV-1 DNA. Anal. Biochem. 2018, 546, 5–9. [Google Scholar] [CrossRef]
- Ling, P.; Lei, J.; Ju, H. Porphyrinic metal-organic framework as electrochemical probe for DNA sensing via triple-helix molecular switch. Biosens. Bioelectron. 2015, 71, 373–379. [Google Scholar] [CrossRef]
- Duan, S.; Huang, Y. Electrochemical sensor using NH2-MIL-88 (Fe)-rGO composite for trace Cd2+, Pb2+, and Cu2+ detection. J. Electroanal. Chem. 2017, 807, 253–260. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, W.; Lv, S.; Han, J.; Xie, G.; Chen, S. A one-step structure-switching electrochemical sensor for transcription factor detection enhanced with synergistic catalysis of PtNi@ MIL-101 and Exo III-assisted cycling amplification. Chem. Commun. 2018, 54, 11901–11904. [Google Scholar] [CrossRef]
- Liu, H.; Ni, T.; Mu, L.; Zhang, D.; Wang, J.; Wang, S.; Sun, B. Sensitive detection of pyrraline with a molecularly imprinted sensor based on metal-organic frameworks and quantum dots. Sens. Actuators B Chem. 2018, 256, 1038–1044. [Google Scholar] [CrossRef]
- Buser, H.; Schwarzenbach, D.; Petter, W.; Ludi, A. The crystal structure of Prussian blue: Fe4[Fe(CN)6]3.xH2O. Inorg. Chem. 1977, 16, 2704–2710. [Google Scholar] [CrossRef]
- Hirai, K.; Sumida, K.; Meilikhov, M.; Louvain, N.; Nakahama, M.; Uehara, H.; Kitagawa, S.; Furukawa, S. Impact of crystal orientation on the adsorption kinetics of a porous coordination polymer–quartz crystal microbalance hybrid sensor. J. Mater. Chem. C 2014, 2, 3336–3344. [Google Scholar] [CrossRef]
- Yang, F.; Li, W.; Tang, B. Facile synthesis of amorphous UiO-66 (Zr-MOF) for supercapacitor application. J. Alloys Compd. 2018, 733, 8–14. [Google Scholar] [CrossRef]
- Zhu, Q.; Chen, Y.; Wang, W.; Zhang, H.; Ren, C.; Chen, H.; Chen, X. A sensitive biosensor for dopamine determination based on the unique catalytic chemiluminescence of metal–organic framework HKUST-1. Sens. Actuators B Chem. 2015, 210, 500–507. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, X.-Y.; Cheng, C.; Shao, Z.-S.; Wang, H.-S. Strategies to fabricate metal–organic framework (MOF)-based luminescent sensing platforms. J. Mater. Chem. C 2019, 7, 10743–10763. [Google Scholar] [CrossRef]
- Lin, W.; Gong, J.; Fang, L.; Jiang, K. A photodynamic system based on endogenous bioluminescence for in vitro anticancer studies. Z. Anorg. Allg. Chem. 2019, 645, 1161–1164. [Google Scholar] [CrossRef]
- Nowroozi-Nejad, Z.; Bahramian, B.; Hosseinkhani, S. A fast and efficient stabilization of firefly luciferase on MIL-53 (Al) via surface adsorption mechanism. Res. Chem. Intermed. 2019, 45, 2489–2501. [Google Scholar] [CrossRef]
- Contag, P.R.; Olomu, I.N.; Stevenson, D.K.; Contag, C.H. Bioluminescent indicators in living mammals. Nat. Med. 1998, 4, 245–247. [Google Scholar] [CrossRef]
- Qiu, P.; Zhao, Y.; Zheng, J.; Wang, J.-F.; Jiang, X.-J. Research on performances of back-illuminated scientific CMOS for astronomical observations. Res. Astron. Astrophys. 2021, 21, 268. [Google Scholar] [CrossRef]
- Bjelošević, M.; Pobirk, A.Z.; Planinšek, O.; Grabnar, P.A. Excipients in freeze-dried biopharmaceuticals: Contributions toward formulation stability and lyophilisation cycle optimisation. Int. J. Pharm. 2020, 576, 119029. [Google Scholar] [CrossRef]
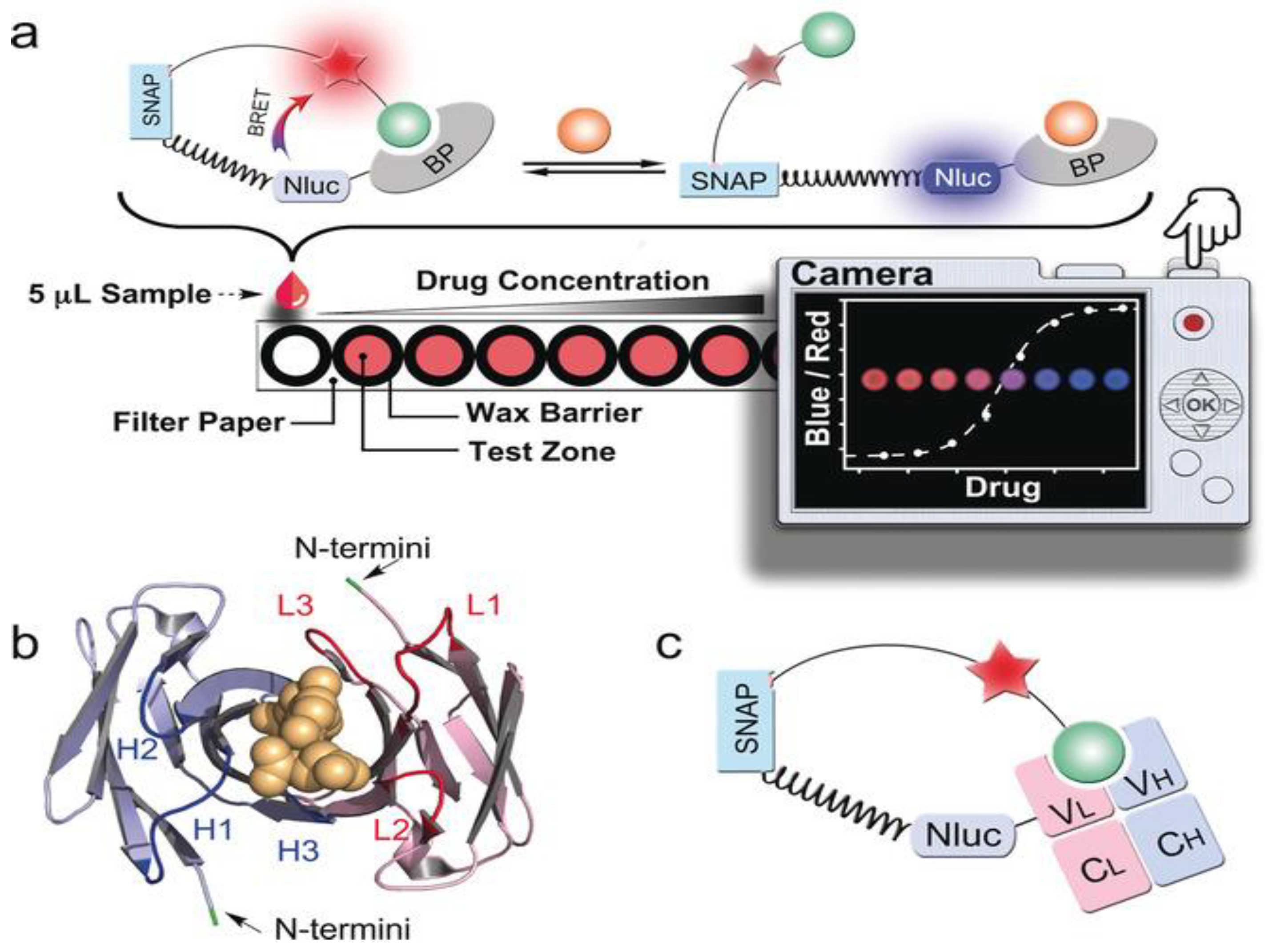
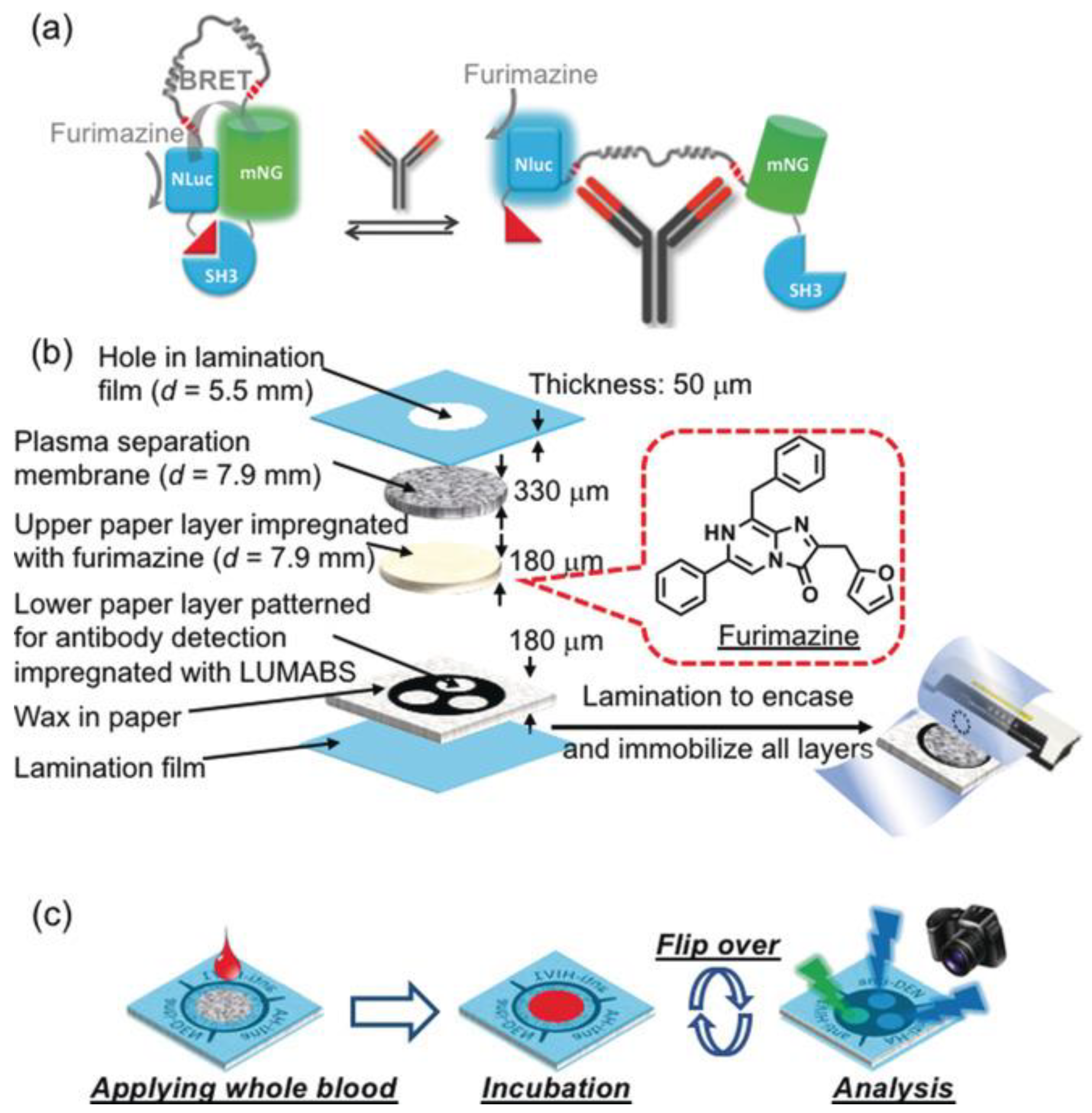
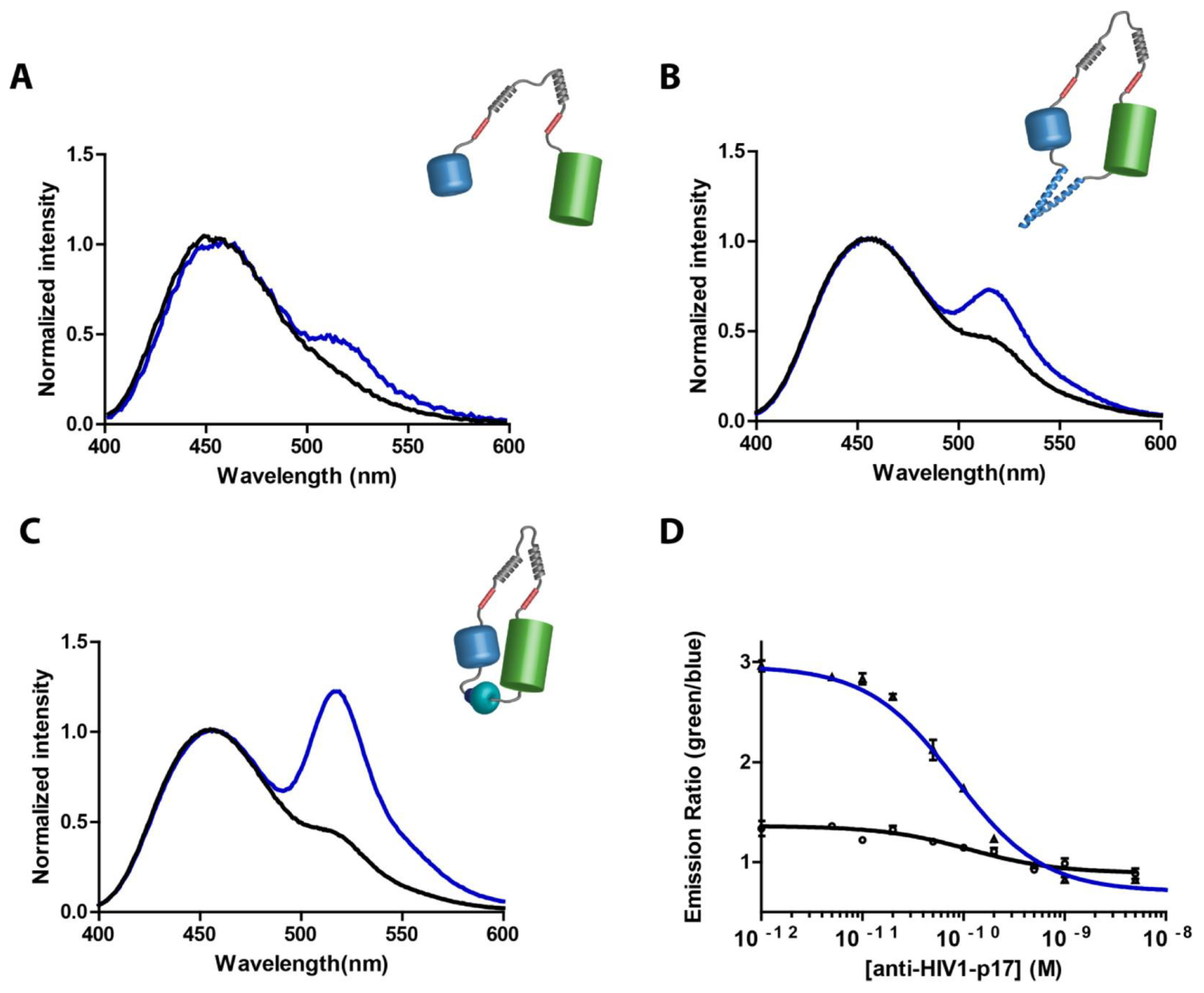

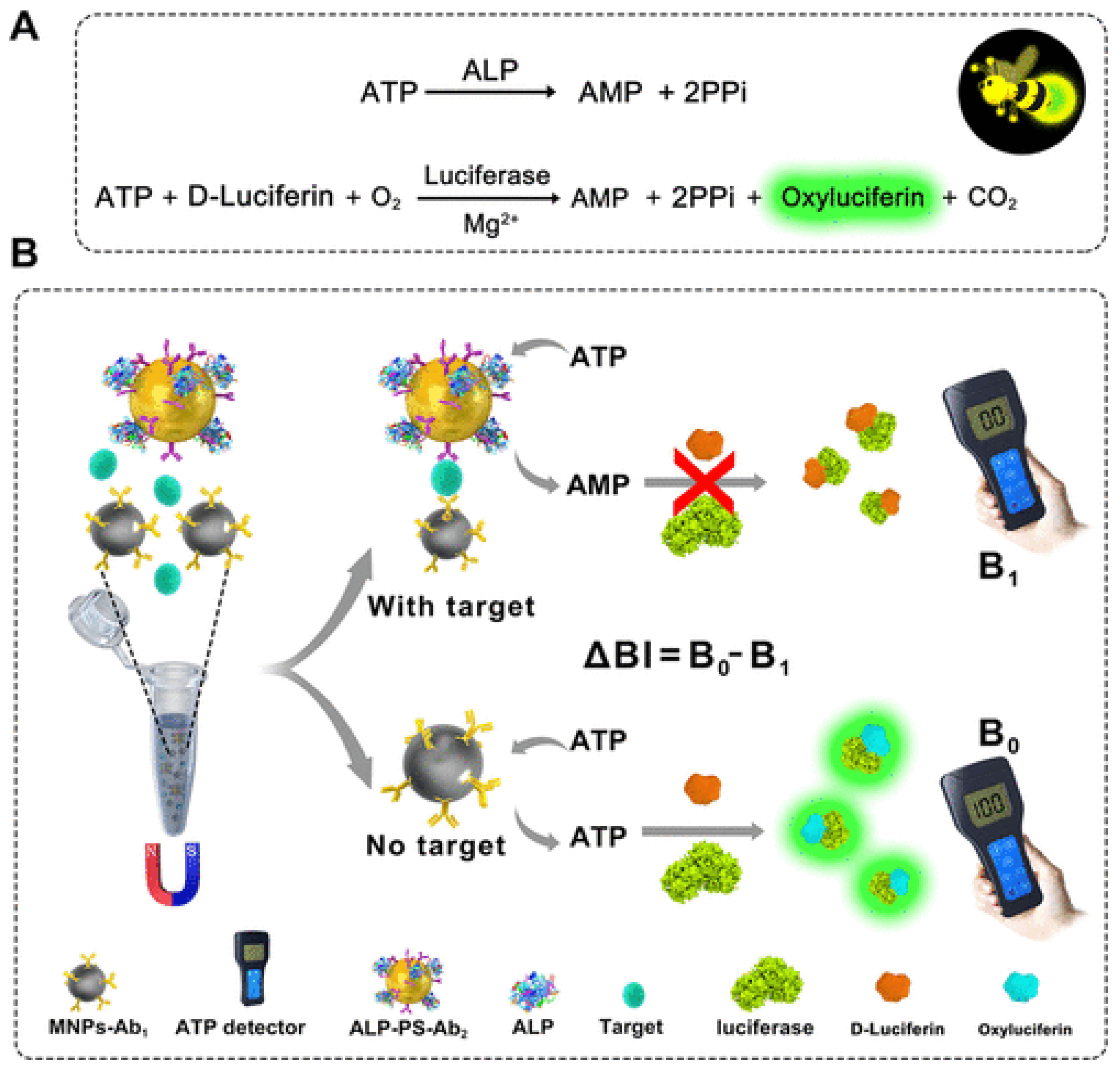

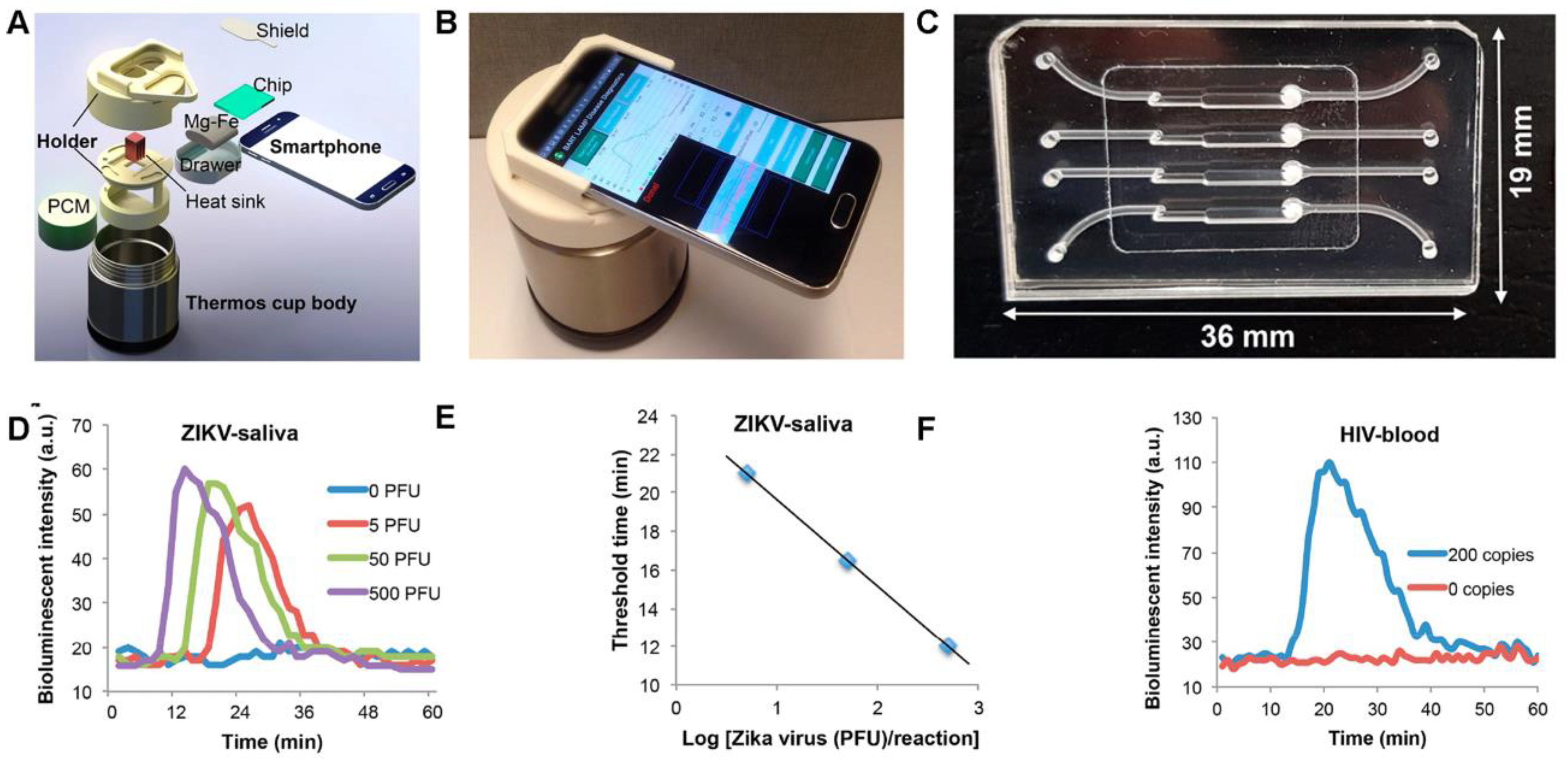
| POC Technology | Analyte Detected | Sensitivity | Detector | Reference |
|---|---|---|---|---|
| LUCIDs (Luciferase-based indicator of drugs) | Immunosuppressants, Anti-epileptics, anti-cancer agents, anti-arrhythmics | Ultra-low analyte detection | Semisynthetic bioluminescent sensor with point-and-shoot camera | [17,109] |
| Paper-based NanoLuc-SNAP antibody | Drug levels in serum/blood | High | Snap-tag, BRET, low-cost digital camera | [109] |
| 3D-µPAD (LUMABS) | Anti-HIV1, anti-HA, anti-DEN1 antibodies | -- | BRET, digital camera | [110] |
| LUMABS with smartphone | HIV-p17, HA-tag, dengue virus type 1 | High (qualitative + quantitative detection) | Smartphone camera | [111] |
| Bio-chemiluminescent paper device | General immunoassay targets, ATP | Improved selectivity and sensitivity | Bioluminescent luciferase + optical detector (smartphone camera) | [65] |
| µTADs (cotton thread) | Anti-HIV, anti-HA, anti-DEN1 antibodies | ~5 µL blood, 5-min detection | Bioluminescent protein + smartphone with 3D lens adapter | [14] |
| ABS immunosensor (ALP + luciferase) | Disease biomarkers | Femto- to pico-molar sensitivity | Portable ATP detector | [112] |
| BAQS (Smartphone-based bioluminescent analyte quantification) | Pseudomonas fluorescence (live bacteria) | ~7.9 × 106 CFU/mL | Smartphone + custom software and sample chamber | [98] |
| BART-LAMP (Bioluminescent Assay) | Chlamydia trachomatis in human urine | 95.6% | Thermostable firefly luciferase | [113] |
| BART-LAMP with Smartphone Integration | Zika virus (ZIKV) in human urine and saliva, HIV in blood | Not specified for ZIKV or HIV | Smartphone app (monitors luciferin emission), ATP sulfurylase converting PPi to ATP | [105] |
| CMOS + impedance spectroscopy | NanoLuc bioluminescence in droplets | 6.7 nA/count resolution (very high) | Integrated CMOS sensor in microfluidic chip | [115] |
| SiPM-based system | General photon detection (for biosensing | 104–105 photons/sec | Silicon photomultiplier (CiPM) | [116] |
| BRET nano Q-body | Methotrexate (MTX) | 0.5, 1.6, 3.7 nM (milk, serum, whole blood)) | Paper + bioluminescence (NanoLuc → TAMRA) | [118] |
| LUNAS (CRISPR + Luciferase) | Pathogenic RNA/DNA (e.g., SARS-CoV-2 | Attomolar sensitivity | Ratiometric luciferase, portable detector | [121] |
| Class | Examples | Definition | Use | Reference |
|---|---|---|---|---|
| Iso-reticular MOFs (IRMOFs) | IRMOF-3, IRMOF-n (n = 1–16) | Octahedral microporous crystal material assembled by [Zn4O]6+ and different ligands | IRMOF-3, recognition of 2,4,6-trinitrophenol in wastewater | [133,134,138] |
| Zeolitic Imidazolate Frameworks (ZIFs) | ZIF-8, ZIF-90, ZIF-L, ZIF-71, ZIF-67, ZIF-7 | Zeolite structure formed by combining transition metal ions and imidazolyl ligands | ZIF-8, detection of HIV-1 DNA | [133,134,139] |
| Porous Coordination Networks (PCNs) | PCN-333, PCN 224, PCN-222, PCN-57 | Stereo octahedrons having a three-dimension structure that has a hole–cage–hole topology | PCN-222, electrochemical sensor to detect DNA | [133,134,140] |
| Materials Institute Lavoisier (MILs) | MIL-101, MIL-100, MIL-53, MIL-88, MIL-125 | Synthesize using various elements that have valence electrons and an organic compound containing dicarboxylic acid ligands | MIL-101 (Cr), Resistive humidity sensors MIL, chemical sensors to immobilize, proteins, quantum dots, and other constituents | [133,134,141,142,143] |
| Porous Coordination Polymers (PCPs) | Prussian blue—PCP, PCP Zn(NO2-ip)(byp) *** | Synthesize by self-assembly of carboxylic acid, pyridine, and their derivatives as ligand (PBU) * and transition metal ions (SBU) ** | PCP Zn(NO2-ip)(byp), organic vapor sensor | [133,134,144,145] |
| University of Oslo (UiO) | UiO-66(Zr) | Based on carboxylic acid (PBU) and Zr6(µ3-O)4(µ3-OH) (SBU) | Supercapacitor electrode material | [134,146] |
| Other MOFs | Northwestern University (NU), Pohang University of Science and Technology (POST-n), Dresden University of Technology (DUT-n family), University of Nottingham (NOTT-n), Hongkong University of Science and Technology (HKUST-n), Christian-Albrechts- University (CAU-n family), and Biological metal–organic frameworks (Bio-MOFs) | Recently discovered MOFs | HKUST-1, used as chemical sensors for detecting dopamine, | [133,134,147] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyes, S.; Rodriguez, R.; Dikici, E.; Daunert, S.; Deo, S. Bioluminescence in Clinical and Point-of-Care Testing. Biosensors 2025, 15, 422. https://doi.org/10.3390/bios15070422
Reyes S, Rodriguez R, Dikici E, Daunert S, Deo S. Bioluminescence in Clinical and Point-of-Care Testing. Biosensors. 2025; 15(7):422. https://doi.org/10.3390/bios15070422
Chicago/Turabian StyleReyes, Sherwin, Raymarcos Rodriguez, Emre Dikici, Sylvia Daunert, and Sapna Deo. 2025. "Bioluminescence in Clinical and Point-of-Care Testing" Biosensors 15, no. 7: 422. https://doi.org/10.3390/bios15070422
APA StyleReyes, S., Rodriguez, R., Dikici, E., Daunert, S., & Deo, S. (2025). Bioluminescence in Clinical and Point-of-Care Testing. Biosensors, 15(7), 422. https://doi.org/10.3390/bios15070422







