Enhanced Visualization of Erythrocytes Through Photoluminescence Using NaYbF4:Yb3+,Er3+ Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. RBC In Vitro Culture
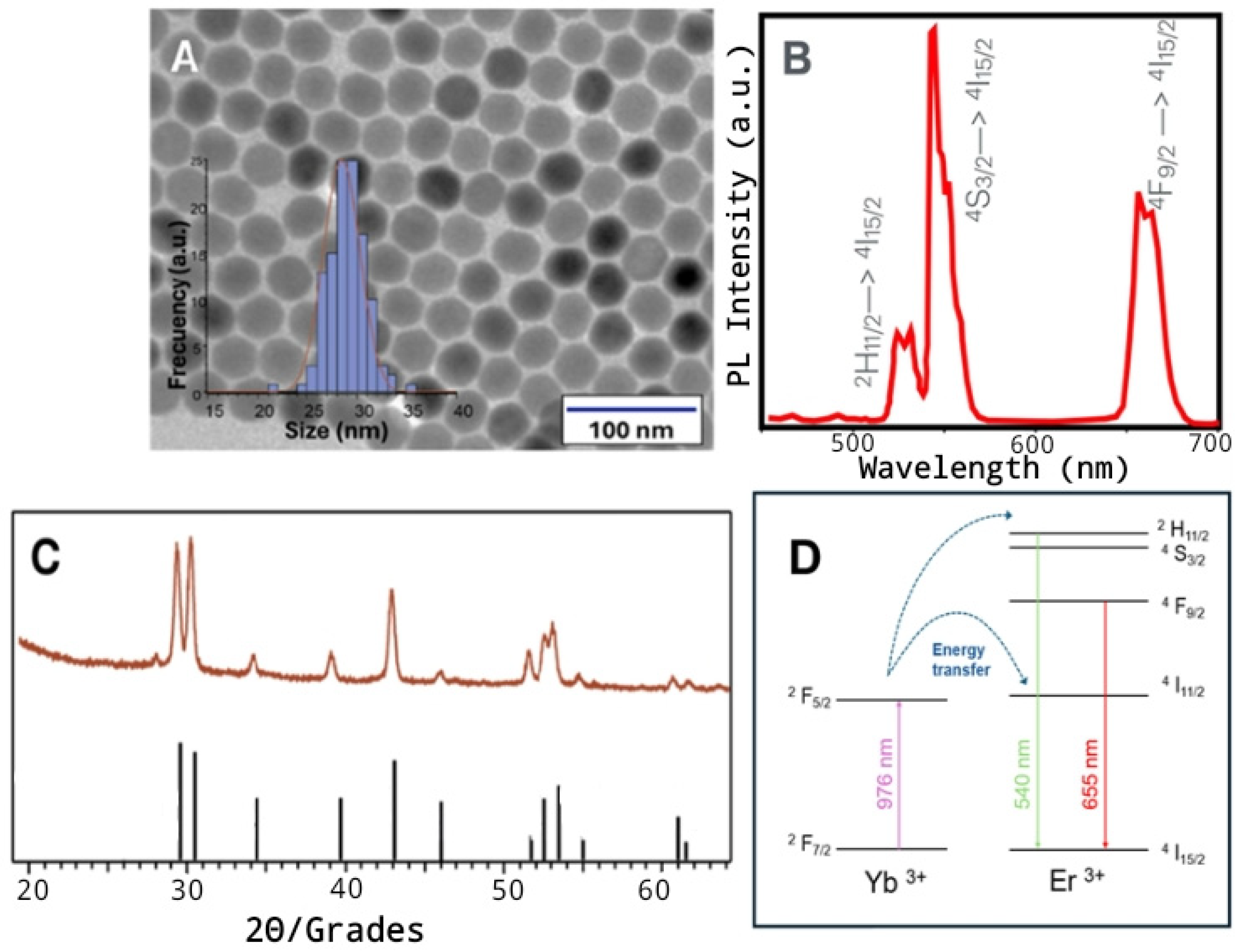
2.2. Synthesis of NaYF4:Yb3+,Er3+ NPs
2.3. Nanoparticle Surface Modification with Polyacrylic Acid
2.4. Morphological Characterization
2.5. Optical Characterization
2.6. Cellular Uptake and Cytotoxicity Studies with Vero Cells
2.7. Hemolysis Bioassay
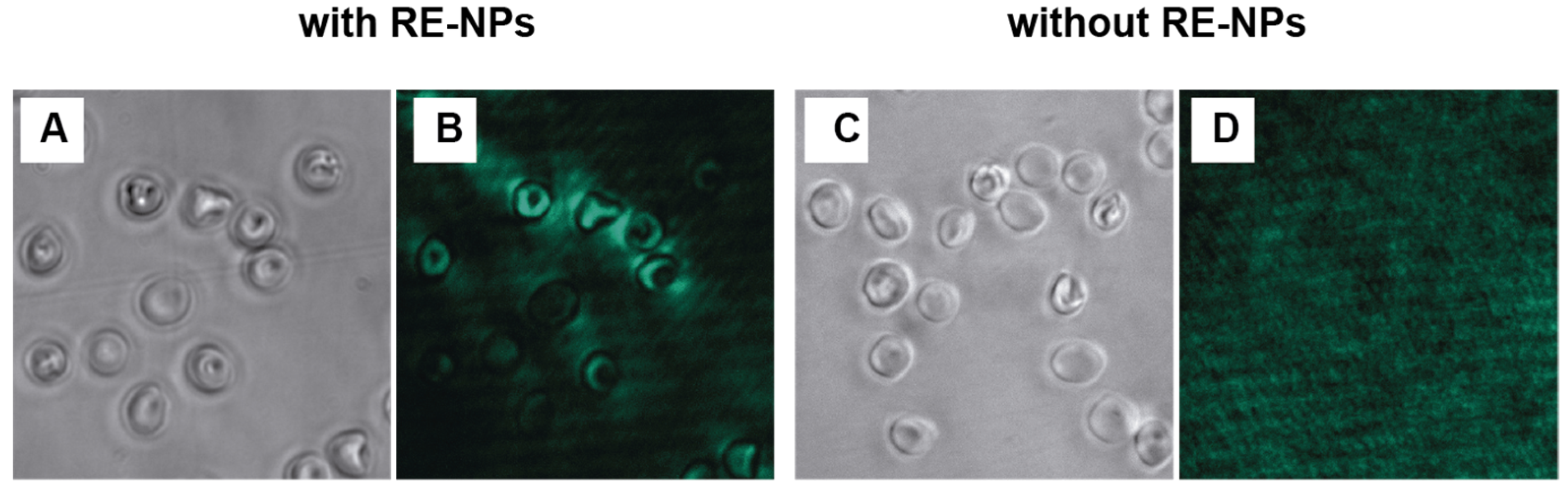
2.8. Colocalization of NaYF4:Yb3+,Er3+ Nanoparticles in Erythrocytes
2.9. Processing and Analysis of Images After Acquisition
3. Results
3.1. Characterization of NaYF4:Yb3+, Er3+- NPs
3.2. Viability Bioassays
3.3. In Vitro Fluorescence Imaging of Human Erythrocytes Using NaYF4:Yb3+, Er3+ NPs
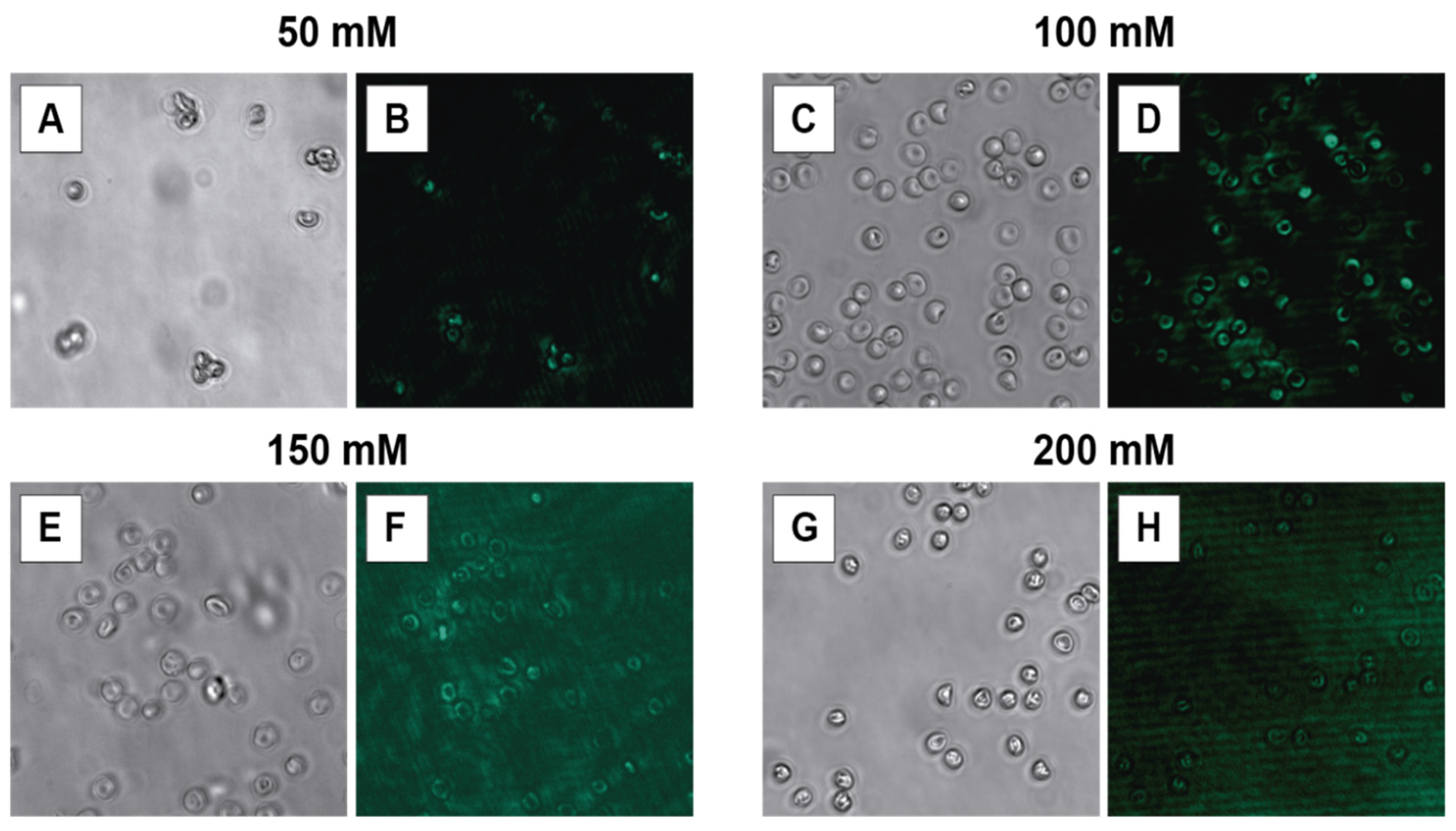
3.3.1. HEPES Concentration
3.3.2. Incubation Time
3.3.3. Laser Current Intensity
3.3.4. NP Concentration
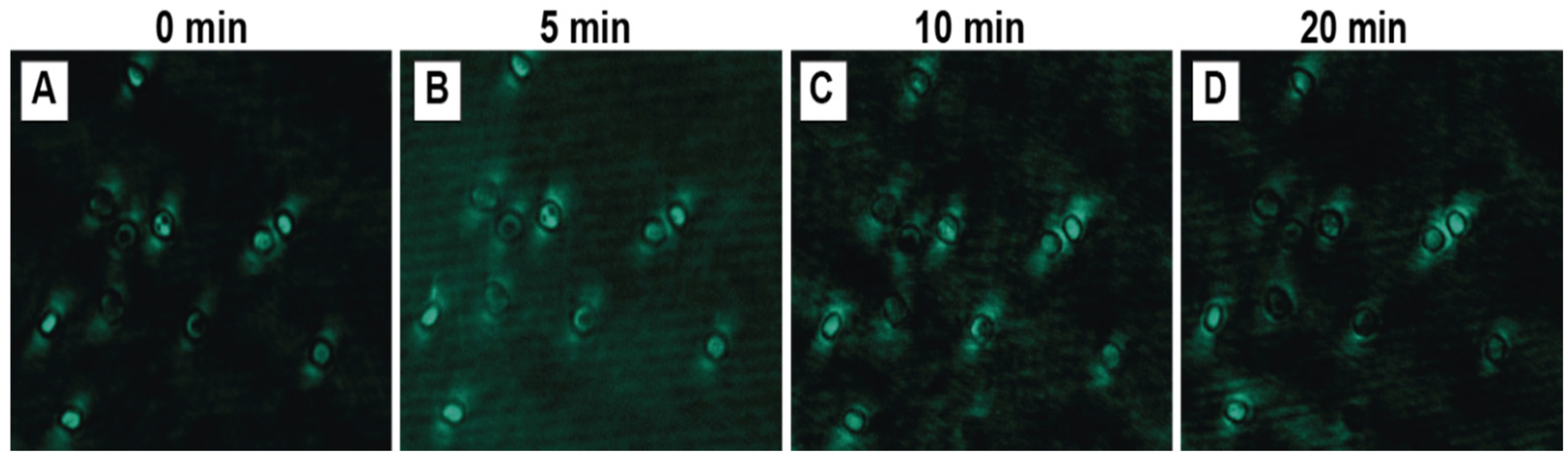
3.3.5. Laser Exposure Time
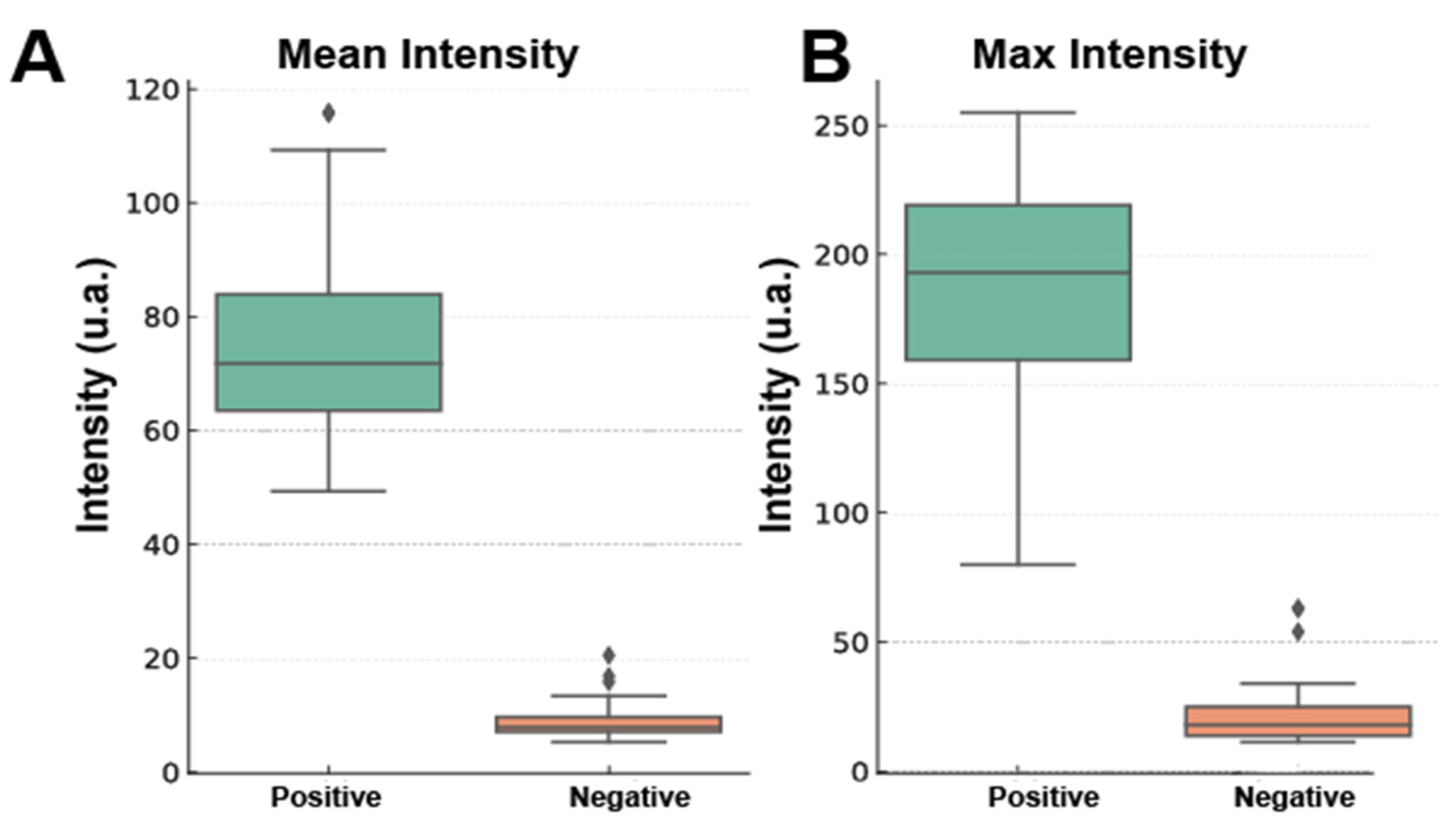
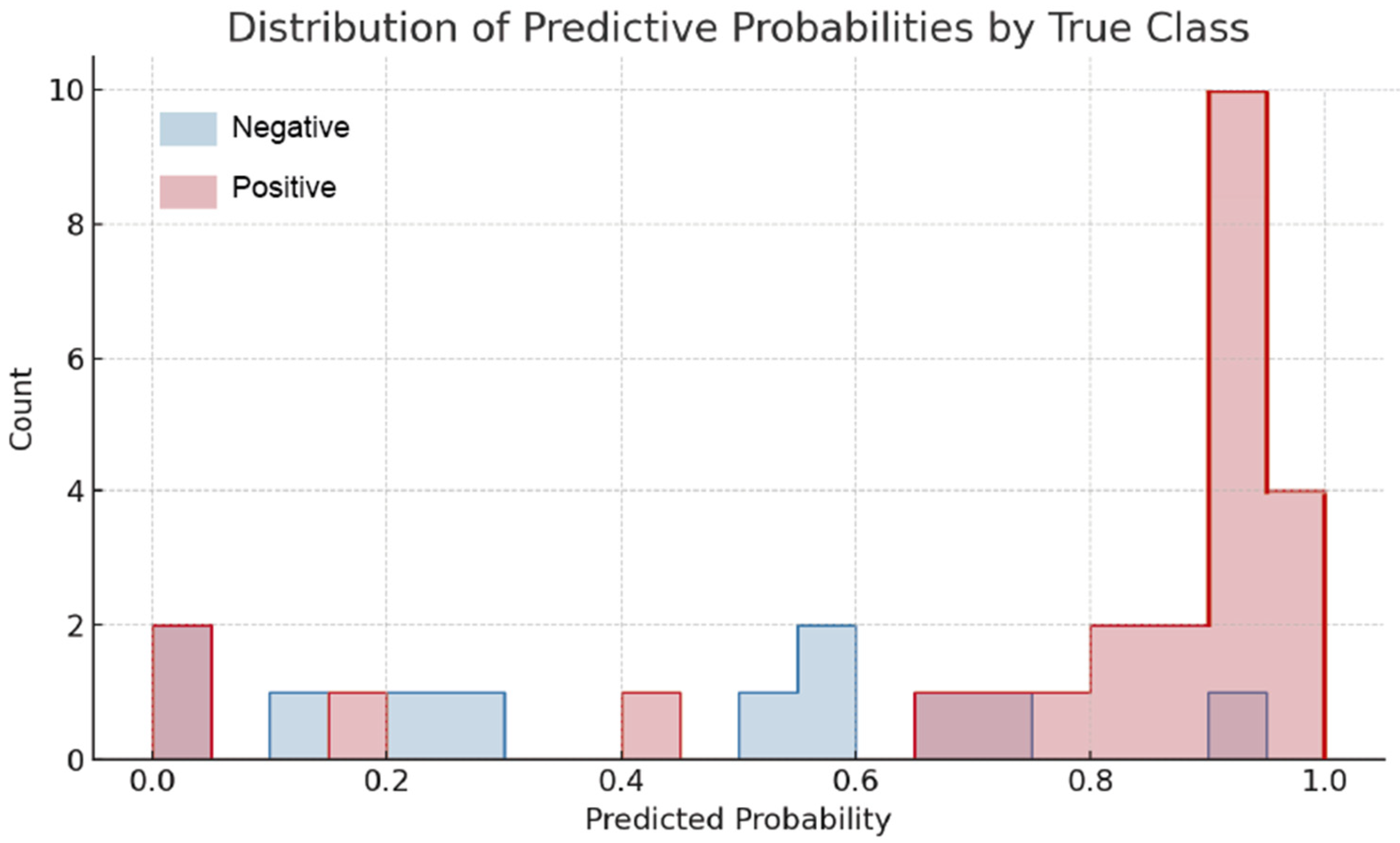
3.3.6. Machine Learning-Based Analysis of Erythrocyte Photoluminescence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NPs | Nanoparticles |
| RBCs | Red blood cells |
| UCNPs | Upconversion nanoparticles |
| ML | Machine learning |
| RE-NPs | Rare-earth nanoparticles |
| TEM | Transmission electron microscopy |
| MTT assay | 3-(4,5- dimethyl-2-thiazolyl)-2,5-di-phenyl-2-H-tetrazolium bromide |
| XRD | X-ray diffraction |
| FT-IR | Fourier-transform infrared spectroscopy |
| TGA | Thermogravimetric analysis |
References
- Barbalato, L.; Pillarisetty, L.S. Histology, Red Blood Cell; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Pretorius, E.; Oore-ofe, O.; Mbotwe, S.; Bester, J. Erythrocytes and Their Role as Health Indicator: Using Structure in a Patient-Orientated Precision Medicine Approach. Blood Rev. 2016, 30, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lin, Y.; Xin, J.; Zhang, Y.; Yang, K.; Luo, Y.; Wang, B. Red Blood Cells in Biology and Translational Medicine: Natural Vehicle Inspires New Biomedical Applications. Theranostics 2024, 14, 220–248. [Google Scholar] [CrossRef] [PubMed]
- Brahm, J. The Permeability of Red Blood Cells to Chloride, Urea and Water. J. Exp. Biol. 2013, 216, 2238–2246. [Google Scholar] [CrossRef] [PubMed]
- Balog, S.; de Almeida, M.S.; Taladriz-Blanco, P.; Rothen-Rutishauser, B.; Petri-Fink, A. Does the Surface Charge of the Nanoparticles Drive Nanoparticle–Cell Membrane Interactions? Curr. Opin. Biotechnol. 2024, 87, 103128. [Google Scholar] [CrossRef]
- Gu, Z.; Yan, L.; Tian, G.; Li, S.; Chai, Z.; Zhao, Y. Recent Advances in Design and Fabrication of Upconversion Nanoparticles and Their Safe Theranostic Applications. Adv. Mater. 2013, 25, 3758–3779. [Google Scholar] [CrossRef]
- González-Béjar, M.; Francés-Soriano, L.; Pérez-Prieto, J. Upconversion Nanoparticles for Bioimaging and Regenerative Medicine. Front. Bioeng. Biotechnol. 2016, 4, 47. [Google Scholar] [CrossRef]
- Altavilla, C. Upconverting Nanomaterials: Perspectives, Synthesis, and Applications; CRC Press: Boca Raton, FL, USA, 2016; ISBN 1498707750. [Google Scholar]
- Haase, M.; Schäfer, H. Upconverting Nanoparticles. Angew. Chem. Int. Ed. 2011, 50, 5808–5829. [Google Scholar] [CrossRef]
- Algar, W.R.; Massey, M.; Rees, K.; Higgins, R.; Krause, K.D.; Darwish, G.H.; Peveler, W.J.; Xiao, Z.; Tsai, H.-Y.; Gupta, R. Photoluminescent Nanoparticles for Chemical and Biological Analysis and Imaging. Chem. Rev. 2021, 121, 9243–9358. [Google Scholar] [CrossRef]
- Johnston, L.J.; Gonzalez-Rojano, N.; Wilkinson, K.J.; Xing, B. Key Challenges for Evaluation of the Safety of Engineered Nanomaterials. NanoImpact 2020, 18, 100219. [Google Scholar] [CrossRef]
- Vera, V.T.; Mendez-Gonzalez, D.; Ramos-Ramos, D.J.; Igalla, A.; Laurenti, M.; Contreras-Caceres, R.; Lopez-Cabarcos, E.; Díaz, E.; Rubio-Retama, J.; Melle, S. The Effects of Dopant Concentration and Excitation Intensity on the Upconversion and Downconversion Emission Processes of β-NaYF 4: Yb 3+, Er 3+ Nanoparticles. J. Mater. Chem. C Mater. 2021, 9, 8902–8911. [Google Scholar] [CrossRef]
- Sirkka, N.; Lyytikäinen, A.; Savukoski, T.; Soukka, T. Upconverting Nanophosphors as Reporters in a Highly Sensitive Heterogeneous Immunoassay for Cardiac Troponin I. Anal. Chim. Acta 2016, 925, 82–87. [Google Scholar] [CrossRef]
- Chávez-García, D.; Juárez-Moreno, K.; Campos, C.H.; Tejeda, E.M.; Alderete, J.B.; Hirata, G.A. Cytotoxicity, Genotoxicity and Uptake Detection of Folic Acid-Functionalized Green Upconversion Nanoparticles Y2O3/Er3+, Yb3+ as Biolabels for Cancer Cells. J. Mater. Sci. 2018, 53, 6665–6680. [Google Scholar] [CrossRef]
- Escala, N.; Pineda, L.M.; Ng, M.G.; Coronado, L.M.; Spadafora, C.; Del Olmo, E. Antiplasmodial Activity, Structure–Activity Relationship and Studies on the Action of Novel Benzimidazole Derivatives. Sci. Rep. 2023, 13, 285. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Kong, W.; Wang, N.; Zhu, G.; Wang, F. Oleylamine-Mediated Synthesis of Small NaYbF4 Nanoparticles with Tunable Size. Chem. Mater. 2019, 31, 4779–4786. [Google Scholar] [CrossRef]
- Ding, M.; Chen, D.; Yin, S.; Ji, Z.; Zhong, J.; Ni, Y.; Lu, C.; Xu, Z. Simultaneous Morphology Manipulation and Upconversion Luminescence Enhancement of β-NaYF4: Yb3+/Er3+ Microcrystals by Simply Tuning the KF Dosage. Sci. Rep. 2015, 5, 12745. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Qiu, P.; Xu, D.; Lin, J.; Tang, Y.; Wang, F.; He, X.; Zhou, Z.; Sun, N.; Zhang, X. Controllable Synthesis and Upconversion Luminescenceof NaYF4: Yb3+, Er3+ Nanocrystals. Ceram. Int. 2015, 41, S713–S718. [Google Scholar] [CrossRef]
- Qian, H.-S.; Zhang, Y. Synthesis of Hexagonal-Phase Core–Shell NaYF4 Nanocrystals with Tunable Upconversion Fluorescence. Langmuir 2008, 24, 12123–12125. [Google Scholar] [CrossRef]
- Rabouw, F.T.; Prins, P.T.; Villanueva-Delgado, P.; Castelijns, M.; Geitenbeek, R.G.; Meijerink, A. Quenching Pathways in NaYF4: Er3+, Yb3+ Upconversion Nanocrystals. ACS Nano 2018, 12, 4812–4823. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Lin, Y.; Huang, Y.; Duan, X. Composition Tuning the Upconversion Emission in NaYF4: Yb/Tm Hexaplate Nanocrystals. Nanoscale 2011, 3, 963–966. [Google Scholar] [CrossRef]
- Francolon, N.; Boyer, D.; Leccia, F.; Jouberton, E.; Walter, A.; Bordeianu, C.; Parat, A.; Felder-Flesch, D.; Begin-Colin, S.; Miot-Noirault, E. Preparation of Core/Shell NaYF4: Yb, Tm@ Dendrons Nanoparticles with Enhanced Upconversion Luminescence for in Vivo Imaging. Nanomedicine 2016, 12, 2107–2113. [Google Scholar] [CrossRef]
- Saleh, M.I.; Rühle, B.; Wang, S.; Radnik, J.; You, Y.; Resch-Genger, U. Assessing the Protective Effects of Different Surface Coatings on NaYF4: Yb3+, Er3+ Upconverting Nanoparticles in Buffer and DMEM. Sci. Rep. 2020, 10, 19318. [Google Scholar] [CrossRef]
- Reddy, K.L.; Prabhakar, N.; Arppe, R.; Rosenholm, J.M.; Krishnan, V. Microwave-Assisted One-Step Synthesis of Acetate-Capped NaYF 4: Yb/Er Upconversion Nanocrystals and Their Application in Bioimaging. J. Mater. Sci. 2017, 52, 5738–5750. [Google Scholar] [CrossRef]
- Zhao, P.; Wu, Y.; Zhu, Y.; Yang, X.; Jiang, X.; Xiao, J.; Zhang, Y.; Li, C. Upconversion Fluorescent Strip Sensor for Rapid Determination of Vibrio Anguillarum. Nanoscale 2014, 6, 3804–3809. [Google Scholar] [CrossRef] [PubMed]
- Sedlmeier, A.; Gorris, H.H. Surface Modification and Characterization of Photon-Upconverting Nanoparticles for Bioanalytical Applications. Chem. Soc. Rev. 2015, 44, 1526–1560. [Google Scholar] [CrossRef] [PubMed]
- Gonzaález Mancebo, D.; Becerro, A.I.; Corral, A.; Moros, M.; Balcerzyk, M.; de la Fuente, J.M.; Ocaña, M. Enhancing Luminescence and X-Ray Absorption Capacity of Eu3+: LaF3 Nanoparticles by Bi3+ Codoping. ACS Omega 2019, 4, 765–774. [Google Scholar] [CrossRef]
- Hou, Z.; Li, C.; Ma, P.; Li, G.; Cheng, Z.; Peng, C.; Yang, D.; Yang, P.; Lin, J. Electrospinning Preparation and Drug-Delivery Properties of an Up-conversion Luminescent Porous NaYF4: Yb3+, Er3+@ Silica Fiber Nanocomposite. Adv. Funct. Mater. 2011, 21, 2356–2365. [Google Scholar] [CrossRef]
- Nahorniak, M.; Patsula, V.; Mareková, D.; Matouš, P.; Shapoval, O.; Oleksa, V.; Vosmanská, M.; Machová Urdzíková, L.; Jendelová, P.; Herynek, V. Chemical and Colloidal Stability of Polymer-Coated NaYF4: Yb, Er Nanoparticles in Aqueous Media and Viability of Cells: The Effect of a Protective Coating. Int. J. Mol. Sci. 2023, 24, 2724. [Google Scholar] [CrossRef]
- Doronkina, A.A.; Kochubey, V.I.; Maksutova, A.V.; Pravdin, A.B.; Mylnikov, A.M.; Navolokin, N.A.; Yanina, I.Y. NaYF4: Yb, Er Upconversion Nanoparticles for Imaging: Effect on Red Blood Cells. Photonics 2023, 10, 1386. [Google Scholar] [CrossRef]
- Markl, S.; Schroter, A.; Hirsch, T. Small and Bright Water-Protected Upconversion Nanoparticles with Long-Time Stability in Complex, Aqueous Media by Phospholipid Membrane Coating. Nano Lett. 2020, 20, 8620–8625. [Google Scholar] [CrossRef]
- Tajon, C.A.; Yang, H.; Tian, B.; Tian, Y.; Ercius, P.; Schuck, P.J.; Chan, E.M.; Cohen, B.E. Photostable and Efficient Upconverting Nanocrystal-Based Chemical Sensors. Opt. Mater. 2018, 84, 345–353. [Google Scholar] [CrossRef]
- Hoang, V.T.; Stępniewski, G.; Czarnecka, K.H.; Kasztelanic, R.; Long, V.C.; Xuan, K.D.; Shao, L.; Śmietana, M.; Buczyński, R. Optical Properties of Buffers and Cell Culture Media for Optofluidic and Sensing Applications. Appl. Sci. 2019, 9, 1145. [Google Scholar] [CrossRef]
- Mendez-Gonzalez, D.; Torres Vera, V.; Zabala Gutierrez, I.; Gerke, C.; Cascales, C.; Rubio-Retama, J.; Calderón, O.G.; Melle, S.; Laurenti, M. Upconverting Nanoparticles in Aqueous Media: Not a Dead-End Road. Avoiding Degradation by Using Hydrophobic Polymer Shells. Small 2022, 18, 2105652. [Google Scholar] [CrossRef] [PubMed]
- Di, W.; Tan, X.; Calderon, I.A.C.; Neal Reilly, A.E.; Niedre, M.; Clark, H.A. Real-Time Particle-by-Particle Detection of Erythrocyte-Camouflaged Microsensor with Extended Circulation Time in the Bloodstream. Proc. Natl. Acad. Sci. USA 2020, 117, 3509–3517. [Google Scholar] [CrossRef] [PubMed]
- Himmelstoß, S.F.; Hirsch, T. Long-term Colloidal and Chemical Stability in Aqueous Media of NaYF4-type Upconversion Nanoparticles Modified by Ligand-exchange. Part. Part. Syst. Charact. 2019, 36, 1900235. [Google Scholar] [CrossRef]
- Tian, Y.; Tian, Z.; Dong, Y.; Wang, X.; Zhan, L. Current Advances in Nanomaterials Affecting Morphology, Structure, and Function of Erythrocytes. RSC Adv. 2021, 11, 6958–6971. [Google Scholar] [CrossRef]
- Yadav, S.; Maurya, P.K. Recent Advances in the Protective Role of Metallic Nanoparticles in Red Blood Cells. 3 Biotech 2022, 12, 28. [Google Scholar] [CrossRef]
- Tan, M.; Li, F.; Cao, N.; Li, H.; Wang, X.; Zhang, C.; Jaque, D.; Chen, G. Accurate in Vivo Nanothermometry through NIR-II Lanthanide Luminescence Lifetime. Small 2020, 16, 2004118. [Google Scholar] [CrossRef]
- López-Peña, G.; Hamraoui, K.; Horchani-Naifer, K.; Gerke, C.; Ortgies, D.H.; Rodríguez, E.M.; Chen, G.; Jaque, D.; Retama, J.R. Lanthanide Doped Nanoheaters with Reliable and Absolute Temperature Feedback. Phys. B Condens. Matter 2022, 631, 413652. [Google Scholar] [CrossRef]
- Hu, S.; Wu, X.; Tang, Z.; Xi, Z.; Chen, Z.; Hu, P.; Yu, Y.; Yan, H.; Liu, Y. Upconversion NaYF4 Nanoparticles for Size Dependent Cell Imaging and Concentration Dependent Detection of Rhodamine B. J. Nanomater. 2015, 2015, 598734. [Google Scholar] [CrossRef]





| Condition | Variation |
|---|---|
| HEPES concentration | 10, 50, 100, 150, and 200 mM |
| Incubation time | 1, 3, 4, 6, and 24 h |
| Photoluminescence detection limit of NaYF4:Yb3+,Er3+-NP concentration * | 0.0001–1 mg/mL |
| Laser current intensity | 50–450 mA |
| Exposure time | 0, 5, 10, and 20 min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Vera, V.; Coronado, L.M.; Valencia, A.P.; Von Chong, A.; Rua, E.; Ng, M.; Rubio-Retama, J.; Spadafora, C.; Correa, R. Enhanced Visualization of Erythrocytes Through Photoluminescence Using NaYbF4:Yb3+,Er3+ Nanoparticles. Biosensors 2025, 15, 396. https://doi.org/10.3390/bios15070396
Torres-Vera V, Coronado LM, Valencia AP, Von Chong A, Rua E, Ng M, Rubio-Retama J, Spadafora C, Correa R. Enhanced Visualization of Erythrocytes Through Photoluminescence Using NaYbF4:Yb3+,Er3+ Nanoparticles. Biosensors. 2025; 15(7):396. https://doi.org/10.3390/bios15070396
Chicago/Turabian StyleTorres-Vera, Vivian, Lorena M. Coronado, Ana Patricia Valencia, Alejandro Von Chong, Esteban Rua, Michelle Ng, Jorge Rubio-Retama, Carmenza Spadafora, and Ricardo Correa. 2025. "Enhanced Visualization of Erythrocytes Through Photoluminescence Using NaYbF4:Yb3+,Er3+ Nanoparticles" Biosensors 15, no. 7: 396. https://doi.org/10.3390/bios15070396
APA StyleTorres-Vera, V., Coronado, L. M., Valencia, A. P., Von Chong, A., Rua, E., Ng, M., Rubio-Retama, J., Spadafora, C., & Correa, R. (2025). Enhanced Visualization of Erythrocytes Through Photoluminescence Using NaYbF4:Yb3+,Er3+ Nanoparticles. Biosensors, 15(7), 396. https://doi.org/10.3390/bios15070396






