Ternary-Emission Molecularly Imprinted Ratiometric Fluorescence Sensor and Kit for the Rapid and Visual Detection of Enrofloxacin
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
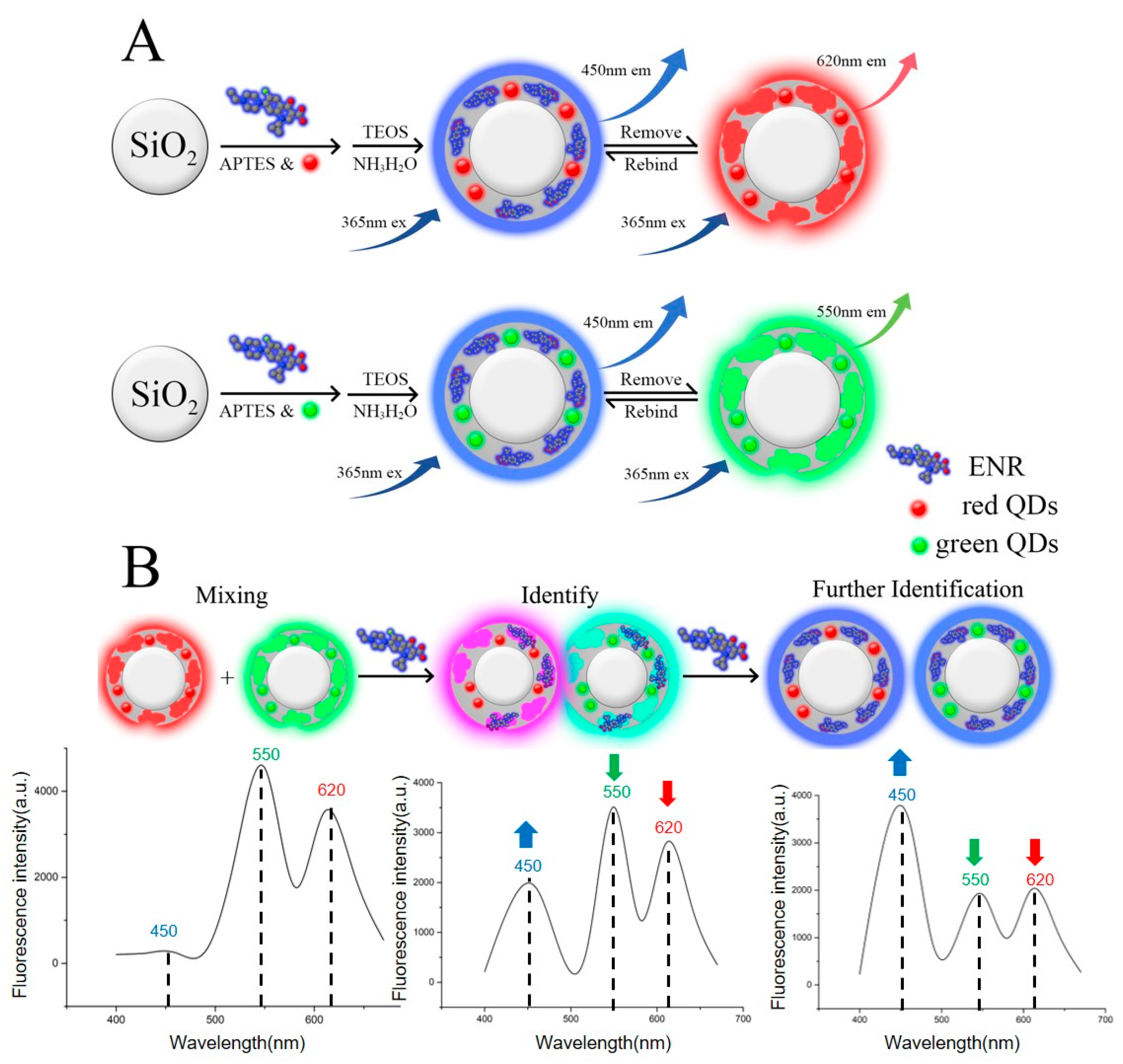
2.2. Preparation of g-MIPs and r-MIPs
2.3. Construction of a Three-Emission MI-RFL Sensor
2.4. Analysis of Real Samples
3. Results and Discussion
3.1. The Construction and Possible Detection Principle of the MI-RFL Sensor
3.2. Characterization of SiO2, MIPs, and NIPs
3.3. Condition Optimization of the MI-RFL Sensor
3.3.1. The pH of the Buffer
3.3.2. Excitation Wavelength
3.3.3. Reaction Time
3.3.4. Mixing Ratio of g- and r-MIPs
3.4. Sensitivity and Selectivity of the MI-RFL Sensor
3.5. Intra- and Inter-Day Variation Study of the MI-RFL Sensor
3.6. Application of the MI-RFL Sensor to Real Samples
3.7. Method Performance Comparison of MI-RFL Sensor
3.8. Analysis of Real Sample by the MI-RFL Sensor Based Kit
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rusu, A.; Hancu, G.; Uivaroşi, V. Fluoroquinolone pollution of food, water and soil, and bacterial resistance. Environ. Chem. Lett. 2015, 13, 21–36. [Google Scholar] [CrossRef]
- Gaber, C.E.; Kinlaw, A.C.; Edwards, J.K.; Lund, J.L. Comparative Effectiveness and Harms of Antibiotics for Outpatient Diverticulitis. Ann. Intern. Med. 2021, 174, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Xi, F. The enrofloxacin pollution control from fish to environment. Mar. Pollut. Bull. 2024, 199, 115923. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Tan, Y.; Xiao, W. Determination of Norfloxacin and Enrofloxacin in milk using deep eutectic solvent-based ferromagnetic fluid by UV-HPLC. J. Dispers. Sci. Technol. 2024, 46, 851–860. [Google Scholar] [CrossRef]
- Cui, Q.; Zhong, Y.; Shang, W.; Deng, F.; Wang, B.; Wu, J.; Wang, P.; Wan, L.; Wang, K.; Fang, L.; et al. Fluorescent Probe Combined with Photoelectric Analysis Technology for Detection of Escherichia coli. Biosensors 2023, 13, 150. [Google Scholar] [CrossRef]
- Chakraborty, S.; Mandal, M.; Rayalu, S. Detection of iron (III) by chemo and fluoro-sensing technology. Inorg. Chem. Commun. 2020, 121, 108189. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, X.; Wang, G.; Zuo, Y. A Rapid Method for Detecting Microplastics Based on Fluorescence Lifetime Imaging Technology (FLIM). Toxics 2022, 10, 118. [Google Scholar] [CrossRef]
- Jia, X.-X.; Li, S.; Han, D.-P.; Chen, R.-p.; Yao, Z.-Y.; Ning, B.-a.; Gao, Z.-X.; Fan, Z.-C. Development and perspectives of rapid detection technology in food and environment. Crit. Rev. Food Sci. Nutr. 2022, 62, 4706–4725. [Google Scholar] [CrossRef]
- Li, X.; Chen, X.; Wu, J.; Liu, Z.; Wang, J.; Song, C.; Zhao, S.; Lei, H.; Sun, Y. Portable, Rapid, and Sensitive Time-Resolved Fluorescence Immunochromatography for On-Site Detection of Dexamethasone in Milk and Pork. Foods 2021, 10, 1339. [Google Scholar] [CrossRef]
- Wen, Y.; Sun, D.; Zhang, Y.; Zhang, Z.; Chen, L.; Li, J. Molecular imprinting-based ratiometric fluorescence sensors for environmental and food analysis. Analyst 2023, 148, 3971–3985. [Google Scholar] [CrossRef]
- Li, J.; Sun, D. Molecularly Imprinted Ratiometric Fluorescence Nanosensors. Langmuir 2022, 38, 13305–13312. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Wen, Y.; Hou, M.; Zhang, Y.; Song, L.; Li, J.; Liu, H. Applications of molecularly imprinted materials for screening and recognition of new pollutants. Chem. Reag. 2023, 45, 1–10. [Google Scholar] [CrossRef]
- Wang, N.; Li, H.; Tian, Y.; Tan, L.; Cheng, S.; Wang, J. Molecularly imprinted ratiometric fluorescence sensor for visual detection of 17β-estradiol in milk: A generalized strategy toward imprinted ratiometric fluorescence construction. Microchim. Acta 2024, 191, 249. [Google Scholar] [CrossRef]
- Wen, Y.; Sun, D.; Fu, X.; Jin, Y.; Yu, J.; Xu, L.; Song, Z.; Chen, L.; Li, J. Molecular Imprinting-Based Ratiometric Fluorescence Nanosensor and Kit for Rapid and Visual Detection of Folic Acid. ACS Appl. Nano Mater. 2024, 7, 9565–9575. [Google Scholar] [CrossRef]
- Yang, Q.; Li, J.; Wang, X.; Xiong, H.; Chen, L. Ternary Emission of a Blue-, Green-, and Red-Based Molecular Imprinting Fluorescence Sensor for the Multiplexed and Visual Detection of Bovine Hemoglobin. Anal. Chem. 2019, 91, 6561–6568. [Google Scholar] [CrossRef]
- Barrera, E.G.; Livotto, P.R.; Santos, J.H.Z.d. Hybrid silica bearing different organosilanes produced by the modified Stöber method. Powder Technol. 2016, 301, 486–492. [Google Scholar] [CrossRef]
- Wang, S.; Yang, G.; Tang, Y.; Wang, Y.; Shen, X.; Si, W.; Yu, H.; Zhai, W.; Fodjo, E.K.; Kong, C. Multi-Residue Screening of Pesticides in Aquatic Products Using High-Performance Liquid Chromatography-Tandem High-Resolution Mass Spectrometry. Foods 2023, 12, 1131. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Y.; Sun, Z.; Cai, T.; Wang, X.; Zhao, S.; Liu, H.; Gong, B. Restricted access media-imprinted nanomaterials based on a metal–organic framework for highly selective extraction of fluoroquinolones in milk and river water. J. Chromatogr. A 2020, 1626, 461364. [Google Scholar] [CrossRef]
- Zu, F.; Yan, F.; Bai, Z.; Xu, J.; Wang, Y.; Huang, Y.; Zhou, X. The quenching of the fluorescence of carbon dots: A review on mechanisms and applications. Microchim. Acta 2017, 184, 1899–1914. [Google Scholar] [CrossRef]
- Yang, Q.; Li, C.; Li, J.; Wang, X.; Arabi, M.; Peng, H.; Xiong, H.; Chen, L. Rational construction of a triple emission molecular imprinting sensor for accurate naked-eye detection of folic acid. Nanoscale 2020, 12, 6529–6536. [Google Scholar] [CrossRef]
- Dadashi-Silab, S.; Doran, S.; Yagci, Y. Photoinduced electron transfer reactions for macromolecular syntheses. Chem. Rev. 2016, 116, 10212–10275. [Google Scholar] [CrossRef] [PubMed]
- Ensafi, A.A.; Nasr-Esfahani, P.; Rezaei, B. Simultaneous detection of folic acid and methotrexate by an optical sensor based on molecularly imprinted polymers on dual-color CdTe quantum dots. Anal. Chim. Acta 2017, 996, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Shao, H.; Xu, H.; Yang, J.; Wang, Y.; Xiong, H.; Wang, P.; Wang, X. Ultrafast synthesis of near-infrared-emitting aqueous CdTe/CdS quantum dots with high fluorescence. Mater. Today Chem. 2021, 20, 100447. [Google Scholar] [CrossRef]
- Su, G.; He, W.; Zhao, M.; Waterhouse, G.I.N.; Sun-Waterhouse, D. Effect of different buffer systems on the xanthine oxidase inhibitory activity of tuna (Katsuwonus pelamis) protein hydrolysate. Food Res. Int. 2018, 105, 556–562. [Google Scholar] [CrossRef]
- Li, Q.; Wang, M.; Jin, Y.; Lu, Y.; Xiong, S.; Wang, M.; Xu, J.; Wei, C.; Li, J. Microfluidic synthesis of pH-responsive molecularly imprinted silica nanospheres for fluorescence sensing target glycoprotein. Food Chem. 2023, 426, 136570. [Google Scholar] [CrossRef]
- Zhang, X.a.; Tang, X.; Yu, J.; Ye, H.; Zhao, L. A novel carbon dots synthesized based on easily accessible biological matrix for the detection of enrofloxacin residues. Microchem. J. 2023, 190, 108690. [Google Scholar] [CrossRef]
- Hamada, H.H.M.; Gamal El-Din, A.A.A.-R.; Samar, H.A.; El-Shimaa, M.N.A. Current Trends and Future Directions of Fluoroquinolones. Curr. Med. Chem. 2019, 26, 3132–3149. [Google Scholar] [CrossRef]
- Liu, X.; Ren, J.; Su, L.; Gao, X.; Tang, Y.; Ma, T.; Zhu, L.; Li, J. Novel hybrid probe based on double recognition of aptamer-molecularly imprinted polymer grafted on upconversion nanoparticles for enrofloxacin sensing. Biosens. Bioelectron. 2017, 87, 203–208. [Google Scholar] [CrossRef]
- Weng, J.; Tian, Y.; Zhang, X.; Chen, F.; Wang, Z.; Sun, M.; He, J. Analysis of trace enrofloxacin in environmental waters by a surface molecular imprinting technique. Langmuir 2024, 40, 23279–23288. [Google Scholar] [CrossRef]
- Ragab, M.T.; Mahmoud, A.M.; Mohamed, H.M. Core-shell molecularly imprinted polymer sensor for enrofloxacin determination in various matrices: A novel, sustainable One Health analytical strategy. Anal. Methods 2024, 16, 6392–6402. [Google Scholar] [CrossRef]
- Tang, Y.; Li, M.; Gao, X.; Liu, X.; Ma, Y.; Li, Y.; Xu, Y.; Li, J. Preconcentration of the antibiotic enrofloxacin using a hollow molecularly imprinted polymer, and its quantitation by HPLC. Microchim. Acta 2016, 183, 589–596. [Google Scholar] [CrossRef]




| Sample | Spiked Concentration (ppm) | Measured Value (ppm) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Seawater | 0 | ND a | – | – |
| 0.25 | 0.290 | 116.0 | 0.91 | |
| 1 | 1.171 | 117.1 | 0.47 | |
| 4 | 3.857 | 96.4 | 1.22 | |
| Bream fish | 0 | ND a | – | – |
| 0.25 | 0.311 | 126.4 | 0.31 | |
| 1 | 1.089 | 108.9 | 0.44 | |
| 4 | 3.773 | 94.3 | 0.71 | |
| Tail fish | 0 | ND a | – | – |
| 0.25 | 0.282 | 112.8 | 2.17 | |
| 1 | 1.021 | 102.1 | 3.80 | |
| 4 | 4.244 | 106.1 | 2.78 | |
| Pomfret fish | 0 | ND a | – | – |
| 0.25 | 0.308 | 123.5 | 0.15 | |
| 1 | 1.213 | 121.3 | 3.97 | |
| 4 | 4.330 | 108.3 | 0.74 |
| Sample | Tagged Value (ppm) | Spiked Concentration (ppm) | MI-RFL Sensor (ppm) | HPLC-UV (ppm) | Measurement Error (%) | RSD (%) |
|---|---|---|---|---|---|---|
| Tail fish | – | 0 | ND a | ND a | – | – |
| 0.25 | 0.282 | 0.303 | 6.93 | 2.18 b | ||
| 1 | 1.081 | 1.160 | 6.81 | 1.19 | ||
| 4 | 4.244 | 4.571 | 7.15 | 1.05 | ||
| Pomfret fish | – | 0 | ND a | ND a | – | – |
| 0.25 | 0.308 | 0.324 | 4.93 | 1.88 | ||
| 1 | 1.213 | 1.179 | 2.88 | 0.51 | ||
| 4 | 4.330 | 4.559 | 5.02 | 0.23 | ||
| Pork | 0.270 | – | 0.253 | – | 6.30 | 3.37 c |
| Chicken | 0.456 | – | 0.510 | – | 11.84 | 0.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Yan, J.; Sun, D.; Peng, S.; Li, J.; Fan, H. Ternary-Emission Molecularly Imprinted Ratiometric Fluorescence Sensor and Kit for the Rapid and Visual Detection of Enrofloxacin. Biosensors 2025, 15, 226. https://doi.org/10.3390/bios15040226
Liu S, Yan J, Sun D, Peng S, Li J, Fan H. Ternary-Emission Molecularly Imprinted Ratiometric Fluorescence Sensor and Kit for the Rapid and Visual Detection of Enrofloxacin. Biosensors. 2025; 15(4):226. https://doi.org/10.3390/bios15040226
Chicago/Turabian StyleLiu, Siwu, Jingyi Yan, Dani Sun, Siyuan Peng, Jinhua Li, and Huaying Fan. 2025. "Ternary-Emission Molecularly Imprinted Ratiometric Fluorescence Sensor and Kit for the Rapid and Visual Detection of Enrofloxacin" Biosensors 15, no. 4: 226. https://doi.org/10.3390/bios15040226
APA StyleLiu, S., Yan, J., Sun, D., Peng, S., Li, J., & Fan, H. (2025). Ternary-Emission Molecularly Imprinted Ratiometric Fluorescence Sensor and Kit for the Rapid and Visual Detection of Enrofloxacin. Biosensors, 15(4), 226. https://doi.org/10.3390/bios15040226






