Study on Detection Method of Sulfamethazine Residues in Duck Blood Based on Surface-Enhanced Raman Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Four Enhancement Substrates
2.3. Duck Blood Sample Preparation
2.4. Optimization of Sample Pretreatment
2.5. Optimization Scheme of SERS Detection Conditions
2.5.1. Optimization of Electrolyte Types
2.5.2. Optimization of Electrolyte Addition Amount
2.5.3. Optimization of Addition Amount of 1 mM Gold Nanocolloids
2.5.4. Optimization of Adsorption Time
2.6. Quantitative Detection Scheme of SM2 in Duck Blood
2.7. Density Functional Theory Calculations and Spectral Acquisition of SM2 Standard
2.8. SERS Spectral Acquisition of SM2 in Duck Blood
2.9. Data Processing Method
3. Results and Discussion
3.1. Analysis of Raman Characteristic Peak Spectrum and Peak Position Attribution of SM2
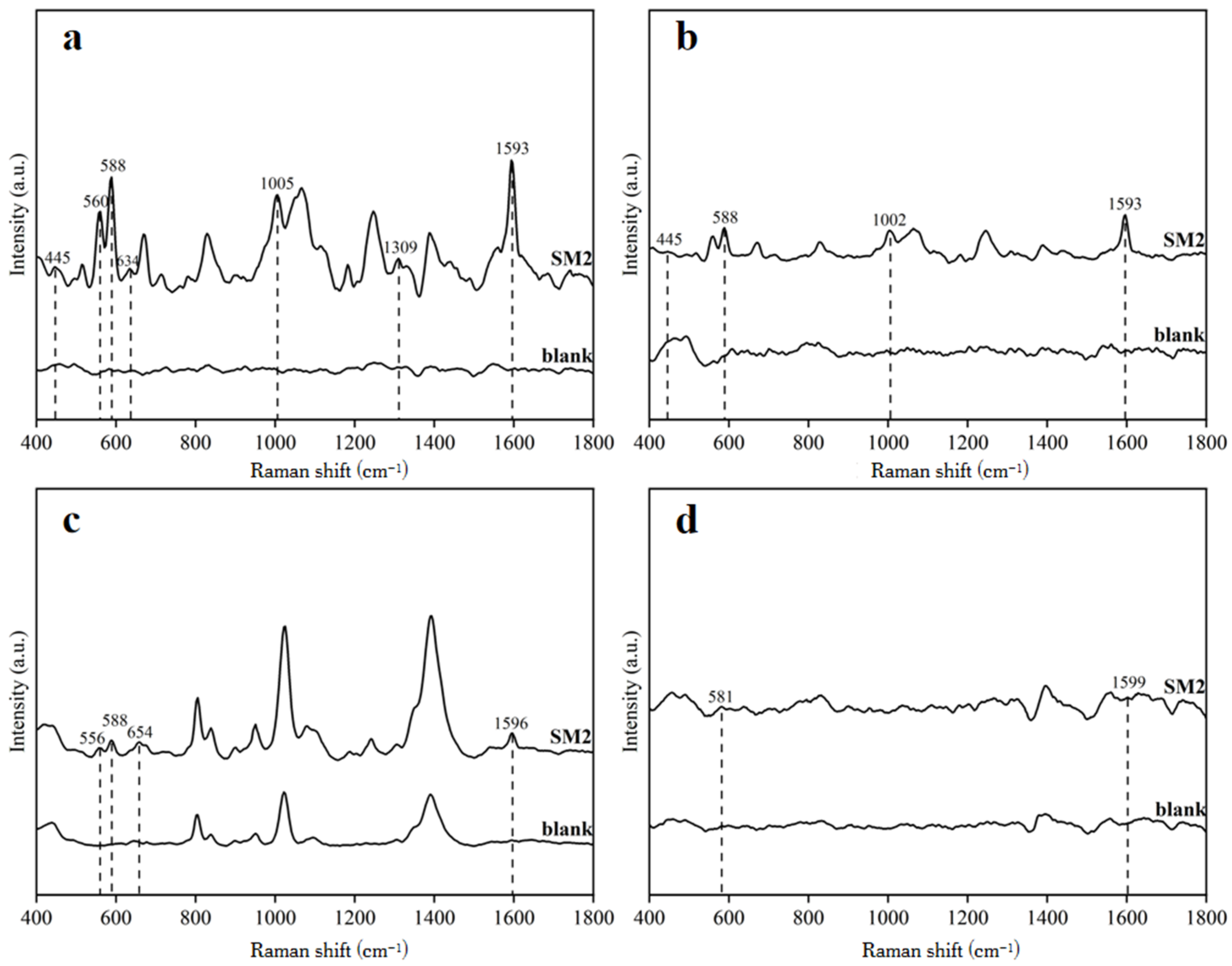
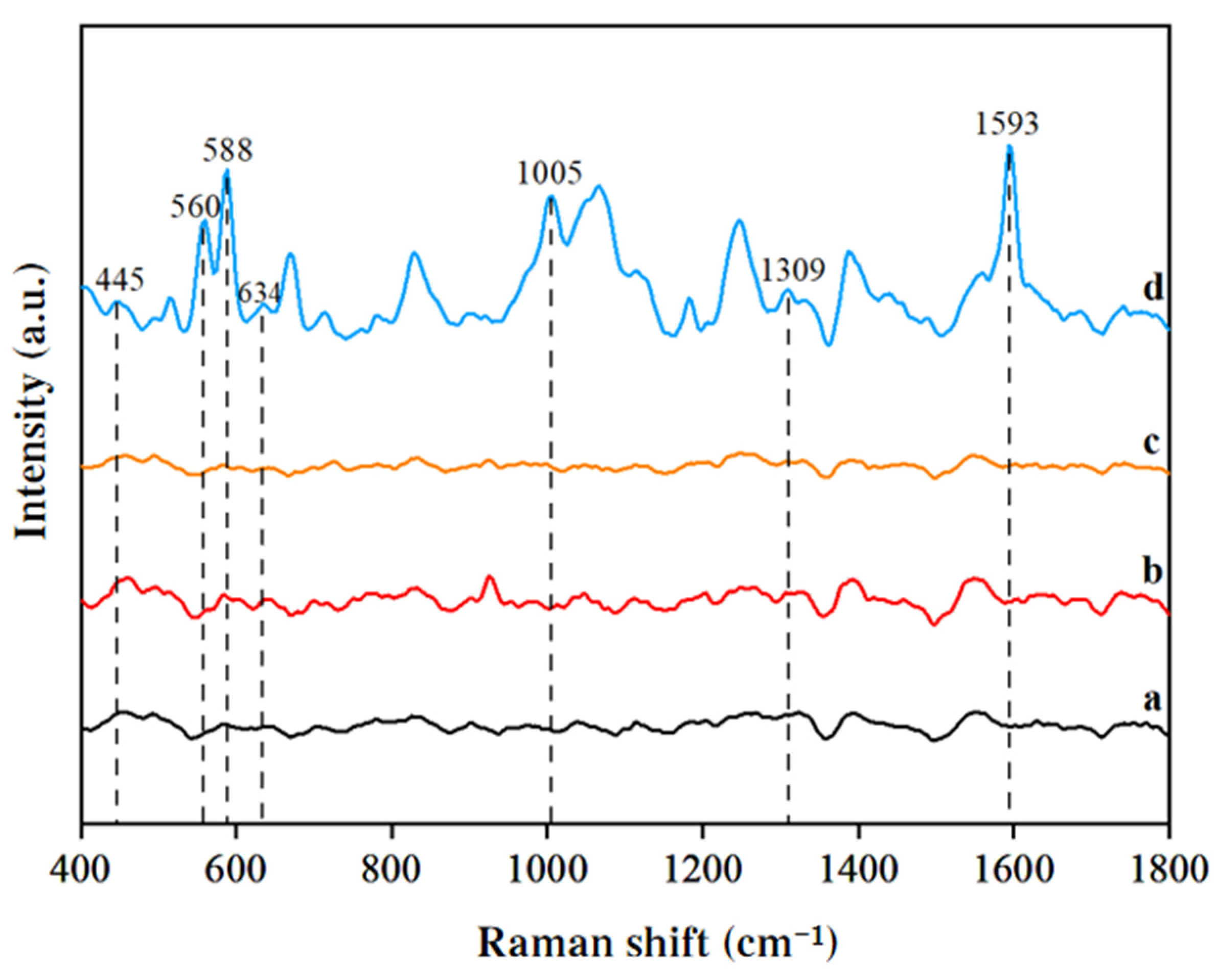
3.2. SERS Signal Analysis of Duck Blood Samples Containing SM2 on Different Enhanced Substrates
3.3. Research on the Optimization of SERS Detection Conditions
3.3.1. SERS Analysis of SM2 in Duck Blood
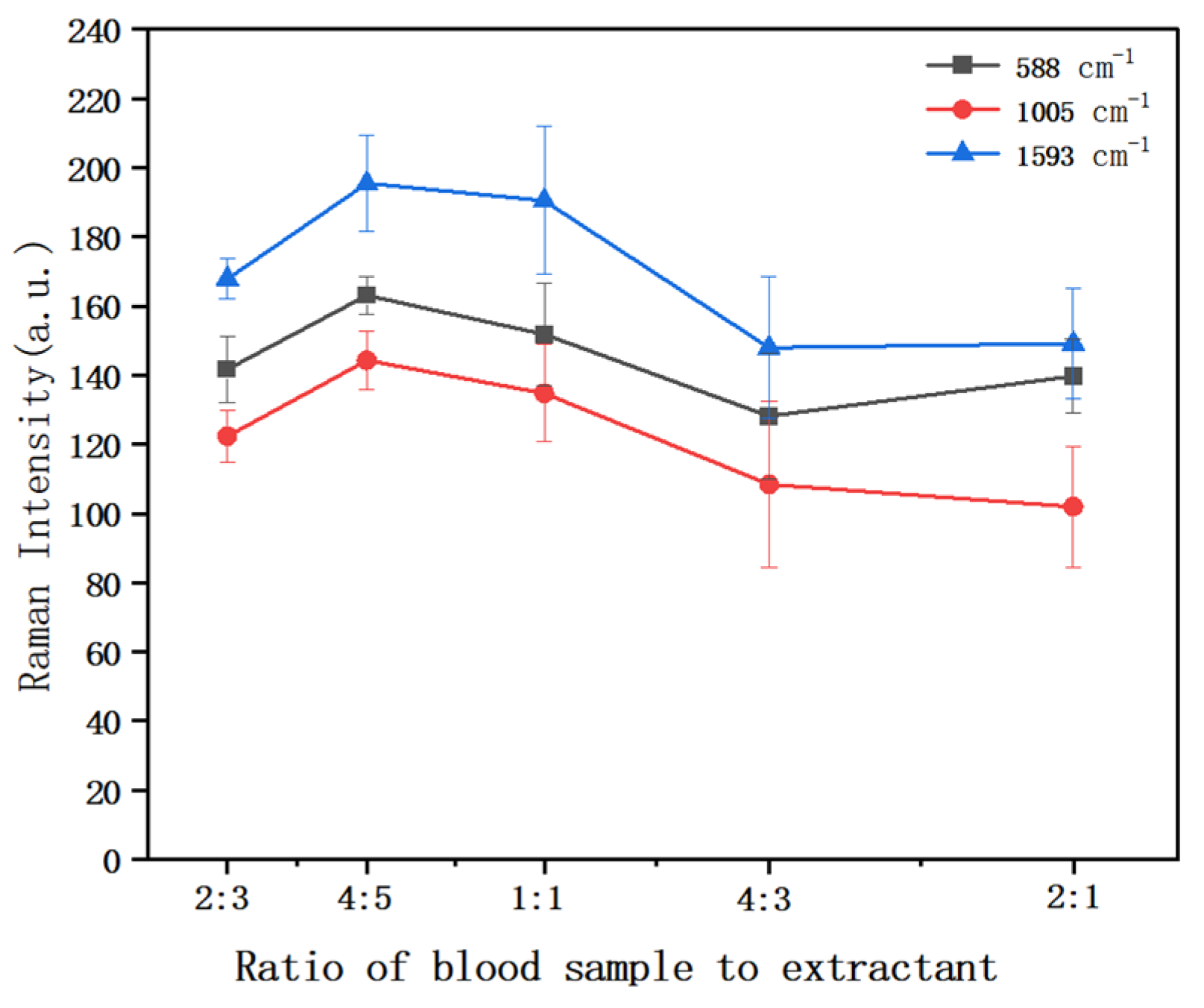
3.3.2. The Effect of the Ratio of Duck Blood Sample to Extractant on Raman Intensity
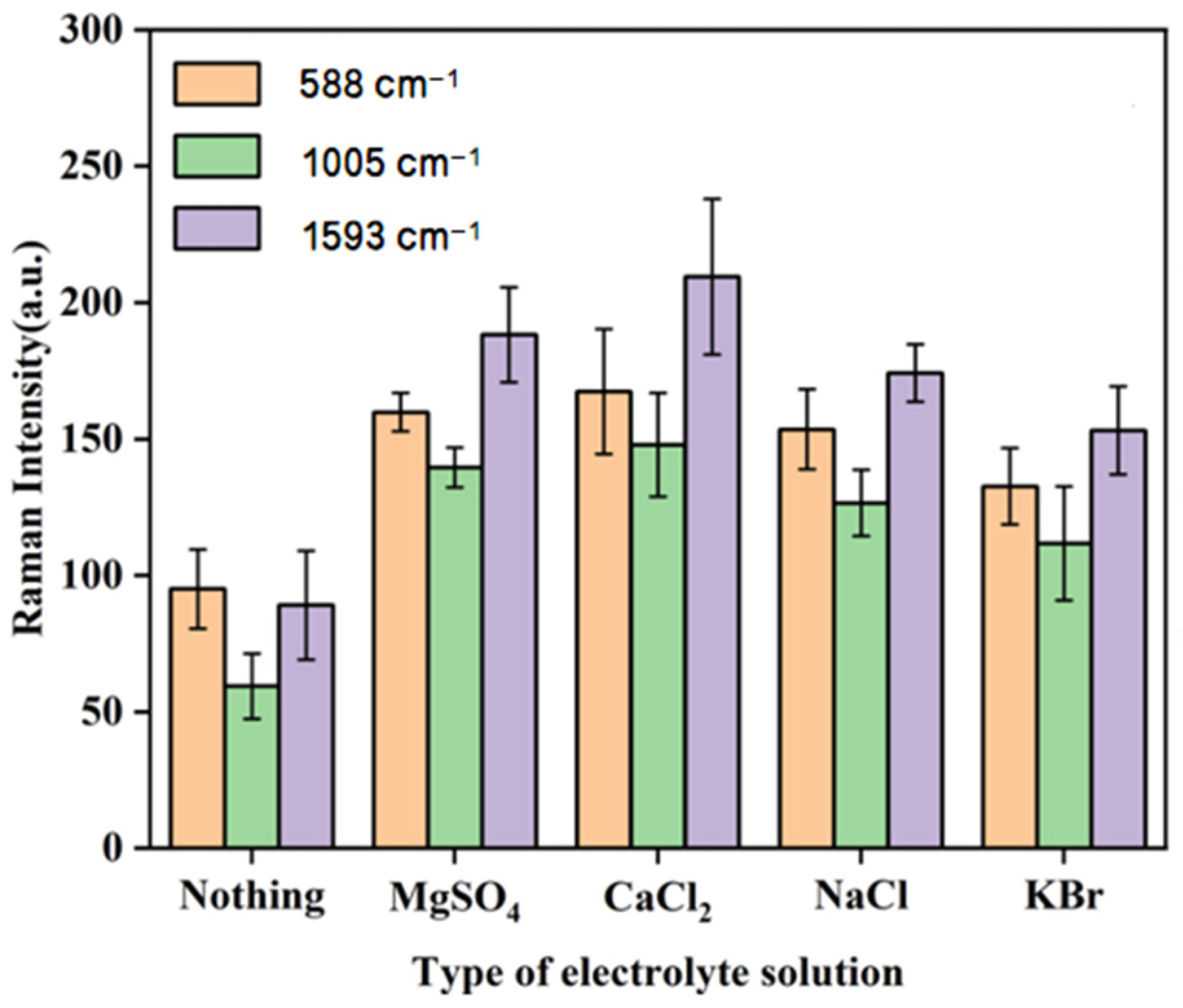
3.3.3. The Effects of Different Types of Agglomerates on Raman Intensity
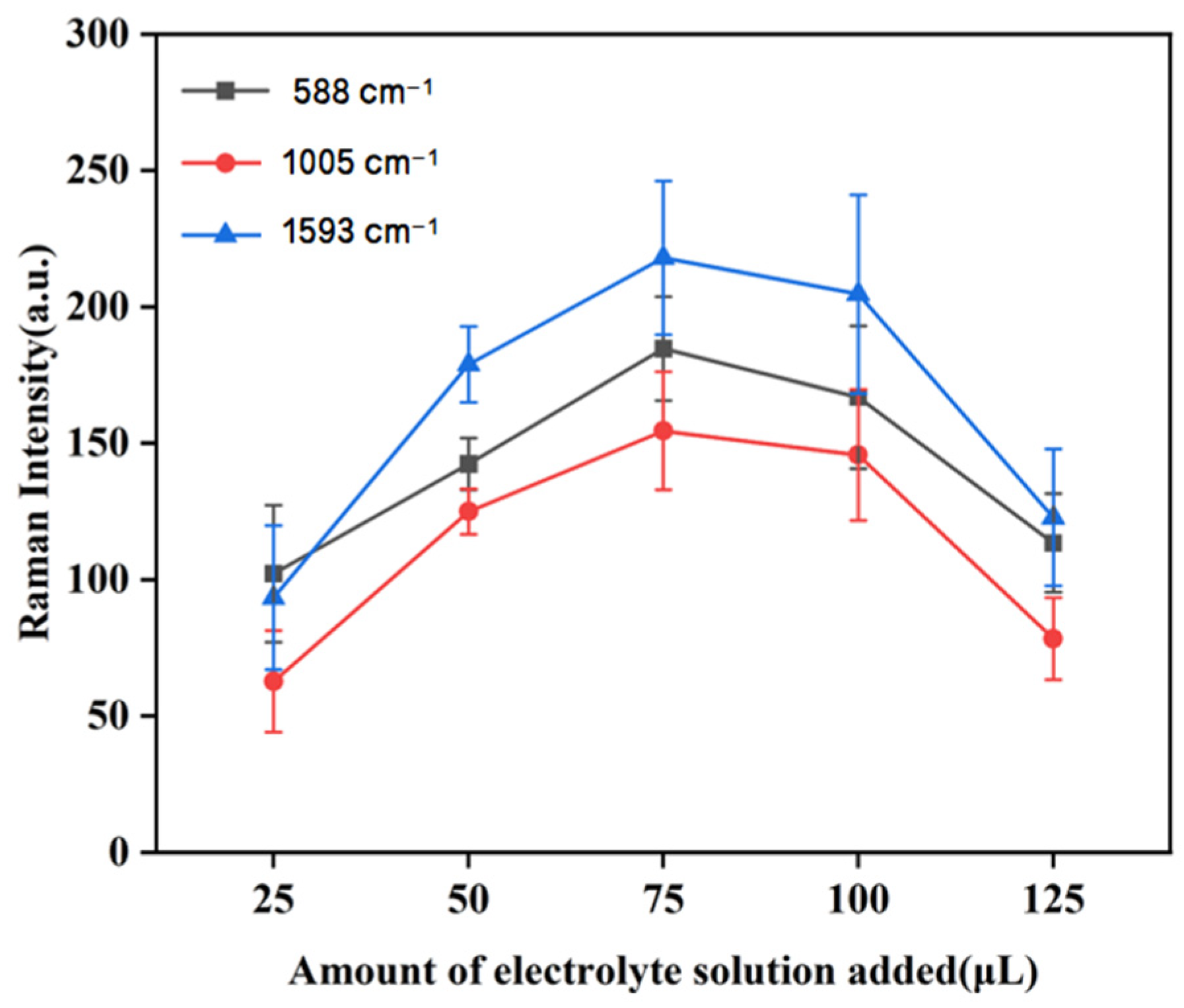
3.3.4. The Effect of the Amount of Electrolyte Agglomeration Agent on Raman Intensity
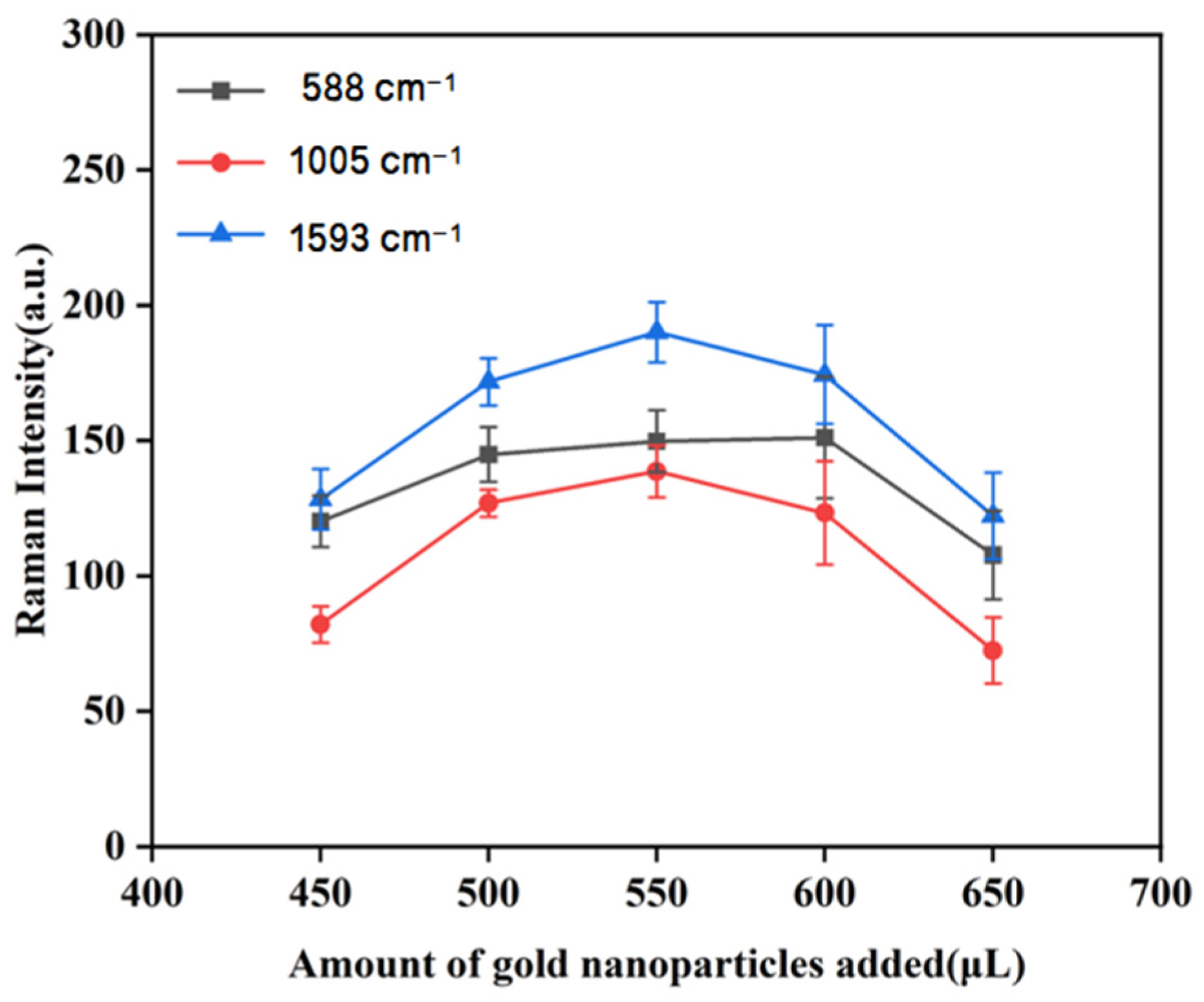
3.3.5. The Effect of Different Amounts of Gold Nanocolloids on Raman Intensity
3.3.6. The Effect of Different Adsorption Times on Raman Intensity
3.4. Quantitative Analysis of SM2 Residues in Duck Blood
3.4.1. Raman Spectroscopy and Detection Limit Analysis of SM2 Residues in Duck Blood
3.4.2. Raman Spectroscopic Pretreatment of SM2 Residues in Duck Blood
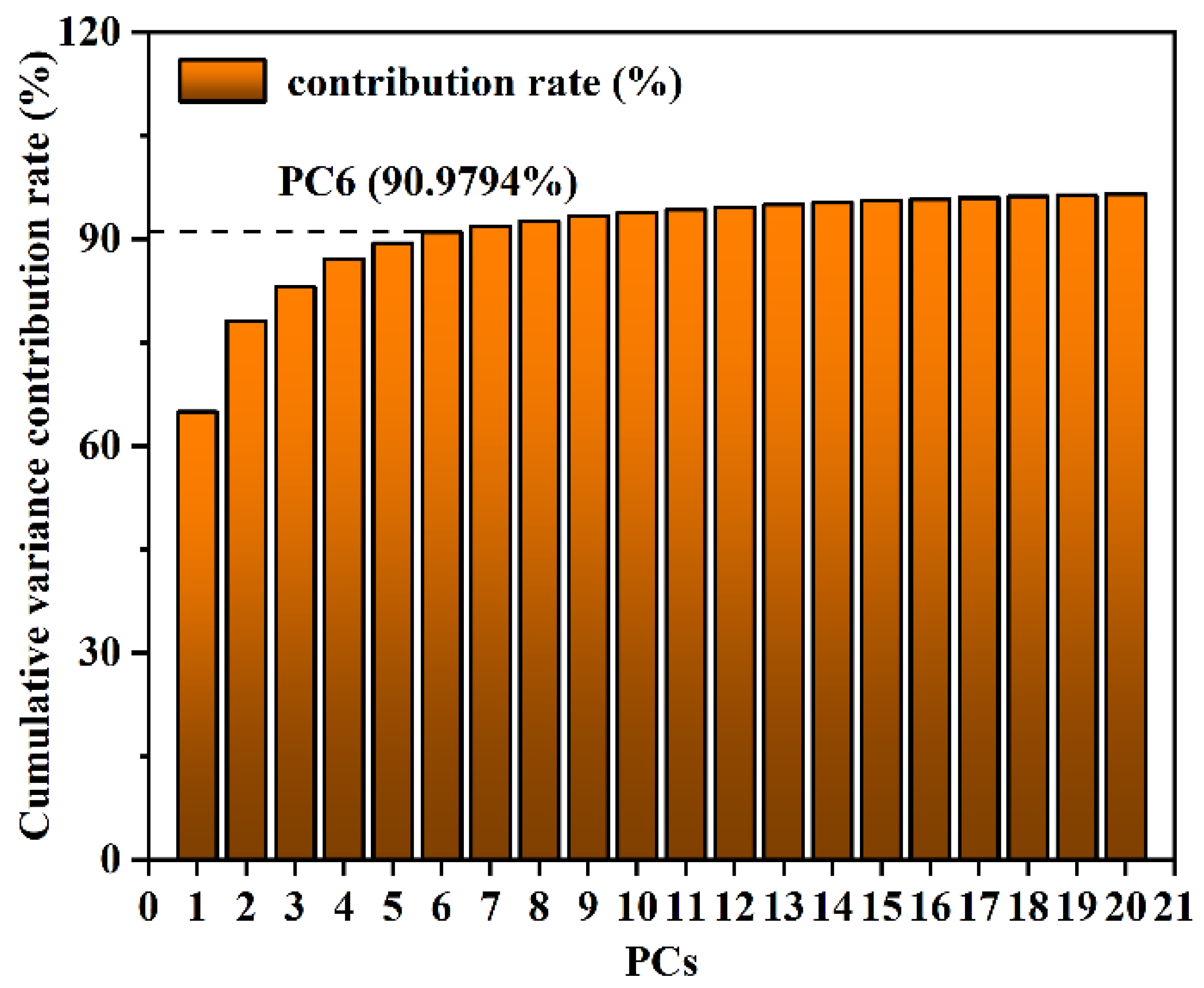
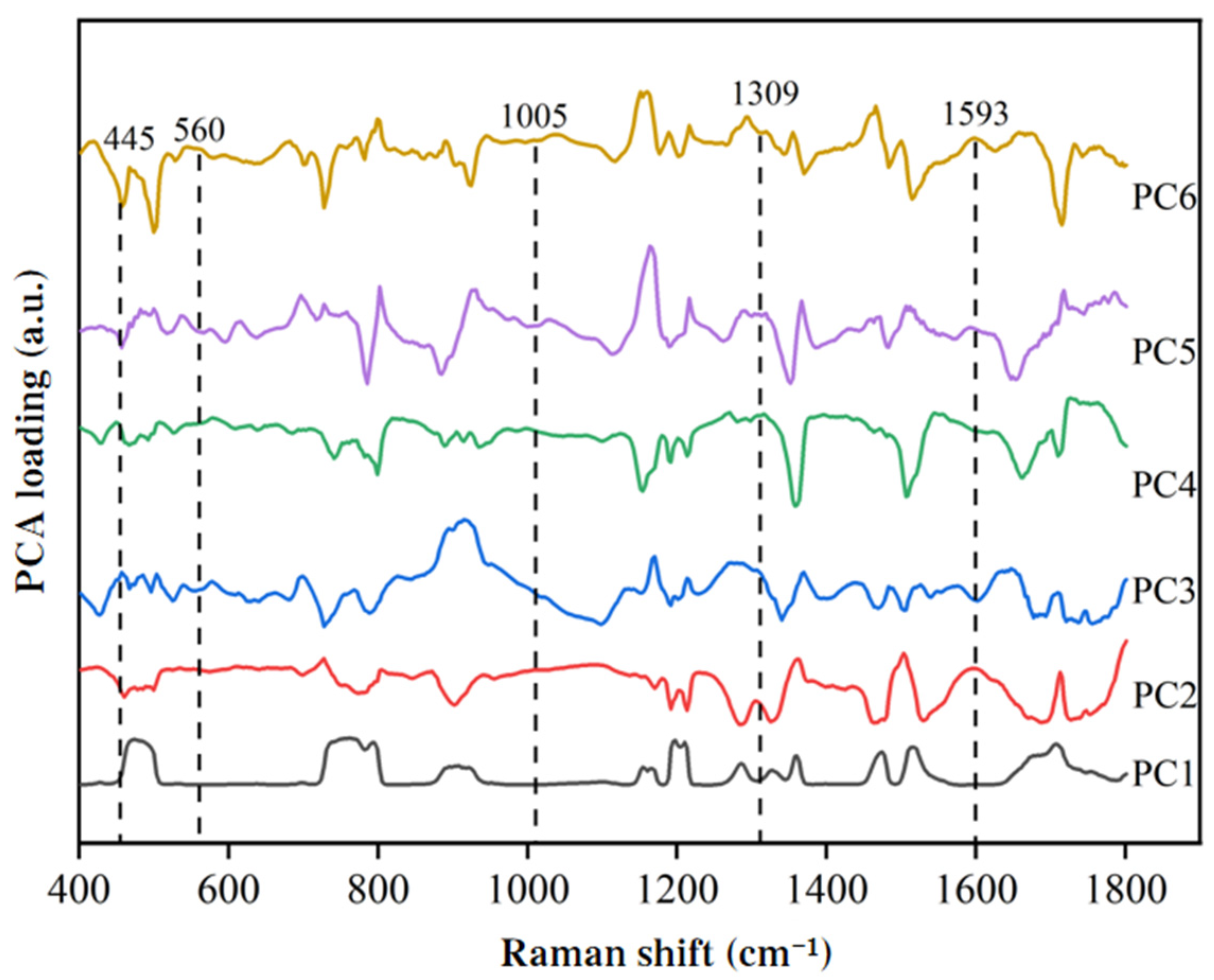
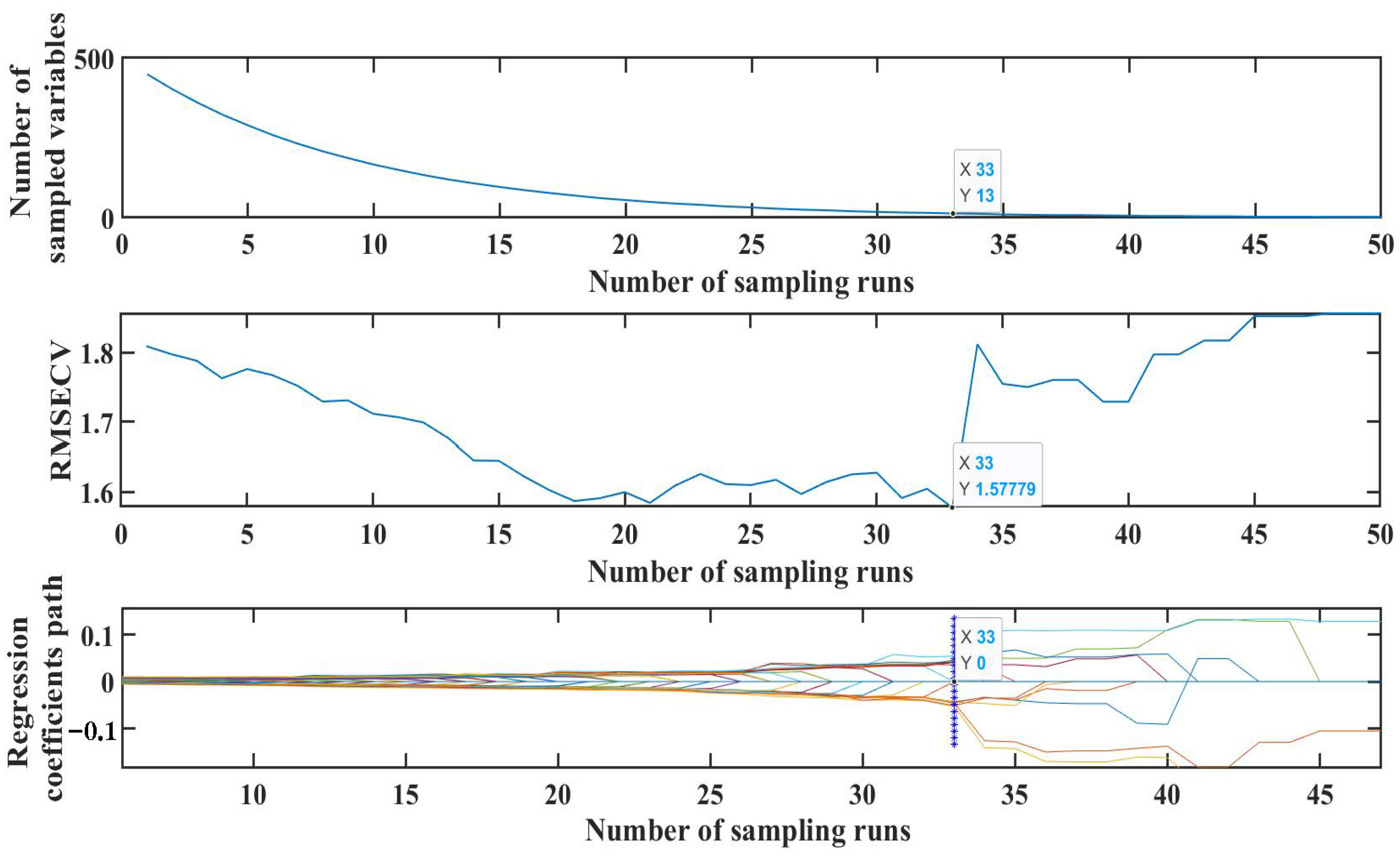
3.4.3. Extraction of Raman Spectral Features for SM2 Residues in Duck Blood
3.4.4. Quantitative Prediction Model of SM2 Residues in Duck Blood
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Menkem, Z.; Bronhilda-Lemalue, N.; Stella-Shinwin-Ateim, T. Antibiotic residues in food animals: Public health concern. Acta Ecol. Sin. 2019, 39, 411–415. [Google Scholar] [CrossRef]
- Wu, Q.; Hanbing, W.; Chenxi, L. ATP bioluminescence-based method for screening of multiclass antibiotic residues in animal derived foods. Microchem. J. 2023, 193, 108981. [Google Scholar] [CrossRef]
- Sartini, I.; Łebkowska-Wieruszewska, B.; Lisowski, A.; Poapolathep, A.; Giorgi, M. Danofloxacin pharmacokinetics and tissue residues in Bilgorajska geese. Res. Vet. Sci. 2021, 136, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.M.; El Zowalaty, M.E.; Lundkvist, Å.; Jarhult, J.D.; Nayem, M.R.K.; Tanzin, A.Z.; Badsha, M.R.; Khan, S.A.; Ashour, H.M. Residual antimicrobial agents in food originating from animals. Trends Food Sci. Technol. 2021, 111, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Aziz, H.; Palaniandy, P.; Naushad, M.; Evik, E.; Khan, N.; Zahmatkesh, S. Pharmaceutical residues in the ecosystem: Antibiotic resistance, health impacts, and removal techniques. Chemosphere 2023, 339, 139647. [Google Scholar] [CrossRef]
- Manikandan, M.; Chun, S.; Kazibwe, Z.; Gopal, J.; Oh, J. Phenomenal Bombardment of Antibiotic in Poultry: Contemplating the Environmental Repercussions. Int. J. Environ. Res. Public Health 2020, 17, 5053. [Google Scholar] [CrossRef]
- Chaijak, P. New method of sulfadiazine residue biodegradation in poultry manure by Spore-bounding laccase. Acta Biol. Colomb. 2023, 28, 547–551. [Google Scholar] [CrossRef]
- Chen, Y.; Xia, S.; Han, X.; Fu, Z. Simultaneous Determination of Malachite Green, Chloramphenicols, Sulfonamides, and Fluoroquinolones Residues in Fish by Liquid Chromatography-Mass Spectrometry. Anal. Methods Chem. 2020, 2020, 3725618. [Google Scholar] [CrossRef]
- Rehm, S.; Rentsch, K.M. A 2D HPLC-MS/MS method for several antibiotics in blood plasma, plasma water, and diverse tissue samples. Anal. Bioanal. Chem. 2020, 412, 715–725. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, W.; Liu, Z.; Fu, X.; Du, D. High-performance liquid chromatography for the sensitive zearalenone determination by the automated immunomagnetic beads purifier for one-step sample pre-treatment. Eur. Food Res. Technol. 2022, 248, 109–117. [Google Scholar] [CrossRef]
- Jiang, L.; Hassan, M.M.; Ali, S.; Sheng, R.; Chen, Q. Evolving trends in SERS-based techniques for food quality and safety: A review. Trends Food Sci. Technol. 2021, 112, 225–242. [Google Scholar] [CrossRef]
- Duan, N.; Chang, B.; Zhang, H.; Wang, Z.; Wu, S. Salmonella typhimurium detection using a surface-enhanced Raman scattering-based aptasensor. Int. J. Food Microbiol. 2016, 218, 38–43. [Google Scholar] [CrossRef]
- Xu, Y.X. Research on the Detection of Three Antibiotics Residues in Poultry Meat Based on SERS Technology. Master’s Thesis, Harbin Institute of Technology, Harbin, China, 2019. [Google Scholar]
- Wang, T. Study on SERS Rapid Identification of Typicalantibiotic Residues in Poultry Meat Based on Stoichiometry. Master’s Thesis, Jiangxi Agricultural University, Nanchang, China, 2021. [Google Scholar]
- Markina, N.E.; Markin, A.V.; Cialla-May, D. Cyclodextrin-assisted SERS determination of fluoroquinolone antibiotics in urine and blood plasma. Talanta 2023, 254, 124083. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Yarbrough, D.; Kozman, M.M.; Herrman, T.J.; Park, J.; Wang, R.; Kurouski, D. Rapid detection and prediction of chlortetracycline and oxytetracycline in animal feed using surface-enhanced Raman spectroscopy (SERS). Food Control 2020, 114, 107243. [Google Scholar] [CrossRef]
- Jiao, Y.; Zou, Z. Guide for the Use of National Food Safety Standards, 1st ed.; China Standards Press: Beijing, China, 2017; pp. 329–333. [Google Scholar]
- Xiong, Y.; Huang, J.; Wu, R.; Geng, X.; Zuo, H.; Wang, X.; Ai, S. Exploring Surface-Enhanced Raman Spectroscopy (SERS) Characteristic Peaks Screening Methods for the Rapid Determination of Chlorpyrifos Residues in Rice. Appl. Spectrosc. 2023, 77, 160–169. [Google Scholar] [CrossRef]
- Hu, W.; Yan, Z.; Li, H.; Qiu, J. Development of a new colloidal gold immunochromatographic strip for rapid detecting subgroup A of avian leukosis virus using colloidal gold nanoparticles. Biochem. Eng. J. 2019, 148, 16–23. [Google Scholar] [CrossRef]
- Bastús, N.G.; Comenge, J.; Puntes, V. Kinetically Controlled Seeded Growth Synthesis of Citrate-Stabilized Gold Nanoparticles of up to 200 nm: Size Focusing versus Ostwald Ripening. Langmuir 2011, 27, 11098–11105. [Google Scholar] [CrossRef]
- Sun, L.; Wang, C. Highly Sensitive and Rapid Surface Enhanced Raman Spectroscopic (SERS) Determination of Thiram on the Epidermis of Fruits and Vegetables Using a Silver Nanoparticle-Modified Fibrous Swab. Anal. Lett. 2020, 53, 973–983. [Google Scholar] [CrossRef]
- Huang, L.; Wang, F.; Weng, S.; Pan, F.; Liang, D. Surface-Enhanced Raman Spectroscopy for Rapid and Accurate Detection of Fenitrothion Residue in Maize. Spectrosc. Spectr. Anal. 2018, 38, 2782–2787. [Google Scholar]
- Chen, W.; Zhu, L.; Han, Y. Application of surface-enhanced Raman spectroscopy to the detection of fenitrothion pesticide residues in watermelon. Food Sci. Technol. 2018, 43, 320–325. [Google Scholar]
- Gao, J.; Zhang, Z.; Li, G. Research progress of quantitative analysis techniques for surface Enhanced Raman Spectroscopy. J. Instrum. Anal. 2016, 35, 1647–1653. [Google Scholar]
- Li, Y.; Sun, J.; Wu, X.; Chen, Q.; Lu, B.; Dai, C. Detection of viability of soybean seed based on fluorescence hyperspectra and cars-svm-adaboost model. Food Process. Preserv. 2019, 43, e14265. [Google Scholar] [CrossRef]
- Barimah, A.O.; Guo, Z.; Agyekum, A.A.; Guo, C.; Chen, P.; El-Seedi, H.R.; Zou, X.; Chen, Q. Sensitive Label-Free Cu2O/Ag Fused Chemometrics SERS Sensor for Rapid Detection of Total Arsenic in Tea. ACS Appl. Mater. Interfaces 2021, 130, 108341. [Google Scholar] [CrossRef]
- Li, L.; Cui, Q.; Li, M.; Li, T.; Cao, S.; Dong, S.; Wang, Y.; Dai, Q.; Ning, M. Rapid detection of multiple colorant adulteration in Keemun black tea based on hemp spherical AgNPs-SERS. Food Chem. 2023, 398, 133841. [Google Scholar] [CrossRef]
- Beattie, J.R.; Esmonde-White, F. Exploration of Principal Component Analysis: Deriving Principal Component Analysis Visually Using Spectra. Appl. Spectrosc. 2021, 75, 361–375. [Google Scholar] [CrossRef]
- Pořízka, P.; Klus, J.; Képeš, E.; Prochazka, D.; Hahn, D.W.; Kaiser, J. On the utilization of principal component analysis in laser-induced breakdown spectroscopy data analysis, a review. Spectrochim. Acta Part B At. Spectrosc. 2018, 148, 65–82. [Google Scholar] [CrossRef]
- Hamed, R.; Shehdeh, J.; Hanbali, G.; Safi, Z.; Berisha, A.; Xhaxhiu, K.; Dagdag, O. Eco-Friendly Synthesis and Characterization of Double-Crossed Link 3D Graphene Oxide Functionalized with Chitosan for Adsorption of Sulfamethazine from Aqueous Solution: Experimental and DFT Calculations. Front. Environ. Sci. 2022, 10, 930693. [Google Scholar] [CrossRef]
- Kumar, M.; Aysha, F.; Meenakshi, S.; Verma, G.; Khanum, G.; Muthu, S.; Ahthubeit, K. Experimental Spectroscopic, Quantum Computational, Hirshfeld Surface, Molecular Docking, and Electronic Excitation Studies on an Antibiotic Agent: SDZ. Polycycl. Aromat. Compd. 2023, 43, 3122–3146. [Google Scholar] [CrossRef]
- Jiang, G.; Liu, L.; Wan, Y.; Li, J.; Pi, F. Surface-enhanced Raman scattering based determination on sulfamethazine using molecularly imprinted polymers decorated with silver nanoparticles. Mikrochim. Acta 2023, 190, 169. [Google Scholar] [CrossRef]
- Hassan, M.M.; Zareef, M.; Jiao, T.; Liu, S.; Xu, Y.; Viswadevarayalu, A.; Li, H.; Chen, Q. Signal Optimized Rough Silver Nanoparticle for Rapid SERS Sensing of Pesticide Residues in Tea. ACS Appl. Mater. Interfaces 2021, 338, 127796. [Google Scholar] [CrossRef]
- Iancu, S.D.; Stefancu, A.; Moisoiu, V.; Leopold, L.F.; Leopold, N. The Role of Ag+, Ca2+, Pb2+, and Al3+ Adions in the SERS Turn-On Effect of Anionic Analytes. Beilstein J. Nanotechnol. 2019, 10, 2338–2345. [Google Scholar] [CrossRef] [PubMed]
- Kline, N.D.; Tripathi, A.; Mirsafavi, R.; Pardoe, L.; Moskovits, M.; Meinhart, C.; Guicheteau, J.A.; Christesen, S.D.; Fountain, A.W. Optimization of Surface-Enhanced Raman Spectroscopy Conditions for Implementation into a Microfluidic Device for Drug Detection. Anal. Chem. 2016, 88, 10513–10522. [Google Scholar] [CrossRef] [PubMed]
- Marion, B.; Antoine, D.; Patrice, P.; Ois-Xavier, L.F.; Ali, T.; Lê, M.M.; Eric, C. Optimization of experimental conditions by surface enhanced Raman Scattering (SERS) spectroscopy with gold nanoparticles suspensions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 268, 120628. [Google Scholar]
- Horne, J.; Bleye, C.D.; Lebrun, P.; Kemik, K.; Laethem, T.V.; Sacré, P.Y.; Hubert, P.; Hubert, C.; Ziemons, E. Optimization of silver nanoparticles synthesis by chemical reduction to enhance SERS quantitative performances: Early characterization using the quality by design approach. J. Pharm. Biomed. Anal. 2023, 233, 115475. [Google Scholar] [CrossRef]
- Santos, J.J.; Toma, S.H.; Corio, P.; Araki, K. Key role of surface concentration on reproducibility and optimization of SERS sensitivity. J. Raman Spectrosc. 2017, 48, 1190–1195. [Google Scholar] [CrossRef]
- Wang, K.; Yue, Z.; Fang, X.; Lin, H.; Wang, L.; Cao, L.; Sui, J.; Ju, L. SERS detection of thiram using polyacrylamide hydrogel-enclosed gold nanoparticle aggregates. Sci. Total Environ. 2023, 856, 159108. [Google Scholar] [CrossRef]
- Yang, T.; Guo, X.; Wang, H.; Fu, S.; Wen, Y.; Yang, H. Magnetically optimized SERS assay for rapid detection of trace drug-related biomarkers in saliva and fingerprints. Biosens. Bioelectron. 2015, 68, 350–357. [Google Scholar] [CrossRef]
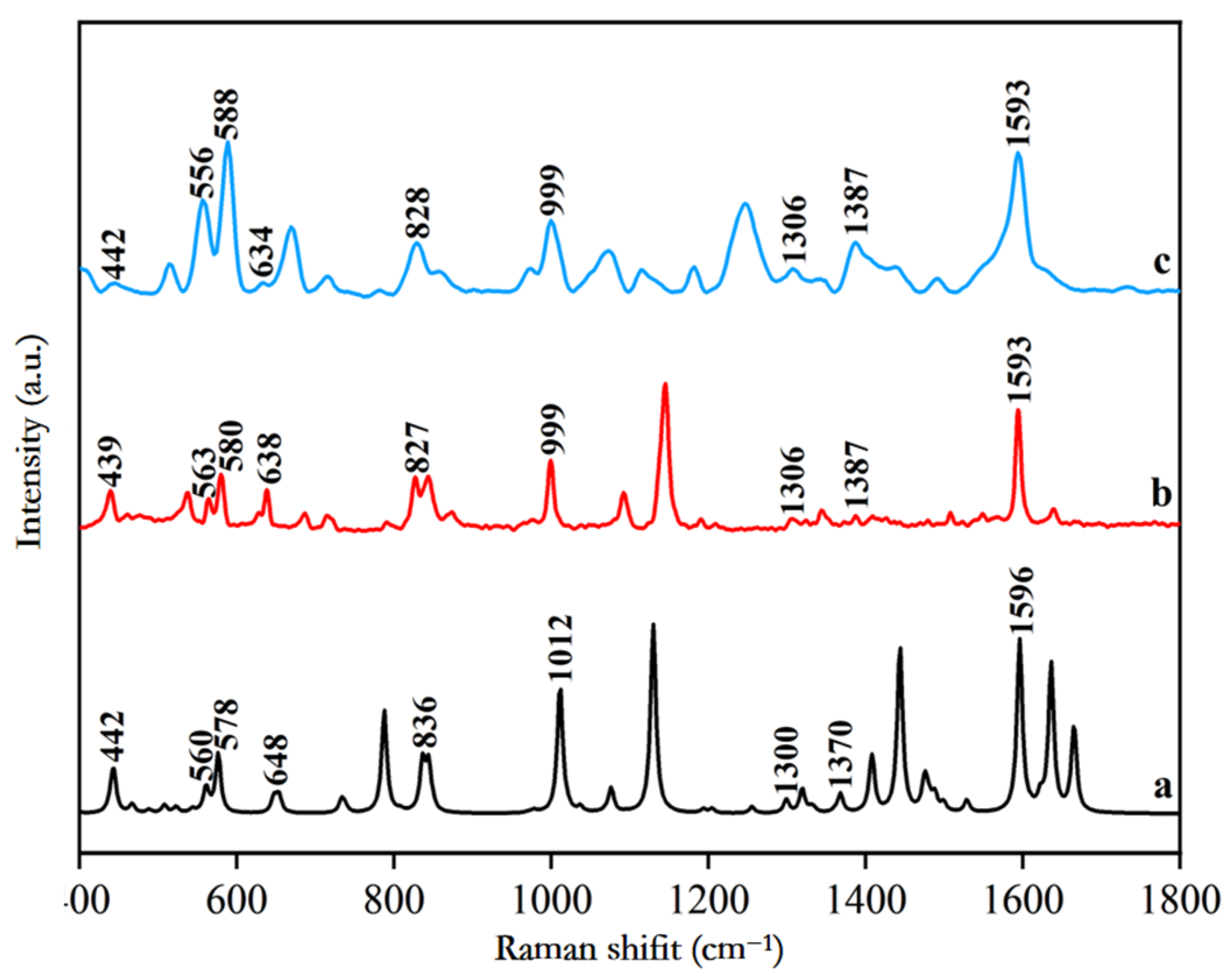
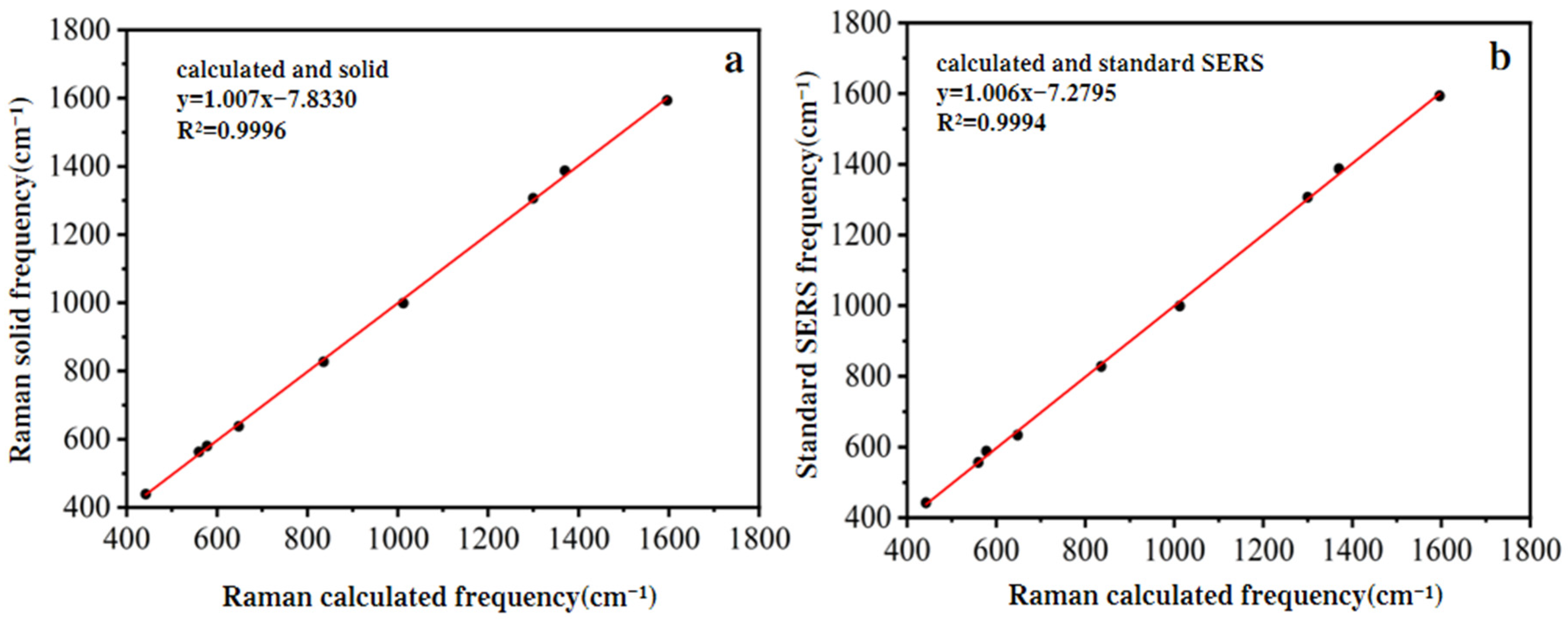



| Theoretical Raman (cm−1) | Solid Standard Raman Spectrum (cm−1) | SERS Spectra of Standard Aqueous Solution (cm−1) | Peak Position Attribution Analysis of SM2 |
|---|---|---|---|
| 442 | 439 | 442 | N-H, C-H in-plane rocking vibration |
| 560 | 563 | 556 | S=O stretching vibration, C-C-C in-plane rocking vibration, C-H out-of-plane rocking vibration |
| 578 | 580 | 588 | C-C-C in-plane rocking vibration, C-H out-of-plane rocking vibration |
| 648 | 638 | 634 | C-C in-plane rocking vibration |
| 836 | 827 | 828 | C-H curling vibration |
| 1012 | 999 | 999 | C-C-N scissoring vibration |
| 1300 | 1306 | 1306 | S=O stretching vibration, N-H in-plane rocking vibration |
| 1370 | 1387 | 1387 | C-C stretching vibration, C-H out-of-plane rocking vibration |
| 1596 | 1593 | 1593 | C-N stretching vibration |
| Pretreatment Methods | Characteristic Number | Total Number of Variables | Rc2 | RMSEC | Rp2 | RMSEP |
|---|---|---|---|---|---|---|
| air-PLS | 13 | 499 | 0.9845 | 1.5561 | 0.9817 | 1.5539 |
| air-PLS + SG | 7 | 499 | 0.9832 | 1.5601 | 0.9804 | 1.5982 |
| air-PLS + first derivative | 4 | 499 | 0.9767 | 1.8011 | 0.9679 | 2.0163 |
| air-PLS + second derivative | 9 | 499 | 0.9792 | 1.7565 | 0.9720 | 1.8830 |
| air-PLS + normalization | 40 | 499 | 0.9716 | 2.6199 | 2.6199 | 5.1382 |
| air-PLS + SNV | 32 | 499 | 0.9620 | 2.8114 | 2.8114 | 4.2437 |
| air-PLS + MSC | 44 | 499 | 0.9814 | 2.2145 | 2.2145 | 4.3097 |
| Feature Extraction Method | Feature Number | Rc2 | RMSEC | Rp2 | RMSEP |
|---|---|---|---|---|---|
| CARS + MLR | 13 | 0.9845 | 1.5561 | 0.9817 | 1.5539 |
| PCA + MLR | 6 | 0.9719 | 2.0011 | 0.9652 | 2.1255 |
| Model | Rc2 | RMSEC | Rp2 | RMSEP | RPD |
|---|---|---|---|---|---|
| PLSR | 0.9670 | 2.0855 | 0.9572 | 2.3369 | 4.7532 |
| MLR | 0.9845 | 1.5561 | 0.9817 | 1.5539 | 7.1953 |
| SVR | 0.9797 | 1.6354 | 0.9724 | 1.9225 | 5.6295 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Zhou, R.; Lin, J.; Chen, Q.; Liu, P.; Huang, S.; Zhao, J. Study on Detection Method of Sulfamethazine Residues in Duck Blood Based on Surface-Enhanced Raman Spectroscopy. Biosensors 2025, 15, 286. https://doi.org/10.3390/bios15050286
Huang J, Zhou R, Lin J, Chen Q, Liu P, Huang S, Zhao J. Study on Detection Method of Sulfamethazine Residues in Duck Blood Based on Surface-Enhanced Raman Spectroscopy. Biosensors. 2025; 15(5):286. https://doi.org/10.3390/bios15050286
Chicago/Turabian StyleHuang, Junshi, Runhua Zhou, Jinlong Lin, Qi Chen, Ping Liu, Shuanggen Huang, and Jinhui Zhao. 2025. "Study on Detection Method of Sulfamethazine Residues in Duck Blood Based on Surface-Enhanced Raman Spectroscopy" Biosensors 15, no. 5: 286. https://doi.org/10.3390/bios15050286
APA StyleHuang, J., Zhou, R., Lin, J., Chen, Q., Liu, P., Huang, S., & Zhao, J. (2025). Study on Detection Method of Sulfamethazine Residues in Duck Blood Based on Surface-Enhanced Raman Spectroscopy. Biosensors, 15(5), 286. https://doi.org/10.3390/bios15050286






