Controlled Synthesis of Tungsten Oxide Nanomaterials with Different Morphologies and Their Gas-Sensing Properties for Formaldehyde in Vegetables
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemical Reagents
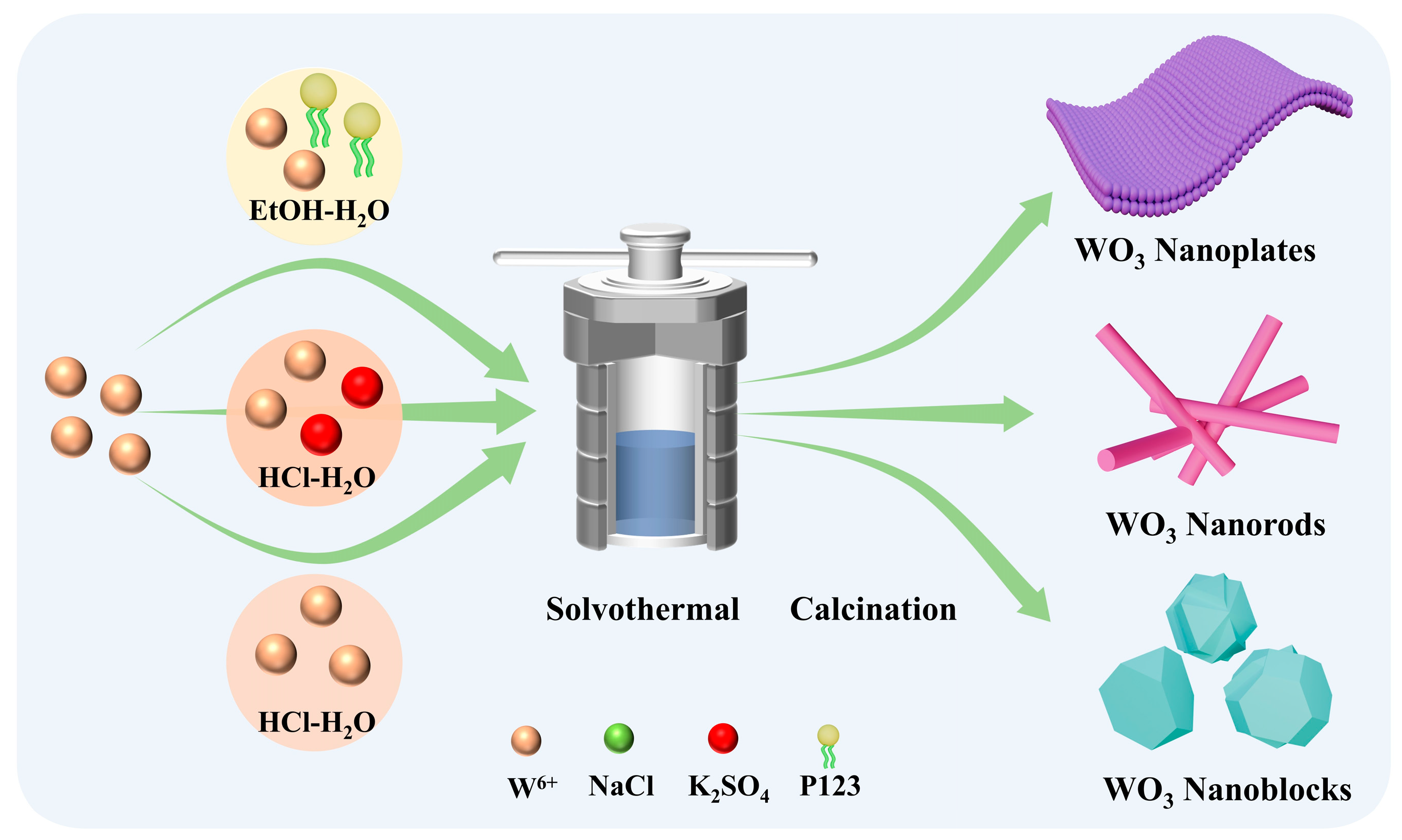
2.2. Synthesis of WO3 with Different Morphologies
2.3. Instruments
2.4. Preparation of the Sensors
3. Results
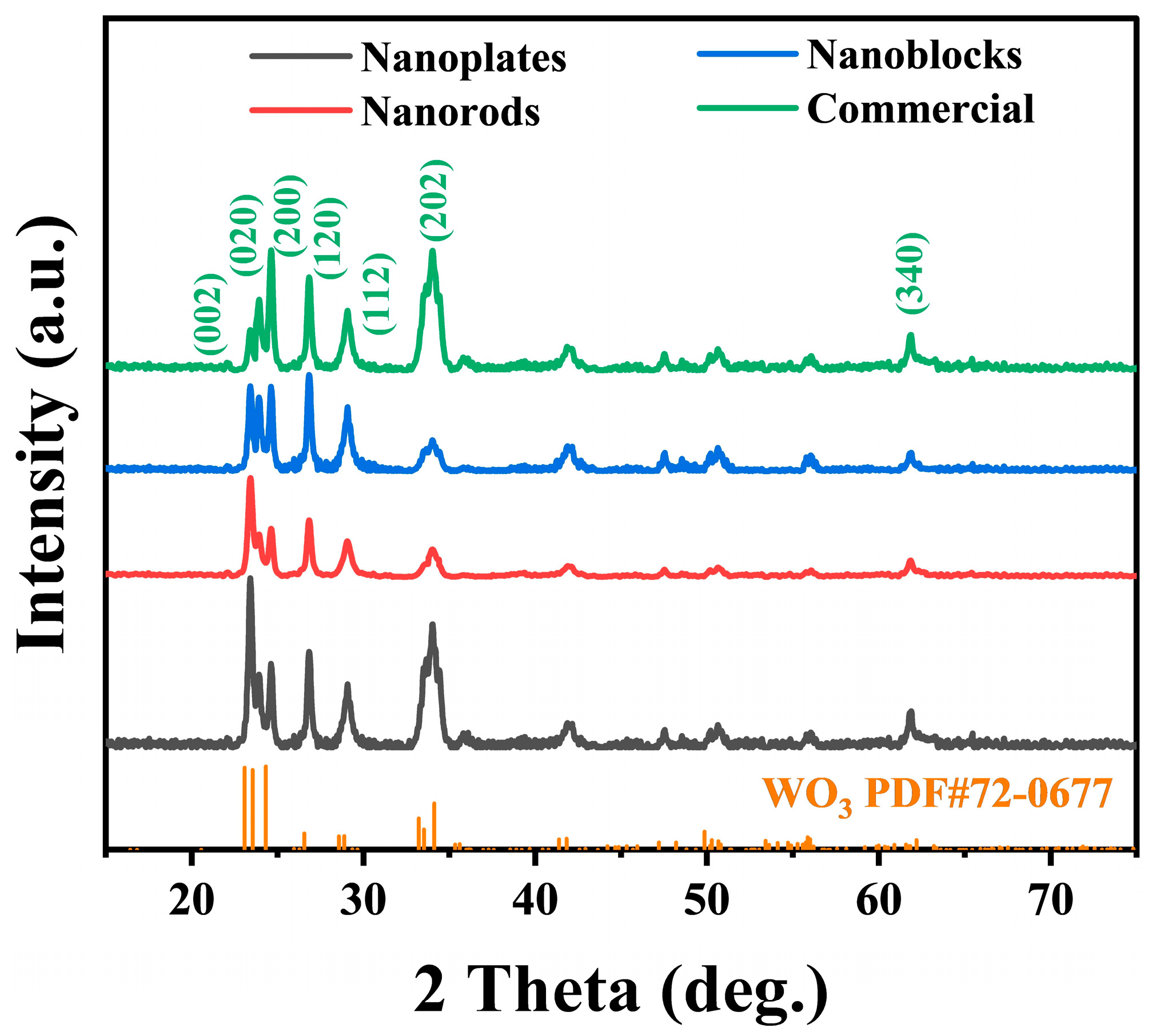
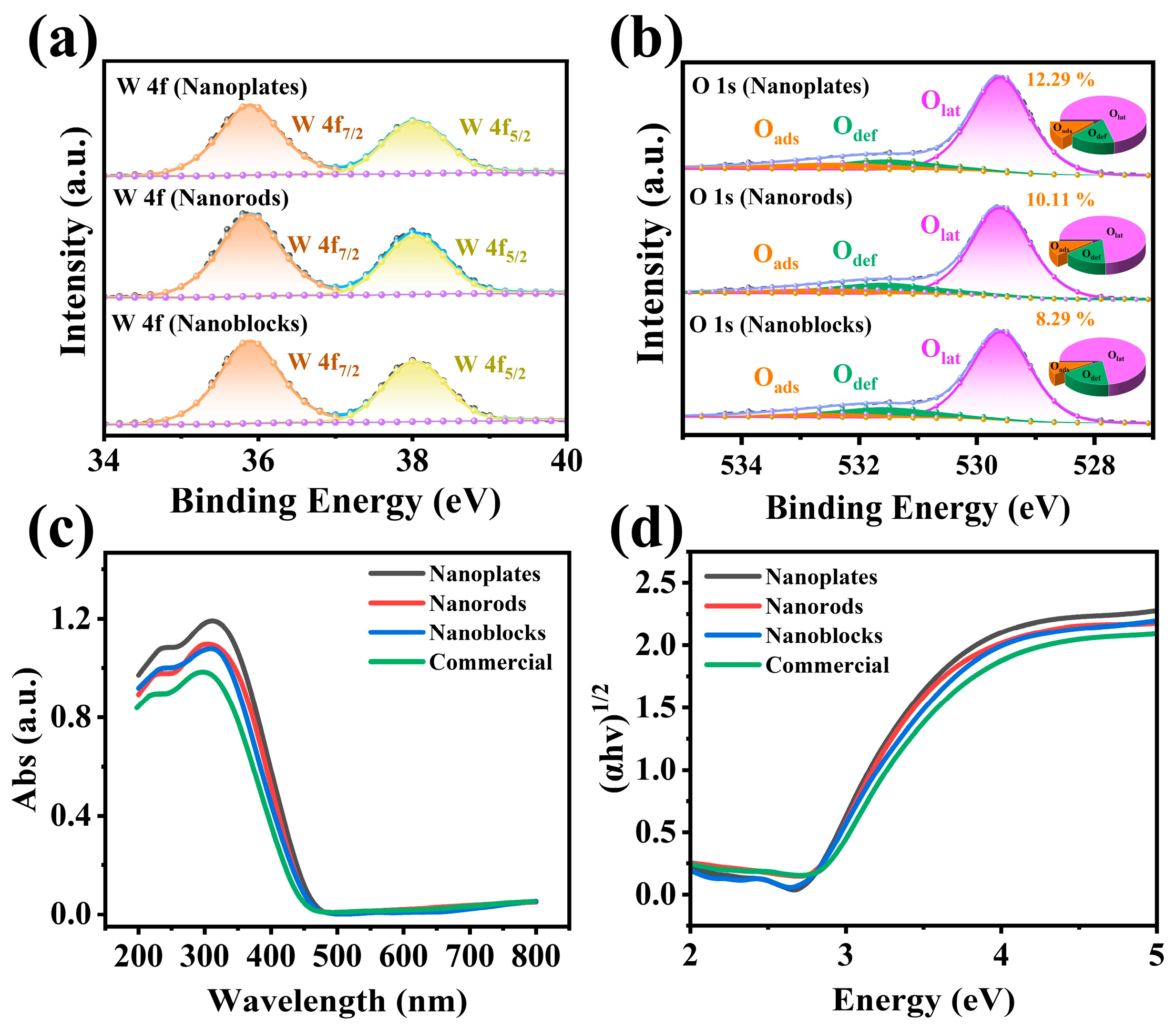
3.1. Material Characterization
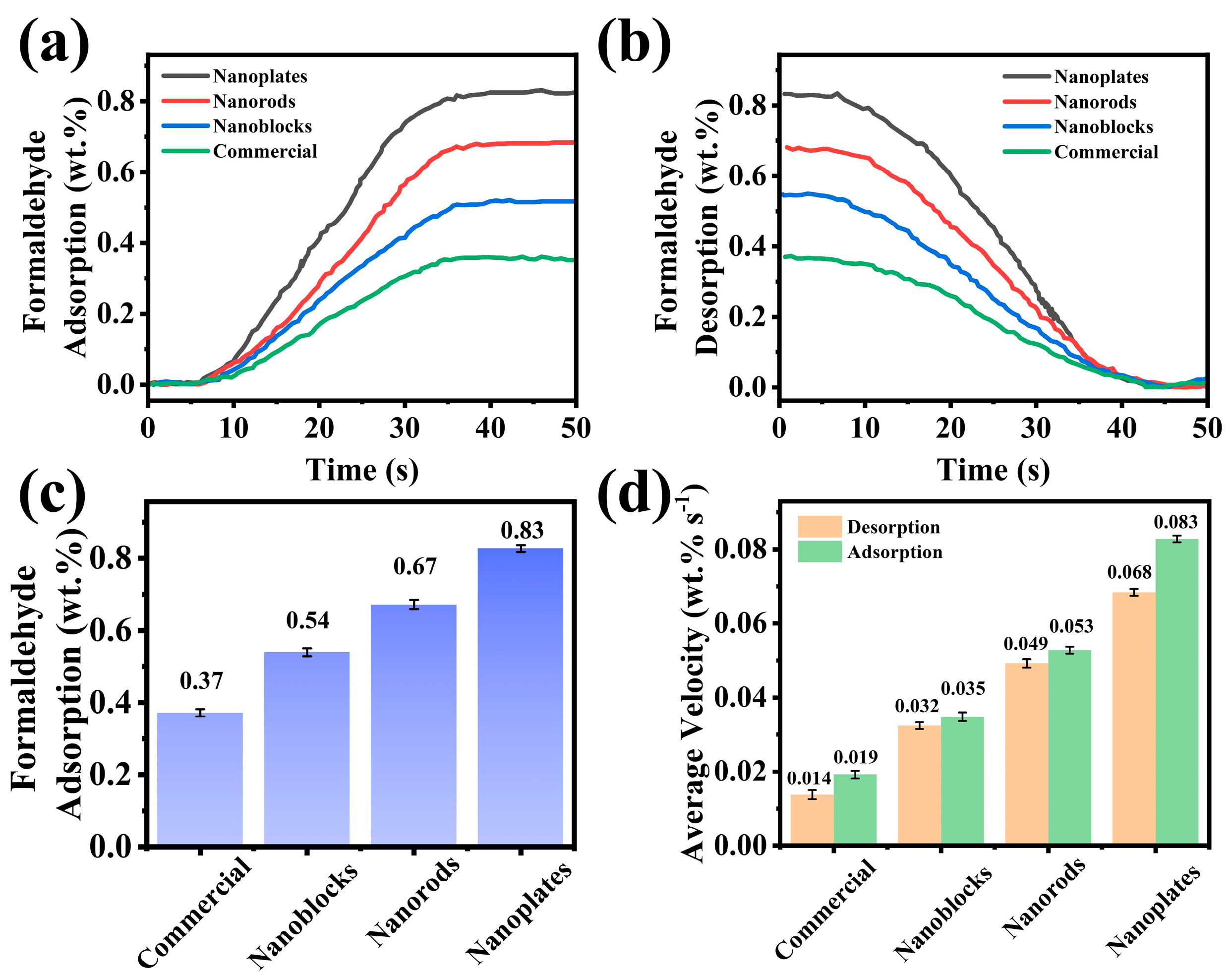
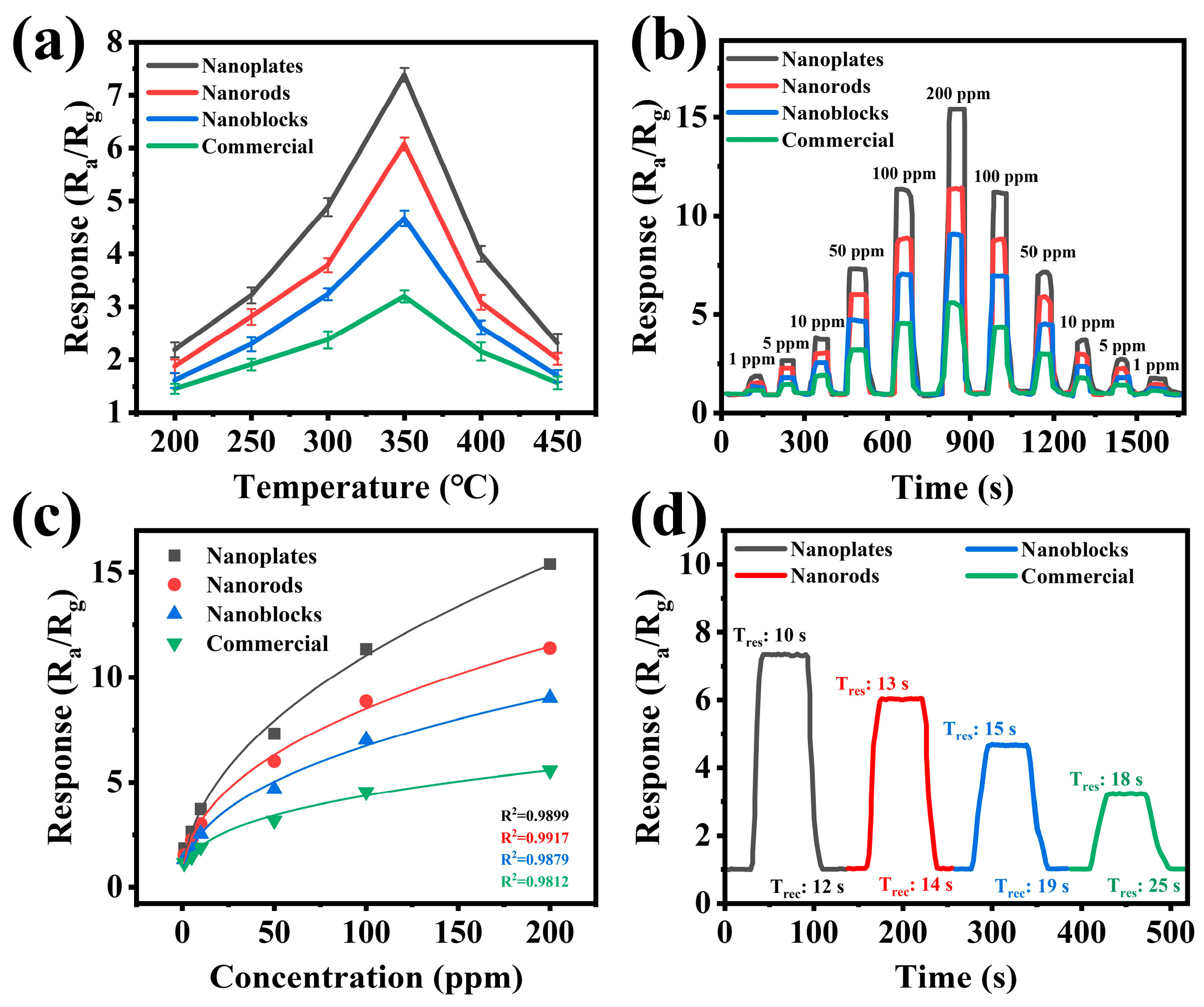
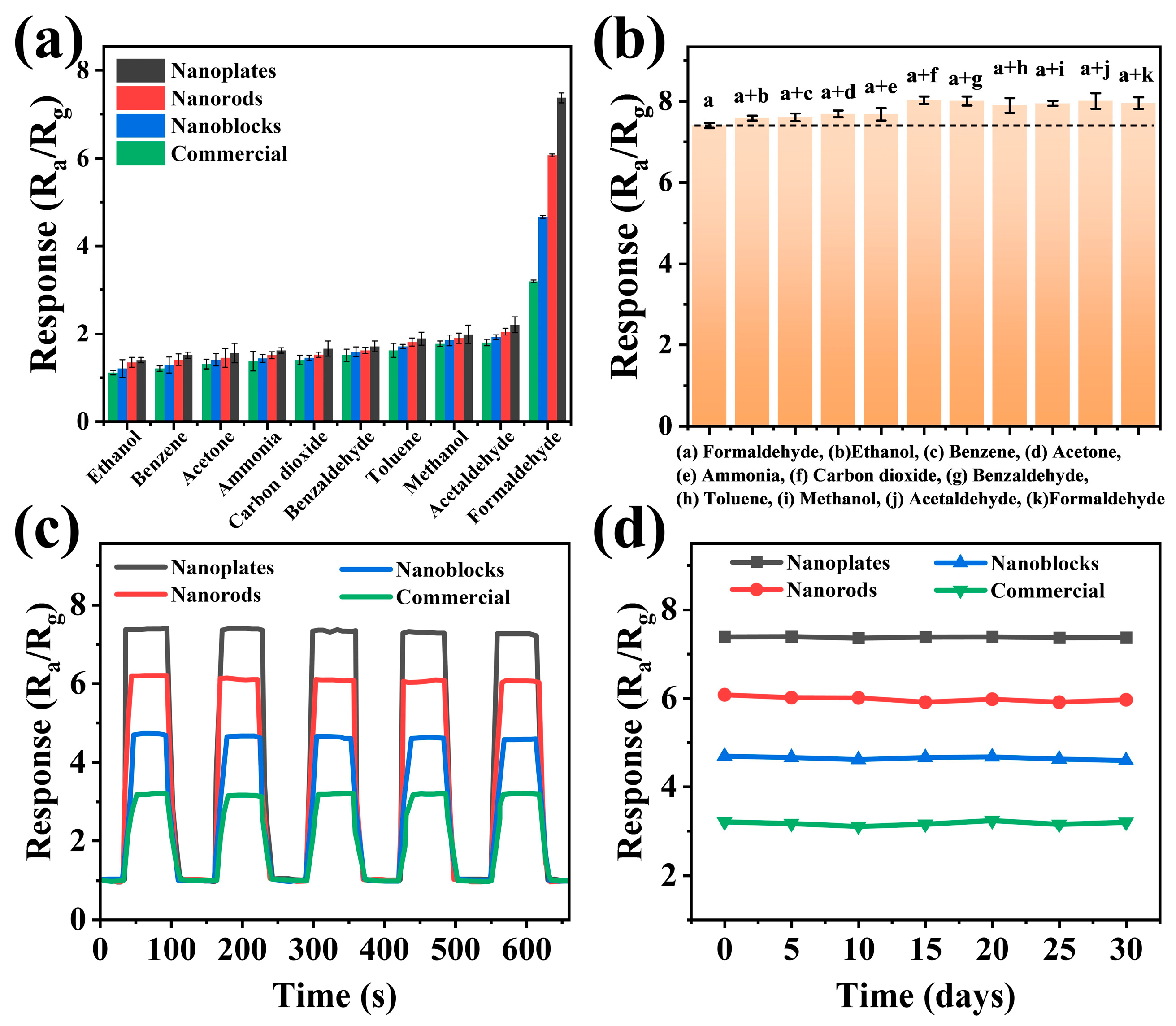
3.2. Sensing Performance
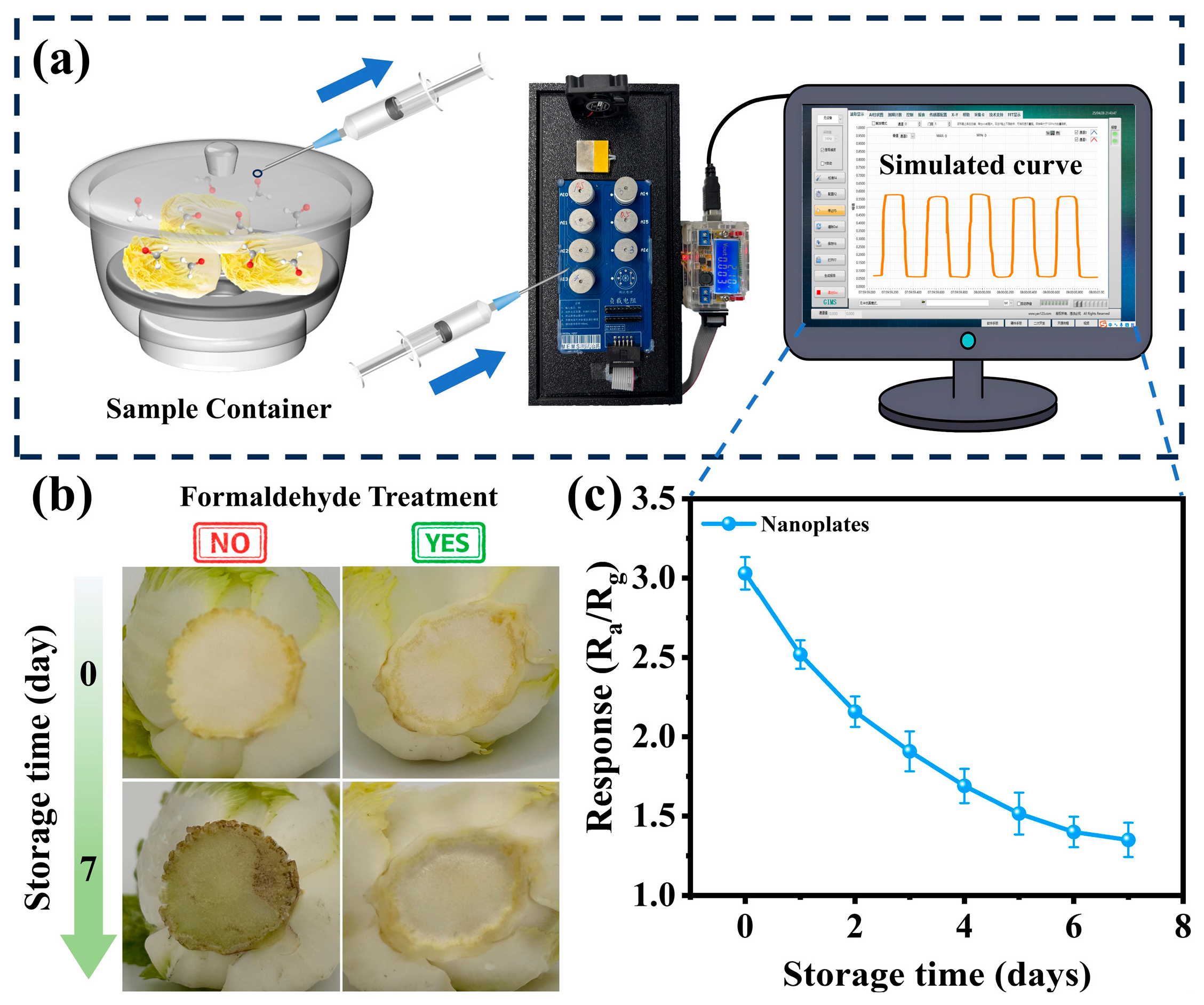
3.3. Practical Application
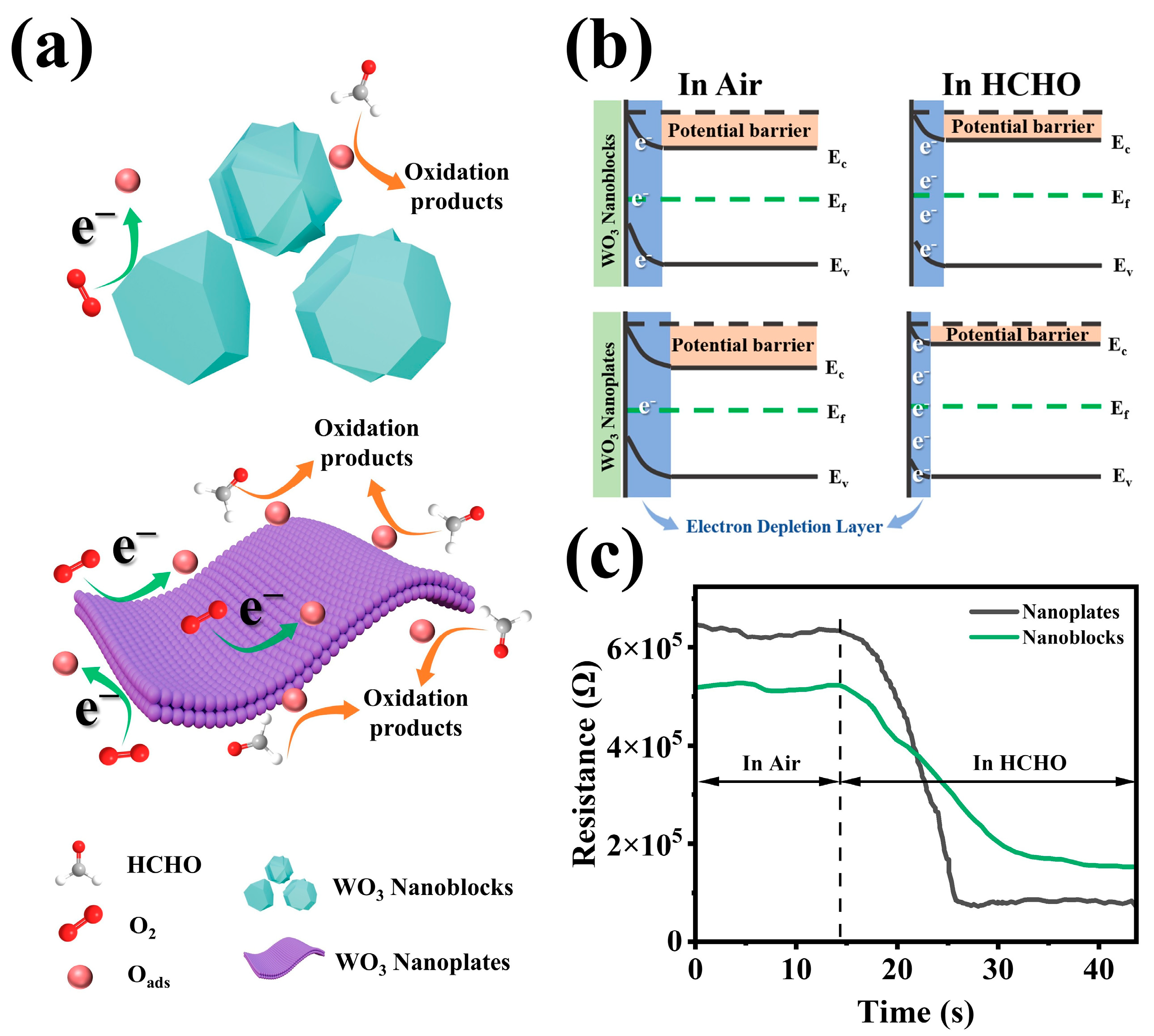
3.4. Sensing Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huff, J.; Infante, P. Identifying Cancer Sites for Human Carcinogens in the IARC Monographs. Occup. Environ. Med. 2009, 66, 140. [Google Scholar] [CrossRef] [PubMed]
- Gullian-Klanian, M.; Terrats-Preciat, M.; Pech-Jiménez, E.C.; Cutz De Ocampo, J. Effect of Frozen Storage on Protein Denaturation and Fatty Acids Profile of the Red Octopus (Octopus maya). J. Food Process. Preserv. 2016, 41, e13072. [Google Scholar] [CrossRef]
- Vohra, P.; Lantz, F.; Kratzer, F.H. Study of the Reaction of Formaldehyde with Vitamin B12. Arch. Biochem. Biophys. 1958, 76, 180–187. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, N.; Wang, S.; Zhang, H. Electronic Structure-Dependent Formaldehyde Gas Sensing Performance of the In2O3/Co3O4 Core/Shell Hierarchical Heterostructure Sensors. J. Colloid Interface Sci. 2020, 577, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Guo, S.; Hu, Z.; Li, T.; Pu, S.; Mao, H.; Cai, H.; Zhu, Z.; Li, H.-Y.; Liu, H. W18O49 Sensitized with Pd Nanoparticles for Ultrasensitive Ppb-Level Formaldehyde Detection. Chem. Eng. J. 2023, 456, 140988. [Google Scholar] [CrossRef]
- Liu, X.; Han, J.; Qiao, X.; Cai, H.; Zhao, Y.; Zhang, Z.; Zhai, B.; Ni, T.; Zhao, C.; Zhu, Y. Bimetallic Au and Pd Nanoparticles Modified WO3 Nanosheets for Enhancing the Sensitivity and Selectivity of Formaldehyde Assessment in Aquatic Products. ACS Appl. Mater. Interfaces 2024, 16, 22155–22165. [Google Scholar] [CrossRef]
- Li, Y.; Chi, C.; Zhao, Y.; Jiang, G.; Wu, J.; Song, J. Formaldehyde Detection Based on Tannin Carbon Dots. Chem. Eng. Sci. 2025, 304, 120914. [Google Scholar] [CrossRef]
- Liu, C.-C.; Wang, Y.-N.; Fu, L.-M.; Chieh, C. Micro-Distillation System for Formaldehyde Concentration Detection. Chem. Eng. J. 2016, 304, 419–425. [Google Scholar] [CrossRef]
- Xu, X.; Yang, E.; Chen, Y. Progress in the Study of Optical Probes for the Detection of Formaldehyde. Crit. Rev. Anal. Chem. 2022, 54, 1146–1172. [Google Scholar] [CrossRef]
- Davenport, J.J.; Hodgkinson, J.; Saffell, J.R.; Tatam, R.P. Non-Dispersive Ultra-Violet Spectroscopic Detection of Formaldehyde Gas for Indoor Environments. IEEE Sens. J. 2018, 18, 2218–2228. [Google Scholar] [CrossRef]
- Guzman, J.M.C.C.; Tayo, L.L.; Liu, C.-C.; Wang, Y.-N.; Fu, L.-M. Rapid Microfluidic Paper-Based Platform for Low Concentration Formaldehyde Detection. Sens. Actuators B 2018, 255, 3623–3629. [Google Scholar] [CrossRef]
- Wang, S.; Cao, J.; Cui, W.; Li, X.; Li, D. Facile Synthesis and Excellent Formaldehyde Gas Sensing Properties of Novel Spindle-like In2O3 Porous Polyhedra. Sens. Actuators B 2016, 237, 944–952. [Google Scholar] [CrossRef]
- Hussain, S.; Liu, T.; Javed, M.S.; Aslam, N.; Zeng, W. Highly Reactive 0D ZnS Nanospheres and Nanoparticles for Formaldehyde Gas-Sensing Properties. Sens. Actuators B 2017, 239, 1243–1250. [Google Scholar] [CrossRef]
- Cao, J.; Wang, S.; Zhang, H. Controllable Synthesis of Zinc Oxide Hierarchical Architectures and Their Excellent Formaldehyde Gas Sensing Performances. Mater. Lett. 2017, 202, 44–47. [Google Scholar] [CrossRef]
- Baghdadi, N.; Salah, N.; Alshahrie, A.; Ansari, A.R.; Koumoto, K. The Effect of Morphological Modification on the Thermoelectric Properties of ZnO Nanomaterials. Ceram. Int. 2021, 47, 6169–6178. [Google Scholar] [CrossRef]
- Hong, S.H.; Kim, Y.K.; Hwang, S.-H.; Seo, H.-J.; Lim, S.K. Effect of Morphology of ZnO on Colorimetric Hydrogen Sensitivity of PdO@ZnO Hybrids. Int. J. Hydrogen Energy 2024, 57, 717–726. [Google Scholar] [CrossRef]
- Fioravanti, A.; Marani, P.; Morandi, S.; Lettieri, S.; Mazzocchi, M.; Sacerdoti, M.; Carotta, M.C. Growth Mechanisms of ZnO Micro-Nanomorphologies and Their Role in Enhancing Gas Sensing Properties. Sensors 2021, 21, 1331. [Google Scholar] [CrossRef]
- Shendage, S.S.; Patil, V.L.; Vanalakar, S.A.; Patil, S.P.; Harale, N.S.; Bhosale, J.L.; Kim, J.H.; Patil, P.S. Sensitive and Selective NO2 Gas Sensor Based on WO3 Nanoplates. Sens. Actuators B 2017, 240, 426–433. [Google Scholar] [CrossRef]
- Wu, C.-H.; Zhu, Z.; Huang, S.-Y.; Wu, R.-J. Preparation of Palladium-Doped Mesoporous WO3 for Hydrogen Gas Sensors. J. Alloys Compd. 2019, 776, 965–973. [Google Scholar] [CrossRef]
- Chen, Y.; Xue, K.; Wang, Z. Controllable Synthesis of Nano-WO3 with {020} Exposure Planes for Toxic Gas Detection. Mater. Sci. Semicond. Process. 2025, 188, 109211. [Google Scholar] [CrossRef]
- Zhai, C.; Zhu, M.; Jiang, L.; Yang, T.; Zhao, Q.; Luo, Y.; Zhang, M. Fast Triethylamine Gas Sensing Response Properties of Nanosheets Assembled WO3 Hollow Microspheres. Appl. Surf. Sci. 2019, 463, 1078–1084. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, Y.; Ma, J.; Cheng, X.; Xie, J.; Xu, P.; Liu, H.; Liu, H.; Zhang, H.; Wu, M.; et al. Mesoporous Tungsten Oxides with Crystalline Framework for Highly Sensitive and Selective Detection of Foodborne Pathogens. J. Am. Chem. Soc. 2017, 139, 10365–10373. [Google Scholar] [CrossRef] [PubMed]
- Maji, B.; Barik, B.; Sahoo, S.J.; Achary, L.S.K.; Kumar Sahoo, K.; Kar, J.P.; Dash, P. Shape Selective Comprehensive Gas Sensing Study of Different Morphological Manganese-Cobalt Oxide Based Nanocomposite as Potential Room Temperature Hydrogen Gas Sensor. Sens. Actuators B 2023, 380, 133348. [Google Scholar] [CrossRef]
- Cheng, Y.; Teng, Y.; Zheng, M.; Zhang, X.; Wang, M.-S.; Gu, L.; Rao, Y.; Dai, X.; Liu, H.; Jing, H.; et al. 3D Urchin-Like WO3-x Based Nanostructures with Abundant Oxygen Vacancies for Ppb-Level Tea Aroma Sensing. Sens. Actuators B 2024, 419, 136293. [Google Scholar] [CrossRef]
- Septiani, N.L.W.; Shukri, G.; Saputro, A.G.; Nugraha; Karim, M.R.; Al-Mubaddel, F.; Hardiansyah, A.; Yamauchi, Y.; Kaneti, Y.V.; Yuliarto, B. Palm Sugar-Induced Formation of Hexagonal Tungsten Oxide with Nanorod-Assembled Three-Dimensional Hierarchical Frameworks for Nitrogen Dioxide Sensing. ACS Sustain. Chem. Eng. 2022, 10, 15035–15045. [Google Scholar] [CrossRef]
- Zhu, K.; Zhu, Z.; Xu, S.; Zhao, C.; Ni, T. Controlled Synthesis of α-Fe2O3 Nanocubes for Gas-Sensing Applications: Feasibility of Assessing Crucian Carp (Carassius Auratus) Freshness via Trimethylamine Levels. Food Chem. 2024, 441, 138361. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Lu, Y.; Liu, Z.; Sui, C.; Wang, Y.; Yang, L.; Liu, F.; Sun, P.; Liu, F.; et al. Preparation of BiOI-Functionalized ZnO Nanorods for Ppb-Level NO2 Detection at Room Temperature. ACS Sens. 2022, 7, 3915–3922. [Google Scholar] [CrossRef]
- Cai, Z.; Park, J.; Park, S. Synthesis of Flower-like ZnO and Its Enhanced Sensitivity towards NO2 Gas Detection at Room Temperature. Chemosensors 2023, 11, 322. [Google Scholar] [CrossRef]
- Van Duy, L.; Nguyet, T.T.; Hung, C.M.; Thanh Le, D.T.; Van Duy, N.; Hoa, N.D.; Biasioli, F.; Tonezzer, M.; Di Natale, C. Ultrasensitive NO2 Gas Sensing Performance of Two Dimensional ZnO Nanomaterials: Nanosheets and Nanoplates. Ceram. Int. 2021, 47, 28811–28820. [Google Scholar] [CrossRef]
- Nagarjuna, Y.; Hsiao, Y.-J. TeO2 Doped ZnO Nanostructure for the Enhanced NO2 Gas Sensing on MEMS Sensor Device. Sens. Actuators B 2024, 401, 134891. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, L.; Ni, W.; Cheng, W.; Yang, Z.; Xu, S.; Wang, T.; Zhang, B.; Xuan, F. NiO/ZnO Nanocomposites for Multimodal Intelligent MEMS Gas Sensors. ACS Sens. 2025, 10, 2531–2541. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Liao, T.; Dou, Y.; Hwang, S.M.; Park, M.-S.; Jiang, L.; Kim, J.H.; Dou, S.X. Generalized Self-Assembly of Scalable Two-Dimensional Transition Metal Oxide Nanosheets. Nat. Commun. 2014, 5, 3813. [Google Scholar] [CrossRef]
- Wang, J.; Khoo, E.; Lee, P.S.; Ma, J. Synthesis, Assembly, and Electrochromic Properties of Uniform Crystalline WO3 Nanorods. J. Phys. Chem. C 2008, 112, 14306–14312. [Google Scholar] [CrossRef]
- Cao, S.; Zhao, C.; Han, T.; Peng, L. Hydrothermal Synthesis, Characterization and Gas Sensing Properties of the WO3 Nanofibers. Mater. Lett. 2016, 169, 17–20. [Google Scholar] [CrossRef]
- Su, P.-G.; Liao, W.-H. Effect of Adding Au Nanoparticles and KOH on the Electrical and Humidity-Sensing Properties of WO3 Particles. Sens. Actuators B 2017, 252, 854–861. [Google Scholar] [CrossRef]
- Doumeng, M.; Makhlouf, L.; Berthet, F.; Marsan, O.; Delbé, K.; Denape, J.; Chabert, F. A Comparative Study of the Crystallinity of Polyetheretherketone by Using Density, DSC, XRD, and Raman Spectroscopy Techniques. Polym. Test. 2021, 93, 106878. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, N.; Zheng, Z.; Chi, H.; Huang, D.; Ye, Z.; Jiang, J.; Zhu, L. Ultrasensitive Detection of H2 Based on WO3 Nanocubes Decorated with PtO Nanoparticles. Int. J. Hydrogen Energy 2024, 94, 1464–1475. [Google Scholar] [CrossRef]
- An, B.; Yang, Y.; Wang, Y.; Li, R.; Wu, Z.; Wang, P.; Zhang, T.; Han, R.; Xie, E. Observation on Switching Properties of WO3 -Based H2 Sensor Regulated by Temperature and Gas Concentration. ACS Sens. 2024, 9, 5179–5187. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Liu, P.-P.; Cai, H.-J.; Li, M.-M.; Deng, C.-H.; Zhao, Y.; Zhang, Z.-H.; Zhu, Y.-H. PdRh Bimetallic-Loaded α-Fe2O3 Nanospindles with Ppb-Limit of 3-Hydroxy-2-Butanone Biomarker Detection: Particle Dimension Regulation and Oxygen Spillover Effect. Chem. Eng. J. 2025, 512, 162686. [Google Scholar] [CrossRef]
- Wu, Z.-Y.; Liu, Y.-F.; Zhang, C.; Zheng, X.-H. Electron Sensitization and Chemical Sensitization of ZnWO4/WO3 Nanorod Heterojunctions for High Performance Triethylamine Sensor. Sens. Actuators B 2025, 424, 136870. [Google Scholar] [CrossRef]
- Cho, S.H.; Suh, J.M.; Jeong, B.; Lee, T.H.; Choi, K.S.; Eom, T.H.; Choi, S.W.; Nam, G.B.; Kim, Y.J.; Jang, H.W. Substantially Accelerated Response and Recovery in Pd-Decorated WO3 Nanorods Gasochromic Hydrogen Sensor. Small 2024, 20, 2309744. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wu, G.; Wu, J.; Shen, J.; Chen, L.; Zhang, J.; Mao, Y.; Cheng, H.; Zhang, M.; Ma, Q.; et al. Ultrathin WO3 Nanosheets/Pd with Strong Metal–Support Interactions for Highly Sensitive and Selective Detection of Mustard-Gas Simulants. ACS Sens. 2024, 9, 3773–3782. [Google Scholar] [CrossRef]
- Zhao, C.; Xu, S.; Wei, J.; Xie, S.; Wei, J.; Han, J.; Zhang, Z.; Liu, H.; Cheng, J.; Zhao, Y.; et al. Enhanced Response for Foodborne Pathogens Detection by Au Nanoparticles Decorated ZnO Nanosheets Gas Sensor. Biosensors 2022, 12, 803. [Google Scholar] [CrossRef]
- Li, R.; Wang, Q.; Wang, Y.; An, B.; Yang, Y.; Wu, Z.; Wang, P.; Zhang, T.; Han, R.; Xie, E. Unraveling the Effect of Oxygen Vacancy on WO3 Surface for Efficient NO2 Detection at Low Temperature. ACS Appl. Mater. Interfaces 2024, 16, 51738–51747. [Google Scholar] [CrossRef]
- Lee, I.; Kannankutty, K.; He, Z.-F.; Wei, T.-C. Facile, Cost-Effective NO2 Gas Sensors Based on Polymer Intercalated Graphene/Reduced Graphene Oxide Materials. J. Taiwan Inst. Chem. Eng. 2024, 157, 105405. [Google Scholar] [CrossRef]
- Li, X.; Zhang, N.; Liu, C.; Adimi, S.; Zhou, J.; Liu, D.; Ruan, S. Enhanced Gas Sensing Properties for Formaldehyde Based on ZnO/Zn2SnO4 Composites from One-Step Hydrothermal Synthesis. J. Alloys Compd. 2021, 850, 156606. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, K.; Liu, S.; Liang, X.; Zhang, M. Reasonable Construction of 2D Porous NiO/Co3O4 Nanosheets for Efficient Detection of Xylene. Sens. Actuators B 2023, 377, 133002. [Google Scholar] [CrossRef]
- Feng, S.; Yu, H.; Zhang, X.; Huo, L.; Gao, R.; Wang, P.; Cheng, X.; Major, Z.; Gao, S.; Xu, Y. Ionic Liquid-Assisted Synthesis of 2D Porous Lotus Root Slice-Shaped NiO Nanomaterials for Selective and Highly Sensitive Detection of N2H4. Sens. Actuators B 2022, 359, 131529. [Google Scholar] [CrossRef]
- Shen, B.; Yuan, T.; Zhang, W.; Chen, Y.; Xu, J. Complex Shell Fe-ZnO Derived from ZIF-8 as High-Quality Acetone MEMS Sensor. Chin. Chem. Lett. 2024, 35, 109490. [Google Scholar] [CrossRef]
- Sun, B.; Liu, M.; Liu, L.; Wang, Q.; Song, P. Synthesis of 1D LaFeO3 Nanofibers/2D MXene Heterostructures for Formaldehyde Detection at Low Temperature. Sens. Actuators B 2024, 415, 136011. [Google Scholar] [CrossRef]
- Kim, K.B.; Sohn, M.S.; Min, S.; Yoon, J.; Park, J.; Li, J.; Moon, Y.K.; Kang, Y.C. Highly Selective and Reversible Detection of Simulated Breath Hydrogen Sulfide Using Fe-Doped CuO Hollow Spheres: Enhanced Surface Redox Reaction by Multi-Valent Catalysts. Small 2024, 20, 2308963. [Google Scholar] [CrossRef]
- Chao, J.; Zhang, K.; Meng, D.; Sun, Y. Au Modified SnO2 Submicron Flowers Sensor for Efficient Detection of Formaldehyde and Its Application in Detection of Green Vegetables. Mater. Res. Bull. 2023, 168, 112481. [Google Scholar] [CrossRef]
- Fappiano, L.; Carriera, F.; Iannone, A.; Notardonato, I.; Avino, P. A Review on Recent Sensing Methods for Determining Formaldehyde in Agri-Food Chain: A Comparison with the Conventional Analytical Approaches. Foods 2022, 11, 1351. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.B.; Hussain, M.; Kabiraz, M.P.; Nordin, N.; Siddiqui, S.A.; Bhowmik, S.; Begum, M. An Update on Formaldehyde Adulteration in Food: Sources, Detection, Mechanisms, and Risk Assessment. Food Chem. 2023, 427, 136761. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.-L.; Zhu, Z.-Y.; Du, L.-L.; Xing, X.-X.; Wang, C.; Chen, J.; Tian, Y.-T.; Yang, D.-C. Improved Sensing Performance of WO3 Nanoparticles Decorated with Ag and Pt Nanoparticles. Rare Met. 2021, 40, 1642–1650. [Google Scholar] [CrossRef]
- Hwan Cho, S.; Min Suh, J.; Jeong, B.; Hyung Lee, T.; Soon Choi, K.; Hoon Eom, T.; Kim, T.; Won Jang, H. Fast Responding and Highly Reversible Gasochromic H2 Sensor Using Pd-Decorated Amorphous WO3 Thin Films. Chem. Eng. J. 2022, 446, 136862. [Google Scholar] [CrossRef]
- Gao, W.; Chang, X.; Ola, O.; Wang, Z.; Liao, Q.; Zhu, X.; Li, J.; Jiang, Y.; Wang, D.; Sun, S. Humidity-Tolerant Ammonia Gas Sensors Based on PrOx/In2O3/WO3 Heterostructure Films. Chem. Eng. J. 2025, 509, 161482. [Google Scholar] [CrossRef]
- Barsan, N.; Weimar, U. Conduction Model of Metal Oxide Gas Sensors. J. Electroceram. 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Li, X.-Y.; Sun, G.-T.; Fan, F.; Li, Y.-Y.; Liu, Q.-C.; Yao, H.-C.; Li, Z.-J. Au25 Nanoclusters Incorporating Three-Dimensionally Ordered Macroporous In2O3 for Highly Sensitive and Selective Formaldehyde Sensing. ACS Appl. Mater. Interfaces 2021, 14, 564–573. [Google Scholar] [CrossRef]
- Yu, H.; Li, J.; Li, Z.; Tian, Y.; Yang, Z. Enhanced Formaldehyde Sensing Performance Based on Ag@WO3 2D Nanocomposite. Powder Technol. 2019, 343, 1–10. [Google Scholar] [CrossRef]
- Li, Z.; Fan, Y.; Zhan, J. In2O3 Nanofibers and Nanoribbons: Preparation by Electrospinning and Their Formaldehyde Gas-Sensing Properties. Eur. J. Inorg. Chem. 2010, 2010, 3348–3353. [Google Scholar] [CrossRef]
- Chen, Z.-W.; Hong, Y.-Y.; Lin, Z.-D.; Liu, L.-M.; Zhang, X.-W. Enhanced Formaldehyde Gas Sensing Properties of ZnO Nanosheets Modified with Graphene. Electron. Mater. Lett. 2017, 13, 270–276. [Google Scholar] [CrossRef]
- Xiao, X.; Xing, X.; Han, B.; Deng, D.; Cai, X.; Wang, Y. Enhanced Formaldehyde Sensing Properties of SnO2 Nanorods Coupled with Zn2SnO4. RSC Adv. 2015, 5, 42628–42636. [Google Scholar] [CrossRef]
- Dong, C.; Liu, X.; Han, B.; Deng, S.; Xiao, X.; Wang, Y. Nonaqueous Synthesis of Ag-Functionalized In2O3/ZnO Nanocomposites for Highly Sensitive Formaldehyde Sensor. Sens. Actuators B 2016, 224, 193–200. [Google Scholar] [CrossRef]
- Gong, F.; Liu, H.; Liu, C.; Gong, Y.; Zhang, Y.; Meng, E.; Li, F. 3D Hierarchical In2O3Nanoarchitectures Consisting of Nanocuboids and Nanosheets for Chemical Sensors with Enhanced Performances. Mater. Lett. 2016, 163, 236–239. [Google Scholar] [CrossRef]
- Zhu, L.-Y.; Yuan, K.; Yang, J.-G.; Ma, H.-P.; Wang, T.; Ji, X.-M.; Feng, J.-J.; Devi, A.; Lu, H.-L. Fabrication of Heterostructured P-CuO/n-SnO2 Core-Shell Nanowires for Enhanced Sensitive and Selective Formaldehyde Detection. Sens. Actuators B 2019, 290, 233–241. [Google Scholar] [CrossRef]
- San, X.; Zhao, G.; Wang, G.; Shen, Y.; Meng, D.; Zhang, Y.; Meng, F. Assembly of 3D Flower-like NiO Hierarchical Architectures by 2D Nanosheets: Synthesis and Their Sensing Properties to Formaldehyde. RSC Adv. 2017, 7, 3540–3549. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, W.; Yang, Y.; Zhao, C.; Wang, X.; Xie, Y.; Jiang, K.; Feng, H.; Zhu, Y. Controlled Synthesis of Tungsten Oxide Nanomaterials with Different Morphologies and Their Gas-Sensing Properties for Formaldehyde in Vegetables. Biosensors 2025, 15, 400. https://doi.org/10.3390/bios15070400
Wu W, Yang Y, Zhao C, Wang X, Xie Y, Jiang K, Feng H, Zhu Y. Controlled Synthesis of Tungsten Oxide Nanomaterials with Different Morphologies and Their Gas-Sensing Properties for Formaldehyde in Vegetables. Biosensors. 2025; 15(7):400. https://doi.org/10.3390/bios15070400
Chicago/Turabian StyleWu, Weihao, Yaochong Yang, Cheng Zhao, Xingyu Wang, Yitong Xie, Kexin Jiang, Huafeng Feng, and Yongheng Zhu. 2025. "Controlled Synthesis of Tungsten Oxide Nanomaterials with Different Morphologies and Their Gas-Sensing Properties for Formaldehyde in Vegetables" Biosensors 15, no. 7: 400. https://doi.org/10.3390/bios15070400
APA StyleWu, W., Yang, Y., Zhao, C., Wang, X., Xie, Y., Jiang, K., Feng, H., & Zhu, Y. (2025). Controlled Synthesis of Tungsten Oxide Nanomaterials with Different Morphologies and Their Gas-Sensing Properties for Formaldehyde in Vegetables. Biosensors, 15(7), 400. https://doi.org/10.3390/bios15070400





