Advancements in Single-Molecule Fluorescence Detection Techniques and Their Expansive Applications in Drug Discovery and Neuroscience
Abstract
1. Introduction
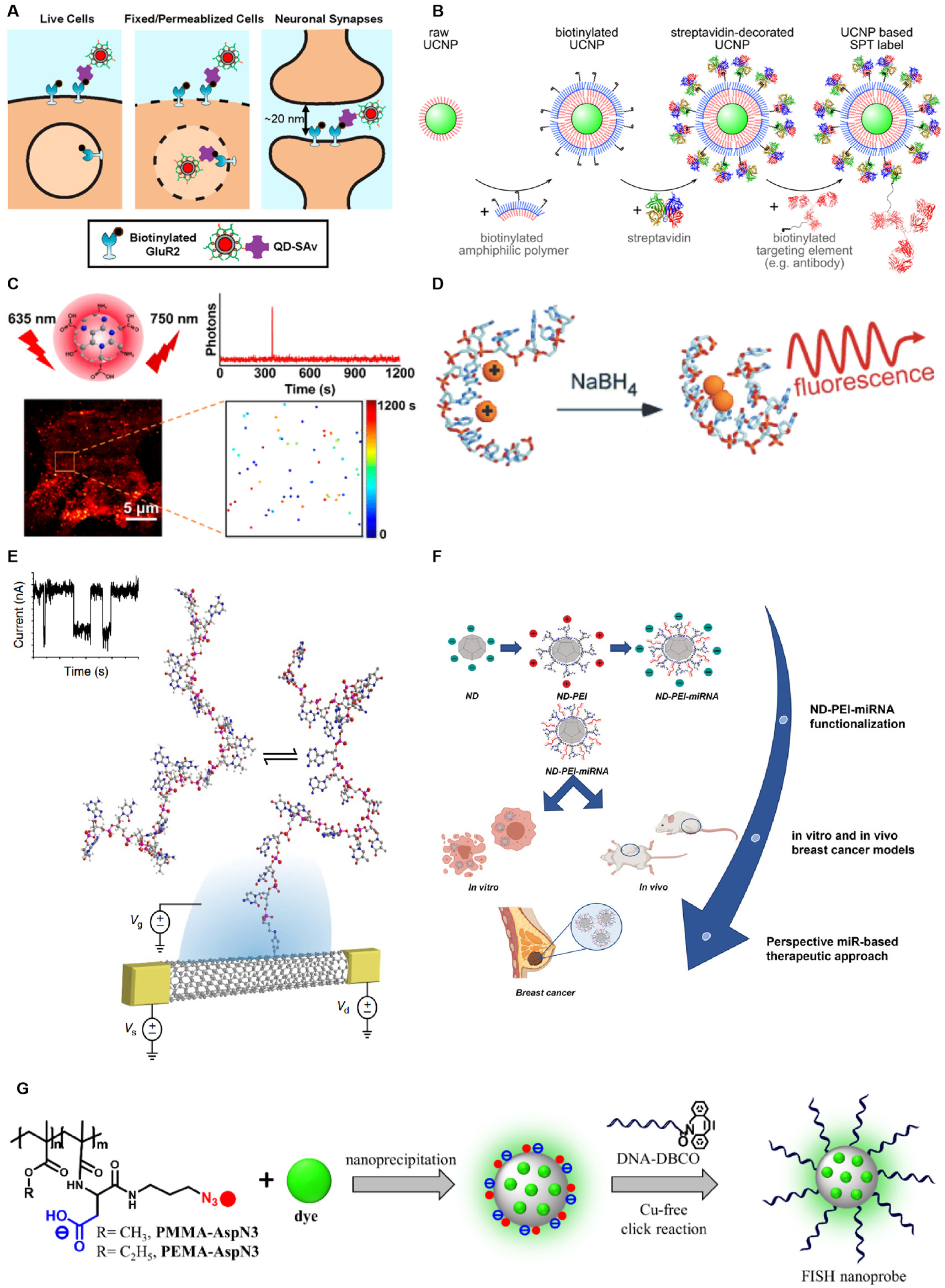
2. Single-Molecule Fluorescent Probe
3. Single-Molecule Fluorescence Microscopy System
3.1. Total Internal Reflection Fluorescence Microscopy
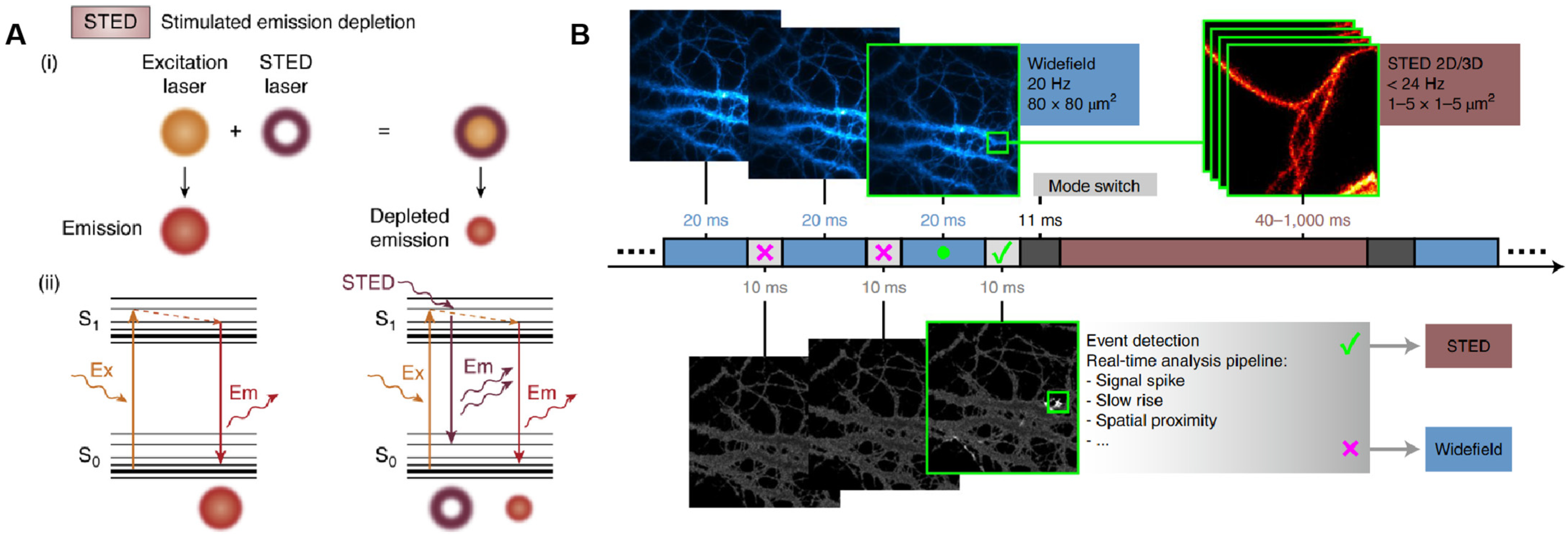
3.2. Stimulated Emission Depletion Microscopy
3.3. Single-Molecule Localization Microscopy
4. Single-Molecule Fluorescent Biosensors
5. Applications of Single-Molecule Fluorescence Detection in Drug Discovery and Neuroscience
5.1. Drug Discovery
5.2. Neuroscience
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ha, T.; Kaiser, C.; Myong, S.; Wu, B.; Xiao, J. Next Generation Single-Molecule Techniques: Imaging, Labeling, and Manipulation in Vitro and in Cellulo. Mol. Cell 2022, 82, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Blank, K.; De Cremer, G.; Hofkens, J. Fluorescence-Based Analysis of Enzymes at the Single-Molecule Level. Biotechnol. J. 2009, 4, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Widom, J.R.; Walter, N.G. Life under the Microscope: Single-Molecule Fluorescence Highlights the RNA World. Chem. Rev. 2018, 118, 4120–4155. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Li, Y.; Tang, B.; Zhang, C.-Y. Fluorescent Biosensors Based on Single-Molecule Counting. Acc. Chem. Res. 2016, 49, 1722–1730. [Google Scholar] [CrossRef] [PubMed]
- Betzig, E.; Chichester, R.J. Single Molecules Observed by Near-Field Scanning Optical Microscopy. Science 1993, 262, 1422–1425. [Google Scholar] [CrossRef] [PubMed]
- Funatsu, T.; Harada, Y.; Tokunaga, M.; Saito, K.; Yanagida, T. Imaging of Single Fluorescent Molecules and Individual ATP Turnovers by Single Myosin Molecules in Aqueous Solution. Nature 1995, 374, 555–559. [Google Scholar] [CrossRef]
- Grohmann, D.; Werner, F.; Tinnefeld, P. Making Connections--Strategies for Single Molecule Fluorescence Biophysics. Curr. Opin. Chem. Biol. 2013, 17, 691–698. [Google Scholar] [CrossRef]
- Gül, O.T.; Pugliese, K.M.; Choi, Y.; Sims, P.C.; Pan, D.; Rajapakse, A.J.; Weiss, G.A.; Collins, P.G. Single Molecule Bioelectronics and Their Application to Amplification-Free Measurement of DNA Lengths. Biosensors 2016, 6, 29. [Google Scholar] [CrossRef]
- Schirripa Spagnolo, C.; Luin, S. Choosing the Probe for Single-Molecule Fluorescence Microscopy. Int. J. Mol. Sci. 2022, 23, 14949. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.K.; Bumb, A.; Mills, M.; Neuman, K.C. SnapShot: Single-Molecule Fluorescence. Cell 2013, 153, 1408–1408.e1. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Wu, C.; Saqib, M.; Hao, R. Single-Molecule Fluorescence Methods for Protein Biomarker Analysis. Anal. Bioanal. Chem. 2023, 415, 3655–3669. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Qin, G.; Xia, T.; Fang, X. Single-Molecule Imaging of Protein Interactions and Dynamics. Annu. Rev. Anal. Chem. 2020, 13, 337–361. [Google Scholar] [CrossRef] [PubMed]
- Akkilic, N.; Geschwindner, S.; Höök, F. Single-Molecule Biosensors: Recent Advances and Applications. Biosens. Bioelectron. 2020, 151, 111944. [Google Scholar] [CrossRef] [PubMed]
- Shashkova, S.; Leake, M.C. Single-Molecule Fluorescence Microscopy Review: Shedding New Light on Old Problems. Biosci. Rep. 2017, 37, BSR20170031. [Google Scholar] [CrossRef]
- Ding, D.; Li, K.; Liu, B.; Tang, B.Z. Bioprobes Based on AIE Fluorogens. Acc. Chem. Res. 2013, 46, 2441–2453. [Google Scholar] [CrossRef]
- Freidus, L.G.; Pradeep, P.; Kumar, P.; Choonara, Y.E.; Pillay, V. Alternative Fluorophores Designed for Advanced Molecular Imaging. Drug Discov. Today 2018, 23, 115–133. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, F.; Wang, Y.; Li, H.; Zhang, J.; Sun, Z.; Jiang, Y. Fluorescent Organic Small Molecule Probes for Bioimaging and Detection Applications. Molecules 2022, 27, 8421. [Google Scholar] [CrossRef]
- Yang, S.K.; Shi, X.; Park, S.; Ha, T.; Zimmerman, S.C. A Dendritic Single-Molecule Fluorescent Probe That Is Monovalent, Photostable and Minimally Blinking. Nat. Chem. 2013, 5, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Mehwish, N.; Dou, X.; Zhao, Y.; Feng, C.-L. Supramolecular Fluorescent Hydrogelators as Bio-Imaging Probes. Mater. Horiz. 2019, 6, 14–44. [Google Scholar] [CrossRef]
- Jiang, C.; Song, Z.; Yu, L.; Ye, S.; He, H. Fluorescent Probes Based on Macrocyclic Hosts: Construction, Mechanism and Analytical Applications. TrAC Trends Anal. Chem. 2020, 133, 116086. [Google Scholar] [CrossRef]
- Wu, Q.; Zhou, Z.; Xu, L.; Zhong, H.; Xiong, B.; Ren, T.; Li, Z.; Yuan, L.; Zhang, X.-B. Multivalent Supramolecular Fluorescent Probes for Accurate Disease Imaging. Sci. Adv. 2024, 10, eadp8719. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, Y.; Liu, X.; Lu, C. Nanoparticle-Based Single Molecule Fluorescent Probes. Luminescence 2022, 37, 1808–1821. [Google Scholar] [CrossRef]
- Sobhanan, J.; Rival, J.V.; Anas, A.; Sidharth Shibu, E.; Takano, Y.; Biju, V. Luminescent Quantum Dots: Synthesis, Optical Properties, Bioimaging and Toxicity. Adv. Drug Deliv. Rev. 2023, 197, 114830. [Google Scholar] [CrossRef] [PubMed]
- Dukhno, O.; Ghosh, S.; Greiner, V.; Bou, S.; Godet, J.; Muhr, V.; Buchner, M.; Hirsch, T.; Mély, Y.; Przybilla, F. Targeted Single Particle Tracking with Upconverting Nanoparticles. ACS Appl. Mater. Interfaces 2024, 16, 11217–11227. [Google Scholar] [CrossRef] [PubMed]
- Dolai, J.; Joshi, P.; Mondal, P.P.; Maity, A.; Mukherjee, B.; Jana, N.R. Blinking Carbon Dots as a Super-Resolution Imaging Probe. ACS Appl. Mater. Interfaces 2024, 16, 16003–16010. [Google Scholar] [CrossRef]
- Rashi; Kaur, V.; Devi, A.; Bain, D.; Sen, T.; Patra, A. Probing the Fluorescence Intermittency of Bimetallic Nanoclusters Using Single-Molecule Fluorescence Spectroscopy. J. Phys. Chem. Lett. 2023, 14, 10166–10172. [Google Scholar] [CrossRef] [PubMed]
- Sorgenfrei, S.; Chiu, C.; Gonzalez, R.L.; Yu, Y.-J.; Kim, P.; Nuckolls, C.; Shepard, K.L. Label-Free Single-Molecule Detection of DNA-Hybridization Kinetics with a Carbon Nanotube Field-Effect Transistor. Nat. Nanotechnol. 2011, 6, 126–132. [Google Scholar] [CrossRef]
- Miller, B.S.; Bezinge, L.; Gliddon, H.D.; Huang, D.; Dold, G.; Gray, E.R.; Heaney, J.; Dobson, P.J.; Nastouli, E.; Morton, J.J.L.; et al. Spin-Enhanced Nanodiamond Biosensing for Ultrasensitive Diagnostics. Nature 2020, 587, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Le, P.; Vaidya, R.; Smith, L.D.; Han, Z.; Zahid, M.U.; Winter, J.; Sarkar, S.; Chung, H.J.; Perez-Pinera, P.; Selvin, P.R.; et al. Optimizing Quantum Dot Probe Size for Single-Receptor Imaging. ACS Nano 2020, 14, 8343–8358. [Google Scholar] [CrossRef]
- Mao, J.; Xue, M.; Guan, X.; Wang, Q.; Wang, Z.; Qin, G.; He, H. Near-Infrared Blinking Carbon Dots Designed for Quantitative Nanoscopy. Nano Lett. 2023, 23, 124–131. [Google Scholar] [CrossRef]
- Vosch, T.; Antoku, Y.; Hsiang, J.-C.; Richards, C.I.; Gonzalez, J.I.; Dickson, R.M. Strongly Emissive Individual DNA-Encapsulated Ag Nanoclusters as Single-Molecule Fluorophores. Proc. Natl. Acad. Sci. USA 2007, 104, 12616–12621. [Google Scholar] [CrossRef]
- Vernick, S.; Trocchia, S.M.; Warren, S.B.; Young, E.F.; Bouilly, D.; Gonzalez, R.L.; Nuckolls, C.; Shepard, K.L. Electrostatic Melting in a Single-Molecule Field-Effect Transistor with Applications in Genomic Identification. Nat. Commun. 2017, 8, 15450. [Google Scholar] [CrossRef] [PubMed]
- Abate, M.; Lombardi, A.; Luce, A.; Porru, M.; Leonetti, C.; Bocchetti, M.; Campani, V.; De Rosa, G.; Graziano, S.F.; Nele, V.; et al. Fluorescent Nanodiamonds as Innovative Delivery Systems for MiR-34a Replacement in Breast Cancer. Mol. Ther. Nucleic Acids 2023, 33, 127–141. [Google Scholar] [CrossRef]
- Egloff, S.; Melnychuk, N.; Cruz Da Silva, E.; Reisch, A.; Martin, S.; Klymchenko, A.S. Amplified Fluorescence in Situ Hybridization by Small and Bright Dye-Loaded Polymeric Nanoparticles. ACS Nano 2022, 16, 1381–1394. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.S.M.; LaGrow, A.P.; Prior, J.A.V. Quantum Dots for Cancer-Related miRNA Monitoring. ACS Sens. 2022, 7, 1269–1299. [Google Scholar] [CrossRef]
- García de Arquer, F.P.; Talapin, D.V.; Klimov, V.I.; Arakawa, Y.; Bayer, M.; Sargent, E.H. Semiconductor Quantum Dots: Technological Progress and Future Challenges. Science 2021, 373, eaaz8541. [Google Scholar] [CrossRef]
- Kesrevani, R.K.; Sharma, A.K. 2—Nanoarchitectured Biomaterials: Present Status and Future Prospects in Drug Delivery. In Nanoarchitectonics for Smart Delivery and Drug Targeting; Holban, A.M., Grumezescu, A.M., Eds.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 35–66. ISBN 978-0-323-47347-7. [Google Scholar]
- Kalyani, N.T.; Dhoble, S.J. 1—Introduction to Nano Materials. In Quantum Dots; Thejo Kalyani, N., Dhoble, S.J., Michalska-Domańska, M., Vengadaesvaran, B., Nagabhushana, H., Arof, A.K., Eds.; Woodhead Publishing Series in Electronic and Optical Materials; Woodhead Publishing: Oxford, UK, 2023; pp. 3–40. ISBN 978-0-323-85278-4. [Google Scholar]
- He, H.; Liu, L.; Chen, X.; Wang, Q.; Wang, X.; Nau, W.M.; Huang, F. Carbon Dot Blinking Enables Accurate Molecular Counting at Nanoscale Resolution. Anal. Chem. 2021, 93, 3968–3975. [Google Scholar] [CrossRef]
- Peng, M.; Fang, Z.; Na, N.; Ouyang, J. A Versatile Single-Molecule Counting-Based Platform by Generation of Fluorescent Silver Nanoclusters for Sensitive Detection of Multiple Nucleic Acids. Nanoscale 2019, 11, 16606–16613. [Google Scholar] [CrossRef]
- Wagner, A.M.; Knipe, J.M.; Orive, G.; Peppas, N.A. Quantum Dots in Biomedical Applications. Acta Biomater. 2019, 94, 44–63. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Kim, D.-H.; Zhou, K.; Jeong, M.G.; Park, S.; Kwon, Y.; Hong, T.M.; Noh, J.; Ryu, S.H. Improved Resolution in Single-Molecule Localization Microscopy Using QD-PAINT. Exp. Mol. Med. 2021, 53, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Carlson, L.J.; Krauss, T.D. Photophysics of Individual Single-Walled Carbon Nanotubes. Acc. Chem. Res. 2008, 41, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Del Bonis-O’Donnell, J.T.; Beyene, A.; Chio, L.; Demirer, G.; Yang, D.; Landry, M.P. Engineering Molecular Recognition with Bio-Mimetic Polymers on Single Walled Carbon Nanotubes. J. Vis. Exp. 2017, 119, e55030. [Google Scholar] [CrossRef]
- Campo, J.; Cambré, S.; Botka, B.; Obrzut, J.; Wenseleers, W.; Fagan, J.A. Optical Property Tuning of Single-Wall Carbon Nanotubes by Endohedral Encapsulation of a Wide Variety of Dielectric Molecules. ACS Nano 2021, 15, 2301–2317. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Schrage, C.A.; Gretz, J.; Akhtar, A.; Sistemich, L.; Schnitzler, L.; Li, H.; Tschulik, K.; Flavel, B.S.; Kruss, S. Stochastic Formation of Quantum Defects in Carbon Nanotubes. ACS Nano 2023, 17, 15989–15998. [Google Scholar] [CrossRef]
- Prabhakar, N.; Peurla, M.; Shenderova, O.; Rosenholm, J.M. Fluorescent and Electron-Dense Green Color Emitting Nanodiamonds for Single-Cell Correlative Microscopy. Molecules 2020, 25, 5897. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.-S.; Cho, K.-J.; Seol, Y.; Takagi, Y.; Dittmore, A.; Roche, P.A.; Neuman, K.C. Polydopamine Encapsulation of Fluorescent Nanodiamonds for Biomedical Applications. Adv. Funct. Mater. 2018, 28, 1801252. [Google Scholar] [CrossRef]
- Jung, H.-S.; Neuman, K.C. Surface Modification of Fluorescent Nanodiamonds for Biological Applications. Nanomaterials 2021, 11, 153. [Google Scholar] [CrossRef]
- Jiang, Y.; McNeill, J. Light-Harvesting and Amplified Energy Transfer in Conjugated Polymer Nanoparticles. Chem. Rev. 2017, 117, 838–859. [Google Scholar] [CrossRef]
- Archontakis, E.; Deng, L.; Zijlstra, P.; Palmans, A.R.A.; Albertazzi, L. Spectrally PAINTing a Single Chain Polymeric Nanoparticle at Super-Resolution. J. Am. Chem. Soc. 2022, 144, 23698–23707. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Rackers, W.H.; Sadtler, B. Getting the Most Out of Fluorogenic Probes: Challenges and Opportunities in Using Single-Molecule Fluorescence to Image Electro- and Photocatalysis. Chem. Biomed. Imaging 2023, 1, 692–715. [Google Scholar] [CrossRef]
- Webmaster the Nobel Prize in Chemistry. 2014. Available online: https://nbi.ku.dk/moed-os/niels_bohr_lectures/2014/nobel_prize_in_chemistry_2014/ (accessed on 16 October 2024).
- Colson, L.; Kwon, Y.; Nam, S.; Bhandari, A.; Maya, N.M.; Lu, Y.; Cho, Y. Trends in Single-Molecule Total Internal Reflection Fluorescence Imaging and Their Biological Applications with Lab-on-a-Chip Technology. Sensors 2023, 23, 7691. [Google Scholar] [CrossRef]
- Reck-Peterson, S.L.; Derr, N.D.; Stuurman, N. Imaging Single Molecules Using Total Internal Reflection Fluorescence Microscopy (TIRFM). Cold Spring Harb. Protoc. 2010, 2010, pdb.top73. [Google Scholar] [CrossRef] [PubMed]
- Schneckenburger, H. Total Internal Reflection Fluorescence Microscopy: Technical Innovations and Novel Applications. Curr. Opin. Biotechnol. 2005, 16, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Hon, J.; Lu, Y. Single-Molecule Methods for Measuring Ubiquitination and Protein Stability. Methods Enzymol. 2019, 619, 225–247. [Google Scholar] [CrossRef] [PubMed]
- Rief, M.; Žoldák, G. Single-Molecule Mechanical Studies of Chaperones and Their Clients. Biophys. Rev. 2022, 3, 041301. [Google Scholar] [CrossRef]
- Walker, G.; Brown, C.; Ge, X.; Kumar, S.; Muzumdar, M.D.; Gupta, K.; Bhattacharyya, M. Oligomeric Organization of Membrane Proteins from Native Membranes at Nanoscale Spatial and Single-Molecule Resolution. Nat. Nanotechnol. 2024, 19, 85–94. [Google Scholar] [CrossRef]
- Asada, R.; Dominguez, A.; Montpetit, B. Single-Molecule Quantitation of RNA-Binding Protein Occupancy and Stoichiometry Defines a Role for Yra1 (Aly/REF) in Nuclear mRNP Organization. Cell Rep. 2023, 42, 113415. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Kuai, Y.; Tang, X.; Zhang, Y.; Zhang, D. Chip-Based Wide Field-of-View Total Internal Reflection Fluorescence Microscopy. Opt. Lett. 2022, 47, 4303–4306. [Google Scholar] [CrossRef]
- Li, Q.; Hulleman, C.N.; Moerland, R.J.; Mailvaganam, E.; Ganapathy, S.; Brinks, D.; Stallinga, S.; Rieger, B. Waveguide-Based Total Internal Reflection Fluorescence Microscope Enabling Cellular Imaging under Cryogenic Conditions. Opt. Express 2021, 29, 34097–34108. [Google Scholar] [CrossRef] [PubMed]
- Sw, H. Nanoscopy with Focused Light (Nobel Lecture). Angew. Chem. 2015, 54, 8054–8066. [Google Scholar] [CrossRef]
- Jahr, W.; Velicky, P.; Danzl, J.G. Strategies to Maximize Performance in STimulated Emission Depletion (STED) Nanoscopy of Biological Specimens. Methods 2020, 174, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Valli, J.; Garcia-Burgos, A.; Rooney, L.M.; Vale de Melo E Oliveira, B.; Duncan, R.R.; Rickman, C. Seeing beyond the Limit: A Guide to Choosing the Right Super-Resolution Microscopy Technique. J. Biol. Chem. 2021, 297, 100791. [Google Scholar] [CrossRef] [PubMed]
- Blom, H.; Widengren, J. Stimulated Emission Depletion Microscopy. Chem. Rev. 2017, 117, 7377–7427. [Google Scholar] [CrossRef] [PubMed]
- Puchkov, D.; Müller, P.M.; Lehmann, M.; Matthaeus, C. Analyzing the Cellular Plasma Membrane by Fast and Efficient Correlative STED and Platinum Replica EM. Front. Cell Dev. Biol. 2023, 11, 1305680. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Maraspini, R.; Beutel, O.; Zehtabian, A.; Eickholt, B.; Honigmann, A.; Ewers, H. Expansion Stimulated Emission Depletion Microscopy (ExSTED). ACS Nano 2018, 12, 4178–4185. [Google Scholar] [CrossRef] [PubMed]
- Alvelid, J.; Damenti, M.; Sgattoni, C.; Testa, I. Event-Triggered STED Imaging. Nat. Methods 2022, 19, 1268–1275. [Google Scholar] [CrossRef]
- Lelek, M.; Gyparaki, M.T.; Beliu, G.; Schueder, F.; Griffié, J.; Manley, S.; Jungmann, R.; Sauer, M.; Lakadamyali, M.; Zimmer, C. Single-Molecule Localization Microscopy. Nat. Rev. Methods Primers 2021, 1, 39. [Google Scholar] [CrossRef] [PubMed]
- Tholen, M.M.E.; Tas, R.P.; Wang, Y.; Albertazzi, L. Beyond DNA: New Probes for PAINT Super-Resolution Microscopy. Chem. Commun. 2023, 59, 8332–8342. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.; van Engelenburg, S.B.; Lippincott-Schwartz, J. Superresolution Imaging of Biological Systems Using Photoactivated Localization Microscopy. Chem. Rev. 2014, 114, 3189–3202. [Google Scholar] [CrossRef] [PubMed]
- Betzig, E.; Patterson, G.H.; Sougrat, R.; Lindwasser, O.W.; Olenych, S.; Bonifacino, J.S.; Davidson, M.W.; Lippincott-Schwartz, J.; Hess, H.F. Imaging Intracellular Fluorescent Proteins at Nanometer Resolution. Science 2006, 313, 1642–1645. [Google Scholar] [CrossRef]
- Rust, M.J.; Bates, M.; Zhuang, X. Sub-Diffraction-Limit Imaging by Stochastic Optical Reconstruction Microscopy (STORM). Nat. Methods 2006, 3, 793–795. [Google Scholar] [CrossRef]
- Sharonov, A.; Hochstrasser, R.M. Wide-Field Subdiffraction Imaging by Accumulated Binding of Diffusing Probes. Proc. Natl. Acad. Sci. USA 2006, 103, 18911–18916. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Ji, C.; Wang, Y.; Tan, J.; Yuan, Q.; Tan, W. Nucleic Acid Probes for Single-Molecule Localization Imaging of Cellular Biomolecules. Chem. Biomed. Imaging 2023, 1, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Lemmer, P.; Gunkel, M.; Baddeley, D.; Kaufmann, R.; Urich, A.; Weiland, Y.; Reymann, J.; Müller, P.; Hausmann, M.; Cremer, C. SPDM: Light Microscopy with Single-Molecule Resolution at the Nanoscale. Appl. Phys. B 2008, 93, 1–12. [Google Scholar] [CrossRef]
- Burnette, D.T.; Sengupta, P.; Dai, Y.; Lippincott-Schwartz, J.; Kachar, B. Bleaching/Blinking Assisted Localization Microscopy for Superresolution Imaging Using Standard Fluorescent Molecules. Proc. Natl. Acad. Sci. USA 2011, 108, 21081–21086. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Chen, K.; Xu, K. Single Molecules Are Your Quanta: A Bottom-Up Approach toward Multidimensional Super-Resolution Microscopy. ACS Nano 2021, 15, 12483–12496. [Google Scholar] [CrossRef]
- Velas, L.; Brameshuber, M.; Huppa, J.B.; Kurz, E.; Dustin, M.L.; Zelger, P.; Jesacher, A.; Schütz, G.J. Three-Dimensional Single Molecule Localization Microscopy Reveals the Topography of the Immunological Synapse at Isotropic Precision below 15 Nm. Nano Lett. 2021, 21, 9247–9255. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Weihs, T.; Wurm, C.A.; Jansen, I.; Rehman, J.; Sahl, S.J.; Hell, S.W. MINFLUX Nanometer-Scale 3D Imaging and Microsecond-Range Tracking on a Common Fluorescence Microscope. Nat. Commun. 2021, 12, 1478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, O.; Guo, Z.; He, Y.; Wu, T.; Vahey, M.D.; Lew, M.D. Six-Dimensional Single-Molecule Imaging with Isotropic Resolution Using a Multi-View Reflector Microscope. Nat. Photonics 2023, 17, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Mitta, G.; Sezgin, Y.; Wang, W.; MacKinnon, R. Freestanding Bilayer Microscope for Single-Molecule Imaging of Membrane Proteins. Sci. Adv. 2024, 10, eado4722. [Google Scholar] [CrossRef] [PubMed]
- Levene, M.J.; Korlach, J.; Turner, S.W.; Foquet, M.; Craighead, H.G.; Webb, W.W. Zero-Mode Waveguides for Single-Molecule Analysis at High Concentrations. Science 2003, 299, 682–686. [Google Scholar] [CrossRef]
- Wang, H.; Lee, D.; Cao, Y.; Bi, X.; Du, J.; Miao, K.; Wei, L. Bond-Selective Fluorescence Imaging with Single-Molecule Sensitivity. Nat. Photonics 2023, 17, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Steves, M.A.; He, C.; Xu, K. Single-Molecule Spectroscopy and Super-Resolution Mapping of Physicochemical Parameters in Living Cells. Annu. Rev. Phys. Chem. 2024, 75, 163–183. [Google Scholar] [CrossRef]
- Jares-Erijman, E.A.; Jovin, T.M. FRET Imaging. Nat. Biotechnol. 2003, 21, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Miyawaki, A.; Llopis, J.; Heim, R.; McCaffery, J.M.; Adams, J.A.; Ikura, M.; Tsien, R.Y. Fluorescent Indicators for Ca2+ Based on Green Fluorescent Proteins and Calmodulin. Nature 1997, 388, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Fijen, C.; Montón Silva, A.; Hochkoeppler, A.; Hohlbein, J. A Single-Molecule FRET Sensor for Monitoring DNA Synthesis in Real Time. Phys. Chem. Chem. Phys. 2017, 19, 4222–4230. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, K.M.; Kanak, M.A.; Harrell, J.C.; Dhakal, S. Single-Molecule Sensor for High-Confidence Detection of miRNA. ACS Sens. 2022, 7, 1086–1094. [Google Scholar] [CrossRef]
- Kaur, A.; Sapkota, K.; Dhakal, S. Multiplexed Nucleic Acid Sensing with Single-Molecule FRET. ACS Sens. 2019, 4, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Megalathan, A.; Wijesinghe, K.M.; Dhakal, S. Single-Molecule FRET-Based Dynamic DNA Sensor. ACS Sens. 2021, 6, 1367–1374. [Google Scholar] [CrossRef]
- Khanna, K.; Mandal, S.; Blanchard, A.T.; Tewari, M.; Johnson-Buck, A.; Walter, N.G. Rapid Kinetic Fingerprinting of Single Nucleic Acid Molecules by a FRET-Based Dynamic Nanosensor. Biosens. Bioelectron. 2021, 190, 113433. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, C.; Liauw, B.W.-H.; Vafabakhsh, R. Visualizing the Conformational Dynamics of Membrane Receptors Using Single-Molecule FRET. J. Vis. Exp. JoVE 2022, 186. [Google Scholar] [CrossRef]
- Sapkota, K.; Dhakal, S. FRET-Based Aptasensor for the Selective and Sensitive Detection of Lysozyme. Sensors 2020, 20, 914. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Qiu, X.; Hildebrandt, N. When Nanoworlds Collide: Implementing DNA Amplification, Nanoparticles, Molecules, and FRET into a Single MicroRNA Biosensor. Nano Lett. 2021, 21, 4802–4808. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Lai, T.; Tao, G.; Hong, H.; Liu, F.; Li, N. Ultraspecific Multiplexed Detection of Low-Abundance Single-Nucleotide Variants by Combining a Masking Tactic with Fluorescent Nanoparticle Counting. Anal. Chem. 2018, 90, 4226–4233. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-H.; Wang, C.-R.; Liu, W.-J.; Xu, Q.; Zhang, C.-Y. Development of a Single Quantum Dot-Mediated FRET Biosensor for Amplification-Free Detection of Ten-Eleven Translocation 2. Talanta 2022, 239, 123135. [Google Scholar] [CrossRef]
- Huang, H.; Dong, F.; Tian, Y. Mitochondria-Targeted Ratiometric Fluorescent Nanosensor for Simultaneous Biosensing and Imaging of O2•− and pH in Live Cells. Anal. Chem. 2016, 88, 12294–12302. [Google Scholar] [CrossRef]
- Liu, M.; Zhong, N.; Zhang, L.; Zhang, Q.; Tian, X.; Ma, F.; Zhang, C.-Y. Single Probe-Based Catalytic Quantum Dot FRET Nanosensor for Human Alkyladenine DNA Glycosylase Detection. Talanta 2024, 266, 125089. [Google Scholar] [CrossRef]
- Krainer, G.; Saar, K.L.; Arter, W.E.; Welsh, T.J.; Czekalska, M.A.; Jacquat, R.P.; Peter, Q.; Traberg, W.C.; Pujari, A.; Jayaram, A.K.; et al. Direct Digital Sensing of Protein Biomarkers in Solution. Nat. Commun. 2023, 14, 653. [Google Scholar] [CrossRef] [PubMed]
- Gunnarsson, A.; Dexlin, L.; Wallin, P.; Svedhem, S.; Jönsson, P.; Wingren, C.; Höök, F. Kinetics of Ligand Binding to Membrane Receptors from Equilibrium Fluctuation Analysis of Single Binding Events. J. Am. Chem. Soc. 2011, 133, 14852–14855. [Google Scholar] [CrossRef] [PubMed]
- Cardellini, J.; Balestri, A.; Montis, C.; Berti, D. Advanced Static and Dynamic Fluorescence Microscopy Techniques to Investigate Drug Delivery Systems. Pharmaceutics 2021, 13, 861. [Google Scholar] [CrossRef]
- Belfiore, L.; Spenkelink, L.M.; Ranson, M.; van Oijen, A.M.; Vine, K.L. Quantification of Ligand Density and Stoichiometry on the Surface of Liposomes Using Single-Molecule Fluorescence Imaging. J. Control. Release 2018, 278, 80–86. [Google Scholar] [CrossRef]
- Joyce, P.; Jõemetsa, S.; Isaksson, S.; Hossain, S.; Larsson, P.; Bergström, C.; Höök, F. TIRF Microscopy-Based Monitoring of Drug Permeation Across a Lipid Membrane Supported on Mesoporous Silica. Angew. Chem. Int. Ed. Engl. 2021, 60, 2069–2073. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, N.; Näreoja, T.; von Haartman, E.; Karaman, D.Ş.; Jiang, H.; Koho, S.; Dolenko, T.A.; Hänninen, P.E.; Vlasov, D.I.; Ralchenko, V.G.; et al. Core-Shell Designs of Photoluminescent Nanodiamonds with Porous Silica Coatings for Bioimaging and Drug Delivery II: Application. Nanoscale 2013, 5, 3713–3722. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, L.; Li, X.; Hu, X.; Han, Y.; Luo, Y.; Wang, Z.; Li, Q.; Aldalbahi, A.; Wang, L.; et al. Size-Dependent Regulation of Intracellular Trafficking of Polystyrene Nanoparticle-Based Drug-Delivery Systems. ACS Appl. Mater. Interfaces 2017, 9, 18619–18625. [Google Scholar] [CrossRef] [PubMed]
- Andrian, T.; Muela, Y.; Delgado, L.; Albertazzi, L.; Pujals, S. A Super-Resolution and Transmission Electron Microscopy Correlative Approach to Study Intracellular Trafficking of Nanoparticles. Nanoscale 2023, 15, 14615–14627. [Google Scholar] [CrossRef] [PubMed]
- Kota, D.; Kang, L.; Rickel, A.; Liu, J.; Smith, S.; Hong, Z.; Wang, C. Low Doses of Zeolitic Imidazolate Framework-8 Nanoparticles Alter the Actin Organization and Contractility of Vascular Smooth Muscle Cells. J. Hazard. Mater. 2021, 414, 125514. [Google Scholar] [CrossRef]
- Yanagawa, M.; Sako, Y. Workflows of the Single-Molecule Imaging Analysis in Living Cells: Tutorial Guidance to the Measurement of the Drug Effects on a GPCR. Methods Mol. Biol. 2021, 2274, 391–441. [Google Scholar] [CrossRef]
- Wang, L.; Wasserman, M.R.; Feldman, M.B.; Altman, R.B.; Blanchard, S.C. Mechanistic Insights into Antibiotic Action on the Ribosome through Single-Molecule Fluorescence Imaging. Ann. N. Y. Acad. Sci. 2011, 1241, E1–E16. [Google Scholar] [CrossRef] [PubMed]
- Silva-Del Toro, S.L.; Allen, L.-A.H. Microtubules and Dynein Regulate Human Neutrophil Nuclear Volume and Hypersegmentation During H. Pylori Infection. Front. Immunol. 2021, 12, 653100. [Google Scholar] [CrossRef] [PubMed]
- Beri, D.; Singh, M.; Rodriguez, M.; Goyal, N.; Rasquinha, G.; Liu, Y.; An, X.; Yazdanbakhsh, K.; Lobo, C.A. Global Metabolomic Profiling of Host Red Blood Cells Infected with Babesia Divergens Reveals Novel Antiparasitic Target Pathways. Microbiol. Spectr. 2023, 11, e0468822. [Google Scholar] [CrossRef]
- Conze, C.; Trushina, N.I.; Holtmannspötter, M.; Rierola, M.; Attanasio, S.; Bakota, L.; Piehler, J.; Brandt, R. Super-Resolution Imaging and Quantitative Analysis of Microtubule Arrays in Model Neurons Show That Epothilone D Increases the Density but Decreases the Length and Straightness of Microtubules in Axon-like Processes. Brain Res. Bull. 2022, 190, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Weinelt, N.; Karathanasis, C.; Smith, S.; Medler, J.; Malkusch, S.; Fulda, S.; Wajant, H.; Heilemann, M.; van Wijk, S.J.L. Quantitative Single-Molecule Imaging of TNFR1 Reveals Zafirlukast as Antagonist of TNFR1 Clustering and TNFα-Induced NF-ĸB Signaling. J. Leukoc. Biol. 2021, 109, 363–371. [Google Scholar] [CrossRef]
- Hartley, J.M.; Zhang, R.; Gudheti, M.; Yang, J.; Kopeček, J. Tracking and Quantifying Polymer Therapeutic Distribution on a Cellular Level Using 3D dSTORM. J. Control. Release 2016, 231, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Tobin, S.J.; Wakefield, D.L.; Terenius, L.; Vukojević, V.; Jovanović-Talisman, T. Ethanol and Naltrexone Have Distinct Effects on the Lateral Nano-Organization of Mu and Kappa Opioid Receptors in the Plasma Membrane. ACS Chem. Neurosci. 2019, 10, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Yadav, J.; Kumar, Y.; Singaraju, G.S.; Patil, S. Interaction of Chloramphenicol with Titin I27 Probed Using Single-Molecule Force Spectroscopy. J. Biol. Phys. 2021, 47, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Krainer, G.; Treff, A.; Hartmann, A.; Stone, T.A.; Schenkel, M.; Keller, S.; Deber, C.M.; Schlierf, M. A Minimal Helical-Hairpin Motif Provides Molecular-Level Insights into Misfolding and Pharmacological Rescue of CFTR. Commun. Biol. 2018, 1, 154. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.; Sreenivasa, K.; Schenkel, M.; Chamachi, N.; Schake, P.; Krainer, G.; Schlierf, M. An Automated Single-Molecule FRET Platform for High-Content, Multiwell Plate Screening of Biomolecular Conformations and Dynamics. Nat. Commun. 2023, 14, 6511. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Elgeti, M.; O’Brien, E.S.; Sár, C.P.; Daibani, A.E.I.; Heng, J.; Sun, X.; White, E.; Che, T.; Hubbell, W.L.; et al. Ligand Efficacy Modulates Conformational Dynamics of the Μ-Opioid Receptor. Nature 2024, 629, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.B. Biochemistry and Neuroscience: The Twain Need to Meet. Curr. Opin. Neurobiol. 2017, 43, 79–86. [Google Scholar] [CrossRef]
- Miklosi, A.G.; Del Favero, G.; Bulat, T.; Höger, H.; Shigemoto, R.; Marko, D.; Lubec, G. Super-Resolution Microscopical Localization of Dopamine Receptors 1 and 2 in Rat Hippocampal Synaptosomes. Mol. Neurobiol. 2018, 55, 4857–4869. [Google Scholar] [CrossRef] [PubMed]
- Heller, J.P.; Odii, T.; Zheng, K.; Rusakov, D.A. Imaging Tripartite Synapses Using Super-Resolution Microscopy. Methods 2020, 174, 81–90. [Google Scholar] [CrossRef]
- Buxbaum, A.R.; Yoon, Y.J.; Singer, R.H.; Park, H.Y. Single-Molecule Insights into mRNA Dynamics in Neurons. Trends Cell Biol. 2015, 25, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Lim, H.; Yoon, Y.J.; Follenzi, A.; Nwokafor, C.; Lopez-Jones, M.; Meng, X.; Singer, R.H. Visualization of Dynamics of Single Endogenous mRNA Labeled in Live Mouse. Science 2014, 343, 422–424. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Eliscovich, C.; Yoon, Y.J.; Singer, R.H. Translation Dynamics of Single mRNAs in Live Cells and Neurons. Science 2016, 352, 1430–1435. [Google Scholar] [CrossRef] [PubMed]
- Bingham, D.; Jakobs, C.E.; Wernert, F.; Boroni-Rueda, F.; Jullien, N.; Schentarra, E.-M.; Friedl, K.; Da Costa Moura, J.; van Bommel, D.M.; Caillol, G.; et al. Presynapses Contain Distinct Actin Nanostructures. J. Cell Biol. 2023, 222, e202208110. [Google Scholar] [CrossRef]
- Frost, N.A.; Shroff, H.; Kong, H.; Betzig, E.; Blanpied, T.A. Single-Molecule Discrimination of Discrete Perisynaptic and Distributed Sites of Actin Filament Assembly within Dendritic Spines. Neuron 2010, 67, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Zhong, G.; Zhuang, X. Actin, Spectrin, and Associated Proteins Form a Periodic Cytoskeletal Structure in Axons. Science 2013, 339, 452–456. [Google Scholar] [CrossRef]
- Dani, A.; Huang, B.; Bergan, J.; Dulac, C.; Zhuang, X. Superresolution Imaging of Chemical Synapses in the Brain. Neuron 2010, 68, 843–856. [Google Scholar] [CrossRef] [PubMed]
- Glebov, O.O.; Jackson, R.E.; Winterflood, C.M.; Owen, D.M.; Barker, E.A.; Doherty, P.; Ewers, H.; Burrone, J. Nanoscale Structural Plasticity of the Active Zone Matrix Modulates Presynaptic Function. Cell Rep. 2017, 18, 2715–2728. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Chen, Q.; Zhao, H.; Yang, Q.; Goudappagouda; Gelléri, M.; Ritz, S.; Ng, D.; Koynov, K.; Parekh, S.H.; et al. Intrinsic Burst-Blinking Nanographenes for Super-Resolution Bioimaging. J. Am. Chem. Soc. 2024, 146, 5195–5203. [Google Scholar] [CrossRef]
- Laine, R.F.; Kaminski Schierle, G.S.; van de Linde, S.; Kaminski, C.F. From Single-Molecule Spectroscopy to Super-Resolution Imaging of the Neuron: A Review. Methods Appl. Fluoresc. 2016, 4, 022004. [Google Scholar] [CrossRef]
- Uversky, V.N. Intrinsically Disordered Proteins and Their (Disordered) Proteomes in Neurodegenerative Disorders. Front. Aging Neurosci. 2015, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Horrocks, M.H.; Lee, S.F.; Gandhi, S.; Magdalinou, N.K.; Chen, S.W.; Devine, M.J.; Tosatto, L.; Kjaergaard, M.; Beckwith, J.S.; Zetterberg, H.; et al. Single-Molecule Imaging of Individual Amyloid Protein Aggregates in Human Biofluids. ACS Chem. Neurosci. 2016, 7, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Rice, L.J.; Ecroyd, H.; van Oijen, A.M. Illuminating Amyloid Fibrils: Fluorescence-Based Single-Molecule Approaches. Comput. Struct. Biotechnol. J. 2021, 19, 4711–4724. [Google Scholar] [CrossRef] [PubMed]
- Paredes, J.M.; Casares, S.; Ruedas-Rama, M.J.; Fernandez, E.; Castello, F.; Varela, L.; Orte, A. Early Amyloidogenic Oligomerization Studied through Fluorescence Lifetime Correlation Spectroscopy. Int. J. Mol. Sci. 2012, 13, 9400–9418. [Google Scholar] [CrossRef]
- Ferreon, A.C.M.; Gambin, Y.; Lemke, E.A.; Deniz, A.A. Interplay of Alpha-Synuclein Binding and Conformational Switching Probed by Single-Molecule Fluorescence. Proc. Natl. Acad. Sci. USA 2009, 106, 5645–5650. [Google Scholar] [CrossRef]
- Elbaum-Garfinkle, S.; Rhoades, E. Identification of an Aggregation-Prone Structure of Tau. J. Am. Chem. Soc. 2012, 134, 16607–16613. [Google Scholar] [CrossRef]
- Warner, J.B.; Ruff, K.M.; Tan, P.S.; Lemke, E.A.; Pappu, R.V.; Lashuel, H.A. Monomeric Huntingtin Exon 1 Has Similar Overall Structural Features for Wild-Type and Pathological Polyglutamine Lengths. J. Am. Chem. Soc. 2017, 139, 14456–14469. [Google Scholar] [CrossRef]
- Narayan, P.; Orte, A.; Clarke, R.W.; Bolognesi, B.; Hook, S.; Ganzinger, K.A.; Meehan, S.; Wilson, M.R.; Dobson, C.M. The Extracellular Chaperone Clusterin Sequesters Oligomeric Forms of the Amyloid-β(1-40) Peptide. Nat. Struct. Mol. Biol. 2011, 19, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Michel, C.H.; Kumar, S.; Pinotsi, D.; Tunnacliffe, A.; St George-Hyslop, P.; Mandelkow, E.; Mandelkow, E.-M.; Kaminski, C.F.; Kaminski Schierle, G.S. Extracellular Monomeric Tau Protein Is Sufficient to Initiate the Spread of Tau Protein Pathology. J. Biol. Chem. 2014, 289, 956–967. [Google Scholar] [CrossRef]
- Pinotsi, D.; Michel, C.H.; Buell, A.K.; Laine, R.F.; Mahou, P.; Dobson, C.M.; Kaminski, C.F.; Kaminski Schierle, G.S. Nanoscopic Insights into Seeding Mechanisms and Toxicity of α-Synuclein Species in Neurons. Proc. Natl. Acad. Sci. USA 2016, 113, 3815–3819. [Google Scholar] [CrossRef] [PubMed]
- Codron, P.; Letournel, F.; Marty, S.; Renaud, L.; Bodin, A.; Duchesne, M.; Verny, C.; Lenaers, G.; Duyckaerts, C.; Julien, J.-P.; et al. STochastic Optical Reconstruction Microscopy (STORM) Reveals the Nanoscale Organization of Pathological Aggregates in Human Brain. Neuropathol. Appl. Neurobiol. 2021, 47, 127–142. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, J.; Cheng, L.; Li, Y.; Wang, R.; Wang, J. Advancements in Single-Molecule Fluorescence Detection Techniques and Their Expansive Applications in Drug Discovery and Neuroscience. Biosensors 2025, 15, 283. https://doi.org/10.3390/bios15050283
Yan J, Cheng L, Li Y, Wang R, Wang J. Advancements in Single-Molecule Fluorescence Detection Techniques and Their Expansive Applications in Drug Discovery and Neuroscience. Biosensors. 2025; 15(5):283. https://doi.org/10.3390/bios15050283
Chicago/Turabian StyleYan, Jing, Lin Cheng, Yitong Li, Ru Wang, and Jie Wang. 2025. "Advancements in Single-Molecule Fluorescence Detection Techniques and Their Expansive Applications in Drug Discovery and Neuroscience" Biosensors 15, no. 5: 283. https://doi.org/10.3390/bios15050283
APA StyleYan, J., Cheng, L., Li, Y., Wang, R., & Wang, J. (2025). Advancements in Single-Molecule Fluorescence Detection Techniques and Their Expansive Applications in Drug Discovery and Neuroscience. Biosensors, 15(5), 283. https://doi.org/10.3390/bios15050283




