Abstract
This work presents the development of a biosensing platform for hydrogen peroxide (H2O2) electrochemical reduction. The developed platform uses a multi-walled carbon nanotube paste (PMWCNT) and the enzyme cholesterol oxidase (ChOx). The supramolecular architecture of the PMWCNT/ChOx platform was characterized using cyclic voltammetry, electrochemical impedance spectroscopy, and amperometry. The results indicated that the presence of ChOx enhances the sensitivity of electrochemical detection for H2O2 by 21 times compared to that without ChOx. The designed electrochemical sensing bio-platform for H2O2 shows a sensitivity of 26.15 µA/mM in the linear range from 0.4 to 4.0 mM, an LOD of 0.43 µM, and an LOQ of 1.31 µM. Furthermore, in silico studies (molecular dynamics simulations, molecular docking assays, and binding free energy calculations (ΔGb)) were carried out to characterize and validate the molecular interaction between ChOx and H2O2. The computed data confirmed that the binding is spontaneous, and the type of labile interaction promotes a rapid electrochemical reduction of H2O2.
1. Introduction
Hydrogen peroxide (H2O2) is the most common and stable type of reactive oxygen species (ROS) [1,2]. It is produced as a by-product of biochemical reactions, including several enzymatic reactions, mitochondrial respiration, and metabolic processes in living organisms [3,4,5]. However, elevated levels of H2O2 in the human body can lead to protein and DNA damage; it is also associated with some pathological conditions such as asthma, atherogenesis, cancer, Parkinson’s, and Alzheimer’s diseases, and cardiovascular dysfunctions [5]. Additionally, H2O2 is produced and detected in different fields, including environmental science, chemistry, food processing, textile, pharmaceutical, and biomedicine, where it can be used either as a reducing or as an oxidizing agent [6,7,8,9,10].
Due to the significance of H2O2 in various fields, its determination in aqueous and non-aqueous samples is commonly conducted using analytical techniques as colorimetry, chemiluminescence, UV-vis spectroscopy, fluorescence, high-performance liquid chromatography (HPLC), and electroanalysis [11,12,13,14,15,16,17,18,19,20]. Among these techniques, electrochemical sensors for H2O2 represent an interesting alternative regarding cost, time efficiency, sample pre-treatment, sensitivity, and selectivity. When designing and constructing these electrochemical sensors, various materials are utilized. There are two main types of electrochemical sensors: the non-enzymatic and the enzymatic sensors. On the one hand, the former has utilized nanomaterials such as carbon nanotubes (CNTs), graphene, carbon dots, metal–organic frameworks (MOFs), or metal nanoparticles [21,22,23,24,25,26,27,28]. Additionally, the use of redox mediators combined with enzymes like horseradish peroxidase (HRP), catalase, or heme prosthetic groups has been the initial point for the design of H2O2 biosensors. These biosensors allow the quantification of the current changes generated by the oxidation or reduction of H2O2 [29,30,31,32,33,34,35,36]. The literature indicates that biosensors based on oxidoreductase enzymes have been intensively researched over the past decade, driven by the need for faster and more accurate protocols. Oxidoreductase enzymes are typically used as recognition agents in the development of biosensors of different analytes; in those cases, enzymes such as glucose oxidase, glutamate oxidase, lactate oxidase, uricase, pyruvate oxidase, alcohol oxidase, or cholesterol oxidase, are employed to catalyze the oxidation of the respective substrate in the presence of oxygen, generating H2O2 as a by-product. The generated H2O2 can be oxidized or reduced at the electrode surface, adjusting the produced current. The current changes can be correlated with the analyte concentration in the tested sample [37,38,39,40,41,42,43,44].
The importance of studying oxidoreductases is that they form a class of enzymes specifically involved in the catalysis of oxidation–reduction reactions. These enzymes enable the transfer of electrons from the reductant to the oxidant [45]. The enzyme cholesterol oxidase (ChOx), also known as 3β-hydroxysterol oxidase, is an alcohol flavoenzyme that functions as an oxidoreductase. This means that it contains flavin adenine dinucleotide (FAD), which allows it to catalyze the transfer of electrons between molecules, the donor CH-OH group with oxygen as acceptor [45,46]. The ChOx enzyme is known for its simplicity, specificity; it possesses remarkable stability in extreme conditions such as high temperatures and very acidic and alkaline pH values. The thermal stability of this enzyme is favorable for clinical applications. Cholesterol oxidase has gained significant attention recently due to its increasing applications in clinical practice, particularly for cholesterol determination in serum. Additionally, it plays a role in the synthesis and production of various steroids. Beyond these uses, ChOx has demonstrated strong insecticidal activity and can help regulate cholesterol levels within cells. Moreover, ChOx is implicated in the development of several diseases of bacterial origin, such as tuberculosis, as well as viral (HIV) and non-viral prion-related diseases like Alzheimer’s disease [46,47,48].
Enzymes that have FAD as a cofactor in their active site have redox properties. Currently, the enzymes employed to detect H2O2 are those that have metal ions in their active center, such as Fe (HRP, cytochrome C, cytochrome P411). This paper presents a study on a new application of the ChOx enzyme to develop an electrochemical biosensor for the electrochemical reduction of H2O2. The design features a multi-walled carbon nanotube paste electrode with immobilized Cholesterol oxidase. Experimental studies were reinforced with in silico studies based on molecular dynamics simulations and ΔGba energy calculations to assess the interaction between ChOx and H2O2.
2. Materials and Methods
2.1. Reagents and Instruments
Multi-walled carbon nanotubes (MWCNTs) (outer diameter: 6–13 nm, length: 2.5–20 μm, purity > 98%), mineral oil, hydrogen peroxide (H2O2, 30% v/v aqueous solution), microbial Cholesterol oxidase (ChOx) (C1235-100UN) lyophilized powder, sodium phosphate monobasic (NaH2PO4), sodium phosphate dibasic (Na2HPO4), and sulfuric acid (H2SO4) were purchased from Sigma-Aldrich (St. Louis, MO, USA). All chemicals were analytical grade and used without purification. All solutions were prepared utilizing 18.2 MΩ·cm deionized water.
Voltammetric and amperometric studies were carried out with a BAS-100 B potentiostat (Version 2.3), and a Biologic SP-150 (Version V11.42) potentiostat was utilized for electrochemical impedance spectroscopy (EIS) experiments. A bar of 5 mm diameter glassy carbon was used as the electrical contact of the working electrode assembly; a graphite cylinder was used as auxiliary electrode; and an Ag/AgCl(sat) was used as reference electrode; all potentials were referred to the Ag/AgCl(sat) reference electrode.
The pH measurements were carried out with a Conductronic PC45 pH meter at room temperature.
2.2. Software
ZView®® version 3.5i was employed for EIS analysis data.
Gromacs 2019.2 is a molecular dynamics program with a wide range of applications for systems with multiple particles and energy functions. It supports multiscale techniques such as QM/MM, MM/CG, and implicit solvents [49].
Charmm-gui server is a web-based platform that provides well-established and reproducible simulation protocols for molecular simulations using various simulation potentials [50,51,52].
Adaptive Poisson–Boltzmann Solver (APBS) calculates electrostatic binding energy by solving Poisson–Boltzmann equations, incorporating both solvation and Coulombic components.
In this work, we use the Visual Molecular Dynamics (VMD) program to calculate the solvent-accessible surface area change for the non-electrostatic component of binding energy.
2.3. Solutions
Sodium phosphate buffer (PB) 0.050 M, pH 7.4, was used as the supporting electrolyte. PB also served as the solvent to prepare the H2O2 stock solution and the 20 U/mL ChOx enzymatic solution, both prepared daily.
2.4. Sensing Bioplatform Preparation
MWCNTs were activated according to [28]. Briefly, the MWCNTs were placed in 1 M nitric acid solution and sonicated for 30 min. After that, the MWCNTs were filtered and placed in 1 M sulfuric acid solution and sonicated for 30 min. This procedure was repeated twice. Finally, the activated MWCNTs were filtered and extensively washed with ethanol and acetone until the washing residues had a neutral pH.
The PMWCNT was prepared by mixing activated MWCNTs and mineral oil in a 70/30 w/w ratio.
The surface of the glassy carbon cylinder was polished with 1 µm and 0.5 µm alumina; after that, it was rinsed with deionized water and sonicated for 1 min to remove any residues. Finally, the glassy carbon was rinsed with deionized water and dried with nitrogen gas, and the PMWCNT was set by placing it onto the glassy carbon (GC) contact. The surface PMWCNT/ChOx biosensing platform was prepared by drop-casting 10 μL of ChOx (20 U/mL) onto the PMWCNT. Finally, it was allowed to dry for 10 min at room temperature before being used (Scheme 1).
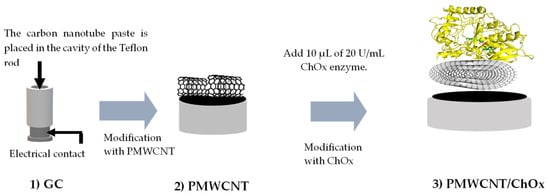
Scheme 1.
Representation of the construction of the PMWCNT/ChOx biosensing platform.
2.5. Characterization of the Electrochemical Sensing Platforms
The PMWCNT and PMWCNT/ChOx platforms were electrochemically characterized by cyclic voltammetry from −0.80 V to 0.20 V at a scan rate of 0.10 V/s in PB. Furthermore, the EIS spectrum was recorded for each sensing platform in PB. All experiments were performed at room temperature, and all potentials were referenced to the Ag/AgCl(sat) electrode.
2.6. Electrochemical Quantification of H2O2
Each sensing platform was used as a working electrode to quantify H2O2 from 0.4 to 4.0 mM through amperometry assay by applying a constant potential in PB solution. Additionally, the analytical parameters were computed for each sensing system.
2.7. Molecular Dynamics Simulations of ChOx
High resolution (PDBID:1N4W) of ChOx from Streptomyces sp. SA-COO [53] was used for molecular simulations. 1N4W structure was processed on the Charmm-gui server [54]. ChOx was immersed in a cubic TIP3 water box with 10 Å of each protein atom to the water edge, containing 0.15 M KCl. A total of 100 ns molecular dynamics simulations by triplicate were performed using Gromacs 2019.2 [55,56,57]. Periodic boundary conditions were used. Electrostatic forces were computed with the Particle Mesh Ewald (PME) method. The Charmm36m force field was used [58,59]. The Gromacs input files were generated and utilized following the recommendations provided by the Charmm-gui server. Subsequently, clustering analysis was carried out using the gmx cluster tool within the Gromacs framework, employing the Gromos clustering method.
2.8. Molecular Docking
A representative structure of the molecular dynamics simulations was used to place the peroxide molecules on the presumable binding site of the ChOx. Cholesterol oxidase of Streptomyces sp. (PDB ID: 1MXT) was used as a reference to define the center of the molecular docking grid box [60]. The center of the grid box was located near the carboxyl group of Glu361 (20.161, −3.652, 5.814) with a size of 7.5 × 7.5 × 7.5 Å3. A total of 100 independent molecular docking experiments were conducted using Autodock Vina 1.2.5 [61,62]. Molecular images were realized on Maestro from Schrödinger 2023-3 [63].
2.9. Binding Energy Calculations Between ChOx and H2O2
The Nathan Baker’s methodology was used to determine the electrostatic components ΔGelec: solvation, ΔGsolv, and Coulombic, ΔGCoul, to the free binding energy ΔGb using the Adaptive Poisson–Boltzmann Solver (APBS) program [64], at pH 7.4 (considering the pH of the electrochemical experiments for the H2O2 detection).
ΔGelec= ΔGsolv + ΔGCoul
The non-electrostatic or non-polar component (ΔGno-polar) was determined by multiplying the solvent-accessible surface area change (∆ASA), calculated using the Visual Molecular Dynamics (VMD) program [65], this ∆ASA was multiplied by the surface tension of the solvent (γ = 5 Cal mol−1 Å−2), as shown in Equation (2) [66,67]:
ΔGnon-polar = γ (ASAComplex − ASAprotein − ASAligand)
ΔGb was determined as follows:
ΔGb = ΔGelec + ΔGnon-polar
Therefore:
ΔGba = ΔGsolv + ΔGCoul + ΔGnon-polar
This methodology has been used in earlier reports to study mainly receptor–ligand interactions [68,69,70,71].
3. Results
3.1. Electrochemical Characterization of the Sensing Platforms
Figure 1A shows the cyclic voltammograms (CV) obtained for the PMWCNT and PMWCNT/ChOx sensing platform; the potential was swept from −0.80 to 0.20 V at a scan rate of 0.10 V/s in PB. As can be observed in the PMWCNT sensing platform, no oxidation or reduction signals were detected. However, the voltammogram for the PMWCNT/ChOx sensing platform exhibited a redox couple, showing an oxidation signal (I) at −0.38 V and a reduction (II) at −0.48 V. These signals can be assigned to the active site of the ChOx enzyme, specifically to the FAD/FADH2 couple. This redox couple shows a conditional potential Similar behavior has been found for glucose oxidase immobilized on different substrates under comparable experimental conditions [72,73,74]. It is important to point out that at the PMWCNT/ChOx interface, a three-dimensional structure is formed, as has been described for other assemblies [48]. This structure enables greater interaction between the sensing platform and the solution.
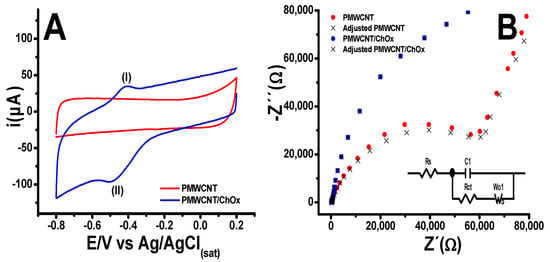
Figure 1.
(A) Cyclic voltammograms for PMWCNT and PMWCNT/ChOx sensing platform in PB at 0.10 V/s scan rate. (B) Impedance spectra (Nyquist plots and equivalent circuit) for each sensing platform at open circuit potential in PB.
The EIS experiments were conducted as a complementary method to study the electron transfer and diffusion processes at the platform/electrolyte interface. The experiments were carried out under a voltage amplitude of 0.010 V and a frequency range from 10 mHz to 200 kHz at open circuit potential (OCP) in PB solution. The Nyquist plots are shown in Figure 1B. The studied sensing platform shows a semicircle at a high-frequency range, which is related to the change transfer resistance (Rct). This is associated with the kinetics of the electron transfer of the redox indicator at the interface electrode/ dissolution. After the semicircle, a linear portion at low frequencies was observed (W01), which can be associated with the control diffusion ions in the PB process and the conductivity of the used materials (PMWCNT or PMWCNT/ChOx). The experimental data were adjusted to an equivalent Randles circuit, as shown in the inset of Figure 1B, where C1 represents the interfacial double layer capacitance, W01 represents the Warburg impedance, and Rs is the bulk solution resistance. From the equivalent circuit fitting, the Rct and the C1 increase at the PMWCNT/ChOx, while W01 remains practically constant. The data for the sensing platform are summarized in Table 1.

Table 1.
Equivalent circuit fitting parameters obtained for the modified electrodes.
The EIS data agree with the results obtained by cyclic voltammetry, showing that the PMWCNT/ChOx assembly displayed a higher capacitance C1 than the PMWCNT platform, while Rct increased by almost twice, and the W01 remained practically constant.
A study at different scan rates was performed; the results are shown in Figure S1A. The plot of current peak vs. scan rate (i vs. v) in Figure S1B showed that the enzyme is adsorbed onto the PMWCNT.
3.2. Electrochemical Detection of Hydrogen Peroxide
The sensing platforms were used for the electrochemical reduction of hydrogen peroxide. Figure 2 shows the results obtained from the cyclic voltammetry of the PMWCT/ChOx platform in PB in the absence and presence of 2 mM H2O2. An experiment under similar conditions for the platform PMWCNT was conducted. It is observed that in the presence of H2O2 at the PMWCNT/ChOx platform, the reduction current increases; therefore, this is associated with the reduction of H2O2 and its interaction with the FAD/FADH2 of the ChOx enzyme. Although the reduction starts at −0.2 V, at −0.6 V it can be guaranteed that H2O2 is completely reduced.
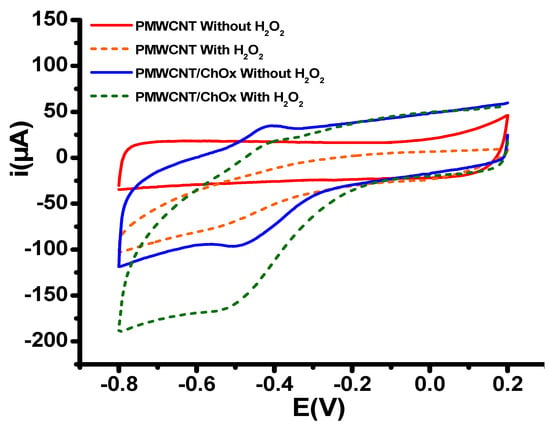
Figure 2.
Cyclic voltammograms in PB at pH 7.4 at the potential window −0.80 to 0.2 V at a scan rate of 0.10 V/s for the PMWCNT and PMWCNT/ChOx in the absence (continuous line) and in the presence of H2O2 (dotted line).
3.3. Determination of H2O2 on the PMWCNT/ChOx
From the results of cyclic voltammetry studies, a constant of −0.6 V was established to ensure a complete reduction of H2O2 in the biosensing platform PMWCNT/ChOx. The H2O2 quantification was conducted in PB from 0.40 to 4.0 mM; five independent experiments were performed. Figure 3A shows a typical amperometric response for the H2O2 detection at both PMWCNT and PMWCNT/ChOx. Figure 3B presents the typical calibration plots for the H2O2 detection at PMWCNT and PMWCNT/ChOx.
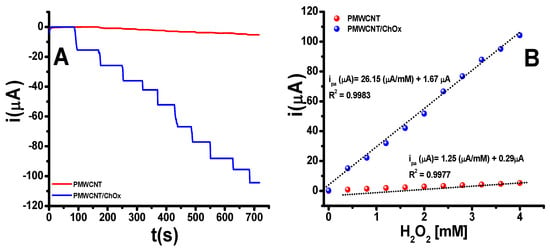
Figure 3.
(A) Amperometric response for the electrochemical reduction of H2O2 at the PMWCNT and PMWCNT/ChOx sensing platforms, conducted at −0.60 V, (B) Calibration plots for H2O2 from 0.4 to 4.0 mM in PBS.
As can be observed from Figure 3A,B, the presence of the ChOx enzyme at the PMWCNT/ChOx sensing bioplatform significantly increases the reduction currents compared to the PMWCNT sensing platform under the same H2O2 concentration. In both systems, linearity in the range from 0.40 to 4.0 mM was achieved. The analytical parameters, including sensitivity, low detection limit (LOD), and limit of quantification (LOQ), have been reported in Table 2. The LOD and LOQ were calculated as 3σ/m and 10σ/m, respectively [75].

Table 2.
Analytical parameters for H2O2 electrochemical reduction at sensing platforms.
The above results indicate that incorporating the ChOx enzyme into the sensing bioplatform enhances the reduction current in the electrochemical determination of H2O2. Specifically, in the PMWCNT/ChOx system, the sensitivity increases by 21 times compared to the PMWCNT sensing platform. The findings suggest that the ChOx enzyme could be a viable alternative to horseradish peroxidase (HRP), catalase, hem prosthetic groups, and/or myeloperoxidase (MPO) in the fabrication of electrochemical biosensors for H2O2 detection [27,28,29,30,31,32,33]. The increment in the reduction current at the amperometry experiments was associated with the interaction at the active site of the ChOx and the H2O2.
The selectivity of the PMWCNT/ChOx was evaluated by conducting amperometric studies with additions of 0.025 mM of Ascorbic acid (AA), and 0.025 mM Uric acid (UA). For either of these, any changes in the electrochemical response were observed, as shown in Figure 4. It is important to mention that both analyzed compounds do not present a response in reduction, while they are commonly detected at oxidation, as mentioned in [76].
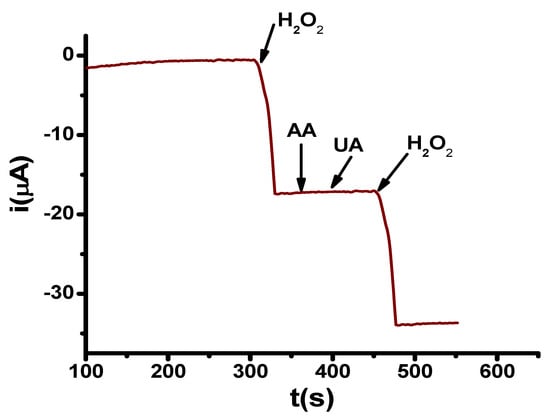
Figure 4.
Amperometric records for additions H2O2 0.4 mM, Ascorbic acid (AA) 0.025 mM, Uric acid (UA) 0.025mM, H2O2 0.4 mM in PB at pH 7.4 in PMWCNT/ChOx at −0.6 V.
To better understand experimental results, the behavior of the ChOx in the sensing bioplatform and the interactions of the molecular ChOx-H2O2 complex interactions were studied by in silico studies (molecular dynamics simulations, docking assays, and binding energy calculations).
3.4. In Silico Studies: Interaction Between ChOx and H2O2
Three independent molecular simulations of Cholesterol oxidase from Streptomyces sp. SA-COO were realized to explore its conformational changes and to obtain a representative relaxed structure. The α-carbon root mean square deviation (RMSD) from the amino acids of the enzyme of all systems was measured for the entire simulated duration. Figure 5 shows the resulting RMSD.
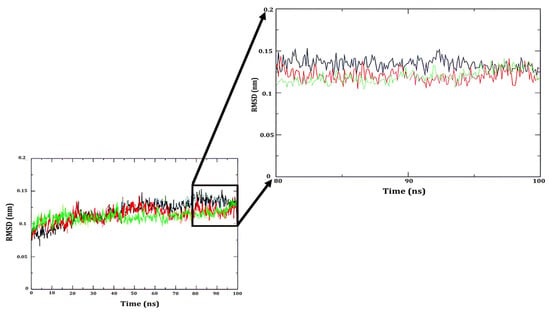
Figure 5.
α-carbon root mean square deviation for all systems studied herein.
As noted above, the α-carbon RMSD presented fluctuations (not higher than 1.6 Å compared to the initial structure) during the first 70 ns, as shown in Figure 5. The last 20 ns of each simulation was used for analysis (80 to 100) ns. Cluster analysis was used to obtain a representative conformation of ChOx. The structure at the center of each cluster was compared against the crystallographic structure, obtaining α-carbon RMSD of 1.32, 1.12, and 1.08 Å. Due to the low value of RMSD, one cluster structure was used to locate the predicted location of the peroxide molecule, the 1MXT structure [60], thus it was used as a template for the peroxide molecule.
From the obtained in silico results, a docking study between ChOx and H2O2 was performed. The H2O2 molecule was docked on a representative structure of the enzyme obtained by molecular dynamics simulations. Figure 6A shows the active site of ChOx, which involves the Asn119, Gly120, Met122, Lys225, Pro344, Glu361, Leu377, His447, Asn485, and Pro486. According to the literature [77,78,79,80,81], the catalytic roles associated with Glu361, His447, and Asn485 have been studied extensively. Furthermore, Chen et al. [82] showed that the binding and release of O2 or H2O2 takes place through the hydrophobic tunnel in ChOx. Notably, the Met122, Glu361, and Asn485 are important residues because they meet the isoalloxazine ring of FAD in the tunnel in an open or closed conformation of ChOx [82].
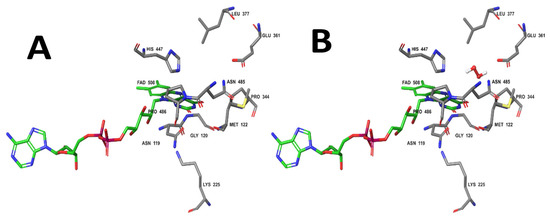
Figure 6.
The cluster representative structure of ChOx and the probable H2O2 location are shown. (A) Probable residues involved in recognizing the H2O2 molecule. (B) H2O2 (center) and those residues at 5 Å. Molecular images were realized on Maestro from Schrödinger 2023-3 program (academic license) [64].
The interaction between the cluster structure of ChOx obtained by molecular dynamics simulations and the H2O2 molecule is shown in Figure 6B. H2O2 was found in the same orientation in 94% of independent experiments with an affinity energy of −2.088 ± 0.004 kcal/mol.
The H2O2 molecule established electrostatic interactions and hydrogen bonds with Glu361, His 447, Asn 485 (Supplementary Materials Figure S2), and some hydrophobic contacts with FAD rings. These interactions are reflected in the contributions of the ΔGb computing values (Table 3).

Table 3.
ΔGb values of ChOx (cluster representative) with H2O2 determined at pH 7.0 by APBS [65] and VMD1.9.1 [66].
Binding energy calculations were performed, and the ΔGb value from H2O2 to ChOx, as shown in Figure 6B, was negative, indicating that the reaction is spontaneous even though peroxide is a small molecule (−1.5 kJ/mol), where the binding was driven by electrostatic interactions, mainly the Coulombic component (−3.9 kJ/mol) and non-polar contacts (−3.3 kJ/mol). The solvation energy was positive because the electrostatic interactions incur a penalty for the desolvation of individual molecules [83,84].
According to the calculated in Equation (4) ΔGba = ΔGsolv + ΔGCoul + ΔGnon-polar, it is verified that the interaction between ChOx-H2O2 is of a labile type that allows the desorption of the product, releasing the active site to continue with a new ChOx-H2O2 interaction cycle. From the experimental data of amperometry, an increase in the peroxide reduction current is observed in the sensing bioplatform. The data help establish that this interaction is determinant in the amperometric response detected in the experimental studies, resulting in greater sensitivity for the electrochemical determination of H2O2.
4. Discussion
The results obtained from this research show that the presence of ChOx in the sensing platform helps enhance the global reduction current of H2O2 determination; thus, the generated biosensor functions like other classical enzymes such as HRP, catalase, or heme prosthetic groups. In this work, we have applied the electrochemical technique of cyclic voltammetryand amperometry to study the capability of ChOx to interact with H2O2, facilitating its electrochemical reduction. When we compared the catalytic activity of the two studied platforms, PMWCNT and PMWCNT/ChOx, we found that the reduction reaction current on the PMWCNT/ChOx bio-platform was 21 times greater than that of the PMWCNT. Through electrochemical characterization using cyclic voltammetry, a redox signal was assigned to the FAD/FADH2 of the enzyme ChOx. Under the experimental conditions studied (phosphate buffer at pH 7.4), the conditional redox potential is , similar to the values reported for this redox pair in other oxidoreductase measured under similar conditions [73,74,75].
The found behavior for the ChOx enzyme may also be similar for other enzymes where the FAD coenzyme is labile, such as in the glucose-methanol-choline (GMC) oxidoreductase class; however, if the study is conducted employing oxidoreductases where the FAD is not labile, as in the group of enzymes of the vanillyl alcohol oxidase(VAO) class, it is expected that the results will be different [45].
To understand the electrochemical response observed on the sensing bio-platform, we conducted an in silico study of the interactions with the molecular complex of ChOx. This study utilized molecular dynamics simulations, docking assays, and binding energy calculations. Through the applied methodology, we elucidated the structure of the active site of ChOx and the protein residues involved (Asn119, Gly120, Met122, Lys225, Pro344, Glu361, Leu377, His447, Asn485, Pro486, and FAD).
According to the literature [78,79,80,81,82], the catalytic roles have been associated with Glu361, His447, and Asn485, and have been studied extensively. Figure 6, presents the ChOx structure, and the residues involved in the interaction with H2O2. The applied methodology enables us to determine the interaction energy between hydrogen peroxide and the active site of ChOx, highlighting the electrostatic interactions and the formation of hydrogen bonds with Glu361, His 447, ASN 485, and hydrophobic interactions with FAD rings.
The computational study yields an interaction energy (ΔGba = −1.5 kJ/mol) between the ChOx and H2O2 complex.
The value of the energy obtained from the calculation is a labile type that allows for the desorption of the product, releasing the active site to continue with a new ChOx-H2O2 interaction cycle. The weak interactions between ChOx-H2O2 explain the increase in the reduction current recorded in amperometric experiments, which is associated with rapid desorption of reaction products, followed by the occupation of the active site by new hydrogen peroxide molecules to continue reacting at this catalytic site.
These results obtained from the electrochemical determination of H2O2 were compared with other measurement systems for hydrogen peroxide reduction, as shown in Table 4.

Table 4.
Comparison of the performance of the hydrogen peroxide biosensors based on HRP.
The data reported in the literature regarding the reduction of H2O2 using HRP-based biosensors combined with other materials present complex architectures. Despite some biosensor platforms showed lower detection limits and narrower linear ranges than those obtained for the sensor in this work, PMWCNT/ChOx is a viable alternative to quantify H2O2 with potential applications in textile, pharmaceutical, and food industrial samples.
5. Conclusions
In this work, a sensing assembly PMWCNT/ChOx, composed of multi-walled carbon nanotubes and Cholesterol oxidase, is utilized for the H2O2 electrochemical reduction. Compared to the sensing assembly without ChOx, the current signal was enhanced 21 times when comparing it with the sensing assembly of PMWCNT without ChOx, and the improvement in the current has been associated with the molecular ChOx–H2O2 complex interactions. The sensitivity value for H2O2 at the PMWCNT/ChOx platform was 26.15 ± 0.49 µA/mM, and the LOD was 0.43 µM.
The interaction between ChOx and H2O2 was investigated through in silico studies. Molecular dynamics simulations, docking assays, and binding energy calculations revealed a ΔGba value of −1.5 kJ/mol. This finding helps to clarify the driving forces behind the interaction of the ChOx-H2O2 complex, which enhances the reduction current of H2O2. Furthermore, our results confirm that ChOx plays an important role in the obtained amperometric response, which is observed by the increments in the reduction current and, ultimately, the improved sensitivity for H2O2 detection. The ChOx enzyme is an alternative to enzyme HRP, or heme prosthetic groups, in the construction of biosensors for the electrochemical detection of H2O2 in potential applications, the biomedical, alimentary, and environmental industries [90,91,92,93]. However, additional studies are necessary before applying the PMWCNT/ChOx biosensor for the determination and quantification of H2O2 in real samples, as these matrices may contain other reactive species that could influence the biosensor’s performance.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/bios15050279/s1, Figure S1. (A) Cyclic voltammograms in PB at pH 7.4 in a potential window of −0.80 to 0.2 V, at different scan rates: 0.01 to 0.50 V/s. (B) Peak current vs. scan rate (i vs. v). Figure S2. Schematic representation of the interaction of the FAD cofactor and H2O2 to cluster representative structure of ChOx: (A) Interaction diagram in 2D, showing hydrogen bonds (green color, dotted line) determined by LigPlot+ program [94]. (B) Interaction map in 3D, showing hydrogen bonds (green color line) determined by PyMOL 2.4 program [95]. HIS 447 changed to HSD 447 for the proton at Nδ on its side chain by PDB2PQR web server [96].
Author Contributions
E.O.-S.: conceptualization, data curation and processing, visualization, writing—original draft, writing—review and editing. G.V.-R.: visualization, writing—original draft, writing—review and editing. I.N.S.: Theoretical calculations, writing—original draft. C.M.-P.: Theoretical calculations, writing—original draft. M.L.L.-C.: conceptualization, writing—original draft. L.G.: conceptualization, writing, review and editing, funding acquisition, project administration, supervision. G.A.R.: writing—review and editing. P.D.: writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
E.O.-S. acknowledges CONAHCYT-Mexico (SECIHTI) for scholarship (765127).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhang, L.; Wang, Y.; Wang, Y.; Guo, M.; Li, Z.; Jin, X.; Du, H. Electrochemical H2O2 sensor based on a Au nanoflower-graphene composite for anticancer drug evaluation. Talanta 2023, 261, 124600. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Q.; Zhai, Q.; Dyson, J.; Gong, S.; Zhao, Y.; Ling, Y.; Chandrasekaran, R.; Dong, D.; Cheng, W. Real-Time and In-Situ Monitoring od H2O2 Release from Living Cells by a Stetchable Electrochemical Biosensor Based on Vertically Aligned Gold Nanowires. Anal. Chem. 2019, 91, 13521–13527. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, H.; Maleki, F.; Khaaki, P.; Kadhom, M.; Kudaibergenov, N.; Khataee, A. Elecrochemical-Based Sensing Platforms for Detection of Glucose and H2O2 by Porous Metal–Organic Frameworks: A Review of Status and Prospects. Biosensors 2023, 13, 347. [Google Scholar] [CrossRef] [PubMed]
- Fatima, B.; Hussain, D.; Bashir, S.; Hussain, H.T.; Aslam, R.; Nawaz, R.; Rashid, H.N.; Bashir, N.; Majeed, S.; Ashiq, M.N.; et al. Catalase immobiliszed antimone quantum dots used as an electrochemical biosensor for quantitative determination of H2O2 from CA-125 diagnosed ovarian cancer samples. Mater. Sci. Eng. C 2020, 117, 111296. [Google Scholar] [CrossRef]
- Yagati, A.K.; Choi, J.-W. Protein Based Electrochemical Biosensors for H2O2 Detection Towards Clinical Diagnostics. Electroanalysis 2014, 26, 1259–1276. [Google Scholar] [CrossRef]
- Villamizar, E.N.; Rios, C.A.; Castillo, J.J. A Hydrogen Peroxide Biosensor Based on the Immobilization of the Highly Stable Royal Palm Tree Peroxidase (Roystonea regia) with Chitosan and Glutaraldehyde on Screen-printed Graphene Electrodes. J. Mex. Chem. Soc. 2016, 60, 135–140. [Google Scholar] [CrossRef]
- Vasconcelos, H.; Matias, A.; Mendes, J.; Araújo, J.; Dias, B.; Jorge, P.A.S.; Saraiva, C.; de Almeida, J.M.M.M.; Coelho, L.C.C. Compact biosensor system for the quantification of hydrogen peroxide in milk. Talanta 2023, 253, 124062. [Google Scholar] [CrossRef]
- Shi, L.; Liu, X.; Niu, W.; Li, H.; Han, S.; Chen, J.; Xu, G. Hydrogen peroxide biosensor based on direct electrochemistry of soybean peroxidase immobilized on single walled carbon nanohorn modified electrode. Biosens. Bioelectron. 2009, 24, 1159–1163. [Google Scholar] [CrossRef]
- Alvarez-Paguay, J.; Fernández, L.; Bolaños-Méndez, D.; González, G.; Espinoza-Montero, P.J. Evaluation of an electrochemical biosensor based on carbon nanotubes, hydroxyapatite and and horseradish peroxidase for the detection of hydrogen peroxide. Sens. Bio-Sens. Res. 2022, 37, 100514. [Google Scholar] [CrossRef]
- Tantawi, O.; Baalbaki, A.; El Asmar, R.; Ghauch, A. A rapid and economical method for the quantification of hydrogen peroxyde (H2O2) using a modified HPLC apparatus. Sci. Total Environ. 2019, 654, 107–117. [Google Scholar] [CrossRef]
- Sobhanie, E.; Hosseini, M.; Faridbod, F.; Ganjali, M.R. Sensitive detection of H2O2 released from cancer cells with electrochemiluminescence sensor based on electrochemically prepared polypyrrole@Ce: Dy tungstate7polyluminol. J. Electroanal. Chem. 2021, 932, 117244. [Google Scholar] [CrossRef]
- Gill, T.M.; Zheng, X. Comparing Methods for Electrochemically Accumulated H2O2. Chem. Mater. 2020, 32, 6285–6294. [Google Scholar] [CrossRef]
- Xu, Y.; Jin, X.; Khan, M.A.; Paiva-Santos, A.C.; Makvandi, P. Electroconductive bioplatform based on dextrin for the immobilization of hemoglobin: Application for electrochemical monitoring of H2O2. Environ. Res. 2023, 235, 116700. [Google Scholar] [CrossRef] [PubMed]
- Campus-Arias, L.; del Olmo, R.; Peřinka, N.; Casado, N.; Vilas-Vilela, J.L.; Mecerreyes, D.; del Campo, F.J.; Lanceros-Méndez, S. PEDOT:PSS-based screen-prentable inks for H2O2 electrochemical detection. Electrochim. Acta. 2023, 439, 141615. [Google Scholar] [CrossRef]
- Zhang, T.; Gu, Y.; Li, C.; Yan, X.; Liu, N.; Zhang, Z.; Zhang, H. Fabrication of Novel Electrochemical Biosensor Based on Graphene Nanohybrid to Detect H2O2 Released from Living Cells with Ultrahigh Performance. ACS Appl. Mater. Interfaces 2017, 9, 37991–37999. [Google Scholar] [CrossRef]
- Cao, Y.; Shi, H.; Zheng, Y.; Tan, Z.; Xie, Z.; Zhang, C.; Chen, Z. Polyaniline/prussian blue nanolayer enhanced electrochemical sensing of H2O2 in EBC using an integrated condensation facemask. Sens. Actuators B Chem. 2023, 393, 134189. [Google Scholar] [CrossRef]
- Harraz, D.M.; Weng, S.; Surendranath, Y. Electrochemically Quantifying Oxygen Reduction Selectivity in Nonaqueous Electrolytes. ACS Catal. 2023, 13, 1462–1469. [Google Scholar] [CrossRef]
- Teodoro, K.B.R.; Migliorini, F.L.; Christinelli, W.A.; Correa, D.S. Detection of hydrogen peroxide (H2O2) using a colorimetric sensor based on cellulose nanowhiskers and silver nanoparticles. Carbohydr. Polym. 2019, 212, 235–241. [Google Scholar] [CrossRef]
- Liu, X.; Feng, H.; Zhang, J.; Zhao, R.; Liu, X.; Wong, D.K.Y. Hydogen peroxide detection at a horseradish peroxidase biosensor with a Au nanoparticle-dotted titanate nanotubeǀhydrophobic ionic liquid scaffold. Biosens. Bioelectron. 2012, 31, 188–194. [Google Scholar] [CrossRef]
- Klun, U.; Zorko, D.; Stojanov, L.; Mirčeski, V.; Jovanoski, V. Amperometric sensor for gaseous H2O2 based on copper redox mediator incorporated electrolyte. Sens. Actuators Rep. 2023, 5, 100144. [Google Scholar] [CrossRef]
- Yin, H.; Zang, C.; Wang, Z.; Bai, X.; Yang, Z.; Liu, Z. A novel sensor based on P-Ru/NC for sensitive electrochemical detection of H2O2. J. Alloys Compd. 2023, 968, 171949. [Google Scholar] [CrossRef]
- Niu, B.; Wang, H.; Zhang, Y.; Nie, B.; Wang, H.; Lian, X.; Li, W. Green synthesis and characterization of Ag nanoparticles in phytic acid/ascorbic acid/sodium hydroxide system and their application in the lectrochemical detection of H2O2. New J. Chem. 2023, 47, 8797–8808. [Google Scholar] [CrossRef]
- Niyitanga, T.; Ahmad, K.; Chaudhary, A.; Kim, H. Carbon dots as efficient electrode material for hydrogen peroxide sensing applications: A mini review. Inorg. Chem. Commun. 2023, 156, 111249. [Google Scholar] [CrossRef]
- Eguilaz, M.; Venegas, C.J.; Gutiérrez, A.; Rivas, G.A.; Bollo, S. Carbon nanotubes non-covalently functionalized with cytochrome c: A new bioanalized with cytochrome c: A new bioanalytical platform for building bienzymatic biosensors. Microchem. J. 2016, 128, 161–165. [Google Scholar] [CrossRef]
- El-Gohary, A.R.M.; Galal, A.; Atta, N.F. CNTs/Graphene Oxide -Nickel Phosphate Nanocomposite-Based Electrochemical Sensors for Detecting H2O2 in Human Serum. ChemistrySelect 2023, 8, 3202301922. [Google Scholar] [CrossRef]
- Wang, X.; Dong, S.; Wei, H. Recent Advances on Nanozyme-based elecyrochemical Biosensors. Electroanalysis 2023, 35, 38–49. [Google Scholar] [CrossRef]
- Lozano, M.L.; Rodríguez, M.C.; Herrasti, P.; Galicia, L.; Rivas, G.A. Amperometric Response of Hydrogen Peroxide at Carbon Nanotubes Paste Electrode Modified with an Electrogenerated Poly(Fe(III)-5amino-phenantroline). Analytical Applications for Glucose Biosensing. Electroanalysis 2010, 22, 128–134. [Google Scholar] [CrossRef]
- Rivas, G.A.; Rubianes, M.D.; Rodrı, M.C.; Ferreyra, N.F.; Luque, G.L.; Pedano, M.L.; Miscoria, S.A.; Parrado, C. Carbon nanotubes for electrochemical biosensing. Talanta 2007, 74, 291–307. [Google Scholar] [CrossRef]
- Li, X.; Shi, F.; Wang, L.; Zhang, S.; Yan, L.; Zhang, X.; Sun, W. Electrochemical Biosensor Based on Horseradish Peroxidase and Blck Phosphorene Quantum Dot Modified Electrode. Molecules 2023, 28, 6151. [Google Scholar] [CrossRef]
- Lee, M.-J.; Song, J.-A.; Choi, J.-H.; Shin, J.-H.; Myeong, J.-W.; Lee, K.-P.; Kim, T.; Park, K.-E.; Oh, B.-K. Horseradish Peroxidase-Encapsulated Fluorescent Bio-Nanoparticle for Ultra-Sensitive and Easy Detection of Hydrogen Peroxide. Biosensors 2023, 13, 289. [Google Scholar] [CrossRef]
- Shin, J.-H.; Lee, M.-J.; Choi, J.-H.; Song, J.-E.; Kim, T.-H.; Oh, B.-K. Electrochemical H2O2 biosensor based on horseradish peroxidase encapsulated protein nanoparticle with reduced graphene oxide-modified gold electrode. Nano Coverg. 2020, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xue, R.; Guo, H.; Wei, Y.; Yang, W. A facil horseradish peroxidase electrochemical biosensor with surface molecular imprinting based on polyaniline nanotubes. J. Electroanal. Chem. 2018, 817, 184–194. [Google Scholar] [CrossRef]
- Bhapkar, S.; Choudhari, U.; Jadhav, U.; Jagtap, S. Evaluation of soybean peroxidase-Cooper phosphate mediated organic-inorganic hybrid for hydrogen peroxide biosensor application. Sens. Int. 2023, 4, 100242. [Google Scholar] [CrossRef]
- Sheikholeslam, M.; Nanda, P.; Sanati, A.; Pritzker, M.; Chen, P. Direct electrochemistry of hemoglobin/peptide-carbon nanotube modified electrode for hydrogen peroxyde biosensing. Mater. Lett. 2023, 335, 133799. [Google Scholar] [CrossRef]
- Sun, M.; Shen, G.; Bai, Z.; Zhang, H.; Liu, H.; Liang, X. Electrochemical Determination of Hydrogen Peroxide Using a Horseradish Peroxidase (HRP) Modified Gold-Nickel Alloy Nanoparticles Glassy Carbon Electrode (GCE). Anal. Lett. 2021, 54, 2565–2573. [Google Scholar] [CrossRef]
- Ahammad, A.J.S. Hydrogen Peroxide Biosensor Based on Horseradish Peroxide and Hemoglobin. J. Biosens. Bioelectron. 2013, 9, 2–11. [Google Scholar] [CrossRef]
- Juska, V.B.; Pemble, M.E. A Critical Review of Electrochemical Glucose Sensing: Evolution of Biosensor Platforms Based on Advanced Nanosystems. Sensors 2020, 20, 6013. [Google Scholar] [CrossRef]
- Pohanka, M. Glucose electrochemical biosensors: The past and current trends. Int. J. Electrochem. Sci. 2021, 16, 210719. [Google Scholar] [CrossRef]
- Garcia-Morales, R.; Zarate-Romero, A.; Wang, J.; Vazquez-Duhalt, R. Bioengineered Lactate Oxidase Mutants for Enhanced Electrochemical Performance at Acidic pH. ChemElectroChem 2023, 10, e202300296. [Google Scholar] [CrossRef]
- Rathee, K.; Dhull, V.; Dhull, R.; Singh, S. Biosensors based on electrochemical lactate detection: A comprehensive review. Biochem. Biophys. 2016, 5, 33–54. [Google Scholar] [CrossRef]
- Lin, Y.; Yang, L.; Ma, Y.; Ye, J. Construction of minitype glutamate sensor for in vivo monitoring of L-glutamate in plant. Microchem. J. 2023, 188, 108505. [Google Scholar] [CrossRef]
- Windmiller, J.R.; Valdés-Ramírez, G.; Zhuo, N.; Zhuo, M.; Miller, P.R.; Jin, C.; Brozik, S.M.; Polsky, R.; Katz, E.; Narayan, R.; et al. Bicomponent Microneedle Array Biosensor for Minimally-Invasive Glutamate Monitoring. Electroanalysis 2011, 23, 2302–2309. [Google Scholar] [CrossRef]
- Campbell, A.S.; Kim, J.; Wang, J. Wearable electrochemical alcohol biosensors. Curr. Opin. Electrochem. 2018, 10, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, A.M.; Prazeres, D.M.F.; Cabral, J.M.S.; Fonseca, L.P. Ethanol biosensors based on alcohol oxidase. Biosens. Bioelectron. 2005, 21, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.; Kanwar, S.S. Cholesterol Oxidase: Source, Properties and Applications. Insights Enzym. Res. 2017, 1, 1–5. [Google Scholar] [CrossRef]
- Kumari, L.; Kanwar, S.S. Cholesterol Oxidase and Its Applications. J. Adv. Microbiol. 2012, 2, 49–65. [Google Scholar] [CrossRef]
- Vrielink, A.; Ghisla, S. Cholesterol Oxidase: Biochemistry and structural features. FEBS J. 2009, 276, 6826–6843. [Google Scholar] [CrossRef]
- Kakhki, S.; Bersan, M.M.; Shams, E.; Brett, C.M.A. New redox and conducting polymer modified electrodes for cholesterol biosensing. Anal. Methods 2013, 5, 1199. [Google Scholar] [CrossRef]
- CHARMM: Home. Available online: https://www.charmm.org/ (accessed on 15 October 2023).
- Sunhwan, J.; Lim, J.B.; Klauda, J.B.; Im, W. CHARMM-GUI Membrane Builder for Mixed Bilayers and Its Application to Yeast Membranes. Biophys. J. 2009, 97, 50–58. [Google Scholar] [CrossRef]
- Lee, J.M.; Cheng, X.; Swails, J.M.; Yeom, M.S.; Eastman, P.K.; Lemkul, J.A.; Wei, S. CHARMM-GUI Input Generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM Simulations Using the CHARMM36 Additive Force Field. J.Chem. Theory Comput. 2016, 12, 405–413. [Google Scholar] [CrossRef]
- Wu, E.L.; Cheng, X.; Jo, S.; Rui, H.; Song, K.C.; Dávila, E.M.; Qi, Y.; Lee, Y.; Monje-Galvan, V.; Venable, R.M.; et al. CHARMM-GUI Membrane Builder toward Realistic Biological Membrane Simulations. J. Comput. Chem. 2014, 35, 1997–2004. [Google Scholar] [CrossRef] [PubMed]
- Lyubimov, A.Y.; Lario, P.I.; Moustafa, I.; Vrielink, A. Atomic resolution crystallography reveals how changes in pH shape the protein microenvironment. Nat. Chem. Biol. 2006, 2, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A web-based graphical user interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef]
- Bekker, H.; Berendsen, H.; Dijkstra, E.; Achterop, S.; Vondrumen, R.; Vanderspoel, D.; Sijbers, A.; Keegstra, H.; Renardus, M. Gromacs—A Parallel Computer for Moleciular—Dynamics Simulations. In Proceedings of the 4th International Conference on Computational Physics (PC 92), Physics Computing ’92, Prague, Czech Republic, 24–28 August 1992; pp. 252–256. [Google Scholar]
- Spoel, D.V.D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J.C. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef]
- Huang, J.; Rauscher, S.; Nawrocki, G.; Ran, T.; Feig, M.; de Groot, B.L.; Grubmüller, H.; MacKerell, A.D. CHARMM36m: An improved force field for folded and intrinsically disordered proteins. Nat. Methods 2017, 14, 71–73. [Google Scholar] [CrossRef]
- Huang, J.; MacKerell, A.D. CHARMM36 all-atom additive protein force field: Validation based on comparison to NMR data. J. Comput. Chem. 2013, 34, 2135–2145. [Google Scholar] [CrossRef]
- Lario, P.I.; Sampson, N.; Vrielink, A. Sub-Atomic Resolution Crystal Structure of Cholesterol Oxidase: What Atomic Resolution Crystallography Reveals about Enzyme Mechanism and the Role of the FAD Cofactor in Redox Activity. J. Mol. Biol. 2003, 326, 1635–1650. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Maestro, Schrödinger, LLC. Maestro Schrödinger Release 2023-3; Maestro, Schrödinger, LLC: New York, NY, USA, 2023; Available online: https://www.schrodinger.com/citations/ (accessed on 20 February 2025).
- Baker, N.A.; Sept, D.; Joseph, S.; Holst, M.J.; McCammon, J.A. Electrostatics of nanosystems: Application to microtubules and the ribosome. Proc. Natl. Acad. Sci. USA 2001, 98, 10037–10041. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Sitkoff, D.; Sharp, K.A.; Honig, B. Accurate Calculation of Hydration Free Energies Using Macroscopic Solvent Models. J. Phys. Chem. 1994, 98, 1978–1988. [Google Scholar] [CrossRef]
- Levy, R.M.; Zhang, L.Y.; Gallicchio, E.; Felts, A.K. On the nonpolar hydration free energy of proteins: Surface area and continuum solvent models for the solute-solvent interaction energy. J. Am. Chem. Soc. 2003, 125, 9523–9530. [Google Scholar] [CrossRef]
- Moral-Rodríguez, A.I.; Leyva-Ramos, R.; Ocampo-Pérez, R.; Mendoza-Barron, R.J.; Serratos-Alvarez, I.N.; Salazar-Rabago, J.J. Removal of ronidazole and sulfamethoxazole from water solutions by adsorption on granular activated carbon: Equilibrium and intraparticle diffusion mechanisms. Adsorption 2016, 22, 89–103. [Google Scholar] [CrossRef]
- Bustos-Terrones, V.; Serratos, I.N.; Castañeda-Villa, N.; Escobar, J.O.V.; Romero-Romo, M.A.; Córdoba, G.; Uruchurtu-Chavarín, J.; Menchaca-Campos, C.; Schulz, J.M.E.; Ortiz, A.D. Functionalized coatings based on organic polymer matrix against the process of corrosion of mild steel in neutral medium. Prog. Org. Coat. 2018, 119, 221–229. [Google Scholar] [CrossRef]
- Bustos-Terrones, V.; Serratos, I.N.; Vargas, R.; Landeros-Rivera, B.C.; Bustos-Terrones, Y.A.; Estrada, S.A.M.; Escobar, J.O.V.; Romo, M.A.R.; Uruchurtu, J.; Menchaca, C.; et al. SBA15-Fluconazole as a Protective Approach Against Mild Steel Corrosion: Synthesis, Characterization, and Computational Studies. ChemistryOpen 2018, 7, 984–994. [Google Scholar] [CrossRef]
- Serratos, I.N.; Luviano, A.S.; Millan-Pacheco, C.; Morales-Corona, J.; Muñoz, E.J.A.; Campos-Terán, J.; Olayo, R. Quartz Crystal Microbalance Application and In Silico Studies to Characterize the Interaction of Bovine Serum Albumin with Plasma Polymerized Pyrrole Surfaces: Implications for the Development of Biomaterials. Langmuir 2023, 39, 1123–11223. [Google Scholar] [CrossRef]
- Shangguan, X.; Zhang, H.; Zheng, J. Direct electrochemistry of glucose oxidase based on its direct immobilization on carbon ionic liquid electrode and glucose sensing. Electrochem. Commun. 2008, 10, 1140–1143. [Google Scholar] [CrossRef]
- Bartlett, P.N.; Al-Lolage, F.A. There is no evidence to support literature claims of direct electron transfer (DET) for native glucose oxidase (GOx) at carbon nanotubes or graphene. J. Electroanal. Chem. 2018, 819, 26–37. [Google Scholar] [CrossRef]
- Wang, G.; Thai, N.M.; Yau, S.T. Preserved enzymatic activity of glucose oxidase immobilized on an unmodified electrode. Electrochem. Commun. 2006, 6, 987–992. [Google Scholar] [CrossRef]
- Alves, T.S.; Santos, J.S.; Fiorucci, A.R.; Arruda, G.J. A new simple electrochemical method for the determination of Bisphenol A using bentonite as modifier. Mater. Sci. Eng. C 2019, 105, 110048. [Google Scholar] [CrossRef]
- Jia, D.; Yang, T.; Wang, K.; Zhou, L.; Wang, E.; Chou, K.; Wang, H.; Hou, X. Facile in-situ synthesis of Ti3C2T /TiO2 nanowires toward simultaneous determination of ascorbic acid, dopamine and uric acid. J. Alloys Compd. 2024, 985, 173392. [Google Scholar] [CrossRef]
- Sampson, N.S.; Kass, I.J. Isomerization, but not oxidation, is suppressed by a single point mutation, E361Q, in the reaction catalyzed by cholesterol oxidase. J. Am. Chem. Soc. 1997, 119, 855–862. [Google Scholar] [CrossRef]
- Kass, I.J.; Sampson, N.S. The importance of Glu361 position in the reaction catalyzed by cholesterol oxidase. Bioorg. Med. Chem. Lett. 1998, 8, 2663–2668. [Google Scholar] [CrossRef]
- Kass, I.J.; Sampson, N.S. Evaluation of the role of His447 in the reaction catalyzed by cholesterol oxidase. Biochemistry 1998, 37, 17990–18000. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, P.; Anderson, R.G.; Sampson, N.S. Construction of a catalytically inactive cholesterol oxidase mutant: Investigation of the interplay between active site-residues glutamate 361 and histidine 447. Arch. Biochem. Biophys. 2002, 402, 235–242. [Google Scholar] [CrossRef]
- Yin, Y.; Sampson, N.S.; Vrielink, A.; Lario, P.I. The presence of a hydrogen bond between asparagine 485 and the π system of FAD modulates the redox potential in the reaction catalyzed by cholesterol oxidase. Biochemistry 2001, 40, 13779–13787. [Google Scholar] [CrossRef]
- Chen, L.; Lyubimov, A.Y.; Brammer, L.; Vrielink, A.; Sampson, N.S. The binding and release of oxygen and hydrogen peroxide are directed by a hydrophobic tunnel in cholesterol oxidase. Biochemistry 2008, 47, 5368–5377. [Google Scholar] [CrossRef]
- Yu, L.J.; Golden, E.; Chen, N. Computational insights for the hydride transfer and distinctive roles of key residues in cholesterol oxidase. Sci. Rep. 2017, 7, 17265. [Google Scholar] [CrossRef]
- Arreola, C.M.R.; Villalobos, A.; Garza, G.; Serratos, I.N. A Putative New Role of Tv-PSP1 Recognizes IRE and ERE Hairpin Structures from Trichomonas vaginalis. Pathogens 2023, 12, 79. [Google Scholar] [CrossRef] [PubMed]
- Thenmozhi, K.; Narayanan, S.S. Horseradish peroxidase and toluidine blue covalently immobilized leak-free sol-gel composite biosensor for hydrogen peroxide. Mater. Sci. Eng. C 2017, 70, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Lu, W.; Zuo, X.; Zhu, Q.; Pan, C.; Niu, X.; Liu, J.; Chen, H.; Chen, X. A novel biosensor based on boronic acid functionalized metal-organic frameworks for the determination of hydrogen peroxide released from living cells. Biosens. Bioelectron. 2017, 95, 131–137. [Google Scholar] [CrossRef]
- Ren, Q.Q.; Wu, J.; Zhang, W.C.; Wang, C.; Qin, X.; Liu, G.C.; Li, Z.X.; Yu, Y. Real-time in vitro detection of cellular H2O2 under camptothecin stress using horseradish peroxidase, ionic liquid, and carbon nanotube-modified carbon fiber ultramicroelectrode. Sens. Actuators B Chem. 2017, 245, 615–662. [Google Scholar] [CrossRef]
- Song, H.; Ni, Y.; Kokot, S. Investigations of an electrochemical platform based on the layered MoS2–graphene and horseradish peroxidase nanocomposite for direct electrochemistry and electrocatalysis. Biosens. Bioelectron. 2014, 56, 137–143. [Google Scholar] [CrossRef]
- Kacar, C.; Dalkiran, B.; Erden, P.E.; Kilic, E. An amperometric hydrogen peroxide biosensor based on Co3O4 nanoparticles and multiwalled carbon nanotube modified glassy carbon electrode. Appl. Surf. Sci. 2014, 311, 139–146. [Google Scholar] [CrossRef]
- Yin, H.; Ai, S.; Shi, W.; Zhu, L. A novel hydrogen peroxide biosensor based on horseradish peroxidase immobilized on gold nanoparticles–silk fibroin modified glassy carbon electrode and direct electrochemistry of horseradish peroxidase. Sens. Actuators B Chem. 2009, 137, 747–753. [Google Scholar] [CrossRef]
- Camacho, C.; Chico, B.; Cao, R.; Matias, J.C.; Hernández, J.; Palchetti, I.; Simpson, B.K.; Palchetti, I.; Mascini, M.; Villalonga, R. Novel enzyme biosensor for hydrogen peroxide via Supramolecular associtions. Biocena Bioeleewen 2009, 24, 2028–2033. [Google Scholar] [CrossRef]
- Yu, Z.; Li, H.; Zhang, X.; Liu, N.; Zhang, X. NiO/graphene nanocomposite for determination of H2O2 with a low detection limit. Talanta 2015, 144, 1–5. [Google Scholar] [CrossRef]
- Wang, L.; Wang, E. A novel hydrogen peroxide sensor based on horseradish peroxidase immobilized on colloidal Au modified ITO electrode. Electrochem. Commun. 2004, 6, 225–229. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple ligand-protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef] [PubMed]
- Schrodinger, LLC. The PyMOL Molecular Graphics System, version 1.3r1; Schrodinger, LLC: New York, NY, USA, 2010. Available online: https://www.pymol.org (accessed on 7 April 2025).
- Jurrus, E.; Engel, D.; Star, K.; Monson, K.; Brandi, J.; Felberg, L.E.; Brookes, D.H.; Wilson, L.; Chen, J.; Liles, K.; et al. Improvements to the APBS biomolecular solvation software suite. Protein Sci. 2018, 27, 112–128. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).