Biosensors for Early Detection of Parkinson’s Disease: Principles, Applications, and Future Prospects
Abstract
1. Introduction
2. Parkinson’s Disease Biomarkers
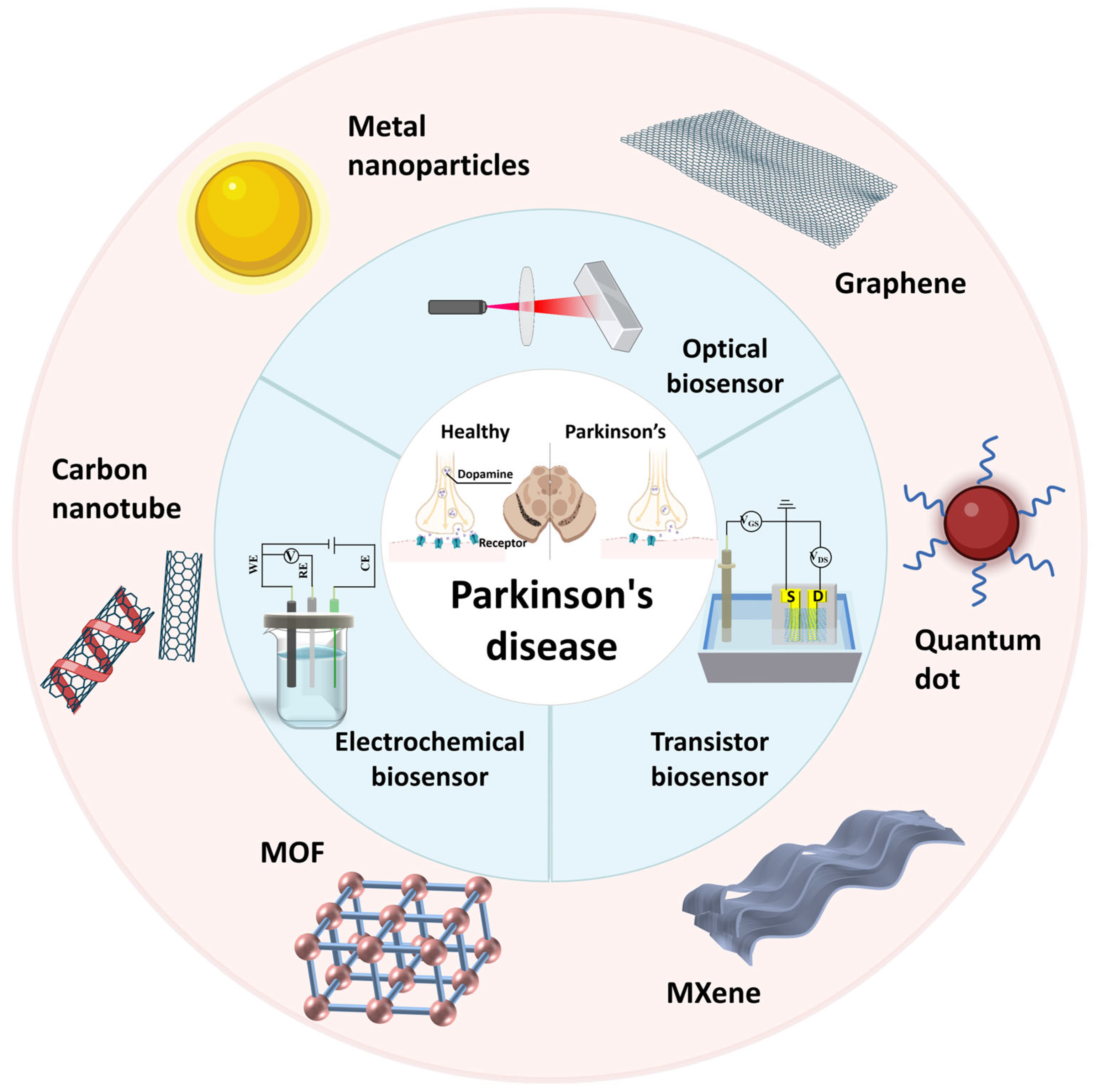
3. Parkinson’s Disease Biosensors
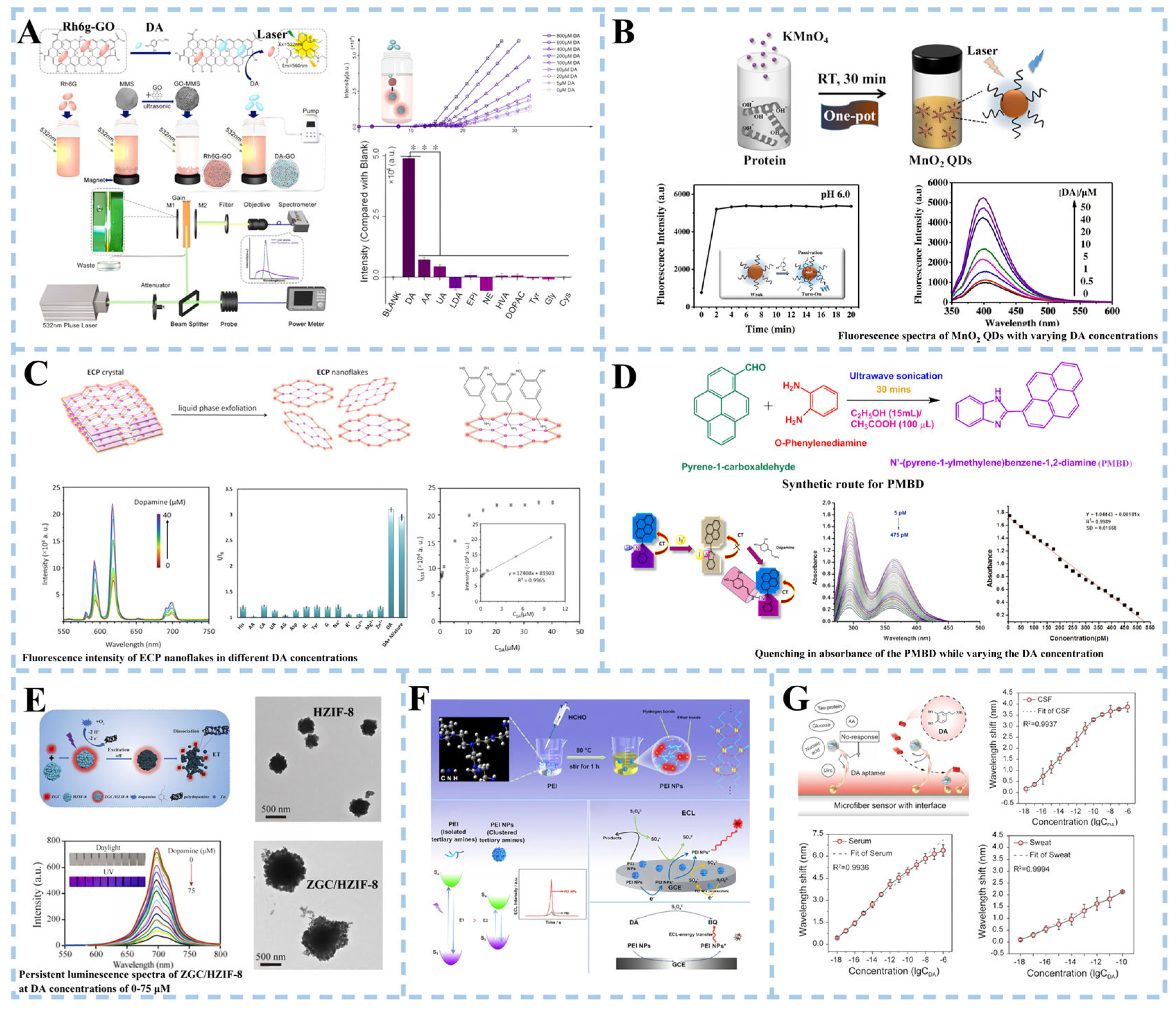
3.1. Optical Biosensors in PD Detection
3.1.1. Dopamine Detection
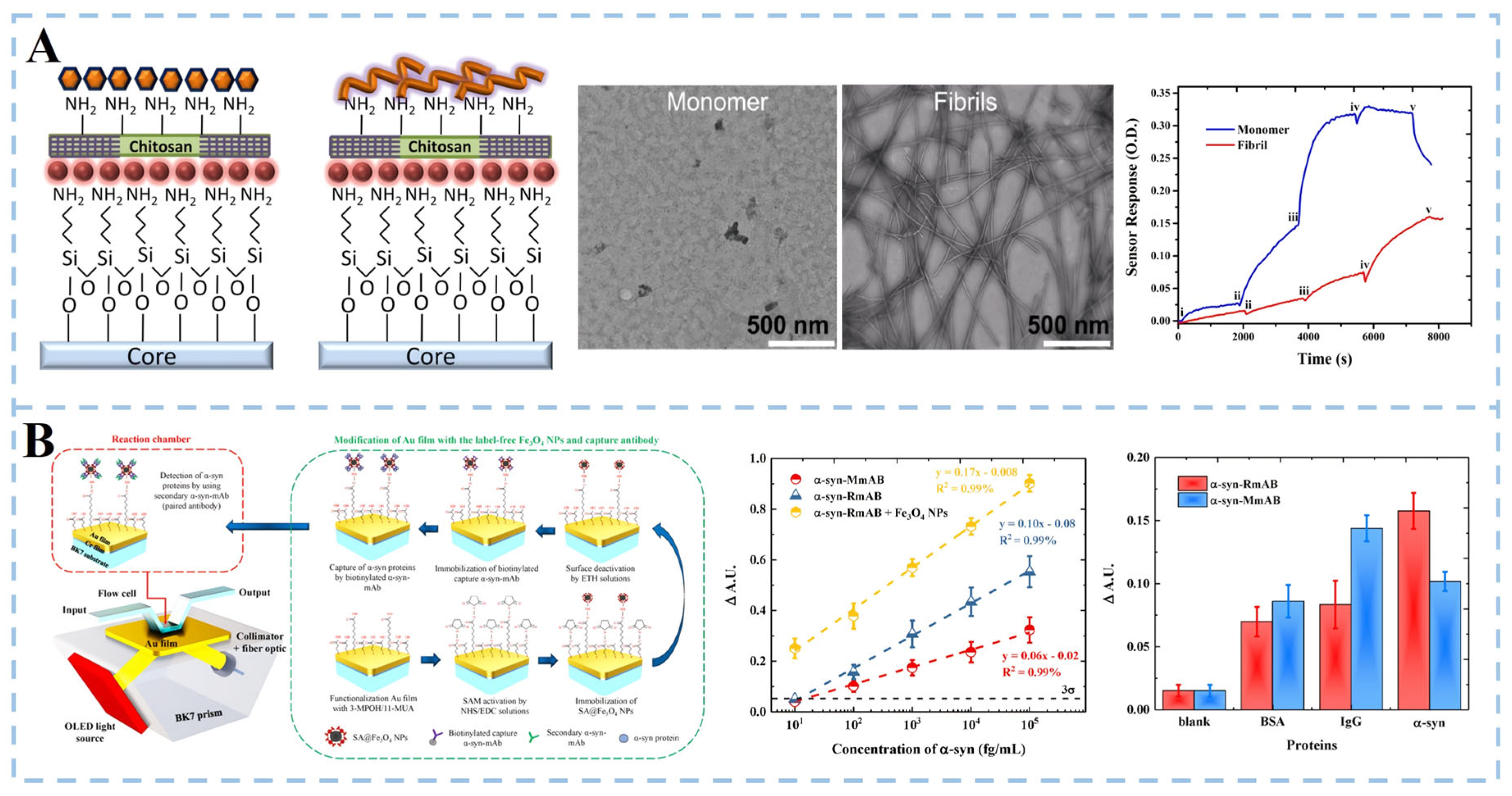
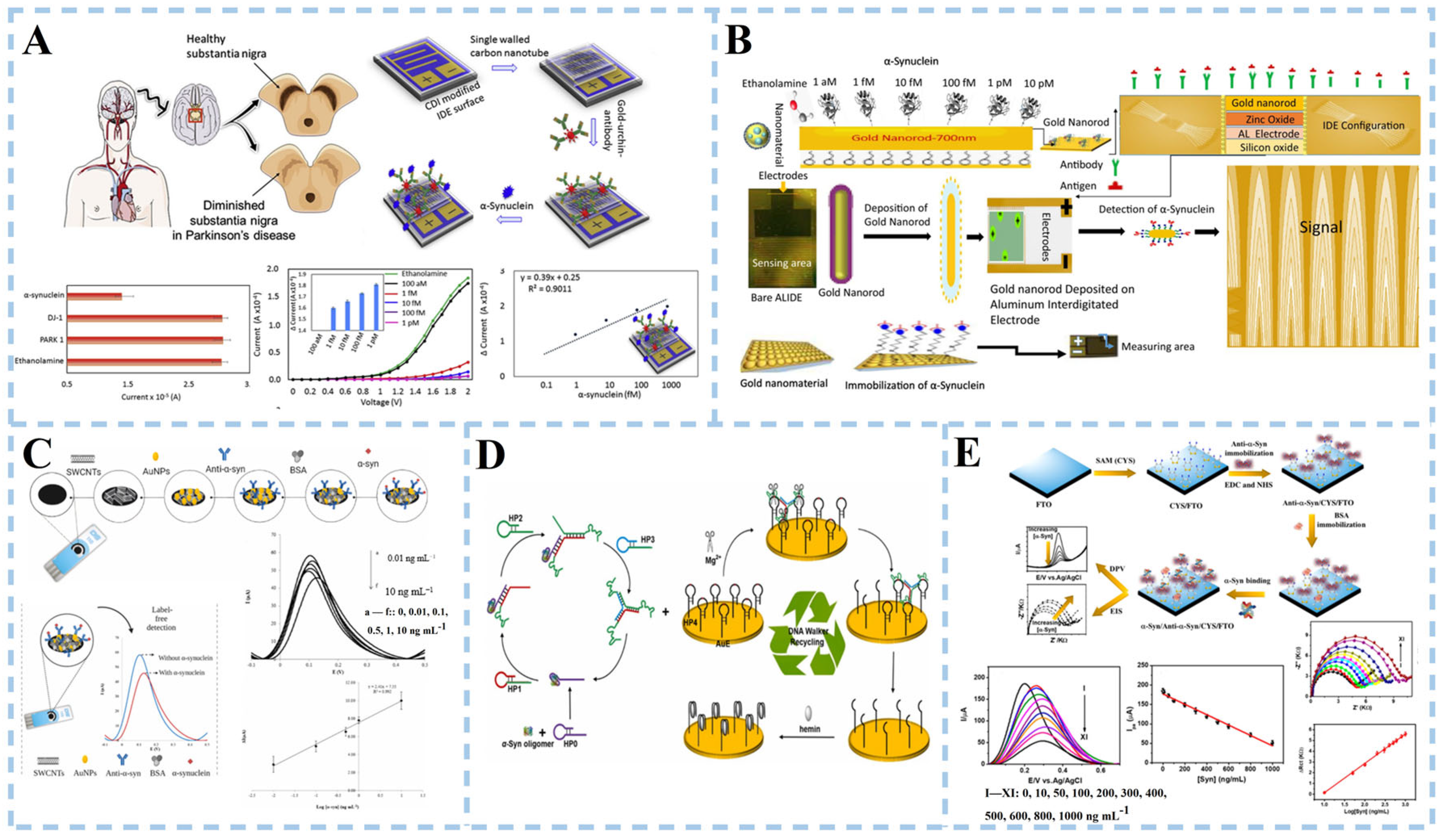
3.1.2. α-Synuclein Detection
3.1.3. Other Biomarker Detection
| Target | Method | Nanoparticles | Response Time | Linear Range | LOD | Selectivity | Stability | Ref |
|---|---|---|---|---|---|---|---|---|
| DA | Fluorescence | Si NPs | - | 1–10 μM, 10–50 μM | 0.22 μM | AA, UA, Tyr, Glu, NE, EP, Gly | 15 days | [44] |
| Fluorescence | ECP | 5 min | 0.1–10 μM | 21 nM | AA, UA, His, Tyr, Glu, Arg, Asp, AL, Na+, K+ | 5 times | [31] | |
| Fluorescence | GO | - | 3–1680 nM | 0.031 nM | Gly, Cys, Glu, Lac | 5 times | [45] | |
| LSPR | Cu2−xS@GO, Au@MoS2 | Seconds to minutes | - | 0.45 aM | UA, AA, Glu, NA, Tau | - | [36] | |
| Colorimetric | - | 2 min | 5–475 pM | 0.02 pM | UA, urea, AA, Glu, creatine, Na+, K+ | 5 times | [33] | |
| ECL | CDs | - | 0.01–100 μM | 62 nM | AA, UA | 14 times | [46] | |
| Colorimetric | CoFe2O4 | 135 s | 5–80 μM | 0.233 μM | Gly, GSH, Lys, AA, UA, Cu2+, Fe2+, Na+, K+ | - | [47] | |
| ECL | PEI NPs | - | 10 nM–10 μM | 43 nM | AA, His, Glu, Cys | 18 times | [35] | |
| ECL | Ir@MXene-PVA | - | 0.01–100 μM | 2 nM | AA, HVA | 8 times | [48] | |
| SERS | Au NPs/GNS/EPR | 3 s | 5–90 μM | 3.3 μM | - | - | [49] | |
| Colorimetric | Bi2Fe4O9 NPs | - | 0.15–50 μM | 51 nM | Cys, Glu, AA, GSH, Lys, Ala, Arg | 4 times | [50] | |
| LSPR | AuNRs@PANI | - | - | 7.11 μM | - | - | [51] | |
| SERS | Ag NPs | - | 1 fM–10 pM | 10 aM | - | 7 days | [52] | |
| Fluorescence | UCNPs | - | 0–200 μM | 83.6 nM | UA, AA, Glu, Gly, Na+, K+, SAM | - | [53] | |
| Fluorescence | N,Cl-CDs | - | 10–90 μM | 0.1212 μM | - | - | [54] | |
| Colorimetric | Co-MOFs/MoS2 | - | 1–10 μM | 0.83 μM | AA, UA, Glu | 12 times | [55] | |
| α-syn | Fluorescence | CDs | 5 min | 0.5–10 μM | 59 nM | Cys, Arg, His, Na+ | - | [56] |
| SPR | Au | - | 50–250 nM | 5.1 nM | - | 5 times | [57] | |
| Liquid crystal | - | - | 50–400 nM | 50 nM | Lysozyme, α-lac, PK, Str | - | [58] | |
| SPR | FpA | - | 7–240 ng/mL | 1.78 ng/mL | AA, Glu, BSA, | - | [40] | |
| SPR | Ti4+@TiP | 1–20 pg/mL 0.1–10 pg/mL (t-αS, p-αS) | 0.07 pg/mL, 0.032 pg/mL (t-αS, p-αS) | Aβ40, Aβ42 | ≥1 month | [41] | ||
| Colorimetric, SPR | Au NPs | 20 nM–3 μM, 0.1 nM–0.5 μM | 10 nM, 8 pM, | BSA, lysozyme, LgG, Thr | - | [59] | ||
| Fluorescence | Eu-MOF@Au | - | 0.0005–1 ng/mL | 0.04 pg/mL | Glu, BSA, lipid, DNase, DNase | - | [60] | |
| GFAP | Optical microfiber | Au NSs | - | 1 aM–0.1 nM | 0.09 aM | Na+, Glu, S100B, IL-6, AFP | - | [42] |
| ECL | CMCS@ATT-AgAu BMNCs | - | 100 ag/mL–160 pg/mL | 73 ag/mL | LgG, CEA, AFP | 10 times | [61] | |
| Fluorescence | MoS2@Ab@AuNCs | 10 min | 31.15–447.76 pg/mL | 1.30 pg/mL | Glu, creatine, BSA, UA, Cys, Arg, Na+, K+ | 5 days | [43] | |
| Fluorescence | CDs | - | 10 pg/mL–10 ng/mL | 2.2 pg/mL | BSA, CPR | 7 days | [62] |
3.2. Electrochemical Biosensors in PD Detection
3.2.1. Dopamine Detection
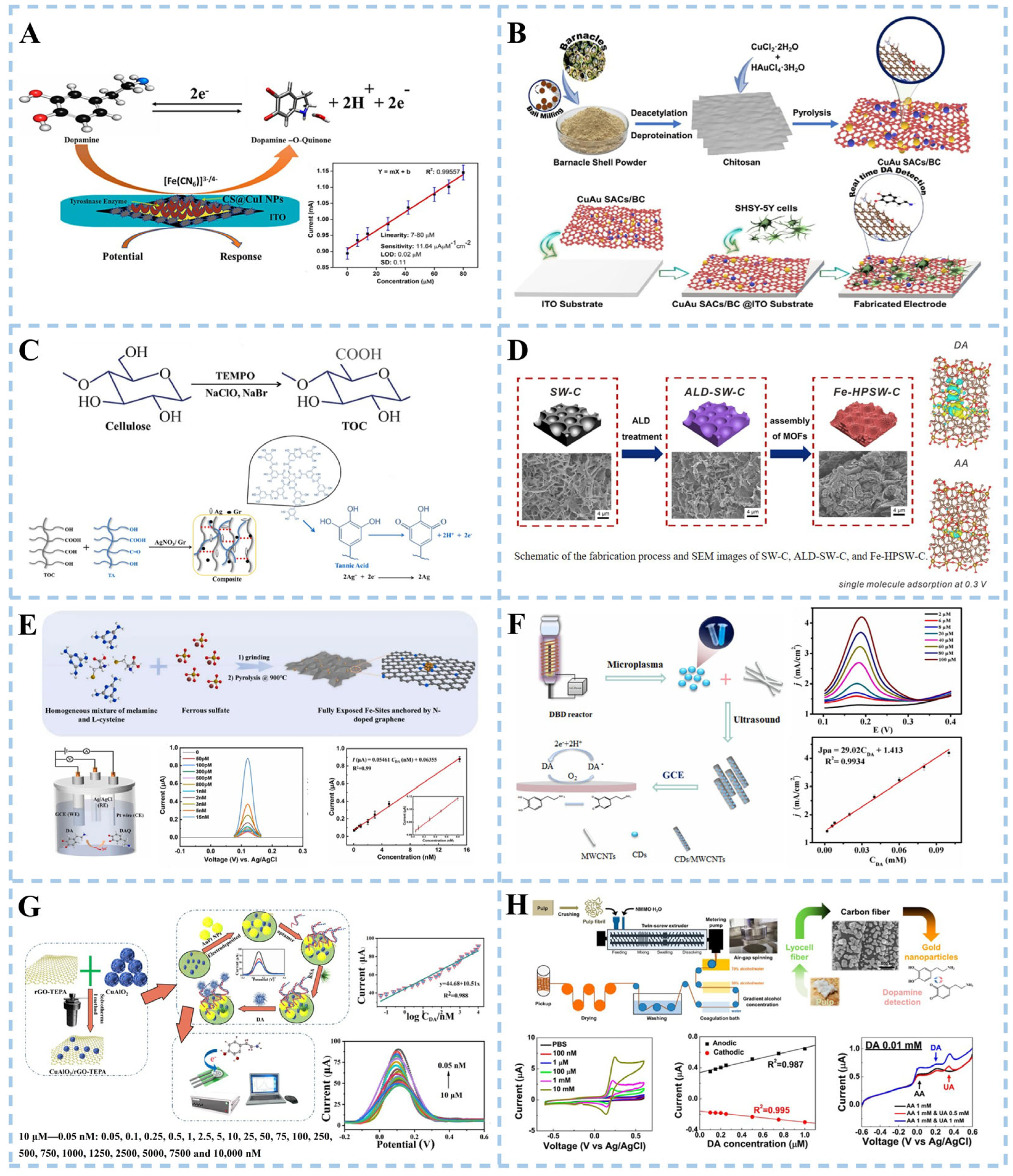
3.2.2. α-Synuclein Detection
3.2.3. Other Biomarker Detection
| Target | Technique | Nanoparticles | Electrode | Response Time | Sensitivity | Linear Range | LOD | Selectivity | Stability | Ref |
|---|---|---|---|---|---|---|---|---|---|---|
| DA | DPV | - | ZTO/ITO | - | 11.057 μAμg−1 mL cm−2 | 0.1–1 μM | 0.013 μM | UA, AA, BSA, | ≥4 years | [84] |
| DPV | MXene-Au | GCE | - | - | 0.1–100 μM | 0.04 μM | UA, AA | 11 times | [85] | |
| CA | MnO2 NSs | GCE | 20 s | - | 0.01–20 μM, 20–100 μM | 4.1 nM | AA, UA | 9 days | [86] | |
| DPV | Au-Sv-MoS2- CNTs | GCE | - | 0.0046 μA nM−1 | 2–900 nM | 2 nM | K+, Na+, Glu, Gly, AA, UA | 30 days | [87] | |
| DPV, Amperometry | TOC/AgNPs/Gr | GCE | - | 0.963 μAmΜ−1 cm−2 | 0.005–250 μM | 0.0005 μM | UA, AA, Glu | - | [67] | |
| DPV | CuAu SACs | ITO | 50 s | 17.15 μAμM−1 cm−2 | 1 nM–1 μΜ, 1–100 μM | 200 pM | AA, UA, Glu, Na+, K+, EP | 10 days | [66] | |
| CV, DPV | CGC-500 | SPCE | - | 13.75 μAμM−1 cm−2, 3.31 μAμM−1 cm−2 | 0.1–10 μM | 0.6 μM, 0.8 μM | UA, AA, Glu | 60 days | [73] | |
| DPV | SiC/graphene | Si | - | 0.86 μAμM−1 cm−2 | 0.5–78 μM | 0.11 μM | UA, AA, Na+, K+ | - | [88] | |
| CV, Amperometry | Fe-HPSW-C | GCE | - | 2084.58 μA mM−1 cm−2 | 1.0–200 μM | 0.73 μM | K+, Na+, THAM, Glu, AA | 15 h | [69] | |
| DPV | Fe/N-GR | GCE | 2.5 s | 0.05461 μA nM−1 | 50 pM–15 nM | 27 pM | Lactate, Glu, urea, adenosine, Thy | 35 days | [70] | |
| DPV | CDs/MWCNTs | GCE | - | 29,020 μAcm−2 mM−1 | 2–100 μM | 11.08 nM | AA, UA, Na+, K+, Cl−, NO3− | - | [71] | |
| DPV | Ni-MoS2 | GCE | - | - | 1 pM–1 mM | 1 pM | Glu, urea, UA, AA, Na+, K+ | 7 days | [68] | |
| DPV | CuAlO2/rGO-TEPA@AuPt | SPCE | Real-time | - | 0.05 nM–10 μM | 0.017 nM | UA, AA, L-cys, Glu | 30 days | [72] | |
| CV | Carbon fiber/gold | - | - | 0.320 ± 0.022 μA μM−1 | 0.1–10 μM | 25 nM | UA, AA | - | [74] | |
| DPV, CA | MPTMS@ Yb2O3 | GE | - | 1.88 μA mM−1 cm−2 | 1–40 μM | 41 nM, 77 nM | Glu, urea, AA | 100 times, 5 days | [89] | |
| CV, DPV | In1−N−C | GCE | - | 8.24 μAμM−1 cm−2 | - | 279 nM | AA, UA | 6 days | [90] | |
| DPV | Ti3C2Tx-MXene | SPCE | - | 0.0134 μA nM−1 | 40–500 nM | 1.3 nM | EP, NE, ST | 15 days | [91] | |
| DA | Amperometry | PtNi@N-GQDs | SPCE | 3 s | 0.279 μAμM−1 cm−2 | 0.0125–952 μM | 0.005 μM | AA, UA, Glu, L-cys | 25 days | [92] |
| DPV | NiTsPc-ZnONPs-CNT | PGE | - | 10 μA μM−1 | - | 7 nM | AA, UA, ST | - | [93] | |
| DPV, | GO-AgNPs@MWCNTs | GCE | 120 s | - | 0.5–6.5 μM | 2.58 nM | AA, UA | 20 days | [94] | |
| DPV | MoTe2/NC | GCE | - | 0.814 μA μM−1 | 100 nM–50 μM | 7.8 nM | UA, AA | 16 days | [95] | |
| CV, EIS, DPV | MIP/4-MPBA/AuNPs | ANE | - | - | 0.5 μM-1 mM | 0.14 μM | AA, UA, Glu | 10 days | [96] | |
| DPV, CA | MXene/DODA | ITO | - | 4.098 μA μM−1 | - | 36.8 nM | UA, AA, Glu, Na⁺, K⁺, Ca2+, Mg2+ | 9 days | [97] | |
| DPV | CoS2-FeS2/HNCC | GCE | - | 91.6 μAμM−1 cm−2 | 0.05–90 μM | 9.3 nM | UA, AA, Glu, H2O2, Na⁺, K⁺ | 21 days | [98] | |
| CA | PCN-333 film | - | - | 4637.78 μA mM−1 cm−2 | 0.5–140 μM | 0.14 μM | AA | - | [99] | |
| CV, DPV, CA | pGr-MoS2 | GCE | - | 4.88 μA mM−1 cm−2 | 0.00001–10 μM | 0.01 nM | AA, UA, EP, NE, Glu, ST | 90 days | [100] | |
| SWV | Ce-MOF m-PdNFs-G4-MBs | GCE | - | 10 pM–100 nM | 6 pM | AA, BSA, Glu, L-cys | 15 days | [101] | ||
| CV | LINC | CPE | 5.78 ± 0.32 nA nM−1 | 10 nM–1 μM | 10 nM | - | - | [102] | ||
| DPV | BNGrD | - | - | 0.21 µA µM−1 | 0.5–500 µM | 0.12 µM | Gl, UA, BSA, urea, Ala, Na+, K+ | 5 times, 48 days | [103] | |
| DPV | H-Mo-CoP/NC | 11.89 μA mM−1 cm−2, 2.509 μA mM−1 cm−2 | 1 μM–50 μΜ, 50 μM–300 μM | 55 nM | UA, AA, Gly, Glu, Na+, urea, SO42−, EP, NE, GSH | 30 times, 30 days | [104] | |||
| α-syn | DPV | - | GE | - | - | 1 fM–10 pM | 0.46 fM | AβO, AβF, IgG, L-cys, BSA, Glu, UA, DA | 14 days | [78] |
| SWV, | AuNPs/SWCNTs | SPCE | - | - | 0.01–10 ng/mL | 4.1 pg/mL | Aβ42, Aβ40 | 1 month | [77] | |
| LSV | SWCN, gold-nanourchin | IDE | - | - | - | 1 fM | DJ-1 | - | [75] | |
| EIS, DPV | - | FTO | 133 μAng−1 mL | 10–1000 ng/mL | 3.62 ng/mL | Chol, BSA, GOX, LgG, DA, AA, UA | 7 days | [79] | ||
| EIS | - | GE | - | - | - | 0.3 pg/mL | CRP, BSA | - | [105] | |
| EIS | - | LIG | 5 s | - | - | - | - | 7 h | [106] | |
| DPV | - | GE | - | - | 1 fg/mL–0.1 ng/mL | 0.57 fg/mL | AβO, AβF, IgG, L-cys | 14 days | [107] | |
| CA | Ag ink | Paper-based electrode | - | - | 0.002–128 ng/mL | 0.002 ng/mL | - | disposable | [108] | |
| EIS | Au NPs | LIG | - | - | 0.01–100 ng/mL | 0.237 pg/mL | Tau, Aβ, BDNF | 10 days | [109] | |
| EIS | ||||||||||
| GFAP | DPV | rGO/PDA-MIP | - | 10 min | - | 1–106 fg/mL | 754.5 ag/mL | AA, UA, urea, vimentin, Gly, Glu | 3 months | [81] |
| NfL | EIS | - | GE | - | - | - | 5.21 ng/L | - | - | [82] |
| DJ-1 | EIS | Au NPs | SPCE | - | 0.167 kΩ nM−1 | 1–500 nM | 1 nM | - | - | [83] |
3.3. Transistor Biosensors in PD Detection
3.3.1. Dopamine Detection
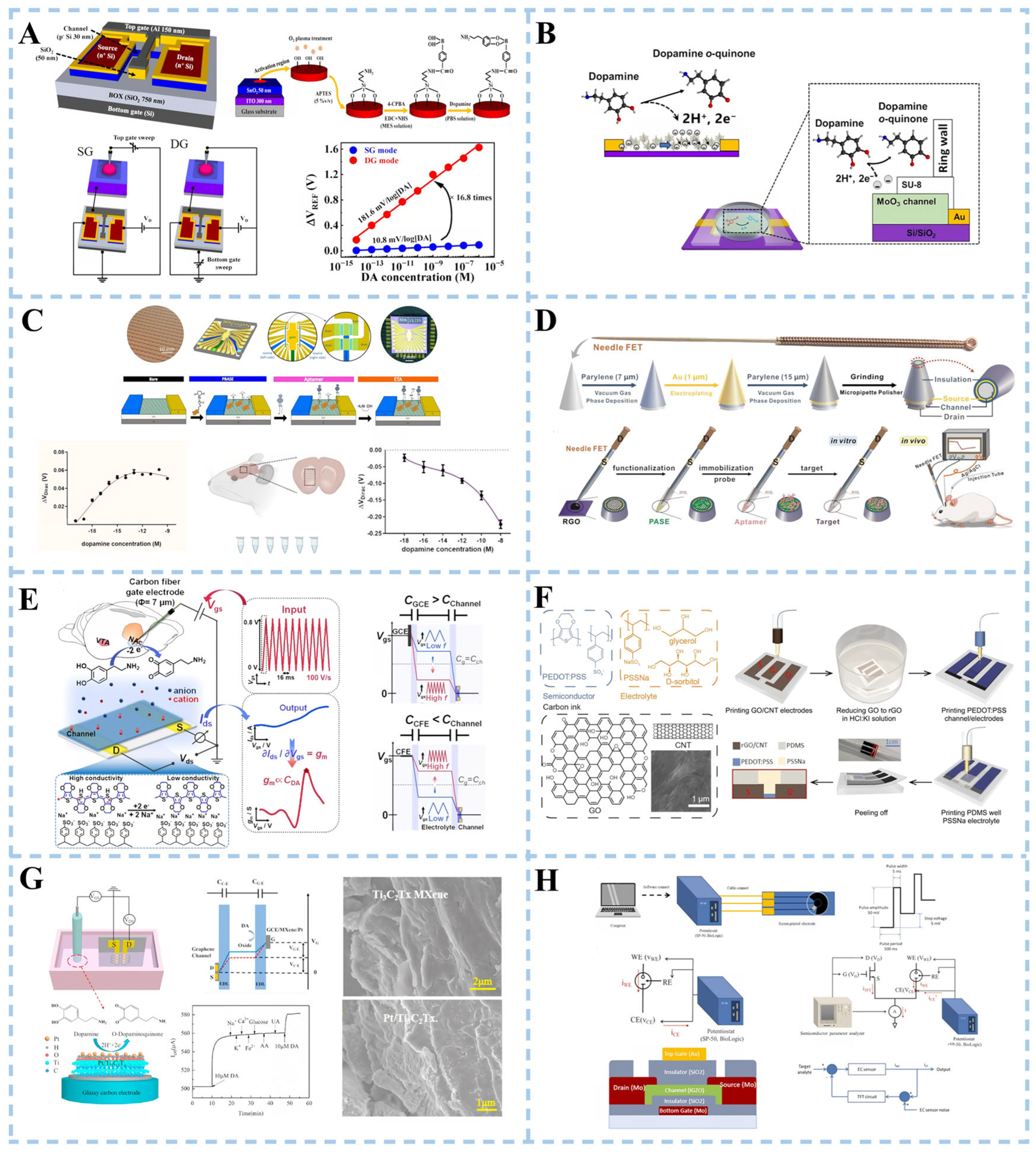
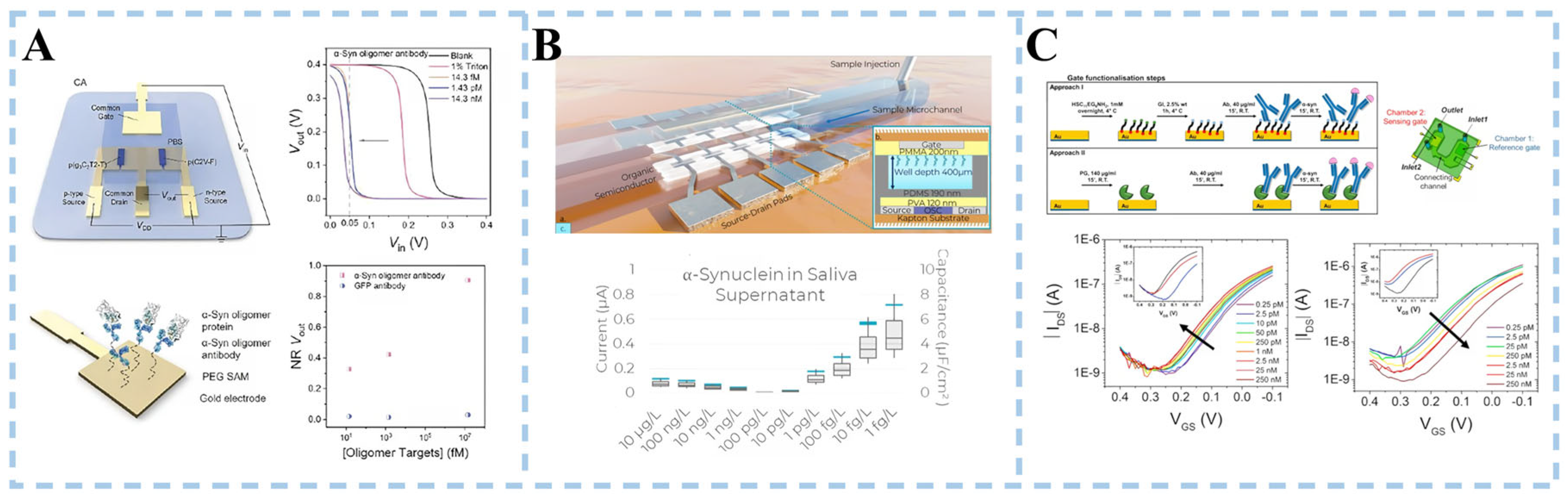
3.3.2. α-Synuclein Detection
3.3.3. Other Biomarker Detection
| Target | Type | Nanoparticles | Response Time | Sensitivity | Linear Range | LOD | Selectivity | Stability | Ref |
|---|---|---|---|---|---|---|---|---|---|
| DA | FET | rGO | A few seconds | - | 1 nM–10 μM | 370 pM | Gl, AA, Ach, EP, NE, K+ | 10 times, 7 days | [112] |
| AC-SGT | Graphene | Second | - | 3 nM–300 μM | 3 nM | AA, UA | 30 days | [122] | |
| OECT | rGO/CNT | - | - | 1–100 μM | 6 μM | - | - | [115] | |
| FET | MoO3 | 1 s | - | - | 100 nM | K+, Na+, Ca2+ | - | [111] | |
| TFT | - | - | 2.91 × 10−2 μA/μM | 2–50 μM | 0.486 μM | UA, AA | - | [117] | |
| FET | - | - | 373.98 mV/log(DA) | 10 fM–1 μM | 10 fM | - | - | [110] | |
| FET | MUA-AuNCs | - | 4200 nA/μM | 0.03–5 μM | 0.01 μM | AA, UA, Gl, Asp, Gly, Na+ | 3 times, 90 min | [123] | |
| IECT | Pt/Ti3C2Tx MXene, graphene | - | - | 50 nM–10 μM, 10 μM–9 mM | 50 nM | Glu, Na+, K+, Ca2+, Fe2+ | 15 days | [116] | |
| OECT | - | Real-time | 0.899 s/M | - | 5 nM | DOPAC, UA, AA | 6 times | [114] | |
| FET | Graphene | - | - | - | 1 aM | AA, L-DOPA, L-Tyr | - | [113] | |
| FET | Fe3O4@AuNPs | - | 3.16 mA/μM | - | 3.3 nM | DOPA, NE, 4-PC, 3,4-DHBA, EP, GA | 15 times, 10 days | [124] | |
| FET | AuNPs/graphene | Real-time | 12.23 mV/decade | 1 × 10−19–1 × 10−11 M | 6 × 10−20 M | Glu, UA, AA | - | [125] | |
| OECT | - | - | - | 1 nM–100 μM | 61 nM | AA, UA | 500 times | [126] | |
| FET | - | - | 10.69 mV/log(DA) | 1 fM–1 nM | 0.523 fM | UA, AA | - | [127] | |
| OECT | CNT/Pt NPs | Real-time | - | - | 5 nM | UA, AA, DOPAC | 7 days | [128] | |
| α-syn | OECT | - | - | - | 14 fM–14 nM | fM level | - | - | [118] |
| FET | - | - | - | 100 fg/L–10 μg/L | 10 fg/L | - | 15 days | [119] | |
| FET | - | - | 37 (±5) mV/decade | 0.25 pM–25 nM | 0.25 pM | - | - | [120] | |
| NfL | FET | - | A few minutes | - | 100 fM–10 nM | 30 fM | - | - | [121] |
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Che, N.N.; Yang, H.Q. Potential use of corneal confocal microscopy in the diagnosis of Parkinson’s disease associated neuropathy. Transl. Neurodegener. 2020, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, D.M.; Kalia, S.K.; Kalia, L.V. Methods for detecting toxic α-synuclein species as a biomarker for Parkinson’s disease. Crit. Rev. Clin. Lab. Sci. 2020, 57, 291–307. [Google Scholar] [CrossRef] [PubMed]
- Nwabufo, C.K.; Aigbogun, O.P. Diagnostic and therapeutic agents that target alpha-synuclein in parkinson’s disease. J. Neurol. 2022, 269, 5762–5786. [Google Scholar] [CrossRef]
- Zhu, B.; Yin, D.; Zhao, H.; Zhang, L. The immunology of Parkinson’s disease. Semin. Immunopathol. 2022, 44, 659–672. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Ru, Q.; Chen, L.; Xu, G.; Wu, Y. Advances in animal models of Parkinson’s disease. Brain Res. Bull. 2024, 215, 111024. [Google Scholar] [CrossRef]
- Mobed, A.; Razavi, S.; Ahmadalipour, A.; Shakouri, S.K.; Koohkan, G. Biosensors in Parkinson’s disease. Clin. Chim. Acta 2021, 518, 51–58. [Google Scholar] [CrossRef]
- Andreescu, S.; Vasilescu, A. Advances in electrochemical detection for probing protein aggregation. Curr. Opin. Electrochem. 2021, 30, 100820. [Google Scholar] [CrossRef]
- Shang, H.; Zhang, X.; Ding, M.; Zhang, A. Dual-mode biosensor platform based on synergistic effects of dual-functional hybrid nanomaterials. Talanta 2023, 260, 124584. [Google Scholar]
- Costa, K.M.; Schoenbaum, G. Dopamine. Curr. Biol. 2022, 32, R817–R824. [Google Scholar] [CrossRef]
- Kujawska, M.; Bhardwaj, S.K.; Mishra, Y.K.; Kaushik, A. Using graphene-based biosensors to detect dopamine for efficient Parkinson’s disease diagnostics. Biosensors 2021, 11, 433. [Google Scholar] [CrossRef]
- Ramezani, M.; Wagenknecht-Wiesner, A.; Wang, T.; Holowka, D.A.; Eliezer, D.; Baird, B.A. Alpha synuclein modulates mitochondrial Ca2+ uptake from er during cell stimulation and under stress conditions. npj Park. Dis. 2023, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Castonguay, A.M.; Gravel, C.; Levesque, M. Treating Parkinson’s disease with antibodies: Previous studies and future directions. J. Park. Dis. 2021, 11, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Mu, C.; Shao, K.; Su, M.; Guo, Y.; Qiu, Y.; Sun, R.; Sun, S.; Sun, Y.; Liu, C.; Wang, W.; et al. Lysophosphatidylcholine promoting α-synuclein aggregation in Parkinson’s disease: Disrupting gcase glycosylation and lysosomal α-synuclein degradation. npj Park. Dis. 2025, 11, 47. [Google Scholar] [CrossRef] [PubMed]
- Rubilar, J.C.; Outeiro, T.F.; Klein, A.D. The lysosomal β-glucocerebrosidase strikes mitochondria: Implications for Parkinson’s therapeutics. Brain 2024, 147, 2610–2620. [Google Scholar] [CrossRef]
- Dobert, J.P.; Bub, S.; Mächtel, R.; Januliene, D.; Steger, L.; Regensburger, M.; Wilfling, S.; Chen, J.X.; Dejung, M.; Plötz, S.; et al. Activation and purification of β-glucocerebrosidase by exploiting its transporter limp-2– implications for novel treatment strategies in gaucher’s and Parkinson’s disease. Adv. Sci. 2024, 11, 2470148. [Google Scholar] [CrossRef]
- Che, N.; Ou, R.; Li, C.; Zhang, L.; Wei, Q.; Wang, S.; Jiang, Q.; Yang, T.; Xiao, Y.; Lin, J.; et al. Plasma GFAP as a prognostic biomarker of motor subtype in early Parkinson’s disease. npj Park. Dis. 2024, 10, 48. [Google Scholar] [CrossRef]
- Fu, Y.; Adler, G.L.; Youssef, P.; Phan, K.; Halliday, G.M.; Dzamko, N.; Kim, W.S. Human endogenous retrovirus k in astrocytes is altered in Parkinson’s disease. Mov. Disord. 2025. online ahead of print. [Google Scholar] [CrossRef]
- Khalil, M.; Teunissen, C.E.; Lehmann, S.; Otto, M.; Piehl, F.; Ziemssen, T.; Bittner, S.; Sormani, M.P.; Gattringer, T.; Abu-Rumeileh, S.; et al. Neurofilaments as biomarkers in neurological disorders—towards clinical application. Nat. Rev. Neurol. 2024, 20, 269–287. [Google Scholar] [CrossRef]
- Lv, L.; Zhang, H.; Tan, J.; Wang, C. Neuroprotective role and mechanistic insights of DJ-1 dimerization in Parkinson’s disease. Cell Commun. Signal. 2025, 23, 129. [Google Scholar] [CrossRef]
- Guo, T.; Zhou, L.; Xiong, M.; Xiong, J.; Huang, J.; Li, Y.; Zhang, G.; Chen, G.; Wang, Z.H.; Xiao, T.; et al. N-homocysteinylation of DJ-1 promotes neurodegeneration in Parkinson’s disease. Aging Cell 2024, 23, e14124. [Google Scholar] [CrossRef]
- Li, S.; Jiao, F.; Li, X.; Xu, Z.; Hu, T.; Liang, X.; Wu, J.; Wang, J.; Zuo, C.; Tang, Y. Plasma GFAP and NfL associate with cerebral glucose metabolism in putative brain-first and body-first Parkinson’s disease subtypes. NPJ Park. Dis. 2025, 11, 54. [Google Scholar] [CrossRef]
- Williams, D.; Glasstetter, L.M.; Jong, T.T.; Chen, T.; Kapoor, A.; Zhu, S.; Zhu, Y.; Calvo, R.; Gehrlein, A.; Wong, K. High-throughput screening for small-molecule stabilizers of misfolded glucocerebrosidase in Gaucher disease and parkinson’s disease. Proc. Natl. Acad. Sci. USA 2024, 121, e2406009121. [Google Scholar] [CrossRef] [PubMed]
- Aftab, S.; Abbas, A.; Iqbal, M.Z.; Hussain, S.; Kabir, F.; Akman, E.; Xu, F.; Hegazy, H. Recent advances in nanomaterials based biosensors. Trends Anal. Chem. 2023, 167, 117223. [Google Scholar] [CrossRef]
- Clark, L.C., Jr.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Wang, Z.; Zhang, W.; Liu, X.; Li, M.; Li, G.; Zhang, B.; Singh, R. Optically active nanomaterials and its biosensing applications—A review. Biosensors 2023, 13, 85. [Google Scholar] [CrossRef]
- Cass, A.E.; Davis, G.; Francis, G.D.; Hill, H.A.O.; Aston, W.J.; Higgins, I.J.; Plotkin, E.V.; Scott, L.D.; Turner, A.P. Ferrocene-mediated enzyme electrode for amperometric determination of glucose. Anal. Chem. 1984, 56, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.P.; Tan, M.T.T. Biosensors for the detection of lung cancer biomarkers: A review on biomarkers, transducing techniques and recent graphene-based implementations. Biosens. Bioelectron. 2023, 237, 115492. [Google Scholar] [CrossRef]
- Lei, Z.L.; Guo, B. 2D material-based optical biosensor: Status and prospect. Adv. Sci. 2021, 9, 2102924. [Google Scholar] [CrossRef]
- Sagar Shrikrishna, N.; Sharma, R.; Sahoo, J.; Kaushik, A.; Gandhi, S. Navigating the landscape of optical biosensors. Chem. Eng. J. 2024, 490, 151661. [Google Scholar] [CrossRef]
- Li, Z.; Liang, L.; Lin, W.; Huang, Y.; Huang, T.; Wang, W.; Ma, J.; Li, J.; Sun, L.-P.; Guan, B.-O. Optofluidic laser sensor for the detection of dopamine. Sens. Actuators B Chem. 2023, 390, 133941. [Google Scholar] [CrossRef]
- Moghzi, F.; Soleimannejad, J.; Sañudo, E.C.; Janczak, J. Dopamine sensing based on ultrathin fluorescent metal–organic nanosheets. ACS Appl. Mater. Interfaces 2020, 12, 44499–44507. [Google Scholar] [CrossRef]
- Zou, W.; Liu, Y.; Li, R.; Guo, R. Ingenious multifunctional MnO2 quantum dot nanozymes with superior catechol oxidase-like activity for highly selective sensing of redox-active dopamine based on an interfacial passivation strategy. ACS Sustain. Chem. Eng. 2022, 10, 10057–10067. [Google Scholar] [CrossRef]
- Servarayan, K.L.; Sundaram, E.; Sivasamy, V.V. Colorimetric sensing of using n′-(pyrene-1-ylmethylene) benzene-1,2-diamine/triiodide ions conjugate in human serum. Sens. Actuators B Chem. 2023, 393, 134202. [Google Scholar] [CrossRef]
- Yao, T.; Dong, G.; Qian, S.; Cui, Y.; Chen, X.; Tan, T.; Li, L. Persistent luminescence nanoparticles/hierarchical porous ZIF-8 nanohybrids for autoluminescence-free detection of dopamine. Sens. Actuators B Chem. 2022, 357, 131470. [Google Scholar] [CrossRef]
- Zhang, G.; Mo, F.; Song, L.; Zhang, L.; Kuang, G.; Yang, Y.; Li, L.; Fu, Y. Cluster-dominated electrochemiluminescence of tertiary amines in polyethyleneimine nanoparticles: Mechanism insights and sensing application. Anal. Chem. 2022, 94, 14682–14690. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, P.; Zhou, L.; Zheng, J.; Wu, H.; Liang, J.; Xiao, A.; Li, J.; Guan, B.O. Plasmonic coupling on an optical microfiber surface: Enabling single-molecule and noninvasive dopamine detection. Adv. Mater. 2023, 35, 2304116. [Google Scholar] [CrossRef]
- Lakshmanakumar, M.; Nesakumar, N.; Kulandaisamy, A.J.; Rayappan, J.B.B.R. Principles and recent developments in optical and electrochemical sensing of dopamine: A comprehensive review. Measurement 2021, 183, 109873. [Google Scholar] [CrossRef]
- Khatri, A.; Punjabi, N.; Ghosh, D.; Maji, S.K.; Mukherji, S. Detection and differentiation of α-synuclein monomer and fibril by chitosan film coated nanogold array on optical sensor platform. Sens. Actuators B Chem. 2018, 255, 692–700. [Google Scholar] [CrossRef]
- Mandala, S.H.S.; Liu, T.-J.; Chen, C.-M.; Liu, K.-K.; Januar, M.; Chang, Y.-F.; Lai, C.-S.; Chang, K.-H.; Liu, K.-C. Enhanced plasmonic biosensor utilizing paired antibody and label-free Fe3O4 nanoparticles for highly sensitive and selective detection of Parkinson’s α-synuclein in serum. Biosensors 2021, 11, 402. [Google Scholar] [CrossRef]
- Hu, X.; Hu, R.; Zhu, H.; Chen, Q.; Lu, Y.; Chen, J.; Liu, Y.; Chen, H. Nanozyme-based cascade SPR signal amplification for immunosensing of nitrated alpha-synuclein. Mikrochim Acta 2022, 189, 367. [Google Scholar] [CrossRef]
- Yin, Z.; Cheng, X.; Wang, G.; Chen, J.; Jin, Y.; Tu, Q.; Xiang, J. SPR immunosensor combined with Ti4+@TiP nanoparticles for the evaluation of phosphorylated alpha-synuclein level. Mikrochim Acta 2020, 187, 509. [Google Scholar] [CrossRef] [PubMed]
- Xiao, A.; Wu, X.; Zheng, J.; Huang, Y.; Xu, A.; Guan, B.-O. Sensitivity evaluation of an optical microfiber featuring interfaces with various gold nanoparticle morphologies: Application to the GFAP detection. Biosens. Bioelectron. 2025, 268, 116901. [Google Scholar] [CrossRef] [PubMed]
- Varghese, S.; Madanan, A.S.; Abraham, M.K.; Shkhair, A.I.; Indongo, G.; Rajeevan, G.; Kala, A.B.K.; George, S. Gold nanocluster–molybdenum disulfide nanosheet couple-based immunoassay probe for the selective detection of glial fibrillary acidic protein (GFAP)─A biomarker for ischemic stroke. ACS Appl. Nano Mater. 2024, 7, 27579–27590. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X.; Chang, X.; Meng, X.; Wang, W.; Zhang, Y.; Yang, A.; Yang, J. A synchronous fluorescence biosensor mediated by hydrogen-bonding interaction for highly selective detection of dopamine. Mikrochim Acta. 2025, 192, 164. [Google Scholar] [CrossRef]
- Teniou, A.; Rhouati, A.; Catanante, G. A simple fluorescent aptasensing platform based on graphene oxide for dopamine determination. Appl. Biochem. Biotechnol. 2022, 194, 1925–1937. [Google Scholar] [CrossRef]
- Zhu, Z.; Niu, H.; Li, R.; Yang, Z.; Wang, J.; Li, X.; Pan, P.; Liu, J.; Zhou, B. One-pot hydrothermal synthesis of fluorescent carbon quantum dots with tunable emission color for application in electroluminescence detection of dopamine. Biosens. Bioelectron. 2022, 10, 100141. [Google Scholar] [CrossRef]
- Sun, P.; Chen, J.; Li, Q.; Luo, M.; Chang, W.; Xue, Z. Self-signaling colorimetric sensor for selective detection of dopamine based on CoFe2O4 nanozyme accelerated dopamine polymerization. Anal. Chim. Acta. 2025, 1338, 243596. [Google Scholar] [CrossRef]
- Xie, Z.; Shao, M.; Liu, Z.; Ren, X.; Gao, M.; Ma, H.; Zhang, N.; Wei, Q. Ultrasensitive aggregation-induced electrochemiluminescence sensor for dopamine detection in polymer hydrogel system. Sens. Actuators B Chem. 2024, 398, 134781. [Google Scholar] [CrossRef]
- Hussein, M.A.; El-Said, W.A.; Abu-Zied, B.M.; Choi, J.W. Nanosheet composed of gold nanoparticle/graphene/epoxy resin based on ultrasonic fabrication for flexible dopamine biosensor using surface-enhanced raman spectroscopy. Nano Converg. 2020, 7, 15. [Google Scholar] [CrossRef]
- Razavi, M.; Barras, A.; Ifires, M.; Swaidan, A.; Khoshkam, M.; Szunerits, S.; Kompany-Zareh, M.; Boukherroub, R. Colorimetric assay for the detection of dopamine using bismuth ferrite oxide (Bi(2)Fe(4)O(9)) nanoparticles as an efficient peroxidase-mimic nanozyme. J. Colloid Interface Sci. 2022, 613, 384–395. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, F.; Lu, Y.; Li, Y.; Liu, G.; Shan, J.; Liu, Q. Bioelectronic modulation of single-wavelength localized surface plasmon resonance (LSPR) for the detection of electroactive biomolecules. Chin. Chem. Lett. 2022, 33, 3144–3150. [Google Scholar] [CrossRef]
- Li, L.; Lu, Y.; Qian, Z.; Yang, Z.; Yang, K.; Zong, S.; Wang, Z.; Cui, Y. Ultra-sensitive surface enhanced raman spectroscopy sensor for in-situ monitoring of dopamine release using zipper-like ortho-nanodimers. Biosens. Bioelectron. 2021, 180, 113100. [Google Scholar] [CrossRef]
- Zhou, B.; Guo, J.; Yang, C.; Kong, L. Upconversion-luminescent hydrogel optical probe for in situ dopamine monitoring. Photonics Res. 2020, 8, 1800–1807. [Google Scholar]
- Treepet, S.; Duangmanee, T.; Chokradjaroen, C.; Kim, K.; Saito, N.; Watthanaphanit, A. Solution plasma synthesis of nitrogen-doped carbon dots from glucosamines: Comparative fluorescence modulation for dopamine detection. Carbon 2025, 231, 119705. [Google Scholar] [CrossRef]
- Nandhini, C.; Huang, C.-H.; Arul, P.; Huang, S.-T.; Lin, C.-M. Fabrication of ternary hybrid composites of Co-MOFs/MoS2/PEDOTs for sensitive detection of dopamine and norepinephrine in biological samples. Sens. Actuators B Chem. 2024, 417, 136147. [Google Scholar]
- Zhang, J.; Luo, W.C.; Zhang, Y.; Li, X.; Jiang, M.; Huang, K.; Yu, X.; Xu, L. Red emissive fluorescent carbon dots based on ternary carbon source for imaging α-synuclein fibrils. J. Colloid Interface Sci. 2024, 670, 576–584. [Google Scholar] [CrossRef]
- Giarola, J.F.; Santos, J.; Estevez, M.C.; Ventura, S.; Pallares, I.; Lechuga, L.M. An α-helical peptide-based plasmonic biosensor for highly specific detection of α-synuclein toxic oligomers. Anal. Chim. Acta. 2024, 1304, 342559. [Google Scholar] [CrossRef]
- Yang, X.; Li, H.; Zhao, X.; Liao, W.; Zhang, C.X.; Yang, Z. A novel, label-free liquid crystal biosensor for Parkinson’s disease related alpha-synuclein. Chem. Commun. 2020, 56, 5441–5444. [Google Scholar] [CrossRef]
- Sun, K.; Xia, N.; Zhao, L.; Liu, K.; Hou, W.; Liu, L. Aptasensors for the selective detection of alpha-synuclein oligomer by colorimetry, surface plasmon resonance and electrochemical impedance spectroscopy. Sens. Actuators B Chem. 2017, 245, 87–94. [Google Scholar]
- Li, Y.; Ren, H.X.; Chi, C.Y.; Miao, Y.B. Artificial intelligence-guided gut-microenvironment-triggered imaging sensor reveals potential indicators of Parkinson’s disease. Adv. Sci. 2024, 11, e2307819. [Google Scholar]
- Wang, X.; Zhu, X.; Shi, X.; Zhou, Y.; Chai, Y.; Yuan, R. Electrostatic interaction-induced aggregation-induced emission-type AgAu bimetallic nanoclusters as a highly efficient electrochemiluminescence emitter for ultrasensitive detection of glial fibrillary acidic protein. Anal. Chem. 2023, 95, 3452–3459. [Google Scholar] [PubMed]
- Liu, B.; Shao, S.; Cai, J.; Zhang, Z.; Tian, F.; Yang, K.; Li, F. Signal cascade amplification of streptavidin-biotin-modified immunofluorescence nanocapsules for ultrasensitive detection of glial fibrillary acidic protein. Chin. Chem. Lett. 2025, 36, 109814. [Google Scholar]
- Zhai, Q.; Cheng, W. Soft and stretchable electrochemical biosensors. Mater. Today Nano 2019, 7, 10041. [Google Scholar] [CrossRef]
- Cesewski, E.; Johnson, B.N. Electrochemical biosensors for pathogen detection. Biosens. Bioelectron. 2020, 159, 112214. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.R.; Singh, P.; Mallick, S.; Singh, J.; Pandey, S.S. Chitosan stabilized copper iodide nanoparticles enabled nano-bio-engineered platform for efficient electrochemical biosensing of dopamine. Int. J. Biol. Macromol. 2023, 253, 127587. [Google Scholar] [CrossRef]
- Chellasamy, G.; Arumugasamy, S.K.; Nam, M.J.; Venkateswarlu, S.; Varathan, E.; Sekar, K.; Manokaran, K.; Choi, M.-J.; Govindaraju, S.; Yun, K. Experimental and simulation studies of bioinspired Au-enhanced copper single atom catalysts towards real-time expeditious dopamine sensing on human neuronal cell. Chem. Eng. J. 2023, 471, 144842. [Google Scholar] [CrossRef]
- Al Kiey, S.A.; Khalil, A.M.; Kamel, S. Insight into TEMPO-oxidized cellulose-based composites as electrochemical sensors for dopamine assessment. Int. J. Biol. Macromol. 2023, 239, 124302. [Google Scholar] [CrossRef]
- Sun, X.; Chen, C.; Xiong, C.; Zhang, C.; Zheng, X.; Wang, J.; Gao, X.; Yu, Z.-Q.; Wu, Y. Surface modification of MoS2 nanosheets by single ni atom for ultrasensitive dopamine detection. Nano Res. 2022, 16, 917–924. [Google Scholar] [CrossRef]
- Ke, X.; Zhao, Z.; Huang, J.; Liu, C.; Huang, G.; Tan, J.; Zhu, H.; Xiao, Z.; Liu, X.; Mei, Y.; et al. Growth control of metal-organic framework films on marine biological carbon and their potential-dependent dopamine sensing. ACS Appl. Mater. Interfaces 2023, 15, 12005–12016. [Google Scholar] [CrossRef]
- Sun, Z.; Sun, S.; Jiang, X.; Ai, Y.; Xu, W.; Xie, L.; Sun, H.-b.; Liang, Q. Oligo-layer graphene stabilized fully exposed Fe-sites for ultra-sensitivity electrochemical detection of dopamine. Biosens. Bioelectron. 2022, 211, 114367. [Google Scholar] [CrossRef]
- Zhou, J.; Xia, Y.; Zou, Z.; Yang, Q.; Jiang, X.; Xiong, X. Microplasma-enabled carbon dots composited with multi-walled carbon nanotubes for dopamine detection. Anal. Chim. Acta 2023, 1237, 340631. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Gu, M.; Xiao, H.; Cao, L.; Zhao, F.; Chen, Z. Electrochemical DNA aptamer platform based on CuAlO2/rGo-TEPA@AuPt nanocomposites for dopamine detection. Mater. Today Chem. 2022, 26, 101248. [Google Scholar] [CrossRef]
- Rana, D.S.; Sharma, R.; Gupta, N.; Sharma, V.; Thakur, S.; Singh, D. Development of metal free carbon catalyst derived from parthenium hysterophorus for the electrochemical detection of dopamine. Environ. Res. 2023, 231, 116151. [Google Scholar] [CrossRef]
- Lim, T.; Won, S.; Nam, I.-W.; Choi, J.S.; Kim, C.H.; Kim, T.H.; Kim, J.H.; Yeo, S.Y.; Zhang, H.; Yeang, B.J. Gold nanoparticle/carbon fiber hybrid structure from the eco-friendly and energy-efficient process for electrochemical biosensing. ACS Sustain. Chem. Eng. 2022, 10, 8815–8824. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, S.; Huang, X.; Yang, Y.; Fan, H.; Yang, F.; Li, J.; Dong, X.; Feng, S.; Anbu, P.; et al. Gold-nanourchin seeded single-walled carbon nanotube on voltammetry sensor for diagnosing neurogenerative Parkinson’s disease. Anal. Chim. Acta 2020, 1094, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Adam, H.; Gopinath, S.C.B.; Arshad, M.K.M.; Parmin, N.A.; Hashim, U. Distinguishing normal and aggregated alpha-synuclein interaction on gold nanorod incorporated zinc oxide nanocomposite by electrochemical technique. Int. J. Biol. Macromol. 2021, 171, 217–224. [Google Scholar] [CrossRef]
- Carneiro, P.; Loureiro, J.A.; Delerue-Matos, C.; Morais, S.; Pereira, M.d.C. Nanostructured label–free electrochemical immunosensor for detection of a Parkinson’s disease biomarker. Talanta 2023, 252, 123838. [Google Scholar] [CrossRef]
- Gong, Y.; Fu, M.; Li, L.; Yin, Y.; Tang, Q.; Zhou, W.; Zhang, G.; Liao, X.; Gao, F. Dnazyme-driven tripedal DNA walker mediated signal-on and label-free for electrochemical detection of α-synuclein oligomers. Sens. Actuators B Chem. 2023, 378, 133150. [Google Scholar] [CrossRef]
- Ge, C.Y.; Rahman, M.M.; Zhang, W.; Lopa, N.S.; Jin, L.; Yoon, S.; Jang, H.; Xu, G.R.; Kim, W. An electrochemical immunosensor based on a self-assembled monolayer modified electrode for label-free detection of alpha-synuclein. Sensors 2020, 20, 617. [Google Scholar] [CrossRef]
- Gao, S.; Wang, Z.; Huang, Y.; Yang, G.; Wang, Y.; Yi, Y.; Zhou, Q.; Jian, X.; Zhao, G.; Li, B.; et al. Early detection of Parkinson’s disease through multiplex blood and urine biomarkers prior to clinical diagnosis. NPJ Park. Dis. 2025, 11, 35. [Google Scholar] [CrossRef]
- Li, Y.; Luo, L.; Senicar, L.; Asrosa, R.; Kizilates, B.; Xing, K.; Torres, E.; Xu, L.; Li, D.; Graham, N.; et al. An ultrasensitive molecularly imprinted point-of-care electrochemical sensor for detection of glial fibrillary acidic protein. Adv. Healthc. Mater. 2024, 13, e2401966. [Google Scholar] [CrossRef] [PubMed]
- Özgür, E.; Uzunçakmak Uyanık, H.; Şenel, S.; Uzun, L. Immunoaffinity biosensor for neurofilament light chain detection and its use in Parkinson’s diagnosis. Mater. Sci. Eng. B 2020, 256, 114545. [Google Scholar] [CrossRef]
- Dhinesh Kumar, M.; Karthikeyan, M.; Sharma, N.; Raju, V.; Vatsalarani, J.; Kalivendi, S.V.; Karunakaran, C. Molecular imprinting synthetic receptor based sensor for determination of Parkinson’s disease biomarker DJ-1. Microchem. J. 2022, 183, 107959. [Google Scholar] [CrossRef]
- Yurttaş, B.; Maral, M.; Erdem, A.; Özyüzer, L. Development of single-use thin film electrodes based on Zn2SnO4 on In2O3:SnO2 substrates with their biosensing applications. Mater. Today Commun. 2022, 33, 104906. [Google Scholar] [CrossRef]
- Jing, W.J.; Li, F.F.; Liu, Y.; Ma, R.N.; Zhang, W.; Shang, L.; Li, X.J.; Xue, Q.W.; Wang, H.S.; Jia, L.P. An electrochemical ratiometric biosensor for the detection of dopamine based on an MXene-Au nanocomposite. Chem. Commun. 2023, 59, 12911–12914. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Z.; Zhang, X.; Wang, M.; Lin, Z.; Zhang, Y.; Liu, A. MnO2 nanosheets based catechol oxidase mimics for robust electrochemical sensor: Synthesis, mechanism and its application for ultrasensitive and selective detection of dopamine. Chem. Eng. J. 2024, 493, 152656. [Google Scholar] [CrossRef]
- Hua, H.; Chen, B.; Ji, Z.; Wang, J. Sulfur-vacancy-enriched mos2-cnts with highly dispersed Au particles for sensitive dopamine detection. Appl. Surf. Sci. 2023, 639, 158244. [Google Scholar] [CrossRef]
- Li, C.; Cai, Y.; Hu, J.; Liu, J.; Dai, H.; Xu, Q.; Zhang, C.; Zhang, X.; Liu, K.; Kosinova, M.L.; et al. SiC/graphene film by laser CVD as an implantable sensor material for dopamine detection. ACS Appl. Mater. Interfaces 2023, 15, 27399–27410. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Ram Chaudhary, G.; Chaudhary, S. Designing of surface engineered ytterbium oxide nanoparticles as effective electrochemical sensing platform for dopamine. ACS Appl. Mater. Interfaces 2022, 355, 118929. [Google Scholar] [CrossRef]
- Li, R.; Guo, W.; Zhu, Z.; Zhai, Y.; Wang, G.; Liu, Z.; Jiao, L.; Zhu, C.; Lu, X. Single-atom indium boosts electrochemical dopamine sensing. Anal. Chem. 2023, 95, 7195–7201. [Google Scholar] [CrossRef]
- Chavan, S.G.; Rathod, P.R.; Koyappayil, A.; Go, A.; Lee, M.-H. “Two-step” signal amplification for ultrasensitive detection of dopamine in human serum sample using Ti3C2TX-MXene. Sens. Actuators B Chem. 2024, 404, 135308. [Google Scholar] [CrossRef]
- Panda, A.K.; Murugan, K.; Sakthivel, R.; Dhawan, U.; Lin, L.Y.; Duann, Y.F.; He, J.H.; Chung, R.J. A biocompatible electrochemical sensor based on PtNi alloy nanoparticles-coupled N-GQDs for in situ monitoring of dopamine in glioma cells. Mater. Today Chem. 2023, 27, 101283. [Google Scholar] [CrossRef]
- da Carvalho Silva, V.N.; Farias, E.A.O.; Araujo, A.R.; Xavier Magalhaes, F.E.; Neves Fernandes, J.R.; Teles Souza, J.M.; Eiras, C.; da Alves Silva, D.; Hugo do Vale Bastos, V.; Teixeira, S.S. Rapid and selective detection of dopamine in human serum using an electrochemical sensor based on zinc oxide nanoparticles, nickel phthalocyanines, and carbon nanotubes. Biosens. Bioelectron. 2022, 210, 114211. [Google Scholar] [CrossRef]
- Tchekep, A.G.K.; Suryanarayanan, V.; Pattanayak, D.K. Alternative approach for highly sensitive and free-interference electrochemical dopamine sensing. Carbon 2023, 204, 57–69. [Google Scholar] [CrossRef]
- Du, Y.; Dai, L.; Yang, F.; Zhang, Y.; An, C. In situ polymerization confinement synthesis of ultrasmall MoTe2 nanoparticles for the electrochemical detection of dopamine. Inorg. Chem. Front. 2022, 9, 4121–4126. [Google Scholar] [CrossRef]
- Xu, C.; Gu, C.; Xiao, Q.; Chen, J.; Yin, Z.-Z.; Liu, H.; Fan, K.; Li, L. A highly selective and sensitive biosensor for dopamine based on a surface molecularly imprinted layer to coordinate nano-interface functionalized acupuncture needle. Chem. Eng. J. 2022, 436, 135203. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, Y.; Han, Y.; Xu, K.; Yao, S.; Shi, L.; Zhu, M. 3D porous structure assembled from NXene via breath figure method for electrochemical detection of dopamine. Chem. Eng. J. 2023, 452, 139414. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Yan, L.; Ding, Y.; Wang, X.; Liu, X.; Li, L.; Ju, J.; Zhan, T. Prussian blue analogue-derived iron sulfide–cobalt sulfide nanoparticle-decorated hollow nitrogen-doped carbon nanocubes for the selective electrochemical detection of dopamine. ACS Sustain. Chem. Eng. 2022, 10, 17230–17240. [Google Scholar] [CrossRef]
- Zhao, Z.; Kong, Y.; Huang, G.; Liu, C.; You, C.; Xiao, Z.; Zhu, H.; Tan, J.; Xu, B.; Cui, J.; et al. Area-selective and precise assembly of metal organic framework particles by atomic layer deposition induction and its application for ultra-sensitive dopamine sensor. Nano Today 2022, 42, 101347. [Google Scholar] [CrossRef]
- Arya Nair, J.S.; Saisree, S.; Aswathi, R.; Sandhya, K.Y. Ultra-selective and real-time detection of dopamine using molybdenum disulphide decorated graphene-based electrochemical biosensor. Sens. Actuators B Chem. 2022, 354, 131254. [Google Scholar] [CrossRef]
- Zhang, C.; You, X.; Li, Y.; Zuo, Y.; Wang, W.; Li, D.; Huang, S.; Hu, H.; Yuan, F.; Shao, F.; et al. A novel electrochemical aptasensor for serum dopamine detection based on methylene blue-integrated m-PDNFs signal material. Sens. Actuators B Chem. 2022, 354, 131233. [Google Scholar] [CrossRef]
- Nam, K.H.; Abdulhafez, M.; Castagnola, E.; Tomaraei, G.N.; Cui, X.T.; Bedewy, M. Laser direct write of heteroatom-doped graphene on molecularly controlled polyimides for electrochemical biosensors with nanomolar sensitivity. Carbon 2022, 188, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Jiang, D.; Li, M.; Xuan, X.; Li, H. Brain–computer interface and electrochemical sensor based on boron–nitrogen Co-doped graphene–diamond microelectrode for EEG and dopamine detection. ACS Sens. 2025, 10, 868–880. [Google Scholar] [CrossRef]
- Xu, L.; Ling, C.; Ou, L.; Jin, Y.; Tan, C.; Gao, Y.; Xiong, X. Mo-doped CoP nanoparticles embedded bamboo-like N-doped carbon nanotube modified hollow carbon nanocage for electrochemical sensing of dopamine in human serum and meat samples. Food Chem. 2025, 464, 141847. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Jiang, C.; Tofaris, G.K.; Davis, J.J. Facile impedimetric analysis of neuronal exosome markers in Parkinson’s disease diagnostics. Anal. Chem. 2020, 92, 13647–13651. [Google Scholar] [CrossRef]
- Balsamo, J.M.; Zhou, K.; Kammarchedu, V.; Ebrahimi, A.; Bess, E.N. Mechanistic insight into intestinal α-synuclein aggregation in Parkinson’s disease using a laser-printed electrochemical sensor. ACS Chem. Neurosci. 2024, 15, 2623–2632. [Google Scholar] [CrossRef]
- Luo, Q.; Qiu, Z.; Liang, H.; Huang, F.; Wei, C.; Cui, J.; Song, Z.; Tang, Q.; Liao, X.; Liu, Z.; et al. Proximity hybridization induced molecular machine for signal-on electrochemical detection of alpha-synuclein oligomers. Talanta 2024, 271, 125720. [Google Scholar] [CrossRef]
- Saadati, A.; Baghban, H.N.; Hasanzadeh, M.; Shadjou, N. An innovative transportable immune device for the recognition of alpha-synuclein using KCC-1-nPr-CS2 modified silver nano-ink: Integration of pen-on-paper technology with biosensing toward early-stage diagnosis of Parkinson’s disease. RSC Adv. 2024, 14, 8810–8818. [Google Scholar] [CrossRef]
- Jeong, S.; Park, S.-H.; Lee, S.; Cho, H.; Lee, K.-y.; Ju, B.K.; Lee, Y.J.; Lee, S.H. Electrochemical biosensor based on gold nanoparticles/laser induced graphene for diagnosis of Parkinson’s disease by detecting phosphorylated α-synuclein in human blood. Chem. Eng. J. 2025, 509, 161329. [Google Scholar] [CrossRef]
- Hyun, T.H.; Cho, W.J. High-performance FET-based dopamine-sensitive biosensor platform based on SOI substrate. Biosensors 2023, 13, 516. [Google Scholar] [CrossRef]
- Tran, D.M.; Son, J.W.; Ju, T.S.; Hwang, C.; Park, B.H. Dopamine-regulated plasticity in MoO3 synaptic transistors. ACS Appl. Mater. Interfaces 2023, 15, 49329–49337. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, B.; Lei, Y.; Tang, L.; Li, T.; Yu, S.; Zhang, G.J.; Li, Y.T. Acupuncture needle-based transistor neuroprobe for in vivo monitoring of neurotransmitter. Small 2022, 18, e2204142. [Google Scholar] [CrossRef]
- Abrantes, M.; Rodrigues, D.; Domingues, T.; Nemala, S.S.; Monteiro, P.; Borme, J.; Alpuim, P.; Jacinto, L. Ultrasensitive dopamine detection with graphene aptasensor multitransistor arrays. J. Nanobiotechnol. 2022, 20, 495. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Jin, J.; Xiong, T.; Yu, P.; Mao, L. Fast-scanning potential-gated organic electrochemical transistors for highly sensitive sensing of dopamine in living rat brain. Angew. Chem. Int. Ed. 2022, 61, e202204134. [Google Scholar] [CrossRef] [PubMed]
- Massetti, M.; Zhang, S.; Harikesh, P.C.; Burtscher, B.; Diacci, C.; Simon, D.T.; Liu, X.; Fahlman, M.; Tu, D.; Berggren, M. Fully 3D-printed organic electrochemical transistors. npj Flex. Electron. 2023, 7, 11. [Google Scholar] [CrossRef]
- Zhou, R.; Tu, B.; Xia, D.; He, H.; Cai, Z.; Gao, N.; Chang, G.; He, Y. High-performance Pt/Ti3C2Tx MXene based graphene electrochemical transistor for selective detection of dopamine. Anal. Chim. Acta 2022, 1201, 339653. [Google Scholar] [CrossRef]
- Tsai, K.-Y.; Peng, H.-F.; Huang, J.-J. Nafion modified electrochemical sensor integrated with a feedback-loop indium-gallium-zinc oxide thin-film transistor for enhancing dopamine detection limit. Sens. Actuators A Phys. 2023, 354, 114287. [Google Scholar] [CrossRef]
- Wang, Y.; Koklu, A.; Zhong, Y.; Chang, T.; Guo, K.; Zhao, C.; Castillo, T.C.H.; Bu, Z.; Xiao, C.; Yue, W.; et al. Acceptor functionalization via green chemistry enables high-performance n-type organic electrochemical transistors for biosensing, memory applications. Adv. Funct. Mater. 2023, 34, 2304103. [Google Scholar] [CrossRef]
- Massey, R.S.; McConnell, E.M.; Chan, D.; Holahan, M.R.; DeRosa, M.C.; Prakash, R. Non-invasive monitoring of alpha-synuclein in saliva for Parkinson’s disease using organic electrolyte-gated FET aptasensor. ACS Sens. 2023, 8, 3116–3126. [Google Scholar] [CrossRef]
- Ricci, S.; Casalini, S.; Parkula, V.; Selvaraj, M.; Saygin, G.D.; Greco, P.; Biscarini, F.; Mas-Torrent, M. Label-free immunodetection of alpha-synuclein by using a microfluidics coplanar electrolyte-gated organic field-effect transistor. Biosens. Bioelectron. 2020, 167, 112433. [Google Scholar] [CrossRef]
- Solodka, K.; Berto, M.; Ferraro, D.; Menozzi, C.; Borsari, M.; Bortolotti, C.A.; Biscarini, F.; Pinti, M. Detection of neurofilament light chain with label-free electrolyte-gated organic field-effect transistors. Adv. Mater. Interfaces 2022, 9, 2102341. [Google Scholar] [CrossRef]
- Xi, X.; Tang, W.; Wu, D.; Shen, C.; Ji, W.; Li, J.; Su, Y.; Guo, X.; Liu, R.; Yan, F. All-carbon solution-gated transistor with low operating voltages for highly selective and stable dopamine sensing. ACS Sens. 2023, 8, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Sun, P.; Cao, S.; Yang, Y.; Wang, X.; Xiao, G.; Yan, G.; Bi, J.; Ji, J.; Yue, Z. Multi-channel detection of dopamine and glucose utilizing graphene field effect transistor electrochemical sensor and efficient data fusion algorithm. J. Electroanal. Chem. 2023, 950, 117901. [Google Scholar] [CrossRef]
- Liu, N.; Xiang, X.; Fu, L.; Cao, Q.; Huang, R.; Liu, H.; Han, G.; Wu, L. Regenerative field effect transistor biosensor for in vivo monitoring of dopamine in fish brains. Biosens. Bioelectron. 2021, 188, 113340. [Google Scholar] [CrossRef]
- Tian, M.; Li, C.; Yu, R.; Shen, C.; Wang, J.; Lu, J.; Liu, G.; Wang, Z.; Wang, T.; Zhao, X.; et al. Ultrasensitive detecting of dopamine in complex components by field effect transistor sensor based on the synergistic enhancement effect and overcoming debye length limitations. Results Phys. 2024, 58, 107487. [Google Scholar] [CrossRef]
- Tseng, H.S.; Chen, Y.L.; Zhang, P.Y.; Hsiao, Y.S. Additive blending effects on PEDOT:PSS composite films for wearable organic electrochemical transistors. ACS Appl. Mater. Interfaces 2024, 16, 13384–13398. [Google Scholar] [CrossRef]
- Palit, S.; Singh, K.; Lou, B.-S.; Her, J.-L.; Pang, S.-T.; Pan, T.-M. Ultrasensitive dopamine detection of indium-zinc oxide on PET flexible based extended-gate field-effect transistor. Sens. Actuators B Chem. 2020, 310, 127850. [Google Scholar] [CrossRef]
- Wu, X.; Feng, J.; Deng, J.; Cui, Z.; Wang, L.; Xie, S.; Chen, C.; Tang, C.; Han, Z.; Yu, H.; et al. Fiber-shaped organic electrochemical transistors for biochemical detections with high sensitivity and stability. Sci. China Chem. 2020, 63, 1281–1288. [Google Scholar] [CrossRef]
| Sensor Type | Advantages | Challenges |
|---|---|---|
| Optics |
|
|
|
| |
| ||
| Electrochemistry |
|
|
|
| |
| ||
| Transistor |
|
|
|
| |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, P.; Gao, N.; Chang, G.; Wu, Y. Biosensors for Early Detection of Parkinson’s Disease: Principles, Applications, and Future Prospects. Biosensors 2025, 15, 280. https://doi.org/10.3390/bios15050280
Jiang P, Gao N, Chang G, Wu Y. Biosensors for Early Detection of Parkinson’s Disease: Principles, Applications, and Future Prospects. Biosensors. 2025; 15(5):280. https://doi.org/10.3390/bios15050280
Chicago/Turabian StyleJiang, Panpan, Nan Gao, Gang Chang, and Yuxiang Wu. 2025. "Biosensors for Early Detection of Parkinson’s Disease: Principles, Applications, and Future Prospects" Biosensors 15, no. 5: 280. https://doi.org/10.3390/bios15050280
APA StyleJiang, P., Gao, N., Chang, G., & Wu, Y. (2025). Biosensors for Early Detection of Parkinson’s Disease: Principles, Applications, and Future Prospects. Biosensors, 15(5), 280. https://doi.org/10.3390/bios15050280





