Design and In Vivo Evaluation of an Intraocular Electrode for Ciliary Muscle Biopotential Measurement in a Non-Human Primate Model of Human Accommodation
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrode Design
2.2. Manufacturing—Laser Cutting
2.2.1. Manufacturing—Electrode Surface Wiring Integration
2.2.2. Manufacturing—Electrode Surface Coating
2.3. Testing—Estimated Post-Implantation Stability Through Accelerated Aging
2.3.1. Testing—Assessing Long-Term Electrical Stability via Electrochemical Impedance Spectroscopy
2.3.2. Testing—In Vivo Experiment
3. Results and Discussion
3.1. Electrode Fabrication
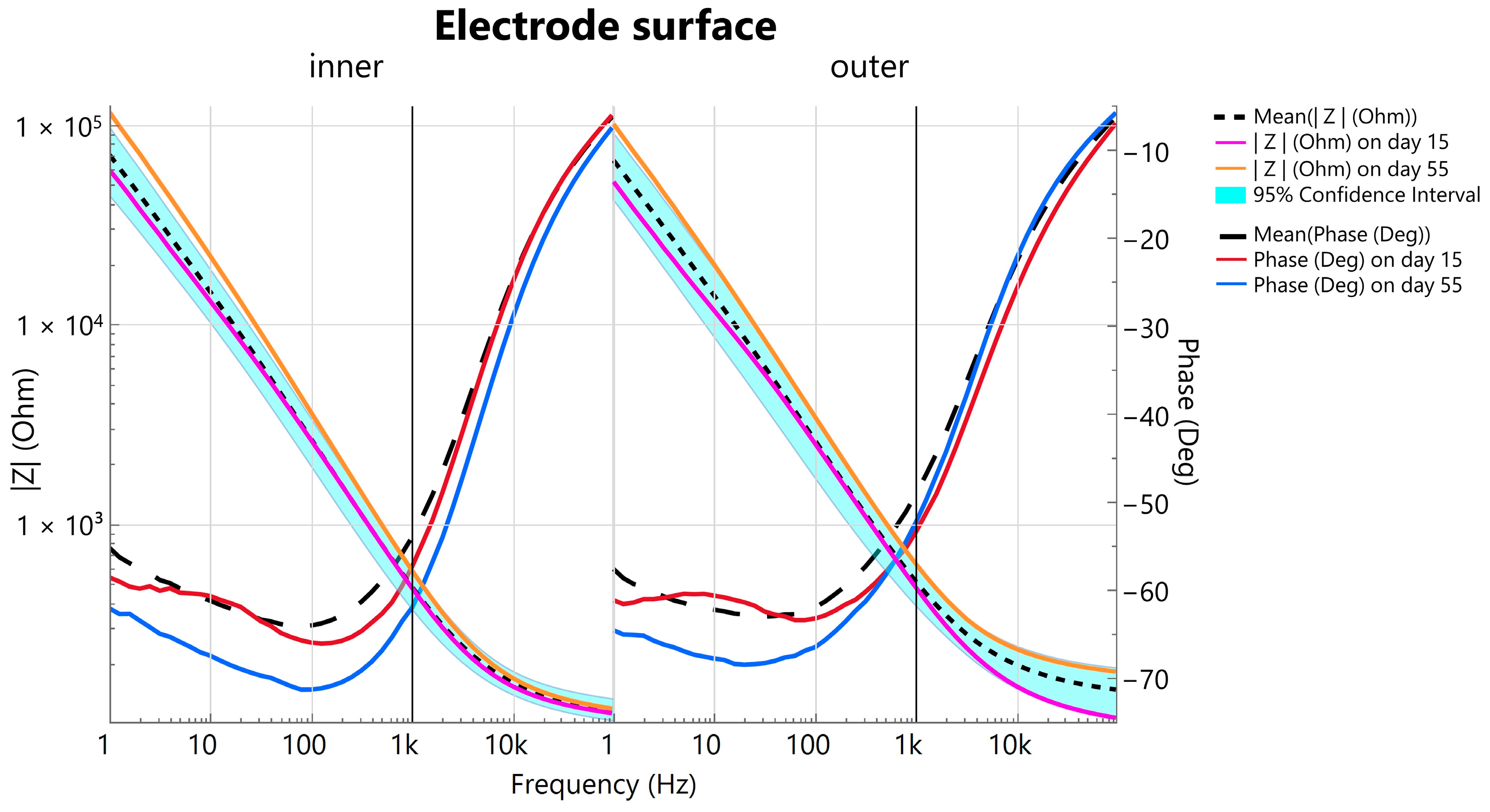
3.1.1. Long-Term Stability During Accelerated Aging
3.1.2. Histology and In Vivo Recording
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ECG | electrocardiogram |
| VEP | visually evoked potentials |
| ERG | electroretinogram |
| EOG | electrooculogram |
| EMG | electromyography |
| ECoG | electrocorticography |
| PET | polyethylenterephthalat |
| CTR | capsular tension ring |
| PFA | perfluoroalkoxy alkanes |
| IPA | isopropyl alcohol |
| Ti | titanium |
| Au | gold |
| Ar | argon |
| PVD | physical vapor deposition |
| PBS | phosphate-buffered saline |
| EIS | electrochemical impedance spectroscopy |
| CVD | chemical vapor deposition |
| SNDR | signal-to-noise-and-distortion ratio |
| SNR | signal-to-noise ratio |
| PMMA | polymethylmethacrylate |
| SEM | scanning electron microscope |
| CAD | computer-aided design |
| IOL | Intraocular lens |
| UGH | uveitis-glaucoma-hyphema |
Appendix A
| Day | ||||||||
| Electrode | 1 | 7 | 15 | 22 | 29 | 39 | 48 | 55 |
| #1 | 0.0150 g | 0.0149 g | 0.0149 g | 0.0134 × g | 0.0135 g | 0.0134 g | 0.0134 g | 0.0134 g |
| #2 | 0.0146 g | 0.0146 g | 0.0146 g | 0.0144 g | 0.0145 g | 0.0144 g | 0.0144 g | 0.0144 g |
| #3 | 0.0169 g | 0.0169 g | 0.0169 g | 0.0168 g | 0.0168 g | 0.0169 g | 0.0168 g | 0.0168 g |
| #4 | 0.0151 g | 0.0149 g | 0.0148 g | 0.0146 g | 0.0145 g | 0.0145 g | - | - |
| The change in weight in grams during the accelerated aging of each electrode, illustrated in tabular form. (×) A small piece of the cable was torn off when electrode #1 was improperly removed from the EIS measurement setup (day 22). Electrode #4 broke on a spoke on day 48 of accelerated aging testing and was not further considered for analysis. | ||||||||
References
- AlGhatrif, M.; Lindsay, J. A Brief Review: History to Understand Fundamentals of Electrocardiography. J. Community Hosp. Intern. Med. Perspect. 2012, 2, 14383. [Google Scholar] [CrossRef]
- Creel, D.J. Visually Evoked Potentials. Available online: http://webvision.med.utah.edu/book/electrophysiology/visually-evoked-potentials/ (accessed on 1 September 2015).
- Robson, A.G.; Nilsson, J.; Li, S.; Jalali, S.; Fulton, A.B.; Tormene, A.P.; Holder, G.E.; Brodie, S.E. ISCEV Guide to Visual Electrodiagnostic Procedures. Doc. Ophthalmol. 2018, 136, 1–26. [Google Scholar] [CrossRef]
- Swegmark, G.; Olsson, T. Impedance Cyclography a New Method for Accommodation Recording. Acta Ophthalmol. 1968, 46, 946–968. [Google Scholar] [CrossRef] [PubMed]
- Bornschein, H.; Schubert, G. Bestandpotential Und Akkommodationszustand Des Menschlichen Auges. Albrecht Von Graefes Arch. Ophthalmol. 1957, 159, 45–51. [Google Scholar] [CrossRef]
- Schubert, G. Aktionspotentiale Des M. Ciliaris Beim Menschen. Albrecht Von Graefes Arch. Ophthalmol. Ver. Arch. Augenheilkd. 1955, 157, 116–121. [Google Scholar] [CrossRef]
- Jacobson, J.H.; Romaine, H.H.; Halberg, G.P.; Stephens, G. The Electric Activity of the Eye During Accommodation. Am. J. Ophthalmol. 1958, 46, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Alpern, M.; Ellen, P.; Goldsmith, R. The Electrical Response of the Human Eye in Far-to-Near Accommodation. AMA Arch. Ophthalmol. 1958, 60, 592–602. [Google Scholar] [CrossRef]
- Hagiwara, H.; Ishikawa, S. The Action Potential of the Ciliary Muscle. Ophthalmologica 1962, 144, 323–340. [Google Scholar] [CrossRef]
- Bishop, S.; Nyboer, J. Electrical Impedance of the Anterior Eye Chamber. Ann. N. Y. Acad. Sci. 1970, 170, 793–800. [Google Scholar] [CrossRef]
- Adel, N.L. Electromyographic and Entopic Studies Suggesting a Theory of Action of the Ciliary Muscle in Accommodation for Near and Its Influence on the Development of Myopia. Optom. Vis. Sci. 1966, 43, 27–38. [Google Scholar] [CrossRef]
- Toates, F.M. Accommodation Function of the Human Eye. Physiol. Rev. 1972, 52, 828–863. [Google Scholar] [CrossRef] [PubMed]
- Ahkami, B.; Ahmed, K.; Thesleff, A.; Hargrove, L.; Ortiz-Catalan, M. Electromyography-Based Control of Lower Limb Prostheses: A Systematic Review. IEEE Trans. Med. Robot. Bionics 2023, 5, 547–562. [Google Scholar] [CrossRef] [PubMed]
- Asghar, A.; Jawaid Khan, S.; Azim, F.; Shakeel, C.S.; Hussain, A.; Niazi, I.K. Review on Electromyography Based Intention for Upper Limb Control Using Pattern Recognition for Human-Machine Interaction. Proc. Inst. Mech. Eng. H 2022, 236, 628–645. [Google Scholar] [CrossRef]
- Chen, Z.; Min, H.; Wang, D.; Xia, Z.; Sun, F.; Fang, B. A Review of Myoelectric Control for Prosthetic Hand Manipulation. Biomimetics 2023, 8, 328. [Google Scholar] [CrossRef]
- Vilela, M.; Hochberg, L.R. Applications of Brain-Computer Interfaces to the Control of Robotic and Prosthetic Arms. In Handbook of Clinical Neurology; Aminoff, M.J., Boller, F., Swaab, D.F., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2020; pp. 87–99. [Google Scholar]
- Kaphle, D.; Schmid, K.L.; Davies, L.N.; Suheimat, M.; Atchison, D.A. Ciliary Muscle Dimension Changes with Accommodation Vary in Myopia and Emmetropia. Investig. Opthalmology Vis. Sci. 2022, 63, 24. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Zrenner, E.; Strasser, T. Emmetropes and Myopes Differ Little in Their Accommodation Dynamics but Strongly in Their Ciliary Muscle Morphology. Vision Res. 2019, 163, 42–51. [Google Scholar] [CrossRef]
- Tabernero, J.; Chirre, E.; Hervella, L.; Prieto, P.; Artal, P. The Accommodative Ciliary Muscle Function Is Preserved in Older Humans. Sci. Rep. 2016, 6, 25551. [Google Scholar] [CrossRef]
- Sheppard, A.L.; Davies, L.N. The Effect of Ageing on In Vivo Human Ciliary Muscle Morphology and Contractility. Investig. Opthalmology Vis. Sci. 2011, 52, 1809. [Google Scholar] [CrossRef]
- Glasser, A.; Campbell, M.C.W. Presbyopia and the Optical Changes in the Human Crystalline Lens with Age. Vision Res. 1998, 38, 209–229. [Google Scholar] [CrossRef]
- Koretz, J.F.; Cook, C.A.; Kaufman, P.L. Accommodation and Presbyopia in the Human Eye: Changes in the Anterior Segment and Crystalline Lens with Focus. Investig. Ophthalmol. Vis. Sci. 1997, 38, 569–578. [Google Scholar]
- Duane, A. Normal Values of the Accommodation at All Ages. J. Am. Med. Assoc. 1912, 59, 1010–1013. [Google Scholar] [CrossRef]
- Ma, X.; Ahadian, S.; Liu, S.; Zhang, J.; Liu, S.; Cao, T.; Lin, W.; Wu, D.; de Barros, N.R.; Zare, M.R.; et al. Smart Contact Lenses for Biosensing Applications. Adv. Intell. Syst. 2021, 3, 2000263. [Google Scholar] [CrossRef]
- Han, F.; Ge, P.; Wang, F.; Yang, Y.; Chen, S.; Kang, J.; Ren, Y.; Liu, H.; Wei, Z.; He, Y.; et al. Smart Contact Lenses: From Rational Design Strategies to Wearable Health Monitoring. Chem. Eng. J. 2024, 497, 154823. [Google Scholar] [CrossRef]
- Stingl, K.; Bartz-Schmidt, K.U.; Besch, D.; Braun, A.; Bruckmann, A.; Gekeler, F.; Greppmaier, U.; Hipp, S.; Hortdorfer, G.; Kernstock, C.; et al. Artificial Vision with Wirelessly Powered Subretinal Electronic Implant Alpha-IMS. Proc. R. Soc. B Biol. Sci. 2013, 280, 20130077. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.G.; Rebscher, S.; Harrison, W.; Sun, X.; Feng, H. Cochlear Implants: System Design, Integration, and Evaluation. IEEE Rev. Biomed. Eng. 2008, 1, 115–142. [Google Scholar] [CrossRef] [PubMed]
- Macherey, O.; Carlyon, R.P. Cochlear Implants. Curr. Biol. 2014, 24, R878–R884. [Google Scholar] [CrossRef]
- Weber, C.H.; Cionni, R.J. All about Capsular Tension Rings. Curr. Opin. Ophthalmol. 2015, 26, 10–15. [Google Scholar] [CrossRef]
- Chen, J.; Lan, L.; Tang, Y.; Lu, Y.; Jiang, Y. Placement of Dual Capsular Tension Rings for the Combined Management of Traumatic Cyclodialysis Cleft and Zonular Dialysis. Eye Vis. 2020, 7, 54. [Google Scholar] [CrossRef]
- Glasser, A.; Kaufman, P.L. The Mechanism of Accommodation in Primates. Ophthalmology 1999, 106, 863–872. [Google Scholar] [CrossRef]
- Törnqvist, G. Accommodation in Monkeys: Some Pharmacological and Physiological Aspects. Acta Ophthalmol. 1967, 45, 429–460. [Google Scholar] [CrossRef]
- Mehdi, M.G.; Alireza, A.-S.; Pourazizi, P.M.; Ghoreishi, M.; Abdi-Shahshahani, M.; Peyman, Á.A.; Pourazizi, Á.M.; Pourazizi, M. A Model for Predicting Sulcus-to-Sulcus Diameter in Posterior Chamber Phakic Intraocular Lens Candidates: Correlation between Ocular Biometric Parameters. Int. Ophthalmol. 2019, 39, 661–666. [Google Scholar] [CrossRef]
- Greenbaum, S.; Lee, P.Y.; Howard-Williams, J.; Podos, S.M. The Optically Determined Corneal and Anterior Chamber Volumes of the Cynomolgus Monkey. Curr. Eye Res. 1985, 4, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Crewther, S.; Brennan Johnson, N.; Vision, J.; Catherine Madigan, M. A Comparison of Ocular Development of the Cynomolgus Monkey and Man. Clin. Vis. Sci. 1987, 1, 269–280. [Google Scholar]
- Simon, N.; Schmid, M.; Blendinger, F.; Bucher, V. Long Term Evaluation of the Barrier Properties of Polymer/Metal Oxide Hybrid Layers for Use in Medical Implants. Curr. Dir. Biomed. Eng. 2022, 8, 435–438. [Google Scholar] [CrossRef]
- Boehler, C.; Carli, S.; Fadiga, L.; Stieglitz, T.; Asplund, M. Tutorial: Guidelines for Standardized Performance Tests for Electrodes Intended for Neural Interfaces and Bioelectronics. Nat. Protoc. 2020, 15, 3557–3578. [Google Scholar] [CrossRef]
- DIN EN ISO 10993-13; Biological Evaluation of Medical Devices-Part 13: Identification and Quantification of Degradation Products from Polymeric Medical Devices. DIN Deutsches Institut für Normung: Berlin, Germany, 2010.
- Hemmerich, K.J. General Aging Theory and Simplified Protocol for Accelerated Aging of Medical Devices. Med. Plast. Biomater. 1998, 5, 16–23. [Google Scholar]
- Oldroyd, P.; Gurke, J.; Malliaras, G.G. Stability of Thin Film Neuromodulation Electrodes under Accelerated Aging Conditions. Adv. Funct. Mater. 2023, 33, 2208881. [Google Scholar] [CrossRef]
- Rubehn, B.; Stieglitz, T. In Vitro Evaluation of the Long-Term Stability of Polyimide as a Material for Neural Implants. Biomaterials 2010, 31, 3449–3458. [Google Scholar] [CrossRef]
- Podrazký, O.; Mrázek, J.; Jasim, A.A.; Proboštová, J.; Vytykáčová, S.; Kašík, I.; Pitrová, Š. Ex-Vivo Measurement of the Ph in Aqueous Humor Samples by a Tapered Fiber-Optic Sensor. Sensors 2021, 21, 5075. [Google Scholar] [CrossRef]
- Shinoda, K.; Yagura, K.; Matsumoto, S.; Terauchi, G.; Mizota, A.; Miyake, Y. Intraocular Temperature at Different Sites in Eye Measured at the Beginning of Vitreous Surgery. J. Clin. Med. 2021, 10, 3412. [Google Scholar] [CrossRef]
- Margo, C.E.; Lee, A. Fixation of Whole Eyes: The Role of Fixative Osmolarity in the Production of Tissue Artifact. Graefe’s Arch. Clin. Exp. Ophthalmol. 1995, 233, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Kaltenstadler, S.; Sigdel, B.; Schumayer, S.; Steinhoff, R.; Straßer, T.; Rothermel, A. An Implantable Ciliary Muscle LFP Recording and Transmitting System. In Proceedings of the 46th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 15–19 July 2024; pp. 1–4. [Google Scholar] [CrossRef]
- Menapace, R.; Findl, O.; Georgopoulos, M.; Rainer, G.; Vass, C.; Schmetterer, K. The Capsular Tension Ring: Designs, Applications, and Techniques. J. Cataract Refract. Surg. 2000, 26, 898–912. [Google Scholar] [CrossRef]
- Koutsonas, A.; Walter, P.; Roessler, G.; Plange, N. Implantation of a Novel Telemetric Intraocular Pressure Sensor in Patients with Glaucoma (ARGOS Study): 1-Year Results. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1063–1069. [Google Scholar] [CrossRef]
- Bernardino, C.R.; Chang, E.L.; Hatton, M.P.; Rubin, P.A.D.; Dohlman, C.H. Glaucoma Drainage Devices: A Systematic Literature Review and Current Controversies. Surv. Ophthalmol. 2005, 50, 411. [Google Scholar] [CrossRef] [PubMed]
- Neuman, M.R. Biopotential Electrodes. In Medical Instrumentation: Application and Design, 4th ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 1998; pp. 189–240. [Google Scholar]
- Lewis, C.M.; Boehler, C.; Liljemalm, R.; Fries, P.; Stieglitz, T.; Asplund, M. Recording Quality Is Systematically Related to Electrode Impedance. Adv. Healthc. Mater. 2024, 13, e2303401. [Google Scholar] [CrossRef] [PubMed]
- Fuglevand, A.J.; Winter, D.A.; Patla, A.E.; Stashuk, D. Detection of Motor Unit Action Potentials with Surface Electrodes: Influence of Electrode Size and Spacing. Biol. Cybern. 1992, 67, 143–153. [Google Scholar] [CrossRef]
- Mehta, R.; Aref, A.A. Intraocular Lens Implantation in the Ciliary Sulcus: Challenges and Risks. Clin. Ophthalmol. 2019, 13, 2317–2323. [Google Scholar] [CrossRef]
- Jaffe, N.S. Polyethylene Terephthalate (Dacron®) in Intraocular Surgery. Ophthalmology 1981, 88, 955–958. [Google Scholar] [CrossRef]
- Hukins, D.W.L.; Mahomed, A.; Kukureka, S.N. Accelerated Aging for Testing Polymeric Biomaterials and Medical Devices. Med. Eng. Phys. 2008, 30, 1270–1274. [Google Scholar] [CrossRef]
- Schiavone, G.; Kang, X.; Fallegger, F.; Gandar, J.; Courtine, G.; Lacour, S.P. Guidelines to Study and Develop Soft Electrode Systems for Neural Stimulation. Neuron 2020, 108, 238–258. [Google Scholar] [CrossRef]
- Chang, D.F.; Masket, S.; Miller, K.M.; Braga-Mele, R.; Little, B.C.; Mamalis, N.; Oetting, T.A.; Packer, M. Complications of Sulcus Placement of Single-Piece Acrylic Intraocular Lenses. Recommendations for Backup IOL Implantation Following Posterior Capsule Rupture. J. Cataract Refract. Surg. 2009, 35, 1445–1458. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schumayer, S.; Zahrani, E.G.; Azarhoushang, B.; Bucher, V.; Straßer, T. Design and In Vivo Evaluation of an Intraocular Electrode for Ciliary Muscle Biopotential Measurement in a Non-Human Primate Model of Human Accommodation. Biosensors 2025, 15, 247. https://doi.org/10.3390/bios15040247
Schumayer S, Zahrani EG, Azarhoushang B, Bucher V, Straßer T. Design and In Vivo Evaluation of an Intraocular Electrode for Ciliary Muscle Biopotential Measurement in a Non-Human Primate Model of Human Accommodation. Biosensors. 2025; 15(4):247. https://doi.org/10.3390/bios15040247
Chicago/Turabian StyleSchumayer, Sven, Esmaeil Ghadiri Zahrani, Bahman Azarhoushang, Volker Bucher, and Torsten Straßer. 2025. "Design and In Vivo Evaluation of an Intraocular Electrode for Ciliary Muscle Biopotential Measurement in a Non-Human Primate Model of Human Accommodation" Biosensors 15, no. 4: 247. https://doi.org/10.3390/bios15040247
APA StyleSchumayer, S., Zahrani, E. G., Azarhoushang, B., Bucher, V., & Straßer, T. (2025). Design and In Vivo Evaluation of an Intraocular Electrode for Ciliary Muscle Biopotential Measurement in a Non-Human Primate Model of Human Accommodation. Biosensors, 15(4), 247. https://doi.org/10.3390/bios15040247






