Polypyrrole Coatings as Possible Solutions for Sensing and Stimulation in Bioelectronic Medicines
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Conductivity of PPy/N Coatings and Films
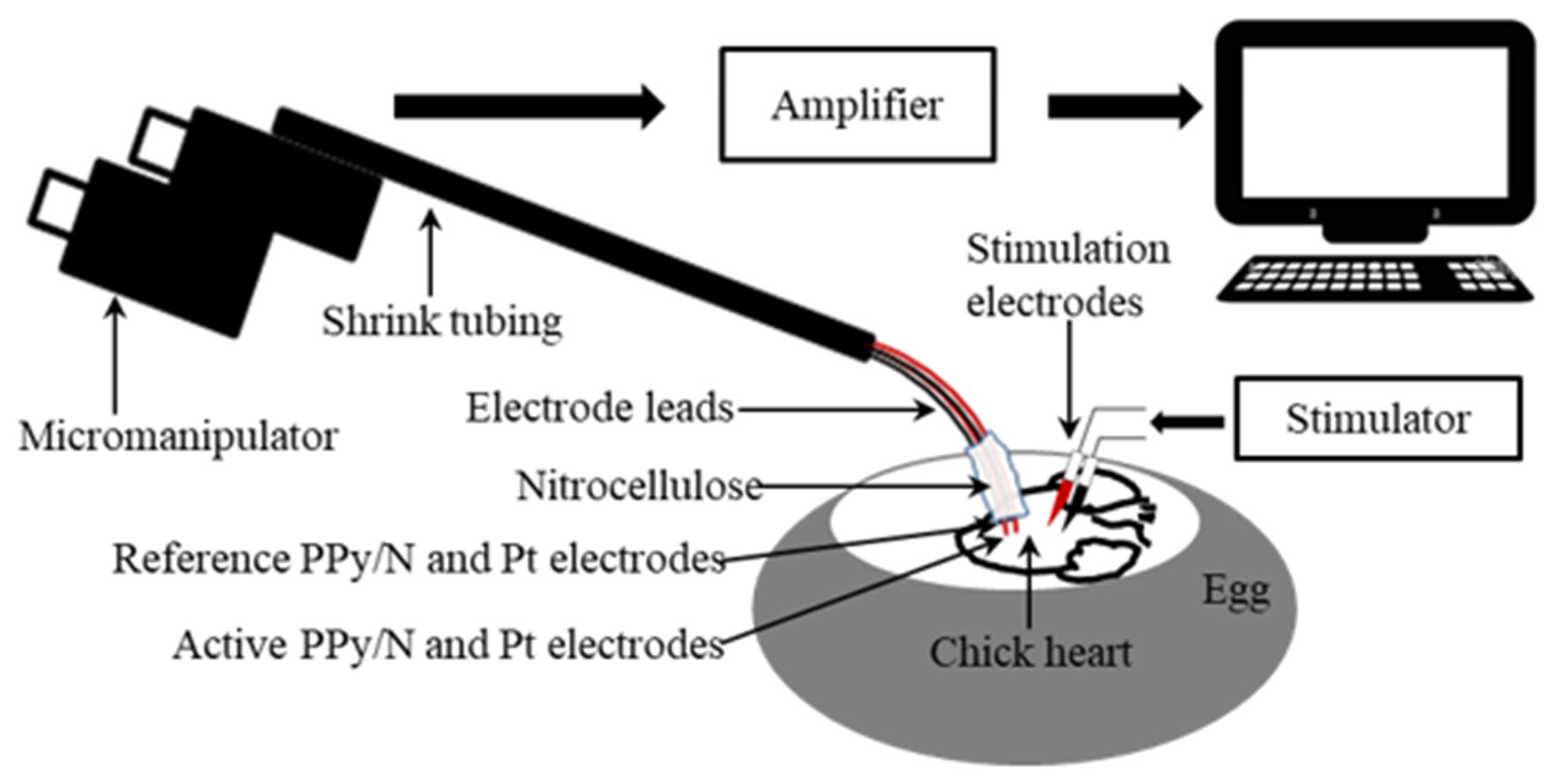
2.2. Recording and Stimulation Experiments
2.3. Cell Culture Tests
3. Results
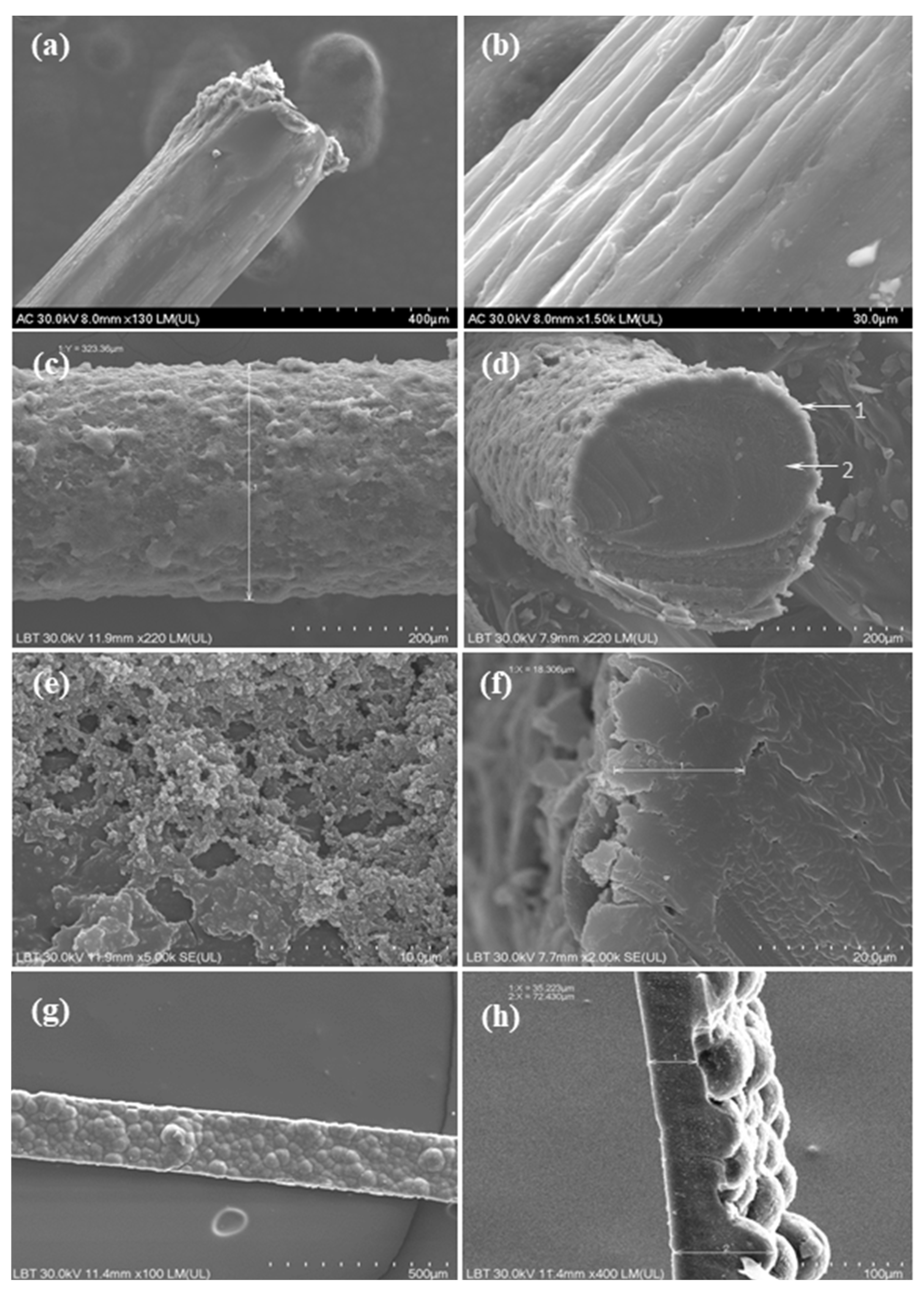
3.1. Morphological and Conductivity Characterization of the PPy/N Coating and Strips
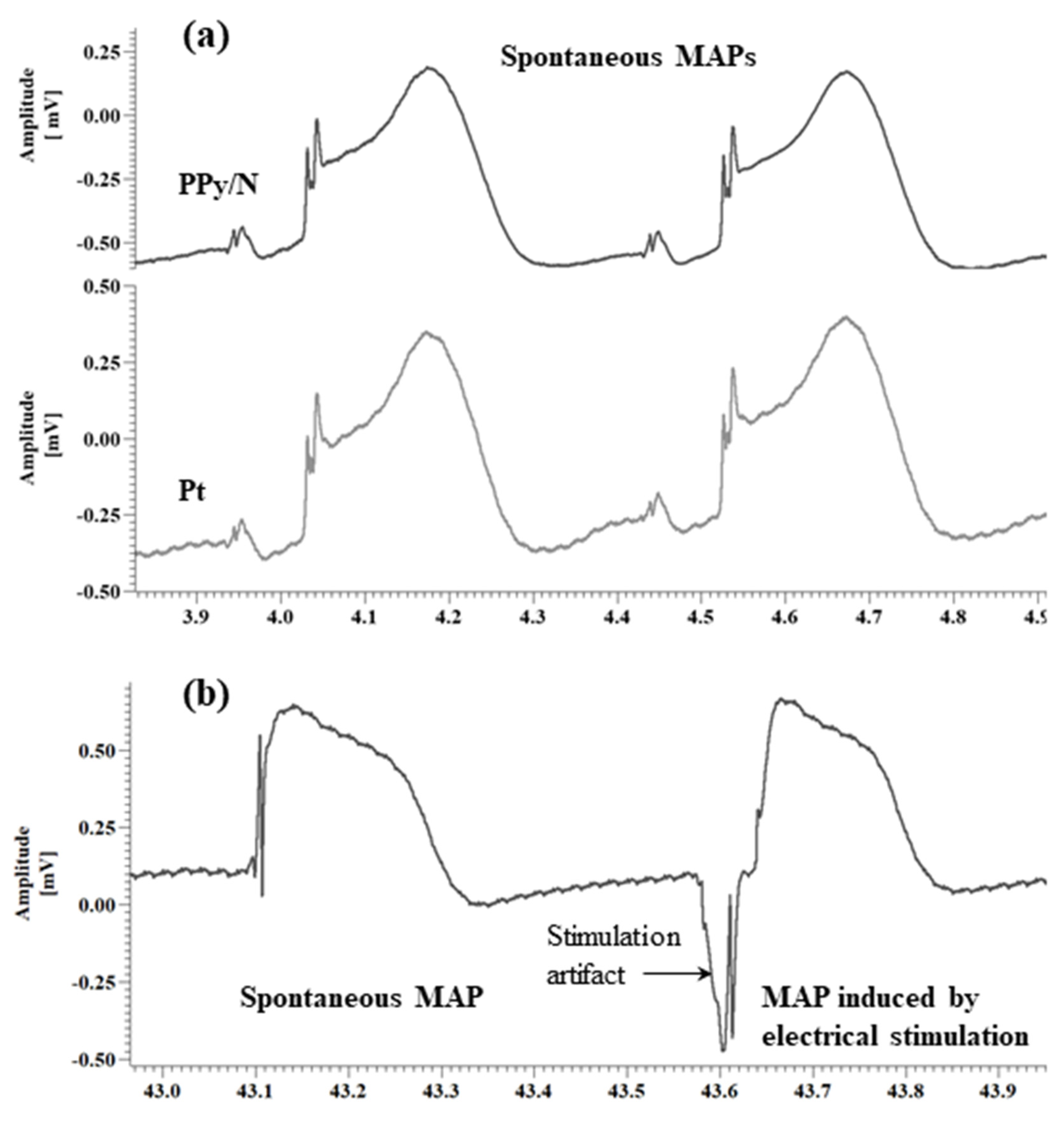
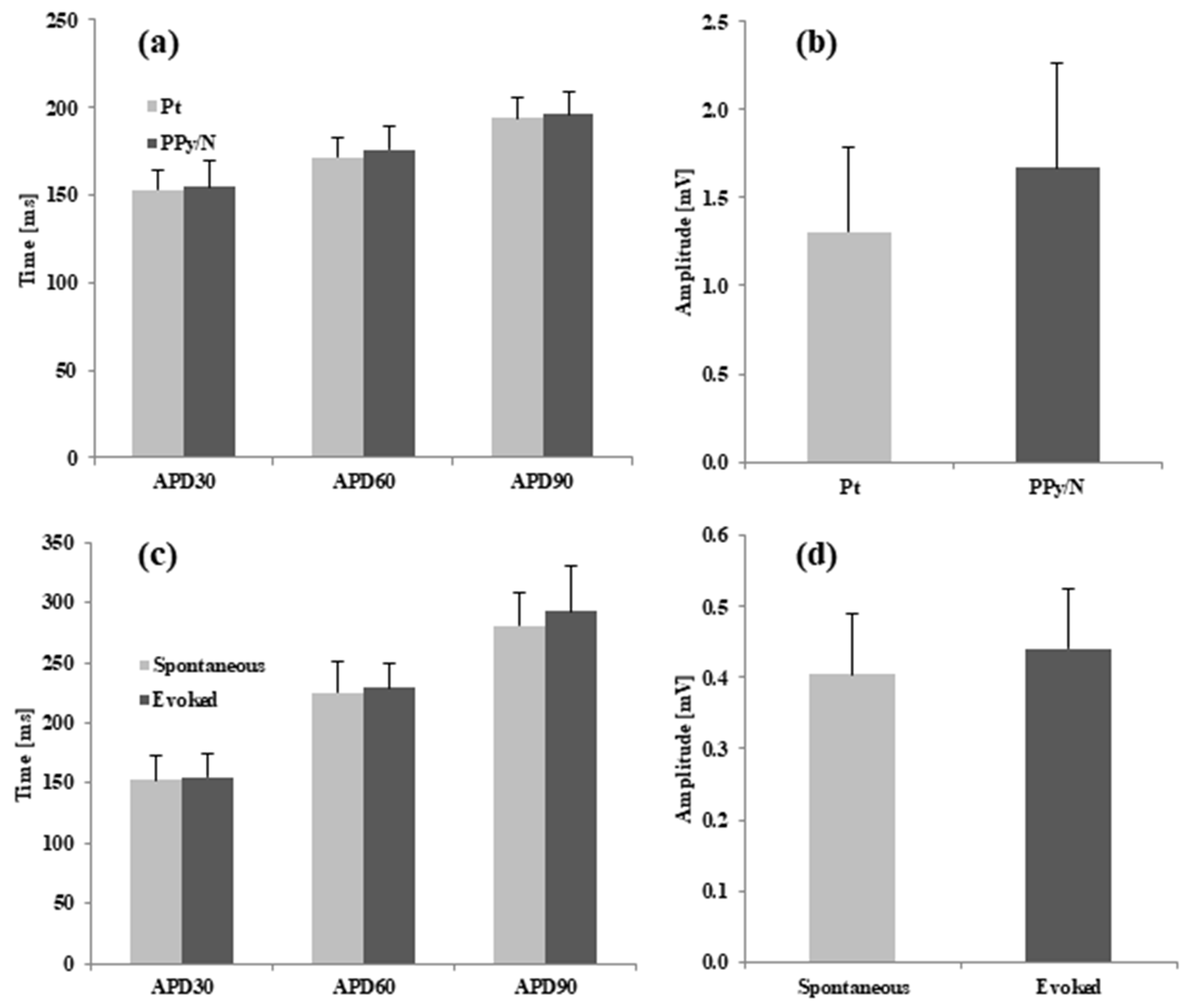
3.2. Recording of Spontaneous MAPs with Pt vs. PPy/N Electrodes
3.3. Spontaneous MAPs vs. MAPs Evoked by Electrical Stimulation with PPy/N Electrodes
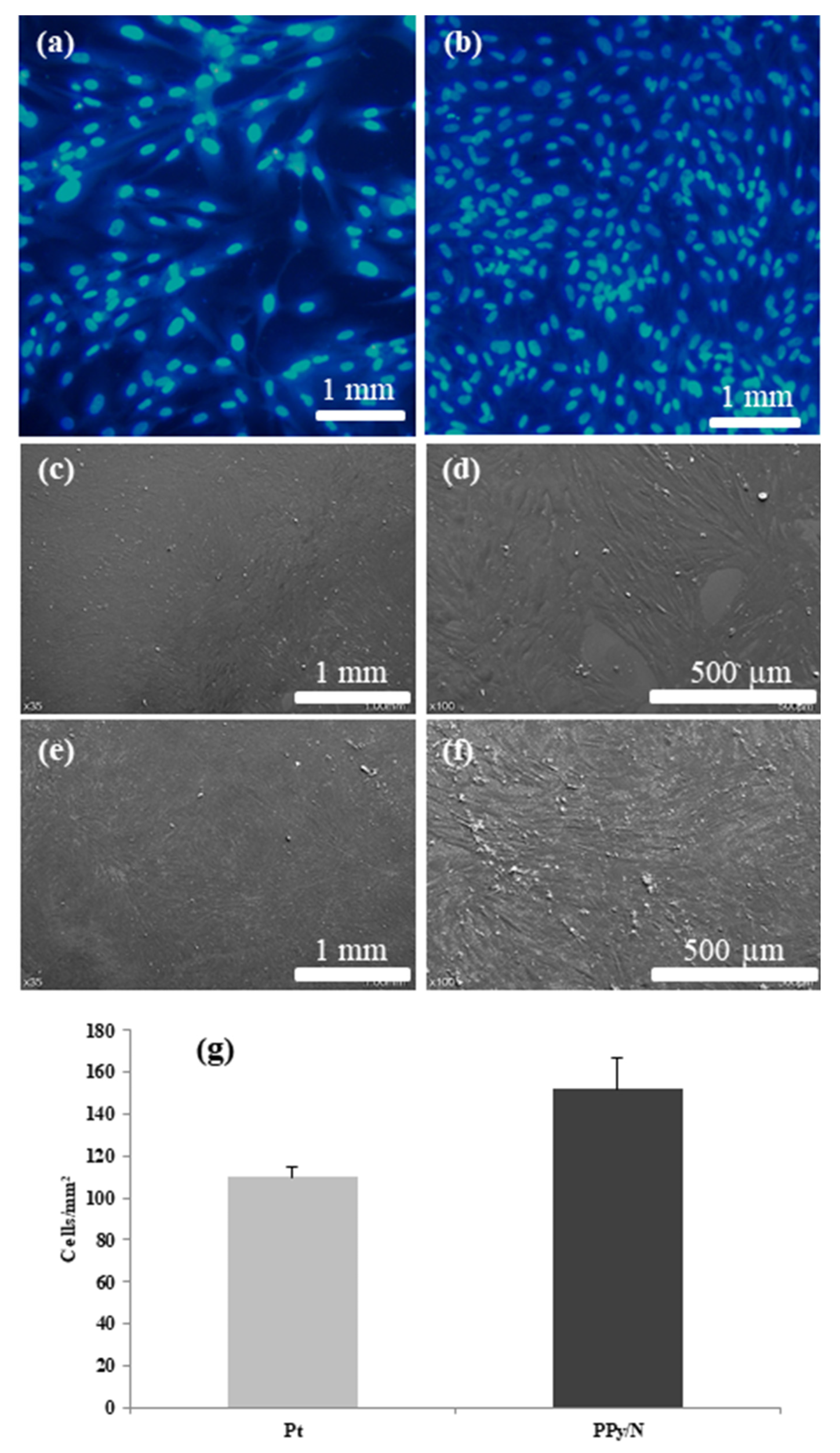
3.4. Cell Culture Results
4. Discussion
4.1. Morphology and Conductivity of the Tested PPy/N Coatings and Strips
4.2. Recording of MAP Signals with PPy/N Electrodes
4.3. MAP Signals Evoked by Electrical Stimulation with PPy Electrodes
4.4. Biocompatibility of PPy/N Films
4.5. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mulpuru, S.K.; Madhavan, M.; McLeod, C.J.; Cha, Y.M.; Friedman, P.A. Cardiac Pacemakers: Function, Troubleshooting, and Management: Part 1 of a 2-Part Series. J. Am. Coll. Cardiol. 2017, 69, 189–210. [Google Scholar] [CrossRef] [PubMed]
- Bouthour, W.; Megevand, P.; Donoghue, J.; Luscher, C.; Birbaumer, N.; Krack, P. Biomarkers for closed-loop deep brain stimulation in Parkinson disease and beyond. Nat. Rev. Neurol. 2019, 15, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Perez-Carbonell, L.; Faulkner, H.; Higgins, S.; Koutroumanidis, M.; Leschziner, G. Vagus nerve stimulation for drug-resistant epilepsy. Pract. Neurol. 2020, 20, 189–198. [Google Scholar] [CrossRef]
- Mekhail, N.; Levy, R.M.; Deer, T.R.; Kapural, L.; Li, S.; Amirdelfan, K.; Hunter, C.W.; Rosen, S.M.; Costandi, S.J.; Falowski, S.M.; et al. Long-term safety and efficacy of closed-loop spinal cord stimulation to treat chronic back and leg pain (Evoke): A double-blind, randomised, controlled trial. Lancet Neurol. 2020, 19, 123–134. [Google Scholar] [CrossRef]
- Sevcencu, C.; Nielsen, T.N.; Struijk, J.J. A neural blood pressure marker for bioelectronic medicines for treatment of hypertension. Biosens. Bioelectron. 2017, 98, 1–6. [Google Scholar] [CrossRef]
- Bouton, C. Cracking the neural code, treating paralysis and the future of bioelectronic medicine. J. Intern. Med. 2017, 282, 37–45. [Google Scholar] [CrossRef]
- Pavlov, V.A.; Tracey, K.J. Bioelectronic medicine: Updates, challenges and paths forward. Bioelectron. Med. 2019, 5, 1. [Google Scholar] [CrossRef]
- Figee, M.; Mayberg, H. The future of personalized brain stimulation. Nat. Med. 2021, 27, 196–197. [Google Scholar] [CrossRef]
- Sisterson, N.D.; Wozny, T.A.; Kokkinos, V.; Constantino, A.; Richardson, R.M. Closed-Loop Brain Stimulation for Drug-Resistant Epilepsy: Towards an Evidence-Based Approach to Personalized Medicine. Neurotherapeutics 2019, 16, 119–127. [Google Scholar] [CrossRef]
- Paff, M.; Loh, A.; Sarica, C.; Lozano, A.M.; Fasano, A. Update on Current Technologies for Deep Brain Stimulation in Parkinson’s Disease. J. Mov. Disord. 2020, 13, 185–198. [Google Scholar] [CrossRef]
- Sevcencu, C. Single-interface bioelectronic medicines-concept, clinical applications and preclinical data. J. Neural Eng. 2022, 19, 031001. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.T.; Pohlmeyer, E.; Gather, M.C.; Kemere, C.; Kitching, J.E.; Malliaras, G.G.; Marblestone, A.; Shepard, K.L.; Stieglitz, T.; Xie, C. Developing Next-generation Brain Sensing Technologies—A Review. IEEE Sens. J. 2019, 19, 10163–10175. [Google Scholar] [CrossRef] [PubMed]
- Green, R.A.; Williams, C.M.; Lovell, N.H.; Poole-Warren, L.A. Novel neural interface for implant electrodes: Improving electroactivity of polypyrrole through MWNT incorporation. J Mater Sci. Mater Med. 2008, 19, 1625–1629. [Google Scholar] [CrossRef] [PubMed]
- Branner, A.; Stein, R.B.; Fernandez, E.; Aoyagi, Y.; Normann, R.A. Long-term stimulation and recording with a penetrating microelectrode array in cat sciatic nerve. IEEE Trans. Biomed Eng. 2004, 51, 146–157. [Google Scholar] [CrossRef]
- Green, R.A.; Baek, S.; Poole-Warren, L.A.; Martens, P.J. Conducting polymer-hydrogels for medical electrode applications. Sci. Technol. Adv. 2010, 11, 014107. [Google Scholar] [CrossRef]
- Zhang, Z.; Rouabhia, M.; Moulton, S.E. Conductive Polymers: Electrical Interactions in Cell Biology and Medicine; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Povlich, L.K.; Feldman, K.E.; Shim, B.S.; Martin, D.C. 1.130—Electroactive Polymeric Biomaterials. In Comprehensive Biomaterials; Ducheyne, P., Ed.; Elsevier: Oxford, UK, 2011; pp. 547–561. [Google Scholar]
- Wang, X.S.; Xu, J.K.; Shi, G.Q.; Lu, X. Microstructure-mechanical properties relationship in conducting polypyrrole films. J. Mater. Sci. 2002, 37, 5171–5176. [Google Scholar] [CrossRef]
- Cui, S.; Mao, J.; Rouabhia, M.; Elkoun, S.; Zhang, Z. A biocompatible polypyrrole membrane for biomedical applications. RSC. Adv. 2021, 11, 16996–17006. [Google Scholar] [CrossRef]
- Lee, S.; Park, S.; Park, J.; Lee, J.Y. Implantable polypyrrole bioelectrodes inducing anti-inflammatory macrophage polarization for long-term in vivo signal recording. Acta Biomater. 2023, 168, 458–469. [Google Scholar] [CrossRef]
- Cui, X.; Hetke, J.F.; Wiler, J.A.; Anderson, D.J.; Martin, D.C. Electrochemical deposition and characterization of conducting polymer polypyrrole/PSS on multichannel neural probes. Sens. Actuators A Phys. 2001, 93, 8–18. [Google Scholar] [CrossRef]
- Kim, S.; Jang, L.K.; Jang, M.; Lee, S.; Hardy, J.G.; Lee, J.Y. Electrically Conductive Polydopamine-Polypyrrole as High Performance Biomaterials for Cell Stimulation in Vitro and Electrical Signal Recording in Vivo. ACS Appl. Mater. Interfaces. 2018, 10, 33032–33042. [Google Scholar] [CrossRef]
- Forciniti, L.; Ybarra, J., III; Zaman, M.H.; Schmidt, C.E. Schwann cell response on polypyrrole substrates upon electrical stimulation. Acta Biomater. 2014, 10, 2423–2433. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Park, J.; Kim, S.; Ok, J.; Yoo, J.I.; Kim, Y.S.; Ahn, Y.; Kim, T.I.; Ko, H.C.; Lee, J.Y. High-Performance Implantable Bioelectrodes with Immunocompatible Topography for Modulation of Macrophage Responses. ACS Nano. 2022, 16, 7471–7485. [Google Scholar] [CrossRef] [PubMed]
- Schroder, D.K. Resistivity. In Semiconductor Material and Device Characterization, 3rd ed.; IEEE Press: Piscataway, NJ, USA, 2005; pp. 1–59. [Google Scholar]
- Knollmann, B.C.; Katchman, A.N.; Franz, M.R. Monophasic action potential recordings from intact mouse heart: Validation, regional heterogeneity, and relation to refractoriness. J. Cardiovasc. Electrophysiol. 2001, 12, 1286–1294. [Google Scholar] [CrossRef]
- Lieberman, M.; Paes de Carvalho, A. The Electrophysiological Organization of the Embryonic Chick Heart. J. Gen. Physiol. 1965, 49, 351–363. [Google Scholar] [CrossRef]
- Nouchi, H.; Kiryu, N.; Kimata, M.; Tsuneoka, Y.; Hamaguchi, S.; Namekata, I.; Takahara, A.; Shigenobu, K.; Tanaka, H. Developmental Changes in Action Potential Prolongation by K(+)-Channel Blockers in Chick Myocardium. J. Pharmacol. Sci. 2011, 115, 235–238. [Google Scholar] [CrossRef]
- Sugiyama, T.; Miyazaki, H.; Saito, K.; Shimada, H.; Miyamoto, K. Chick embryos as an alternative experimental animal for cardiovascular investigations: Stable recording of electrocardiogram of chick embryos in ovo on the 16th day of incubation. Toxicol. Appl. Pharmacol. 1996, 138, 262–267. [Google Scholar] [CrossRef]
- Kollmansperger, S.; Anders, M.; Werner, J.; Saller, A.M.; Weiss, L.; Suss, S.C.; Reiser, J.; Schneider, G.; Schusser, B.; Baumgartner, C.; et al. Nociception in Chicken Embryos, Part II: Embryonal Development of Electroencephalic Neuronal Activity In Ovo as a Prerequisite for Nociception. Animals 2023, 13, 2839. [Google Scholar] [CrossRef]
- Suss, S.C.; Werner, J.; Saller, A.M.; Weiss, L.; Reiser, J.; Ondracek, J.M.; Zablotski, Y.; Kollmansperger, S.; Anders, M.; Potschka, H.; et al. Nociception in Chicken Embryos, Part III: Analysis of Movements before and after Application of a Noxious Stimulus. Animals 2023, 13, 2859. [Google Scholar] [CrossRef]
- Weiss, L.; Saller, A.M.; Werner, J.; Suss, S.C.; Reiser, J.; Kollmansperger, S.; Anders, M.; Potschka, H.; Fenzl, T.; Schusser, B.; et al. Nociception in Chicken Embryos, Part I: Analysis of Cardiovascular Responses to a Mechanical Noxious Stimulus. Animals 2023, 13, 2710. [Google Scholar] [CrossRef]
- Franz, M.R. Current status of monophasic action potential recording: Theories, measurements and interpretations. Cardiovasc. Res. 1999, 41, 25–40. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.; Zhao, G. Morphology, structure, and conductivity of polypyrrole prepared in the presence of mixed surfactants in aqueous solutions. J. Appl. Polym. Sci. 2007, 104, 1987–1996. [Google Scholar] [CrossRef]
- Carquigny, S.P.; Lakard, B.; Lakard, S.; Moutarlier, V.; Hihn, J.Y.; Viau, L. Investigation of pharmaceutically active ionic liquids as electrolyte for the electrosynthesis of polypyrrole and active component in controlled drug delivery. Electrochim. Acta 2016, 211, 950–961. [Google Scholar] [CrossRef]
- Bae, W.J.; Ruddy, B.P.; Richardson, A.G.; Hunter, I.W.; Bizzi, E. Cortical recording with polypyrrole microwire electrodes. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2008, 2008, 5794–5797. [Google Scholar]
- Meng, L.; Fu, Q.; Hao, S.; Xu, F.; Yang, J. Self-adhesive, biodegradable silk-based dry electrodes for epidermal electrophysiological monitoring. Chem. Eng. J. 2022, 427, 131999. [Google Scholar] [CrossRef]
- Lindsey, S.E.; Street, G.B. Conductive composites from polyvinyl alcohol and polypyrrole. Synth. Met. 1984, 10, 67–69. [Google Scholar] [CrossRef]
- Cui, X.; Lee, V.A.; Raphael, Y.; Wiler, J.A.; Hetke, J.F.; Anderson, D.J.; Martin, D.C. Surface modification of neural recording electrodes with conducting polymer/biomolecule blends. J. Biomed. Mater. Res. 2001, 56, 261–272. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sevcencu, C.; Crăciunescu, I.; Andrei, A.-A.; Suciu, M.; Macavei, S.; Barbu-Tudoran, L. Polypyrrole Coatings as Possible Solutions for Sensing and Stimulation in Bioelectronic Medicines. Biosensors 2025, 15, 366. https://doi.org/10.3390/bios15060366
Sevcencu C, Crăciunescu I, Andrei A-A, Suciu M, Macavei S, Barbu-Tudoran L. Polypyrrole Coatings as Possible Solutions for Sensing and Stimulation in Bioelectronic Medicines. Biosensors. 2025; 15(6):366. https://doi.org/10.3390/bios15060366
Chicago/Turabian StyleSevcencu, Cristian, Izabella Crăciunescu, Alin-Alexandru Andrei, Maria Suciu, Sergiu Macavei, and Lucian Barbu-Tudoran. 2025. "Polypyrrole Coatings as Possible Solutions for Sensing and Stimulation in Bioelectronic Medicines" Biosensors 15, no. 6: 366. https://doi.org/10.3390/bios15060366
APA StyleSevcencu, C., Crăciunescu, I., Andrei, A.-A., Suciu, M., Macavei, S., & Barbu-Tudoran, L. (2025). Polypyrrole Coatings as Possible Solutions for Sensing and Stimulation in Bioelectronic Medicines. Biosensors, 15(6), 366. https://doi.org/10.3390/bios15060366






