Recent Progress in MXene-Based Materials for Supercapacitors and Electrochemical Sensing Applications
Abstract
1. Introduction
2. Synthetic Procedures and Properties of MXene
2.1. Etching Approaches (Direct HF, In Situ HF and Molten Salt Etching)
2.2. Hydrothermal/Solvothermal Approaches
2.3. Other Methods
3. SC Applications
3.1. MXenes and Functionalized/Doped MXenes Based SCs
3.2. MXenes/Metal Oxides Based SCs
3.3. MXenes/Metal Sulfides/Selenides Based SCs
3.4. MXenes/Polymers Based SCs
3.5. MXenes/Carbon-Based Materials for SCs
3.6. MXenes/Hydroxides/MOFs/LDH
3.7. Others
4. Electrochemical Sensors
4.1. MXenes/Metal Oxides Based Sensors
4.2. MXenes/Metal Sulfides Based Sensors
4.3. MXenes/Polymers Based Sensors
4.4. MXenes/Carbon-Based Materials for Sensors
4.5. MXenes/MOFs/LDH Based Sensors
4.6. MXenes/Metal Nanoparticles/Others
5. Conclusions, Challenges and Future Perspectives
- i.
- MXene suffers from the restacking of the nanosheets, which is due to the van der Waals forces, which can significantly affect the surface area and ion movements. Thus, the electrochemical performance of the MXene-based electrode materials can be influenced for SCs and sensing studies.
- ii.
- MXene also faces structural degradation and compromised electrochemical performance for long-term stability and cycles for SCs and sensing applications.
- iii.
- The synthesis of MXene involves harsh conditions, such as the use of HF, increasing the cost of the preparation of MXene and resulting in negative impacts on the environment. Thus, etching-based synthetic methods for the preparation of MXene restrict their potential applications to large-scale production.
- iv.
- The surface chemistry for the modification and functionalization of MXene is still unknown and needs in-depth investigations.
- v.
- Scalability is also another challenge for large-scale production.
- vi.
- The toxicity of the fluoride groups on the MXene surface is another challenge.
- vii.
- The preparation of MXene with uniform morphological characteristics and controlled surface properties remains a challenge.
- viii.
- MXene can be oxidized in alkaline electrolyte solution-based systems for long-term operations.
- (a)
- Novel fluoride etching-free and green synthesis methods should be developed for the preparation of MXene instead of conventional methods with harsh conditions (HF etching).
- (b)
- The surface passivation strategy may be applied to improve the structural stability of MXene by incorporating carbon-based or protective polymer-based layers.
- (c)
- In-depth studies are required to understand the mechanism of the formation of MXene-based composites and their charge transfer properties for electrochemical applications.
- (d)
- The synergistic interactions need to be studied in-depth.
- (e)
- The introduction of ionic liquid-based electrolytes or novel electrolyte additives may be useful to improve the stability of MXene-based materials for long-term cycles.
- (f)
- MXene-based materials can be employed for the construction of flexible SCs and sensors due to their excellent mechanochemical properties.
Author Contributions
Funding
Conflicts of Interest
References
- Velický, M.; Toth, P.S. From two-dimensional materials to their heterostructures: An electrochemist’s perspective. Appl. Mater. Today 2017, 8, 68–103. [Google Scholar]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.Q.; Gogotsi, Y.; Barsoum, M.W. Conductive two-dimensional titanium carbide ‘clay’with high volumetric capacitance. Nature 2014, 516, 78–81. [Google Scholar] [CrossRef]
- Chen, W.; Tang, J.; Cheng, P.; Ai, Y.; Xu, Y.; Ye, N. 3D Porous MXene (Ti3C2Tx) Prepared by Alkaline-Induced Flocculation for Supercapacitor Electrodes. Materials 2022, 15, 925. [Google Scholar] [CrossRef]
- Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional transition metal carbides. ACS Nano 2012, 6, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yao, L.; Liu, Q.; Gu, J.; Luo, R.; Li, J.; Yan, X.; Wang, W.; Liu, P.; Chen, B. Fluorine-Free Synthesis of High-Purity Ti3C2Tx (T = OH, O) via Alkali Treatment. Angew. Chem. Int. Ed. 2018, 57, 6115–6119. [Google Scholar]
- Suganthi, S.; Ahmad, K.; Oh, T.H. Progress in MXenes and Their Composites as Electrode Materials for Electrochemical Sensing and Dye-Sensitized Solar Cells. Molecules 2024, 29, 5233. [Google Scholar] [CrossRef]
- Kim, H.; Jung, Y.; Lee, W.; Jeon, Y.-P.; Hong, J.-Y.; Lee, J.U. Sustainable MXene Synthesis via Molten Salt Method and Nano-Silicon Coating for Enhanced Lithium-Ion Battery Performance. Molecules 2025, 30, 812. [Google Scholar] [CrossRef] [PubMed]
- Niyitanga, T.; Chaudhary, A.; Ahmad, K.; Kim, H. Titanium Carbide (Ti3C2Tx) MXene as Efficient Electron/Hole Transport Material for Perovskite Solar Cells and Electrode Material for Electrochemical Biosensors/Non-Biosensors Applications. Micromachines 2023, 14, 1907. [Google Scholar] [CrossRef]
- Zhang, L.; Song, W.; Liu, H.; Ding, H.; Yan, Y.; Chen, R. Influencing Factors on Synthesis and Properties of MXene: A Review. Processes 2022, 10, 1744. [Google Scholar] [CrossRef]
- Bahutair, W.N.; Alhajar, A.; Al Othman, A.; Tawalbeh, M. The role of MXenes and MXene composites in enhancing dye-sensitized solar cells characteristics. Process Saf. Environ. Prot. 2024, 191, 490–504. [Google Scholar] [CrossRef]
- Navitski, I.; Ramanaviciute, A.; Ramanavicius, S.; Pogorielov, M.; Ramanavicius, A. MXene-Based Chemo-Sensors and Other Sensing Devices. Nanomaterials 2024, 14, 447. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Wei, Z.; Ma, C.; Xing, X.; Li, Z.; Luo, D. MXene Boosted CoNi-ZIF-67 as Highly Efficient Electrocatalysts for Oxygen Evolution. Nanomaterials 2019, 9, 775. [Google Scholar] [CrossRef]
- Sulaiman, R.R.R.; Hanan, A.; Wong, W.Y.; Mohamad Yunus, R.; Shyuan Loh, K.; Walvekar, R.; Chaudhary, V.; Khalid, M. Structurally Modified MXenes-Based Catalysts for Application in Hydrogen Evolution Reaction: A Review. Catalysts 2022, 12, 1576. [Google Scholar] [CrossRef]
- Li, X.; Li, M.; Yang, Q.; Liang, G.; Huang, Z.; Ma, L.; Wang, D.; Mo, F.; Dong, B.; Huang, Q.; et al. In Situ Electrochemical Synthesis of MXenes without Acid/Alkali Usage in/for an Aqueous Zinc Ion Battery. Adv. Energy Mater. 2020, 10, 2001791. [Google Scholar] [CrossRef]
- Luo, B.; Wang, X.; Tuokedaerhan, K.; Wang, S.; Wang, C.; Shi, X.; Yu, Z.; Shen, X. MXenes in Perovskite Solar Cells: Emerging Applications and Performance Enhancements. Coatings 2024, 14, 1564. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, R.; Matulis, V.E.; Vladimir, K. Structure, Synthesis, and Catalytic Performance of Emerging MXene-Based Catalysts. Molecules 2024, 29, 1286. [Google Scholar] [CrossRef]
- Gajdzik, B.; Wolniak, R.; Nagaj, R.; Žuromskaitė-Nagaj, B.; Grebski, W.W. The Influence of the Global Energy Crisis on Energy Efficiency: A Comprehensive Analysis. Energies 2024, 17, 947. [Google Scholar] [CrossRef]
- Holechek, J.L.; Geli, H.M.E.; Sawalhah, M.N.; Valdez, R. A Global Assessment: Can Renewable Energy Replace Fossil Fuels by 2050? Sustainability 2022, 14, 4792. [Google Scholar] [CrossRef]
- Azni, M.A.; Md Khalid, R.; Hasran, U.A.; Kamarudin, S.K. Review of the Effects of Fossil Fuels and the Need for a Hydrogen Fuel Cell Policy in Malaysia. Sustainability 2023, 15, 4033. [Google Scholar] [CrossRef]
- Zimon, G.; Pattak, D.C.; Voumik, L.C.; Akter, S.; Kaya, F.; Walasek, R.; Kochański, K. The Impact of Fossil Fuels, Renewable Energy, and Nuclear Energy on South Korea’s Environment Based on the STIRPAT Model: ARDL, FMOLS, and CCR Approaches. Energies 2023, 16, 6198. [Google Scholar] [CrossRef]
- Hiris, D.; Balan, M.C.; Bode, F.I. A Comprehensive Review on Enhancing Seasonal Energy Storage Systems through Energy Efficiency Perspectives. Processes 2024, 12, 1623. [Google Scholar] [CrossRef]
- Subasinghage, K.; Gunawardane, K.; Padmawansa, N.; Kularatna, N.; Moradian, M. Modern Supercapacitors Technologies and Their Applicability in Mature Electrical Engineering Applications. Energies 2022, 15, 7752. [Google Scholar] [CrossRef]
- Mehra, P.; Saxena, S.; Bhullar, S. A Comprehensive Analysis of Supercapacitors and Their Equivalent Circuits—A Review. World Electr. Veh. J. 2024, 15, 332. [Google Scholar] [CrossRef]
- Czagany, M.; Hompoth, S.; Keshri, A.K.; Pandit, N.; Galambos, I.; Gacsi, Z.; Baumli, P. Supercapacitors: An Efficient Way for Energy Storage Application. Materials 2024, 17, 702. [Google Scholar] [CrossRef]
- Yan, J.; Lu, J.; Sheng, Y.; Sun, Y.; Zhang, D. Research Progress in the Preparation of Transition Metal Sulfide Materials and Their Supercapacitor Performance. Micromachines 2024, 15, 849. [Google Scholar] [CrossRef]
- Liang, R.; Du, Y.; Xiao, P.; Cheng, J.; Yuan, S.; Chen, Y.; Yuan, J.; Chen, J. Transition Metal Oxide Electrode Materials for Supercapacitors: A Review of Recent Developments. Nanomaterials 2021, 11, 1248. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, M.G.; Ahmmed, A.S.; Lübben, J.F. Review on Conductive Polymer Composites for Supercapacitor Applications. J. Compos. Sci. 2024, 8, 53. [Google Scholar] [CrossRef]
- Chen, S.; Wang, W.; Zhang, X.; Wang, X. High-Performance Supercapacitors Based on Graphene/Activated Carbon Hybrid Electrodes Prepared via Dry Processing. Batteries 2024, 10, 195. [Google Scholar] [CrossRef]
- Chaluvachar, P.; Mahesha, G.T.; Sudhakar, Y.N.; Nair, V.; Pai, D. A Review on Graphitic Carbon Nitride and Conducting Polymer Nanocomposite Electrodes for Supercapacitors. Eng. Proc. 2023, 59, 154. [Google Scholar]
- Shen, B.; Hao, R.; Huang, Y.; Guo, Z.; Zhu, X. Research Progress on MXene-Based Flexible Supercapacitors: A Review. Crystals 2022, 12, 1099. [Google Scholar] [CrossRef]
- Yan, A.-L.; Wang, X.-C.; Cheng, J.-P. Research Progress of NiMn Layered Double Hydroxides for Supercapacitors: A Review. Nanomaterials 2018, 8, 747. [Google Scholar] [CrossRef]
- Nahirniak, S.; Ray, A.; Saruhan, B. Challenges and Future Prospects of the MXene-Based Materials for Energy Storage Applications. Batteries 2023, 9, 126. [Google Scholar] [CrossRef]
- Yang, M.; Wang, L.; Lu, H.; Dong, Q. Advances in MXene-Based Electrochemical (Bio)Sensors for Neurotransmitter Detection. Micromachines 2023, 14, 1088. [Google Scholar] [CrossRef] [PubMed]
- Chia, H.L.; Mayorga-Martinez, C.C.; Antonatos, N.; Sofer, Z.; Gonzalez-Julian, J.J.; Webster, R.D.; Pumera, M. MXene Titanium Carbide-based Biosensor: Strong Dependence of Exfoliation Method on Performance. Anal. Chem. 2020, 92, 2452–2459. [Google Scholar] [CrossRef]
- Tran, V.A.; Tran, N.T.; Doan, V.D.; Nguyen, T.-Q.; Pham Thi, H.H.; Vo, G.N.L. Application Prospects of MXenes Materials Modifications for Sensors. Micromachines 2023, 14, 247. [Google Scholar] [CrossRef] [PubMed]
- Bounegru, A.V.; Dinu Iacob, A.; Iticescu, C.; Georgescu, P.L. Electrochemical Sensors and Biosensors for the Detection of Pharmaceutical Contaminants in Natural Waters—A Comprehensive Review. Chemosensors 2025, 13, 65. [Google Scholar] [CrossRef]
- Baranwal, J.; Barse, B.; Gatto, G.; Broncova, G.; Kumar, A. Electrochemical Sensors and Their Applications: A Review. Chemosensors 2022, 10, 363. [Google Scholar] [CrossRef]
- Ferreira, M.d.P.; Yamada-Ogatta, S.F.; Teixeira Tarley, C.R. Electrochemical and Bioelectrochemical Sensing Platforms for Diagnostics of COVID-19. Biosensors 2023, 13, 336. [Google Scholar] [CrossRef]
- Marco, A.; Canals, A.; Morallón, E.; Aguirre, M.Á. Electrochemical Sensor for the Determination of Methylthiouracil in Meat Samples. Sensors 2022, 22, 8842. [Google Scholar] [CrossRef]
- Yuan, M.; Wang, L.; Liu, X.; Du, X.; Zhang, G.; Chang, Y.; Xia, Q.; Hu, Q.; Zhou, A. 3D printing quasi-solid-state micro-supercapacitors with ultrahigh areal energy density based on high concentration MXene sediment. Chem. Eng. J. 2023, 451, 138686. [Google Scholar] [CrossRef]
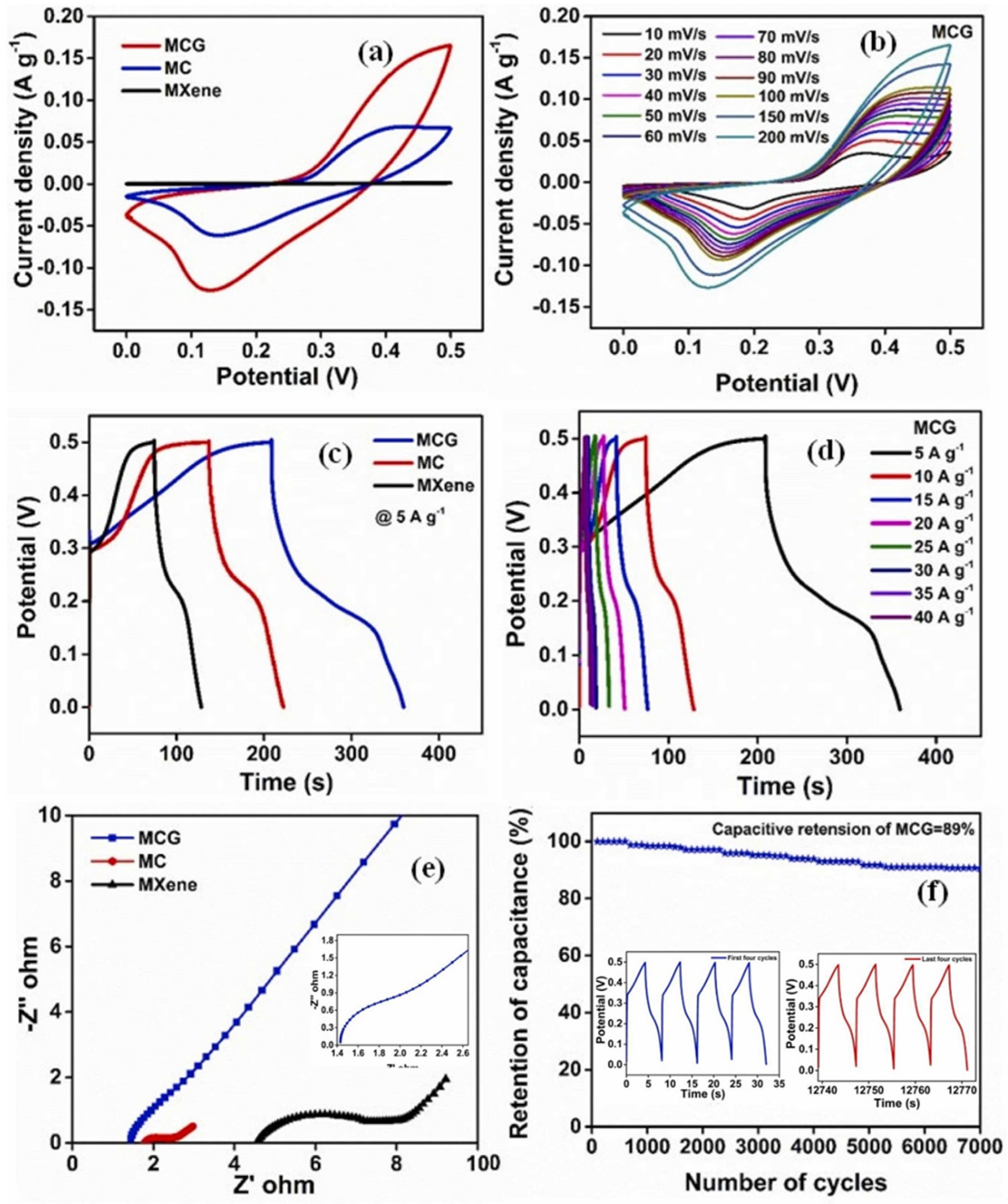
- Wang, X.; Wang, S.; Li, C.; Cui, Y.; Yong, Z.; Liang, D.; Chi, Y.; Wang, Z. Flexible supercapacitor based on MXene cross-linked organic gel electrolyte with wide working temperature. Int. J. Hydrogen Energy 2023, 48, 4921–4930. [Google Scholar] [CrossRef]
- Mateen, A.; Ahmad, Z.; Ali, S.; Hassan, N.U.; Ahmed, F.; Alshgari, R.A.; Mushab, M.; Eldin, S.M.; Ansari, M.Z.; Javed, M.S.; et al. Silicon intercalation on MXene nanosheets towards new insights into a superior electrode material for high-performance Zn-ion supercapacitor. J. Energy Storage 2023, 71, 108151. [Google Scholar] [CrossRef]
- Mu, J.; Zhao, Y.; Guo, Z.; Zhang, Z.; Che, H.; Wang, Y.; Zhang, X.; Wang, G.; Mu, J.; Wang, L. Rational design of new, efficient, and suitable nickel phthalocyanine reinforced MXene electrodes for supercapacitors. J. Energy Storage 2023, 61, 106768. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Dong, S.; Zhang, X.; Lv, L.; He, S. Room-temperature prepared MXene foam via chemical foaming methods for high-capacity supercapacitors. J. Alloys Compd. 2023, 945, 169279. [Google Scholar] [CrossRef]
- Cai, M.; Wei, X.; Huang, H.; Yuan, F.; Li, C.; Xu, S.; Liang, X.; Zhou, W.; Guo, J. Nitrogen-doped Ti3C2Tx MXene prepared by thermal decomposition of ammonium salts and its application in flexible quasi-solid-state supercapacitor. Chem. Eng. J. 2023, 458, 141338. [Google Scholar] [CrossRef]
- Yang, W.; Yang, Z.; Wang, J.; Lu, W.; Wang, W. A bean catching double pigeons: Sonication assisted modification of Nb2C MXenes composites by O-doping porous biomass-carbons for supercapacitors and zinc-ion batteries. J. Energy Storage 2023, 65, 107334. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, X.; Chen, Z.; Zhao, Y.; Yao, W.; Xu, J. Ti3−yNbyC2Tx MXenes as high-rate and ultra-stable electrode materials for supercapacitors. J. Alloys Compd. 2023, 954, 170128. [Google Scholar] [CrossRef]
- Shen, B.; Liao, X.; Hu, X.; Ren, H.T.; Lin, J.H.; Lou, C.W.; Li, T.T. OH-WCNT/Nb2CTx MXene sponge for flexible free-standing high -performance supercapacitors. Electrochim. Acta 2023, 464, 142921. [Google Scholar] [CrossRef]
- Cai, C.; Liu, J.; Wei, Z.; Huang, Y.; Fu, Y. Aestivum-inspired photothermal 3D-MXene-unity membrane-aerogel for flexible supercapacitor with solar enhanced performance. Compos. Part B Eng. 2023, 260, 110748. [Google Scholar] [CrossRef]
- Lu, G.; Xing, X.; Zhang, J.; Xu, X.; Zhang, X.; Liu, H.; Fan, J.; Zhang, B. Rational design of cobalt ion chelated quasi-monolayer Ti3C2Tx MXene with abundant surface loading for high-performance asymmetric supercapacitor. Colloids Surf. A Physicochem. Eng. Asp. 2023, 671, 131694. [Google Scholar] [CrossRef]
- Cui, Y.; Shi, Q.; Liu, Z.; Lv, J.; Wang, C.; Xie, X.; Zhang, S. MXene/biomass/chitosan carbon aerogel (MBC) with shared cathode and anode for the construction of high-efficiency asymmetric supercapacitor. Chem. Eng. J. 2023, 472, 144701. [Google Scholar] [CrossRef]
- Zhao, D.; Chen, Z.; Fu, N.; Yang, Z. Hexaazatriphenylene-doped MXene films for the high-performance micro-supercapacitors and wearable pressure sensor system. Mater. Today Sustain. 2023, 24, 100523. [Google Scholar] [CrossRef]
- Yan, S.; Shi, C.; Tian, Z.; Li, D.; Chen, Y.; Guo, L.; Wang, Y. Self-standing porous MXene film via in situ formed microgel induced by 2- methylimidazole for high rate supercapacitor. Chem. Eng. J. 2023, 478, 147393. [Google Scholar] [CrossRef]
- Kumar, J.; Soomro, R.A.; Fan, B.; Tan, J.; Sun, N.; Xu, B. Butanedioic acid unlock shelf-stable Ti3C2Tx (MXene) dispersions and their electrochemical performance in supercapacitor. J. Alloys Compd. 2024, 1009, 176749. [Google Scholar] [CrossRef]
- Wan, J.; Wu, R.; Chen, Y.; Zhang, H.; Li, H.; Wang, B.; Liskiewicz, T.; Shi, S. Amino modification of Ti3C2 MXenes for high-performance supercapacitors. Appl. Surf. Sci. 2024, 678, 161154. [Google Scholar] [CrossRef]
- Liang, Y.; Shen, H.; Li, J.; Zhao, H.; Xu, J.; Huang, H.; Xu, S.; Liang, X.; Zhou, W.; Guo, J. Tailoring surface terminals of Ti3C2Tx MXene microgels via interlayer domain-confined polyphosphate ammonium for flexible supercapacitor applications. Chem. Eng. J. 2024, 497, 154775. [Google Scholar] [CrossRef]
- Cai, D.; Wu, S.; Guo, L.; Wang, Y. Mg2+ induced Ti3C2Tx MXene@microfibrillated cellulose into composite aerogels in a framework of melamine sponge for high-rate performance supercapacitors. J. Energy Storage 2024, 102, 114039. [Google Scholar] [CrossRef]
- Rafique, M.; Anwar, S.; Irshad, M.; Khan, M.I.; Tahir, M.B.; Nabi, G.; Assiri, M.A. Novel synthesis and electrochemical performance of 2D molybdenum nitride (MXene) for supercapacitor application. J. Energy Storage 2024, 103, 114354. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, Y.; Wang, J.; Han, X.; Zhang, G.; Sun, X. Cu1.5Mn1.5O4 hollow sphere decorated Ti3C2Tx MXene for flexible all-solid-state supercapacitor and electromagnetic wave absorber. Carbon 2023, 209, 118006. [Google Scholar] [CrossRef]
- Vetrikarasan, B.T.; Nair, A.R.; Karthick, T.; Shinde, S.K.; Kim, D.Y.; Sawant, S.N.; Jagadale, A.D. Co-precipitation synthesis of pseudocapacitive λ-MnO2 for 2D MXene (Ti3C2Tx) based asymmetric flexible supercapacitor. J. Energy Storage 2023, 72, 108403. [Google Scholar] [CrossRef]
- Ghaemi, S.P.; Masoudpanah, S.M.; Heidari, P. Facile synthesis of Ni0.5Zn0.5Fe2O4/MXene composite powders for high-performance asymmetric supercapacitors. Chem. Eng. J. 2023, 72, 108439. [Google Scholar] [CrossRef]
- Wei, X.; Cai, M.; Yuan, F.; Li, C.; Huang, H.; Xu, S.; Liang, X.; Zhou, W.; Guo, J. Construction of CoMoO4 nanosheets arrays modified by Ti3C2Tx MXene and their enhanced charge storage performance for hybrid supercapacitor. Colloids Surf. A Physicochem. Eng. Asp. 2023, 658, 130637. [Google Scholar] [CrossRef]
- Pathak, M.; Mane, P.; Chakraborty, B.; Rout, C.S. Facile in-situ grown spinel MnCo2O4/MWCNT and MnCo2O4/Ti3C2 MXene composites for high-performance asymmetric supercapacitor with theoretical insight. J. Energy Storage 2023, 66, 107475. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Y.; Zhang, S.; Du, X.; Duan, Z.; Sun, X. Hollow Co9S8 cores encapsulated in hierarchical MXene@Bi2O3 multiple shells for constructing binder-free electrodes of foldable supercapacitors. J. Mater. Science Technol. 2023, 147, 112–123. [Google Scholar] [CrossRef]
- Luo, L.; Meng, W.; Wang, G.; Qin, J.; He, H.; Huang, H. MnO2 nanoflowers-decorated MXene nanosheets with enhanced supercapacitor performance. J. Alloys Compd. 2023, 957, 170411. [Google Scholar] [CrossRef]
- Luo, W.; Sun, Y.; Lin, Z.; Li, X.; Han, Y.; Ding, J.; Li, T.; Hou, C.; Ma, Y. Flexible Ti3C2Tx MXene/V2O5 composite films for high-performance all-solid supercapacitors. J. Energy Storage 2023, 62, 106807. [Google Scholar] [CrossRef]
- Karmur, R.S.; Gogoi, D.; Biswas, A.; Prathibha, C.; Das, M.R.; Ghosh, N.N. Nanocomposite having hierarchical architecture of MXene-WO3 nanorod@rGOsponge and porous carbon for cathode and anode materials for high-performance flexible all-solid-state asymmetric supercapacitor device. Appl. Surf. Sci. 2023, 623, 157042. [Google Scholar] [CrossRef]
- Ashraf, I.; Ahmad, S.; Dastan, D.; Iqbal, M. Ni-doped CeO2 decorated delaminated layered titanium carbonitride (d-Ti3CN) MXene for potential applications in symmetric supercapacitors. J. Alloys Compd. 2023, 952, 170043. [Google Scholar] [CrossRef]
- Beknalkar, S.A.; Teli, A.M.; Khot, A.C.; Mane, S.M.; Shin, J.C. Preparation of CuMn2O4/Ti3C2 MXene composite electrodes for supercapacitors with high energy density and study on their charge transfer kinetics. Ceram. Int. 2023, 49, 31236–31247. [Google Scholar] [CrossRef]
- Reina, M.; Serrapede, M.; Zaccagnini, P.; Pedico, A.; Castellino, M. Decoration of laser induced graphene with MXene and manganese oxide for fabrication of a hybrid supercapacitor. Electrochim. Acta 2023, 468, 143163. [Google Scholar] [CrossRef]
- Althubiti, N.A.; Aman, S.; Taha, T.A.M. Synthesis of MnFe2O4/MXene/NF nanosized composite for supercapacitor application. Ceram. Int. 2023, 49, 27496–27505. [Google Scholar] [CrossRef]
- Nikhil, P.; Vasanth, S.; Ponpandian, N.; Viswanathan, C. Synthesis effect on surface functionalized Ti3C2Tx MXene supported nickel oxide nanocomposites with enhanced specific capacity for supercapacitor application. J. Energy Storage 2023, 72, 108414. [Google Scholar] [CrossRef]
- Ammar, A.U.; Bakan-Misirlioglu, F.; Aleinawi, M.H.; Franzo, G.; Condorelli, G.G.; Yesilbag, F.N.T.; Yesilbag, Y.O.; Mirabella, S.; Erdem, E. All-in-one supercapacitors with high performance enabled by Mn/Cu doped ZnO and MXene. Mater. Res. Bull. 2023, 165, 112334. [Google Scholar] [CrossRef]
- Sun, L.; Su, X.; Chen, Y.; Zhuo, K.; Li, H.; Sun, D.; Wang, J. Ferric ion-assisted assembly of MXene/TiO2-graphene aerogel for ionic liquid-based supercapacitors. Chem. Eng. J. 2023, 476, 146731. [Google Scholar] [CrossRef]
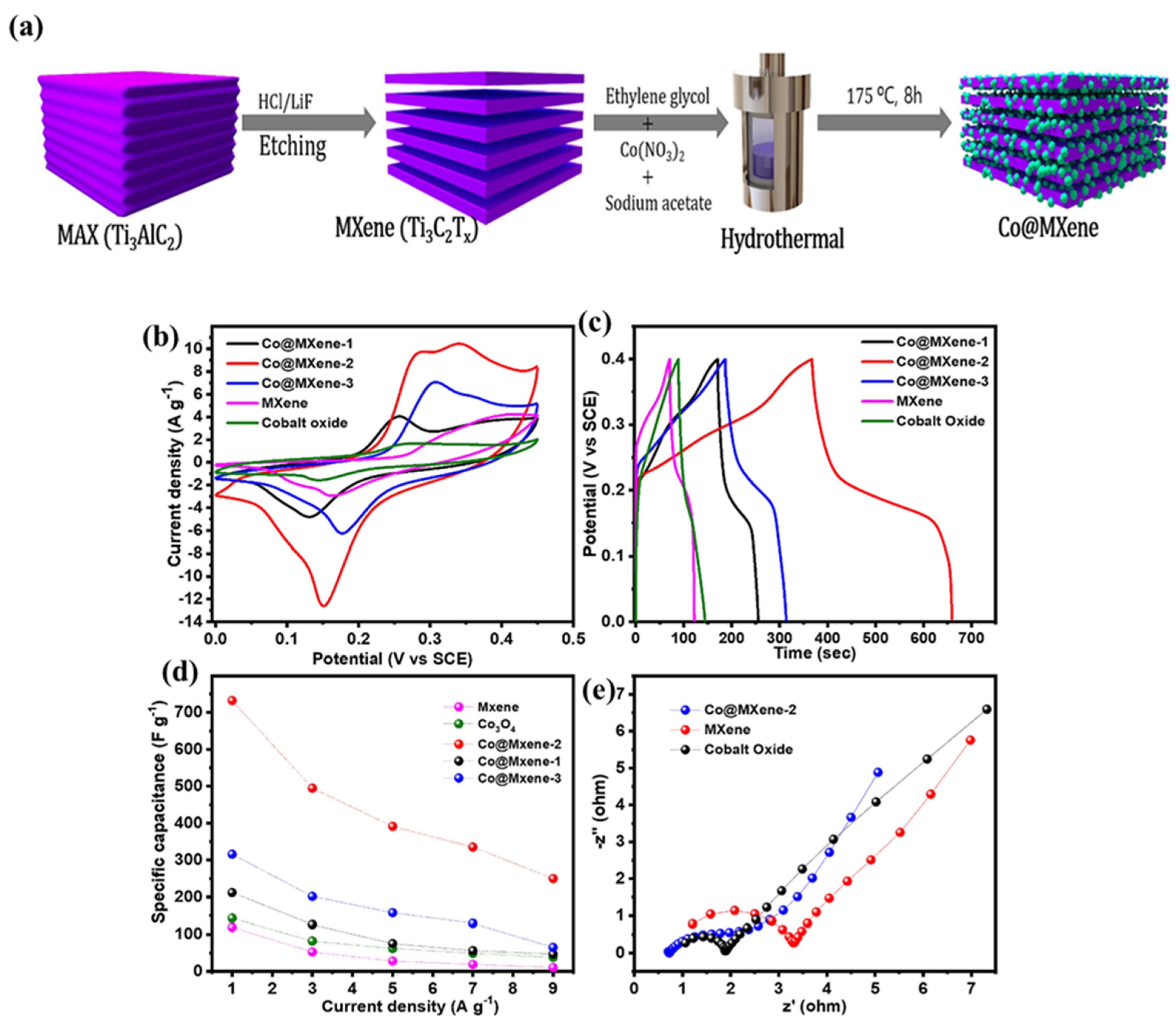
- Kunwar, J.; Acharya, D.; Chhetri, K.; Karki, B.; Deo, B.; Bhattarai, R.M.; Neupane, S.; Adhikari, M.P.; Yadav, A.P. Cobalt oxide decorated 2D MXene: A hybrid nanocomposite electrode for high-performance supercapacitor application. J. Electroanal. Chem. 2023, 950, 117915. [Google Scholar] [CrossRef]
- Bin, X.; Sheng, M.; Luo, Y.; Que, W. Heterostructures of MoO3 nanobelts assembled on delaminated V4C3Tx MXene nanosheets for supercapacitors with excellent room/high temperature performance. Electrochim. Acta 2023, 446, 142070. [Google Scholar] [CrossRef]
- Prabhakar, N.; Humayun, A.; Nagamony, P.; Rajaji, U.; Alshgari, R.A.; Liu, T.Y.; Chinnuswamy, V. Effect on delamination of Nb4C3Tx MXene supported tungsten oxide for high-performance supercapacitor. J. Energy Storage 2024, 98, 112830. [Google Scholar] [CrossRef]
- Wang, W.; Chen, G.; Kong, W.; Chen, J.; Pu, L.; Gong, J.; Zhang, H.; Dai, Y. Sandwich-like high-performance Ti3C2Tx MXene/NiCo2O4 nanosphere composites for asymmetric supercapacitor application. J. Energy Storage 2024, 86, 111097. [Google Scholar] [CrossRef]
- Xu, Q.; Chen, Y.; Huang, Y.; Xu, C.; Hu, C.; Jiang, N.; Yang, L.; Yin, S. g-C3N4/MoO3 heterostructure decorated Ti3C2Tx MXene film as a flexible electrode for supercapacitors with high energy density and low temperature tolerance. J. Energy Storage 2024, 101, 113968. [Google Scholar] [CrossRef]
- Li, B.; Zhang, L.; Zhao, Z.; Zou, Y.; Chen, B.; Fu, X.; Wang, F.; Long, S.; Guo, W.; Liang, J.; et al. Unraveling hierarchical hollow NiCo2S4/MXene/N-doped carbon microspheres via dual templates for high-performance hybrid supercapacitors. Chem. Eng. J. 2024, 487, 150730. [Google Scholar] [CrossRef]
- Shahid, A.; Kayani, Z.N.; Riaz, S.; Naseem, S. Enhanced supercapacitor potential of CeO2/MXene nanocomposites with tunable conductive polyaniline concentrations. Diam. Relat. Mater. 2024, 146, 111167. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, M.; Min, Y. Construction of CuCo2O4 hollow microspheres/Ti3C2Tx MXene composite for electrode material of hybrid supercapacitors. Colloids Surf. A Physicochem. Eng. Asp. 2024, 695, 134315. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; An, L.; Zou, Z.; Cong, Z.; Liu, M.; Yang, J.; Zhai, S.; An, Q.; Wang, K.; et al. Synergistic MXene/NiCo2S4 composite for high-performance flexible all-solid-state supercapacitors. J. Energy Storage 2024, 93, 112398. [Google Scholar] [CrossRef]
- Ikram, M.; Baig, M.M.; Shakir, I.; Irshad, A.; ALOthman, Z.A.; Warsi, M.F.; Lee, S.G. Boosting the electrochemical properties of MoO3/MnFe2O4/MXene for high-performance supercapacitor applications. J. Energy Storage 2024, 90, 111746. [Google Scholar] [CrossRef]
- Mathew, S.; Devi K.R., S.; Saravanakumar, B.; Pinheiro, D. Strategic design of MXene/CoFe2O4/g-C3N4 electrode for high-energy asymmetric supercapacitors. J. Alloys Compd. 2024, 1008, 176488. [Google Scholar] [CrossRef]
- Akbar, S.; Baig, M.M.; Khan, M.N.; ALOthman, Z.A.; Shakir, I.; Lee, S.G.; Warsi, M.F. Ag-doped MgCo2O4/MXene composite: Tailoring the electrochemical properties via 2D layered material for next generation supercapacitor study. J. Alloys Compd. 2024, 1009, 176813. [Google Scholar] [CrossRef]
- Khan, A.J.; Ding, H.; Zhang, Y.; Zhang, D.; Gao, L.; Zhao, G. NiCo2O4@MXene composite electrodes: Unveiling high-performance asymmetric supercapacitor capabilities through enhanced redox activity. Chem. Eng. J. 2025, 506, 160287. [Google Scholar] [CrossRef]
- Verma, K.D.; Kar, K.K. Cost-effective synthesis route for ultra-high purity of Ti3AlC2 MAX phase with enhanced performance of Ti3C2Tx MXene and MXene/NiO composite for supercapacitor application. Chem. Eng. J. 2025, 504, 158938. [Google Scholar] [CrossRef]
- Meenakshi, G.A.; Agalya, M.; Surya, R.; Sakthinathan, S.; Kiruthika, S.; Chiu, T.W.; Sukumaran, S. Construction of novel bifunctional electrocatalyst CuCoTiO2/Mxene for supercapacitor and sensor application. Ceram. Int. 2025. (accepted). [Google Scholar] [CrossRef]
- Hussain, S.; Vikraman, D.; Sheikh, Z.A.; Mehran, M.T.; Shahzad, F.; Batoo, K.M.; Kim, H.S.; Kim, D.K.; Ali, M.; Jung, J. WS2-embedded MXene/GO hybrid nanosheets as electrodes for asymmetric supercapacitors and hydrogen evolution reactions. Chem. Eng. J. 2023, 452, 139523. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Gong, T.; Liang, C.; Li, L.; Lin, X.; Ying, Z.; Liu, H. One-step hydrothermal preparation of a novel 2D MXene-based composite electrode material synergistically modified by CuS and carbon dots for supercapacitors. J. Alloys Compd. 2023, 947, 169400. [Google Scholar] [CrossRef]
- Chen, X.; Cai, J.; Qiu, C.; Liu, W.; Xia, Y. High-performance solid-state asymmetric supercapacitor based on Ti3C2Tx MXene/VS2 cathode and Fe3O4@rGO hydrogel anode. Electrochim. Acta 2023, 438, 141572. [Google Scholar] [CrossRef]
- De, S.; Maity, C.K.; Kim, M.J.; Nayak, G.C. Tin(IV) selenide anchored-biowaste derived porous carbon-Ti3C2Tx (MXene) nanohybrid: An ionic electrolyte enhanced high performing flexible supercapacitor electrode. Electrochim. Acta 2023, 463, 142811. [Google Scholar] [CrossRef]
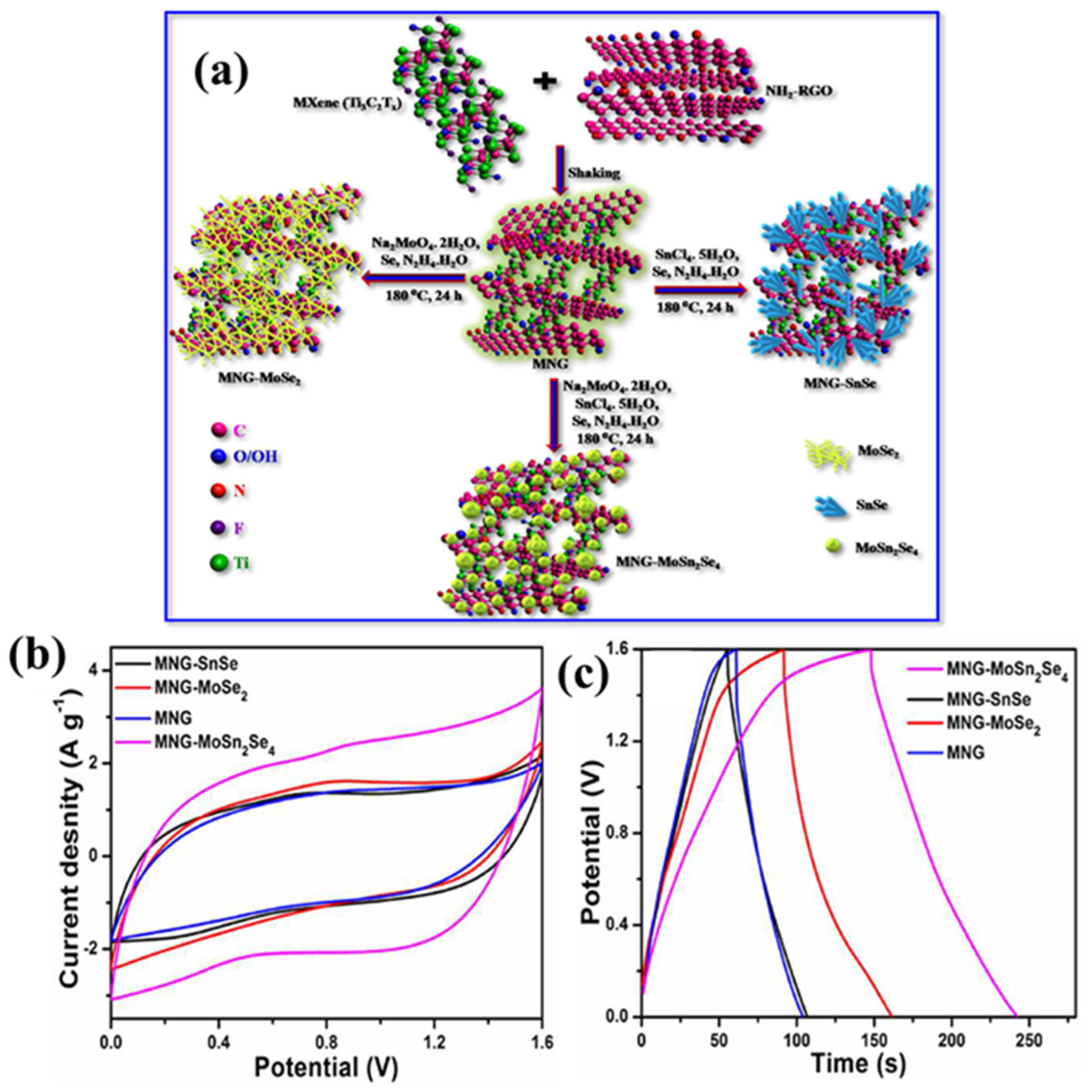
- De, S.; Roy, S.; Nayak, G.C. MoSn2Se4-decorated MXene/functionalized RGO nanohybrid for ultrastable supercapacitor and oxygen evolution catalyst. Mater. Today Nano 2023, 22, 100337. [Google Scholar] [CrossRef]
- Adil, M.; Olabi, A.G.; Abdelkareem, M.A.; Alawadhi, H.; Bahaa, A.; ElSaid, K.; Rodriguez, C. In-situ grown metal-organic framework derived CoS-MXene pseudocapacitive asymmetric supercapacitors. J. Energy Storage 2023, 60, 106537. [Google Scholar] [CrossRef]
- Chetana, S.; Upadhyay, S.; Joshi, N.C.; Kumar, N.; Choudhary, P.; Sharma, N.; Thakur, V.N. A facile supercritical fluid synthesis of cobalt sulfide integrated with MXene and PANI/PEDOT nanocomposites as electrode material for supercapacitor applications. FlatChem 2023, 37, 100456. [Google Scholar]
- Zhang, M.; Shahnavaz, Z.; Yan, X.; Huang, X.; Wu, S.; Zhang, W.; Dou, A.; Pan, J.; Li, T.; Yu, X. Mild synthesis of flower-like CuS modified MXene nanocomposites for high-performance supercapacitors and efficient microwave absorption. J. Alloys Compd. 2023, 960, 170914. [Google Scholar] [CrossRef]
- Chen, X.; Ge, H.; Yang, W.; Liu, J.; Yang, P. Construction of high-performance solid-state asymmetric supercapacitor based on Ti3C2Tx MXene/CuS positive electrode and Fe2O3@rGO negative electrode. J. Energy Storage 2023, 68, 107700. [Google Scholar] [CrossRef]
- Sun, P.; Liu, J.; Liu, Q.; Yu, J.; Chen, R.; Zhu, J.; Sun, G.; Li, Y.; Liu, P.; Wang, J. An inkjet-printing ink based on porous NiS/N-MXene for high-performance asymmetric micro-supercapacitors and self-powered microelectronics. Chem. Eng. J. 2023, 474, 145466. [Google Scholar] [CrossRef]
- Arulkumar, C.; Gandhi, R.; Vadivel, S. Ultra-thin nanosheets of Ti3C2Tx MXene/MoSe2 nanocomposite electrode for asymmetric supercapacitor and electrocatalytic water splitting. Electrochim. Acta 2023, 462, 142742. [Google Scholar] [CrossRef]
- Kim, M.C.; Saeed, G.; Alam, A.; Choi, Y.; Zhang, L.; Lee, D.; Kwon, S.H.; Mathur, S.; Kim, K.H. Ultrafine nanoparticles of tin-cobalt-sulfide decorated over 2D MXene sheets as a cathode material for high-performance asymmetric supercapacitor. J. Ind. Eng. Chem. 2023, 124, 294–303. [Google Scholar]
- Qiao, Y.; Sun, W.; Yu, F.; Yu, J.; Yao, P.; Zhu, C.; Xu, J. Exploration of high performance and highly flexible supercapacitor configuration with MXene/1T-MoS2 composite paper electrode. Electrochim. Acta 2023, 464, 142929. [Google Scholar] [CrossRef]
- Ranjith, K.S.; Lee, S.Y.; Ghoreishian, S.M.; Chodankar, N.R.; Raju, G.S.R.; Patil, S.J.; Huh, Y.S.; Park, S.J.; Han, Y.K. Defect and interface engineering of MXene-tagged N,F-doped carbon-CoSe2 heterostructure for superior hydrogen evolution reactions and supercapacitors. Carbon 2023, 206, 246–259. [Google Scholar] [CrossRef]
- Jiao, L.; Zhao, M.; Su, Z.; Shi, M.; Li, M.; Li, F. ZIF-67 derived high stability CoNi2S4/carbon/MXene composites as an enhanced electrode for asymmetric supercapacitor. Electrochim. Acta 2024, 479, 143888. [Google Scholar] [CrossRef]
- Hayat, H.P.; Kılıç Dokan, F.; Onses, M.S.; Yılmaz, E.; Duran, A.; Sahmetlioglu, E. Flexible electrodes composed of flower-like MoS2 and MXene for supercapacitor applications. Mater. Res. Bull. 2024, 175, 112747. [Google Scholar] [CrossRef]
- Liang, X.; Chen, Y.; Jiao, Z.; Demir, M.; Du, M.; Han, J. MXene-transition metal sulfide composite electrodes for supercapacitors: Synthesis and electrochemical characterization. J. Energy Storage 2024, 88, 111634. [Google Scholar] [CrossRef]
- Kumar, S.; Kaliamurthy, A.K.; Lee, Y.; Lee, S. High-performance positive electrode material of MXene/FeNi2S4 nanocomposite for flexible supercapacitor with large potential window. J. Energy Storage 2024, 95, 112643. [Google Scholar] [CrossRef]
- Chen, J.; Song, T.; Sun, M.; Qi, S.; Chen, C.; Zhao, Y.; Wu, X. Cobalt nickel selenide with MXene and graphene dual support system to enhance electrochemical activity and stability for supercapacitors. J. Alloys Compd. 2024, 1009, 176786. [Google Scholar] [CrossRef]
- Dey, N.; Yadav, A.; Ray, S.K.; Guha, P.K. Printed MXene-NiSe asymmetric micro-supercapacitors for flexible energy storage devices. J. Energy Storage 2024, 98, 112831. [Google Scholar] [CrossRef]
- Pınar, P.T.; Gülcan, M.; Yardım, Y. Synthesis and characterization of a nanocomposite consisting of Ti3C2Tx (MXene) and WS2 nanosheets for potential use in supercapacitors. J. Alloys Compd. 2025, 1010, 177656. [Google Scholar] [CrossRef]
- Zheng, W.; Yang, Y.; Fan, L.; Ye, D.; Xu, W.; Xu, J. Ultralight PPy@PVA/BC/MXene composite aerogels for high-performance supercapacitor eltrodes and pressure sensors. Appl. Surf. Sci. 2023, 624, 157138. [Google Scholar] [CrossRef]
- Lin, Z.; Li, X.; Li, S.; Li, B.; Ding, J.; Han, Y.; Li, T.; Hou, C.; Ma, Y. Highly flexible, foldable carbon cloth/MXene/polyaniline/CoNi layered double hydroxide electrode for high-performance all solid-state supercapacitors. J. Energy Storage 2023, 64, 107116. [Google Scholar] [CrossRef]
- Luo, W.; Sun, Y.; Han, Y.; Ding, J.; Li, T.; Hou, C.; Ma, Y. Flexible Ti3C2Tx MXene/polypyrrole composite films for high-performance all-solid asymmetric supercapacitors. Electrochim. Acta 2023, 441, 141818. [Google Scholar] [CrossRef]
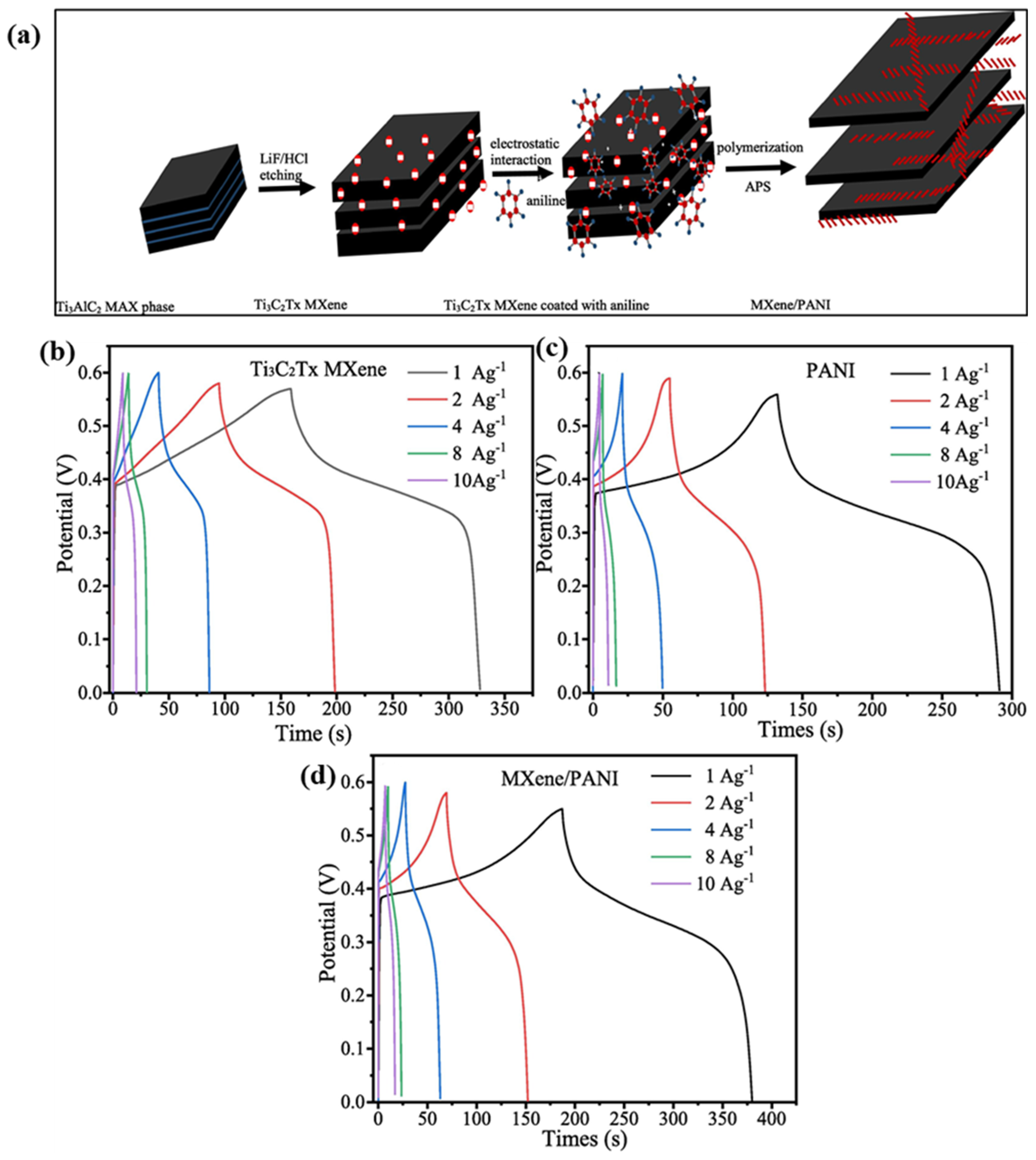
- Yuan, T.; Zhang, Z.; Liu, Q.; Liu, X.-T.; Miao, Y.-N.; Yao, C. MXene (Ti3C2Tx)/cellulose nanofiber/polyaniline film as a highly conductive and flexible electrode material for supercapacitors. Carbohydr. Polym. 2023, 304, 120519. [Google Scholar] [CrossRef]
- Chen, T.; Li, M.; Li, Y.; Song, S.; Kim, J.; Bae, J. Synthesis of hierarchical structure MXene/PANI composite hybrid electrodes for supercapacitors. Mater. Sci. Eng. B 2023, 290, 116354. [Google Scholar] [CrossRef]
- Pathak, I.; Acharya, D.; Chhetri, K.; Lohani, P.C.; Ko, T.H.; Muthurasu, A.; Subedi, S.; Kim, T.; Saidin, S.; Dahal, B.; et al. Ti3C2Tx MXene integrated hollow carbon nanofibers with polypyrrole layers for MOF-derived freestanding electrodes of flexible asymmetric supercapacitors. Chem. Eng. J. 2023, 469, 143388. [Google Scholar] [CrossRef]
- Wang, S.; Guo, X.; Liao, S.; Chen, J.; Wei, Q. Aramid nanofiber-reinforced MXene/PEDOT:PSS hybrid fibers for high-performance fiber-shaped supercapacitors. Electrochim. Acta 2023, 466, 143062. [Google Scholar] [CrossRef]
- Xie, H.; Ma, S.; He, Z. Facile preparation of PANI/MoOx nanowires decorated MXene film electrodes for electrochemical supercapacitors. Electrochim. Acta 2023, 448, 142173. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, P.; Ding, B.; Tang, J.; Hong, X.; Liu, Z.; Xia, W.; Zhou, X.; Wang, Z.; Zou, L.; et al. Coaxial-helix MXene/PANI-based core-spun yarn towards strain-insensitive conductor and supercapacitor. Mater. Today Commun. 2023, 36, 106788. [Google Scholar] [CrossRef]
- Bai, W.; Yong, Z.; Wang, S.; Wang, X.; Li, C.; Pan, F.; Liang, D.; Cui, Y.; Wang, Z. Polyaniline-MXene composite electrode with excellent electrochemical properties for all-solid flexible supercapacitors. J. Energy Storage 2023, 71, 108053. [Google Scholar] [CrossRef]
- Fei, H.; Joseph, N.; Vargun, E.; Zandraa, O.; Omastová, M.; Sáha, P. Fabrication and floating test of an asymmetric supercapacitor based on polyaniline and MXene. Synth. Met. 2023, 300, 117490. [Google Scholar] [CrossRef]
- Mohammadi, S.; Ahmadi, S.; Navid, H.; Azadvari, R.; Ghafari, M.; Sanaee, Z.; Moeini, M. High-capacity freestanding supercapacitor electrode based on electrospun Ti3C2Tx MXene/PANI/PVDF composite. Heliyon 2024, 10, e40482. [Google Scholar] [CrossRef]
- Lima, J.V.M.; Lemos, H.G.; Silva, R.A.; Rossato, J.H.H.; Boratto, M.H.; Graeff, C.F.O. Tailoring interlayer spacing of Ti3C2Tx MXene with organic acid doped polyaniline and polypyrrole for more stable supercapacitors. J. Alloys Compd. Commun. 2024, 3, 100007. [Google Scholar] [CrossRef]
- Bai, W.; Ma, G.; Zhou, X.; Gao, T.; Luo, Y.; Yu, D.; Wang, S. Highly stable MXene/g-PPy@sulfonated cellulose composite electrodes for flexible supercapacitors. J. Energy Storage 2024, 89, 111743. [Google Scholar] [CrossRef]
- Khan, N.; Ullah, R.; Khan, M. Enhanced electrochemical performances of ternary polypyrrole/MXene/Gum arabic composites as supercapacitors electrode materials. Electrochim. Acta 2024, 505, 144988. [Google Scholar] [CrossRef]
- Han, L.; Li, Y.; Chen, C.; Liu, L.; Lu, Z. Multifunctional enhanced energy density of flexible wide-temperature supercapacitors based on MXene/PANI conductive hydrogel. Chem. Eng. J. 2024, 485, 149951. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, K.; Liu, X.; Yao, S.; Li, X.; Si, Y.; Li, X. 3D porous PEDOT/MXene scaffold toward high-performance supercapacitors. Chem. Eng. J. 2024, 496, 154348. [Google Scholar] [CrossRef]
- Ma, G.; Bai, W.; Zhou, X.; Guan, X.; Zhang, S.; Wu, W.; Li, C.; Wang, S. Sulfonated polyaniline/MXene composite electrode with high cycling stability for anti-freezing flexible supercapacitor. Chem. Eng. J. 2024, 496, 153730. [Google Scholar] [CrossRef]
- Bejjanki, D.; Puttapati, S.K. PANI/WO3/MXene nanocomposite material for high-performance solid state symmetric supercapacitor. Diam. Relat. Mater. 2024, 146, 111248. [Google Scholar] [CrossRef]
- He, T.; Li, X.; Sun, B.; Lin, L.; Guo, F.; Diao, G.; Piao, Y.; Zhang, W. Preparation of cyclodextrin polymer-functionalized polyaniline/MXene composites for high-performance supercapacitor. RSC Adv. 2024, 14, 13685–13693. [Google Scholar] [CrossRef]
- Varghese, S.M.; Mohan, V.V.; Suresh, S.; Gowd, E.B.; Rakhi, R.B. Synergistically modified Ti3C2Tx MXene conducting polymer nanocomposites as efficient electrode materials for supercapacitors. J. Alloys Compd. 2024, 973, 172923. [Google Scholar] [CrossRef]
- Li, Z.; Li, J.; Wu, B.; Wei, H.; Guo, H.; El-Bahy, Z.M.; Liu, B.; He, M.; Melhi, S.; Shi, X.; et al. Interfacial-engineered robust and high performance flexible polylactic acid/polyaniline/MXene electrodes for high-perfarmance supercapacitors. J. Mater. Sci. Technol. 2024, 203, 201–210. [Google Scholar] [CrossRef]
- Wang, G.; Jiang, N.; Xu, Y.; Zhang, Z.; Wang, G.; Cheng, K. Solvent-assisted assembly of reduced graphene oxide/MXene-polypyrrole composite film for flexible supercapacitors. J. Colloid Interface Sci. 2023, 630, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Olatoye, A.G.; Li, W.; Fagbohun, E.O.; Zeng, X.; Zheng, Y.; Cui, Y. High-performance asymmetric supercapacitor based on nickel-MOF anchored MXene//NPC/rGO. J. Electroanal. Chem. 2023, 928, 117036. [Google Scholar] [CrossRef]
- An, N.; Li, W.; Shao, Z.; Zhou, L.; He, Y.; Sun, D.; Dong, X.; Hu, Z. Graphene oxide coated polyaminoanthraquinone@MXene based flexible film electrode for high-performance supercapacitor. J. Energy Storage 2023, 57, 106180. [Google Scholar] [CrossRef]
- Liu, Z.; Xue, F.; Zhao, X.; Chen, Z.; Zheng, H.; Ding, R.; Li, P.; Xu, L.; Xiong, J.; Peng, Q.; et al. Anti-stacking synthesis of MXene-reduced graphene oxide sponges for aqueous zinc-ion hybrid supercapacitor with improved performance. J. Mater. Sci. Technol. 2023, 154, 22–29. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Q.; Liu, L.; Wang, J.; Zhang, L.; Shi, M.; Chen, X. Ultra-stable sandwich shaped flexible MXene/CNT@Ni films for high performance supercapacitor. J. Alloys Compd. 2023, 941, 168963. [Google Scholar] [CrossRef]
- Murugan, N.; Thangarasu, S.; Venkatachalam, P.; Bhosale, M.; Kamalakannan, S.; Prakash, M.; Seo, S.B.; Choi, Y.R.; Kang, M.; Oh, T.H.; et al. Holey carbon-nanotube-wrapped MXene for hydrogen evolution reactions and supercapacitor applications. Int. J. Hydrogen Energy 2023, 48, 38584–38601. [Google Scholar] [CrossRef]
- Jorn-am, T.; Pholauyphon, W.; Supchocksoonthorn, P.; Sirisit, N.; Chanthad, C.; Manyam, J.; Liang, X.; Song, S.; Paoprasert, P. High-performance supercapacitors using synergistic hierarchical Ni-doped copper compounds/activated carbon composites with MXenes and carbon dots as simultaneous performance enhancers. Electrochim. Acta 2023, 447, 142147. [Google Scholar] [CrossRef]
- Nasrin, K.; Arunkumar, M.; Kumar, N.K.; Sudharshan, V.; Rajasekar, S.; Mukhilan, D.; Arshad, M.; Sathish, M. A rationally designed hetero-assembly of 2D/2D Nitrogen-doped MXene/Graphene via supercritical fluid processing for high energy durable supercapacitors. Chem. Eng. J. 2023, 474, 145505. [Google Scholar] [CrossRef]
- Zhu, Y.; Ni, Z.; Gao, J.; Zhang, D.; Wang, S.; Zhao, J. Fabrication of flexible planar micro-supercapacitors using laser scribed graphene electrodes incorporated with MXene. J. Phys. Chem. Solids 2023, 183, 111619. [Google Scholar] [CrossRef]
- Lyu, S.; Chang, H.; Zhang, L.; Wang, S.; Li, S.; Lu, Y.; Li, S. High specific surface area MXene/SWCNT/cellulose nanofiber aerogel film as an electrode for flexible supercapacitors. Compos. Part B Eng. 2023, 264, 110888. [Google Scholar] [CrossRef]
- Shi, C.; Liu, Z.; Tian, Z.; Li, D.; Chen, Y.; Guo, L.; Wang, Y. Fabrication of 3D MXene@graphene hydrogel with high ion accessibility via Al-induced self-assembly and reduction for high-performance supercapacitors. Electrochim. Acta 2023, 464, 142892. [Google Scholar] [CrossRef]
- Luo, W.; Liu, Q.; Zhang, B.; Li, J.; Li, R.; Li, T.; Sun, Z.; Ma, Y. Binder-free flexible Ti3C2Tx MXene/reduced graphene oxide/carbon nanotubes film as electrode for asymmetric supercapacitor. Chem. Eng. J. 2023, 474, 145553. [Google Scholar] [CrossRef]
- Yao, W.; Zheng, D.; Li, Z.; Wang, Y.; Tan, H.; Zhang, Y. MXene@ carbonized wood monolithic electrode with hierarchical porous framework for high-performance supercapacitors. Appl. Surf. Sci. 2023, 638, 158130. [Google Scholar] [CrossRef]
- Nasrin, K.; Mukhilan, D.; Arshad, M.; Arunkumar, M.; Sathish, M. Intralayered ostwald ripening impelled nanoarchitectonics of MXene nanorods on MXene nanosheets with boron carbon nitride for robust supercapacitor application. Electrochim. Acta 2023, 469, 143251. [Google Scholar] [CrossRef]
- Dharmasiri, B.; Usman, K.A.S.; Qin, S.A.; Razal, J.M.; Tran, N.T.; Coia, P.; Harte, T.; Henderson, L.C. Ti3C2Tx MXene coated carbon fibre electrodes for high performance structural supercapacitors. Chem. Eng. J. 2023, 476, 146739. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, H.; Xie, Y.; Jiang, F.; Gao, X.; Bai, H.; Yao, F.; Yue, H. Vertically aligned graphene-MXene nanosheets based electrodes for high electrochemical performance asymmetric supercapacitor. Chem. Eng. J. 2024, 82, 149063. [Google Scholar] [CrossRef]
- Chen, J.; Feng, M.; Lian, X.; Zheng, F.; Xu, C.; Wang, K.; Niu, H. Rational design of MXene/MWCNT/TOCNF film for flexible supercapacitor. Ceram. Int. 2024, 50, 29895–29903. [Google Scholar] [CrossRef]
- Sun, L.; Chai, L.; Jing, L.; Chen, Y.; Zhuo, K.; Wang, J. 2,6-Diaminoanthraquinone modified MXene (Ti3C2Tx)/graphene as the negative electrode materials for ionic liquid-based asymmetric supercapacitors. Green Energy Environ. 2024, 10, 845–853. [Google Scholar] [CrossRef]
- Yang, S.; Fu, P.; Chen, Z.; Chen, Y.; Feng, Y.; Wu, J.; Lu, H.; Hou, C. MXene-reduced graphene oxide heterostructures as binder-free cathodes for zinc-ion hybrid supercapacitors with superior performance. J. Power Sources 2024, 623, 235466. [Google Scholar] [CrossRef]
- Alharbi, N. Cylindrical janus Mosse@CNT intercalated into Mxene: A high-performance supercapacitor electrode with high volumetric capacity and flexibility. J. Alloys Compd. 2024, 1008, 176273. [Google Scholar] [CrossRef]
- Jin, Y.; Geng, J.; Wang, Y.; Zhao, Z.; Chen, Z.; Guo, Z.; Pei, L.; Ren, F.; Sun, Z.; Ren, P.G. Flexible all-solid-state asymmetric supercapacitor based on Ti3C2Tx MXene/graphene/carbon nanotubes. Surf. Interfaces 2024, 53, 104999. [Google Scholar] [CrossRef]
- Rundla, A.; Kumar, B.; Tripathi, P.; Kumar, P.; Singh, K. Ti3C2Tx MXene@rGO composite electrodes for high-performance supercapacitor applications. J. Power Sources 2025, 632, 236408. [Google Scholar] [CrossRef]
- Liu, L.; Huang, L.; Yan, S.; Shi, W.; Li, S.; Chen, X.; Liu, J. Heteroatom doped S, N-MXene/rGO flexible film for supercapacitor applications. Chem. Eng. J. 2025, 506, 160320. [Google Scholar] [CrossRef]
- Liu, X.; Fu, Q.; Li, Z.; Wang, H.; Ren, J. Modulating surface electron density of hydrophilic/high-conductive MXene/Ni(OH)2/NF heterostructures for efficient asymmetric supercapacitors. Diam. Relat. Mater. 2023, 140, 110474. [Google Scholar] [CrossRef]
- Zhu, C.; Pei, C.; Park, H.S.; Yu, X. Design of 2D/2D heterostructure by coupling cobalt hydroxides with Mxene on nickel foam for high energy density supercapacitors. J. Alloys Compd. 2023, 948, 169809. [Google Scholar] [CrossRef]
- Huang, X.; Huang, Y.; Zhao, J.; Xu, G.; Wang, X. Facile design and synthesis of nickel foam@Mxene@NiCo layered hydroxides core-sheath nanostructure for high mass-loading of supercapacitors. Electrochim. Acta 2023, 461, 142657. [Google Scholar] [CrossRef]
- Kitchamsetti, N.; Kim, D. A facile method for synthesizing MOF derived ZnCo2O4 particles on MXene nanosheets as a novel anode material for high performance hybrid supercapacitors. Electrochim. Acta 2023, 441, 141824. [Google Scholar] [CrossRef]
- Ma, S.; Wang, W.; Che, X.; Ren, Q.; Li, Y.; Hou, C. Fabrication of nickel foam/MXene/CoAl-layered double hydroxide by electrodeposition as electrode material for high-performance asymmetric supercapacitor. Synth. Met. 2024, 305, 117613. [Google Scholar] [CrossRef]
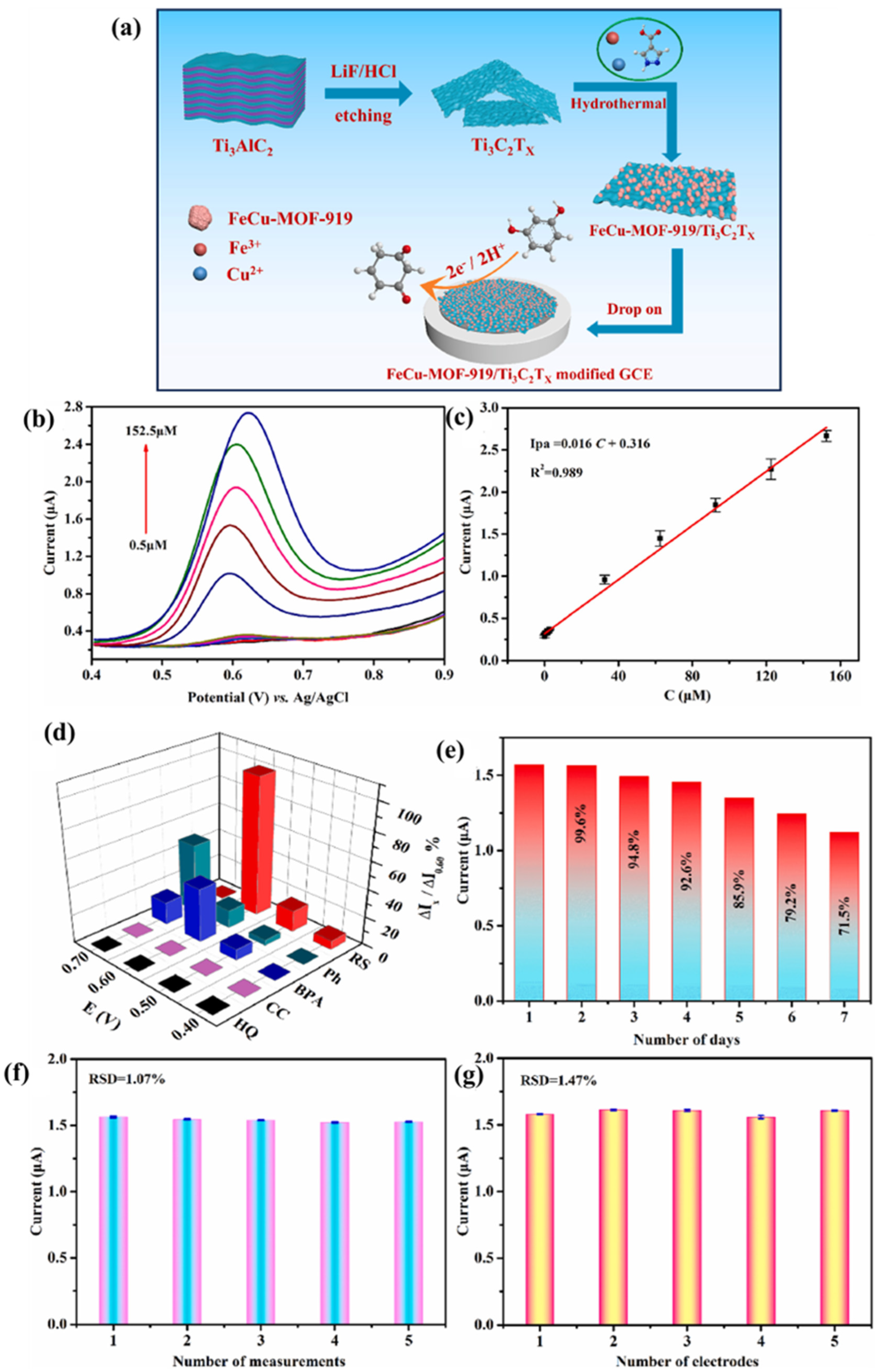
- Adil, M.; Olabi, A.G.; Abdelkareem, M.A.; Alawadhi, H.; Bahaa, A.; ElSaid, K.; Rodriguez, C. In-situ grown bimetallic FeCu MOF-MXene composite for solid-state asymmetric supercapacitors. J. Energy Storage 2023, 68, 107817. [Google Scholar] [CrossRef]
- Yue, L.; Chen, L.; Wang, X.; Lu, D.; Zhou, W.; Shen, D.; Yang, Q.; Xiao, S.; Li, Y. Ni/Co-MOF@aminated MXene hierarchical electrodes for high-stability supercapacitors. Chem. Eng. J. 2023, 451, 138687. [Google Scholar] [CrossRef]
- Xiang, G.T.; Chen, N.; Lu, B.; Xu, J.L.; Rodriguez, R.D.; Sheremet, E.; Hu, Y.D.; Chen, J.J. Flexible solid-state Zn-Co MOFs@MXene supercapacitors and organic ion hydrogel sensors for self-powered smart sensing applications. Nano Energy 2023, 118, 108936. [Google Scholar] [CrossRef]
- Ji, Y.; Li, W.; You, Y.; Xu, G. In situ synthesis of M (Fe, Cu, Co and Ni)-MOF@MXene composites for enhanced specific capacitance and cyclic stability in supercapacitor electrodes. Chem. Eng. J. 2024, 496, 154009. [Google Scholar] [CrossRef]
- Shivade, D.S.; Kurade, A.N.; Bhosale, R.K.; Kundale, S.S.; Shelake, A.R.; Patil, A.D.; Waifalkar, P.P.; Kamat, R.K.; Teli, A.M.; Dongale, T.D. Folic acid-assisted in situ solvothermal synthesis of Ni-MOF/MXene composite for high-performance supercapacitors. J. Energy Storage 2024, 100, 113754. [Google Scholar] [CrossRef]
- Shalini, S.S.; Fu, Y.-P.; Bose, A.C. Synergetic Interplay of MnNi-MOF Composite with 2D MXene for Improved Supercapacitor Application. Chem. Eng. J. 2024, 500, 156751. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Wang, Y.; Wan, S.; Pei, D.; Li, K.; Lu, H. High-performance supercapacitor materials based on NiMn-LDH layered structures with MXene layers. J. Alloys Compd. 2024, 1008, 176785. [Google Scholar] [CrossRef]
- Lan, Y.; Wang, S.; Yuan, Z.; Zhao, W. NiCo layered double hydroxide anchored on MXene/tannin carbon cryogel composite with 3D hierarchical sea urchin-like structure for asymmetric supercapacitors. Chem. Eng. J. 2024, 501, 157471. [Google Scholar] [CrossRef]
- Jiang, R.; Ding, L.; Li, S.; Zhang, Q.; Zhao, Z.; Zhou, C.; Cheng, Z.; Mario, G.; Wang, Q.; Chen, H.; et al. In-situ growth of FeCo layered double hydroxide nanorods on MXene for high-performance supercapacitor electrodes. J. Energy Storage 2024, 102, 114126. [Google Scholar] [CrossRef]
- Chen, W.; Li, Z.; Yang, K.; Zhang, D.; Luo, M.; Ling, Y.; Liu, C.; Zhou, X. Low-temperature carbonized MXene/protein-based eggshell membrane composite as free-standing electrode for flexible supercapacitors. Int. J. Biol. Macromol. 2023, 226, 588–596. [Google Scholar] [CrossRef]
- Falak, U.; Rasheed, A.; Rasheed, T.; Dastgeer, G.; Lee, S.G.; Ajmal, S. Synthesis of stable titanium carbide MXene nanocomposites using crosslinker nanoparticles for flexible supercapacitors: A novel and cost-effective approach. Ceram. Int. 2023, 49, 21026–21035. [Google Scholar] [CrossRef]
- Yesilbag, F.N.T.; Yesilbag, Y.O.; Huseyin, A.; Salih, A.J.S.; Ertugrul, M. Rational design of MXene/Boron Carbon Nitride nanotube composite electrode for supercapacitors with improved electrochemical properties. J. Energy Storage 2023, 72, 108446. [Google Scholar] [CrossRef]
- Beknalkar, S.A.; Teli, A.M.; Khot, A.C.; Dongale, T.D.; Yewale, M.A.; Nirmal, K.A.; Shin, J.C. A new path to high-performance supercapacitors: Utilizing Ag-embedded CoFe-phosphate and Ti3C2 MXene as hybrid electrodes. J. Energy Storage 2023, 72, 108272. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, Y.; Wang, J.; Zhang, S.; Han, X.; Sun, X. Co-TCPP/MXene film electrodes and patterned interdigital electrodes with high performance for flexible all-solid supercapacitors. J. Energy Storage 2023, 70, 107973. [Google Scholar] [CrossRef]
- Pathak, I.; Dahal, B.; Acharya, D.; Chhetri, K.; Muthurasu, A.; Rosyara, Y.R.; Kim, T.; Saidin, S.; Ko, T.H.; Kim, H.Y. Integrating V-doped CoP on Ti3C2Tx MXene-incorporated hollow carbon nanofibers as a freestanding positrode and MOF-derived carbon nanotube negatrode for flexible supercapacitors. Chem. Eng. J. 2023, 475, 146351. [Google Scholar] [CrossRef]
- Das, P.; Mondal, T.K.; Bera, S.; Das, S.; Hsu, H.-L.; Su, Y.-K.; Saha, S.K. Facile in-situ growth of spore-like silica on layered MXene sheets for potential application in Supercapacitor. Electrochim. Acta 2023, 465, 142983. [Google Scholar] [CrossRef]
- Chu, D.; Qu, X.; Zhang, S.; Liu, Z.; Wang, J.; Zhou, L.; Fu, B.; Jin, H.; Yang, Y. Polyoxometalate/MXene hybrid film with a 3D porous structure for high-performance electrochromic supercapacitors. Mater. Today Chem. 2023, 30, 101561. [Google Scholar] [CrossRef]
- Baasanjav, E.; Raj, K.A.S.; Hakkeem, H.; Rout, C.S.; Jeong, S.M. High-performance asymmetric supercapacitors based on 2D MXene/NiCoP hybrid and ZIF derived porous nanocarbon. J. Mater. Sci. Technol. 2025, 228, 42–53. [Google Scholar] [CrossRef]
- Myndrul, V.; Coy, E.; Babayevska, N.; Zahorodna, V.; Balitskyi, V.; Baginskiy, I.; Gogotsi, O.; Bechelany, M.; Giardi, M.T.; Iatsunskyi, I. MXene nanoflakes decorating ZnO tetrapods for enhanced performance of skin-attachable stretchable enzymatic electrochemical glucose sensor. Biosens. Bioelectron. 2022, 207, 114141. [Google Scholar] [CrossRef]
- Thukkaram, M.P.; Chakravorty, A.; Mini, A.A.; Ramesh, K.; Grace, A.N.; Raghavan, V. Titanium carbide MXene and V2O5 composite-based electrochemical sensor for detection of bisphenol A. Microchem. J. 2023, 193, 109004. [Google Scholar] [CrossRef]
- Wu, P.; Zhao, X.; Ding, Y.; Huang, Y.; Lin, D.; Xie, L.; Li, Z.; Zhu, Z.; Zhao, H.; Lan, M. Self-assembled multilayer Nb2C MXene/MnFe2O4 electrochemical sensor with Schottky junctions for the detection of acetaminophen and dopamine. Colloids Surf. A Physicochem. Eng. Asp. 2023, 667, 131377. [Google Scholar] [CrossRef]
- Alanazi, N.; Selvi Gopal, T.; Muthuramamoorthy, M.; Alobaidi, A.A.E.; Alsaigh, R.A.; Aldosary, M.H.; Pandiaraj, S.; Almutairi, M.; Grace, A.N.; Alodhayb, A. Cu2O/MXene/rGO ternary nanocomposites as sensing electrodes for nonenzymatic glucose sensors. ACS Appl. Nano Mater. 2023, 6, 12271–12281. [Google Scholar] [CrossRef]
- Selvi Gopal, T.; James, J.T.; Gunaseelan, B.; Ramesh, K.; Raghavan, V.; Malathi, A.C.J.; Amarnath, K.; Kumar, V.G.; Rajasekaran, S.J.; Pandiaraj, S.; et al. MXene-embedded porous carbon-based Cu2O nanocomposites for non-enzymatic glucose sensors. ACS Omega 2024, 9, 8448–8456. [Google Scholar] [CrossRef]
- Prasanna, S.B.; Jagtap, A.A.; Kumar, G.S.; Ningappa, K.S.; Lin, Y.C.; Lu, Y.C.; Sakthivel, R.; Liu, T.Y.; Chung, R.J. A developed highly selective and sensitive electrochemical sensor using gold nanoparticles on Cerium Stannate/MXene as an electrode matrix for detection of metol in various environmental and biological samples. Electrochim. Acta 2024, 499, 144703. [Google Scholar] [CrossRef]
- Baskaran, N.; Prasanna, S.B.; Jeyaram, K.; Lin, Y.C.; Govindasamy, M.; Wei, Y.; Chung, R.J. 2D sheet structure of zinc molybdate decorated on MXene for highly selective and sensitive electrochemical detection of the arsenic drug Roxarsone in water samples. Chemosphere 2024, 364, 143188. [Google Scholar] [CrossRef]
- Guo, W.; Chen, Z.; Zhao, Y.; Zhao, C.; Lu, X.; Gao, F. Hierarchical Cu3V2O8/Cu6Mo5O18/MXene nanostructures by ultrathin nanosheets for highly sensitive electrochemical detection of organophosphorus pesticides. Microchem. J. 2024, 201, 110601. [Google Scholar] [CrossRef]
- Ganjeh, A.A.; Arvand, M.; Habibi, M.F. Electrostatically self-assembled magnetized MXene/copper ferrite nanospheres hybrids: Evaluation of molecularly imprinted electrochemical sensor for the quercetin antioxidant supplement. Microchem. J. 2024, 207, 112026. [Google Scholar] [CrossRef]
- Sundaresan, P.; Lee, T.Y. Electrochemical sensor for selective diquat detection based on samarium-stannate-nanoparticle-anchored titanium aluminum carbide MXene nanocomposites. Food Chem. 2025, 477, 143487. [Google Scholar] [CrossRef]
- Baskaran, N.; Sakthivel, R.; Karthik, C.S.; Lin, Y.C.; Liu, X.; Wen, H.W.; Yang, W.; Chung, R.J. Polydopamine-modified 3D flower-like ZnMoO4 integrated MXene-based label-free electrochemical immunosensor for the food-borne pathogen Listeria monocytogenes detection in milk and seafood. Talanta 2025, 282, 127008. [Google Scholar] [CrossRef]
- Zhou, K.; Li, Y.; Zhuang, S.; Ren, J.; Tang, F.; Mu, J.; Wang, P. A novel electrochemical sensor based on CuO-CeO2/MXene nanocomposite for quantitative and continuous detection of H2O2. J. Electroanal. Chem. 2022, 921, 116655. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Liu, X.; Liu, Y.; Cheng, Y.; Cui, D.; Chen, F.; Cao, W. A MXene/MoS2 heterostructure based biosensor for accurate sweat ascorbic acid detection. FlatChem 2023, 39, 100503. [Google Scholar] [CrossRef]
- Zhao, J.; He, C.; Wu, W.; Yang, H.; Peng, L.; Wen, L.; Hu, Z.; Hou, C.; Huo, D. MXene- MoS2 carbon-fiber-based flexible electrochemical interface for multiple bioanalysis in biofluids. Chem. Eng. Sci. 2022, 446, 136841. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, Y.; Qian, L.; Yin, Y.; Yuan, Z.; Dai, Y.; Zhang, T.; Yang, D.; Qiu, F. Lamellar Ti3C2 MXene composite decorated with platinum-doped MoS2 nanosheets as electrochemical sensing functional platform for highly sensitive analysis of organophosphorus pesticides. Food Chem. 2024, 459, 140379. [Google Scholar] [CrossRef]
- Gopi, P.K.; Sanjayan, C.G.; Akhil, S.; Ravikumar, C.H.; Thitamadee, S.; Kongpatanakul, S.; Balakrishna, R.G.; Surareungchai, W. Silver bismuth sulphide (AgBiS2)-MXene composite as high-performance electrochemical sensing platform for sensitive detection of pollutant 4-nitrophenol. Electrochim. Acta 2024, 498, 144616. [Google Scholar] [CrossRef]
- Mashhadizadeh, M.H.; Ghalkhani, M.; Sohouli, E. Synthesis and characterization of N-MPG/CuS flower-like/MXene to modify a screen-printed carbon electrode for electrochemical determination of nalbuphine. J. Electroanal. Chem. 2024, 957, 118130. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, X.; Zhang, J.; Guo, X.; Xu, Y.; Chen, L. Ti3C2 MXene/MoS2@AuNPs ternary nanocomposite for highly sensitive electrochemical detection of phoxim residues in fruits. Food Chem. 2025, 462, 140939. [Google Scholar] [CrossRef] [PubMed]
- Neampet, S.; Ruecha, N.; Qin, J.; Wonsawat, W.; Chailapakul, O.; Rodthongkum, N. A nanocomposite prepared from platinum particles, polyaniline and a Ti3C2 MXene for amperometric sensing of hydrogen peroxide and lactate. Microchim. Acta 2019, 186, 752. [Google Scholar] [CrossRef]
- Cheng, H.; Yang, J. Preparation of Ti3C2-PANI composite as sensor for electrochemical determination of mercury ions in water. Int. J. Electrochem. Sci. 2020, 15, 2295–2306. [Google Scholar] [CrossRef]
- Lu, Z.; Wei, K.; Ma, H.; Duan, R.; Sun, M.; Zou, P.; Yin, J.; Wang, X.; Wang, Y.; Wu, C.; et al. Bimetallic MOF synergy molecularly imprinted ratiometric electrochemical sensor based on MXene decorated with polythionine for ultra-sensitive sensing of catechol. Anal. Chim. Acta 2023, 1251, 340983. [Google Scholar] [CrossRef]
- Zhang, L.; Li, C.; Chen, Y.; Li, S.; Wang, X.; Li, F. A polyoxometalate/chitosan-Ti3C2Tx MXene nanocomposite constructed by electrostatically mediated strategy for electrochemical detecting L-tryptophan in milk. Food Chem. 2024, 458, 140309. [Google Scholar] [CrossRef] [PubMed]
- Saraswathi, K.A.; Reddy, M.S.B.; Jayarambabu, N.; Harish, C.M.; Rao, K.V.; Rao, T.V. NiO Embedded PANI/Ti3C2Tx MXene Detector for Electrochemical Enzyme Free Glucose Detection. Surf. Interfaces 2025, 56, 105728. [Google Scholar] [CrossRef]
- Khaleque, M.A.; Ali, M.R.; Aly, M.A.S.; Hossain, M.I.; Tan, K.H.; Zaed, M.A.; Saidur, R.; Rahman, M.M.; Mubarak, N.M.; Khan, M.Z.H. Highly sensitive electrochemical detection of ciprofloxacin using MXene (Ti3C2Tx)/poly (rutin) composite as an electrode material. Diam. Relat. Mater. 2024, 150, 111749. [Google Scholar] [CrossRef]
- Huang, R.; Liao, D.; Chen, S.; Yu, J.; Jiang, X. Self-assembled Ti3C2/MWCNTs nanocomposites modified glassy carbon electrode for electrochemical simultaneous detection of hydroquinone and catechol. Sens. Actuators B Chem. 2020, 320, 128386. [Google Scholar] [CrossRef]
- Nah, J.S.; Barman, S.C.; Zahed, M.A.; Sharifuzzaman, M.; Yoon, H.; Park, C.; Yoon, S.; Zhang, S.; Park, J.Y. A wearable microfluidics-integrated impedimetric immunosensor based on Ti3C2Tx MXene incorporated laser-burned graphene for noninvasive sweat cortisol detection. Sens. Actuators B Chem. 2021, 329, 129206. [Google Scholar] [CrossRef]
- Chen, S.; Xu, J.; Shi, M.; Yu, Y.; Xu, Q.; Duan, X.; Gao, Y.; Lu, L. Polydopamine bridged MXene and NH2-MWCNTs nanohybrid for high-performance electrochemical sensing of Acetaminophen. Appl. Surf. Sci. 2021, 570, 151149. [Google Scholar] [CrossRef]
- Zhang, X.; An, D.; Bi, Z.; Shan, W.; Zhu, B.; Zhou, L.; Yu, L.; Zhang, H.; Xia, S.; Qiu, M. Ti3C2-MXene@N-doped carbon heterostructure-based electrochemical sensor for simultaneous detection of heavy metals. J. Electroanal. Chem. 2022, 911, 116239. [Google Scholar] [CrossRef]
- Ni, M.; Chen, J.; Wang, C.; Wang, Y.; Huang, L.; Xiong, W.; Zhao, P.; Xie, Y.; Fei, J. A high-sensitive dopamine electrochemical sensor based on multilayer Ti3C2 MXene, graphitized multi-walled carbon nanotubes and ZnO nanospheres. Microchem. J. 2022, 178, 107410. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, P.; Liang, Y.; Ma, Y.; Liu, Y.; Zhao, J.; Hou, J.; Hou, C.; Huo, D. A sensitive electrochemical sensor based on 3D porous melamine-doped rGO/MXene composite aerogel for the detection of heavy metal ions in the environment. Talanta 2023, 256, 124294. [Google Scholar] [CrossRef]
- Qu, G.; Zhang, Y.; Zhou, J.; Tang, H.; Ji, W.; Yan, Z.; Pan, K.; Ning, P. Simultaneous electrochemical detection of dimethyl bisphenol A and bisphenol A using a novel Pt@SWCNTs-MXene-rGO modified screen-printed sensor. Chemosphere 2023, 337, 139315. [Google Scholar] [CrossRef]
- Dong, J.; Li, X.; Wen, L.; Ma, Y.; Xu, J.; Luo, S.; Hou, J.; Hou, C.; Huo, D. A novel electrochemical strategy based on MXene@rGO composite aerogel-doped UiO-66-NH2 for simultaneous detection of cadmium and lead in grain and water samples. Food Chem. 2024, 437, 137835. [Google Scholar] [CrossRef] [PubMed]
- Ankitha, M.; Shamsheera, F.; Rasheed, P.A. MXene-integrated single-stranded carbon yarn-based wearable sensor patch for on-site monitoring of dopamine. ACS Appl. Electron. Mater. 2024, 6, 599–610. [Google Scholar] [CrossRef]
- Hou, Y.; Yan, F.; Bai, X.; Wei, X.; Chen, L.; Fu, Y. Designing advanced 0D/2D heterojunctions of N, S-co-doped carbon dots/Ti3C2Tx MXene nanosheets in electrochemical sensor applications. Ceram. Int. 2024, 50, 10735–10745. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Chu, M.; Xin, J.; Liu, Y.; Pang, H.; Yang, G.; Ma, H. Construction of defect-rich bimetallic MOF loaded on N, S-codoped MXene QDs/rGO for electrochemical detection of catechol. Anal. Chim. Acta 2025, 1346, 343770. [Google Scholar] [CrossRef]
- Facure, M.H.M.; Sampaio, B.S.; Mercante, L.A.; Correa, D.S. The beneficial impact of MXene on the electrochemical performance of graphene quantum dots for dopamine detection. Mater. Today Commun. 2025, 42, 111197. [Google Scholar] [CrossRef]
- Mohammadi, A.; Ranjith, K.S.; Ezhil Vilian, A.T.; Lee, S.; Won, J.; Huh, Y.S.; Han, Y.K. Synergistic on engineering layered N-doped carbon/MXene heterostructure: A potential scaffold for simultaneous electrochemical detection of Cu2+ and Hg2+ ions. Sens. Actuators B Chem. 2025, 422, 136661. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Liu, Y.-Q.; Kou, X. Confinement synthesis of few-layer MXene-cobalt@N-doped carbon and its application for electrochemical sensing. Talanta 2025, 281, 126887. [Google Scholar] [CrossRef]
- Chen, Y.; Li, S.; Zhang, L.; Jing, T.; Wang, J.; Zhao, L.; Li, F.; Li, C.; Sun, J. Facile and fast synthesis of three-dimensional Ce-MOF/Ti3C2Tx MXene composite for high performance electrochemical sensing of L-Tryptophan. J. Solid State Chem. 2022, 308, 122919. [Google Scholar] [CrossRef]
- Xiao, P.; Zhu, G.; Shang, X.; Hu, B.; Zhang, B.; Tang, Z.; Yang, J.; Liu, J. An Fe-MOF/MXene-based ultra-sensitive electrochemical sensor for arsenic (III) measurement. J. Electroanal. Chem. 2022, 916, 116382. [Google Scholar] [CrossRef]
- Paul, J.; Kim, J. Reticular synthesis of a conductive composite derived from metal- organic framework and MXene for the electrochemical detection of dopamine. Appl. Surf. Sci. 2023, 613, 156103. [Google Scholar] [CrossRef]
- Zhang, L.; Han, Y.; Sun, M.; Li, F.; Li, S.; Gui, T. Facile design of FeCu metal-organic frameworks anchored on layer Ti3C2Tx MXene for high-performance electrochemical sensing of resorcinol. Talanta 2024, 275, 126100. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Chen, Y.; Li, Q.; Dang, X.; Chen, H. A novel ratiometric electrochemical sensing platform combined with molecularly imprinted polymer and Fe-MOF-NH2/CNTs-NH2/MXene composite for efficient detection of ofloxacin. Anal. Chim. Acta 2024, 1316, 342876. [Google Scholar] [CrossRef]
- Boruah, P.K.; Sharma, N.; Das, M.R.; Ohtani, R.; Le Ouay, B.; Ohba, M. Metal–organic framework/Nb4C3Tx MXene composites for ultrasensitive detection of dopamine. Chem. Commun. 2024, 60, 7307–7310. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Dong, F.; Rodas-Gonzalez, A.; Wang, G.; Yang, L.; Chen, S.; Zheng, H.-B.; Wang, S. Simultaneous detection of heavy metal ions in food samples using a hypersensitive electrochemical sensor based on APTES-incubated MXene-NH2@CeFe-MOF-NH2. Food Chem. 2025, 475, 143362. [Google Scholar] [CrossRef]
- Xu, Q.; Shi, L.; Kong, W.; Wang, J.; Xiao, P.; Liu, J. A novel bacillus-shaped CoNi ZIF-MXene@MWCNT carbon cloth electrochemical sensor for sensitive detection of salidroside. Microchem. J. 2024, 204, 111055. [Google Scholar] [CrossRef]
- Hou, S.; Wen, M.; Wei, J.; Li, L.; Liu, G. In-situ grown MXene@PDA/MOF composites constructed electrochemical sensor for detecting trace l-cysteine. J. Mol. Struct. 2025, 1321, 140076. [Google Scholar] [CrossRef]
- Liu, M.; Zhe, T.; Li, F.; Zhu, L.; Ouyang, S.; Wang, L. An ultrasensitive electrochemical sensor based on NiFe-LDH-MXene and ruthenium nanoparticles composite for detection of nitrofurantoin in food samples. Food Chem. 2024, 461, 140915. [Google Scholar] [CrossRef] [PubMed]
- Shahparast, S.; Asadpour-Zeynali, K. Development of a novel and highly sensitive electrochemical sensor based on FeCu-LDH@MXene nanocomposite for the selective determination of clonazepam. Microchem. J. 2025, 211, 113095. [Google Scholar] [CrossRef]
- Liu, J.; Fan, Y.; Chen, G.; Liu, Y. Highly sensitive glutamate biosensor based on platinum nanoparticles decorated MXene-Ti3C2Tx for L-glutamate determination in foodstuffs. LWT-Food Sci. Technol. 2021, 148, 111748. [Google Scholar] [CrossRef]
- Rasheed, P.A.; Pandey, R.P.; Jabbar, K.A.; Mahmoud, K.A. Platinum nanoparticles/Ti3C2Tx (MXene) composite for the effectual electrochemical sensing of Bisphenol A in aqueous media. J. Electroanal. Chem. 2021, 880, 114934. [Google Scholar] [CrossRef]
- Chandran, M.; Aswathy, E.; Shamna, I.; Vinoba, M.; Kottappara, R.; Bhagiyalakshmi, M. Laccase immobilized on Au confined MXene-based electrode for electrochemical detection of catechol. Mater. Today Proc. 2021, 46, 3136–3143. [Google Scholar] [CrossRef]
- Kumar, J.; Soomro, R.A.; Neiber, R.R.; Ahmed, N.; Medany, S.S.; Albaqami, M.D.; Nafady, A. Ni nanoparticles embedded Ti3C2Tx-MXene nanoarchitectures for electrochemical sensing of methylmalonic acid. Biosensors 2022, 12, 231. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Sun, J.; Ye, Y.; Ji, J.; Sheng, L.; Yang, D.; Sun, X. Metabolic pathway-based self-assembled Au@MXene liver microsome electrochemical biosensor for rapid screening of aflatoxin B1. Bioelectrochemistry 2023, 151, 108378. [Google Scholar] [CrossRef]
- Khoshfetrat, S.M.; Moradi, M.; Zhaleh, H.; Hosseini, M. Multifunctional methyl orange-delaminated Ti3C2 MXene for non-enzymatic/metal-free electrochemical detection of hydrogen peroxide and hydrazine. Microchem. J. 2024, 205, 111382. [Google Scholar] [CrossRef]
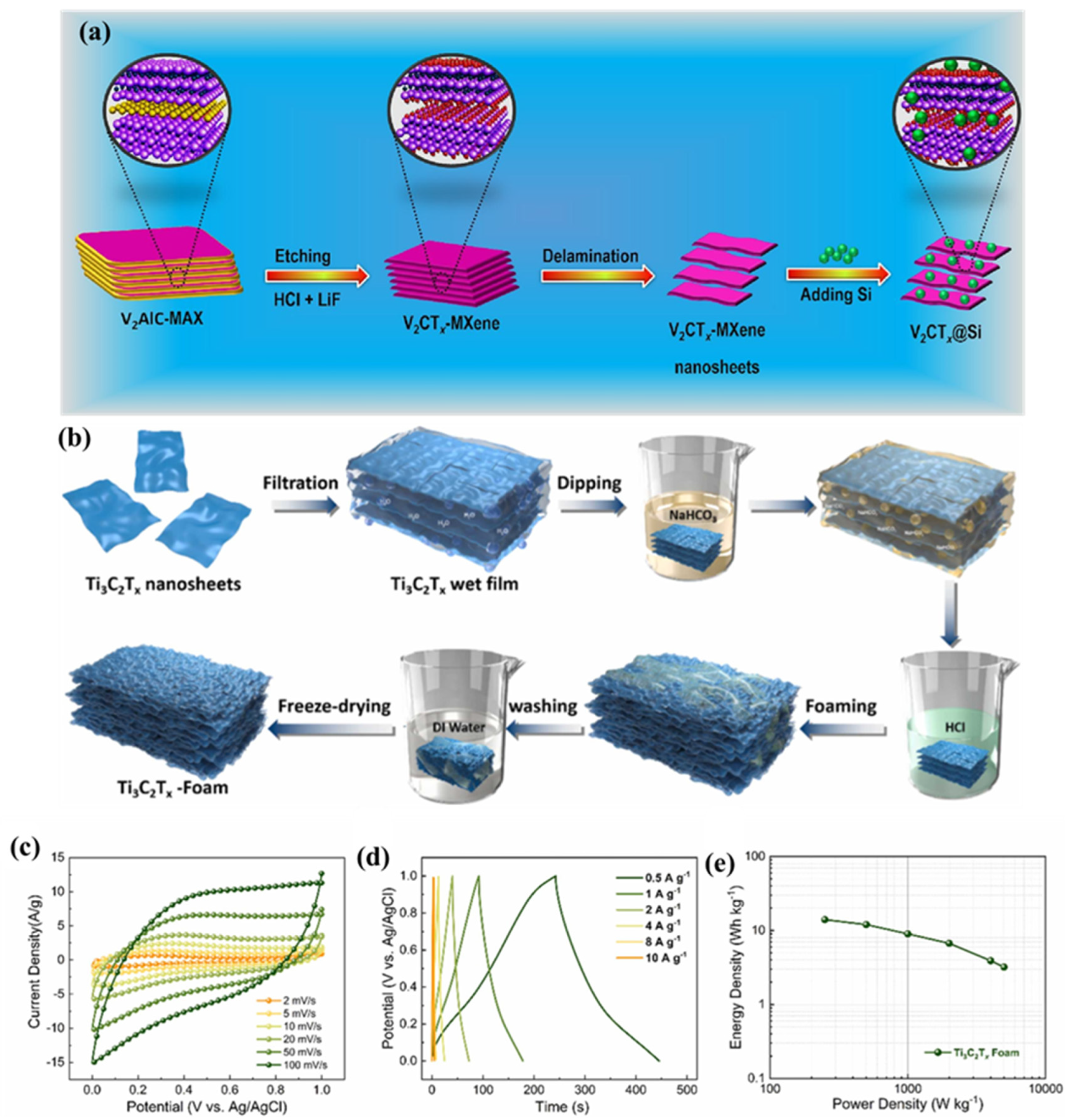
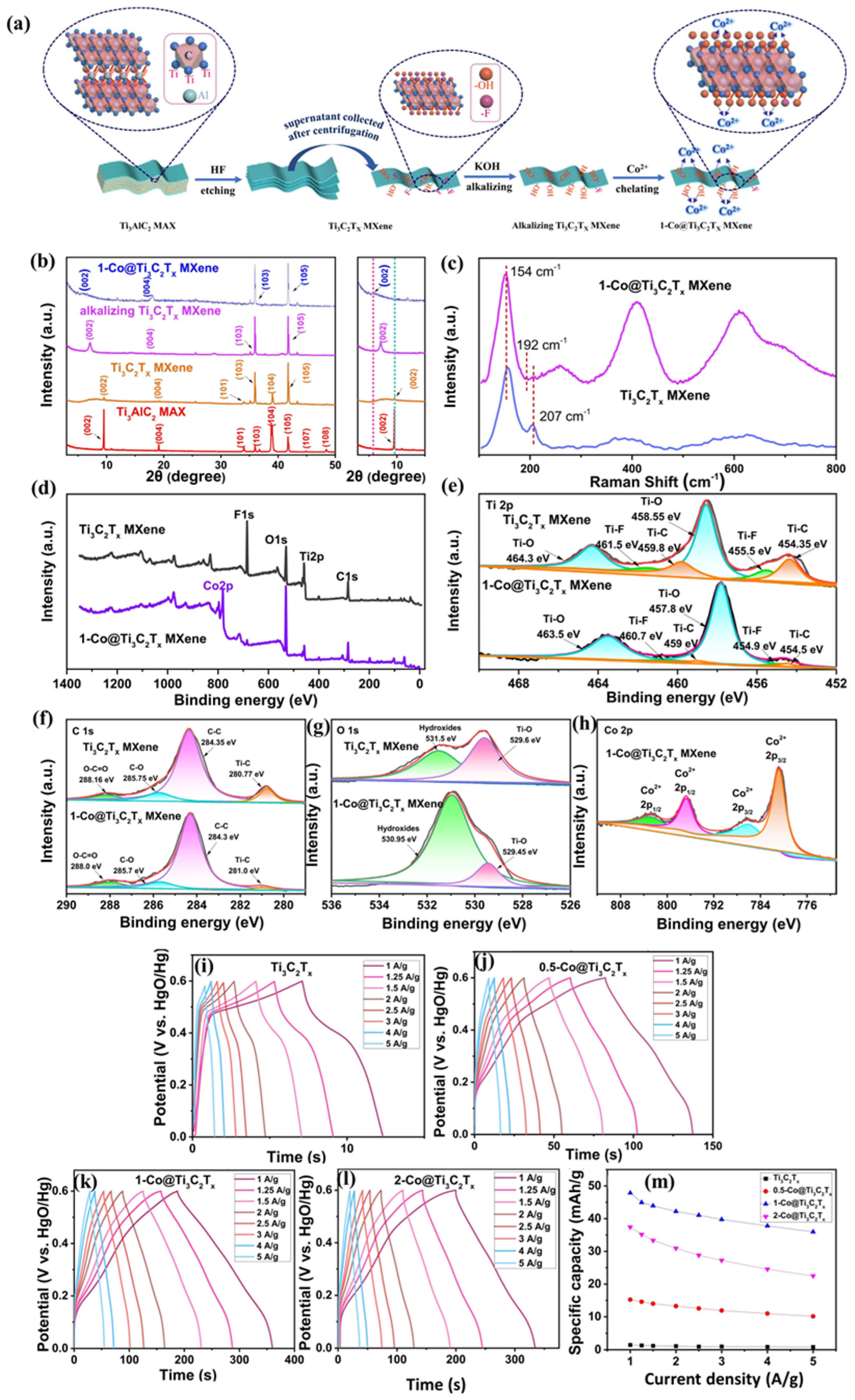

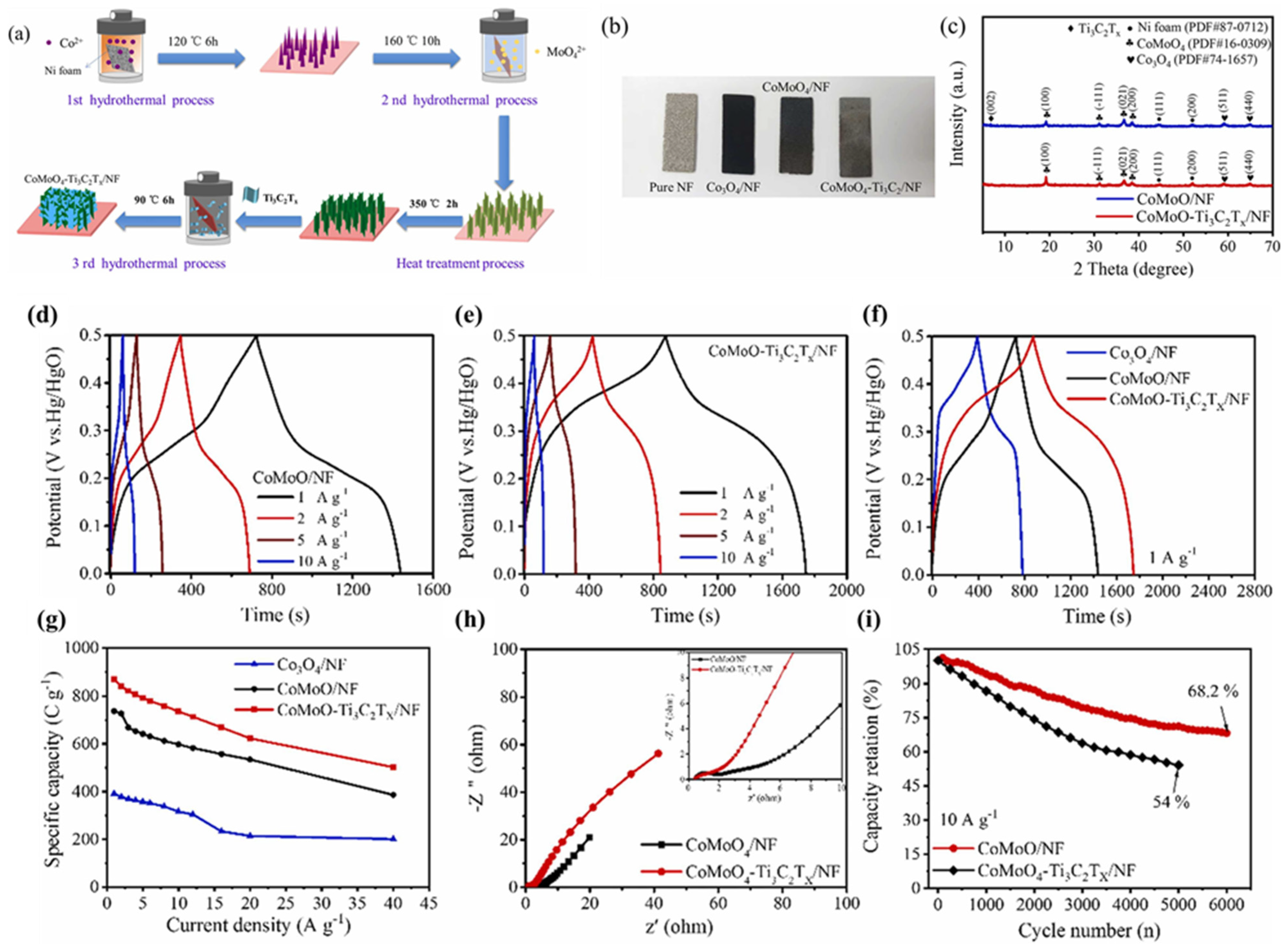
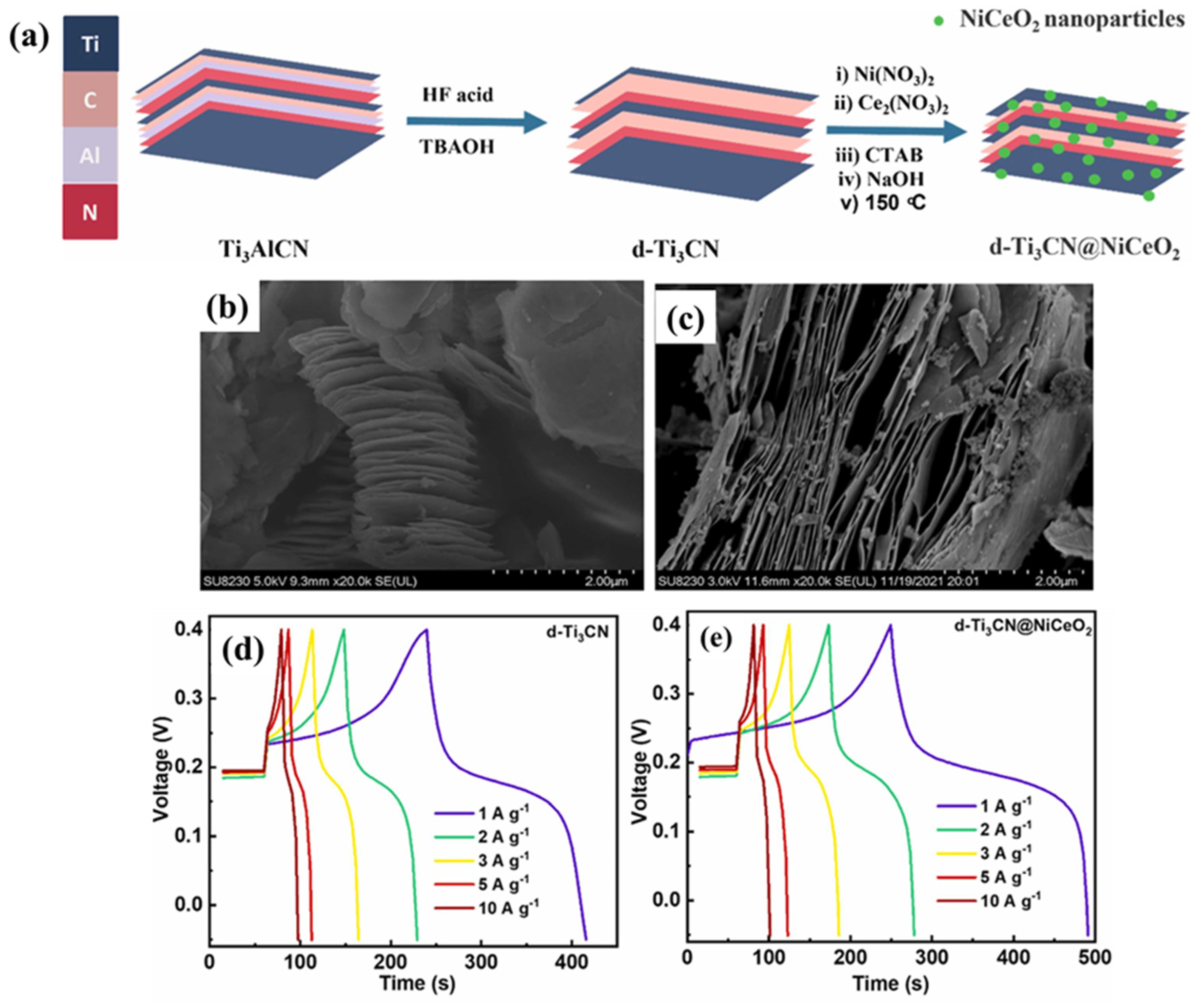
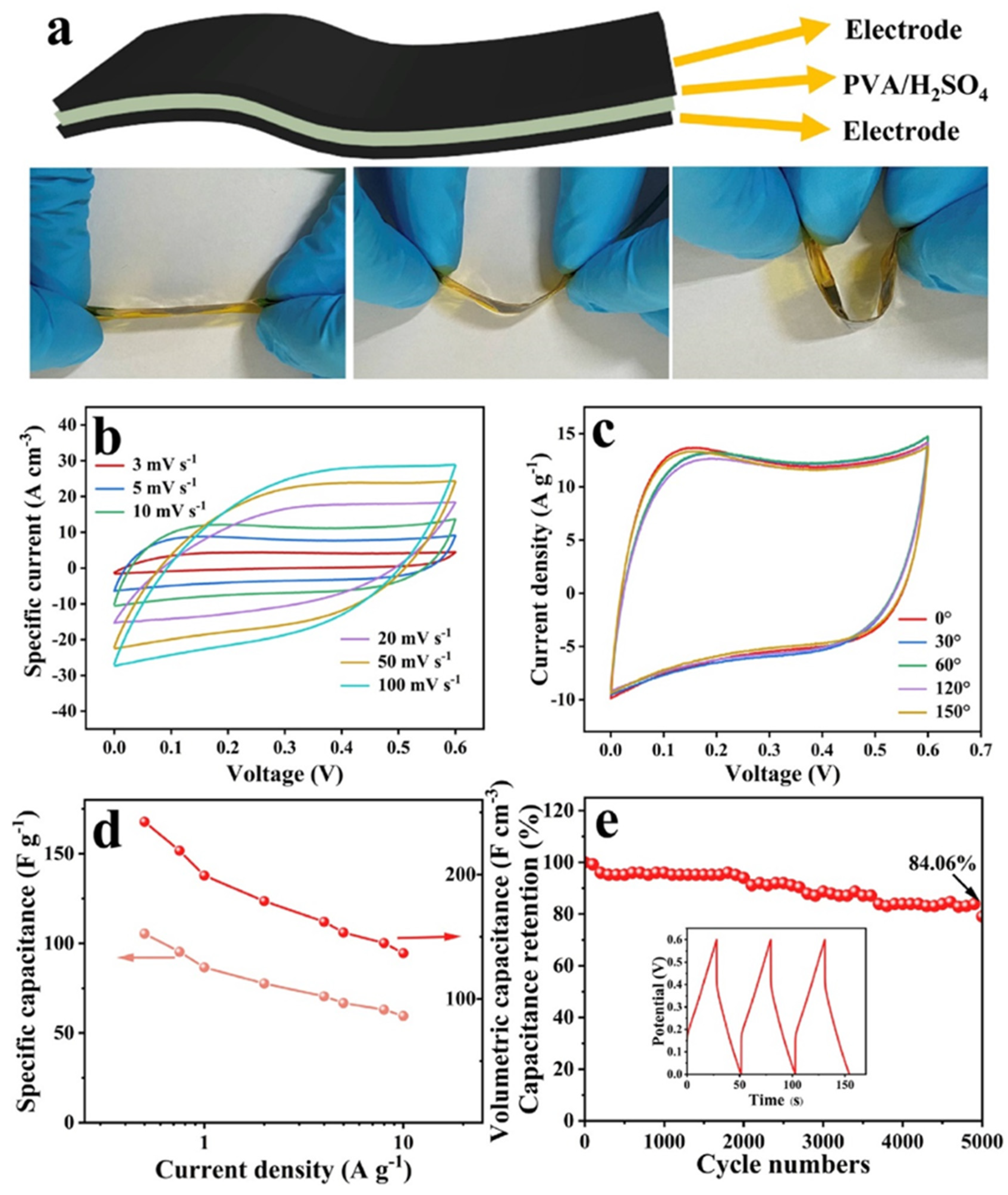

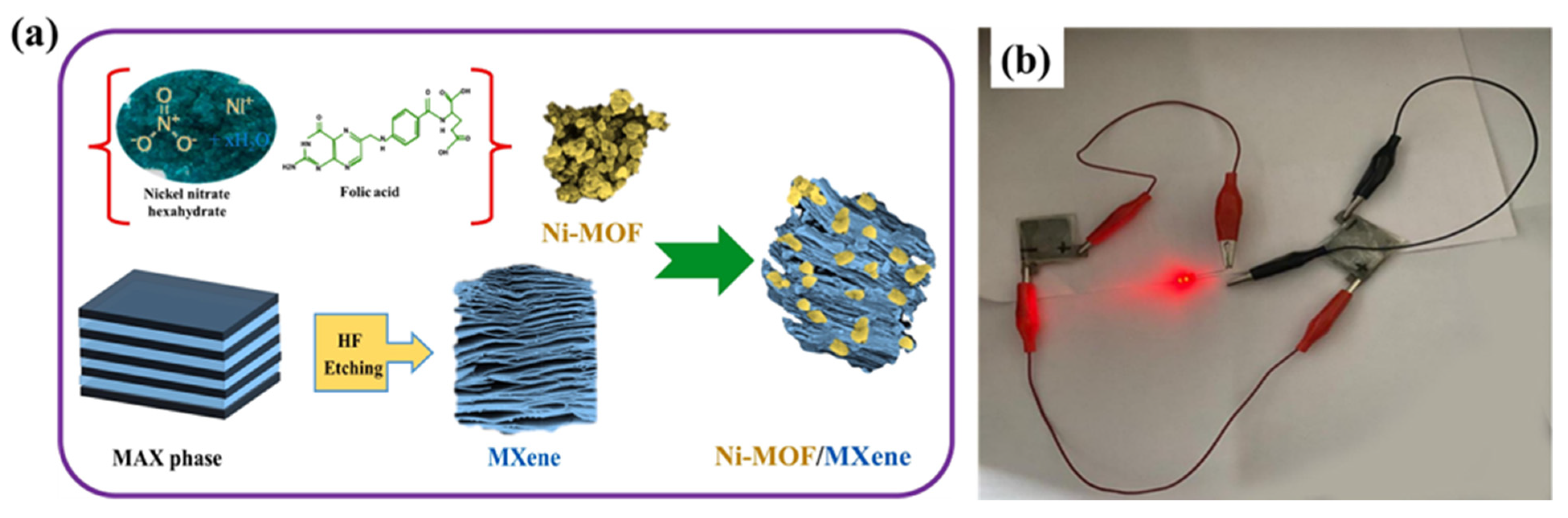
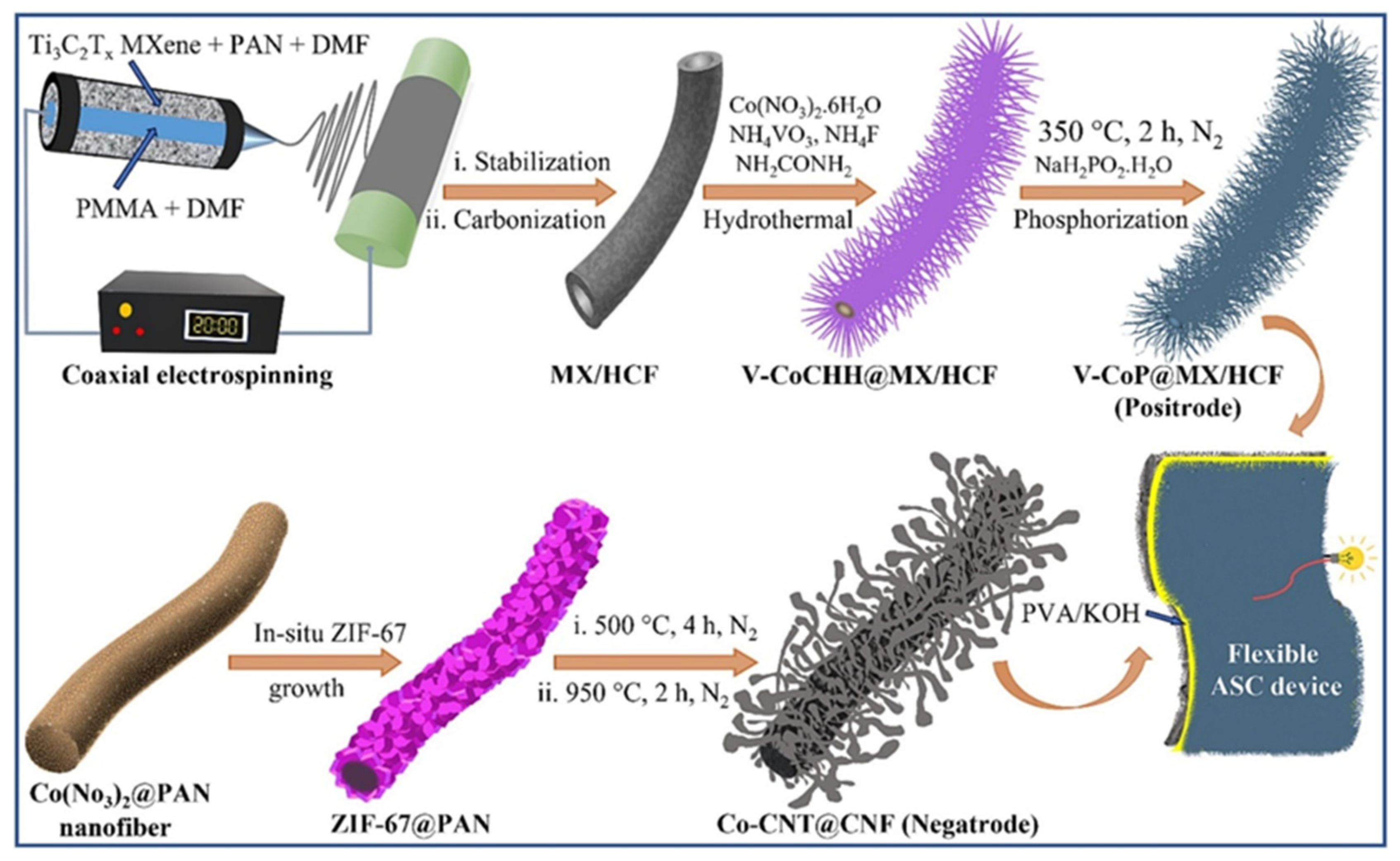
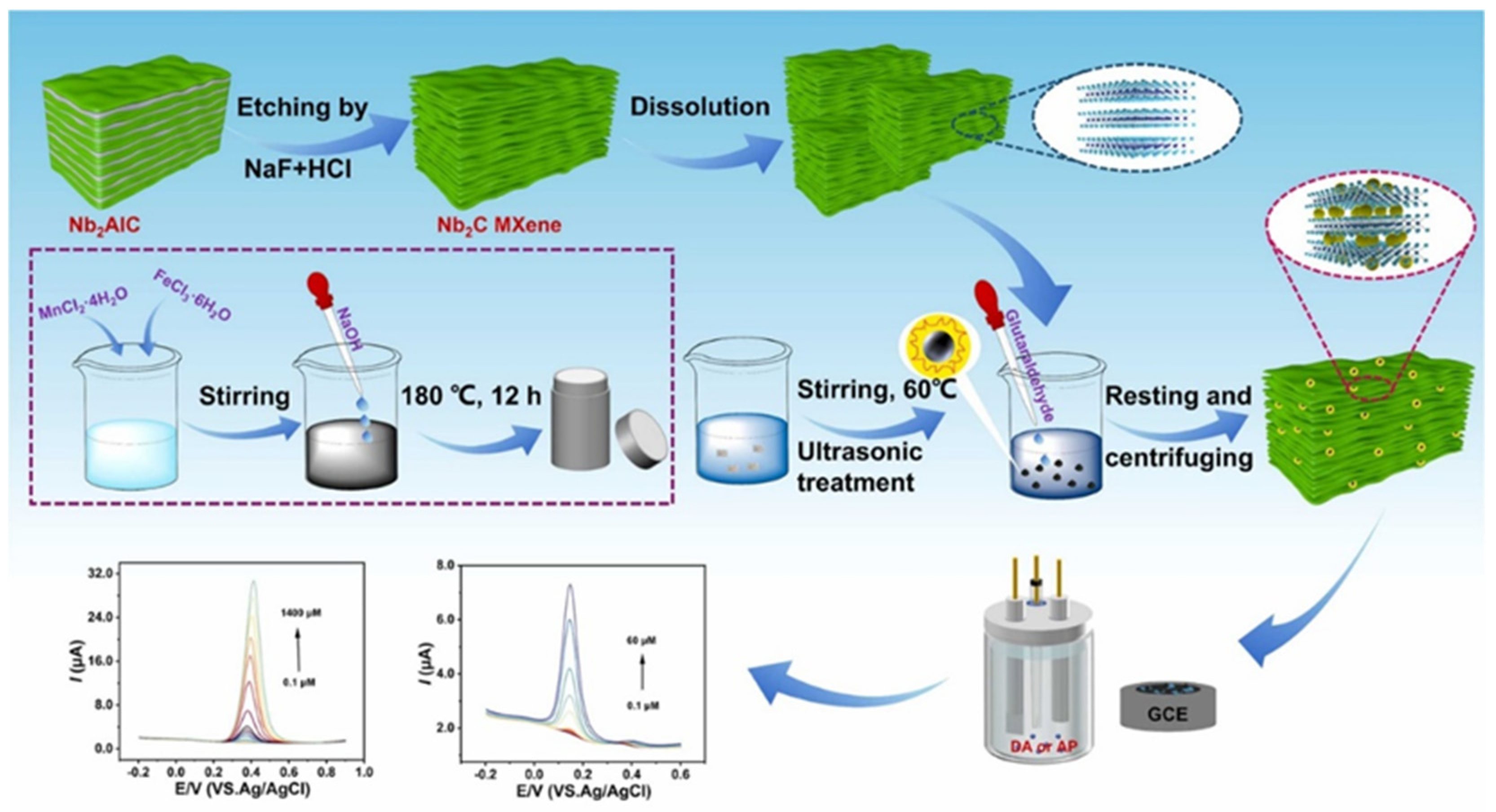

| Electrode Modifier | Electrolyte | Retention@Stability Cycles | Csp (F/g) | Current Density (A/g) | Refs. |
|---|---|---|---|---|---|
| MXene/NiPc | 3 M KOH | 95.1%@5000 | 792 | 1 | [44] |
| Ti3C2Tx | 3 M H2SO4 | 92%@10,000 | 426 | 1 | [45] |
| N-Ti3C2Tx | 2 M H2SO4 | 80.4%@8000 | 449 | 1 | [46] |
| Nb2C-PCarbons CPs | 6 M KOH | 93.93%@10,000 | 465.6 | 0.5 | [47] |
| Ti2.9Nb0.1C2Tx | 1 M H2SO4 | 92.89%@10,000 | 1014 F/cm | 2 mV/s | [48] |
| 1-Co@Ti3C2Tx MXene | 1 M KOH | 5000 | 48 mAh/g | 1 | [51] |
| Biomass/MXene/Cs aerogel | 3 M H2SO4 | 82%@50,000 | 1526.4 mF·cm3 | 2 mA/cm3 | [52] |
| SSA@Ti3C2Tx | 3 M H2SO4 | 90.1%@20,000 | 321 | 1 | [55] |
| Mg-10%MFMX@MS | 3 M H2SO4 | 10,000 | 685.77 mF/cm2 | 10 mV/s | [58] |
| Mo2N MXene | 2 M KOH | 93.9%@5000 | 1272.45 | 10 mV/s | [59] |
| CoMoO4-Ti3C2Tx | 6 M KOH | 68.2%@6000 | 870.7 C/g | 1 | [63] |
| MNF/Ti3C2Tx | 1 M Na2SO4 | 91.9%@5000 | 348.5 | 0.5 | [66] |
| MXene-WO3@rGOsp | 2 M KOH + 0.1 M K4[Fe(CN)6] | 86%@3000 | 774.4 | 5 | [68] |
| d-Ti3CN@NiCeO2 | 2 M KOH | 79%@8000 | 941 | 1 | [69] |
| CuMn2O4/Ti3C2 | 2 M KOH | 80%@10,000 | 628 mF/cm2 | 4 mA/cm2 | [70] |
| MnFe2O4/MXene | 2 M KOH | 97.8%@5000 | 1263 | 1 | [72] |
| MXene/TiO2-G | EmimTFSI-ACN-LiTFSI | 85.1%@5000 | 196.2 | 1 | [75] |
| d- V4C3TxMoO3 | 3 M H2SO4 | 97%@10,000 | 6450 C/g | 1 | [77] |
| 40 wt% g-C3N4/MoO3 | 1 M H2SO4 | 96.8%@5000 | 1168 | 1 | [80] |
| (CeO2/MXene)/PANI(40%:60%) | 2 M KOH | 96.3%@6000 | 2247.962 | 2 | [82] |
| MXene/NiCo2S4 | 3 M KOH | 96.51%@10,000 | 2675 | 1 | [84] |
| MXene/CoFe2O4/g-C3N4 | 3 M KOH | 89%@5000 | 1506.2 | 5 | [86] |
| NiCo2O4@MXene | 3 M KOH | 89.4%@10,000 | 777.7 | 1 | [88] |
| WS2@MXene/GO | 1 M KOH | 5000 | 1111 | 2 | [91] |
| Ti3C2Tx/NH2-RGO | 1 M KOH | 97%@10,000 | 120.2 | 1 | [95] |
| CoS/MXene/PANI | 1 M H2SO4 | 97%@10,000 | 246 | 2 | [97] |
| MXene/CuS | 3 M KOH | 93.5%@10,000 | 2569.3 | 1 | [99] |
| CNS/C/MXene | 6 M KOH | 71.17%@30,000 | 1221.6 | 1 | [105] |
| MXene/FeNi2S4 | 6 M KOH | 90%@2000 | 673 | 1 | [108] |
| Ti3C2Tx (MXene)/WS2 | 1 M H2SO4 | - | 373 | 0.4 | [111] |
| MXene/PANI | 1 M KOH | 95.5%@1000 | 458.3 | 5 mV/s | [116] |
| P-M10 | H2SO4 | 98.5%@10,000 | 1196.5 | 1 mA/cm2 | [121] |
| PPy/Mxene/GA | 1 M H2SO4 | 94%@2000 | 657.64 | 1 | [126] |
| PANI-WO3/MXene | 1 M H2SO4 | 82%@3000 | 741 | 1 | [130] |
| rGO/MXene-PPy | 1 M H2SO4 | 67.3%@10,000 | 408.2 | 10 | [134] |
| MXene@h-CNT | 2 M KOH | 80%@4000 | 404 | 4 | [139] |
| MXene/rGO/CNTs | 1 M H2SO4 | 92.9%@8000 | 463.5 | 1 | [145] |
| CF-PhNO2/oPD-MX | 1 M H2SO4 | 94%@5000 | 157 | 5 mV/s | [148] |
| MoSSe@CNTs | 1 M H2SO4 | - | 585 | - | [153] |
| NF@Mxene@NiCo-LDH | 3 M KOH | 87%@10,000 | 22.6 F/cm2 | 5 mA/cm2 | [159] |
| Ni-MOF/MXene | 1 M KOH | 87.20%@2000 | 716.19 | 1 | [166] |
| FeCo-LDH/MXene | 3 M KOH | 82.4%@5000 | 2058.2 | 1 | [170] |
| MXene/BCN10 (m-MX/BCN10) | KOH | 95%@10,000 | 678 | 0.5 | [173] |
| MXene/Ni-Co phosphide | 2 M KOH | 93.8%@10,000 | 1754 | 3 mA/cm2 | [179] |
| Electrode Modifier | Sensing Method | LOD | Linear Range | Sensitivity | Analyte | Refs. |
|---|---|---|---|---|---|---|
| ZnO TPs/MXene | CA | 17 μM | 0.05 to 0.7 mM | 29 μA mM−1 cm−2 | Glu | [180] |
| MXene/V2O5 | DPV | 87 nM | 414 nM to 31.2 µM | - | BPA | [181] |
| Nb2C/MnFe2O4 | DPV | 0.079 μM | 0.1 to 1000 μM | - | AP | [182] |
| Nb2C/MnFe2O4 | DPV | 0.070 μM | 0.1 to 60 μM | - | DA | [182] |
| Cu2O/MXene/rGO | CA | 1.1 μM | 0.1 to 14 and 15 to 40 mM | 264.52 μA mM−1 cm−2 | Glu | [183] |
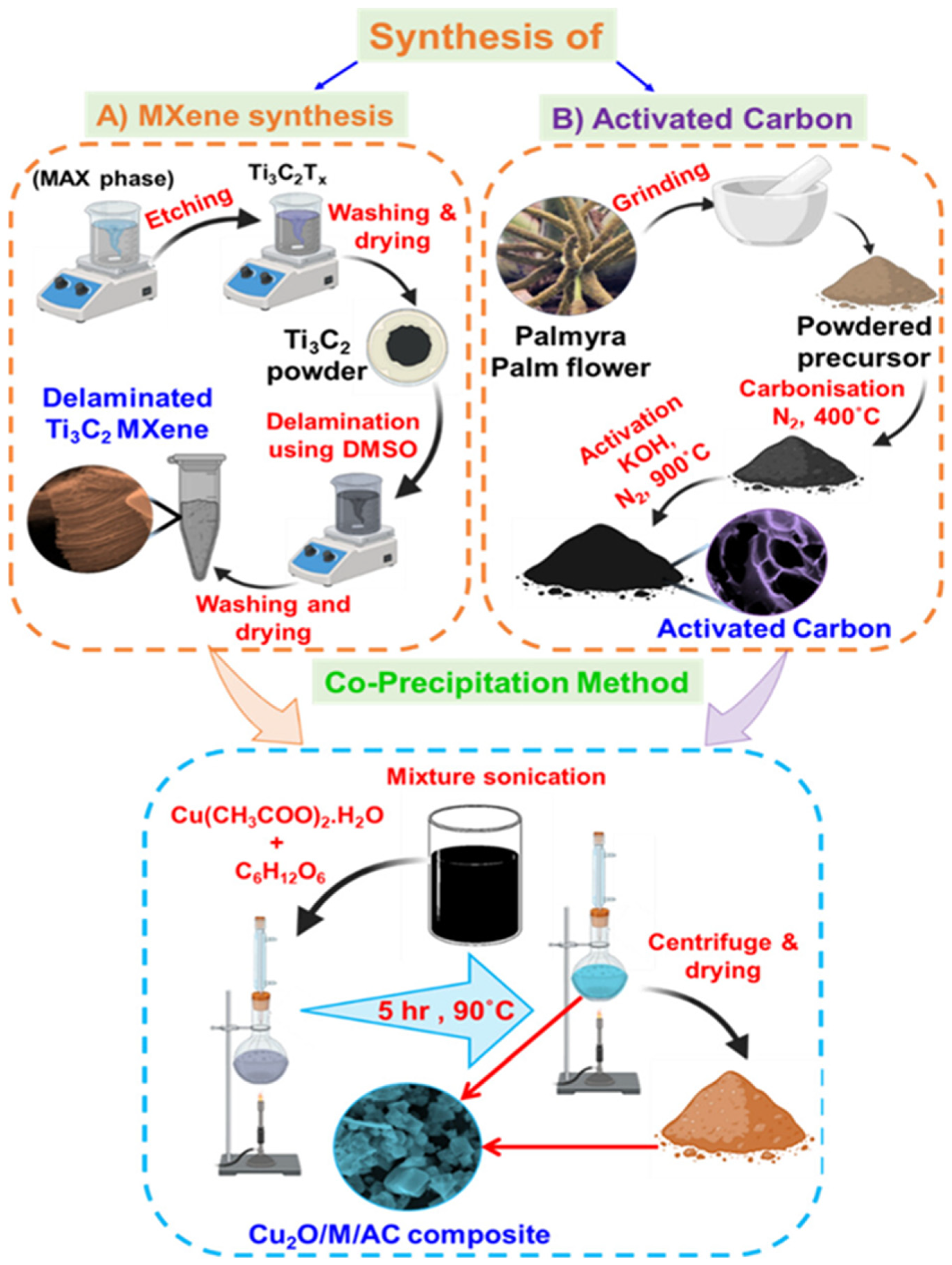
| Cu2O/MXene/AC | CA | 1.96 μM | 0.004 to 13.3 mM and 15.3 to 28.4 mM | 430.3 μA mM−1 cm−2 | Glu | [184] |
| Au@Ce2Sn2O7/MXene | DPV | 5.63 nM | 0.00125 to 1021.96 μM | 0.403 μA·μM–1·cm–2 | MTL | [185] |
| ZnMoO4/MXene | DPV | 0.0081 μM | 10.65 to 605.65 μM | 10.413 μA·μM–1·cm–2 | ROX | [186] |
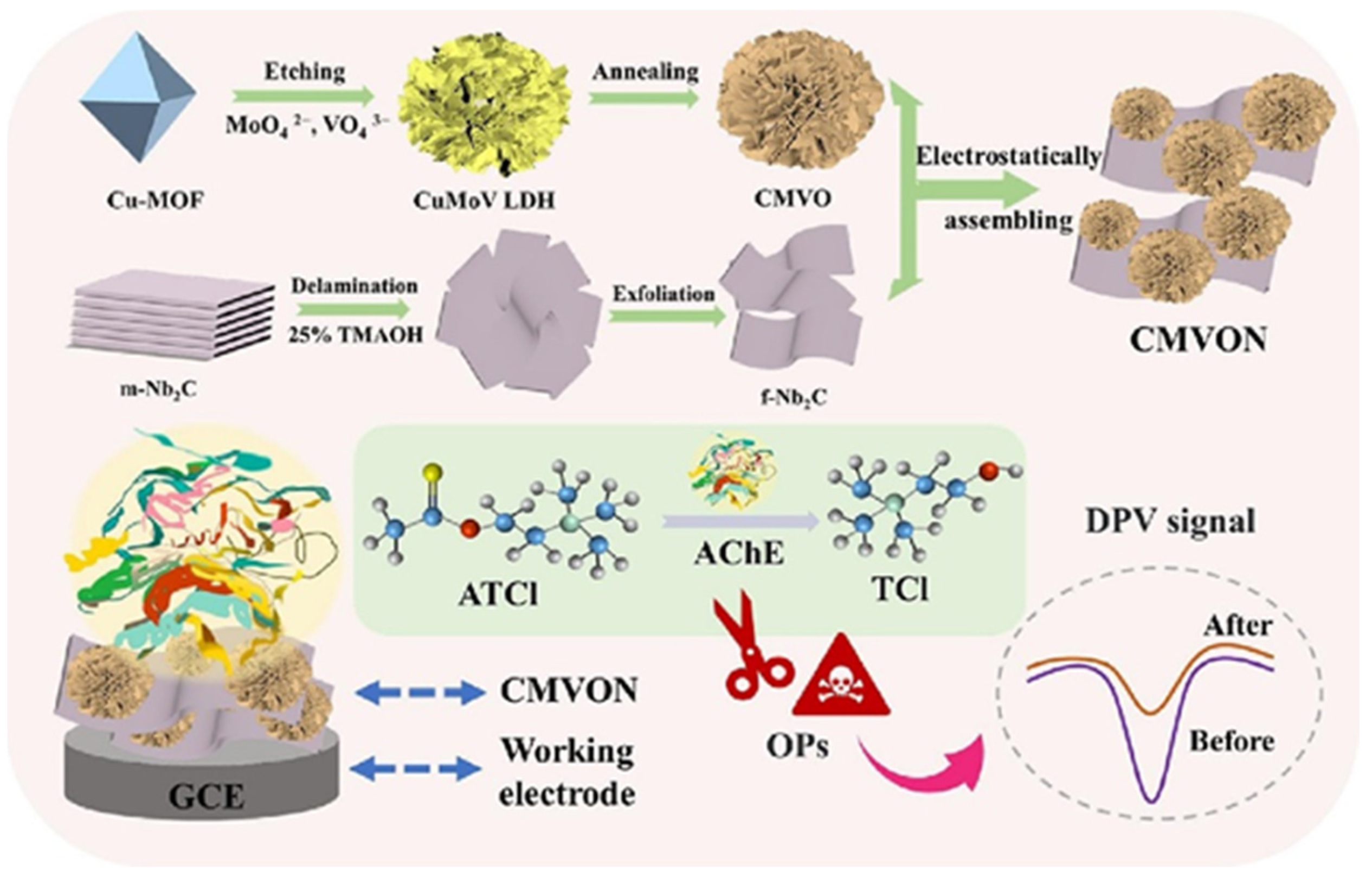
| AChE-CS/CMVON | DPV | 2.3 × 10−14 M | 3.6 × 10−13 to 3.6 × 10−8 M | - | fenitrothion | [187] |
| NbC@Mo NC | DPV | 1.5 × 10−10 M | 1.0 × 10−6 to 1.9 × 10−3 M | - | fenitrothion | [187] |
| MIP/CFO/MXene/MCPE | SWV | 1.6 nM | 0.005 to 0.7 μM and 0.7 to 10 μM | - | quercetin | [188] |
| ZnMoO4/MXene | DPV | 12 CFU/ml | 10 to 107 CFU/ml | - | L. monocytogenes | [190] |
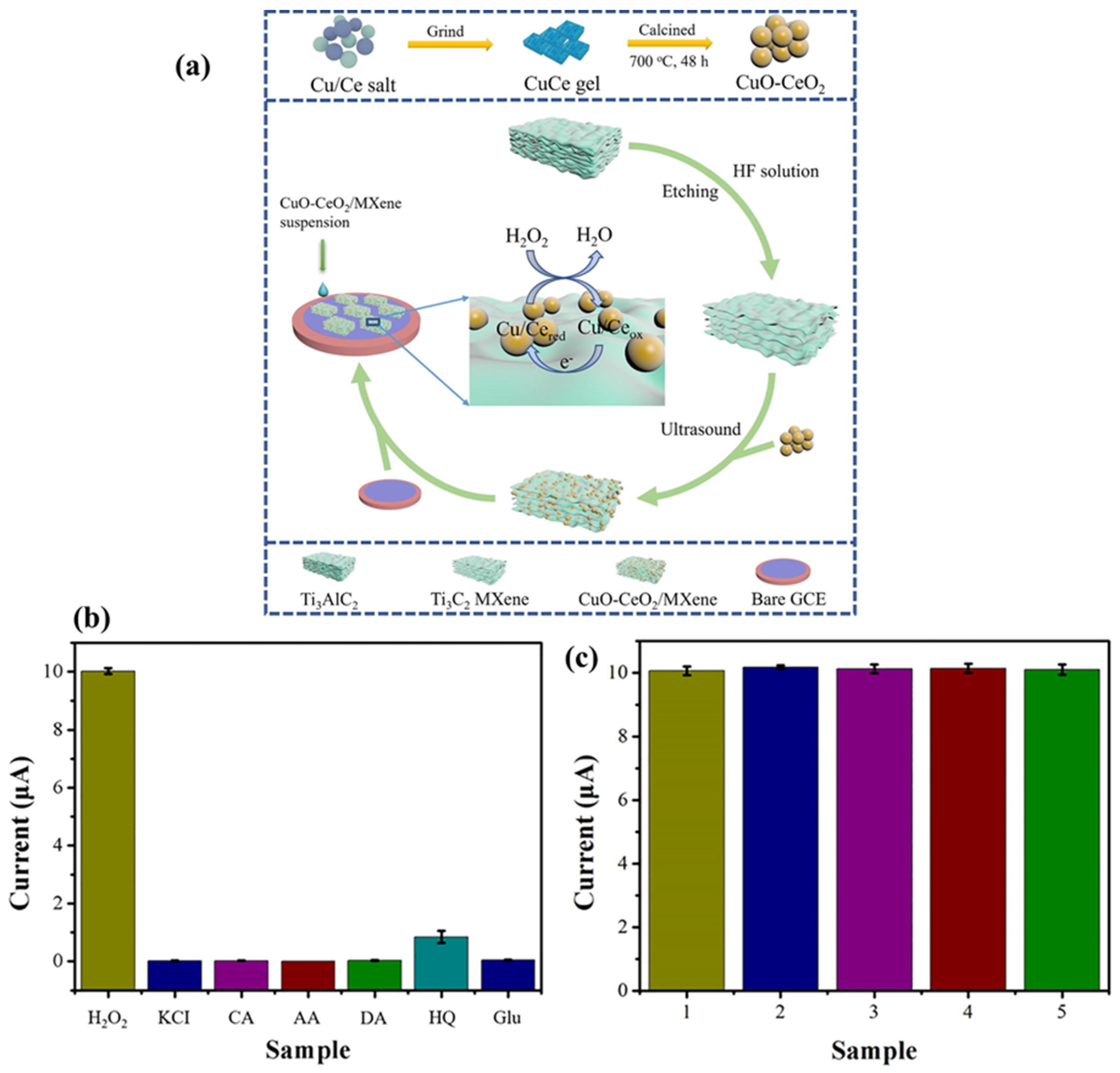
| CuO-CeO2/MXene | CA | 1.67 μM | 5.0 to 100 μM | - | H2O2 | [191] |
| MXene/MoS2 | CA | 4.2 μM | - | 54.6 nAμM−1 | AA | [192] |
| CFP-MXene-MoS2 | CA | 1.47 μM | 10 to 3000 μM | - | AA | [193] |
| CFP-MXene-MoS2 | CA | 0.27 μM | 0.5 to 1000 μM | - | DA | [193] |
| CFP-MXene-MoS2 | CA | 0.38 μM | 0.5 to 1000 μM | - | UA | [193] |
| AChE-Chit/Pt/MoS2/TM | DPV | 4.71 × 10−13 M | 10−6 to 1 μM | - | chlorpyrifos | [194] |
| Mxene-AgBiS2 | DPV | 2.54 nM | 0.02 to 5 and 10 to 78 μM | - | 4-NP | [195] |
| N-MPG/CuS flower-like/MXene | DPV | 1.6 μM | 5 to 150 μM | - | NAL | [196] |
| Ti3C2MXene/MoS2@AuNPs/AChE | DPV | 5.29 × 10−15 M | 1 × 10−13 to 1 × 10−7 M | - | phoxim | [197] |
| LOx/Pt/PANI/MXene | Amperometry | 5.0 μM | 0.005 to 5 mM | - | lactate | [198] |
| PANI-Ti3C2 | ASV | 0.017 μg/L | 0.1 to 20 μg/L | - | Hg2+ | [199] |
| MIP/pTHi/MXene/Fe@Ti-MOF-NH2 | SWV | 0.54 μM | 0.1 to 4000 μM | - | CC | [200] |
| (P2Mo17V/Cs-Ti3C2Tx)2 | DPV | 0.08 μM | 0.1 to 103 μM | 0.141 μA·μM–1·cm–2 | L-Trp | [201] |
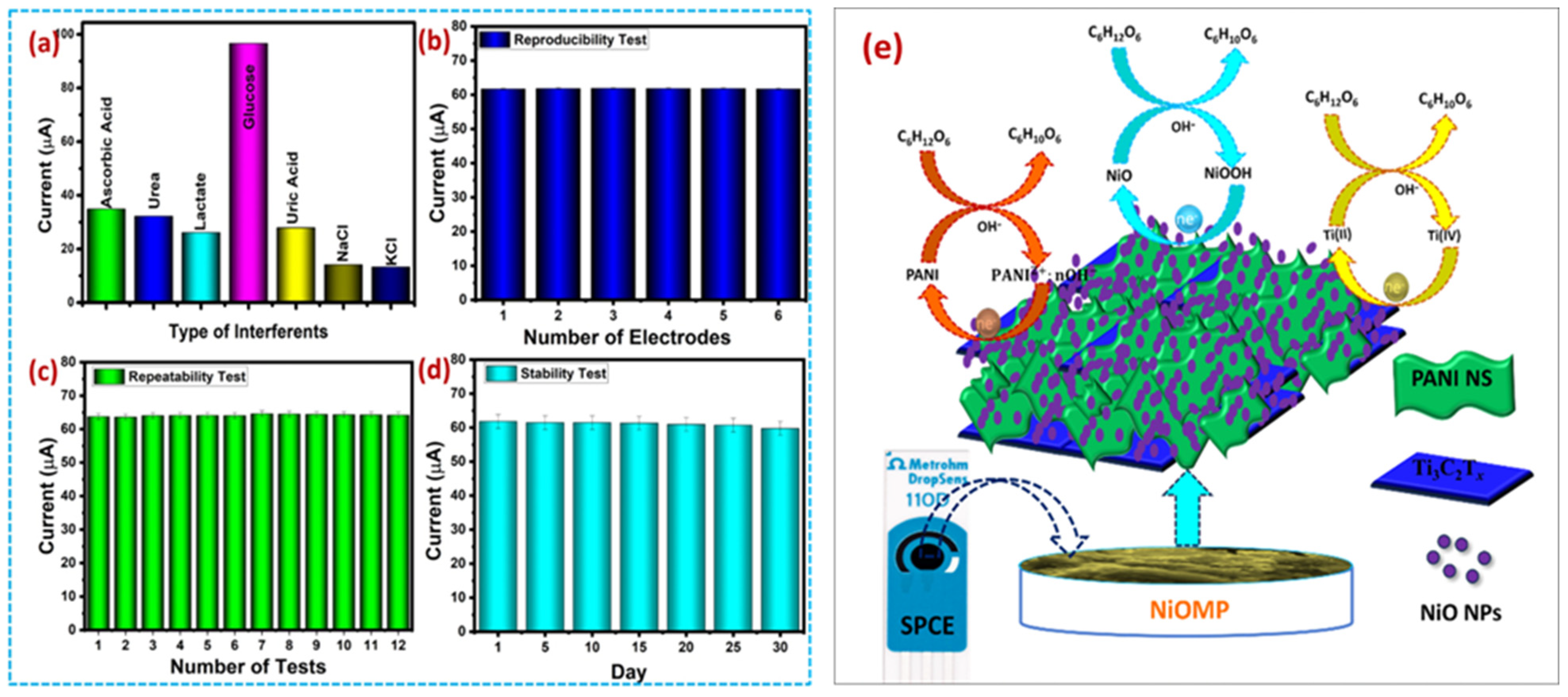
| Ti3C2Tx | DPV | 0.031 µM | 10 to 500µM | 564.30 μA mM−1 cm−2 | Glu | [202] |
| Ti3C2Tx/poly(rutin) | DPV | 1 nM | 1.0 × 10−9 to 1.0 × 10−4 M | 0.49 μA·μM–1·cm–2 | ciprofloxacin | [203] |
| Ti3C2-MWCNT | DPV | 0.0066 µM | 2 to 150 | - | HQ | [204] |
| Ti3C2-MWCNT | DPV | 0.0039 µM | 2 to 150 | - | CC | [204] |
| MXene@PDA/NH2-MWCNTs | DPV | 1 nM | 0.005 to 10.0 and 10.0 to 60.0 µM | - | AP | [206] |
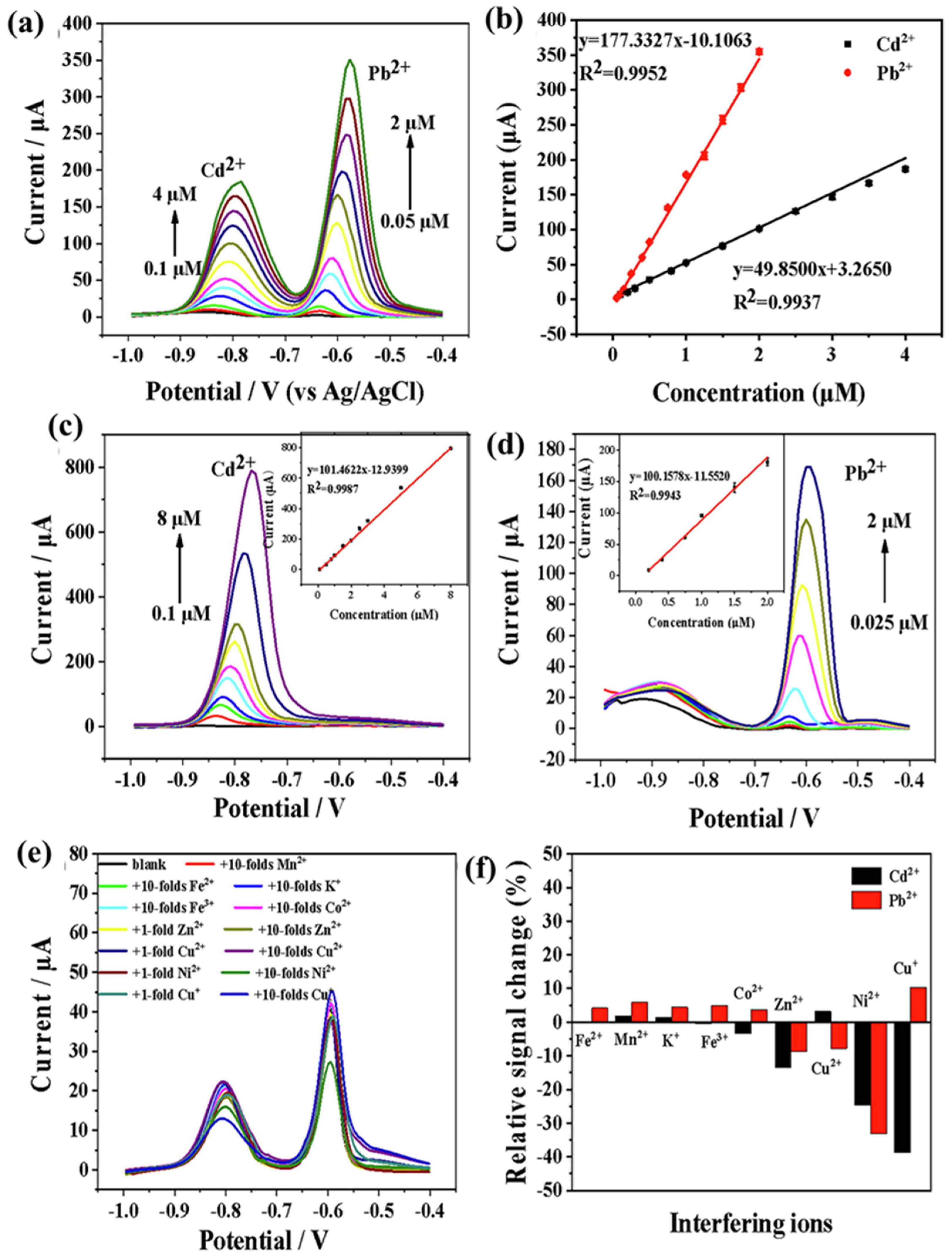
| Ti3C2@N-C | SWASV | 2.55 nM | 0.1 to 4 µM | 49.85 μM μA−1 | Cd2+ | [207] |
| Ti3C2@N-C | SWASV | 1.10 nM | 0.05 to 2 µM | 177.33 μM μA−1 | Pb2+ | [207] |
| Ti3C2/G-MWCNTs/ZnO | DPV | 3.2 nM | 0.01 to 30 µM | 16 A/M | DA | [208] |
| 3D MGMA | DPASV | 0.48 μg L−1 | 3 to 900 μg L−1 | - | Zn2+ | [209] |
| 3D MGMA | DPASV | 0.45 μg L−1 | 3 to 900 μg L−1 | - | Cd2+ | [209] |
| 3D MGMA | DPASV | 0.29 μg L−1 | 3 to 900 μg L−1 | - | Pb2+ | [209] |
| Pt@SWCNTs-Ti3C2-rGO | DPV | 2.5 nM | 0.006 to 11.4 µM | 1.941 μA (μmol L−3)−1 cm −2 | BPA | [210] |
| N, S-CDs/Ti3C2Tx | DPV | 0.91 μM and 3.71 μM | 1 to 1000 μM | - | DA | [213] |
| Ti3C2:GQDs(1:3) | DPV | 1.8 μM | 40 to 400 μM | - | DA | [215] |
| layered N-doped carbon/MXene | SWASV | 0.019 μM | - | 114.54 µA µM−1 cm−2 | Cu2+ | [216] |
| layered N-doped carbon/MXene | SWASV | 0.056 μM | - | 64.317 µA µM−1 cm−2 | Hg2+ | [216] |
| Ce-MOF/Ti3C2TX MXene | DPV | 0.19 μM | 0.2 to 139 μM | - | L-Trp | [218] |
| MOF-Ti3C2 | DPV | 110 nM | 90 to 130 nM | - | DA | [220] |
| Fe-MOF-NH2/CNTs-NH2/MXene | DPV | 13.2 nM | 0.1 to 100 μM | - | ofloxacin | [222] |
| MXene-NH2@CeFe-MOF-NH2 | DPV | 0.95 nM | 5 to 50 nM | - | Pb2+ | [224] |
| MXene-NH2@CeFe-MOF-NH2 | DPV | 0.32 nM | 1 to 35 nM | - | Hg2+ | [224] |
| MXene@PDA/MOF | DPV | 0.00374 μM | 0.01 to 5 μM | - | L-Cys | [226] |
| Ru/NiFe-LDH-MXen | LSV | 2.2 nM | 0.01 to 275 μM | 152.44 μA·μM–1·cm–2 | NFT | [227] |
| FeCu-LDH@MXene | DPV | 0.09 μM | 0.66 to 418 μM | - | CLZP | [228] |
| PtNP@MXene-Ti3C2Tx | AMP | 0.45 μM | 10 to 110 μM | 1.5906 nA/μM | L-glutamate | [229] |
| MXene-Ni NPs | DPV | 0.12 pM | 0.001 to 0.017 μM | - | MMA | [232] |
| MO/Ti3C2 | CA | 0.05 μM | 0.33 to 1200 μM | - | H2O2 | [234] |
| MO/Ti3C2 | CA | 0.01 μM | 0.1 to 1350 μM | - | N2H4 | [234] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, K.; Oh, T.H. Recent Progress in MXene-Based Materials for Supercapacitors and Electrochemical Sensing Applications. Biosensors 2025, 15, 288. https://doi.org/10.3390/bios15050288
Ahmad K, Oh TH. Recent Progress in MXene-Based Materials for Supercapacitors and Electrochemical Sensing Applications. Biosensors. 2025; 15(5):288. https://doi.org/10.3390/bios15050288
Chicago/Turabian StyleAhmad, Khursheed, and Tae Hwan Oh. 2025. "Recent Progress in MXene-Based Materials for Supercapacitors and Electrochemical Sensing Applications" Biosensors 15, no. 5: 288. https://doi.org/10.3390/bios15050288
APA StyleAhmad, K., & Oh, T. H. (2025). Recent Progress in MXene-Based Materials for Supercapacitors and Electrochemical Sensing Applications. Biosensors, 15(5), 288. https://doi.org/10.3390/bios15050288





