A Mobile Sperm Analyzer with User-Friendly Microfluidic Chips for Rapid On-Farm Semen Evaluation
Abstract
1. Introduction
2. Materials and Methods
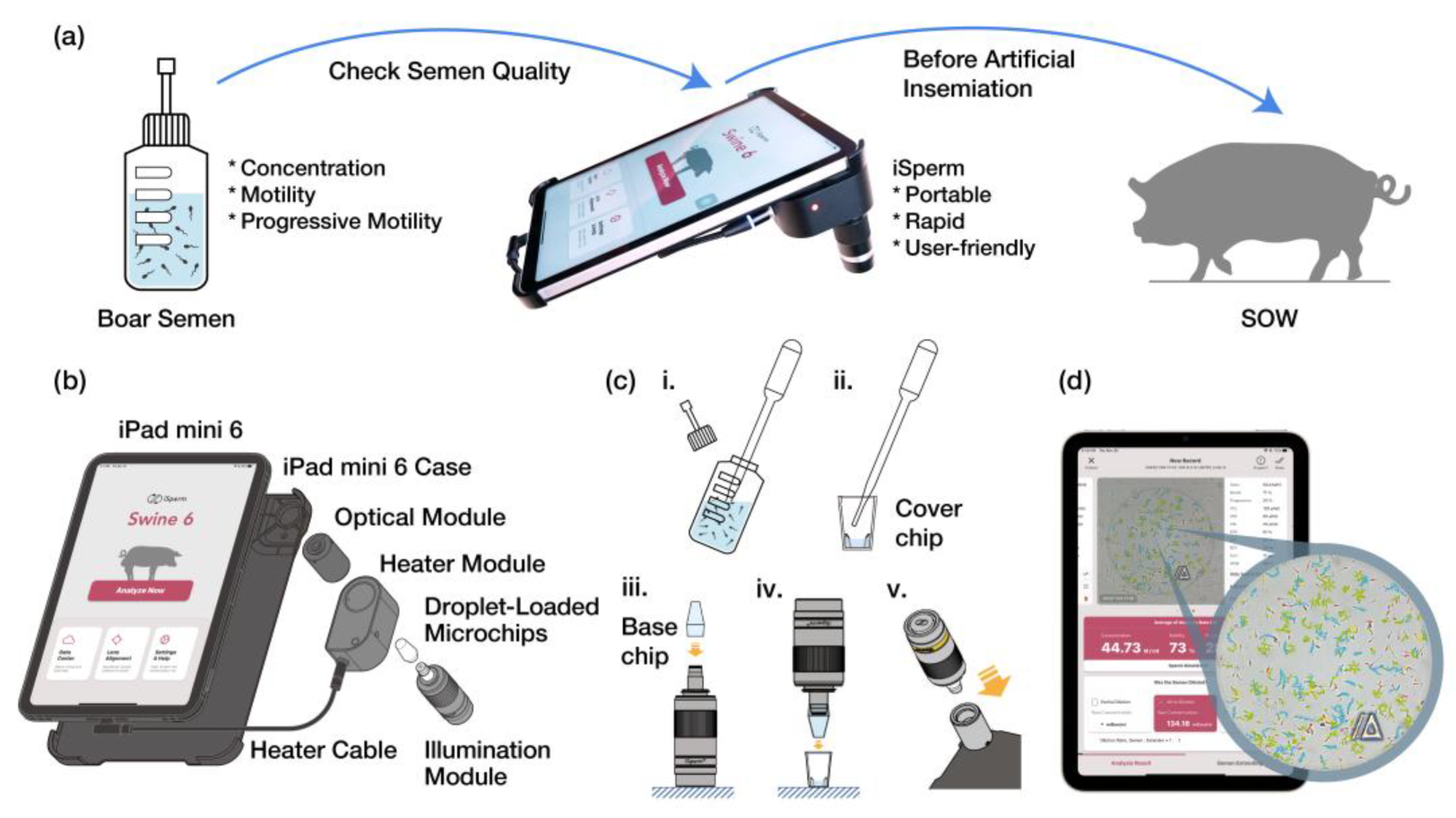
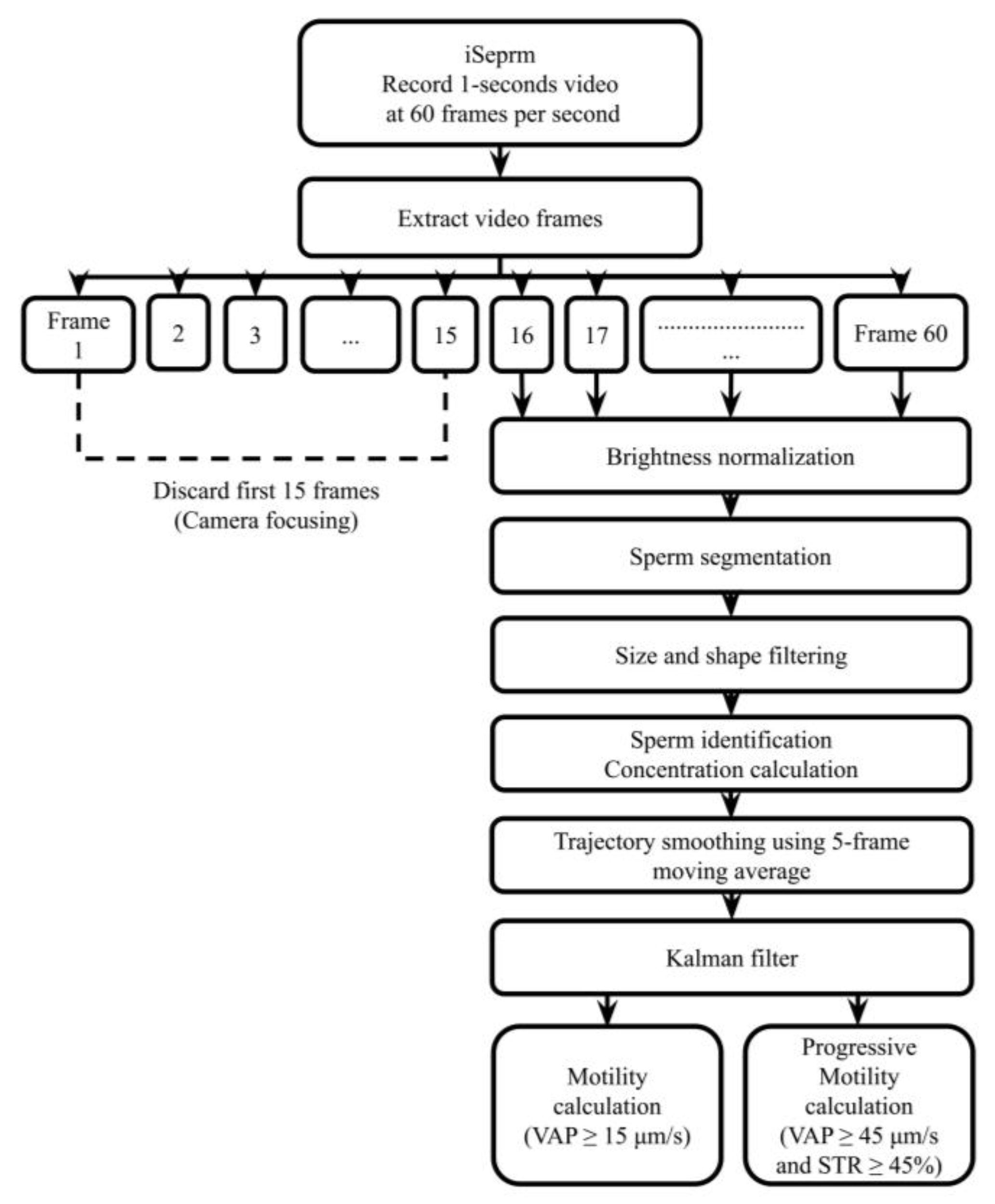
2.1. Design of the iSperm Analyzer
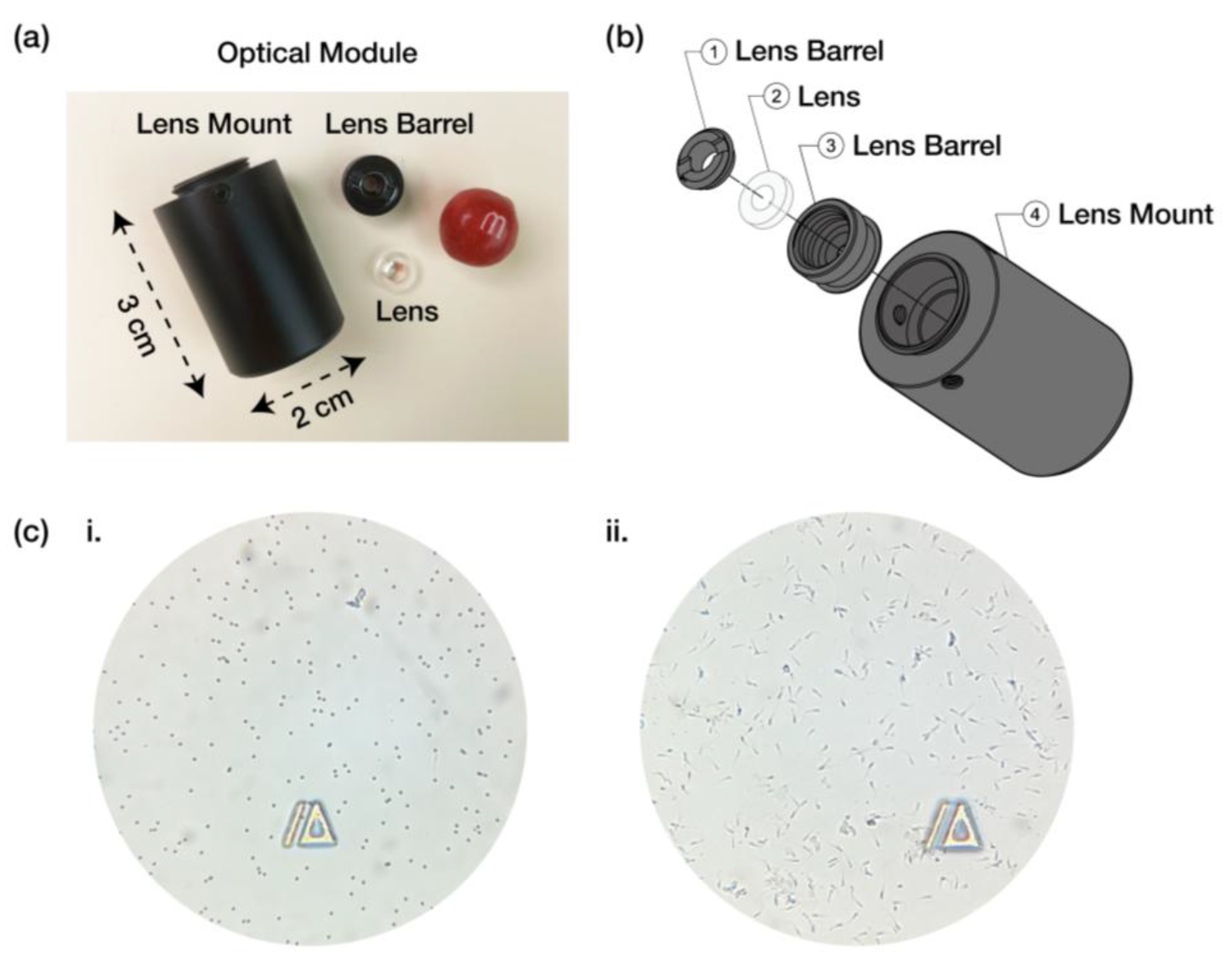
2.2. Optical Module
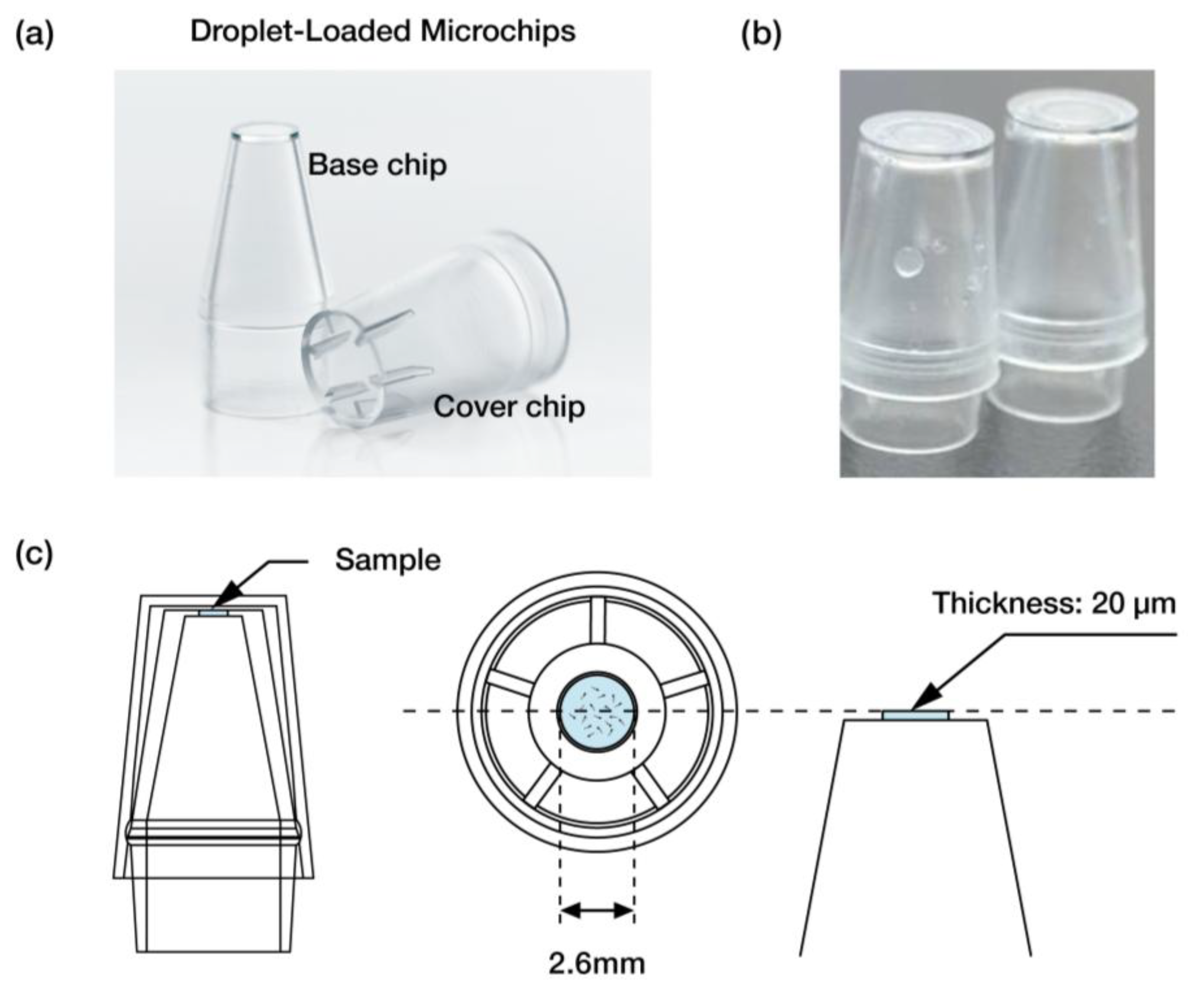
2.3. Droplet-Loaded Microchips
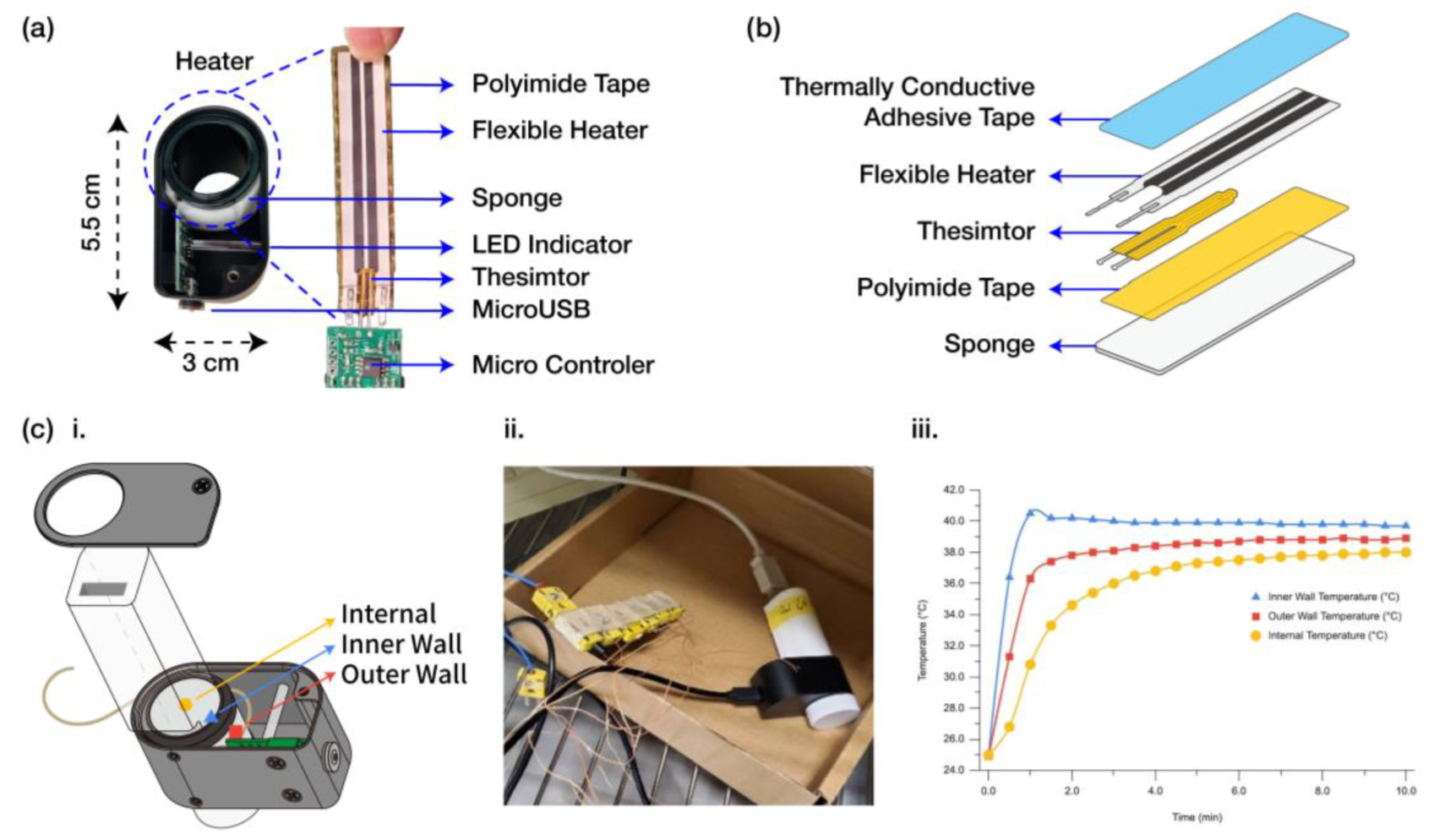
2.4. Heater
2.5. Software Application
2.6. Precision and User Variability Assessment
2.6.1. Precision Comparison
2.6.2. User Variability Assessment
2.6.3. Analysis Procedure
2.7. CASA Comparisons to Validate the iSperm Analyzer
3. Results and Discussion
3.1. System Design and Characterization
3.2. Precision and User Variability Testing
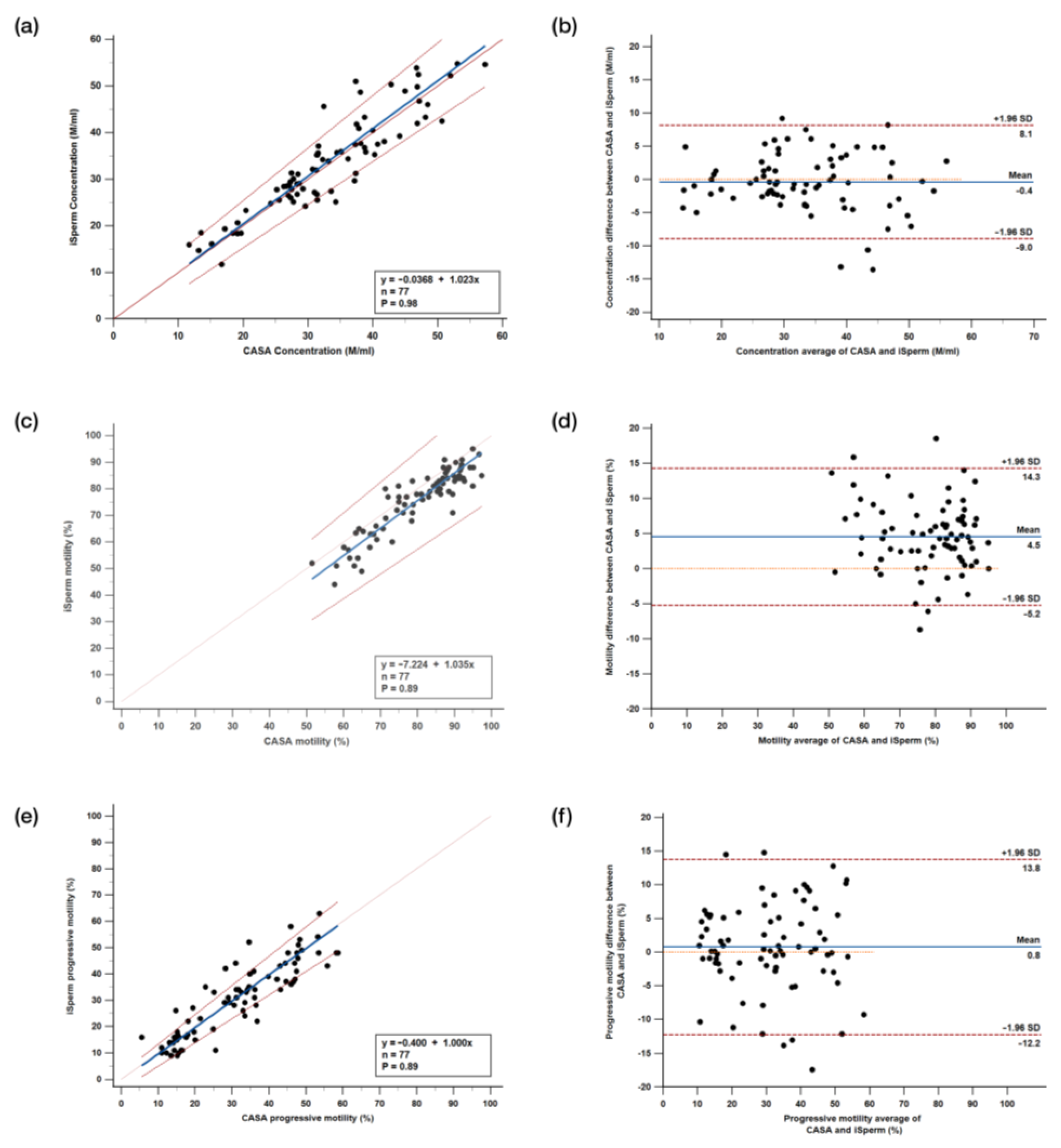
3.3. Comparative Analysis Between iSperm and CASA
3.3.1. Passing–Bablok Regression Analysis
3.3.2. Bland–Altman Analysis
- Frame rate and number of analyzed frames;
- Geometry and optical depth of the specimen chamber;
- Algorithmic definitions and threshold settings (e.g., VAP, STR);
- Operator handling and environmental conditions (e.g., temperature).
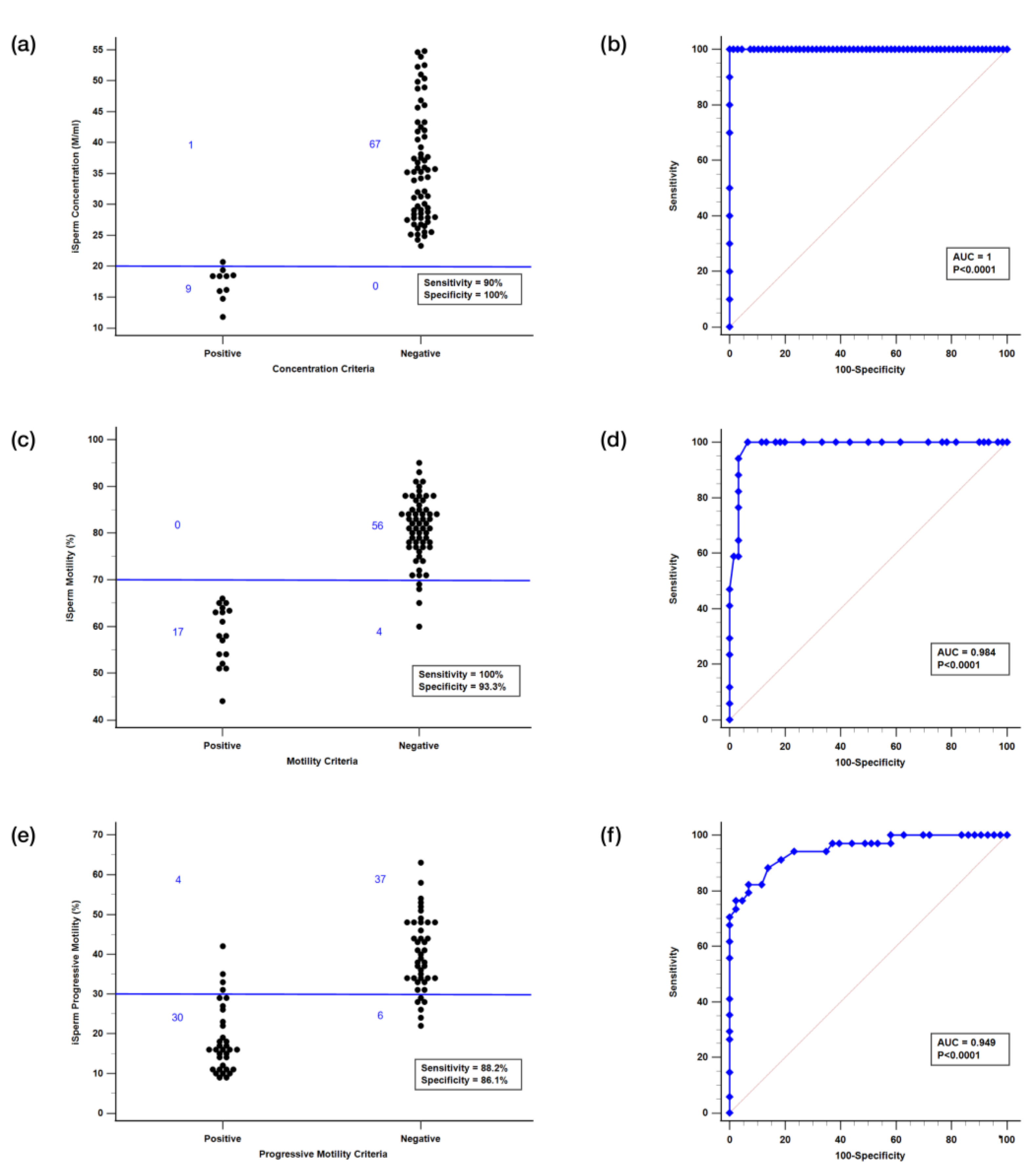
3.4. Effect Evaluation
3.4.1. Scatter Plot Analysis
- The current iSperm algorithm employs fixed thresholds for binary classification. Minor deviations in borderline cases can lead to misclassification. To improve flexibility, we plan to implement adjustable cutoff options (e.g., user-defined thresholds for progressive motility) to accommodate field-specific requirements.
- Future versions will also incorporate a confidence interval-based “gray zone” near classification thresholds to reduce volatility in borderline classification and enhance diagnostic robustness.
3.4.2. ROC Curve Analysis
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schulze, M.; Jung, M.; Hensel, B. Science-based quality control in boar semen production. Mol. Reprod. Dev. 2023, 90, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.A.; Weitze, K.F.; Fiser, P.; Maxwell, W.M.C. Storage of boar semen. Anim. Reprod. Sci. 2000, 62, 143–172. [Google Scholar] [CrossRef]
- Roca, J.; Parrilla, I.; Bolarin, A.; Martinez, E.A.; Rodriguez-Martinez, H. Will AI in pigs become more efficient? Theriogenology 2016, 86, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Contreras, M.J.; Núñez-Montero, K.; Bruna, P.; García, M.; Leal, K.; Barrientos, L.; Weber, H. Bacteria and boar semen storage: Progress and challenges. Antibiotics 2022, 11, 1796. [Google Scholar] [CrossRef]
- Lopez Rodriguez, A.; Van Soom, A.; Arsenakis, I.; Maes, D. Boar management and semen handling factors affect the quality of boar extended semen. Porc. Health Manag. 2017, 3, 15. [Google Scholar] [CrossRef]
- Brito, L.F.C.; Althouse, G.C.; Aurich, C.; Chenoweth, P.J.; Eilts, B.E.; Love, C.C.; Luvoni, G.C.; Mitchell, J.R.; Peter, A.T.; Pugh, D.G.; et al. Andrology laboratory review: Evaluation of sperm concentration. Theriogenology 2016, 85, 1507–1527. [Google Scholar] [CrossRef]
- Maside, C.; Recuero, S.; Salas-Huetos, A.; Ribas-Maynou, J.; Yeste, M. An update on the methods for semen quality evaluation in swine–from farm to the lab. Animal 2023, 17, 100720. [Google Scholar] [CrossRef] [PubMed]
- Tanga, B.M.; Qamar, A.Y.; Raza, S.; Bang, S.; Fang, X.; Yoon, K.; Cho, J. Semen evaluation: Methodological advancements in sperm quality-specific fertility assessment—A review. Anim. Biosci. 2021, 34, 1253–1270. [Google Scholar] [CrossRef]
- Broekhuijse, M.L.W.J.; Šoštarić, E.; Feitsma, H.; Gadella, B.M. Application of computer-assisted semen analysis to explain variations in pig fertility. J. Anim. Sci. 2012, 90, 779–789. [Google Scholar] [CrossRef]
- Mortimer, S.T.; van der Horst, G.; Mortimer, D. The future of computer-aided sperm analysis. Asian J. Androl. 2015, 17, 545–553. [Google Scholar] [CrossRef]
- Valverde, A.; Barquero, V.; Soler, C. The application of computer-assisted semen analysis (CASA) technology to optimise semen evaluation: A review. J. Anim. Feed Sci. 2020, 29, 189–198. [Google Scholar] [CrossRef]
- Amann, R.P.; Waberski, D. Computer-assisted sperm analysis (CASA): Capabilities and potential developments. Theriogenology 2014, 81, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.C.; Huang, Y.F.; Lü, N.Q. Computer-aided sperm analysis: Past, present and future. Andrologia 2014, 46, 329–338. [Google Scholar] [CrossRef]
- Ehlers, J.; Behr, M.; Bollwein, H.; Beyerbach, M.; Waberski, D. Standardization of computer-assisted semen analysis using an e-learning application. Theriogenology 2011, 76, 448–454. [Google Scholar] [CrossRef]
- Kuster, C. Sperm concentration determination between hemacytometric and CASA systems: Why they can be different. Theriogenology 2005, 64, 614–617. [Google Scholar] [CrossRef]
- Douglas-Hamilton, D.H.; Smith, N.G.; Kuster, C.E.; Vermeiden, J.P.W.; Althouse, G.C. Capillary-Loaded Particle Fluid Dynamics: Effect on Estimation of Sperm Concentration. J. Androl. 2005, 26, 115–122. [Google Scholar] [CrossRef]
- Douglas-Hamilton, D.H.; Smith, N.G.; Kuster, C.E.; Vermeiden, J.P.W.; Althouse, G.C. Particle Distribution in Low-Volume Capillary-Loaded Chambers. J. Androl. 2005, 26, 107–114. [Google Scholar] [CrossRef]
- Amini, H.; Lee, W.; Di Carlo, D. Inertial microfluidic physics. Lab Chip 2014, 14, 2739–2761. [Google Scholar] [CrossRef] [PubMed]
- Gączarzewicz, D. Influence of chamber type integrated with computer-assisted semen analysis (CASA) system on the results of boar semen evaluation. J. Vet. Sci. 2015, 18, 817–824. [Google Scholar] [CrossRef]
- Del Gallego, R.; Sadeghi, S.; Blasco, E.; Soler, C.; Yániz, J.L.; Silvestre, M.A. Effect of chamber characteristics, loading and analysis time on motility and kinetic variables analysed with the CASA-mot system in goat sperm. Anim. Reprod. Sci. 2017, 177, 97–104. [Google Scholar] [CrossRef]
- Broekhuijse, M.L.W.J.; Šoštarić, E.; Feitsma, H.; Gadella, B.M. Additional value of computer assisted semen analysis (CASA) compared to conventional motility assessments in pig artificial insemination. Theriogenology 2011, 76, 1473–1486. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yin, H.; Zhou, C.; Zhou, W.; Huan, Z.; Ma, W. A Hand-Held Platform for Boar Sperm Viability Diagnosis Based on Smartphone. Biosensors 2023, 13, 978. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Trujillo, A.; Kandula, H.; Kumar, J.; Devi, A.; Shirley, L.; Thirumalaraju, P.; Kanakasabapathy, M.K.; Shafiee, H.; Hart, L. Validation of a smartphone-based device to measure concentration, motility, and morphology in swine ejaculates. Transl. Anim. Sci. 2022, 6, 1–8. [Google Scholar] [CrossRef]
- Kanakasabapathy, M.K.; Sadasivam, M.; Singh, A.; Preston, C.; Thirumalaraju, P.; Venkataraman, M.; Bormann, C.L.; Draz, M.S.; Petrozza, J.C.; Shafiee, H. An automated smartphone-based diagnostic assay for point-of-care semen analysis. Sci. Transl. Med. 2017, 9, eaai7863. [Google Scholar] [CrossRef]
- Huang, X.; Xu, D.; Chen, J.; Liu, J.; Li, Y.; Song, J.; Ma, X.; Guo, J. Smartphone-based analytical biosensors. Analyst 2018, 143, 5339–5351. [Google Scholar] [CrossRef]
- Banik, S.; Melanthota, S.K.; Arbaaz; Vaz, J.M.; Kadambalithaya, V.M.; Hussain, I.; Dutta, S.; Mazumder, N. Recent trends in smartphone-based detection for biomedical applications: A review. Anal. Bioanal. Chem. 2021, 413, 2389–2406. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Kang, Y.; Xu, Z.; Xue, P. Development and prospects of microfluidic platforms for sperm inspection. Anal. Methods 2019, 11, 4547–4560. [Google Scholar] [CrossRef]
- Yaghoobi, M.; Azizi, M.; Mokhtare, A.; Abbaspourrad, A. Progressive bovine sperm separation using parallelized microchamber-based microfluidics. Lab Chip 2021, 21, 2791–2804. [Google Scholar] [CrossRef]
- Dadkhah, E.; Hajari, M.A.; Abdorahimzadeh, S.; Shahverdi, A.; Esfandiari, F.; Ziarati, N.; Taghipoor, M.; Montazeri, L. Development of a novel cervix-inspired tortuous microfluidic system for efficient, high-quality sperm selection. Lab Chip 2023, 23, 3080–3091. [Google Scholar] [CrossRef]
- Passing, H.; Bablok, W. A new biometrical procedure for testing the equality of measurements from two different analytical methods. J. Clin. Chem. Clin. Biochem. 1983, 21, 709–720. [Google Scholar]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Knox, R.V. Boar Management. Merck Veterinary Manual; Merck & Co., Inc.: Rahway, NJ, USA, 2021. [Google Scholar]
- PIC. Boar Stud Management Guidelines; PIC: Hendersonville, TN, USA, 2017. [Google Scholar]
- Medica, A.J.; Lambourne, S.; Aitken, R.J. Predicting the Outcome of Equine Artificial Inseminations Using Chilled Semen. Animals 2023, 13, 1203. [Google Scholar] [CrossRef]
- Dini, P.; Troch, L.; Lemahieu, I.; Deblende, P.; Daels, P. Validation of a portable device (iSperm®) for the assessment of stallion sperm motility and concentration. Reprod. Domest. Anim. 2019, 54, 1113–1120. [Google Scholar] [CrossRef]
- Moraes, C.R.; Runcan, E.E.; Blawut, B.; Coutinho da Silva, M.A. Technical note: The use of iSperm technology for on-farm measurement of equine sperm motility and concentration. Transl. Anim. Sci. 2019, 3, 1513–1520. [Google Scholar] [CrossRef]
- Aitken, R.J.; Lambourne, S.; Medica, A.J. Predicting the outcome of Thoroughbred stallion matings on the basis of dismount semen sample analyses. Reproduction 2023, 165, 281–289. [Google Scholar] [CrossRef]
- Bulkeley, E.; Collins, C.; Foutouhi, A.; Gonzales, K.; Power, H.; Meyers, S. Assessment of an iPad-based sperm motility analyzer for determination of canine sperm motility. Transl. Anim. Sci. 2021, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Domain, G.; Banchi, P.; Hassan, H.A.; Eilers, A.; Lannoo, J.; Wydooghe, E.; Niżański, W.; Van Soom, A. Sperm Gone Smart: A Portable Device (iSperm®) to Assess Semen Concentration and Motility in Dogs. Animals 2022, 12, 652. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, F.; Soler, C.; Araya-Zúñiga, I.; Barquero, V.; Roldan, E.R.S.; Valverde, A. Are There Differences between Methods Used for the Objective Estimation of Boar Sperm Concentration and Motility? Animals 2023, 13, 1622. [Google Scholar] [CrossRef]
- Matsuura, K.; Wang, W.-H.; Ching, A.; Chen, Y.; Cheng, C.-M. Paper-Based Resazurin Assay of Inhibitor-Treated Porcine Sperm. Micromachines 2019, 10, 495. [Google Scholar] [CrossRef]
- Matsuura, K.; Huang, H.-W.; Chen, M.-C.; Chen, Y.; Cheng, C.-M. Relationship between Porcine Sperm Motility and Sperm Enzymatic Activity Using Paper-Based Devices. Sci. Rep. 2017, 7, 46213. [Google Scholar] [CrossRef]
- Jorge-Neto, P.N.; Silva, M.C.C.; Csermak-Júnior, A.C.; Salmão-Júnior, J.A.; Araújo, G.R.; Oliveira, G.; Leuzinger, L.; Pizzutto, C.S.; Deco-Souza, T. Cryptorchidism in Free-Living Jaguar (Panthera onca): First Case Report. Anim. Reprod. 2020, 17, e20200555. [Google Scholar] [CrossRef] [PubMed]
- Rispoli, L.A.; Roth, T.L. Validation of the iSperm for Assessing Rhinoceros Sperm. Theriogenology Wild 2023, 3, 100048. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.-S.; Chen, C.-Y.; Lin, C.-M.; Chiang, T.-C.; Tang, Y.-S.; Yeh, C.-C.; Hsu, W.-F.; Wo, A.M. A Mobile Sperm Analyzer with User-Friendly Microfluidic Chips for Rapid On-Farm Semen Evaluation. Biosensors 2025, 15, 394. https://doi.org/10.3390/bios15060394
Lin S-S, Chen C-Y, Lin C-M, Chiang T-C, Tang Y-S, Yeh C-C, Hsu W-F, Wo AM. A Mobile Sperm Analyzer with User-Friendly Microfluidic Chips for Rapid On-Farm Semen Evaluation. Biosensors. 2025; 15(6):394. https://doi.org/10.3390/bios15060394
Chicago/Turabian StyleLin, Shu-Sheng, Chang-Yu Chen, Cheng-Ming Lin, Tsun-Chao Chiang, Yu-Siang Tang, Chang-Ching Yeh, Wei-Fan Hsu, and Andrew M. Wo. 2025. "A Mobile Sperm Analyzer with User-Friendly Microfluidic Chips for Rapid On-Farm Semen Evaluation" Biosensors 15, no. 6: 394. https://doi.org/10.3390/bios15060394
APA StyleLin, S.-S., Chen, C.-Y., Lin, C.-M., Chiang, T.-C., Tang, Y.-S., Yeh, C.-C., Hsu, W.-F., & Wo, A. M. (2025). A Mobile Sperm Analyzer with User-Friendly Microfluidic Chips for Rapid On-Farm Semen Evaluation. Biosensors, 15(6), 394. https://doi.org/10.3390/bios15060394



